Stereoselective Asymmetric Syntheses of Molecules with a 4,5-Dihydro-1H-[1,2,4]-Triazoline Core Possessing an Acetylated Carbohydrate Appendage: Crystal Structure, Spectroscopy, and Pharmacology
Abstract
1. Introduction
2. Results and Discussion
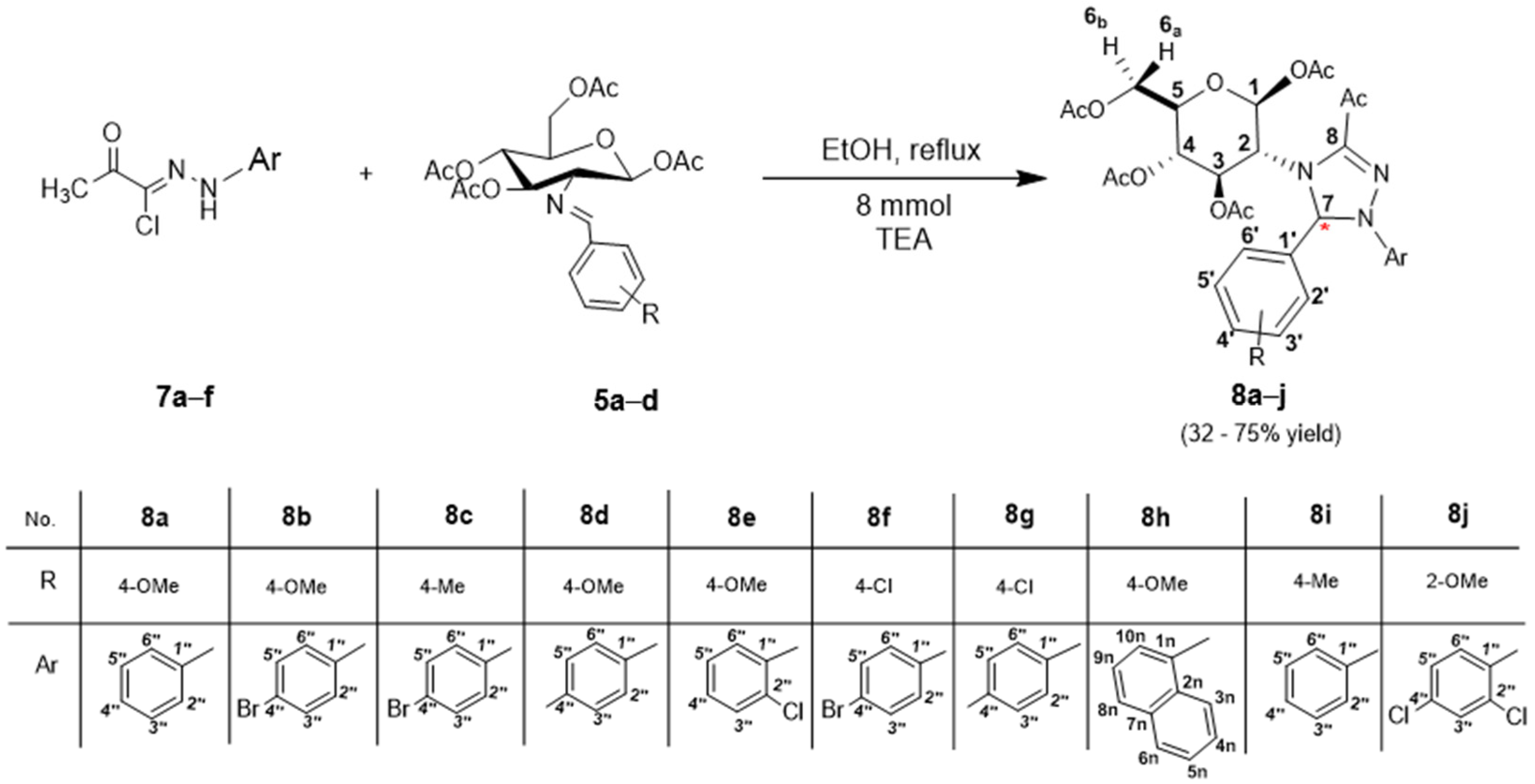
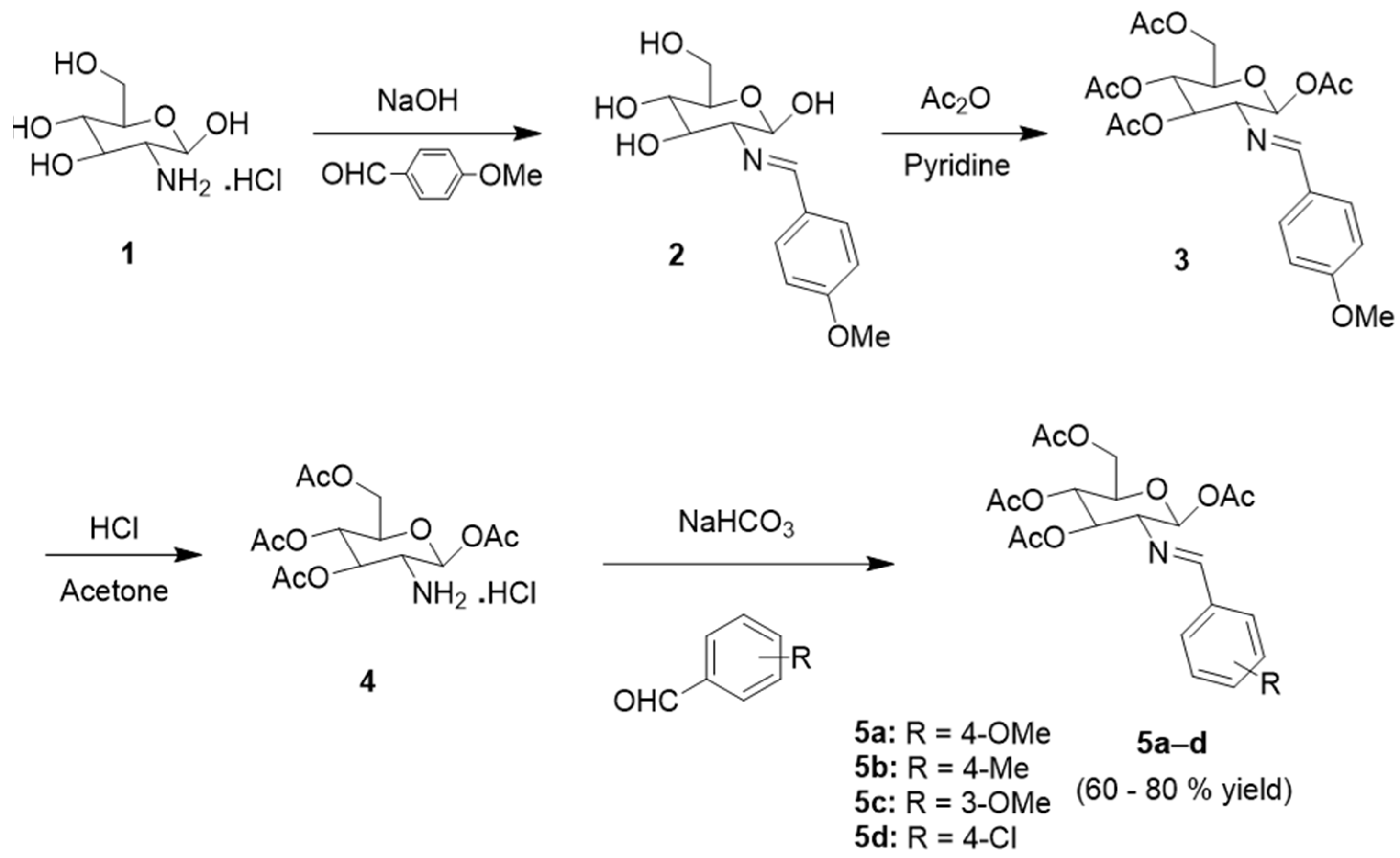
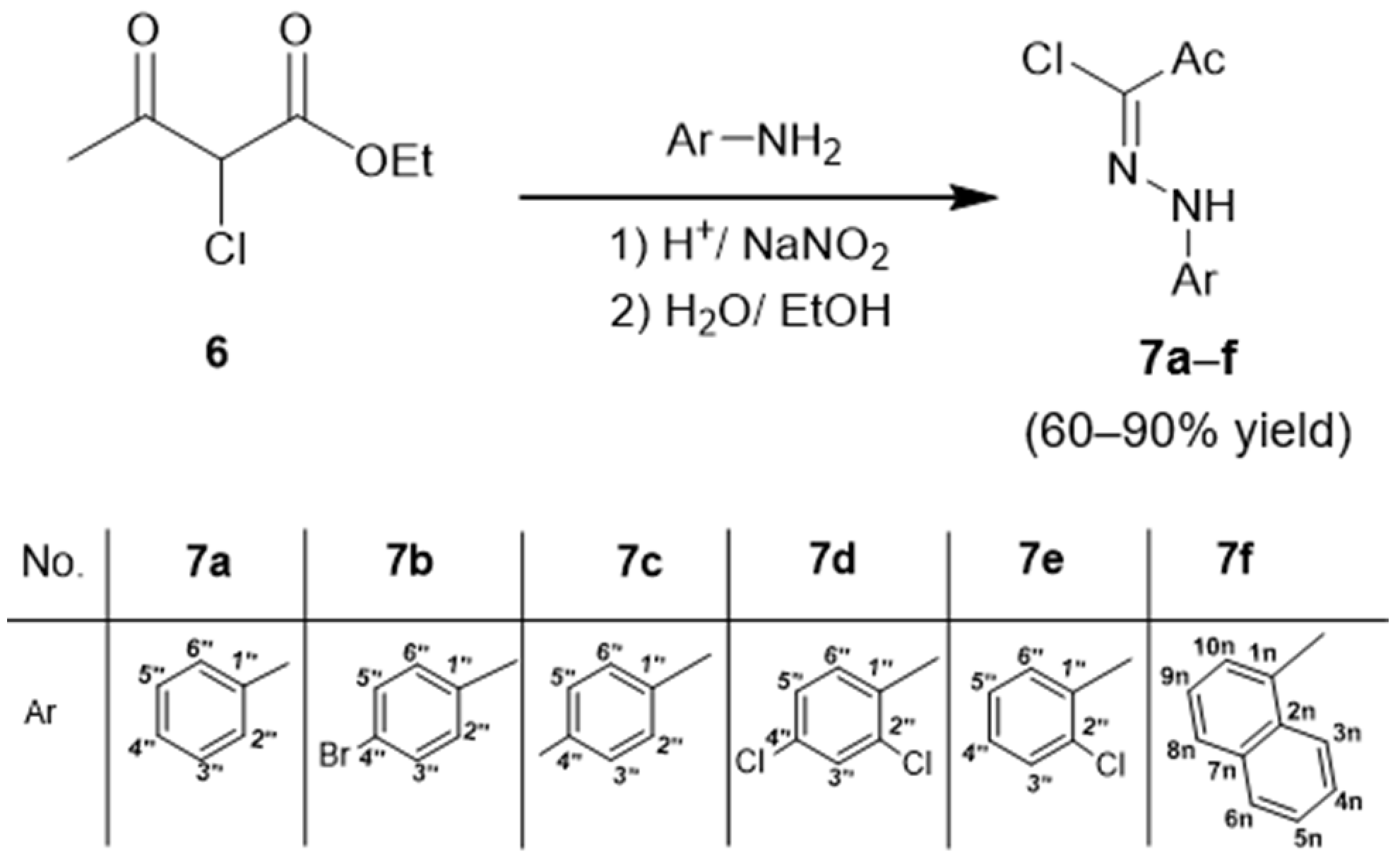
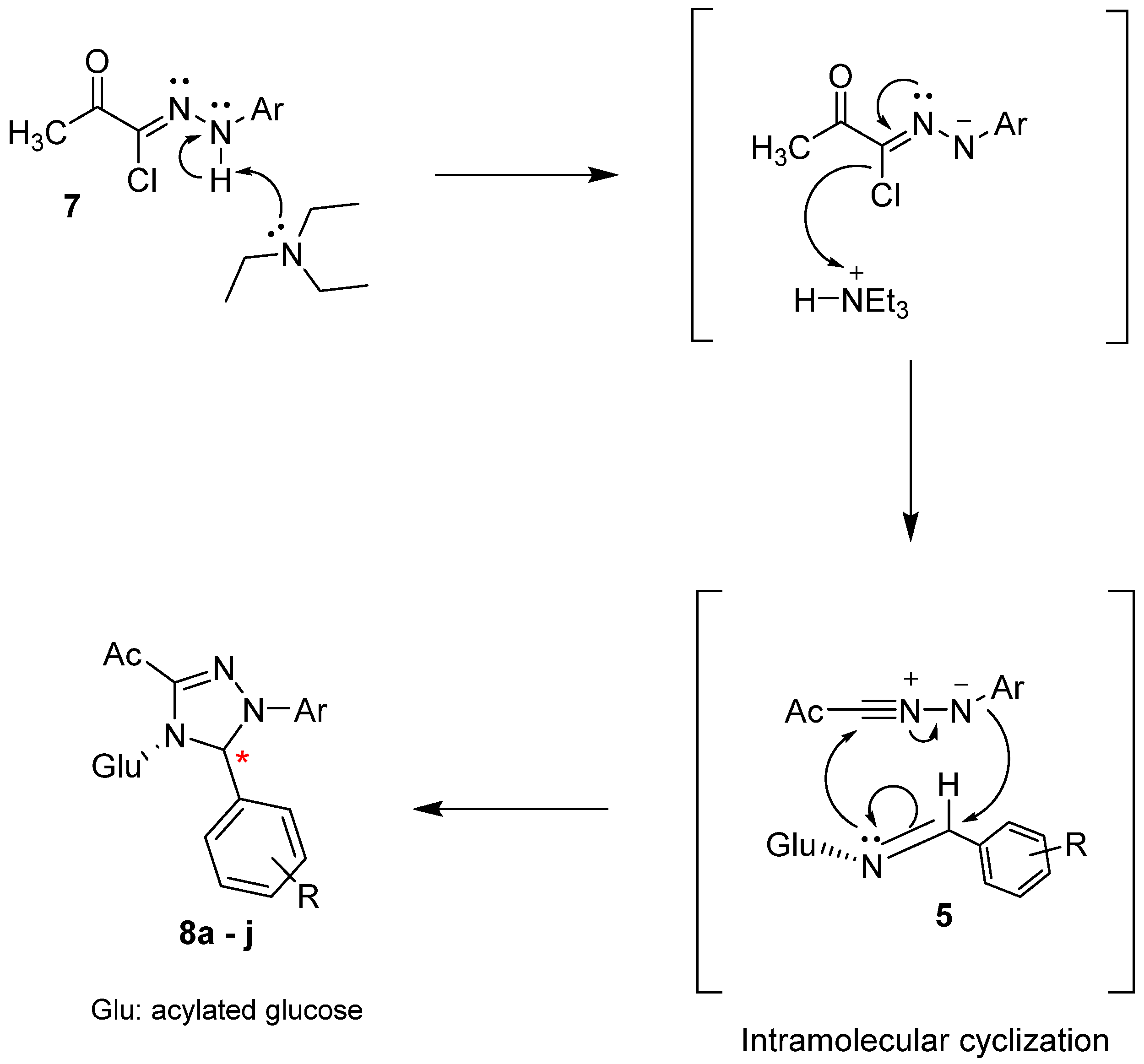
2.1. Chemical Synthesis
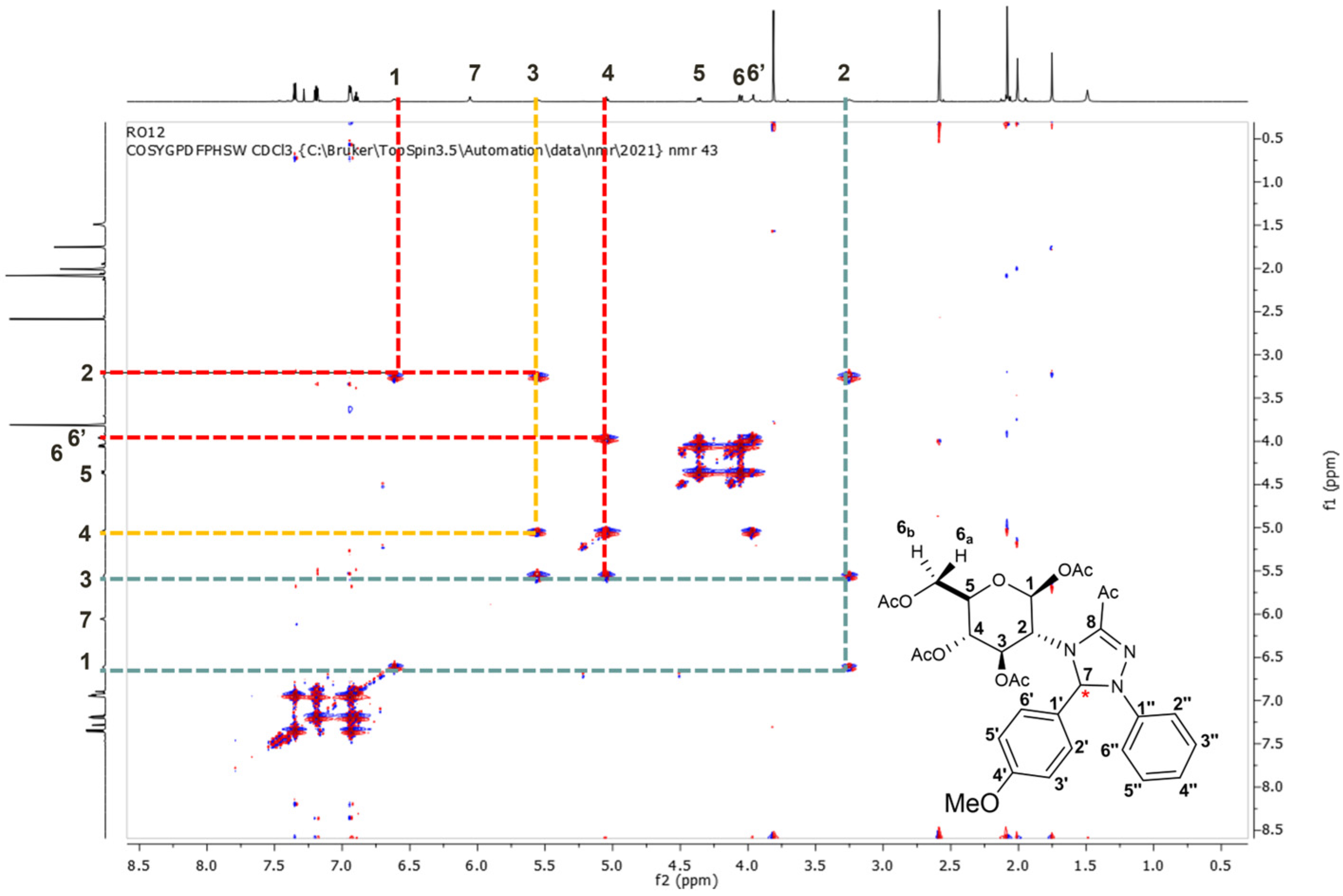
2.2. 1H NMR and 13C NMR Spectroscopic Characterization
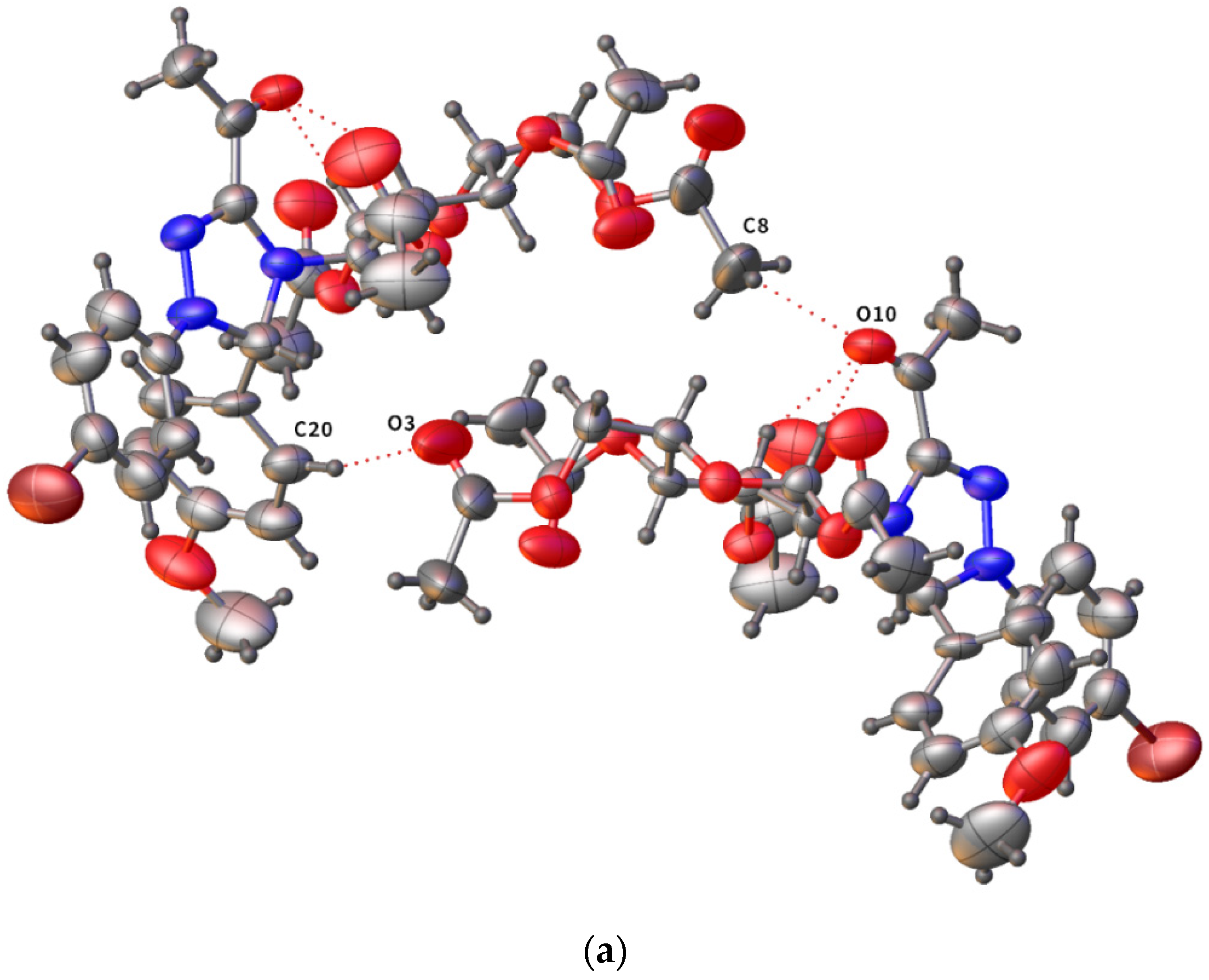
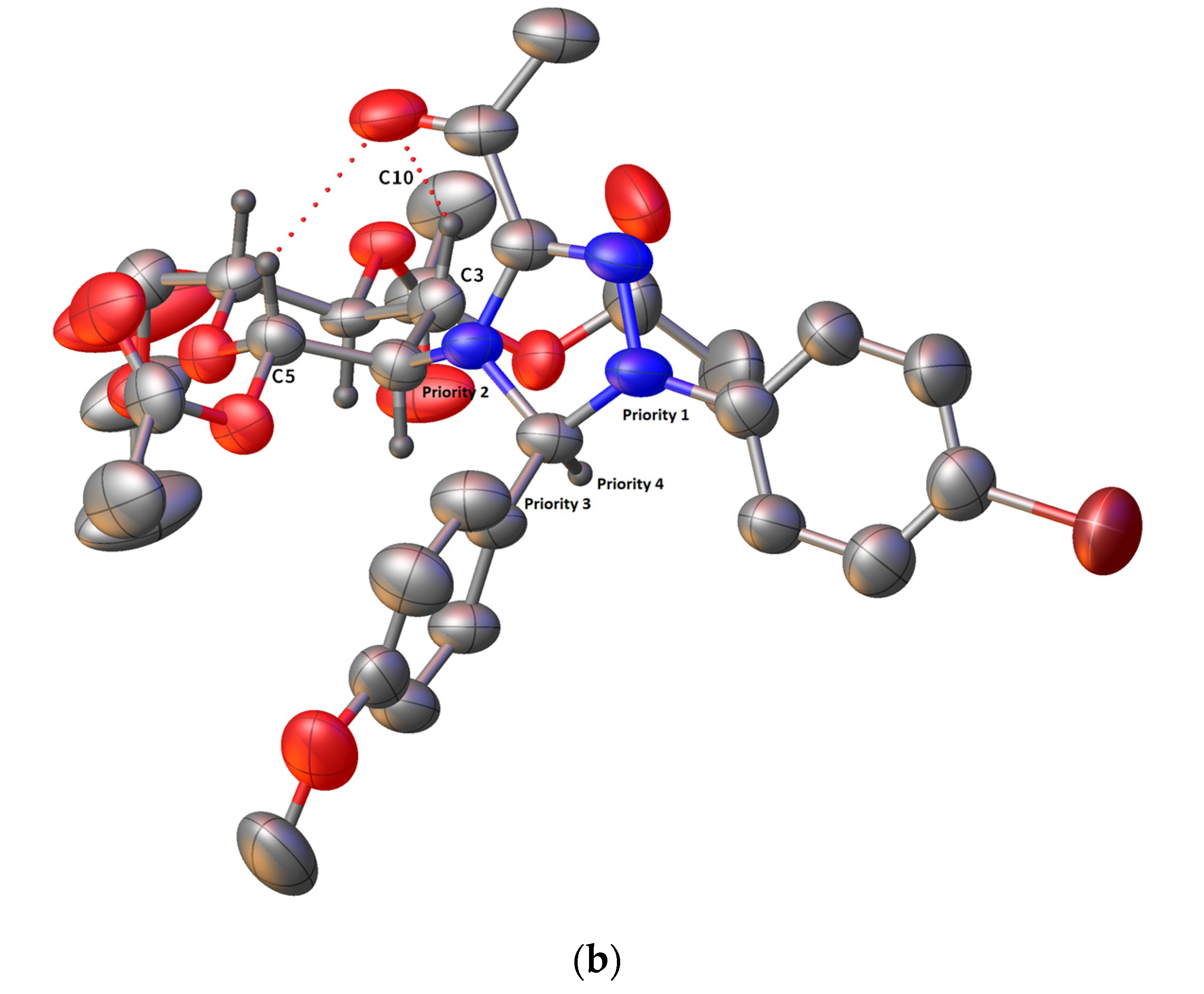
2.3. Crystallographic Analysis of Derivative 8b
2.4. FTIR Spectroscopy
2.5. Biological Assay
2.5.1. Anti-Tumor Activity
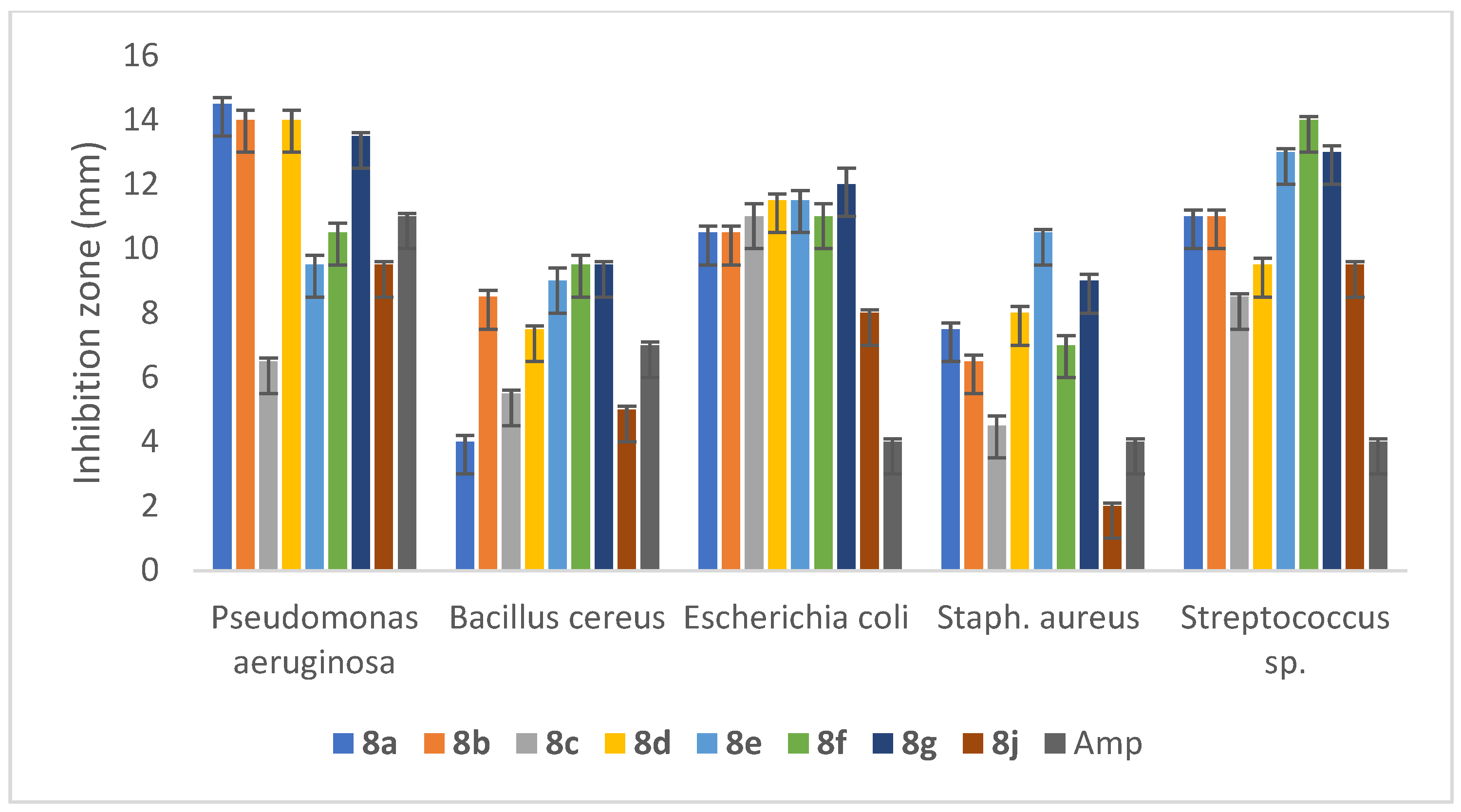
2.5.2. Anti-Bacterial Activity
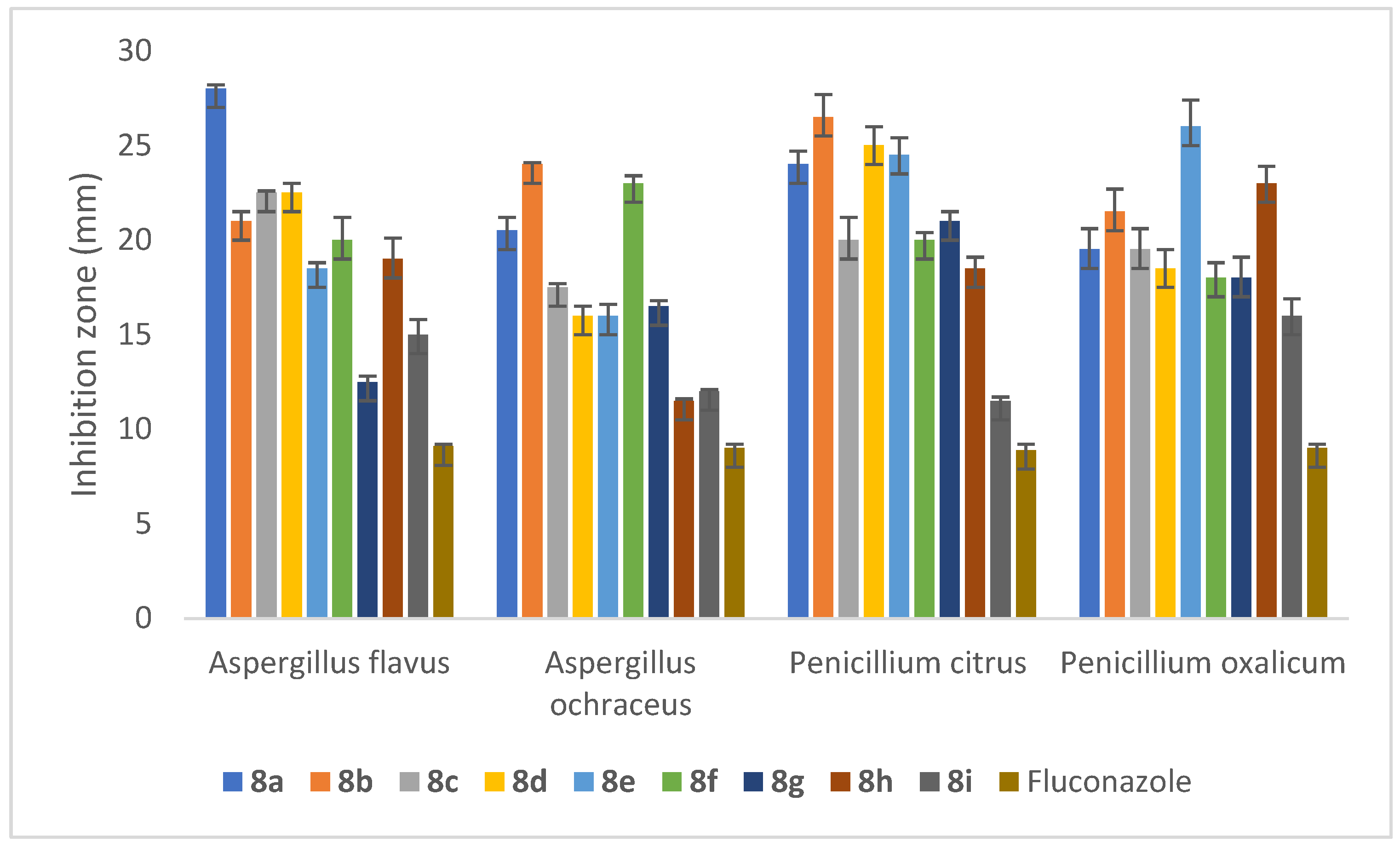
2.5.3. Anti-Fungal Activity
3. Experimental Setup
3.1. General
3.2. Physical Techniques of Characterization
3.3. Single-Crystal X-ray Analysis
3.4. Anti-Tumor Activity
3.5. Anti-Bacterial Activity
3.6. Anti-Fungal Activity
3.7. General Synthesis of 4,5-Dihydro-1H-[1,2,4] Triazoline Derivatives
3.7.1. 2-(3-Acetyl-1-(phenyl)-5-((R)-4-methoxyphenyl)-1,2,4-triazolo-4-yl)-2-deoxy-1,3,4,6-tetraacetyl-β-ᴅ-glucose (8a)
3.7.2. 2-(3-Acetyl-1-(4-bromophenyl)-5-((S)-4-methoxyphenyl)-1,2,4-triazolo-4-yl)-2-deoxy-1,3,4,6-tetraacetyl-β-ᴅ-glucose (8b)
3.7.3. 2-(3-Acetyl-1-(4-bromophenyl)-5-((S)-4-methylphenyl)-1,2,4-triazolo-4-yl)-2-deoxy-1,3,4,6-tetraacetyl-β-ᴅ-glucose (8c)
