Recent Progress in the Conversion of Methylfuran into Value-Added Chemicals and Fuels
Abstract
1. Introduction
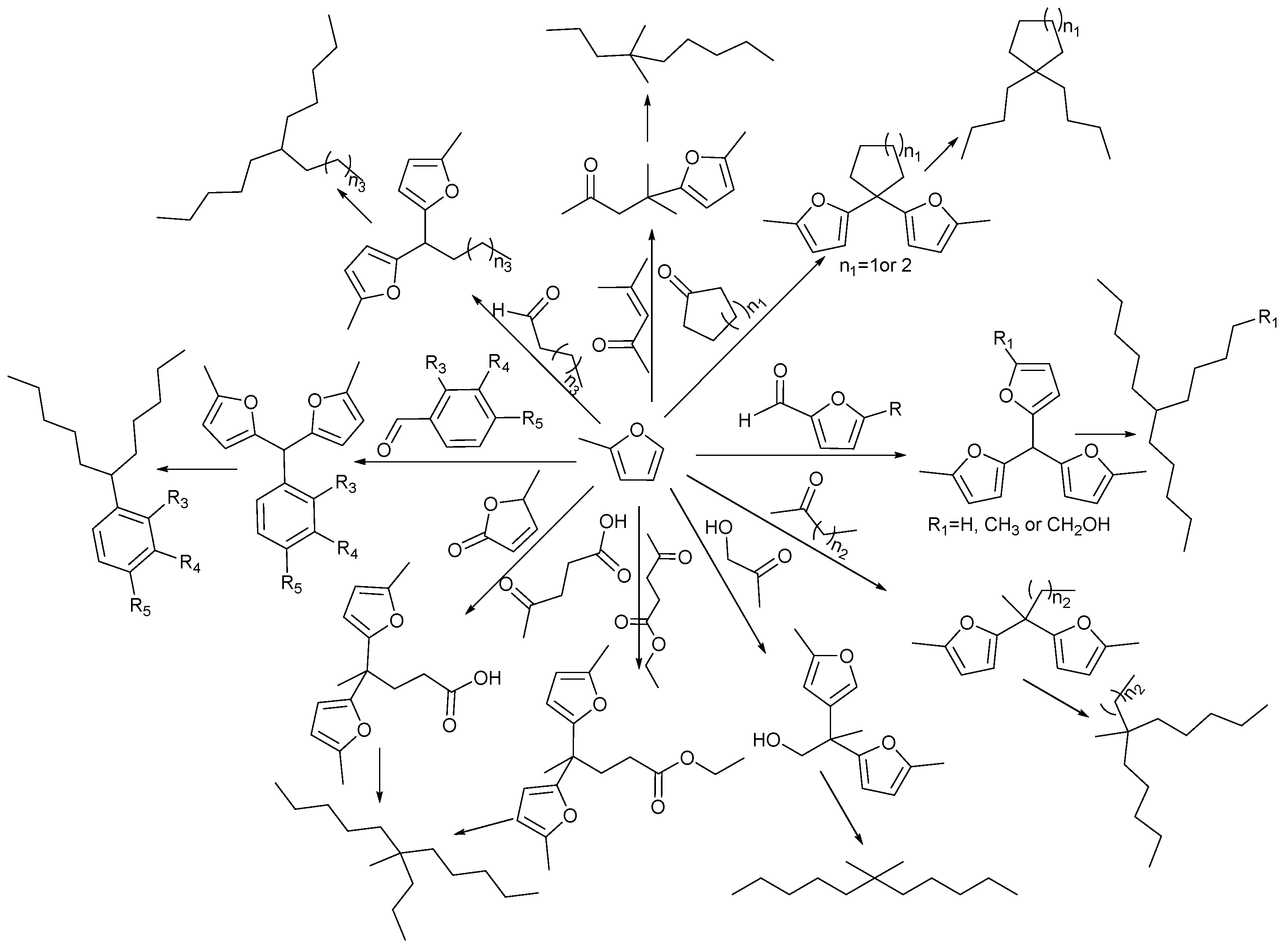
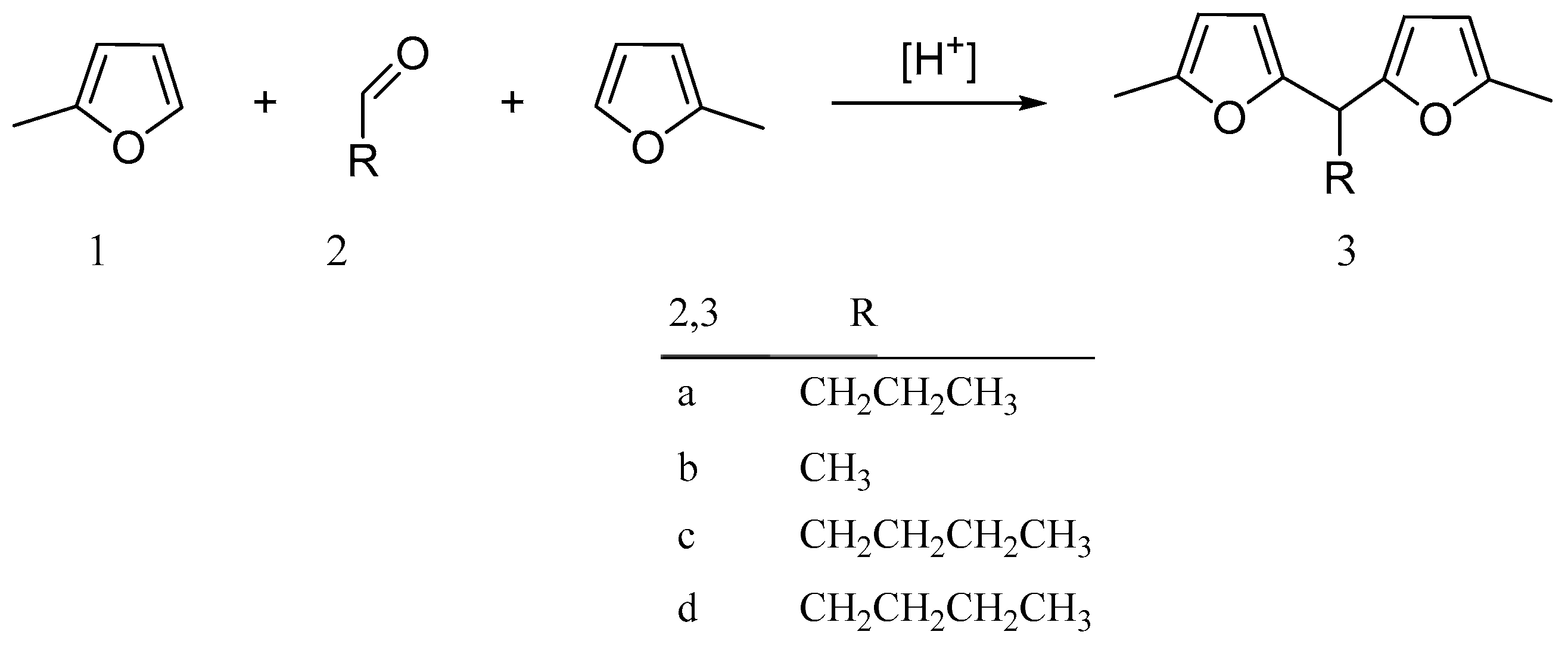
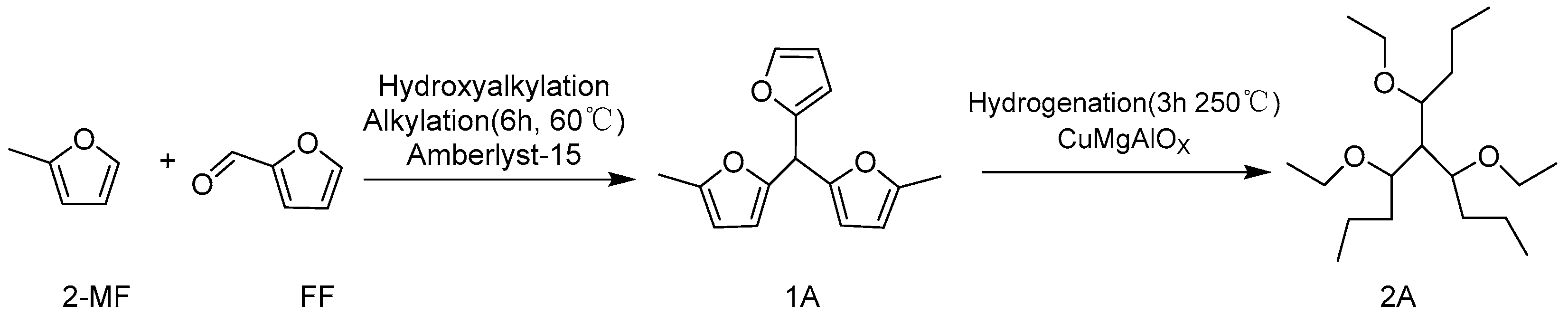
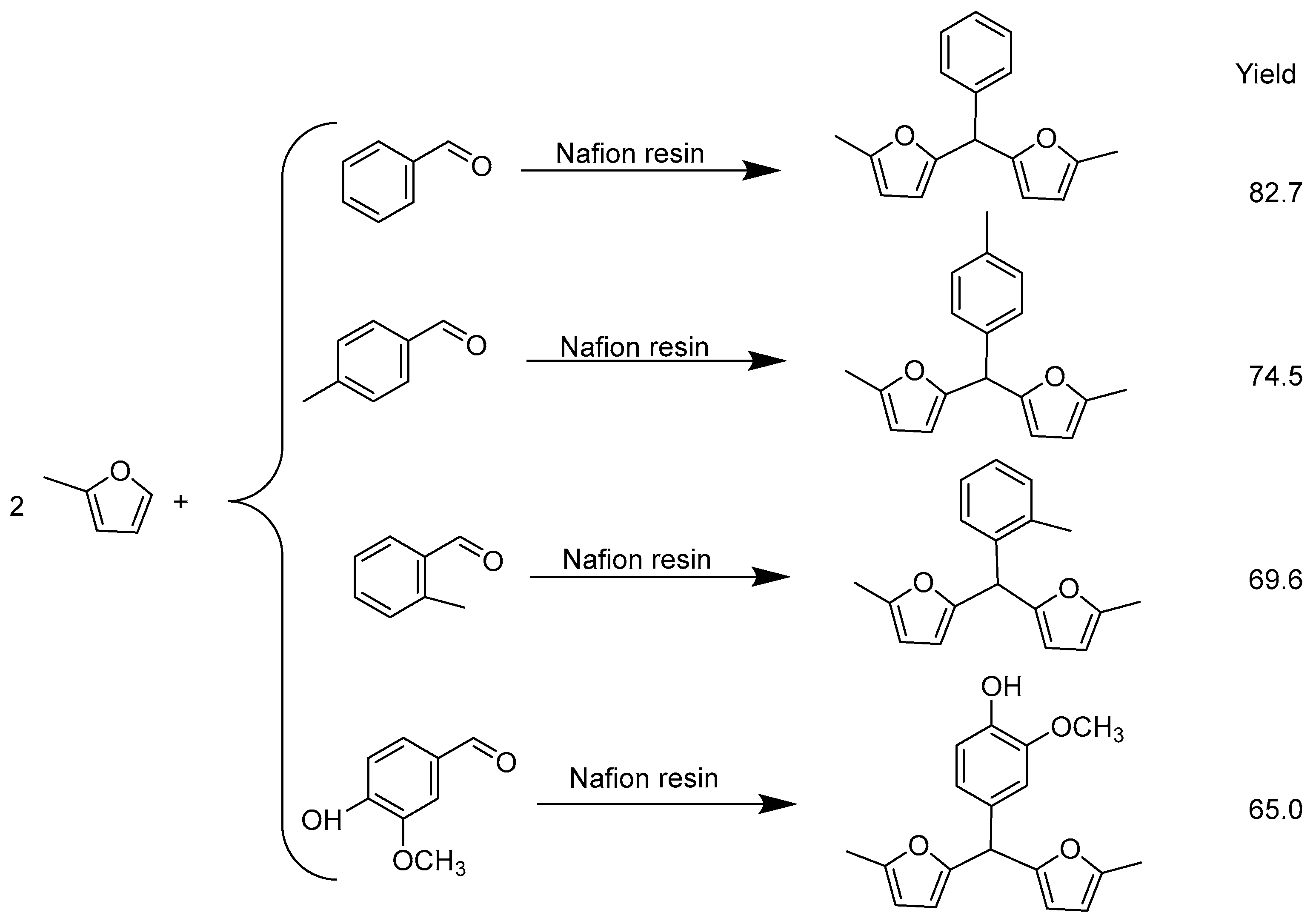
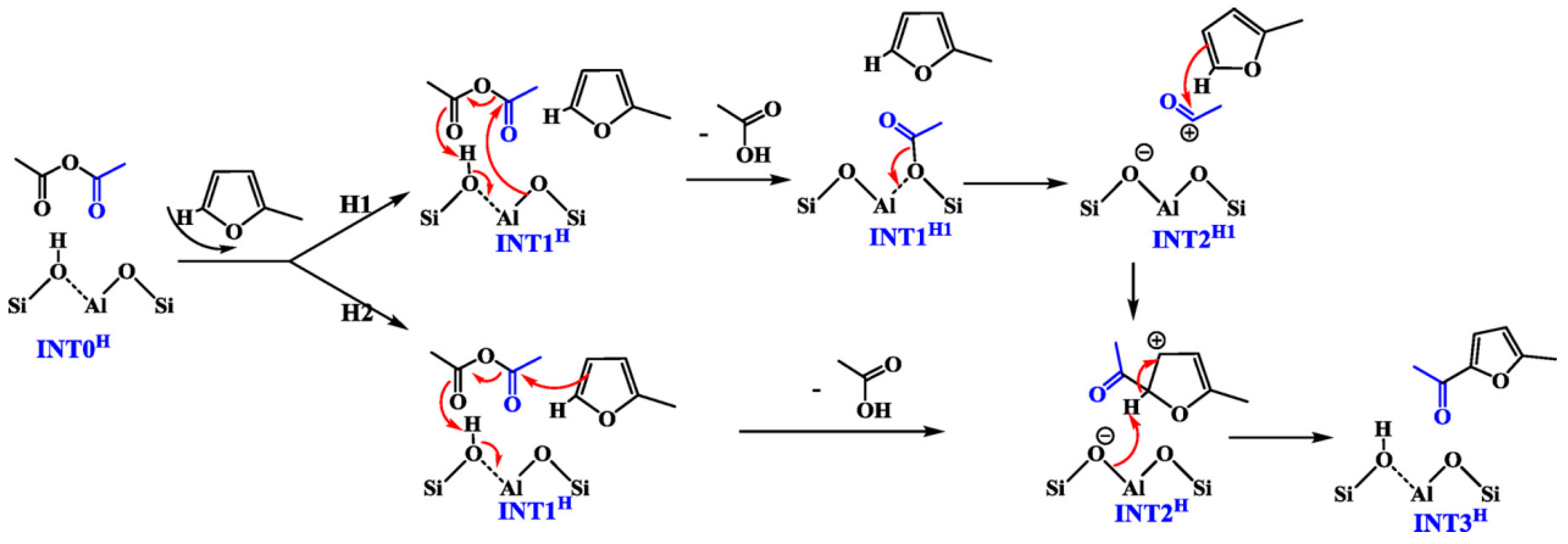
2. Hydroxyalkylation/Alkylation of Methylfuran
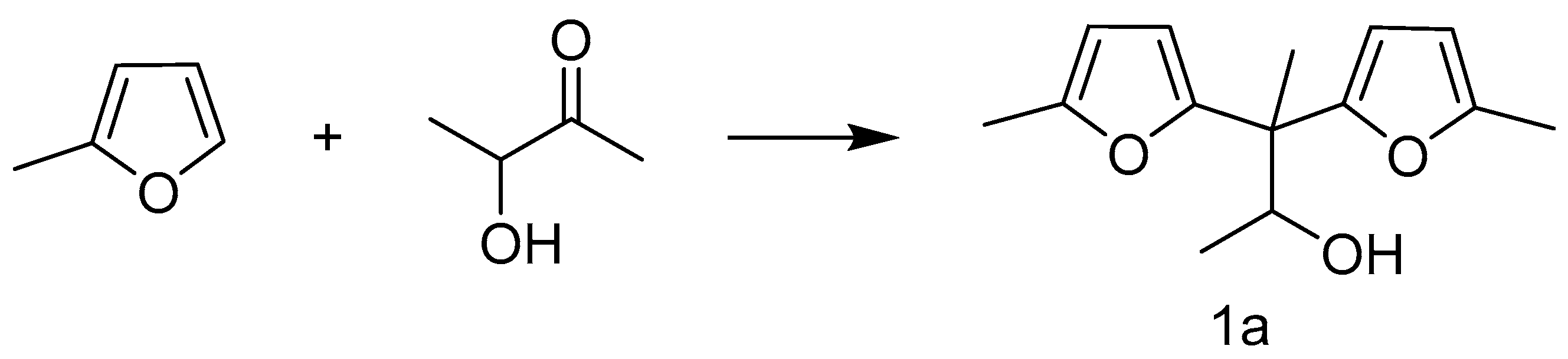
Hydroxylalkylation/Alkylation Reaction of Methylfuran and Cyclopentanone
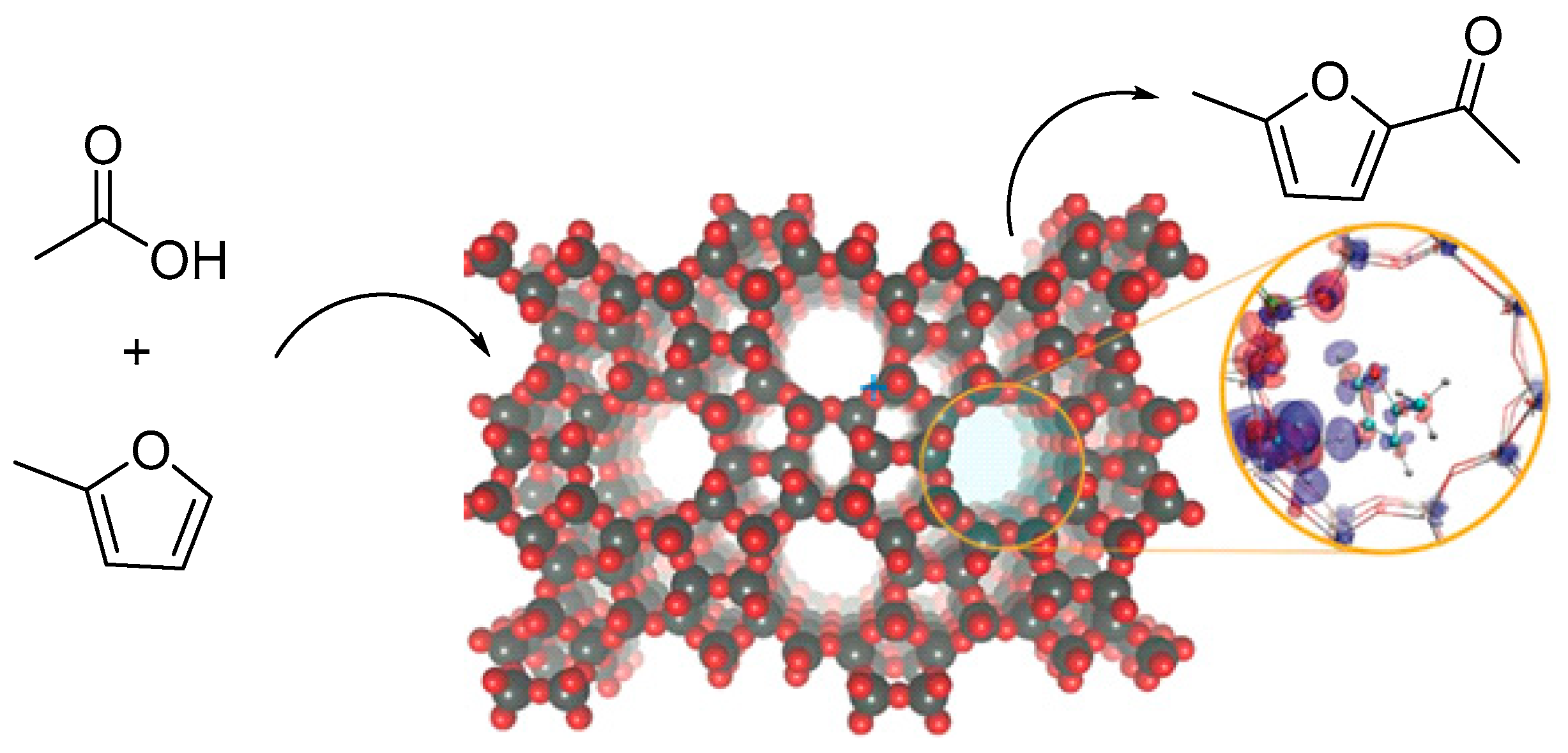
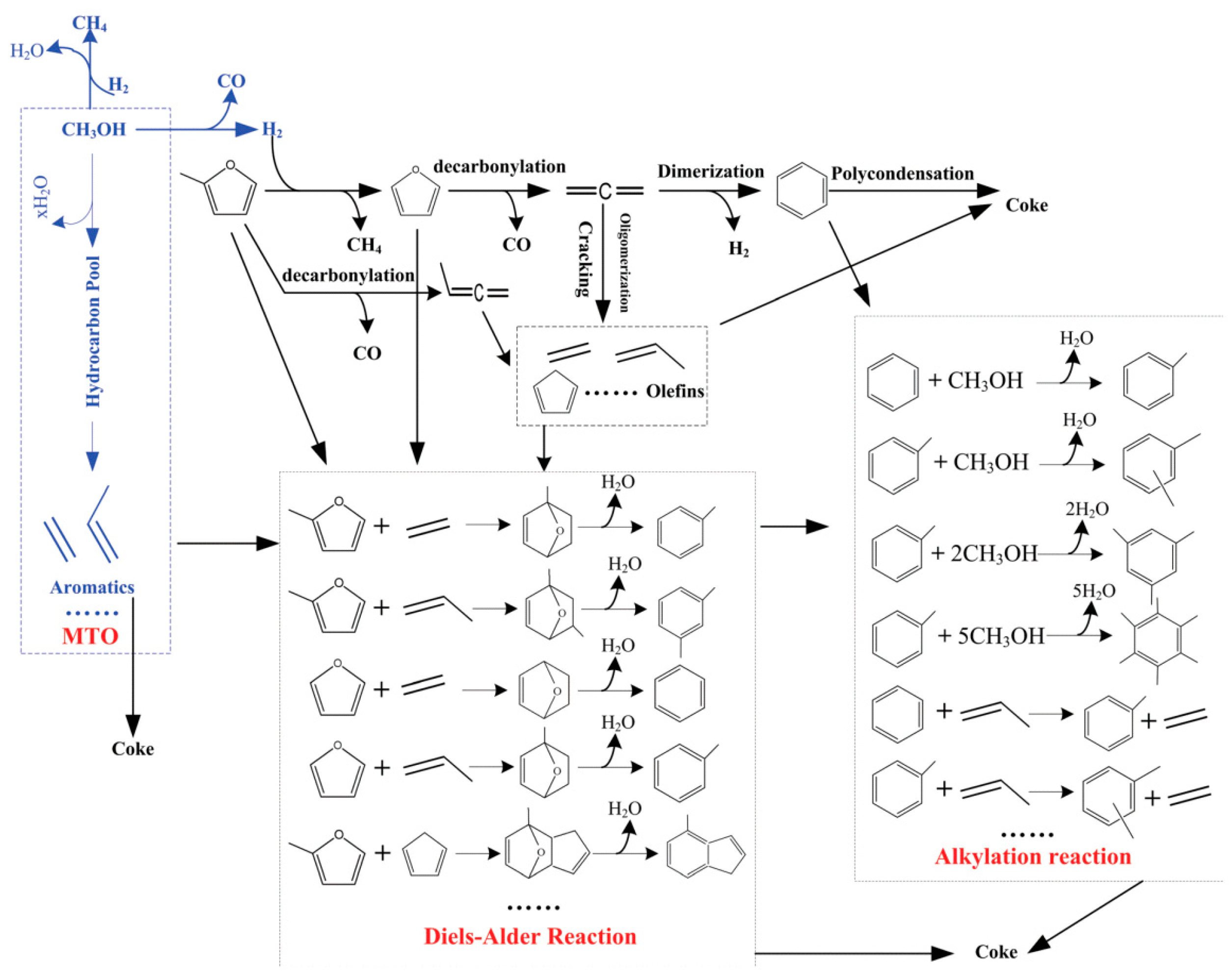
3. Acetylation Reaction of Methylfuran
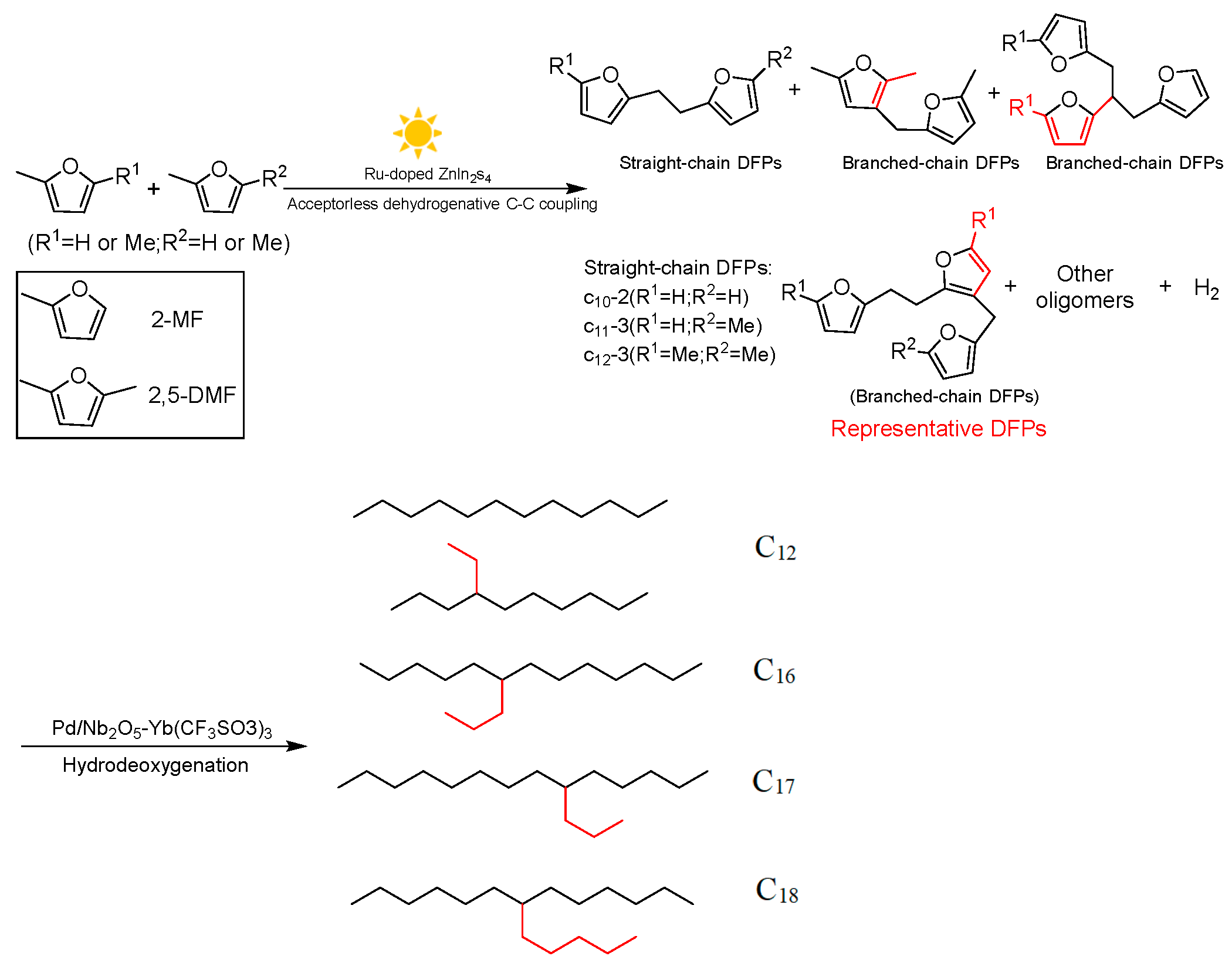
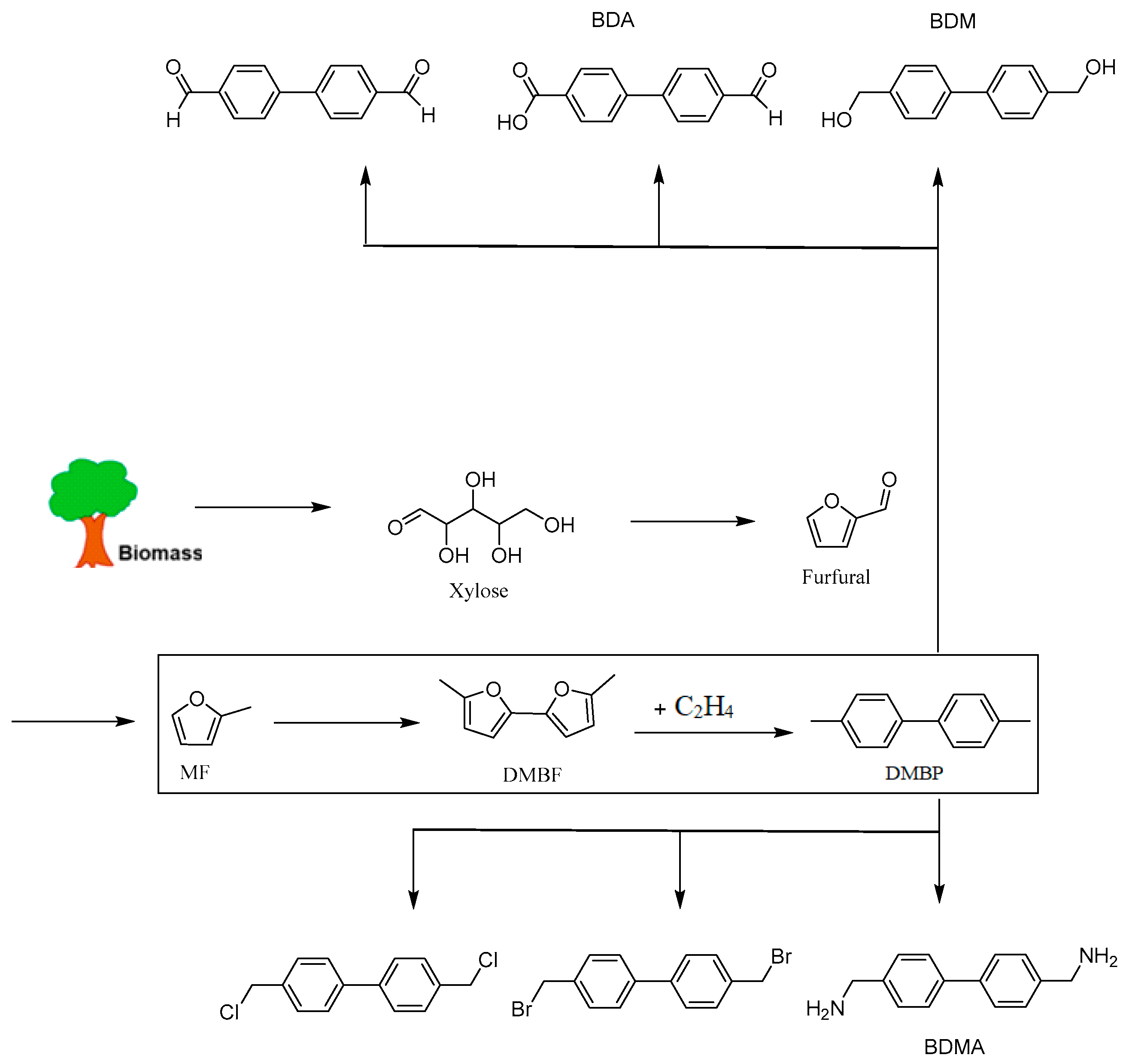
4. Diels–Alder Reaction of Methylfuran and Olefin
4.1. Diels–Alder Reaction of 2-Methylfuran and Ethylene, Propylene
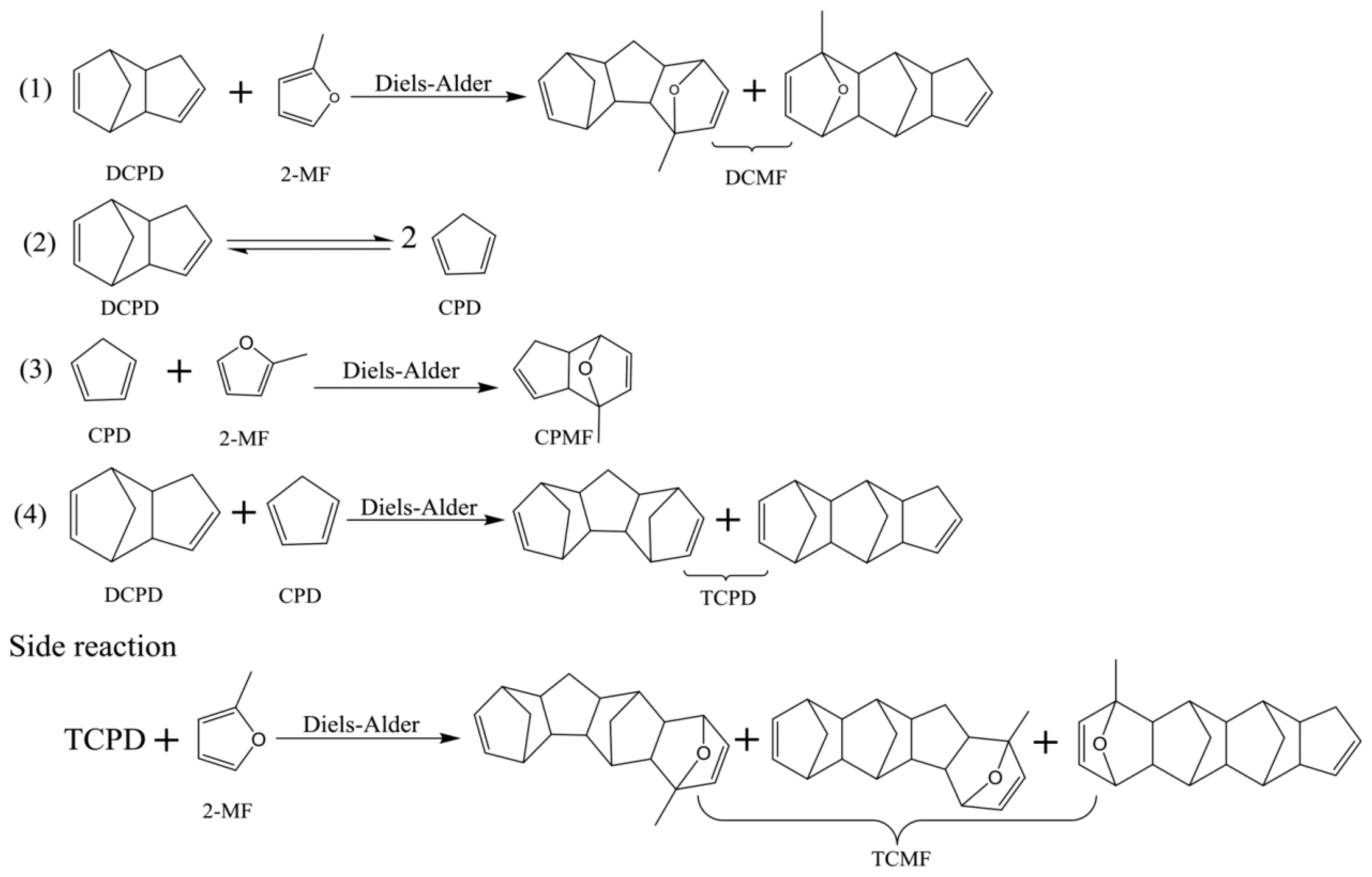
4.2. Diels–Alder Reaction of 2-Methylfuran and Dicyclopentadiene
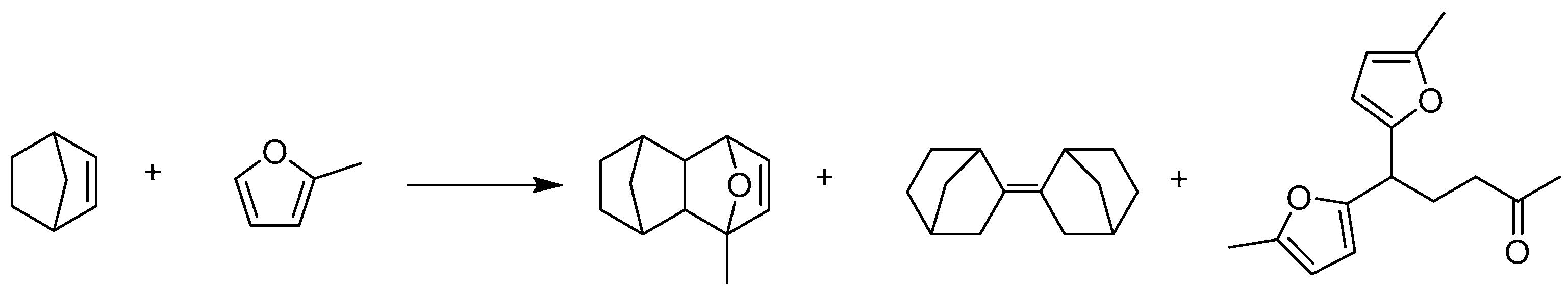
4.3. Diels–Alder Reaction of 2-Methylfuran and Norbornene
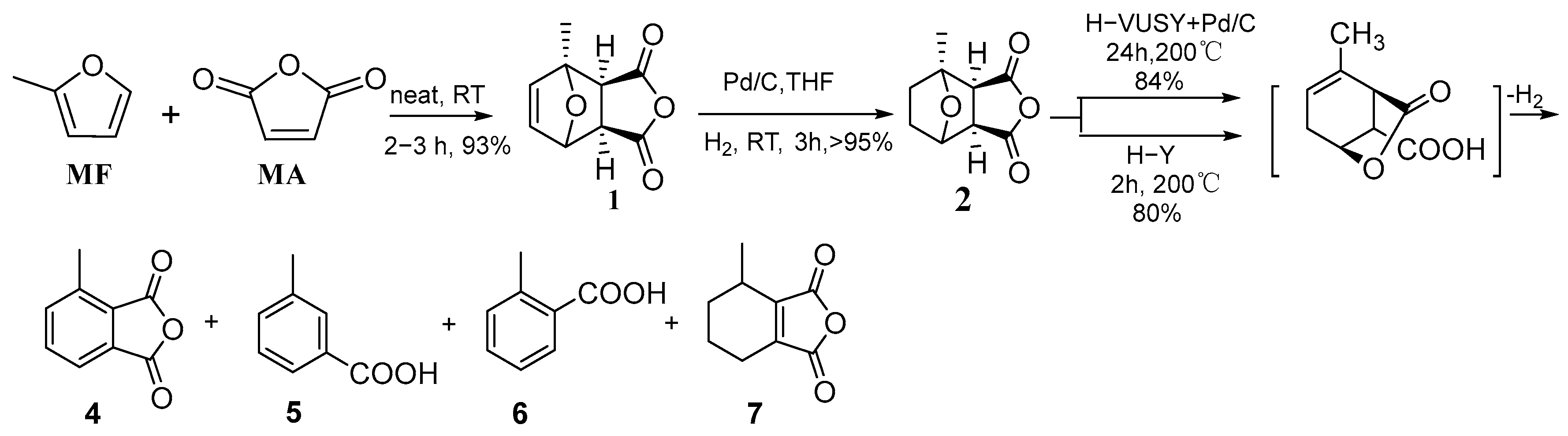
4.4. Diels–Alder Reaction of 2-Methylfuran and Maleic Anhydride
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melero, J.A.; Iglesias, J.; Garcia, A. Biomass as renewable feedstock in standard refinery units. Feasibility, opportunities and challenges. Energy Environ. Sci. 2012, 5, 7393. [Google Scholar] [CrossRef]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, S.; Zhang, L.; Liu, X.; Wang, J.; Pan, X.; Li, N.; Wang, A.; Cong, Y.; Wang, X.; et al. Hydrodeoxygenation of furans over Pd-FeOx/SiO2 catalyst under atmospheric pressure. Appl. Catal. B Environ. 2017, 201, 266–277. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Li, G.; Wang, R.; Pang, J.; Wang, A.; Li, N.; Zhang, T. Production of Renewable Hydrocarbon Biofuels with Lignocellulose and Its Derivatives over Heterogeneous Catalysts. Chem. Rev. 2024, 124, 2889–2954. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.W.; Chheda, J.N.; Barrett, C.J.; Dumesic, J.A. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates. Science 2005, 308, 1446–1450. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gärtner, C.A.; Dumesic, J.A. Catalytic Conversion of Biomass to Monofunctional Hydrocarbons and Targeted Liquid-Fuel Classes. Science 2008, 322, 417–421. [Google Scholar] [CrossRef]
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Xia, X.; Lin, C.-X.; Tong, D.-S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588. [Google Scholar] [CrossRef] [PubMed]
- Thewes, M.; Muether, M.; Pischinger, S.; Budde, M.; Brunn, A.; Sehr, A.; Adomeit, P.; Klankermayer, J. Analysis of the Impact of 2-Methylfuran on Mixture Formation and Combustion in a Direct-Injection Spark-Ignition Engine. Energy Fuels 2011, 25, 5549–5561. [Google Scholar] [CrossRef]
- Singh, E.; Shankar, V.S.B.; Tripathi, R.; Pitsch, H.; Sarathy, S.M. 2-Methylfuran: A bio-derived octane booster for spark-ignition engines. Fuel 2018, 225, 349–357. [Google Scholar] [CrossRef]
- Banerjee, D.; Sahu, A.K.; Clegg, J.K.; Upadhyayula, S. Recent advances in 2-methylfuran production via catalytic transfer hydrogenation of biomass-derived furfural. Chem. Eng. J. 2024, 493, 152552. [Google Scholar] [CrossRef]
