The Composition and Biochemical Properties of Strophantus (Apocynaceae), with a Focus on S. sarmentosus
Abstract
1. Introduction
2. Bioactive Compounds
2.1. Cardiac Glycosides and Their Activity
- The ouabain group: S. gardeniiflorus, S. gratus, S. thollonii;
- The sarmentogenin/sarverogenin group: S. welwitschii, S. amboensis, S. gerrardii, S. congoensis, S. petersianus, S. courmontii, S. sarmentosus;
- The strophanthidin/strophanthidol/periplogenin group: S. arnoldianus, S. hispidus, S. mirabilis, S. barteri, S. hypoleucos, S. mortehanii, S. eminii, S. kombe, S. nicholsonii, S. gracilis, S. ledienii, S. preussii;
- The divaricoside/caudoside group: S. caudatus, S. divaricatus, S. wightianus.
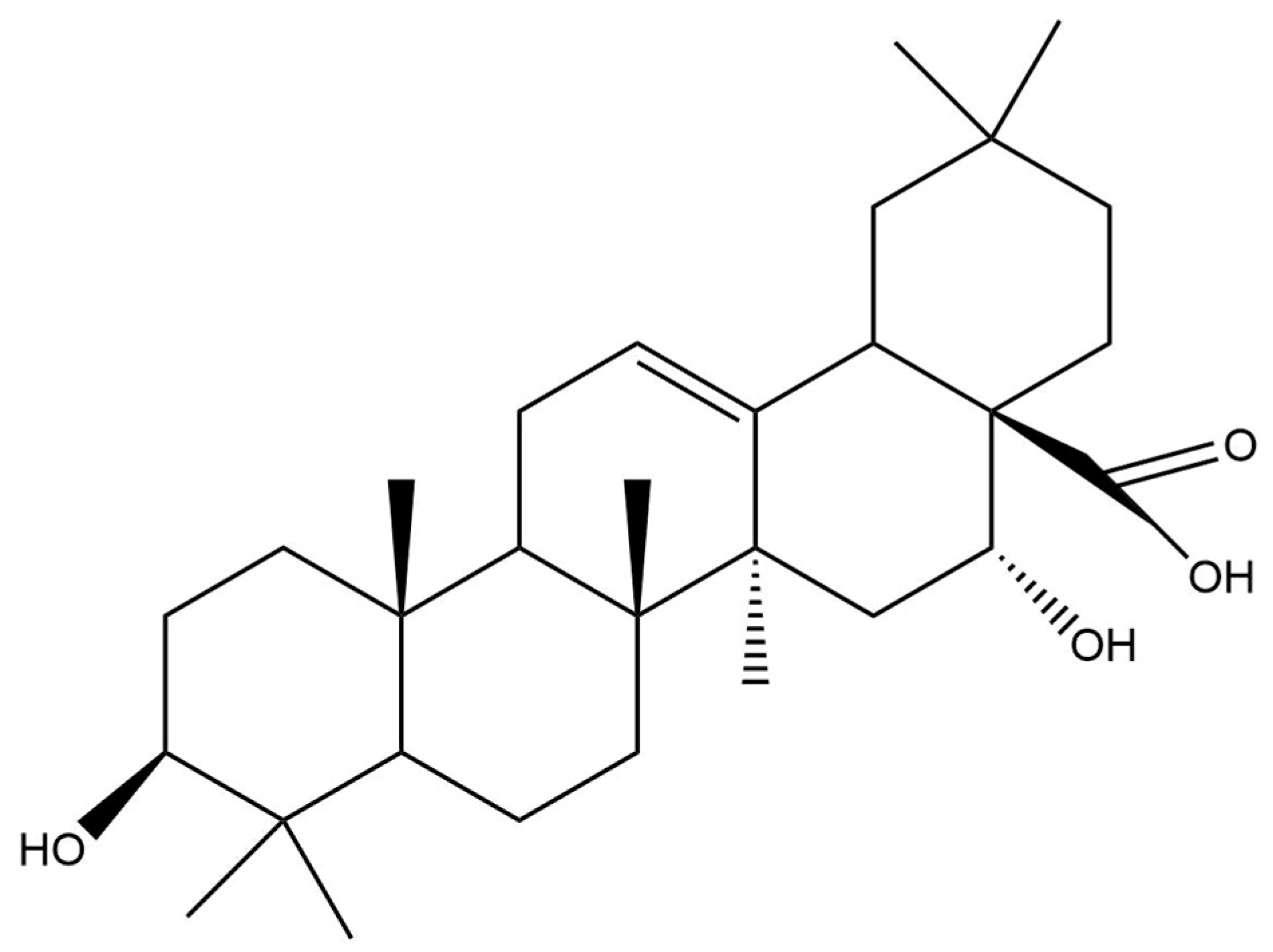
2.2. Triterpene Glycosides and Other Substances
3. Bioactivity
3.1. Toxicity and Mutagenicity
3.2. Anti-Inflammatory, Antibacterial, and Antioxidant Activity
3.3. Hypoglycemic Effects
3.4. Anti-Nociceptive Effects
3.5. Anti-Venomous Activity
3.6. Anti-Phytoviral, Anti-Herpetic, Anti-Trypanasomal, Anti-Protozoal, Anti-Malarial, and Hydroxynitrile Lyase Activities
4. Methods
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Beentje, H.J. A Monograph on Strophanthus DC. (Apocynaceae); Wageningen University and Research: Wageningen, The Netherlands, 1982. [Google Scholar]
- Włodarczyk, M.; Gleńsk, M. An in-depth look into a well-known herbal drug: Fingerprinting, isolation, identification, and content estimation of saponins in different Strophanthus seeds. Planta Medica 2022, 88, 576–586. [Google Scholar] [CrossRef]
- Vickery, M. Plant poisons: Their occurrence, biochemistry and physiological properties. Sci. Prog. 2010, 93, 181–221. [Google Scholar] [CrossRef] [PubMed]
- Makgobole, M.U.; Mpofana, N.; Ajao, A.A. Medicinal Plants for Dermatological Diseases: Ethnopharmacological Significance of Botanicals from West Africa in Skin Care. Cosmetics 2023, 10, 167. [Google Scholar] [CrossRef]
- Famewo, E.B.; Clarke, A.M.; Afolayan, A.J. Toxicological Evaluation of Polyherbal Medicines used for the Treatment of Tuberculosis in Eastern Cape, South Africa. Int. J. Pharmacol. 2017, 13, 91–97. [Google Scholar]
