Fluorinated Fullerenes as Electrolyte Additives for High Ionic Conductivity Lithium-Ion Batteries
Abstract
1. Introduction
2. Results and Discussion
3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Li, M.; Fang, S.; Wang, Y.; He, H.; Wang, C.; Zhang, Z.; Yuan, B.; Jiang, L.; Baughman, R.H.; et al. Water-induced strong isotropic MXene-bridged graphene sheets for electrochemical energy storage. Science 2024, 383, 771–777. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, M.; Viswanathan, V.V.; Swart, B.; Shao, Y.; Wu, G.; Zhou, C. 3D printing technologies for electrochemical energy storage. Nano Energy 2017, 40, 418–431. [Google Scholar] [CrossRef]
- Wan, H.; Wang, Z.; Zhang, W.; He, X.; Wang, C. Interface design for all-solid-state lithium batteries. Nature 2023, 623, 739–744. [Google Scholar] [CrossRef]
- Quilty, C.D.; Wu, D.; Li, W.; Bock, D.C.; Wang, L.; Housel, L.M.; Abraham, A.; Takeuchi, K.J.; Marschilok, A.C.; Takeuchi, E.S. Electron and ion transport in lithium and lithium-ion battery negative and positive composite electrodes. Chem. Rev. 2023, 123, 1327–1363. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Yu, W.; Xiao, R.; Huang, F.; Tian, H.; Wang, C.; Chen, X.; Shao, J. Scalable fabrication of turbostratic graphene with high density and high ion conductivity for compact capacitive energy storage. Matter 2023, 6, 4032–4049. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Liu, L.; Lang, J.; Qiu, J. A low-concentration and high ionic conductivity aqueous electrolyte toward ultralow-temperature zinc-ion hybrid capacitors. Small Struct. 2023, 4, 2200345. [Google Scholar] [CrossRef]
- Shi, P.; Ma, J.; Liu, M.; Guo, S.; Huang, Y.; Wang, S.; Zhang, L.; Chen, L.; Yang, K.; Liu, X.; et al. A dielectric electrolyte composite with high lithium-ion conductivity for high-voltage solid-state lithium metal batteries. Nat. Nanotechnol. 2023, 18, 602–610. [Google Scholar] [CrossRef]
- Wee, G.; Larsson, O.; Srinivasan, M.; Berggren, M.; Crispin, X.; Mhaisalkar, S. Effect of the ionic conductivity on the performance of polyelectrolyte-based supercapacitors. Adv. Funct. Mater. 2010, 20, 4344–4350. [Google Scholar] [CrossRef]
- Lu, D.-L.; Zhao, R.-R.; Wu, J.-L.; Ma, J.-M.; Huang, M.-L.; Yao, Y.-B.; Tao, T.; Liang, B.; Zhai, J.-W.; Lu, S.-G. Investigations on the properties of Li3xLa2/3−xTiO3 based all-solid-state supercapacitor: Relationships between the capacitance, ionic conductivity, and temperature. J. Eur. Ceram. Soc. 2020, 40, 2396–2403. [Google Scholar] [CrossRef]
- Hu, E.; Xu, K. An electrolyte additive allows stable high-voltage cycling of a nickel-rich layered cathode. Nat. Energy 2022, 7, 482–483. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Pollard, T.P.; Li, Q.; Tan, S.; Hou, S.; Wan, H.; Chen, F.; He, H.; Hu, E.; et al. Electrolyte design for Li-ion batteries under extreme operating conditions. Nature 2023, 614, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Tölle, P.; Köhler, C.; Marschall, R.; Sharifi, M.; Wark, M.; Frauenheim, T. Proton transport in functionalised additives for PEM fuel cells: Contributions from atomistic simulations. Chem. Soc. Rev. 2012, 41, 5143–5159. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xie, X.; Xing, Z.; Chen, X.; Fang, G.; Lu, B.; Zhou, J.; Liang, S.; Fan, H.J. Mechanistic insights of Mg2+-electrolyte additive for high-energy and long-life zinc-ion hybrid capacitors. Adv. Energy Mater. 2021, 11, 2101158. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Liu, A.; Zhang, Y.; Li, Z.; Chen, H.; Shi, Z. Effect of LiOH solution additives on ionic conductivity of Li6.25Al0.25La3Zr2O12 electrolytes prepared by cold sintering. J. Mater. Sci. Mater. Electron. 2022, 33, 19187–19194. [Google Scholar] [CrossRef]
- Kim, S.; Kwon, Y.M.; Cho, K.Y.; Yoon, S. Metal iodides (LiI, MgI2, AlI3, TiI4, and SnI4) potentiality as electrolyte additives for Li−S batteries. Electrochim. Acta 2021, 391, 138927. [Google Scholar] [CrossRef]
- Goo, D.E.; Lee, G.R.; Hong, S.H.; Moon, H.C. Effect of novel ionic additives on the performance of lithium batteries. J. Ind. Eng. Chem. 2024, 132, 546–551. [Google Scholar] [CrossRef]
- Palluzzi, M.; Tsurumaki, A.; Mozhzhukhina, N.; Rizell, J.; Matic, A.; D’Angelo, P.; Navarra, M.A. Ionic liquids as cathode additives for high voltage lithium batteries. Batter. Supercaps 2024, e202400068. [Google Scholar] [CrossRef]
- Huang, Q.; Weng, J.; Ouyang, D.; Chen, M.; Wang, X.; Wang, J. Comparative studies on the combustion characteristics of electrolytes and carbonate mixed solvents with flame retardant additives under low pressures. Case Stud. Therm. Eng. 2023, 43, 102810. [Google Scholar] [CrossRef]
- Chen, M.; Mei, J.; Wang, S.; Chen, Q.; Zhao, L.; Kong, Q.; Wu, X. Comparative studies on the combustion characters of the lithium-ion battery electrolytes with composite flame-retardant additives. J. Energy Chem. 2022, 47, 103642. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Z.; Fu, S.; Zhang, Y.; Wang, R.; Mu, H.; Lian, C.; Wang, W.; Wang, G. A multifunctional electrolyte additive for zinc-ion capacitors with low temperature resistant and long lifespan. J. Energy Chem. 2024, 94, 477–485. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.-J. Electrolyte additives for lithium ion battery electrodes: Progress and perspectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Liu, L.; Meng, H.; Chai, Y.; Chen, X.; Xu, J.; Liu, X.; Liu, W.; Guldi, D.M.; Zhu, Y. Enhancing built-in electric fields for efficient photocatalytic hydrogen evolution by encapsulating C60 fullerene into zirconium-based metal-organic frameworks. Angew. Chem. Int. Ed. 2023, 62, e202217897. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, Y.; Li, Y.; Wang, Y.; Peng, B.; Davey, K.; Sun, L.; Li, G.; Zhang, S.; Guo, Z. C60 and derivatives boost electrocatalysis and photocatalysis: Electron buffers to heterojunctions. Adv. Energy Mater. 2023, 13, 2302438. [Google Scholar] [CrossRef]
- Shen, W.; Azmy, A.; Li, G.; Mishra, A.; Syrgiannis, Z.; Zheng, W.; Volonakis, G.; Kepenekian, M.; Even, J.; Wojtas, L.; et al. A crystalline 2D fullerene-based metal halide semiconductor for efficient and stable ideal-bandgap perovskite solar cells. Adv. Energy Mater. 2024, 14, 2400582. [Google Scholar] [CrossRef]
- Szala-Bilnik, J.; Costa Gomes, M.F.; Pádua, A.A.H. Solvation of C60 fullerene and C60F48 fluorinated fullerene in molecular and ionic liquids. J. Phys. Chem. C 2016, 120, 19396–19408. [Google Scholar] [CrossRef]
- Okino, F.; Yajima, S.; Suganuma, S.; Mitsumoto, R.; Seki, K.; Touhara, H. Fluorination of fullerene C60 and electrochemical properties of C60Fx. Synth. Met. 1995, 70, 1447–1448. [Google Scholar] [CrossRef]
- Oreshkin, A.I.; Muzychenko, D.A.; Oreshkin, S.I.; Panov, V.I.; Bakhtizin, R.Z.; Petukhov, M.N. Fluorinated fullerene molecule on Cu(001) surface as a controllable source of fluorine atoms. J. Phys. Chem. C 2018, 122, 24454–24458. [Google Scholar] [CrossRef]
- Kalika, E.B.; Katin, K.P.; Kochaev, A.I.; Kaya, S.; Elik, M.; Maslov, M.M. Fluorinated carbon and boron nitride fullerenes for drug delivery: Computational study of structure and adsorption. J. Mol. Liq. 2022, 353, 118773. [Google Scholar] [CrossRef]
- Li, G.; Duan, X.; Liu, X.; Zhan, R.; Wang, X.; Du, J.; Chen, Z.; Li, Y.; Cai, Z.; Shen, Y.; et al. Locking active Li metal through localized redistribution of fluoride enabling stable Li-metal batteries. Adv. Mater. 2023, 35, 2207310. [Google Scholar] [CrossRef]
- Li, L.F.; Lee, H.S.; Li, H.; Yang, X.Q.; Huang, X.J. A pentafluorophenylboron oxalate additive in non-aqueous electrolytes for lithium batteries. Electrochem. Commun. 2009, 11, 2296–2299. [Google Scholar] [CrossRef]
- Wang, R.; Parent, L.R.; Zhong, Y. Sulfur poisoning mechanism of LSCF cathode material in the presence of SO2: A computational and experimental study. J. Mater. Inf. 2023, 3, 3. [Google Scholar] [CrossRef]
- Ma, B.; Yu, F.; Zhou, P.; Wu, X.; Zhao, C.; Lin, C.; Gao, M.; Lin, T.; Sa, B. Machine learning accelerated discovery of high transmittance in (K0.5Na0.5)NbO3-based ceramics. J. Mater. Inf. 2023, 3, 13. [Google Scholar] [CrossRef]
- Bauer, B.; Bravyi, S.; Motta, M.; Chan, G.K.-L. Quantum algorithms for quantum chemistry and quantum materials science. Chem. Rev. 2020, 120, 12685–12717. [Google Scholar] [CrossRef]
- Goryunkov, A.A.; Kareev, I.E.; Ioffe, I.N.; Popov, A.A.; Kuvychko, I.V.; Markov, V.Y.; Goldt, I.V.; Pimenova, A.S.; Serov, M.G.; Avdoshenko, S.M.; et al. Reaction of C60 with KMnF4: Isolation and characterization of a new isomer of C60F8 and re-evaluation of the structures of C60F7(CF3) and the known isomer of C60F8. J. Fluor. Chem. 2006, 127, 1423–1435. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, Z.; Si, Y.; Sa, B.; Li, H.; Yu, T.; Wen, C.; Wu, B. Structural, electronic, and nonlinear optical properties of C66H4 and C70Cl6 encapsulating Li and F atoms. ACS Omega 2021, 6, 16234–16240. [Google Scholar] [CrossRef]
- Raghavachari, K. Perspective on “Density functional thermochemistry. III. The role of exact exchange”. Theor. Chem. Acc. 2000, 103, 361–363. [Google Scholar] [CrossRef]
- Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Maxwell, D.S.; Tirado-Rives, J. Development and testing of the opls all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996, 118, 11225–11236. [Google Scholar] [CrossRef]
- Wang, C.L.W.; Liao, K.; Wang, Z.; Wang, Y.; Gong, K. AuToFF Program; Version 1.0; Hzwtech: Shanghai, China, 2023. [Google Scholar]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Lai, P.; Huang, B.; Deng, X.; Li, J.; Hua, H.; Zhang, P.; Zhao, J. A localized high concentration carboxylic ester-based electrolyte for high-voltage and low temperature lithium batteries. Chem. Eng. J. 2023, 461, 141904. [Google Scholar] [CrossRef]
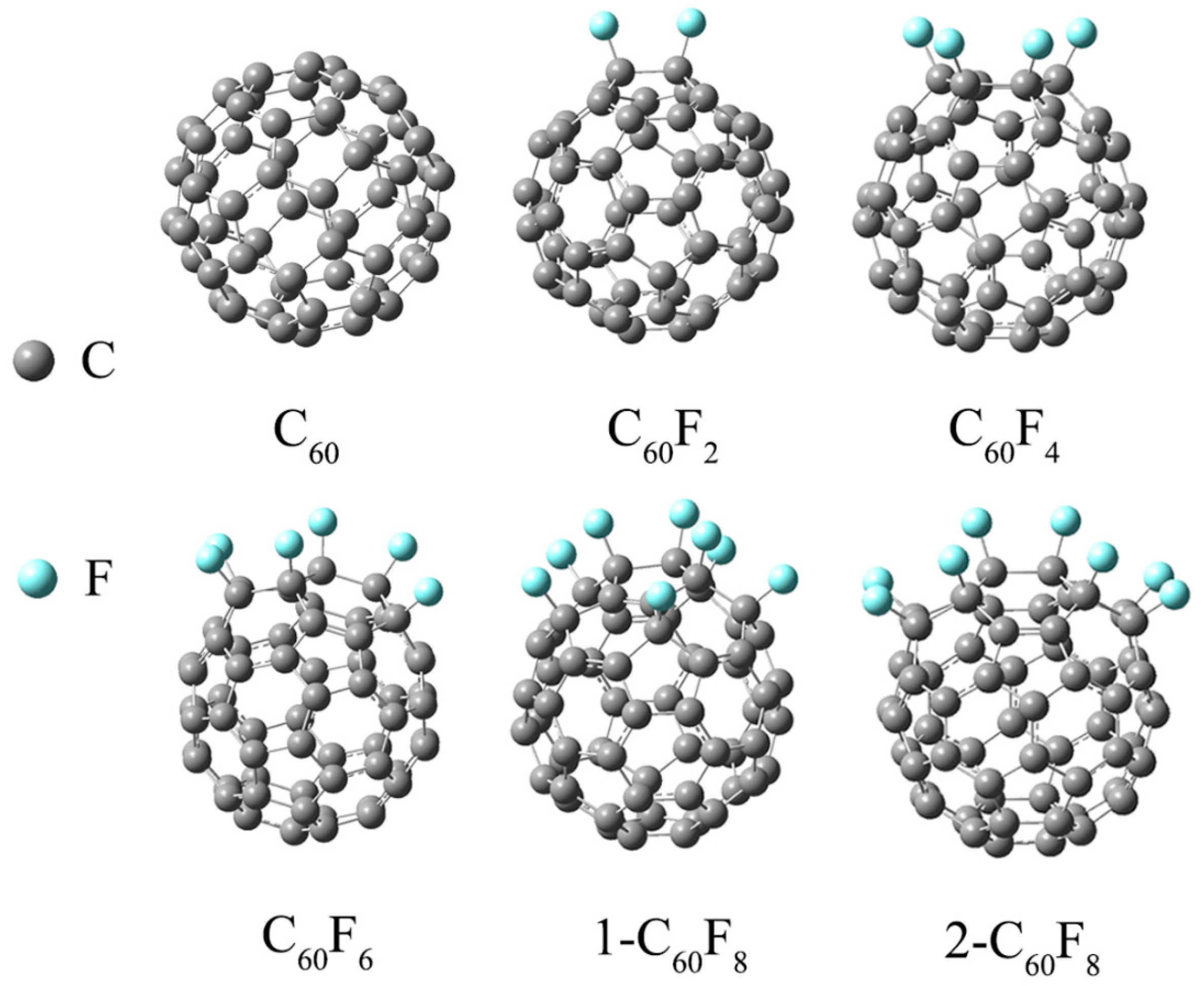

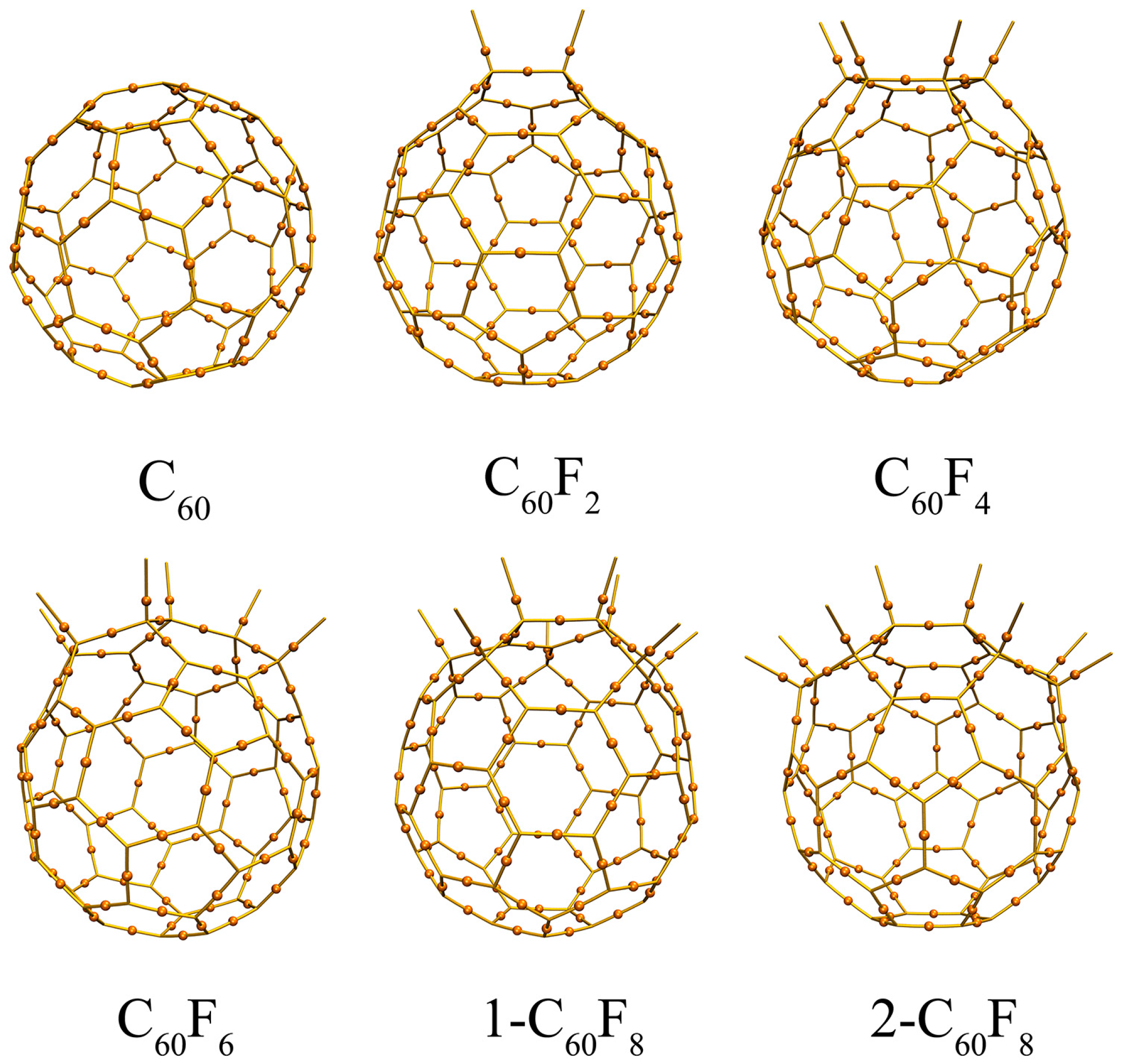
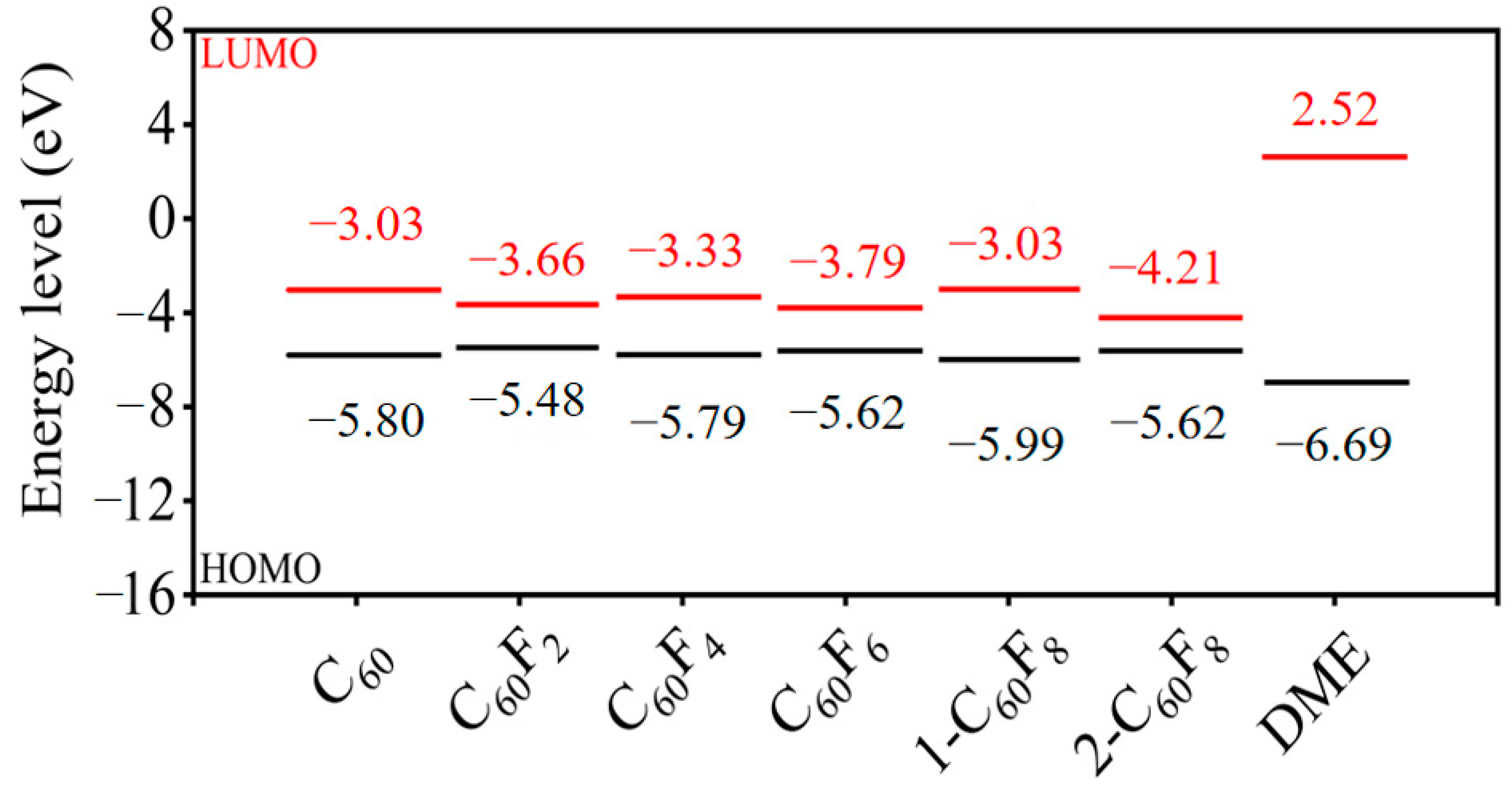

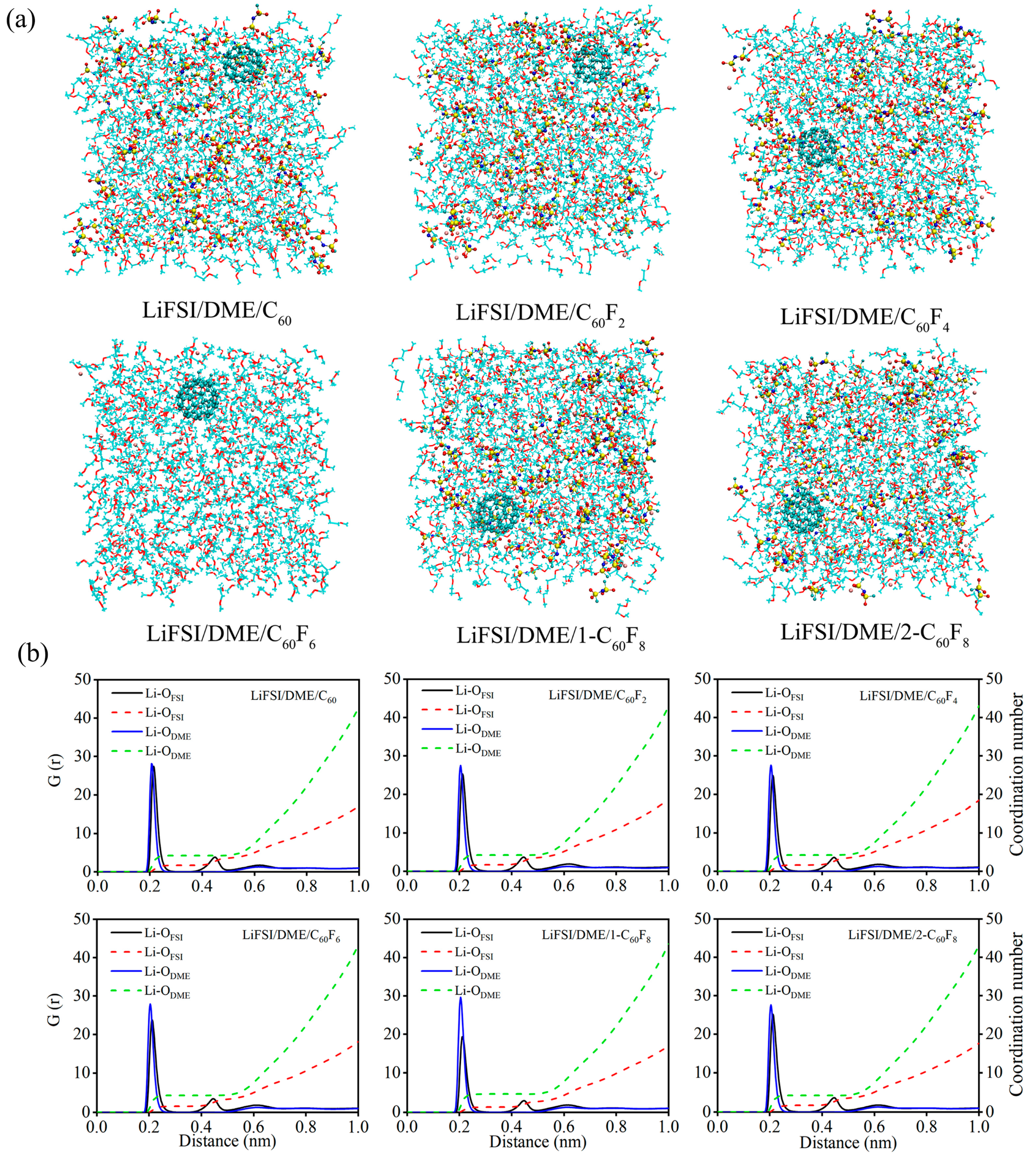
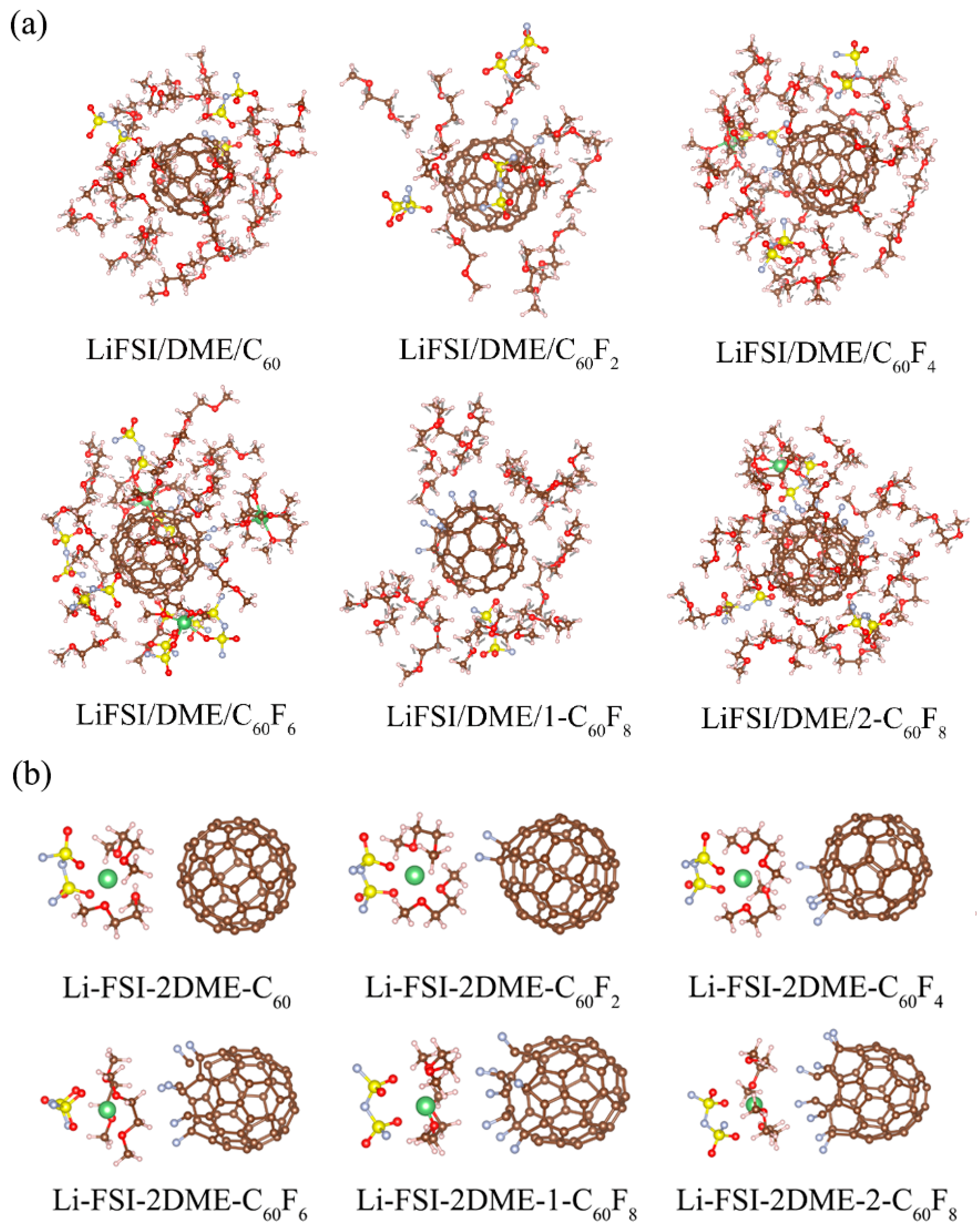
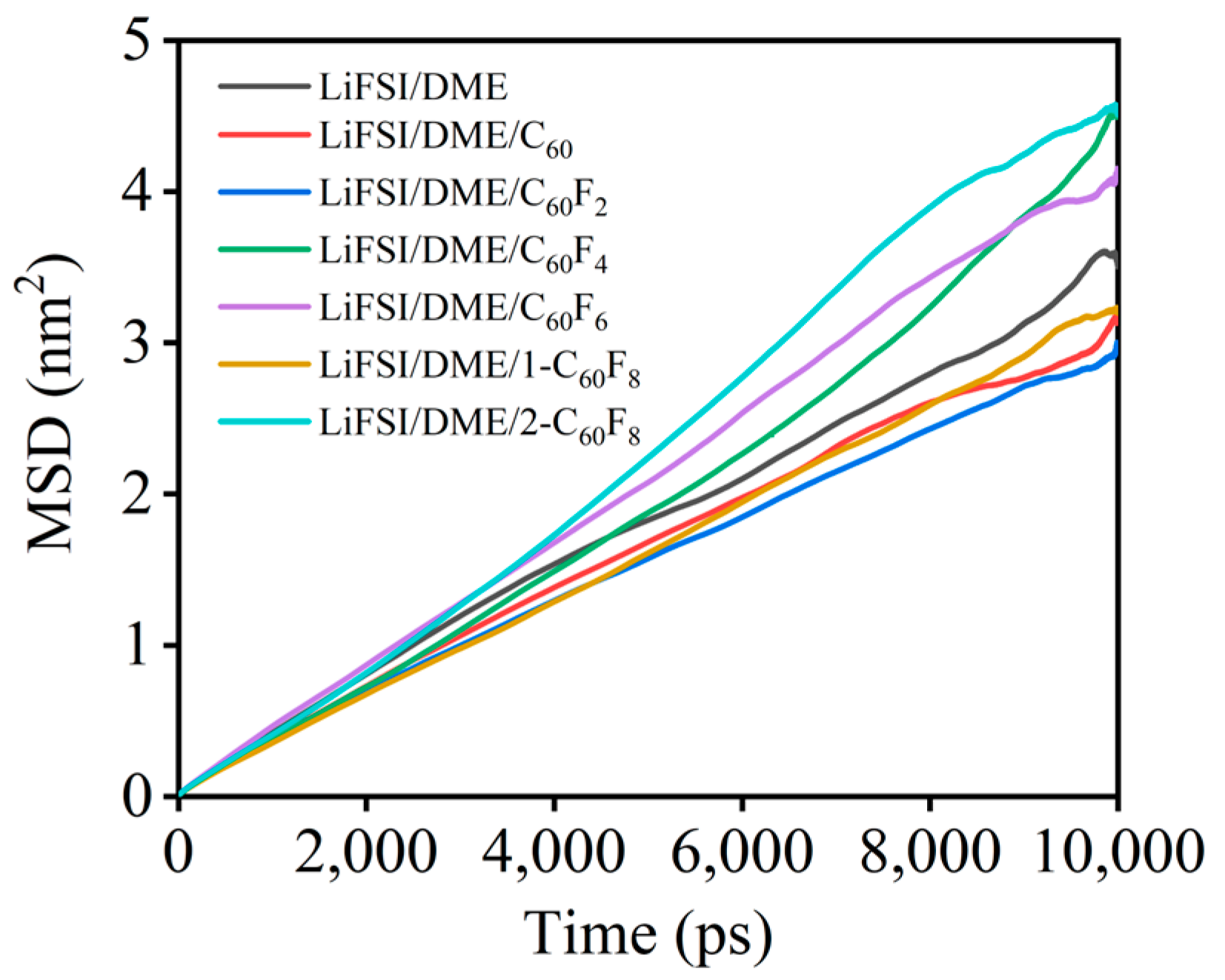
| Features | C60 | C60F2 | C60F4 | C60F6 | 1-C60F8 | 2-C60F8 | |
|---|---|---|---|---|---|---|---|
| C-C bonding | L (Å) | 1.45 | 1.62 | 1.60 | 1.62 | 1.58 | 1.58 |
| ρBCP | 0.28 | 0.22 | 0.26 | 0.22 | 0.23 | 0.21 | |
| HBCP | −0.25 | −0.16 | −0.21 | −0.16 | −0.18 | −0.15 | |
| |VBCP|/GBCP | 4.11 | 4.67 | 4.60 | 4.67 | 4.87 | 4.71 | |
| MBO | 1.16 | 0.87 | 0.89 | 0.89 | 0.89 | 0.88 | |
| C-F bonding | L (Å) | / | 1.39 | 1.38 | 1.39 | 1.36 | 1.38 |
| ρBCP | / | 0.24 | 0.24 | 0.24 | 0.25 | 0.24 | |
| HBCP | / | −0.32 | −0.34 | −0.33 | −0.35 | −0.33 | |
| |VBCP|/GBCP | / | 2.12 | 2.05 | 2.07 | 2.04 | 2.07 | |
| MBO | / | 0.83 | 0.81 | 0.82 | 0.84 | 0.83 | |
| F atom | NPA charge | / | −0.37 | −0.36 | −0.35 | −0.35 | −0.35 |
| Electrolytes | CN-Li+-OFSI− | CN-Li+-ODME |
|---|---|---|
| LiFSI/DME | 1.90 | 4.07 |
| LiFSI/DME/C60 | 1.74 | 4.25 |
| LiFSI/DME/C60F2 | 1.73 | 4.25 |
| LiFSI/DME/C60F4 | 1.68 | 4.29 |
| LiFSI/DME/C60F6 | 1.61 | 4.37 |
| LiFSI/DME/1-C60F8 | 1.33 | 4.65 |
| LiFSI/DME/2-C60F8 | 1.73 | 4.24 |
| Electrolytes | Box Size (nm) | Molar Ratio | Viscosity (103 μPa·s) | Diffusion Coefficient (1 × 107 cm2/s) |
|---|---|---|---|---|
| LiFSI/DME | 4.81 × 4.81 × 4.81 | 100:602 | 3.64 | 5.30 |
| LiFSI/DME/C60 | 4.82 × 4.82 × 4.82 | 100:602:1 | 3.59 | 5.16 |
| LiFSI/DME/C60F2 | 4.81 × 4.81 × 4.81 | 100:602:1 | 4.46 | 4.78 |
| LiFSI/DME/C60F4 | 4.81 × 4.81 × 4.81 | 100:602:1 | 4.41 | 6.43 |
| LiFSI/DME/C60F6 | 4.81 × 4.81 × 4.81 | 100:602:1 | 3.57 | 6.79 |
| LiFSI/DME/1-C60F8 | 4.81 × 4.81 × 4.81 | 100:602:1 | 4.92 | 5.26 |
| LiFSI/DME/2-C60F8 | 4.82 × 4.82 × 4.82 | 100:602:1 | 3.99 | 8.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, H.; Yang, Z.; Chen, J.; Li, H.; Wen, C.; Sa, B. Fluorinated Fullerenes as Electrolyte Additives for High Ionic Conductivity Lithium-Ion Batteries. Molecules 2024, 29, 2955. https://doi.org/10.3390/molecules29132955
Pan H, Yang Z, Chen J, Li H, Wen C, Sa B. Fluorinated Fullerenes as Electrolyte Additives for High Ionic Conductivity Lithium-Ion Batteries. Molecules. 2024; 29(13):2955. https://doi.org/10.3390/molecules29132955
Chicago/Turabian StylePan, Haoyu, Zhanlin Yang, Jianhui Chen, Hengyi Li, Cuilian Wen, and Baisheng Sa. 2024. "Fluorinated Fullerenes as Electrolyte Additives for High Ionic Conductivity Lithium-Ion Batteries" Molecules 29, no. 13: 2955. https://doi.org/10.3390/molecules29132955
APA StylePan, H., Yang, Z., Chen, J., Li, H., Wen, C., & Sa, B. (2024). Fluorinated Fullerenes as Electrolyte Additives for High Ionic Conductivity Lithium-Ion Batteries. Molecules, 29(13), 2955. https://doi.org/10.3390/molecules29132955