3.7.4. 2-(3-Acetyl-1-(4-methylphenyl)-5-(4-methoxyphenyl)-1,2,4-triazolo-4-yl)-2-deoxy-1,3,4,6-tetraacetyl-β-ᴅ-glucose (8d)
3.7.5. 2-(3-Acetyl-1-(3-chlorophenyl)-5-(4-methoxyphenyl)-1,2,4-triazolo-4-yl)-2-deoxy-1,3,4,6-tetraacetyl-β-ᴅ-glucose (8e)
3.7.6. 2-(3-Acetyl-1-(4-bromophenyl)-5-((S)-4-chlorophenyl)-1,2,4-triazolo-4-yl)-2-deoxy-1,3,4,6-tetraacetyl-β-ᴅ-glucose (8f)
3.7.7. 2-(3-Acetyl-1-(p-tolyl)-5-((R)-4-chlorophenyl)-1,2,4-triazolo-4-yl)-2-deoxy-1,3,4,6-tetraacetyl-β-ᴅ-glucose (8g)
3.7.8. 2-(3-Acetyl-1-(naphthalen-1-yl)-5-(4-methylphenyl)-1,2,4-triazolo-4-yl)-2-deoxy-1,3,4,6-tetraacetyl-β-ᴅ-glucose (8h)
3.7.9. 2-(3-Acetyl-1-(phenyl)-5-((R)-p-tolyl)-1,2,4-triazolo-4-yl)-2-deoxy-1,3,4,6-tetraacetyl-β-ᴅ-glucose (8i)
3.7.10. 2-(3-Acetyl-1-(3,4-dichlorophenyl)-5-(3-methylphenyl)-1,2,4-triazolo-4-yl)-2-deoxy-1,3,4,6-tetraacetyl-β-ᴅ-glucose (8j)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fakhreddin, J. Stereochemically pure drugs: An overview. In Drug Stereochemistry: Analytical Methods and Pharmacology; Irving, W., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1993; pp. 375–382. [Google Scholar]
- Ötvös, S.B.; Kappe, C.O. Continuous flow asymmetric synthesis of chiral active pharmaceutical ingredients and their advanced intermediates. Green Chem. 2021, 23, 6117–6138. [Google Scholar] [CrossRef] [PubMed]