- Sitthisa, S.; An, W.; Resasco, D.E. Selective conversion of furfural to methylfuran over silica-supported NiFe bimetallic catalysts. J. Catal. 2011, 284, 90–101. [Google Scholar] [CrossRef]
- Wang, C.; Wu, C.; Deng, L.; Zhang, R.; Zhou, S.; Wang, Z.; Qiao, C.; Tian, Y. Ni−Promoted Cu/ZSM-5 for selective hydrodeoxygenation of furfural to produce 2-Methylfuran. Fuel 2023, 353, 129233. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Y.; Li, Y.-W.; Jiao, H. Mechanisms of Mo2C(101)-Catalyzed Furfural Selective Hydrodeoxygenation to 2-Methylfuran from Computation. ACS Catal. 2016, 6, 6790–6803. [Google Scholar] [CrossRef]
- Wang, B.; Li, C.; He, B.; Qi, J.; Liang, C. Highly stable and selective Ru/NiFe2O4 catalysts for transfer hydrogenation of biomass-derived furfural to 2-methylfuran. J. Energy Chem. 2017, 26, 799–807. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Panagiotopoulou, P.; Mironenko, A.V.; Jenness, G.R.; Vlachos, D.G.; Xu, B. Mechanistic Insights into Metal Lewis Acid-Mediated Catalytic Transfer Hydrogenation of Furfural to 2-Methylfuran. ACS Catal. 2015, 5, 3988–3994. [Google Scholar] [CrossRef]
- Grazia, L.; Lolli, A.; Folco, F.; Zhang, Y.; Albonetti, S.; Cavani, F. Gas-phase cascade upgrading of furfural to 2-methylfuran using methanol as a H-transfer reactant and MgO based catalysts. Catal. Sci. Technol. 2016, 6, 4418–4427. [Google Scholar] [CrossRef]
- Dohade, M.G.; Dhepe, P.L. One pot conversion of furfural to 2-methylfuran in the presence of PtCo bimetallic catalyst. Clean Technol. Environ. Policy 2017, 20, 703–713. [Google Scholar] [CrossRef]
- Date, N.S.; Hengne, A.M.; Huang, K.W.; Chikate, R.C.; Rode, C.V. Single pot selective hydrogenation of furfural to 2-methylfuran over carbon supported iridium catalysts. Green Chem. 2018, 20, 2027–2037. [Google Scholar] [CrossRef]
- Niu, H.; Luo, J.; Li, C.; Wang, B.; Liang, C. Transfer Hydrogenation of Biomass-Derived Furfural to 2-Methylfuran over CuZnAl Catalysts. Ind. Eng. Chem. Res. 2019, 58, 6298–6308. [Google Scholar] [CrossRef]
- Kalong, M.; Hongmanorom, P.; Ratchahat, S.; Koo-amornpattana, W.; Faungnawakij, K.; Assabumrungrat, S.; Srifa, A.; Kawi, S. Hydrogen-free hydrogenation of furfural to furfuryl alcohol and 2-methylfuran over Ni and Co-promoted Cu/γ-Al2O3 catalysts. Fuel Process. Technol. 2021, 214, 106721. [Google Scholar] [CrossRef]
- Gong, X.; Li, N.; Li, Y.; Hu, R. The catalytic hydrogenation of furfural to 2-methylfuran over the Mg-Al oxides supported Co-Ni bimetallic catalysts. Mol. Catal. 2022, 531, 112651. [Google Scholar] [CrossRef]
- Kalong, M.; Srifa, A.; Hongmanorom, P.; Cholsuk, C.; Klysubun, W.; Ratchahat, S.; Koo-Amornpattana, W.; Khemthong, P.; Assabumrungrat, S.; Kawi, S. Catalytic transfer hydrogenation of furfural to furfuryl alcohol and 2-methylfuran over CuFe catalysts: Ex situ observation of simultaneous structural phase transformation. Fuel Process. Technol. 2022, 231, 107256. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, S.; Wang, Y.; Li, T.; Sun, Z.; Liu, Y.-Y.; Wang, A. Selective Hydrodeoxygenation of Furfural to 2-Methylfuran over Silica-Supported MoP Catalysts under Mild Conditions. Ind. Eng. Chem. Res. 2023, 62, 17681–17690. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Zhang, X.; Wang, F.; Wang, Z.; Li, Y.; Wang, X.; Ahishakiye, R.; Zhang, X. Single pot selective conversion of furfural into 2-methylfuran over a Co-CoOx/AC bifunctional catalyst. Appl. Surf. Sci. 2023, 612, 155871. [Google Scholar] [CrossRef]
- Dou, S.; Ma, L.; Dong, Y.; Zhu, Q.; Kong, X. Hydrodeoxygenation of furfural to 2-methylfuran over Cu-Co confined by hollow carbon cage catalyst enhanced by optimized charge transfer and alloy structure. J. Colloid Interface Sci. 2024, 663, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Kalong, M.; Praikaew, W.; Ratchahat, S.; Chaiwat, W.; Koo-amornpattana, W.; Klysubun, W.; Limphirat, W.; Assabumrungrat, S.; Srifa, A. Continuous Furfural Hydrogenolysis into 2-Methylfuran and 2-Methyltetrahydrofuran over Cu/γ–Al2O3 with ReOx and WOx as Catalyst Boosters. Energy Fuels 2024, 38, 9836–9848. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Cai, C.; Wang, H.; Huang, Y.; Li, S.; Yang, B.; Wang, C.; Liao, Y.; Ma, L. Tandem conversion xylose to 2-methylfuran with NiCu/C catalyst. Catal. Commun. 2023, 175, 106625. [Google Scholar] [CrossRef]
- Wang, T.; Cheng, Y.; Niu, H.; Li, S.; Luo, J.; Liang, C. Construction of a sol–gel derived ternary CuZn/FeOx nanostructure for catalytic transfer hydrogenation of furfural. Sustain. Energy Fuels 2023, 7, 1187–1195. [Google Scholar] [CrossRef]
- Corma, A.; de la Torre, O.; Renz, M.; Villandier, N. Production of high-quality diesel from biomass waste products. Angew. Chem. Int. Ed. 2011, 50, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; de la Torre, O.; Renz, M. High-quality diesel from hexose- and pentose-derived biomass platform molecules. ChemSusChem 2011, 4, 1574–1577. [Google Scholar] [CrossRef]
- Corma, A.; de la Torre, O.; Renz, M. Production of high quality diesel from cellulose and hemicellulose by the Sylvan process: Catalysts and process variables. Energy Environ. Sci. 2012, 5, 6328–6344. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Yang, J.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable diesel with the 2-methylfuran, butanal and acetone derived from lignocellulose. Bioresour. Technol. 2013, 134, 66–72. [Google Scholar] [CrossRef]
- Li, S.; Li, N.; Li, G.; Li, L.; Wang, A.; Cong, Y.; Wang, X.; Xu, G.; Zhang, T. Protonated titanate nanotubes as a highly active catalyst for the synthesis of renewable diesel and jet fuel range alkanes. Appl. Catal. B Environ. 2015, 170–171, 124–134. [Google Scholar] [CrossRef]
- Li, S.; Li, N.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of diesel range alkanes with 2-methylfuran and mesityl oxide from lignocellulose. Catal. Today 2014, 234, 91–99. [Google Scholar] [CrossRef]
- Li, S.; Li, N.; Wang, W.; Li, L.; Wang, A.; Wang, X.; Zhang, T. Synthesis of jet fuel range branched cycloalkanes with mesityl oxide and 2-methylfuran from lignocellulose. Sci. Rep. 2016, 6, 32379. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Shen, T.; Liu, D.; Wu, J.; Chen, Y.; Wang, L.; Guo, K.; Ying, H.; Ouyang, P. Production of liquid hydrocarbon fuels with acetoin and platform molecules derived from lignocellulose. Green Chem. 2016, 18, 2165–2174. [Google Scholar] [CrossRef]