- Boakye-Yiadom, M.; Kumadoh, D.; Adase, E.; Woode, E. Medicinal Plants with Prospective Benefits in the Management of Peptic Ulcer Diseases in Ghana. BioMed Res. Int. 2021, 2021, 5574041. [Google Scholar] [CrossRef]
- Traore, M.S.; Diane, S.; Diallo, M.S.T.; Balde, E.S.; Balde, M.A.; Camara, A.; Diallo, A.; Keita, A.; Cos, P.; Maes, L.; et al. In vitro antiprotozoal and cytotoxic activity of ethnopharmacologically selected guinean plants. Planta Medica 2014, 80, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Faboro, E.O.; Wichitnithad, W.; Fadare, O.A.; Akinpelu, D.A.; Obafemi, C.A. Antibacterial and antioxidant activities and phytochemical screening of aqueous methanol extracts of eight Nigerian medicinal and aromatic Plants. J. Pharm. Res. 2016, 10, 523–532. [Google Scholar]
- John Kenneth, M.; Amina, I.; Yakubu, J. Co-extract mixture from Strophanthus hispidus (roots) and Aframomum meleguta (seeds) show phytochemical synergy in its anti-inflammatory activity. Arch. Pharm. Pharma Sci. 2019, 3, 89–100. [Google Scholar] [CrossRef]
- Ojiako, O.; Igwe, C. A Time-Trend Hypoglycemic Study of Ethanol and Chloroform Extracts of Strophanthus hispidus. J. Herbs Spices Med. Plants 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Tuyg’unovna, S.S. Chemical composition of medicinal plants and classification. EJMMP 2023, 3, 33–35. [Google Scholar]
- Alamgir, A.N.M. Medicinal, Non-medicinal, Biopesticides, Color- and Dye-Yielding Plants; Secondary Metabolites and Drug Principles; Significance of Medicinal Plants; Use of Medicinal Plants in the Systems of Traditional and Complementary and Alternative Medicines (CAMs). Ther. Use Med. Plants Their Extr. Vol. 1 2017, 73, 61–104. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, R.A. Tincture of strophanthus and strophanthin. JAMA 1909, 52, 5–10. [Google Scholar] [CrossRef][Green Version]
- Wormer, E.J. Strophanthin—Comeback Eines Herzmittels; Narayana Verlag GmbH: Kandern, Germany, 2015; Available online: https://www.narayana-verlag.de/Strophanthin-Eberhard-J-Wormer/b20490 (accessed on 29 April 2024).
- Norton, S.A. Useful plants of dermatology. III. Corticosteroids, Strophanthus, and Dioscorea. J. Am. Acad. Dermatol. 1998, 38, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Youngken, H.W.; Simonian, V.H. A study of the seeds of Strophanthus sarmentosus and some related species of Strophanthus. J. Am. Pharm. Assoc. 1950, 39, 615–620. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, B.-E. A review of commercially important African medicinal plants. J. Ethnopharmacol. 2015, 176, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Tamura, S.; Okada, M.; Kato, S.; Shinoda, Y.; Shioda, N.; Fukunaga, K.; Ui-Tei, K.; Ueda, M. Ouabagenin is a naturally occurring LXR ligand without causing hepatic steatosis as a side effect. Sci. Rep. 2018, 8, 2305. [Google Scholar] [CrossRef] [PubMed]
- Fuhrer, H.; Zürcher, R.F.; Reichstein, T. Sarverogenin, vermutliche Struktur. Glykoside und Aglykone, 314. Mitteilung. Helv. Chim. Acta 1969, 52, 616–621. [Google Scholar] [CrossRef]
- Schmelzer, G.H.; Gurib-Fakim, A.; Arroo, R.; Lemmens, R.H.M.J. Medicinal Plants 1; Fondation PROTA: Wageningen, The Netherlands, 2008; ISBN 9789057822049. [Google Scholar]
- Cardiac Glycosides 1785–1985; SpringerLink: Berlin/Heidelberg, Germany, 2013; Available online: https://link.springer.com/book/10.1007/978-3-662-11292-2 (accessed on 30 April 2024).
- Orhan, I.E.; Gokbulut, A.; Senol, F.S. Adonis sp., Convallaria sp., Strophanthus sp., Thevetia sp., and Leonurus sp.—Cardiotonic Plants with Known Traditional Use and a Few Preclinical and Clinical Studies. Curr. Pharm. Des. 2017, 23, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Strobach, H.; Wirth, K.E.; Rojsathaporn, K. Absorption, metabolism and elimination of strophanthus glycosides in man. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1986, 334, 496–500. [Google Scholar] [CrossRef]
- Kaplan, J.H. The sodium pump and hypertension: A physiological role for the cardiac glycoside binding site of the Na,K-ATPase. Proc. Natl. Acad. Sci. USA 2005, 102, 15723–15724. [Google Scholar] [CrossRef]
- v. Euw, J.; Gürtler, J.; Lardon, A.; Mohr, K.; Reber, F.; Richter, R.; Schindler, O.; Reichstein, T. Die Glykoside von Strophanthus sarmentosus P. DC. 8. Mitteilung. Untersuchung von Einzelpflanzen der “Sarmentogenin-produzierenden Variante b” Strophanthus sarmentosus var. senegambiae (A. DC.) Monachino. Glykoside und Aglykone, 183. Mitteilung. Helv. Chim. Acta 1957, 40, 2079–2109. [Google Scholar] [CrossRef]
- Richter, R.; Mohr, K.; Reichstein, T. Sarmutosid und Musarosid. Glykoside der Samen von Strophanthus sarmentosus A.P.DC. 4. Mitteilung. Glykoside und Aglykone. 113. Mitteilung. Helv. Chim. Acta 1953, 36, 1073–1088. [Google Scholar] [CrossRef]
- Knittel, D.N.; Huber, U.; Stintzing, F.C.; Kammerer, D.R. Effect of extraction, microbial fermentation and storage on the cardenolide profile of Strophanthus kombé Oliv. seed preparations. J. Pharm. Biomed. Anal. 2016, 129, 96–104. [Google Scholar] [CrossRef]
- Chen, R.-F.; Abe, F.; Yamauchi, T.; Taki, M. Cardenolide glycosides of Strophanthus divaricatus. Phytochemistry 1987, 26, 2351–2355. [Google Scholar] [CrossRef]
- Makarevich, I.F.; Kovalev, S.V. Cardiac glycosides from Strophanthus kombe. Chem. Nat. Compd. 2006, 42, 189–193. [Google Scholar] [CrossRef]
- Knittel, D.N.; Lorenz, P.; Huber, U.; Stintzing, F.C.; Kammerer, D.R. Characterization of the cardiac glycoside and lipid profiles of Strophanthus kombé Oliv. seeds. Z. Naturforsch. C J. Biosci. 2016, 71, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Grosa, G.; Allegrone, G.; Del Grosso, E. LC-ESI-MS/MS characterization of strophanthin-K. J. Pharm. Biomed. Anal. 2005, 38, 79–86. [Google Scholar] [CrossRef]
- Beentje, H.; Cooke, D. 401. Strophanthus sarmentosus: Apocynaceae. Curtis’s Bot. Mag. 2000, 17, 202–207. [Google Scholar] [CrossRef]
- Fechtig, B.; v. Euw, J.; Schindler, O.; Reichstein, T. Die Struktur der Sarmentoside. Glykoside von Strophanthus sarmentosus P. DC. 11. Mitteilung. Glykoside und Aglykone, 219. Mitteilung. Helv. Chim. Acta 1960, 43, 1570–1584. [Google Scholar] [CrossRef]
- Owonubi, M.O.; Iwalewa, E.O.; Shok, M. Cardio-Activity of Sarmentoside—A from Strophanthus sarmentosus Seeds. Niger. J. Nat. Prod. Med. NJNPM 1997, 1, 16–18. [Google Scholar] [CrossRef]
- Laurente, M.R.; Ysrael, M.C. The cardioactive screening of the extract from the bark of Strophanthus cumingii a.dc. (apocynaceae) using isolated frog heart. Int. J. Pharm. 2015, 5, 1048–1050. [Google Scholar]
- Ojo, O.O.; Emoghwa, A.R. Methanol extracts of Strophanthus hispidus exhibit anti-apoptotic effects via alteration of cytochrome c and caspase 3 levels in rats with myocardial infarction. Chem. Pap. 2020, 74, 521–528. [Google Scholar] [CrossRef]
- Gundamaraju, R.; Vemuri, R.C.; Singla, R.K.; Manikam, R.; Rao, A.R.; Sekaran, S.D. Strophanthus hispidus attenuates the Ischemia-Reperfusion induced myocardial Infarction and reduces mean arterial pressure in renal artery occlusion. Pharmacogn. Mag. 2014, 10, S557–S562. [Google Scholar] [CrossRef] [PubMed]
- Karkare, S.; Adou, E.; Cao, S.; Brodie, P.; Miller, J.S.; Andrianjafy, N.M.; Razafitsalama, J.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. Cytotoxic cardenolide glycosides of Roupellina (Strophanthus) boivinii from the Madagascar rainforest. J. Nat. Prod. 2007, 70, 1766–1770. [Google Scholar] [CrossRef][Green Version]
- Pezzani, R.; Rubin, B.; Redaelli, M.; Radu, C.; Barollo, S.; Cicala, M.V.; Salvà, M.; Mian, C.; Mucignat-Caretta, C.; Simioni, P.; et al. The antiproliferative effects of ouabain and everolimus on adrenocortical tumor cells. Endocr. J. 2014, 61, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Chen, Y.; Lu, Y.; Wang, Y.; Ding, L.; Jiang, M. Cardenolides from the Apocynaceae family and their anticancer activity. Fitoterapia 2016, 112, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Zuhrotun, A.; Suganda, A.G.; Wirasutisna, K.R.; Wibowo, M.S. Anticancer Screening of Selected Apocynaceae, Simaroubaceae and Magnoliaceae of Indonesian Plants using Mechanism-Based Yeast Bioassay. Int. J. Pharm. Sci. Rev. Res. 2015, 35, 90–94. [Google Scholar]
- Weng, J.-R.; Bai, L.-Y.; Chiu, S.-J.; Chiu, C.-F.; Lin, W.-Y.; Hu, J.-L.; Shieh, T.-M. Divaricoside Exerts Antitumor Effects, in Part, by Modulating Mcl-1 in Human Oral Squamous Cell Carcinoma Cells. Comput. Struct. Biotechnol. J. 2019, 17, 151–159. [Google Scholar] [CrossRef]
- AlQathama, A.; Ezuruike, U.F.; Mazzari, A.L.D.A.; Yonbawi, A.; Chieli, E.; Prieto, J.M. Effects of Selected Nigerian Medicinal Plants on the Viability, Mobility, and Multidrug-Resistant Mechanisms in Liver, Colon, and Skin Cancer Cell Lines. Front. Pharmacol. 2020, 11, 546439. [Google Scholar] [CrossRef]
- An, P.-P.; Cui, Y.-S.; Shi, Q.-Y.; Ren, Y.-H.; Wu, P.-Q.; Liu, Q.-F.; Liu, H.-C.; Zhou, B.; Yue, J.-M. Pregnane steroids from the twigs and leaves of Strophanthus divaricatus and their cytotoxic activities. Tetrahedron Lett. 2022, 93, 153691. [Google Scholar] [CrossRef]
- Ran, H.-L.; Huang, S.-Z.; Wang, H.; Yang, L.; Gai, C.-J.; Duan, R.-J.; Dai, H.-F.; Guan, Y.-L.; Mei, W.-L. Cytotoxic steroids from the stems of Strophanthus divaricatus. Phytochemistry 2023, 210, 113668. [Google Scholar] [CrossRef]
- BROWER, L.P.; MOFFITT, C.M. Palatability dynamics of cardenolides in the monarch butterfly. Nature 1974, 249, 280–283. [Google Scholar] [CrossRef]
- Yu, H.; Li, W.; Cao, X.; Wang, X.; Zhao, Y.; Song, L.; Chen, J.; Wang, S.; Chen, B.; Xu, Y. Echinocystic acid, a natural plant extract, alleviates cerebral ischemia/reperfusion injury via inhibiting the JNK signaling pathway. Eur. J. Pharmacol. 2019, 861, 172610. [Google Scholar] [CrossRef]
- Abiola, J.L.; Aiyelaagbe, O.O. Phytochemical, Antimicrobial and Cytotoxic Activities of Strophanthus sarmentosus DC. Biol. Med. Nat. Prod. Chem. 2022, 12, 119–126. [Google Scholar] [CrossRef]
- Muhammad, K.T.; Mann, A.; Kabiru, A.Y.; Busari, M.B. Phytochemical Evaluation and Anti-Inflammatory Activity of the Leaf, Stem and Root Bark Extracts of Strophanthus sarmentosus dc. World J. Pharm. Res. 2015, 4, 2097–2108. [Google Scholar]
- Gunstone, F.D. Vegetable oils. I.—The component acids of Strophanthus sarmentosus seed oil. J. Sci. Food Agric. 1952, 3, 185–189. [Google Scholar] [CrossRef]
- Gunstone, F.D.; Qureshi, M.I. Glyceride studies. VIII.—The component glycerides of four Strophanthus oils containing an unsaturated hydroxy acid. J. Sci. Food Agric. 1968, 19, 386–388. [Google Scholar] [CrossRef]
- Suhitha, S.; Devi, S.K.; Gunasekaran, K.; Pakyntein, H.C.; Bhattacharjee, A.; Velmurugan, D. Phytochemical analyses and activity of herbal medicinal plants of North-East India for anti-diabetic, anti-cancer and anti-tuberculosis and their docking studies. Curr. Top. Med. Chem. 2015, 15, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Cowan, S.; Stewart, M.; Abbiw, D.K.; Latif, Z.; Sarker, S.D.; Nash, R.J. Lignans from Strophanthus gratus. Fitoterapia 2001, 72, 80–82. [Google Scholar] [CrossRef]
- Nishibe, S.; Hisada, S.; Inagaki, I. The cyclitols of [leaves of] Ochrosia nakaiana, Plumeria acutifolia and Strophanthus gratus. Phytochemistry 1971, 10, 2453. [Google Scholar] [CrossRef]
- Nishibe, S.; Sakushima, A.; Takemura, H.; Takenaka, T.; Noguchi, Y. Cyclitols from Apocynaceae Leaves. Nat. Med. 2001, 55, 268–271. [Google Scholar]
- Ran, H.-L.; Huang, S.-Z.; Wang, H.; Yang, L.; Yu, M.; Gai, C.-J.; Duan, R.-J.; Dai, H.-F.; Guan, Y.-L.; Mei, W.-L. Three new sesquiterpenoids from the stems of Strophanthus divaricatus. Phytochem. Lett. 2021, 46, 100–104. [Google Scholar] [CrossRef]
- Chen, N.-H.; Zhang, Y.-B.; Li, Z.-H.; Jiang, J.-W.; Li, G.-Q.; Wang, G.-C.; Li, Y.-L. Two New Sesquiterpenoids from the Root of Strophanthus divaricatus. Chem. Lett. 2015, 44, 1119–1121. [Google Scholar] [CrossRef]
- Mulula, A.; Bouzina, A.; Mambu, H.B.; Ntumba, J.K.; Nsomue, J.; Tshingamb, M.N.; Kashishi, K.T.; Zaki, A.; Kalulu, T. HPLC Fingerprint profile, In-vitro Cytotoxity and Anti-Herpes Simplex Virus Activity of Methanol Extract from Strophanthus hispidus DC (Stem bark). Microbes Infect. Dis. 2022, 5, 379–388. [Google Scholar] [CrossRef]
- Mulula, A.; Bouzina, A.D.; Mambu, H.B.; Ntumba, J.K.; Taba, K.M. HPLC Fingerprint profile and Antioxidant, Antibacterial Activities of Methanol Extract of Strophanthus hispidus DC (Stem bark). IOSR J. Appl. Chem. 2021, 14, 21–27. [Google Scholar] [CrossRef]
- Mulula, A.; Ntumba, K.; Mifundu, M.M.; Taba, K.M. Phytochemical screening, antibacterial and antioxidant activities of aqueous and organics stem extracts of Strophanthus hispidus dc. Int. J. Pharm. Sci. Res. 2017, 8, 95–100. [Google Scholar]
- Ezuruike, U.F.; Chieli, E.; Prieto, J.M. In Vitro Modulation of Glibenclamide Transport by P-glycoprotein Inhibitory Antidiabetic African Plant Extracts. Planta Medica 2019, 85, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Traoré, M.S.; Camara, T.; Diane, S.; Camara, A.; Baldé, M.A.; Diallo, M.S.T.; Camara, A.K.; Baldé, A.M. Ethnobotanical survey of the adverse and toxic effects of medicinal plants used in Guinean traditional medicine. J. Pharmacogn. Phytochem. 2022, 11, 186–194. [Google Scholar] [CrossRef]
- Fageyinbo, M.S.; Akindele, A.J.; Agbaje, E.O. Sub-chronic toxicological evaluation of Strophanthus hispidus DC (Apocynaceae) aqueous root extract. J. Complement. Integr. Med. 2021, 18, 753–760. [Google Scholar] [CrossRef]
- Osibemhe, M.; Omaji, G.O.; Onoagbe, I.O. Sub-chronic toxicity of extracts of Strophanthus hispidus stem bark in normal rats. Pharmacologyonline 2017, 2, 140–161. [Google Scholar]
- Oppong Bekoe, E.; Agyare, C.; Boakye, Y.D.; Baiden, B.M.; Asase, A.; Sarkodie, J.; Nettey, H.; Adu, F.; Otu, P.B.; Agyarkwa, B.; et al. Ethnomedicinal survey and mutagenic studies of plants used in Accra metropolis, Ghana. J. Ethnopharmacol. 2020, 248, 112309. [Google Scholar] [CrossRef] [PubMed]
- Dzimiri, N.; Fricke, U. Lipophilicity and pharmacodynamics of cardiotonic steroids in guinea-pig isolated heart muscle preparations. Br. J. Pharmacol. 1988, 93, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Baah, S.; Borquaye, L.S. Ethanolic leaf extract from Strophanthus gratus (Hook.) Franch. (Apocynaceae) exhibits anti-inflammatory and antioxidant activities. Cogent Biol. 2019, 5, 1710431. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Olajuyin, A. A Comparative In Vitro Study on the Antioxidant and Anti-acetylcholinesterase Properties of Aerial Parts of Strophanthus preusii Engl & Pax. West Indian Med. J. 2014, 63, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Agyare, C.; Dwobeng, A.S.; Agyepong, N.; Boakye, Y.D.; Mensah, K.B.; Ayande, P.G.; Adarkwa-Yiadom, M. Antimicrobial, Antioxidant, and Wound Healing Properties of Kigelia africana (Lam.) Beneth. and Strophanthus hispidus DC. Adv. Pharmacol. Sci. 2013, 2013, 692613. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Cai, Y.-Z.; Xing, J.; Corke, H.; Sun, M. A Potential Antioxidant Resource: Endophytic Fungi from Medicinal Plants. Econ. Bot. 2007, 61, 14–30. [Google Scholar] [CrossRef]
- Elya, B.; Basah, K.; Mun’im, A.; Yuliastuti, W.; Bangun, A.; Septiana, E.K. Screening of α-glucosidase inhibitory activity from some plants of Apocynaceae, Clusiaceae, Euphorbiaceae, and Rubiaceae. J. Biomed. Biotechnol. 2012, 2012, 281078. [Google Scholar] [CrossRef] [PubMed]
- Sunmonu, T.O.; Ugbaja, R.N.; Ogunlesi, O.; Olafimihan, D.; Shodeinde, T.; Toriola, M.A.; Akinloye, D.I.; Balogun, E.A. Effects of Strophanthus hispidus DC. (Apocynaceae) aqueous root extract on antioxidant status in Streptozotocin-induced diabetic rats. Biokemistri 2015, 27, 89–97. [Google Scholar]
- Fageyinbo, M.S.; Akindele, A.J.; Adenekan, S.O.; Agbaje, E.O. Evaluation of in-vitro and in-vivo antidiabetic, antilipidemic and antioxidant potentials of aqueous root extract of Strophanthus hispidus DC (Apocynaceae). J. Complement. Integr. Med. 2019, 16, 20180055. [Google Scholar] [CrossRef]
- Osibemhe, M.; Ibrahim, M.; Onoagbe, I.O. Anti-diabetic potential assessment of aqueous and ethanol extracts of arrow poison (Strophanthus hispidus) plant stem bark in Wistar male rats. J. Appl. Sci. Environ. Manag. JASEM 2019, 23, 603. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Oyedemi, B.O.; Ijeh, I.I.; Ohanyerem, P.E.; Coopoosamy, R.M.; Aiyegoro, O.A. Alpha-Amylase Inhibition and Antioxidative Capacity of Some Antidiabetic Plants Used by the Traditional Healers in Southeastern Nigeria. Sci. World J. 2017, 2017, 3592491. [Google Scholar] [CrossRef] [PubMed]
- Fageyinbo, S.M.; Agbaje, O.E.; Rotimi, K.; Ikumawoyi, V.; Adeyemi; Fashina, Y. Anti-Nociceptive Activity and Possible Mechanisms of Action of Aqueous Root Extract of Strophanthus hispidus dc (Apocynaceae). Int. J. Pharm. Sci. Res. 2016, 127–135. [Google Scholar] [CrossRef]
- Ishola, I.O.; Awodele, O.; Oreagba, I.A.; Murtala, A.A.; Chijioke, M.C. Antinociceptive, anti-inflammatory and antiulcerogenic activities of ethanol root extract of Strophanthus hispidus DC (Apocynaceae). J. Basic Clin. Physiol. Pharmacol. 2013, 24, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Molander, M.; Nielsen, L.; Søgaard, S.; Staerk, D.; Rønsted, N.; Diallo, D.; Chifundera, K.Z.; van Staden, J.; Jäger, A.K. Hyaluronidase, phospholipase A2 and protease inhibitory activity of plants used in traditional treatment of snakebite-induced tissue necrosis in Mali, DR Congo and South Africa. J. Ethnopharmacol. 2014, 157, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Skari, K.P. The effect on blood clotting of some west African plants used against snakebite. J. Ethnopharmacol. 1994, 44, 99–108. [Google Scholar] [CrossRef]
- Yazdi, S.E.; Mulabisana, J.; Prinsloo, G.; Cloete, M.; Kritzinger, Q. Plants containing cardiac glycosides showing antiphytoviral activity against Potato virus Y (PVYNTN) on tobacco plants. J. Plant Prot. Res. 2023, 58, 397–403. [Google Scholar] [CrossRef]
- Innocent, E.; Moshi, M.J.; Masimba, P.J.; Mbwambo, Z.H.; Kapingu, M.C.; Kamuhabwa, A. Screening of traditionally used plants for in vivo antimalarial activity in mice. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 163–167. [Google Scholar] [CrossRef][Green Version]
- Onotu, C.S.; Abedo, J.A.; Anchua, R.G.; Shettima, F.A.T.; Abdulmalik, U.; Sambo, F. Invivo antitrypanosomal activity of methanolic stem extract of Strophanthus sarmentosus dc on wistar white mice infected with trypanosomal brucei brucei spp (federe strain). Adv. Life Sci. Technol. 2014, 16, 10–16. [Google Scholar]
- Kassim, M.A.; Sooklal, S.A.; Archer, R.; Rumbold, K. Screening for hydroxynitrile lyase activity in non-commercialised plants. S. Afr. J. Bot. 2014, 93, 9–13. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
König, S. The Composition and Biochemical Properties of Strophantus (Apocynaceae), with a Focus on S. sarmentosus. Molecules 2024, 29, 2847. https://doi.org/10.3390/molecules29122847
König S. The Composition and Biochemical Properties of Strophantus (Apocynaceae), with a Focus on S. sarmentosus. Molecules. 2024; 29(12):2847. https://doi.org/10.3390/molecules29122847
Chicago/Turabian StyleKönig, Simone. 2024. "The Composition and Biochemical Properties of Strophantus (Apocynaceae), with a Focus on S. sarmentosus" Molecules 29, no. 12: 2847. https://doi.org/10.3390/molecules29122847
APA StyleKönig, S. (2024). The Composition and Biochemical Properties of Strophantus (Apocynaceae), with a Focus on S. sarmentosus. Molecules, 29(12), 2847. https://doi.org/10.3390/molecules29122847