- Gawley, R.E.; Aubé, J. Principles of Asymmetric Synthesis; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kunz, H.; Stoye, A. Carbohydrates as stereodifferentiating auxiliaries. In Heterocycles as Chiral Auxiliaries in Asymmetric Synthesis; Springer: Cham, Switzerland, 2017; pp. 1–72. [Google Scholar]
- Eswaran, S.; Adhikari, A.V.; Shetty, N.S. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur. J. Med. Chem. 2009, 44, 4637–4647. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Johnson, L.B.; Kauffman, C.A. Voriconazole: A new triazole antifungal agent. Clin. Infect. Dis. 2003, 36, 630–637. [Google Scholar]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, S.; Gao, C.; Fan, J.; Zhao, F.; Lv, Z.-S.; Feng, L.-S. Isatin hybrids and their anti-tuberculosis activity. Chin. Chem. Lett. 2017, 28, 159–167. [Google Scholar] [CrossRef]
- Gilandoust, M.; Harsha, K.B.; Mohan, C.D.; Raquib, A.R.; Rangappa, S.; Pandey, V.; Lobie, P.E.; Rangappa, K.S. Synthesis, characterization and cytotoxicity studies of 1,2,3-triazoles and 1,2,4-triazolo [1,5-a] pyrimidines in human breast cancer cells. Bioorg. Med. Chem. Lett. 2018, 28, 2314–2319. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Chang, L.; Xu, Z.; Yan, X.-F.; Ding, C.; Zhao, F.; Wu, X.; Feng, L.-S. Recent advances of tetrazole derivatives as potential anti-tubercular and anti-malarial agents. Eur. J. Med. Chem. 2019, 163, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Christov, K.; Shilkaitis, A.; Green, A.; Mehta, R.G.; Grubbs, C.; Kelloff, G.; Lubet, R. Cellular responses of mammary carcinomas to aromatase inhibitors: Effects of vorozole. Breast Cancer Res. Treat. 