- Lusardi, M.; Davis, M.E. Sulfonic Acid-Functionalized Zeolite Beta: Bronsted Acid Catalysts for Reactions Involving Liquid Water. ACS Sustain. Chem. Eng. 2021, 9, 17120–17127. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Wang, Z.; Li, C.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of high-quality diesel with furfural and 2-methylfuran from hemicellulose. ChemSusChem 2012, 5, 1958–1966. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Sacia, E.R.; Bell, A.T. Syntheses of Biodiesel Precursors: Sulfonic Acid Catalysts for Condensation of Biomass-Derived Platform Molecules. ChemSusChem 2014, 7, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Niu, X.; Wu, H.; Zhang, H.; Ao, Z.; Zhang, S. Valorization of humin as a glucose derivative to fabricate a porous carbon catalyst for esterification and hydroxyalkylation/alkylation. Waste Manag. 2020, 103, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, N.; Li, G.; Li, L.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Lignosulfonate-based acidic resin for the synthesis of renewable diesel and jet fuel range alkanes with 2-methylfuran and furfural. Green Chem. 2015, 17, 3644–3652. [Google Scholar] [CrossRef]
- Chhabra, T.; Dwivedi, P.; Krishnan, V. Acid functionalized hydrochar as heterogeneous catalysts for solventless synthesis of biofuel precursors. Green Chem. 2022, 24, 898–910. [Google Scholar] [CrossRef]
- Huang, Y.B.; Yan, X.Y.; Huang, Z.H.; Shan, T.X.; Geng, J.Y.; Cao, Z.H.; Lu, Q. Rapid Synthesis of Diesel Precursors from Biomass-Derived Furanics Over Aluminum-Doped Mesoporous Silica Sphere Catalysts. ChemSusChem 2022, 16, e202201677. [Google Scholar] [CrossRef]
- Wang, T.; Li, K.; Liu, Q.; Zhang, Q.; Qiu, S.; Long, J.; Chen, L.; Ma, L.; Zhang, Q. Aviation fuel synthesis by catalytic conversion of biomass hydrolysate in aqueous phase. Appl. Energy 2014, 136, 775–780. [Google Scholar] [CrossRef]
- Konwar, L.J.; Samikannu, A.; Mäki-Arvela, P.; Mikkola, J.-P. Efficient C–C coupling of bio-based furanics and carbonyl compounds to liquid hydrocarbon precursors over lignosulfonate derived acidic carbocatalysts. Catal. Sci. Technol. 2018, 8, 2449–2459. [Google Scholar] [CrossRef]
- Kunamalla, A.; Maity, S.K. Production of green jet fuel from furanics via hydroxyalkylation-alkylation over mesoporous MoO3-ZrO2 and hydrodeoxygenation over Co/γ-Al2O3: Role of calcination temperature and MoO3 content in MoO3-ZrO2. Fuel 2023, 332, 125977. [Google Scholar] [CrossRef]
- Dutta, S.; Bohre, A.; Zheng, W.; Jenness, G.R.; Nu, M.; Saha, B.; Vlachos, D.G. Solventless C–C Coupling of Low Carbon Furanics to High Carbon Fuel Precursors Using an Improved Graphene Oxide Carbocatalyst. ACS Catal. 2017, 7, 3905–3915. [Google Scholar] [CrossRef]
- Xia, Q.; Xia, Y.; Xi, J.; Liu, X.; Zhang, Y.; Guo, Y.; Wang, Y. Selective One-Pot Production of High-Grade Diesel-Range Alkanes from Furfural and 2-Methylfuran over Pd/NbOPO4. ChemSusChem 2016, 10, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Bai, X.; Zhao, W.; Dai, Z.; Zhao, Y.; Li, J. Research on high-carbon ether-based oxygen additives: Catalytic transformation of 2-methylfuran and furfural to ethers via CuMgAlOx catalysts. Fuel 2024, 371, 132028. [Google Scholar] [CrossRef]
- Yan, P.; Wang, H.; Liao, Y.; Wang, C. Synthesis of renewable diesel and jet fuels from bio-based furanics via hydroxyalkylation/alkylation (HAA) over SO42−/TiO2 and hydrodeoxygenation (HDO) reactions. Fuel 2023, 342, 127685. [Google Scholar] [CrossRef]
- Ravi, K.; Biradar, A.V. Highly active and scalable SO3H functionalized carbon catalyst synthesized from bagasse for transformation of bio-based platform chemicals into fuel precursors and its in-depth characterization studies. Fuel 2022, 321, 124008. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Li, S.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of renewable diesel with hydroxyacetone and 2-methyl-furan. Chem. Commun. 2013, 49, 5727–5729. [Google Scholar] [CrossRef] [PubMed]
- Tarade, K.; Shinde, S.; Rode, C. Magnetically separable catalyst for condensation of renewable aldehydes and 2-methylfuran to saturated cyclic oxygenates. Fuel Process. Technol. 2020, 197, 106191. [Google Scholar] [CrossRef]
- Li, H.; Gui, Z.; Yang, S.; Qi, Z.; Saravanamurugan, S.; Riisager, A. Catalytic Tandem Reaction for the Production of Jet and Diesel Fuel Range Alkanes. Energy Technol. 2018, 6, 1060–1066. [Google Scholar] [CrossRef]
- Wang, W.; Li, N.; Li, S.; Li, G.; Chen, F.; Sheng, X.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable diesel with 2-methylfuran and angelica lactone derived from carbohydrates. Green Chem. 2016, 18, 1218–1223. [Google Scholar] [CrossRef]
- Shinde, S.H.; Rode, C.V. An Integrated Production of Diesel Fuel Precursors from Carbohydrates and 2-Methylfuran over Sn-Mont Catalyst. ChemistrySelect 2018, 3, 4039–4046. [Google Scholar] [CrossRef]
- Tarade, K.P.; Kamble, S.P.; Rode, C.V. Novel Sulfonic Acid Functionalized Silica Supported Isonicotinic Acid Catalyst for Conversion of 2-Methylfuran to Diesel Fuel Precursors. Catal. Lett. 2023, 154, 1511–1520. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, Z.; Li, H.; Yang, S. 3-Bromopyridine-Heterogenized Phosphotungstic Acid for Efficient Trimerization of Biomass-Derived 5-Hydroxymethylfurfural with 2-Methylfuran to C21 Fuel Precursor. Adv. Polym. Technol. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Wang, X.; Sheng, X.; Li, S.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Synthesis of Diesel or Jet Fuel Range Cycloalkanes with 2-Methylfuran and Cyclopentanone from Lignocellulose. Energy Fuels 2014, 28, 5112–5118. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, Q.; Han, P.; Xu, J.; Pan, L.; Wang, L.; Zou, J.-J. Hydrophobic mesoporous acidic resin for hydroxyalkylation/alkylation of 2-methylfuran and ketone to high-density biofuel. AIChE J. 2017, 63, 680–688. [Google Scholar] [CrossRef]
- Zhong, Y.; Deng, Q.; Zhang, P.; Wang, J.; Wang, R.; Zeng, Z.; Deng, S. Sulfonic acid functionalized hydrophobic mesoporous biochar: Design, preparation and acid-catalytic properties. Fuel 2019, 240, 270–277. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, P.; Zhu, X.; Li, H.; Deng, Q.; Wang, J.; Zeng, Z.; Zou, J.-J.; Deng, S. Highly Efficient Alkylation Using Hydrophobic Sulfonic Acid-Functionalized Biochar as a Catalyst for Synthesis of High-Density Biofuels. ACS Sustain. Chem. Eng. 2019, 7, 14973–14981. [Google Scholar] [CrossRef]
- Deng, Q.; Han, P.; Xu, J.; Zou, J.-J.; Wang, L.; Zhang, X. Highly controllable and selective hydroxyalkylation/alkylation of 2-methylfuran with cyclohexanone for synthesis of high-density biofuel. Chem. Eng. Sci. 2015, 138, 239–243. [Google Scholar] [CrossRef]
- Lian, L.; Liu, Y.; Yi, X.; Hu, H.; Chen, X.; Li, H.; Chen, W.; Zheng, A.; Song, Y.-F.; Yi, X.; et al. Covalently tethering disulfonic acid moieties onto polyoxometalate boosts acid strength and catalytic performance for hydroxyalkylation/alkylation reaction. Sci. China Chem. 2022, 65, 699–709. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Li, Y.; Shao, X.; Ji, J.; Liu, J.; Wang, W.; Li, Z.; Ji, X. Synthesis of renewable diesel and jet fuel range alkanes using 2-methylfuran and cyclohexanone. RSC Adv. 2022, 12, 12932–12937. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Montini, T.; Zhang, J.; Fornasiero, P.; Fonda, E.; Hou, T.; Nie, W.; Lu, J.; Liu, J.; Heggen, M.; et al. Visible-light-driven coproduction of diesel precursors and hydrogen from lignocellulose-derived methylfurans. Nat. Energy 2019, 4, 575–584. [Google Scholar] [CrossRef]
- Ren, G.; Li, G.; Zhang, Y.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T.; Li, N. Synthesis of jet fuel and diesel range cycloalkanes with 2-methylfuran and benzaldehyde. Sustain. Energy Fuels 2022, 6, 1156–1163. [Google Scholar] [CrossRef]
- Gumidyala, A.; Wang, B.; Crossley, S. Direct carbon-carbon coupling of furanics with acetic acid over Brønsted zeolites. Sci. Adv. 2016, 2, 1601072. [Google Scholar] [CrossRef] [PubMed]
- Koehle, M.; Zhang, Z.; Goulas, K.A.; Caratzoulas, S.; Vlachos, D.G.; Lobo, R.F. Acylation of methylfuran with Brønsted and Lewis acid zeolites. Appl. Catal. A Gen. 2018, 564, 90–101. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Zhao, W.; Yang, S. Low-temperature and solvent-free production of biomass-derived diesel-range C17 precursor via one-pot cascade acylation–alkylation over Sn4+-montmorillonite. J. Ind. Eng. Chem. 2018, 66, 325–332. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Luo, X.; Li, Z.; Zhang, H.; Li, H.; Yang, S. Catalytic cascade acetylation-alkylation of biofuran to C17 diesel precursor enabled by a budget acid-switchable catalyst. Chin. J. Chem. Eng. 2021, 34, 171–179. [Google Scholar] [CrossRef]
- Naik, A.V.; Joseph, K.E.; Shetty, M.; Ardagh, M.A.; Dauenhauer, P.J. Kinetics of 2-Methylfuran Acylation with Fatty Acid Anhydrides for Biorenewable Surfactants. ACS Sustain. Chem. Eng. 2020, 8, 18616–18625. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, Z.; Chang, S.; Huang, Z.; Zhao, K.; Wu, H.; Wang, X.; He, F.; Li, H. Maximum synergistic effect in the coupling conversion of bio-derived furans and methanol over ZSM-5 for enhancing aromatic production. Green Chem. 2014, 16, 2580. [Google Scholar] [CrossRef]
- Xue, Y.; Cui, X.; Shan, J.; Gao, S.; Wang, S.; Zheng, H.; Tang, J.; Niu, Y.; Wang, K.; Wang, X. Tailoring porosity and acidity of Sn-modified ZSM-5 for co-aromatization of methanol with 2-methylfuran. Fuel 2023, 340, 127440. [Google Scholar] [CrossRef]
- Green, S.K.; Patet, R.E.; Nikbin, N.; Williams, C.L.; Chang, C.-C.; Yu, J.; Gorte, R.J.; Caratzoulas, S.; Fan, W.; Vlachos, D.G.; et al. Diels–Alder cycloaddition of 2-methylfuran and ethylene for renewable toluene. Appl. Catal. B Environ. 2016, 180, 487–496. [Google Scholar] [CrossRef]
