Effect of Storage Conditions on the Volatilome, Biochemical Composition and Quality of Golden Delicious and Red Delicious Apple (Malus domestica) Varieties
Abstract
1. Introduction
2. Results
2.1. Effect of Storage Conditions on Firmness, Colour, Total Soluble Solids, Titratable Acidity and pH
2.2. Effect of Storage Conditions on the Malic Acid and Sugars Content
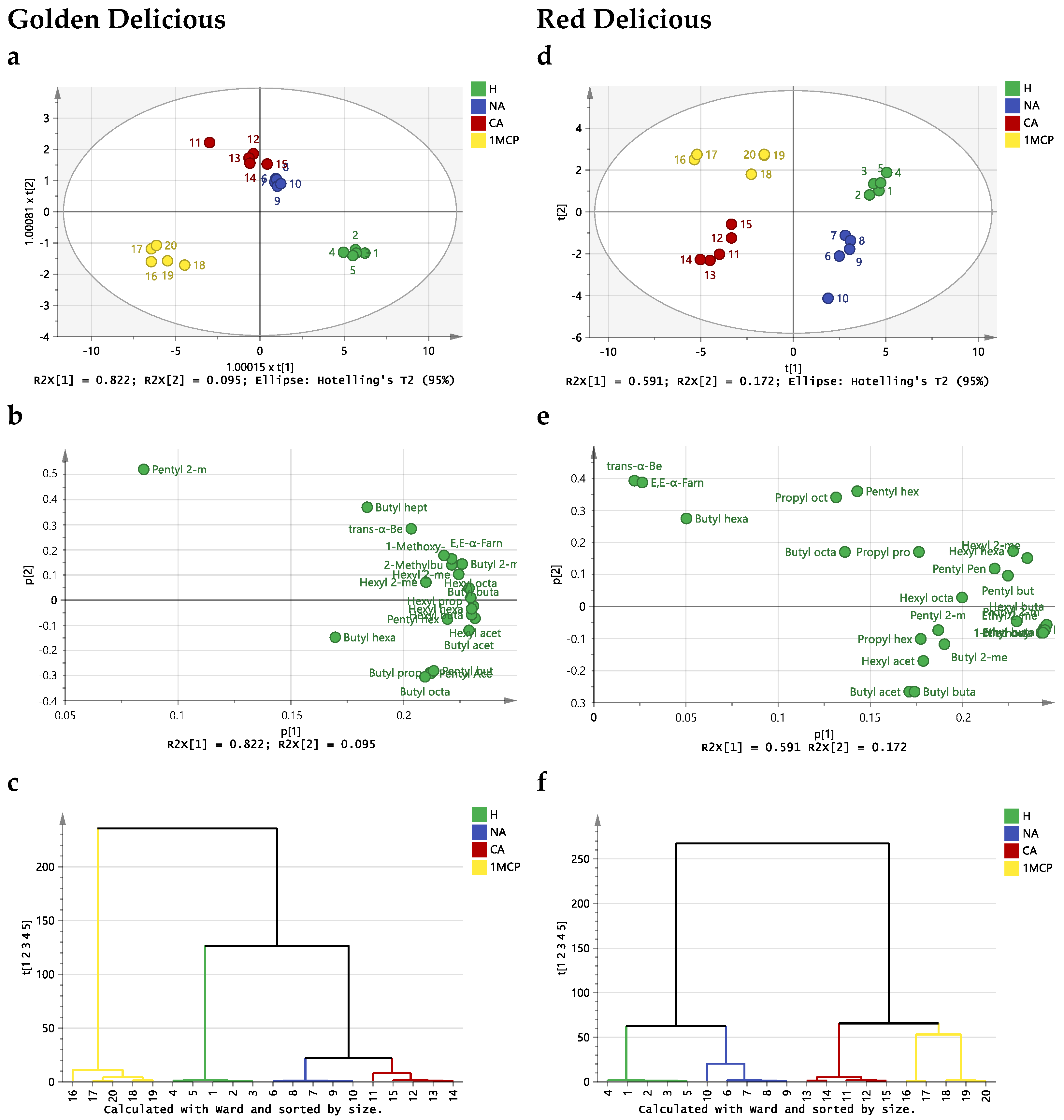
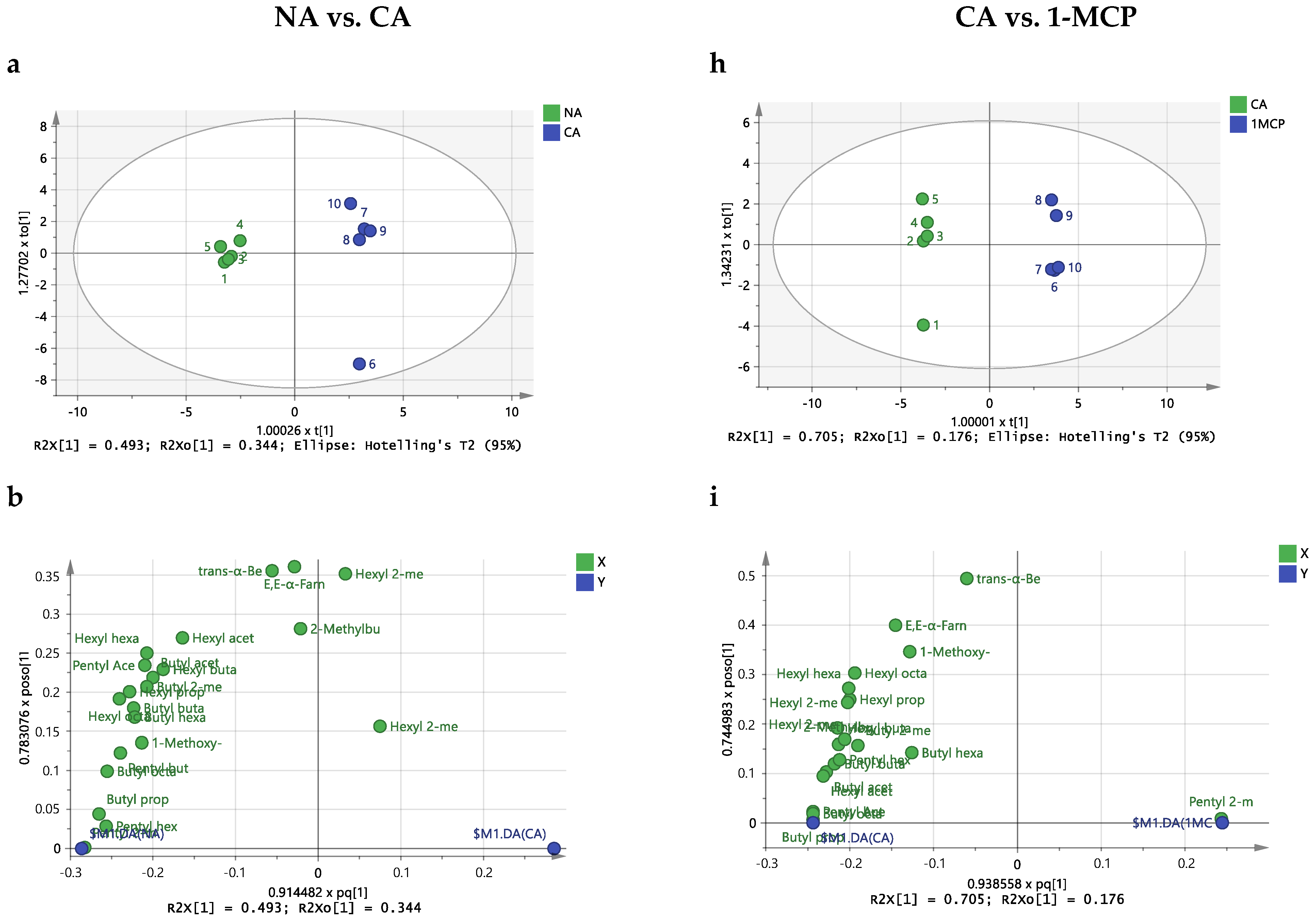
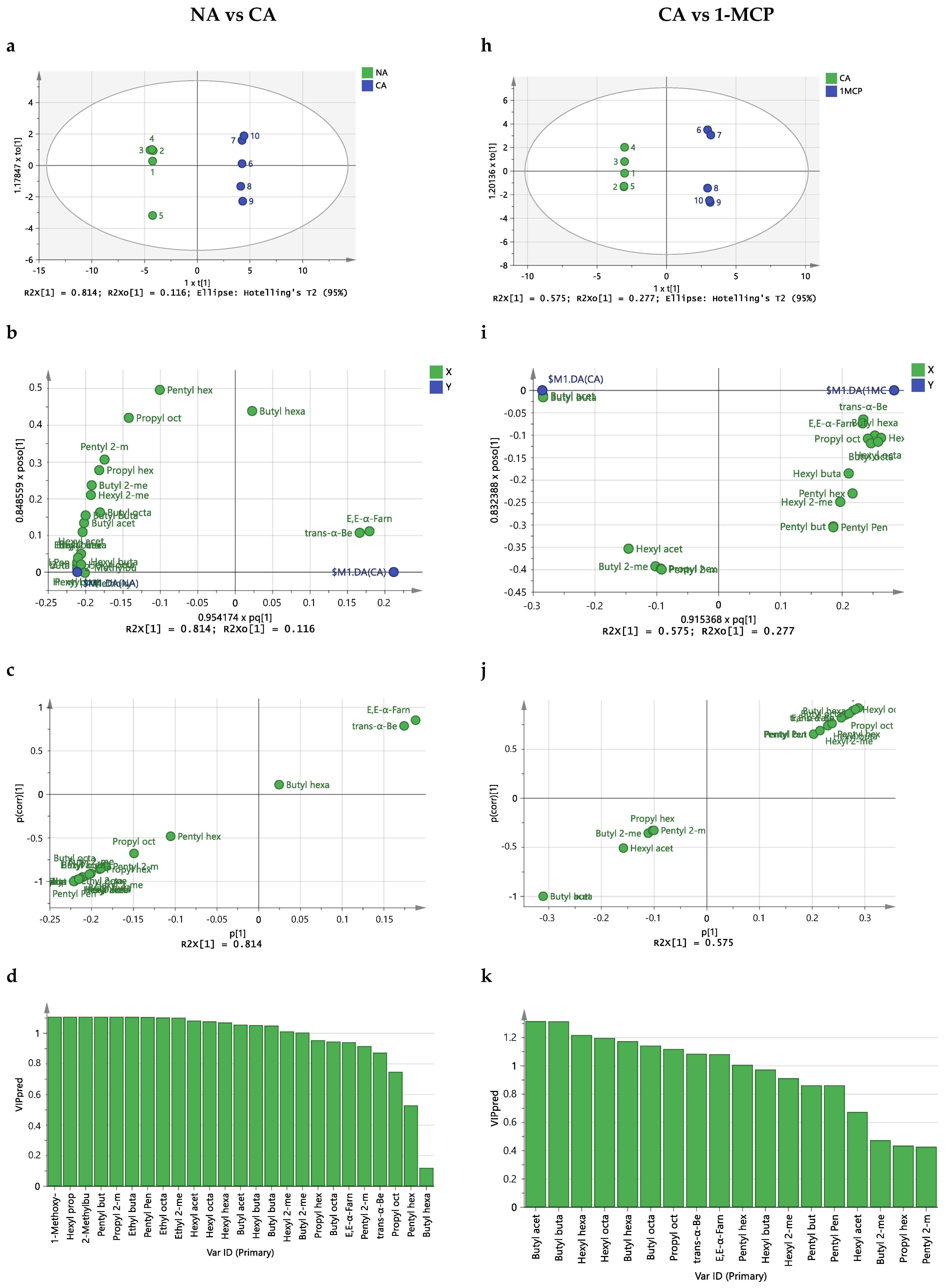
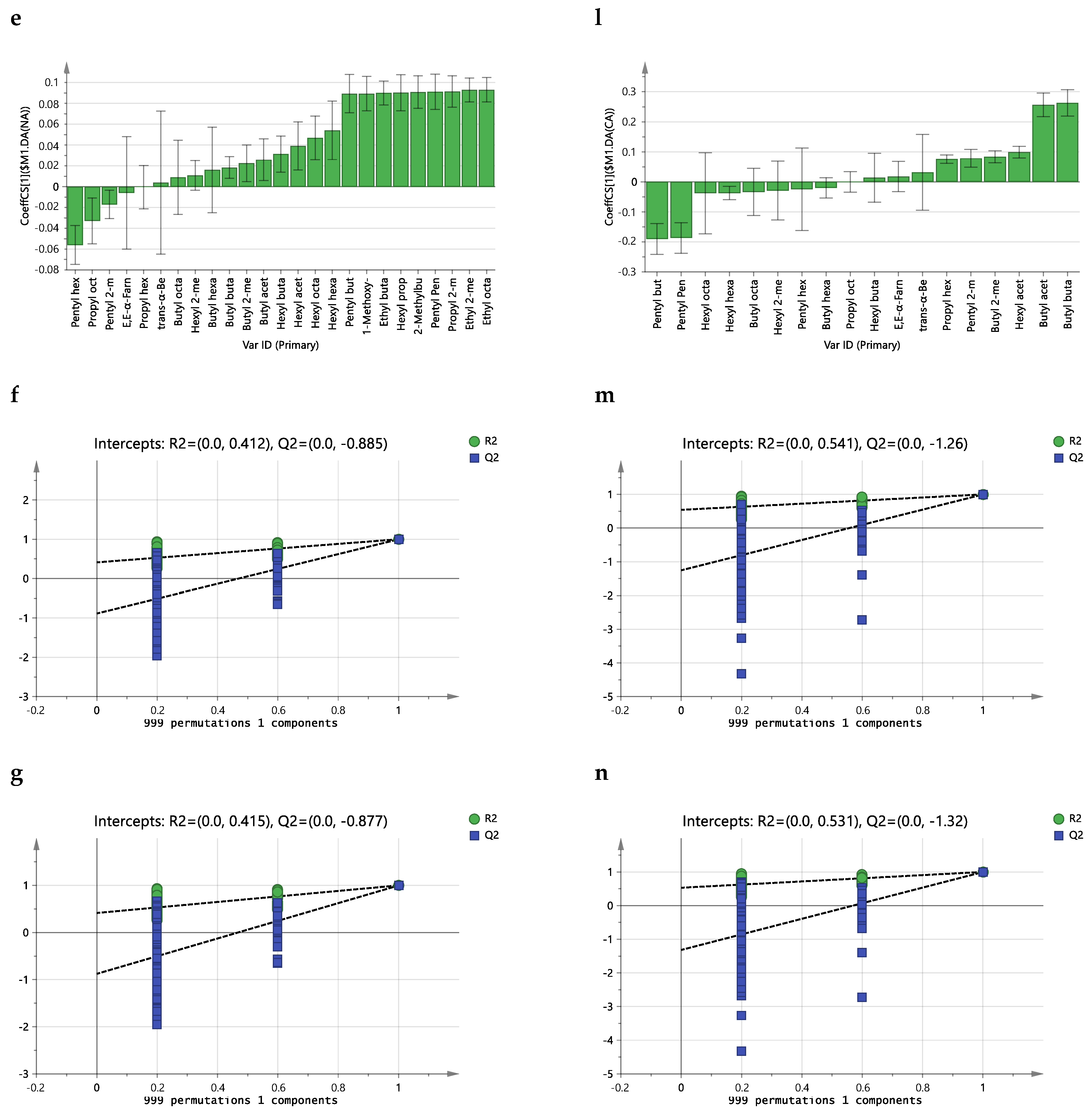
2.3. Effect of Storage Conditions on the Volatilome of Whole Apples
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Vegetable Material
4.3. Firmness
4.4. Total Soluble Solids, pH, Total Acidity
4.5. Chromatic Characteristics
4.6. Determination of Malic Acid
4.7. Determination of Free Sugars by High-Performance Anion Exchange Chromatography with Pulsed Amperometric Detection
4.8. Estimation of Apple Taste Parameter
4.9. Glycemic Index (GI) and Glycemic Load (GL)
4.10. Analysis of the Whole Apples Volatilome by HS-SPME/GC-MS
4.11. Statistical Analysis
4.11.1. Principal Component Analysis (PCA)
4.11.2. Orthogonal Partial Least Squares (OPLS-DA)—Discriminant Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization. FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QL (accessed on 22 May 2023).