2000, 60, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.; Rahman, M.N.; Vukomanovic, D.; Jia, Z.; Nakatsu, K.; Szarek, W.A. Heme oxygenase inhibition by 2-Oxy-substituted 1-Azolyl-4-phenylbutanes: Effect of variation of the azole moiety. X-ray crystal structure of human heme oxygenase-1 in complex with 4-Phenyl-1-(1H-1,2,4-triazol-1-yl)-2-butanone. Chem. Biol. Drug Des. 2010, 75, 68–90. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Ba, Y.; Xu, Z. 1,2,4-Triazole-quinoline/quinolone hybrids as potential anti-bacterial agents. Eur. J. Med. Chem. 2019, 174, 1–8. [Google Scholar]
- Dai, J.; Tian, S.; Yang, X.; Liu, Z. Synthesis methods of 1,2,3-/1,2,4-triazoles: A review. Front. Chem. 2022, 10, 891484. [Google Scholar] [CrossRef] [PubMed]
- Bedekar, A.N.; Naik, A.N.; Pise, A.C. Schiff base derivatives of 2-amino-2-deoxy-1,3,4,6-tetra-O-acetyl-β-ᴅ-glucopyranose. Asian J. Chem. 2009, 21, 6661–6666. [Google Scholar]
- Eliseeva, A.I.; Nesterenko, O.O.; Slepukhin, P.A.; Benassi, E.; Belskaya, N.P. Synthesis and fluorescent behaviour of 2-Aryl-4,5-dihydro-1 H-1,2,4-triazoles. J. Org. Chem. 2017, 82, 86–100. [Google Scholar] [CrossRef]
- Karlsson, S.; Högberg, H.-E. Asymmetric 1,3-dipolar cycloadditions for the construction of enantiomerically pure heterocycles. A review. Org. Prep. Proced. Int. 2001, 33, 103–172. [Google Scholar] [CrossRef]