- Wijaya, Y.P.; Kristianto, I.; Lee, H.; Jae, J. Production of renewable toluene from biomass-derived furans via Diels-Alder and dehydration reactions: A comparative study of Lewis acid catalysts. Fuel 2016, 182, 588–596. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Liu, Y.; Li, Z.; Xiu-Tian-Feng, E.; Xie, J.; Zhang, Y.-C.; Pan, L.; Zou, J.-J. Synthesis of high-density liquid fuel via Diels-Alder reaction of dicyclopentadiene and lignocellulose-derived 2-methylfuran. Catal. Today 2019, 319, 139–144. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; Wang, X.; Xie, J.; Yang, B.; Liang, Y.; Zou, J.-J.; Zhang, Q. Synthesis of JP-10 analogues high-density fuels via one-pot Diels-Alder/hydrodeoxygenation reaction. Fuel 2024, 361, 130738. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Genuino, H.C.; van der Waal, J.C.; de Jong, E.; Weckhuysen, B.M.; van Haveren, J.; Bruijnincx, P.C.; van Es, D.S. A Facile Solid-Phase Route to Renewable Aromatic Chemicals from Biobased Furanics. Angew. Chem. Int. Ed. Engl. 2016, 55, 1368–1371. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.J.; Kuo, M.J.; Ye, M.; Kurz, Y.; Yuan, Y.; Lobo, R.F. Selective Synthesis of 4,4′-Dimethylbiphenyl from 2-Methylfuran. ACS Sustain. Chem. Eng. 2021, 9, 3316–3323. [Google Scholar] [CrossRef]
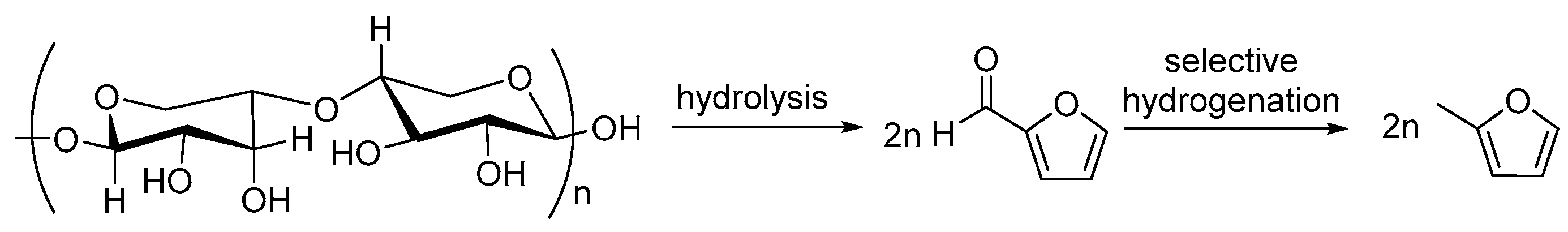




| Entry | Molar Ratio 2-MF/5-Methylfurfural | Catalyst | Mass Ratio p-TosOH/2-MF | Time (h) | Temperature (°C) | Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 5.0 | p-TosOH | 0.0230 | 6 | 50 | 93 | [33] |
| 2 | 5.0 | p-TosOH | 0.0350 | 6 | 50 | 93 | [34] |
| 3 | 5.0 | Sulfuric acid | 0.2449 | 6 | 50 | 83 | [34] |
| Entry | Molar Ratio 2-MF/Butanal | Catalyst | Mass Ratio p-TosOH/2-MF | Time (h) | Temperature (°C) | Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 2.0 | p-TosOH | 0.0324 | 6 | 50 | 79 | [33] |
| 2 | 3.0 | p-TosOH | 0.0222 | 6 | 50 | 84 | [33] |
| 3 | 3.5 | p-TosOH | 0.0192 | 6 | 50 | 88 | [33] |
| 4 | 2.0 | Amberlyst-15 | 0.0331 | 22 | 50 | 59 | [33] |
| 5 | 3.0 | Amberlyst-15 | 0.0222 | 22 | 50 | 69 | [33] |
| 6 | 2.0 | USY | 0.0167 | 8 | 50 | 53 | [35] |
| 7 | 2.0 | Beta(comm) | 0.0167 | 8 | 50 | 67 | [35] |
| 8 | 2.0 | Beta(nano) | 0.0167 | 8 | 50 | 59 | [35] |
| 9 | 2.0 | Beta(OH−) | 0.0167 | 8 | 50 | 34 | [35] |
| 10 | 2.0 | Beta(F−) | 0.0167 | 8 | 50 | 16 | [35] |
| 11 | 2.0 | MCM-41 (Si/Al ratio = 15) | 0.0167 | 8 | 50 | 45 | [35] |
| 12 | 2.0 | MCM-41(Si/Al ratio = 28) | 0.0167 | 8 | 50 | 60 | [35] |
| 13 | 2.0 | ITQ-2 | 0.0167 | 8 | 50 | 86 | [35] |
| 14 | 2.0 | Dowex 50WX2-100 | 0.0167 | 8 | 50 | 80 | [35] |
| 15 | 2.0 | Nafion212 | 0.0915 | 2 | 50 | 89.5 | [36] |
| 16 | 2.0 | PTNT | 0.0457 | 6 | 50 | 77 | [37] |
| Entry | Aldehyde | p-TosOH (mol%) | Time (h) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|---|
| 1 | Ethanol (2b) | 3.3 | 4 | 50 | 87.4 |
| 2 | Ethanol (2b) | 2.8 | 6 | 50 | 76.3 |
| 3 | Ethanol (2b) | 2.0 | 5 | 50 | 66.0 |
| 4 | Propanal (2c) | 2.6 | 6 | 50 | 88.4 |
| 5 | Butanal (2a) | 3.2 | 6 | 50 | 88.4 |
| 6 | Pentanal (2d) | 2.7 | 7.5 | 50 | 87.4 |
| Entry | Catalyst | Catalyst/2-MF (wt%) | Time (h) | Temperature (°C) | Yield (%) | Reference |
|---|---|---|---|---|---|---|
| 1 | p-TosOH | 1.6 | 24 | 30 | 89 | [35] |
| 2 | p-TosOH | 1.4 | 7 | 60 | 80 | [35] |
| 3 | p-TosOH | 1.6 | 24 | 60 | 77 | [35] |
| 4 | PTNT | 4.5 | 4 | 20 | 8.2 | [37] |
| 5 | Nafion212 | 9.1 | 2 | 60 | 70 | [38] |
| 6 | Amberlyst36 | 9.1 | 2 | 60 | 61 | [38] |
| 7 | Amberlyst15 | 9.1 | 2 | 60 | 59 | [38] |
| Entry | Molar Ratio 2-MF/Furfural | Catalyst | Ratio of Catalyst/2MF | Time (h) | Temperature (°C) | Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 2.0 | PTNT | 0.0457 wt% | 4 | 50 | 50 | [37] |
| 2 | 2.0 | Nafion212 | 0.0457 wt% | 2 | 65 | 75 | [42] |
| 3 | 2.0 | Amberlyst36 | 0.0457 wt% | 2 | 50 | 59 | [42] |
| 4 | 2.0 | Amberlyst15 | 0.0457 wt% | 2 | 50 | 60 | [42] |
| 5 | 2.2 | 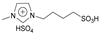 | 5 mol% | 2 | 65 | 72 | [43] |
| 6 | 2.2 | 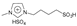 | 5 mol% | 4 | 65 | 89 | [43] |
| 7 | 2.2 | 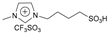 | 5 mol% | 2 | 65 | 86 | [43] |
| 8 | 2.2 |  | 5 mol% | 2 | 65 | 81 | [43] |
| 9 | 2.2 |  | 5 mol% | 4 | 65 | 92 | [43] |
| 10 | 2.2 |  | 5 mol% | 2 | 65 | 92 | [43] |
| 11 | 2.0 | HC573-S423 | 0.0457 wt% | 2 | 50 | 64 | [44] |
| 12 | 2.0 | LF | 0.0457 wt% | 6 | 60 | 85 | [45] |
| 13 | 2.0 | LF | 0.0457 wt% | 12 | 60 | 89 | [45] |
| 14 | 2.0 | SHC | 0.3049 wt% | 5 | 50 | 83 | [46] |
| 15 | 2.0 | Al-MSS20-450 | 0.0067 wt% | 20 | 140 | 94 | [47] |
| 16 | 2.0 | Formic acid | 3.6552 | 16 | 55 | 95 | [48] |
| 17 | 2.0 | 80LS20PS350H+ | 0.0430 | 2 | 60 | 70 | [49] |
| 18 | 2.0 | 60LS40PS350H+ | 0.0430 | 2 | 60 | 88 | [49] |
| 19 | 2.0 | LS-RES | 0.0430 | 4 | 60 | 73 | [49] |
| 20 | 2.0 | Amberlyst®70 | 0.0430 | 24 | 60 | 83 | [49] |
| 21 | 2.0 | Amberlite®IR120 | 0.0430 | 24 | 60 | 84 | [49] |
| Entry | Molar Ratio 2-MF/Formaldehyde | Catalyst | Mass Ratio of Catalyst/2-MF | Time (h) | Temperature (°C) | Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 2.0 | H2SO4 | 0.3048 | 3 | 65 | 74 | [57] |
| 2 | 2.0 | Amberlyst-15 | 0.3048 | 3 | 65 | 83 | [57] |
| 3 | 2.0 | Amberlyst IR-120 | 0.3048 | 3 | 65 | 82 | [57] |
| 4 | 2.0 | Sn-Mont | 0.3048 | 3 | 65 | 67 | [57] |
| 5 | 2.0 | Zr-Mont | 0.3048 | 3 | 65 | 60 | [57] |
| 6 | 2.0 | [Fe3O4@SiO2-Pr-Py-H][2HSO42−] | 0.3048 | 3 | 65 | 86 | [57] |
| 7 | 2.0 | SO42−/SiO2 | 0.3048 | 3 | 65 | 74 | [57] |
| 8 | 2.0 | SO42−/Fe3O4@SiO2 | 0.3048 | 3 | 65 | 79 | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Yan, J.; Sun, M.; Li, X.; Li, Y.; An, L.; Qian, C.; Zhang, X.; Shao, X.; Duan, Y.; et al. Recent Progress in the Conversion of Methylfuran into Value-Added Chemicals and Fuels. Molecules 2024, 29, 2976. https://doi.org/10.3390/molecules29132976
Wang W, Yan J, Sun M, Li X, Li Y, An L, Qian C, Zhang X, Shao X, Duan Y, et al. Recent Progress in the Conversion of Methylfuran into Value-Added Chemicals and Fuels. Molecules. 2024; 29(13):2976. https://doi.org/10.3390/molecules29132976
Chicago/Turabian StyleWang, Wei, Jiamin Yan, Mengze Sun, Xiufeng Li, Yanqing Li, Ling An, Chi Qian, Xing Zhang, Xianzhao Shao, Yanping Duan, and et al. 2024. "Recent Progress in the Conversion of Methylfuran into Value-Added Chemicals and Fuels" Molecules 29, no. 13: 2976. https://doi.org/10.3390/molecules29132976
APA StyleWang, W., Yan, J., Sun, M., Li, X., Li, Y., An, L., Qian, C., Zhang, X., Shao, X., Duan, Y., & Li, G. (2024). Recent Progress in the Conversion of Methylfuran into Value-Added Chemicals and Fuels. Molecules, 29(13), 2976. https://doi.org/10.3390/molecules29132976