- The European Market Potential for Apples. Available online: https://www.cbi.eu/market-information/fresh-fruit-vegetables/apples/market-potential (accessed on 22 May 2023).
- Harker, F.; Kupferman, E.M.; Marin, A.B.; Gunson, F.A.; Triggs, C.M. Eating quality standards for apples based on consumer preferences. Postharvest Biol. Technol. 2008, 50, 70–78. [Google Scholar] [CrossRef]
- Yang, L.; Cong, P.; He, J.; Bu, H.; Qin, S.; Lyu, D. Differential pulp cell wall structures lead to diverse fruit textures in apple (Malus domestica). Protoplasma 2022, 259, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, E.A. Fruit and vegetable flavor. In Handling, Transportation and Storage of Fruits, Vegetables, and Florist and Nursery Stock; Gross, K.C., Wang, C.Y., Saltveit, M.A., Eds.; Agriculture Handbook; United States Department of Agriculture (USDA): Washington, DC, USA, 2002; p. 66. [Google Scholar]
- Pérez, A.G.; Sanz, C. Formation of fruit flavour. In Fruit and Vegetable Flavour; Brückner, B., Wyllie, S.G., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 71–102. [Google Scholar]
- Dandekar, A.M.; Teo, G.; Defilippi, B.G.; Uratsu, S.L.; Passey, A.J.; Kader, A.A.; Stow, J.R.; Colgan, R.J.; James, D.J. Effect of down-regulation of ethylene biosynthesis on fruit flavor complex in apple fruit. Transgenic Res. 2004, 13, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.G.; Acree, T.E.; Barnard, J.; Butt, R.M.; Braell, P.A. Charm analysis of apple volatiles. Food Chem. 1986, 19, 137–147. [Google Scholar] [CrossRef]
- Sanz, C.; Olías, J.M.; Pérez, A.G. Aroma biochemistry of fruits and vegetables. In Phytochemistry of Fruits and Vegetables; Barberán, F.A.T., Robins, R.J., Eds.; Clarendon Press: Oxford, UK, 1997; pp. 125–255. [Google Scholar]
- Dimick, P.S.; Hoskin, J.C. Review of apple flavor-state of the art. Crit. Rev. Food Sci. Nutr. 1983, 18, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, E.; Grosch, W. Character impact odorants of the apple cultivars Elstar and Cox Orange. Nahrung 2002, 46, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Dunemann, F. Towards the development of molecular markers for apple volatiles. Flavour Fragr. J. 2012, 27, 286–289. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Cappellin, L.; Ting, V.; Costa, G.; Biasioli, F.; Costa, F. Dynamic volatile organic compound fingerprinting of apple fruit during processing. LWT-Food Sci. Technol. 2015, 63, 21–28. [Google Scholar] [CrossRef]
- Contreras, C.; Beaudry, R. Lipoxygenase-associated apple volatiles and their relationship with aroma perception during ripening. Postharvest Biol. Technol. 2013, 82, 28–38. [Google Scholar] [CrossRef]
- Holland, D.; Larkov, O.; Bar-Ya’akov, I.; Bar, E.; Zax, A.; Brandeis, E.; Ravid, U.; Lewinsohn, E. Developmental and varietal differences in volatile ester formation and acetyl-CoA:alcohol acetyl transferase activities in apple (Malus domestica Borkh.) fruit. J. Agric. Food Chem. 2005, 53, 7198–7203. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Du, L.; Forney, C.; Campbell-Palmer, L.; Fillmore, S.; Wismer, P.; Zhang, Z. Ethylene and 1-MCP regulate major volatile biosynthetic pathways in apple fruit. Food Chem. 2016, 194, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, C.; López, M.L.; Echeverría, G.; Graell, J. Effect of controlled atmospheres and shelf life period on concentrations of volatile substances released by ‘Pink Lady’ apples and on consumer acceptance. J. Sci. Food Agric. 2009, 89, 1023–1034. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverría, G.; Lopez, M. Fruit color development, anthocyanin content, standard quality, volatile compound emissions and consumer acceptability of several ‘Fuji’ apple strains. Sci. Hortic. 2012, 137, 138–147. [Google Scholar] [CrossRef]
- Marin, A.B.; Colonna, A.E.; Kudo, K.; Kupferman, E.M.; Mattheis, J.P. Measuring consumer response to ‘Gala’ apples treated with 1-methylcyclopropene (1-MCP). Postharvest Biol. Technol. 2009, 51, 73–79. [Google Scholar] [CrossRef]
- Lu, X.; Meng, G.; Jin, W.; Gao, H. Effects of 1-MCP in combination with Ca application on aroma volatiles production and softening of ‘Fuji’ apple fruit. Sci. Hortic. 2018, 229, 91–98. [Google Scholar] [CrossRef]
- Gupta, K.J.; Zabalza, A.; Van Dongen, J.T. Regulation of respiration when the oxygen availability changes. Physiol. Plant. 2009, 137, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Mattheis, J.P. Impact of 1-methylcyclopropene and methyl jasmonate on apple volatile production. J. Agric. Food Chem. 1999, 47, 2847–2853. [Google Scholar] [CrossRef]
- Mehinagic, E.; Royer, G.; Symoneaux, R.; Jourjon, F.; Prost, C. Characterization of Odor-Active Volatiles in Apples: Influence of Cultivars and Maturity Stage. J. Agric. Food Chem. 2006, 54, 2678–2687. [Google Scholar] [CrossRef] [PubMed]
- Ferenczi, A.; Song, J.; Tian, M.; Vlachonasios, K.; Dilley, D.; Beaudry, R. Volatile ester suppression and recovery following 1-methylcyclopropene application to apple fruit. J. Am. Soc. Hortic. Sci. 2006, 131, 691–701. [Google Scholar] [CrossRef]
- Zhu, Y.; Rudell, D.R.; Mattheis, J.P. Characterization of cultivar differences in alcohol acyltransferase and 1-aminocyclopropane-1-carboxylate synthase gene expression and volatile ester emission during apple fruit maturation and ripening. Postharvest Biol. Technol. 2008, 49, 330–339. [Google Scholar] [CrossRef]
- Rowan, D.D.; Lane, H.P.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of 2-methylbutyl, 2-methyl-2-butenyl, and 2-methylbutanoate esters in Red Delicious and Granny Smith apples using deuterium-labeled substrates. J. Agric. Food Chem. 1996, 44, 3276–3285. [Google Scholar] [CrossRef]
- Rowan, D.D.; Allen, J.M.; Fielder, S.; Hunt, M.B. Biosynthesis of straight-chain ester volatiles in Red Delicious and Granny Smith apples using deuterium-labeled precursors. J. Agric. Food Chem. 1999, 47, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, S.; Johansson, E.; Sjöström, L.; Mellerowicz, E.J.; Edlund, U.; Shockcor, J.P.; Gottfries, J.; Moritz, T.; Trygg, J. Visualization of GC/TOF-MS-based metabolomics data for identification of biochemically interesting compounds using OPLS class models. Anal. Chem. 2008, 80, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Xie, G.; Zhou, Z.; Shi, P.; Qiu, Y.; Zheng, X.; Chen, T.; Su, M.; Zhao, A.; Jia, W. Salivary metabolite signatures of oral cancer and leukoplakia. Int. J. Cancer 2011, 129, 2207–2217. [Google Scholar] [CrossRef] [PubMed]
- Cliff, M.A.; Stanich, K.; Lu, R.; Hampson, C.R. Use of descriptive analysis and preference mapping for early-stage assessment of new and established apples. J. Sci. Food Agric. 2016, 96, 2170–2183. [Google Scholar] [CrossRef]
- Brackmann, A.; Streif, J.; Bangerth, F. Relationship between a reduced aroma production and lipid metabolism of apples after long-term controlled-atmosphere storage. J. Am. Soc. Hortic. Sci. 1993, 118, 243–247. [Google Scholar] [CrossRef]
- Sisler, E.; Grichko, V.P.; Serek, M. Interaction of ethylene and other compounds with the ethylene receptor agonists and antagonists. In Ethylene Action in Plants; Serek, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–34. [Google Scholar]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharvest Biol. Technol. 2010, 56, 147–154. [Google Scholar] [CrossRef]
- Ng, J.K.T.; Schroder, R.; Brummell, D.A.; Sutherland, P.W.; Hallet, I.C.; Smith, B.G.; Melton, L.D.; Johnston, J.W. Lower cell wall pectin solubilizations and galactose loss during early fruit development in apple (Malus × domestica) cultivar “Scifresh” are associated with slower softening rate. J. Plant Physiol. 2015, 176, 129–137. [Google Scholar] [CrossRef]
- Gwanpua, S.G.; Buggenhout, S.V.; Verlinden, B.E.; Christiaens, S.; Shpigelman, A.; Vicent, V.; Kermani, Z.J.; Nicolai, B.M.; Hendrickx, M.; Geeraerd, A. Pectin modifications and the role of pectin-degrading enzymes during postharvest softening of “Jonagold” apples. Food Chem. 2014, 158, 283–291. [Google Scholar] [CrossRef]
- Ortiz, A.; Graell, J.; Lara, I. Cell wall-modifying enzymes and firmness loss in ripening “Golden Reinders” apples: A comparison between calcium dips and ULO storage. Food Chem. 2011, 128, 1072–1079. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Naing, A.H.; Kwon, J.-G.; Kang, I.-K. 1-Methylcyclopropene (1-MCP) treatment delays modification of cell wall pectin and fruit softening in “Hwangok” and “Picnic” apples during cold storage. Postharvest Biol. Technol. 2021, 180, 111599. [Google Scholar] [CrossRef]
- Suni, M.; Nyman, M.; Eriksson, N.-A.; Bjork, L.; Bjorck, I. Carbohydrate composition and content of organic acids in fresh and stored apples. J. Sci. Food Agric. 2000, 80, 1538–1544. [Google Scholar] [CrossRef]
- Róth, E.; Berna, A.; Beullens, K.; Yarramraju, S.; Lammertyn, J.; Schenk, A.; Nicolai, B. Postharvest quality of integrated and organically produced apple fruit. Postharvest Biol. Technol. 2007, 45, 11–19. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, R.; Li, B.; Tian, S. Characterisation of genes encoding key enzymes involved in sugar metabolism of apple fruit in controlled atmosphere storage. Food Chem. 2013, 141, 3323–3328. [Google Scholar] [CrossRef]
- Akbudak, B.; Ozer, M.; Erturk, U.; Cavusoglu, S. Response of 1-methylcyclopropene treated ‘Granny Smith’ apple fruit to air and controlled atmosphere storage conditions. J. Food Qual. 2009, 32, 18–33. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Murr, D.P.; Paliyath, G.; Skog, L. Inhibitory effect of 1-MCP on ripening and superficial scald development in ‘McIntosh’ and ‘Delicious’ apples. J. Hortic. Sci. Biotech. 2000, 75, 271–276. [Google Scholar] [CrossRef]
- DeEll, J.R.; Murr, D.P.; Porteous, M.D.; Rupasinghe, H.P.V. Influence of temperature and duration of 1-methylcyclopropene (1-MCP) treatment on apple quality. Postharvest Biol. Technol. 2002, 24, 349–353. [Google Scholar] [CrossRef]
- Larrigaudiere, C.; Vilaplana, R.; Soria, Y.; Recasens, I. Comparative study of the effects of 1-MCP treatment on apple quality by instrumental and multivariate analysis. J. Sci. Food Agric. 2008, 88, 1614–1621. [Google Scholar] [CrossRef]
- DeEll, J.R.; Murr, D.P.; Mueller, R.; Wiley, L.; Porteous, M.D. Influence of 1-methylcyclopropene (1-MCP), diphenylamine (DPA), and CO2 concentration during storage on ‘Empire’ apple quality. Postharvest Biol. Technol. 2005, 38, 1–8. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1-MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, M.C.; Ku, K.H. Chemical, physical, and sensory properties of 1-MCP-treated Fuji apple (Malus domestica Borkh.) fruits after long-term cold storage. Appl. Biol. Chem. 2017, 60, 363–374. [Google Scholar] [CrossRef]
- Howard, R. Ratio scales of sugar sweetness. Percept. Psychophys. 1970, 7, 315–320. [Google Scholar] [CrossRef]
- Pancoast, H.M.; Junk, W. Handbook of Sugars; AVI Publishing Co. Inc.: Westport, CT, USA, 1980. [Google Scholar]
- Salmeron, J.; Manson, J.; Stampfer, M.; Colditz, G.; Wing, A.; Willett, W. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA 1997, 277, 472–477. [Google Scholar] [CrossRef]
- Watkins, C.B.; Nock, J.F.; Whitaker, B.D. Responses of early, mid and late season apple cultivars to postharvest application of 1-methylcyclopropene (1-MCP) under air and controlled atmosphere storage conditions. Postharvest Biol. Technol. 2000, 19, 17–32. [Google Scholar] [CrossRef]
- DeLong, J.M.; Prange, R.K.; Harrison, P.A. The influence of 1-methylcyclopropene on ‘Cortland’ and ‘McIntosh’ apple quality following long-term storage. HortScience 2004, 39, 1062–1065. [Google Scholar] [CrossRef]
- Lu, X.; Ma, Y.; Liu, X. Effects of maturity and 1-MCP treatment on postharvest quality and antioxidant properties of ‘Fuji’ apples during long-term cold storage. Hortic. Environ. Biotechnol. 2012, 53, 378–386. [Google Scholar] [CrossRef]
- DeEll, J.R.; Ehsani-Moghaddam, B. Preharvest 1-methylcyclopropene treatment reduces soft scald in ‘Honeycrisp’ apples during storage. HortScience 2010, 45, 414–417. [Google Scholar] [CrossRef]
- DeEll, J.; Ehsani-Moghaddam, B. Effects of rapid consecutive postharvest 1-methylcyclopropene treatments on fruit quality and storage disorders in apples. HortScience 2013, 48, 227–232. [Google Scholar] [CrossRef]
- Neta, E.R.D.C.; Johanningsmeier, S.D.; McFeeters, R.F. The chemistry and physiology of sour taste—A review. J. Food Sci. 2007, 72, R33–R38. [Google Scholar] [CrossRef]
- Harker, F.; Marsh, K.; Young, H.; Murray, S.; Gunson, F.; Walker, S. Sensory interpretation of instrumental measurements 2: Sweet and acid taste of apple fruit. Postharvest Biol. Technol. 2002, 24, 241–250. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Impact of suppression of ethylene action or biosynthesis on flavor metabolites in apple (Malus domestica Borkh) fruits. J. Agric. Food Chem. 2004, 52, 5694–5701. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, Y.; Qin, G.; Tian, S. Molecular basis of 1-methylcyclopropene regulating organic acid metabolism in apple fruit during storage. Postharvest Biol. Technol. 2016, 117, 57–63. [Google Scholar] [CrossRef]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Mignard, P.; Beguería, S.; Giménez, R.; Font i Forcada, C.; Reig, G.; Moreno, M.Á. Effect of genetics and climate on apple sugars and organic acids profiles. Agronomy 2022, 12, 827. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Jia, R.; Yang, N.; Jin, L.; Zhu, L.; Ma, B.; Yao, Y.-X.; Ma, F.; Li, M. Malate metabolism mediated by the cytoplasmic malate dehydrogenase gene MdcyMDH affects sucrose synthesis in apple fruit. Hortic. Res. 2022, 9, uhac194. [Google Scholar] [CrossRef] [PubMed]
- Berüter, J. Carbohydrate metabolism in two apple genotypes that differ in malate accumulation. J. Plant Physiol. 2004, 161, 1011–1029. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.X.; Liu, X.H.; Chen, L.S. Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica Lindl.) cultivars differing in fruit acidity. Food Chem. 2009, 114, 657–664. [Google Scholar] [CrossRef]
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 2009, 70, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Thewes, F.R.; Brackmann, A.; Anese, R.O.; Wagner, R.; Schultz, E.E.; Berghetti, M.R.P. 1-Methylcyclopropene suppresses anaerobic metabolism in apples stored under dynamic controlled atmosphere monitored by respiratory quotient. Sci. Hortic. 2017, 227, 288–295. [Google Scholar] [CrossRef]
- Altisent, R.; Echeverría, G.; Graell, J.; López, L.; Lara, I. Lipoxygenase activity is involved in the regeneration of volatile ester-synthesizing capacity after ultra-low oxygen storage of ‘Fuji’ apple. J. Agric. Food Chem. 2009, 57, 4305–4312. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef]
- Both, V.; Brackmann, A.; Thewes, F.R.; Ferreira, D.D.F.; Wagner, R. Effect of storage under extremely low oxygen on the volatile composition of ‘Royal Gala’ apples. Food Chem. 2014, 156, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Blankenship, S.; Mattheis, J. 1-Methylcyclopropene inhibits apple ripening. J. Am. Soc. Hortic. Sci. 1999, 124, 690–695. [Google Scholar] [CrossRef]
- López, M.L.; Villatoro, C.; Fuentes, T.; Graell, J.; Lara, I.; Echeverría, G. Volatile compounds, quality parameters and consumer acceptance of ‘Pink Lady®’ apples stored in different conditions. Postharvest Biol. Technol. 2007, 43, 55–66. [Google Scholar] [CrossRef]
- Both, V.; Thewes, F.R.; Brackmann, A.; Ferreira, D.D.F.; Pavanello, E.P.; Wagner, R. Effect of low oxygen conditioning and ultra-low oxygen storage on the volatile profile, ethylene production and respiration rate of ‘Royal Gala’ apples. Sci. Hortic. 2016, 209, 156–164. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Kader, A.A.; Dandekar, A.M. Apple aroma: Alcohol acyltransferase, a rate limiting step for ester biosynthesis, is regulated by ethylene. Plant Sci. 2005, 168, 1199–1210. [Google Scholar] [CrossRef]
- Bangerth, F.K.; Song, J.; Streif, J. Physiological impacts of fruit ripening and storage conditions on aroma volatile formation in apple and strawberry fruit: A review. HortScience 2012, 47, 4–10. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, D.; Mi, H.; Pristijono, P.; Ge, Y.; Lv, J.; Li, Y.; Liu, B. Tissue-specific recovery capability of aroma biosynthesis in ‘Golden Delicious’ apple fruit after low oxygen storage. Agronomy 2022, 12, 2794. [Google Scholar] [CrossRef]
- Souleyre, E.J.F.; Greenwood, D.R.; Friel, E.N.; Karunairetnam, S.; Newcomb, R.D. An alcohol acyl transferase from apple (cv. Royal Gala), MpAAT1, produces esters involved in apple fruit flavor. FEBS J. 2005, 272, 3132–3144. [Google Scholar] [CrossRef]
- Watkins, C.B.; Nock, J.F. Effects of delays between harvest and 1-methylcyclopropene treatment, and temperature during treatment, on ripening of air-stored and controlled-atmosphere-stored apples. HortScience 2005, 40, 2096–2101. [Google Scholar] [CrossRef]
- Mir, N.; Curell, E.; Khan, N.; Whitaker, M. Harvest maturity, storage temperature, and 1-MCP application frequency alter firmness retention and chlorophyll fluorescence of ‘Redchief Delicious’ apples. J. Am. Soc. Hortic. Sci. 2001, 126, 618–624. [Google Scholar] [CrossRef]
- Nock, J.F.; Watkins, C.B. Repeated treatment of apple fruit with 1-methylcyclopropene (1-MCP) prior to controlled atmosphere storage. Postharvest Biol. Technol. 2013, 79, 73–79. [Google Scholar] [CrossRef]
- Jung, S.; Watkins, C.B. Internal ethylene concentrations in apple fruit at harvest affect persistence of inhibition of ethylene production after 1-Methylcyclopropene treatment. Postharvest Biol. Technol. 2014, 96, 1–6. [Google Scholar] [CrossRef]
- Dunemann, F.; Ulrich, D.; Malysheva-Otto, L.; Weber, W.E.; Longhi, S.; Velasco, R.; Costa, F. Functional allelic diversity of the apple alcohol acyl-transferase gene MdAAT1 associated with fruit ester volatile contents in apple cultivars. Mol. Breed. 2012, 29, 609–625. [Google Scholar] [CrossRef]
- Espino-Diaz, M.; Sepulveda, D.R.; Gonzalez-Aguilar, G.; Olivas, G.I. Biochemistry of apple aroma. Food Technol. Biotech. 2016, 54, 375–394. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, G.; Graell, J.; Lara, I.; López, M.L. Physicochemical measurements in ‘Mondial Gala®’ apples stored at different atmospheres: Influence on consumer acceptability. Postharvest Biol. Technol. 2008, 50, 135–144. [Google Scholar] [CrossRef]
- Schmidt, S.F.P.; Schultz, E.E.; Ludwig, V.; Berghetti, M.R.P.; Thewes, F.R.; Both, V. Volatile compounds and overall quality of ‘Braeburn’ apples after long-term storage: Interaction of innovative storage technologies and 1-MCP treatment. Sci. Hortic. 2020, 262, 109039. [Google Scholar] [CrossRef]
- Yan, D.; Shi, J.; Ren, X.; Tao, Y.; Ma, F.; Li, R.; Liu, X.; Liu, C. Insights into the aroma profiles and characteristic aroma of ‘Honeycrisp’ apple (Malus × domestica). Food Chem. 2020, 327, 127074. [Google Scholar] [CrossRef]
- Ingle, M.; D’souza, M.C. Physiology and control of superficial scald of apples: A review. HortScience 1998, 24, 28–31. [Google Scholar] [CrossRef]
- Rowan, D.D.; Hunt, M.B.; Fielder, S.; Norris, J.; Sherburn, M.S. Conjugated triene oxidation products of α-farnesene induce symptoms of superficial scald on stored apples. J. Agric. Food Chem. 2001, 49, 2780–2787. [Google Scholar] [CrossRef]
- Whitaker, B.D. Oxidative stress and superficial scald of apple fruit. HortScience 2004, 39, 933–937. [Google Scholar] [CrossRef]
- Watkins, C.B.; Bramlage, W.J.; Cregoe, B.A. Superficial scald of ‘Granny Smith’ apples is expressed as a typical chilling injury. J. Am. Soc. Hortic. Sci. 1995, 120, 88–94. [Google Scholar] [CrossRef]
- Whitaker, B.D.; Saftner, R.A. Temperature-dependent autoxidation of conjugated trienols from apple peel yields 6-methyl-5-hepten-2-one, a volatile implicated in induction of scald. J. Agric. Food Chem. 2000, 48, 2040–2043. [Google Scholar] [CrossRef]
- Shaham, Z.; Lers, A.; Lurie, S. Effect of heat or 1-methylcyclopropene on antioxidative enzyme activities and antioxidants in apples in relation to superficial scald development. J. Am. Soc. Hort. Sci. 2003, 128, 761–766. [Google Scholar] [CrossRef]
- Tsantili, E.; Gapper, N.E.; Arquiza, J.M.R.A.; Whitaker, B.D.; Watkins, C.B. Ethylene and alpha-farnesene metabolism in green and red skin of three apple cultivars in response to 1-methylcyclopropene (1-MCP) treatment. J. Agric. Food Chem. 2007, 55, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Song, J.; Palmer, L.C.; Vinqvist-Tymchuk, M.; Fillmore, S.; Toivonen, P.; Zhang, Z. Tracking the development of the superficial scald disorder and effects of treatments with diphenylamine and 1-MCP using an untargeted metabolomic approach in apple fruit. Food Chem. Mol. Sci. 2021, 2, 100022. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Nock, J.F.; Ma, Y.; Liu, X.; Watkins, C.B. Effects of repeated 1-methylcyclopropene (1-MCP) treatments on ripening and superficial scald of ‘Cortland’ and ‘Delicious’ apples. Postharvest Biol. Technol. 2013, 78, 48–54. [Google Scholar] [CrossRef]
- Magazin, N.; Gvozdenovic, D.; Keserovic, Z.; Milic, B. Fruit quality of Granny Smith apples picked at different harvest times and treated with 1-MCP. Fruits 2010, 65, 191–197. [Google Scholar] [CrossRef][Green Version]
- Tomala, K.; Małachowska, M.; Guzek, D.; Głąbska, D.; Gutkowska, K. The effects of 1-methylcyclopropene treatment on the fruit quality of ‘Idared’ apples during storage and transportation. Agriculture 2020, 10, 490. [Google Scholar] [CrossRef]
- Tomala, K.; Guzek, D.; Głąbska, D.; Małachowska, M.; Widłak, Ł.; Krupa, T.; Gutkowska, K. Maintaining the quality of ‘Red Jonaprince’ apples during storage by 1-methylcyclopropene preharvest and postharvest treatment. Agriculture 2022, 12, 1189. [Google Scholar] [CrossRef]
- Shui, G.; Leong, L.P. Separation and determination of organic acids and phenolic compounds in fruit juices and drinks by high-performance liquid chromatography. J. Chromatogr. A 2002, 977, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, M.C.B.M.; Nunes, F.; Garcia-Viguera, C.; Bennett, R.N.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Industrial processing effects on chestnut fruits (Castanea sativa Mill.) 3. Minerals, free sugars, carotenoids and antioxidant vitamins. Int. J. Food Sci. 2010, 45, 496–505. [Google Scholar] [CrossRef]
- Food and Agriculture Organization; World Health Organization. The role of glycemic index in food choice. In Carbohydrates in Human Nutrition: Report of a Joint FAO/WHO Expert Consultation; Food and Nutrition Paper; WHO: Rome, Italy, 1998; pp. 25–37. [Google Scholar]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- ISO 26642-2010; Food Products–Determination of the Glycaemic Index (GI) and Recommendation for Food Classification. International Organization for Standardization: Geneva, Switzerland, 2010. Available online: https://wwwisoorg/standard/43633html (accessed on 12 April 2024).
- Górecki, T.; Pawliszyn, J. The effect of sample volume on quantitative analysis by solid phase microextraction. Part 1. Theoretical considerations. Analyst 1997, 122, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Matich, A.J.; Rowan, D.D.; Banks, N.H. Solid phase microextraction for quantitative headspace sampling of apple volatiles. Anal. Chem. 1996, 68, 4114–4118. [Google Scholar] [CrossRef]
- Song, J.; Gardner, B.D.; Holland, J.F.; Beaudry, R.M. Rapid analysis of volatile flavor compounds in apple fruit using SPME and GC/Time-of-Flight mass spectrometry. J. Agric. Food Chem. 1997, 45, 1801–1807. [Google Scholar] [CrossRef]
- Reis, S.F.A.R.; Rocha, S.M.; Barros, A.S.; Delgadillo, I.; Coimbra, M.A. Establishment of the volatile profile of ‘Bravo de Esmolfe’ apple variety and identification of varietal markers. Food Chem. 2009, 113, 513–521. [Google Scholar] [CrossRef]
- Rubert, J.; Lacina, O.; Fauhl-Hassek, C.; Hajslova, J. Metabolic fingerprinting based on high-resolution tandem mass spectrometry: A reliable tool for wine authentication? Anal. Bioanal. Chem. 2014, 406, 6791–6803. [Google Scholar] [CrossRef]


| Apple Variety | Storage | Firmness (N) | TSS (%) | TA (g/100 g Malic Acid) | pH | Luminosity (L) | Cromaticity (C) | Hue (h°) |
|---|---|---|---|---|---|---|---|---|
| H | 27.8 ± 6.4 1 b 2 | 13.9 ± 0.6 a | 7.9 ± 1.2 a | 3.1 ± 0.2 d | 74.1 ± 2.1 a | 47.6 ± 2.5 b | 123.2 ± 0.3 a | |
| NA | 26.5 ± 1.9 b | 13.0 ± 0.9 a | 3.8 ± 0.5 c | 4.0 ± 0.1 a | ||||
| GD | CA | 28.5 ± 4.8 b | 12.8 ± 0.5 a | 2.7 ± 0.5 d | 3.8 ± 0.1 b | 73.9 ± 2.4 a | 54.2 ± 2.1 a | 122.7 ± 0.1 a |
| 1-MCP | 35.5 ± 5.4 a | 13.2 ± 0.7 a | 5.7 ± 0.8 b | 3.4 ± 0.1 c | 71 ± 2.7 b | 49.3 ± 2.6 b | 122.8 ± 0.2 a | |
| H | 35.3 ± 3.8 b | 13.2 ± 0.4 b | 4.5 ± 0.4 a | 3.9 ± 0.1 b | 35.4 ± 6.4 b | 39.2 ± 3.8 a | 28.4 ± 6.6 a | |
| NA | 33.6 ± 2.8 b | 14.3 ± 0.6 ab | 3.1 ± 0.5 b | 4.2 ± 0.1 a | ||||
| RD | CA | 32.7 ± 4.5 b | 15.3 ± 0.9 a | 3.0 ± 0.4 b | 3.9 ± 0.1 b | 35.7 ± 4.6 b | 34.2 ± 4.0 c | 28.9 ± 5.7 a |
| 1-MCP | 43.0 ± 6.6 a | 13.1 ± 0.6 b | 3.5 ± 0.4 b | 3.8 ± 0.1 b | 38.4 ± 6.2 a | 34.8 ± 5.3 b | 27.4 ± 5.3 a |
| Apple Variety | Storage | Malic Acid | Sorbitol | Glucose | Sucrose | Frutose | Total Sugars | SI | GI | GL |
|---|---|---|---|---|---|---|---|---|---|---|
| GD | H | 19.5 ± 2.36 1 ab 2 | 0.9 ± 0.1 1 a 2 | 9.1 ± 1.5 bc | 17.1 ± 2.0 a | 43.6 ± 3.2 ab | 70.7 ± 2.7 a | 100.2 | 45 | 4.7 |
| NA | 22.6 ± 3.07 a | 1.4 ± 0.1 a | 6.7 ± 1.9 c | 11.3 ± 2.1 b | 28.6 ± 1.8 c | 48.0 ± 1.9 c | 64.9 | 46 | 3.2 | |
| CA | 17.1 ± 2.68 b | 1.0 ± 0.2 a | 11.2 ± 1.4 b | 10.8 ± 2.7 bc | 40.6 ± 3.8 b | 63.6 ± 3.1 b | 88.2 | 46 | 4.3 | |
| 1-MCP | 13.5 ± 2.15 c | 1.1 ± 0.2 a | 14.4 ± 2.3 a | 8.1 ± 2.0 c | 46.5 ± 2.9 a | 70.1 ± 2.6 a | 98.0 | 46 | 4.8 | |
| RD | H | 12.3 ± 1.74 a | 2.0 ± 1.2 a | 15.9 ± 3.6 b | 15.7 ± 3.1 a | 43.3 ± 2.5 a | 76.9 ± 2.8 a | 101.2 | 50 | 5.7 |
| NA | 12.8 ± 2.29 a | 1.9 ± 0.7 a | 11.0 ± 1.4 c | 14.9 ± 2.6 a | 28.9 ± 2.3 c | 56.7 ± 2.1 c | 72.3 | 52 | 4.3 | |
| CA | 10.1 ± 1.66 ab | 2.3 ± 0.4 a | 18.9 ± 1.0 a | 6.3 ± 1.2 b | 41.4 ± 2.8 a | 68.9 ± 2.1 b | 90.0 | 52 | 5.1 | |
| 1-MCP | 8.67 ± 2.68 b | 1.5 ± 0.5 a | 19.4 ± 3.1 a | 3.1 ± 1.2 c | 37.1 ± 3.7 b | 61.1 ± 3.3 c | 80.7 | 53 | 4.8 |
| Aroma Compounds | KI | ID a | Aroma descriptor | ‘Golden Delicious’ | ‘Red Delicious’ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H | NA | CA | 1-MCP | H | NA | CA | 1-MCP | |||||
| Esters | ||||||||||||
| 23 | Ethyl butanoate | 1058 | A,B,C | oxidized apple, sweet | 2.3 ± 1.4 a,1,2 | 1.0 ± 0.6 a | n.d. b | n.d. b | ||||
| 24 | Propyl propionate | 1059 | B,C | sweet, fruity | 0.2 ± 0.1 a | n.d. b | n.d. b | n.d. b | ||||
| 25 | Ethyl 2-methylbutanoate | 1063 | A,B,C | Fruity | 2.0 ± 1.4 a | 0.8 ± 0.7 a | n.d. b | n.d. b | ||||
| 1 | Butyl acetate | 1082 | A,B,C | fruity, apple | 5.0 ± 1.6 a | 0.9 ± 0.1 b | 0.4 ± 0.3 c | 0.01 ± 0.01 d | 2.5 ± 0.9 a | 0.4 ± 0.1 b | 0.03 ± 0.01 c | n.d. d |
| 2 | 2-Methylbutyl acetate | 1134 | A,B,C | overall aroma, characteristic apple, banana like | 4.2 ± 2.0 a | 0.2 ± 0.1 b | 0.2 ± 0.1 b | 0.05 ± 0.02 c | 10.5 ± 2.8 a | 1.7 ± 0.5 b | n.d. c | n.d. c |
| 3 | Butyl propionate | 1137 | B,C | Fuity | 0.8 ± 0.5 a | 0.2 ± 0.1 b | 0.02 ± 0.01 c | n.d. d | ||||
| 26 | Propyl 2-methylbutanoate | 1151 | B,C | fruity, apple | 3.8 ± 1.5 a | 0.3 ± 0.1 b | n.d. c | n.d. c | ||||
| 4 | Pentyl Acetate | 1168 | A,B,C | fruity, apple, banana-like | 1.0 ± 0.2 a | 0.09 ± 0.01 b | 0.04 ± 0.02 c | n.d. d | ||||
| 5 | Butyl butanoate | 1221 | A,B,C | rotten apple, cheesy | 6.7 ± 1.0 a | 1.2 ± 0.3 b | 0.4 ± 0.3 c | 0.04 ± 0.01 d | 3.5 ± 0.8 a | 0.8 ± 0.3 b | 0.04 ± 0.02 c | n.d. d |
| 6 | Butyl 2-methylbutanoate | 1239 | A,B,C | fruity, apple | 5.3 ± 1.0 a | 0.3 ± 0.1 b | 0.2 ± 0.1 b | 0.04 ± 0.02 c | 9.4 ± 3.4 a | 2.5 ± 1.3 a,b | 0.1 ± 0.1 a,b | 0.3 ± 0.3 b |
| 7 | Hexyl acetate | 1268 | A,B,C | sweet fruity, apple | 29.9 ± 7.2 a | 4.4 ± 0.8 b | 2.8 ± 1.4 b | 0.2 ± 0.1 c | 13.2 ± 3.6 a | 2.6 ± 0.6 a | 0.3 ± 0.1 a,b | 0.1 ± 0.2 b |
| 8 | Pentyl butanoate | 1305 | A,B,C | banana | 1.4 ± 0.1 a | 0.2 ± 0.1 b | 0.04 ± 0.02 c | n.d. d | 0.8 ± 0.3 a | 0.1 ± 0.1 a,b | n.d. c | 0.1 ± 0.1 b |
| 27 | Propyl hexanoate | 1313 | B,C | Fruit | 5.4 ± 1.9 a | 0.9 ± 0.4 a,b | 0.1 ± 0.1 a,b | 0.6 ± 0.6 b | ||||
| 9 | Pentyl 2-methylbutanoate | 1332 | B,C | 1.0 ± 0.2 a | 0.02 ± 0.01 b | n.d. d | 0.01 ± 0.01 c | 2.3 ± 0.8 a | 0.1 ± 0.1 a,b | 0.01 ± 0.01 a,b | 0.1 ± 0.1 b | |
| 11 | Hexyl 2-methylpropanoate | 1339 | B,C | sweet, green, fruity, apple-like | 0.6 ± 0.3 a | 0.1 ± 0.0 b | 0.1 ± 0.0 b | 0.02 ± 0.01 c | ||||
| 10 | Hexyl propionate | 1354 | B,C | fruity, apple | 3.2 ± 2.2 a | 0.5 ± 0.1 b | 0.2 ± 0.1 c | 0.03 ± 0.01 d | 2.4 ± 0.5 a | 0.6 ± 0.2 b | n.d. c | n.d. c |
| 28 | Pentyl pentanoate | 1401 | B,C | ripe, fruity, apple | 1.8 ± 0.5 a | 0.2 ± 0.1 a | n.d. b | 0.6 ± 0.6 a | ||||
| 12 | Butyl hexanoate | 1406 | B,C | green apple | 28.3 ± 4.1 a | 5.8 ± 1.3 a | 1.7 ± 1.2 a,b | 0.1 ± 0.1 b | 32.0 ± 7.4 a | 6.2 ± 3.7 a | 0.8 ± 0.4 a | 3.3 ± 2.0 a |
| 13 | Hexyl butanoate | 1420 | B,C | apple | 20.6 ± 2.5 a | 5.4 ± 1.6 b | 2.7 ± 2.7 c | 0.2 ± 0.1 d | 3.2 ± 1.2 a | 3.9 ± 2.1 a | 0.3 ± 0.1 b | 0.5 ± 0.2 b |
| 29 | Ethyl octanoate | 1420 | A,B,C | fruit, fat | 0.4 ± 0.3 a | 0.4 ± 0.3 a | n.d. b | n.d. b | ||||
| 14 | Hexyl 2-methylbutanoate | 1425 | B,C | apple, grapefruit | 59.4 ± 19.9 a | 3.8 ± 0.4 b | 4.5 ± 1.8 b | 1.0 ± 0.6 c | 75.9 ± 10.0 a | 13.9 ± 7.3 b | 1.4 ± 0.8 d | 5.2 ± 3.0 c |
| 15 | Pentyl hexanoate | 1501 | B,C | sweet, green fruity, apple, fatty | 0.7 ± 0.2 a | 0.5 ± 0.1 a | 0.2 ± 0.0 b | 0.1 ± 0.0 c | 3.7 ± 1.2 a | 0.8 ± 0.4 b,c | 0.4 ± 0.3 c | 2.0 ± 1.1 a,b |
| 31 | Propyl octanoate | 1510 | B,C | coconut, cocoa, fatty | 0.5 ± 0.1 a | 0.2 ± 0.1 a | 0.01 ± 0.01 b | 0.3 ± 0.2 a | ||||
| 16 | Butyl heptanoate | 1514 | B,C | Green, fruity, licorice, grassy | 3.5 ± 0.2 a | n.d. b | n.d. b | n.d. b | ||||
| 17 | Hexyl hexanoate | 1599 | B,C | apple | 34.7 ± 4.1 a | 11.9 ± 1.5 b | 5.2 ± 2.5 c | 0.9 ± 0.5 d | 30.8 ± 8.3 a | 13.3 ± 3.5 b | 1.9 ± 0.6 d | 5.8 ± 3.2 c |
| 18 | Butyl octanoate | 1619 | B,C | Fruit | 6.0 ± 0.9 a | 1.3 ± 0.5 b | 0.2 ± 0.1 c | n.d. d | 2.0 ± 0.4 a | 1.6 ± 0.7 a | 0.2 ± 0.2 b | 0.9 ± 0.5 a |
| 22 | Hexyl octanoate | 1793 | B,C | herb, green, oil | 1.1 ± 0.2 a | 0.3 ± 0.1 b | 0.1 ± 0.0 c | 0.02 ± 0.01 d | 0.5 ± 0.1 b | 1.0 ± 0.4 a | 0.1 ± 0.01 c | 0.2 ± 0.1 b |
| Subtotal | 213 ± 42 a | 37 ± 2 b | 19 ± 9 c | 2.7 ± 1.3 d | 209 ± 41 a | 53 ± 15 b | 5.7 ± 2.6 d | 22 ± 9 c | ||||
| Subtotal (%) | 57 | 48 | 33 | 11 | 52 | 53 | 6.3 | 14 | ||||
| Phenols | ||||||||||||
| 19 | 1-Methoxy-4-(2-propen-1-yl)-benzene (Estragol) | 1685 | B,C | licorice, anise | 1.5 ± 0.5 a | 0.2 ± 0.1 b | 0.1 ± 0.1 c | 0.03 ± 0.01 c | 1.1 ± 0.3 a | 0.5 ± 0.1 b | n.d. c | n.d. c |
| Subtotal | 1.5 ± 0.5 a | 0.2 ± 0.1 b | 0.1 ± 0.1 c | 0.03 ± 0.01 c | 1.1 ± 0.3 a | 0.5 ± 0.1 b | n.d. c | n.d. c | ||||
| Subtotal (%) | 0.4 | 0.3 | 0.1 | 0.1 | 0.3 | 0.5 | 0 | 0 | ||||
| Terpenoids | ||||||||||||
| 20 | trans-α-Bergamotene | 1592 | B,C | fruity, bergamote | 0.8 ± 0.2 a | 0.2 ± 0.0 b | 0.1 ± 0.1 b | 0.1 ± 0.0 b | 0.9 ± 0.1 a | 0.2 ± 0.1 c | 0.4 ± 0.1 b | 0.6 ± 0.2 a |
| 21 | (E,E)-α-Farnesene | 1757 | A,B,C | green, floral, herbal, citrus | 158 ± 17 a | 39.1 ± 5.1 b | 38.9 ± 13.6 b | 21.1 ± 8.2 c | 191 ± 26 a | 46.1 ± 10.8 d | 83.9 ± 16.1 c | 134 ± 23 b |
| Subtotal | 159 ± 17 a | 39. ± 5 b | 39 ± 14 b | 21. ± 8 c | 191 ± 26 a | 46.3 ± 10.8 d | 84.3 ± 16.2 c | 134 ± 23 b | ||||
| Subtotal (%) | 42 | 51 | 67 | 88 | 48 | 46 | 94 | 86 | ||||
| Total | 373 ± 35 a | 77 ± 7 b | 58 ± 22 b | 24 ± 9 c | 402 ± 62 a | 100 ± 20 c | 90 ± 17 c | 156 ± 30 b | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, C.; Ribeiro, C.; Nunes, F.M. Effect of Storage Conditions on the Volatilome, Biochemical Composition and Quality of Golden Delicious and Red Delicious Apple (Malus domestica) Varieties. Molecules 2024, 29, 2954. https://doi.org/10.3390/molecules29132954
Ferreira C, Ribeiro C, Nunes FM. Effect of Storage Conditions on the Volatilome, Biochemical Composition and Quality of Golden Delicious and Red Delicious Apple (Malus domestica) Varieties. Molecules. 2024; 29(13):2954. https://doi.org/10.3390/molecules29132954
Chicago/Turabian StyleFerreira, Cláudio, Carlos Ribeiro, and Fernando M. Nunes. 2024. "Effect of Storage Conditions on the Volatilome, Biochemical Composition and Quality of Golden Delicious and Red Delicious Apple (Malus domestica) Varieties" Molecules 29, no. 13: 2954. https://doi.org/10.3390/molecules29132954
APA StyleFerreira, C., Ribeiro, C., & Nunes, F. M. (2024). Effect of Storage Conditions on the Volatilome, Biochemical Composition and Quality of Golden Delicious and Red Delicious Apple (Malus domestica) Varieties. Molecules, 29(13), 2954. https://doi.org/10.3390/molecules29132954