- El-Abadelah, M.M.; Hussein, A.Q.; Kamal, M.R.; Al-Adhami, K.H. Heterocycles from nitrile imines. I: 1,2,3,4-tetrahydro-1,2,4,5-Tetrazines. Heterocycles 1988, 27, 917–924. [Google Scholar] [CrossRef]
- Jian, F.; Bai, Z.; Xiao, H.; Li, K. 3-Benzyl-4-phenyl-1H-1,2,4-triazole-5 (4H)-thione. Acta Crystallogr. Sect. E Struct. Rep. Online 2005, 61, 557–558. [Google Scholar] [CrossRef]
- Kruszynski, R.; Trzesowska, A.; Przybycin, M.; Gil, K.; Dobosz, M. 2-(3-Methyl-5-thioxo-4,5-dihydro-1H-1,2,4-triazol-4-yl) acetic acid. Acta Crystallogr. Sect. E Struct. Rep. Online 2007, 63, 4378. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zhao, P.-S.; Li, R.-Q.; Zhou, S.-M. Synthesis, crystal structure and quantum chemical study on 3-phenylamino-4-phenyl-1,2,4-triazole-5-thione. Molecules 2009, 14, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Saleem, R.S.Z.; Tepe, J.J. Synthesis of 1,2,4-triazolines and triazoles utilizing oxazolones. J. Org. Chem. 2010, 75, 4330–4332. [Google Scholar] [CrossRef]
- Karczmarzyk, Z.; Pitucha, M.; Wysocki, W.; Fruziński, A.; Olender, E. Ethyl 2-(3-methyl-5-sulfanylidene-4,5-dihydro-1H-1,2,4-triazol-4-yl) acetate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, 3264–3265. [Google Scholar] [CrossRef]
- Boraei, A.T.; Soliman, S.M.; Haukka, M.; El Tamany, E.S.H.; Al-Majid, A.M.; Barakat, A. X-ray single crystal structure, tautomerism aspect, DFT, NBO, and Hirshfeld surface analysis of new Schiff bases based on 4-amino-5-indol-2-yl-1,2,4-triazole-3-thione hybrid. Crystals 2021, 11, 1041. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Haukka, M.; Soliman, S.M.; Barakat, A.; Boraei, A.T.; Aboelmagd, A. Stereoselective synthesis of new 4-aryl-5-indolyl-1,2,4-triazole S-and N-β-galactosides: Characterizations, X-ray crystal structure and hirshfeld surface analysis. Crystals 2023, 13, 797. [Google Scholar] [CrossRef]
- Boraei, A.T.; Eltamany, E.H.; Haukka, M.; Soliman, S.M.; Barakat, A.; Sopaih, M. Synthesis and X-ray crystal structure analysis of substituted 1,2,4-triazolo [4′,3′:2,3] pyridazino [4,5-b] indole and its precursor. Crystals 2023, 13, 1036. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Ji, L. Crystal structure of 4-(acetoxymethyl)-6-(3-acetyl-3-(4-fluorophenyl) thioureido) cyclohex-4-ene-1,2,3-triyl triacetate. Z. Für Krist. New Cryst. Struct. 2018, 234, 189–190. [Google Scholar] [CrossRef]
- Lopyrev, V.; Chipanina, N.; Rozinova, L.; Sarapulova, G.; Sultangareev, R.; Voronkov, M. Synthesis and absorption spectra of 3,5-diaryl-1,2,4-triazoles. Chem. Heterocycl. Compd. 1977, 13, 1346–1349. [Google Scholar] [CrossRef]
- Kahveci, B.; Ikizler, A.A. A study on some 4,5-dihydro-1H-1,2,4-triazol-5-one dervatives. Acta Pol. Pharm. 2000, 57, 119–122. [Google Scholar] [PubMed]
- Sharma, P.; Chen, A.; Wang, D.; Wang, G. Synthesis and self-assembling properties of peracetylated β-1-triazolyl alkyl ᴅ-glucosides and D-galactosides. Chemistry 2021, 3, 935–958. [Google Scholar] [CrossRef]
- Al-Ajely, M.; Al-Ajely, H.; Al-Naib, A. Synthesis of some substituted 1,2,3-triazole derivatives via 1,3-cycloaddition reaction of phenacylazides and some substituted propargyl compounds. Tikrit J. Pure Sci. 2008, 13, 100–106. [Google Scholar]
- Abdel-Jalil, R.J.; Arafeh, M.M.; Shongwe, M.S.; Maichle-Mößmer, C.; Kociok-Köhn, G.; Voelter, W. 1-(Naphthylamino)-1-(p-chlorophenylhydrazono)-2-propanone and 2-(p-tolyldiazenyl)-[1H]-3-methylbenzo [g] indole: Crystallographic and spectroscopic elucidation of the cyclisation of an arylamidrazone. J. Mol. Struct. 2015, 1079, 307–314. [Google Scholar] [CrossRef]
- Sert, Y.; El-Emam, A.A.; Al-Abdullah, E.S.; Al-Tamimi, A.-M.S.; Çırak, Ç.; Ucun, F. Use of vibrational spectroscopy to study 4-benzyl-3-(thiophen-2-yl)-4,5-dihydro-1H-1,2,4-triazole-5-thione: A combined theoretical and experimental approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 126, 280–290. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Kamel, S.; Salama, A.; Tohamy, H.-A.S. Preparation and infrared study of cellulose based amphiphilic materials. Cellul. Chem. Technol. 2018, 52, 193–200. [Google Scholar]
- Rajasekaran, A.; Sivakumar, K.K.; Sureshkumar, K.; Manjushree, M. Design, synthesis, characterisation and in-vitro antimicrobial activity of some hybridized triazole scaffolds. Future J. Pharm. Sci. 2017, 3, 1–10. [Google Scholar] [CrossRef]
- Strzelecka, M.; Świątek, P. 1,2,4-triazoles as important antibacterial agents. Pharmaceuticals 2021, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.; Piscitelli, S.; Walsh, T. Clinical pharmacology of systemic antifungal agents in clinical use, current investigational compounds and putative targets for antifungal drug development. Adv. Pharmacol. 1998, 44, 343–500. [Google Scholar] [PubMed]
- Ji, H.; Zhang, W.; Zhou, Y.; Zhang, M.; Zhu, J.; Song, Y.; Lü, J.; Zhu, J. A three-dimensional model of lanosterol 14α-demethylase of Candida albicans and its interaction with azole antifungals. J. Med. Chem. 2000, 43, 2493–2505. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-H.; Sheng, C.-Q.; Xu, X.-H.; Jiang, Y.-Y.; Zhang, W.-N.; He, C. Identification of Y118 amino acid residue in Candida albicans sterol 14α-demethylase associated with the enzyme activity and selective antifungal activity of azole analogues. Biol. Pharm. Bull. 2007, 30, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Madison, V.; Chau, A.S.; Loebenberg, D.; Palermo, R.E.; McNicholas, P.M. Three-dimensional models of wild-type and mutated forms of cytochrome P450 14α-sterol demethylases from aspergillus fumigatus and candida albicans provide insights into posaconazole binding. Antimicrob. Agents Chemother. 2004, 48, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Shalini, K.; Kumar, N.; Drabu, S.; Sharma, P.K. Advances in synthetic approach to and antifungal activity of triazoles. Beilstein J. Org. