Study on Rapid Non-Destructive Detection Method of Corn Freshness Based on Hyperspectral Imaging Technology
Abstract
1. Introduction
2. Results and Discussion
2.1. Changes in Fatty Acid Values of Corn during Aging and Sample Set Partitioning
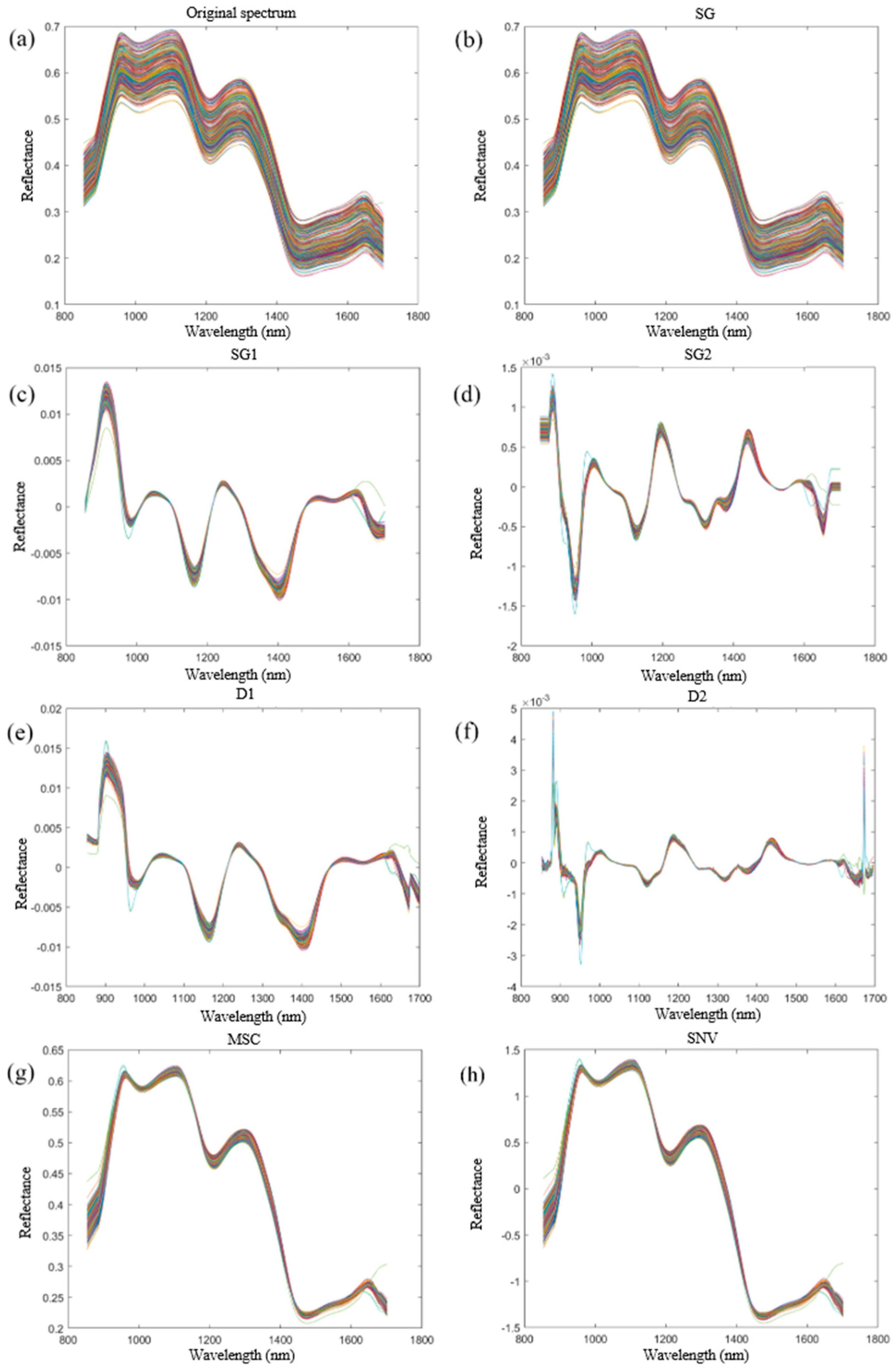
2.2. Data Preprocessing and Extraction of Characteristic Bands
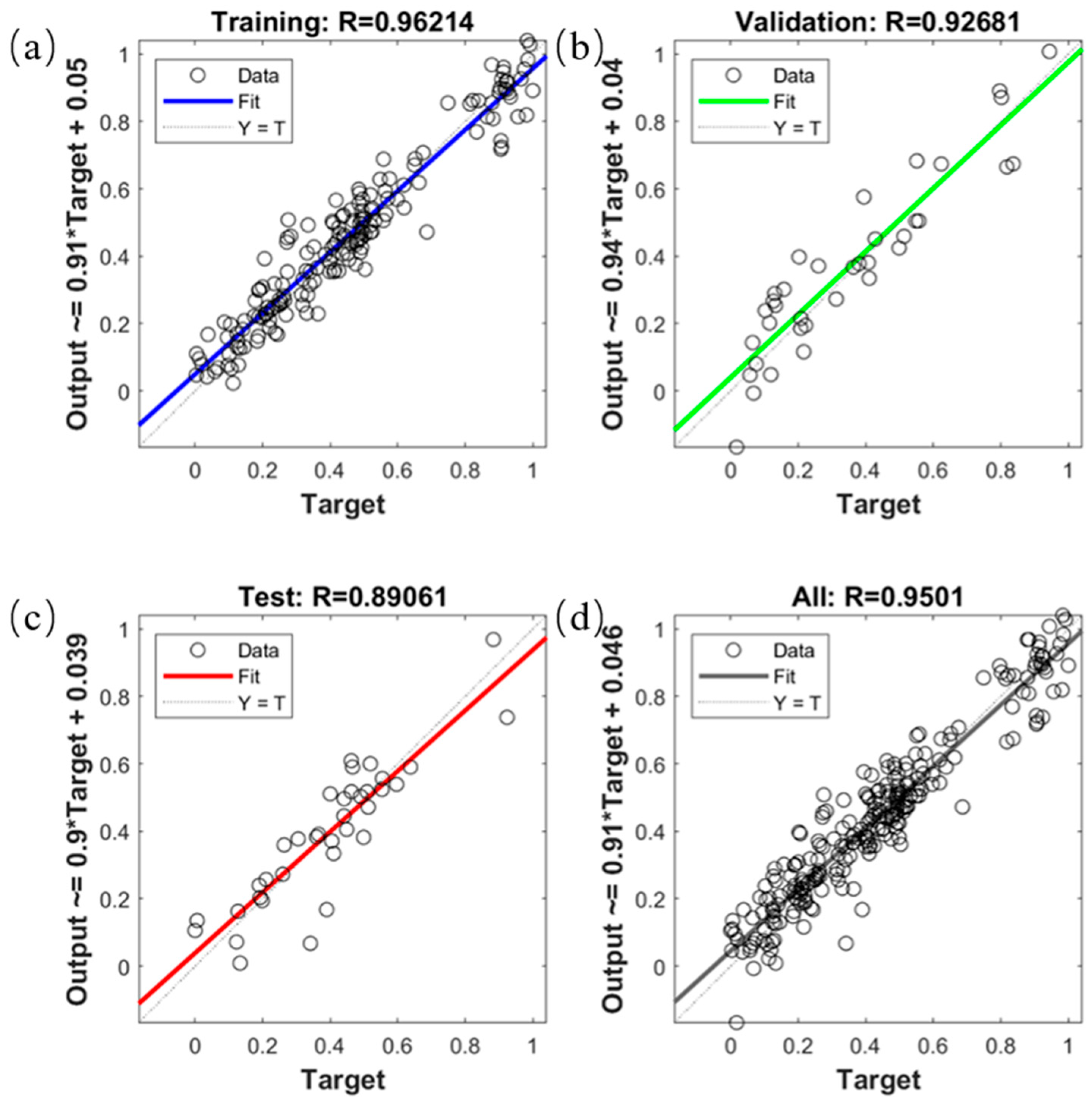
2.3. Model Construction for Predicting Fatty Acid Values of Corn Based on Neural Network
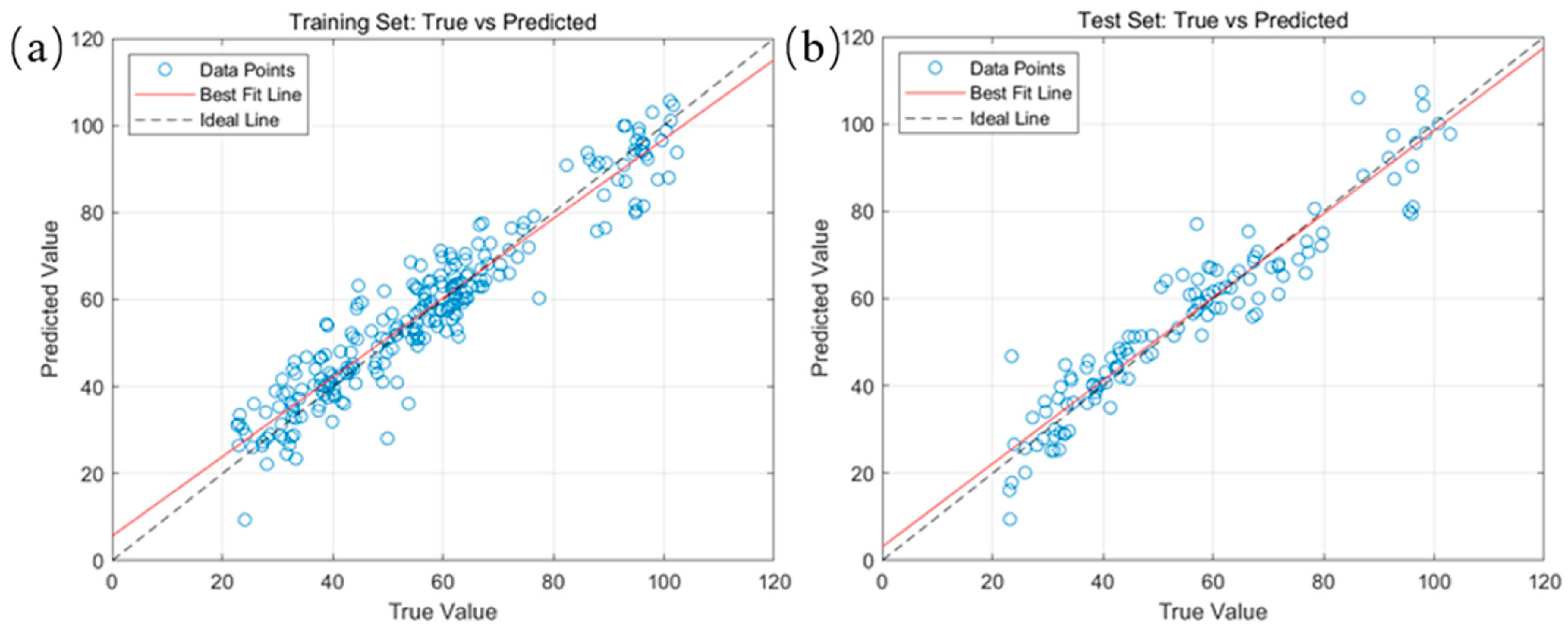
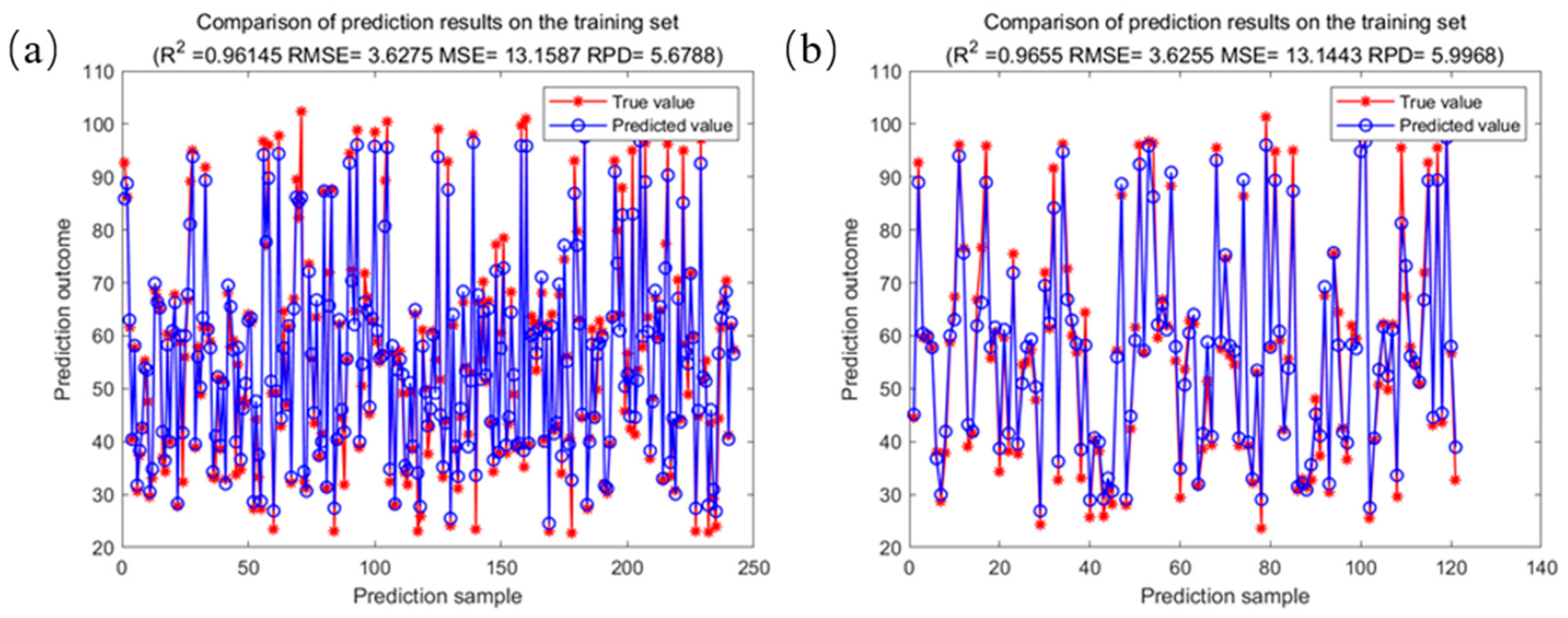
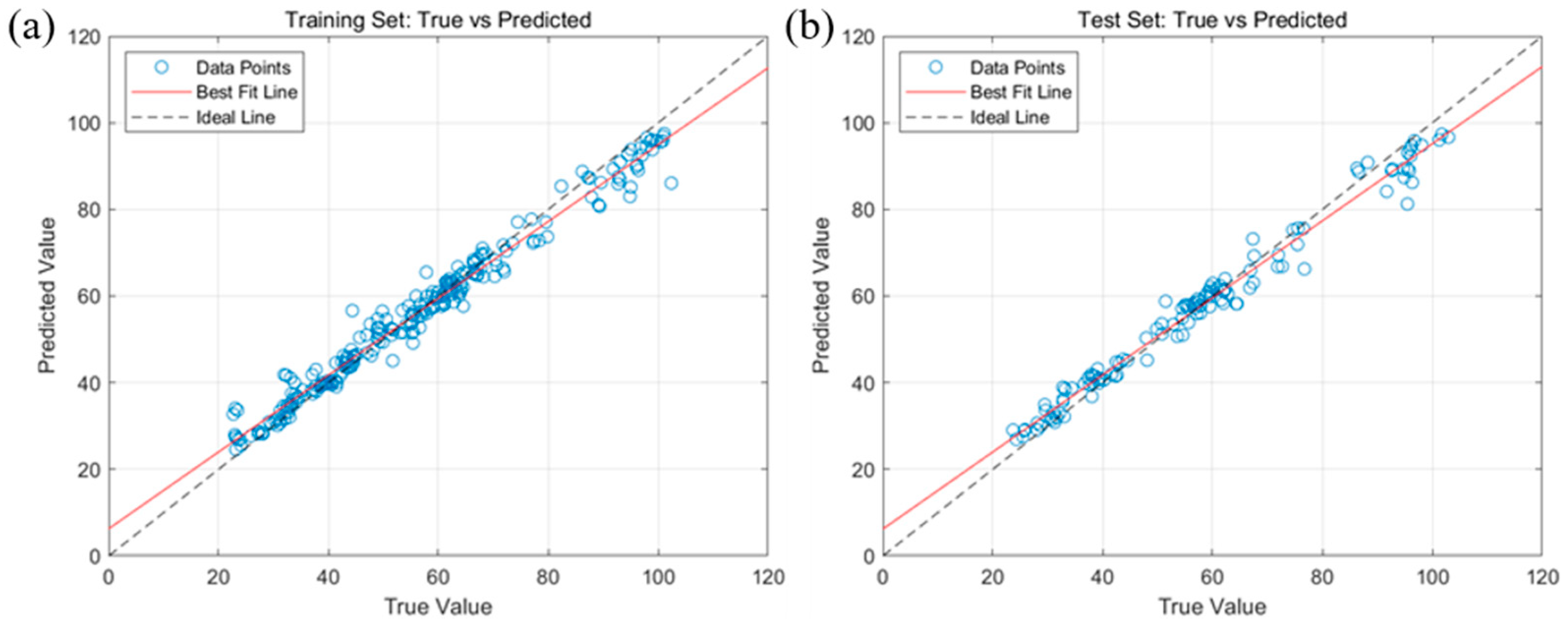
2.4. Model Construction for Predicting Fatty Acid Values of Corn Based on Random Forest
2.5. Visualization of Fatty Acid Values in Corn
3. Materials and Methods
3.1. Test Materials
3.2. Sample Processing
3.3. Determination of Fatty Acid Values
3.4. Image Acquisition and Correction for Hyperspectral Image
3.5. Data Analysis
3.6. Visualization of Fatty Acid Values of Corn
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, H.; Liu, T.; Chen, Q. Quantitative detection of fatty acid value during storage of wheat flour based on a portable near-infrared (NIR) spectroscopy system. Infrared Phys. Technol. 2020, 109, 103423. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, T.; Chen, Q. Dynamic monitoring of fatty acid value in rice storage based on a portable near-infrared spectroscopy system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118620. [Google Scholar] [CrossRef]
- Yin, D.; Yuan, J.; Guo, Y.; Chiba, L.I. Effect of storage time on the characteristics of corn and efficiency of its utilization in broiler chickens. Anim. Nutr. 2017, 3, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hong, T.; Zhao, Z.; Gu, Y.; Guo, Y.; Han, J. Fatty Acid Profiles and Nutritional Evaluation of Fresh Sweet-Waxy Corn from Three Regions of China. Foods 2022, 11, 2636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, K.; Cheng, H.; Hu, J.; Qi, X.; Guo, X. Effect of thermal treatments on volatile profiles and fatty acid composition in sweet corn (Zea mays L.). Food Chem. X 2023, 18, 100743. [Google Scholar]
- Zhang, L.; Yu, Y.; Yu, R. Analysis of metabolites and metabolic pathways in three maize (Zea mays L.) varieties from the same origin using GC–MS. Sci. Rep. 2020, 10, 17990. [Google Scholar]
- GB/T 20570-2015; Guidelines for Evaluation of Maize Storage Character. Standardization Administration of China: Beijing, China, 2015.
- Hussain, N.; Sun, D.-W.; Pu, H. Classical and emerging non-destructive technologies for safety and quality evaluation of cereals: A review of recent applications. Trends Food Sci. Technol. 2019, 91, 598–608. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, H.; Rao, Z.; Ji, H. Non-destructive identification of slightly sprouted wheat kernels using hyperspectral data on both sides of wheat kernels. Biosyst. Eng. 2020, 200, 188–199. [Google Scholar]
- Youngwook, S.; Ahyeong, L.; Balgeum, K.; Jongguk, L. Classification of Rice and Starch Flours by Using Multiple Hyperspectral Imaging Systems and Chemometric Methods. Appl. Sci. 2020, 10, 6724. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Wang, L.; Zhang, H.; Guo, Y.; Yang, X. Monitoring the southern corn rust based on hyperspectral remote sensing. In Proceedings of the 4th International Conference on Geology, Mapping and Remote Sensing (ICGMRS 2023), Wuhan, China, 14–16 April 2023; p. 1297817. [Google Scholar]
- Zhang, L.; Wei, Y.; Liu, J.; An, D.; Wu, J. Maize seed fraud detection based on hyperspectral imaging and one-class learning. Eng. Appl. Artif. Intell. 2024, 133, 108130. [Google Scholar] [CrossRef]
- Singh, T.; Garg, N.M.; Iyengar, S.R.S. Nondestructive identification of barley seeds variety using near-infrared hyperspectral imaging coupled with convolutional neural network. J. Food Process Eng. 2021, 44, e13821. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, S.; Wu, J.; Zhang, C.; Li, J. Application of Long-Wave Near Infrared Hyperspectral Imaging for Determination of Moisture Content of Single Maize Seed. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 254, 119666. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Huang, T.; Zeng, S.; Li, H.; Dong, L.; Zhang, C. A Method for Detection of Corn Kernel Mildew Based on Co-Clustering Algorithm with Hyperspectral Image Technology. Sensors 2022, 22, 5333. [Google Scholar] [CrossRef] [PubMed]
- Timm, N.d.S.; Ramos, A.H.; Ferreira, C.D.; Rios, A.d.O.; Zambiazi, R.C.; de Oliveira, M. Influence of germ storage from different corn genotypes on technological properties and fatty acid, tocopherol, and carotenoid profiles of oil. Eur. Food Res. Technol. 2021, 247, 1449–1460. [Google Scholar] [CrossRef]
- Temba, M.C.; Njobeh, P.B.; Kayitesi, E. Storage stability of maize-groundnut composite flours and an assessment of aflatoxin B1 and ochratoxin A contamination in flours and porridges. Food Control 2017, 71, 178–186. [Google Scholar] [CrossRef]
- Wen, Y.-q.; Xu, L.-l.; Xue, C.-h.; Jiang, X.-m. Effect of Stored Humidity and Initial Moisture Content on the Qualities and Mycotoxin Levels of Maize Germ and Its Processing Products. Toxins 2020, 12, 535. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.Z.; Lu, Q.; Paengkoum, P.; Paengkoum, S. Short communication: Effect of purple corn pigment on change of anthocyanin composition and unsaturated fatty acids during milk storage. J. Dairy Sci. 2020, 103, 7808–7812. [Google Scholar] [CrossRef] [PubMed]
- Marek, A.; Koloman, K.; Ján, J.; Pavol, F.; Milan, K. The effect of conditions and storage time on course of moisture and temperature of maize grains. BIO Web Conf. 2018, 10, 02001. [Google Scholar]
- Roizman, V.; Jonckheere, M.; Pascal, F. Robust clustering and outlier rejection using the Mahalanobis distance distribution. In Proceedings of the 2020 28th European Signal Processing Conference (EUSIPCO), Amsterdam, The Netherlands, 18–21 January 2021. [Google Scholar]
- Vareldzhan, G.; Yurkov, K.; Ushenin, K. Anomaly Detection in Image Datasets Using Convolutional Neural Networks, Center Loss, and Mahalanobis Distance. In Proceedings of the 2021 Ural Symposium on Biomedical Engineering, Radioelectronics and Information Technology (USBEREIT), Yekaterinburg, Russia, 13–14 May 2021. [Google Scholar]
- Li, Y.-h.; Tan, X.; Zhang, W.; Jiao, Q.-b.; Xu, Y.-x.; Li, H.; Zou, Y.-b.; Yang, L.; Fang, Y.-p. Research and Application of Several Key Techniques in Hyperspectral Image Preprocessing. Front. Plant Sci. 2021, 12, 627865. [Google Scholar] [CrossRef]
- Cozzolino, D.; Williams, P.J.; Hoffman, L.C. An overview of pre-processing methods available for hyperspectral imaging applications. Microchem. J. 2023, 193, 109129. [Google Scholar] [CrossRef]
- He, J.; He, J.; Li, G.; Li, W.; Li, Z.; Li, Z. Inversion analysis of soil nitrogen content using hyperspectral images with different preprocessing methods. Ecol. Inform. 2023, 78, 102381. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, X.; Hu, Y.; Wu, X.; Zhang, X.; Wang, P. Visualizing distribution of moisture content in tea leaves using optimization algorithms and NIR hyperspectral imaging. Comput. Electron. Agric. 2019, 160, 153–159. [Google Scholar] [CrossRef]
- Arif, M.; Qi, Y.; Dong, Z.; Wei, H. Rapid retrieval of cadmium and lead content from urban greenbelt zones using hyperspectral characteristic bands. J. Clean. Prod. 2022, 374, 133922. [Google Scholar] [CrossRef]
- Sonobe, R.; Hirono, Y. Applying Variable Selection Methods and Preprocessing Techniques to Hyperspectral Reflectance Data to Estimate Tea Cultivar Chlorophyll Content. Remote Sens. 2023, 15, 19. [Google Scholar] [CrossRef]
- Yang, C.; Feng, M.; Song, L.; Jing, B.; Xie, Y.; Wang, C.; Yang, W.; Xiao, L.; Zhang, M.; Song, X. Study on hyperspectral monitoring model of soil total nitrogen content based on fractional-order derivative. Comput. Electron. Agric. 2022, 201, 107307. [Google Scholar] [CrossRef]
- Xu, X.; Chen, S.; Xu, Z.; Yu, Y.; Zhang, S.; Dai, R. Exploring Appropriate Preprocessing Techniques for Hyperspectral Soil Organic Matter Content Estimation in Black Soil Area. Remote Sens. 2020, 12, 3765. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Liu, J.; Wei, Y.; An, D.; Wu, J. Maize seed variety identification using hyperspectral imaging and self-supervised learning: A two-stage training approach without spectral preprocessing. Expert Syst. Appl. 2024, 238, 122113. [Google Scholar] [CrossRef]
- Pal, M.; Foody, G.M. Feature Selection for Classification of Hyperspectral Data by SVM. IEEE Trans. Geosci. Remote Sens. 2010, 48, 2297–2307. [Google Scholar] [CrossRef]
- Chen, J.; Bai, T.; Zhang, N.; Zhu, L.; Zhang, X. Hyperspectral detection of sugar content for sugar-sweetened apples based on sample grouping and SPA feature selecting methods. Infrared Phys. Technol. 2022, 125, 104240. [Google Scholar]
- Zhang, Y.; Lu, G.; Zhou, X.; Cheng, J.-H. Non-Destructive Hyperspectral Imaging for Rapid Determination of Catalase Activity and Ageing Visualization of Wheat Stored for Different Durations. Molecules 2022, 27, 8648. [Google Scholar] [CrossRef]
- Ge, Y.; Song, S.; Yu, S.; Zhang, X.; Li, X. Rice seed classification by hyperspectral imaging system: A real-world dataset and a credible algorithm. Comput. Electron. Agric. 2024, 219, 108776. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Li, P.; Zeng, F.; Wang, H. Hyperspectral detection of salted sea cucumber adulteration using different spectral preprocessing techniques and SVM method. LWT 2021, 152, 112295. [Google Scholar]
- Chen, Y.; Chen, Z.; Yan, Q.; Liu, Y.; Wang, Q. Non-destructive detection of egg white and yolk morphology transformation and salt content of salted duck eggs in salting by hyperspectral imaging. Int. J. Biol. Macromol. 2024, 262, 130002. [Google Scholar] [CrossRef]
- Wan, G.; Liu, G.; He, J.; Luo, R.; Cheng, L.; Ma, C. Feature wavelength selection and model development for rapid determination of myoglobin content in nitrite-cured mutton using hyperspectral imaging. J. Food Eng. 2020, 287, 110090. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Fan, S.; Jiang, Y.; Li, J. Determination of Moisture Content of Single Maize Seed by Using Long-Wave Near-Infrared Hyperspectral Imaging (LWNIR) Coupled With UVE-SPA Combination Variable Selection Method. IEEE Access 2020, 8, 195229–195239. [Google Scholar] [CrossRef]
- Song, K.; Wang, S.H.; Yang, D.; Shi, T.Y. Combination of spectral and image information from hyperspectral imaging for the prediction and visualization of the total volatile basic nitrogen content in cooked beef. J. Food Meas. Charact. 2021, 15, 4006–4020. [Google Scholar] [CrossRef]
- Thennadil, S.; Blank, T.B.; Troy, T.L. Non-Invasive Method of Determining Skin Thickness and Characterizing Layers of Skin Tissue In Vivo. U.S. Patent US20010041829A1, 15 November 2001. [Google Scholar]
- Zhang, C.; Shi, Y.; Wei, Z.; Wang, R.; Li, T.; Wang, Y.; Zhao, X.; Gu, X. Hyperspectral estimation of the soluble solid content of intact netted melons decomposed by continuous wavelet transform. Front. Phys. 2022, 10, 1034982. [Google Scholar]
- Liu, W.; Li, M.; Zhang, M.; Wang, D.; Guo, Z.; Long, S.; Yang, S.; Wang, H.; Li, W.; Hu, Y.; et al. Estimating leaf mercury content in Phragmites australis based on leaf hyperspectral reflectance. Ecosyst. Health Sustain. 2020, 6, 1726211. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, W.; Zhuang, H.; Yoon, S.C.; Yang, Y.; Zhao, X. Hyperspectral imaging for a rapid detection and visualization of duck meat adulteration in beef. Food Anal. Methods 2019, 12, 2205–2215. [Google Scholar] [CrossRef]






| Sample Set | Sample Size | Fatty Acid Value (mg KOH/100 g) | |||
|---|---|---|---|---|---|
| Maximum | Minimum | Average Value | Standard Deviation | ||
| Training set | 242 | 102.93 | 22.68 | 58.44 | 21.87 |
| Test set | 121 | 98.49 | 23.48 | 51.85 | 18.55 |
| Pretreatment | Training Set | Test Set | ||||
|---|---|---|---|---|---|---|
| Rc2 | RMSEc | MAPEc | Rp2 | RMSEc | MAPEc | |
| RAW-BP | 0.8660 | 5.9845 | 0.3170 | 0.6798 | 0.7438 | 0.4553 |
| SG-BP | 0.8858 | 6.2572 | 0.0982 | 0.8293 | 7.7727 | 0.1266 |
| SG1-BP | 0.8613 | 7.7252 | 0.1312 | 0.8445 | 8.3271 | 0.1377 |
| SG2-BP | 0.9107 | 6.0349 | 0.0899 | 0.7208 | 10.9412 | 0.1361 |
| D1-BP | 0.9315 | 5.5645 | 0.0536 | 0.8150 | 8.7462 | 0.1263 |
| D2-BP | 0.9093 | 6.2641 | 0.0763 | 0.6989 | 13.0575 | 0.1853 |
| MSC-BP | 0.8387 | 8.0021 | 0.1165 | 0.8617 | 8.8030 | 0.1455 |
| SNV-BP | 0.9297 | 5.4476 | 0.0757 | 0.8218 | 8.6234 | 0.1240 |
| RAW-CARS-BP | 0.8119 | 6.7643 | 0.4312 | 0.6915 | 9.5862 | 0.5957 |
| SG-CARS-BP | 0.8685 | 7.9494 | 0.1166 | 0.8394 | 9.1946 | 0.1381 |
| SG1-CARS-BP | 0.9043 | 6.2501 | 0.0917 | 0.8122 | 8.5736 | 0.1384 |
| SG2-CARS-BP | 0.9204 | 5.5003 | 0.0770 | 0.8548 | 8.4467 | 0.1210 |
| D1-CARS-BP | 0.7980 | 8.2932 | 0.4819 | 0.7972 | 8.5857 | 0.5528 |
| D2-CARS-BP | 0.8645 | 5.8363 | 0.2916 | 0.7015 | 9.7550 | 0.4691 |
| MSC-CARS-BP | 0.8236 | 6.5603 | 0.2478 | 0.8017 | 7.0142 | 0.3599 |
| SNV-CARS-BP | 0.8226 | 6.0610 | 0.3097 | 0.8214 | 6.9934 | 0.3574 |
| RAW-SPA-BP | 0.8506 | 8.1238 | 0.1287 | 0.7090 | 11.1226 | 0.1624 |
| SG-SPA-BP | 0.8819 | 6.4897 | 0.1037 | 0.8262 | 8.4327 | 0.1258 |
| SG1-SPA-BP | 0.9241 | 5.5883 | 0.0838 | 0.8820 | 7.5515 | 0.1191 |
| SG2-SPA-BP | 0.8945 | 6.4355 | 0.0989 | 0.7627 | 8.5817 | 0.1377 |
| D1-SPA-BP | 0.9089 | 6.1796 | 0.07762 | 0.8705 | 7.5989 | 0.1033 |
| D2-SPA-BP | 0.8437 | 7.9903 | 0.0959 | 0.6319 | 14.2419 | 0.2258 |
| MSC-SPA-BP | 0.8933 | 6.4775 | 0.1014 | 0.8949 | 7.0581 | 0.1076 |
| SNV-SPA-BP | 0.7989 | 8.5473 | 0.1311 | 0.7074 | 9.017 | 0.1428 |
| Pretreatment | Training Set | Test Set | ||||
|---|---|---|---|---|---|---|
| Rc2 | RMSEc | MAPEc | Rp2 | RMSEc | MAPEc | |
| RAW-RF | 0.8828 | 8.9837 | 0.0911 | 0.8460 | 6.5106 | 0.0979 |
| SG-RF | 0.8689 | 6.1217 | 0.0920 | 0.8534 | 6.6442 | 0.0990 |
| SG1-RF | 0.9695 | 3.4059 | 0.0481 | 0.9354 | 4.1953 | 0.0626 |
| SG2-RF | 0.9537 | 3.9452 | 0.0589 | 0.9567 | 3.8408 | 0.0567 |
| D1-RF | 0.9669 | 3.4372 | 0.0476 | 0.9467 | 4.0964 | 0.0578 |
| D2-RF | 0.9247 | 4.4579 | 0.0682 | 0.9017 | 5.7885 | 0.0922 |
| MSC-RF | 0.9338 | 4.6645 | 0.0649 | 0.8727 | 6.0067 | 0.0953 |
| SNV-RF | 0.9304 | 4.7527 | 0.0689 | 0.8984 | 5.6229 | 0.0854 |
| RAW-CARS-RF | 0.8828 | 5.8947 | 0.0872 | 0.8440 | 6.9076 | 0.1089 |
| SG-CARS-RF | 0.8703 | 6.1707 | 0.0910 | 0.8641 | 6.4221 | 0.0996 |
| SG1-CARS-RF | 0.9562 | 3.8170 | 0.0554 | 0.9504 | 4.1216 | 0.0603 |
| SG2-CARS-RF | 0.9538 | 3.9964 | 0.0569 | 0.9196 | 4.9458 | 0.0746 |
| D1-CARS-RF | 0.9555 | 3.9768 | 0.0497 | 0.9250 | 4.7160 | 0.7012 |
| D2-CARS-RF | 0.9103 | 4.8881 | 0.0738 | 0.9018 | 5.8638 | 0.0972 |
| MSC-CARS-RF | 0.9237 | 4.9561 | 0.0715 | 0.9068 | 5.2608 | 0.0779 |
| SNV-CARS-RF | 0.9224 | 5.0055 | 0.0710 | 0.9176 | 5.2332 | 0.0759 |
| RAW-SPA-RF | 0.8425 | 6.6777 | 0.1035 | 0.8523 | 6.4739 | 0.1030 |
| SG-SPA-RF | 0.8564 | 6.2990 | 0.0973 | 0.8052 | 7.4769 | 0.1181 |
| SG1-SPA-RF | 0.9715 | 3.1123 | 0.0408 | 0.9134 | 4.9158 | 0.0695 |
| SG2-SPA-RF | 0.9615 | 3.6275 | 0.0520 | 0.9655 | 3.6255 | 0.0523 |
| D1-SPA-RF | 0.9617 | 3.5357 | 0.04816 | 0.9462 | 4.5827 | 0.0659 |
| D2-SPA-RF | 0.9015 | 5.5503 | 0.0884 | 0.8338 | 5.4602 | 0.0898 |
| MSC-SPA-RF | 0.9168 | 5.2789 | 0.0733 | 0.9202 | 4.8252 | 0.0716 |
| SNV-SPA-RF | 0.9224 | 5.0055 | 0.0710 | 0.9176 | 5.2332 | 0.0759 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, S.; Zhou, X.; Cheng, J. Study on Rapid Non-Destructive Detection Method of Corn Freshness Based on Hyperspectral Imaging Technology. Molecules 2024, 29, 2968. https://doi.org/10.3390/molecules29132968
Zhang Y, Liu S, Zhou X, Cheng J. Study on Rapid Non-Destructive Detection Method of Corn Freshness Based on Hyperspectral Imaging Technology. Molecules. 2024; 29(13):2968. https://doi.org/10.3390/molecules29132968
Chicago/Turabian StyleZhang, Yurong, Shuxian Liu, Xianqing Zhou, and Junhu Cheng. 2024. "Study on Rapid Non-Destructive Detection Method of Corn Freshness Based on Hyperspectral Imaging Technology" Molecules 29, no. 13: 2968. https://doi.org/10.3390/molecules29132968
APA StyleZhang, Y., Liu, S., Zhou, X., & Cheng, J. (2024). Study on Rapid Non-Destructive Detection Method of Corn Freshness Based on Hyperspectral Imaging Technology. Molecules, 29(13), 2968. https://doi.org/10.3390/molecules29132968







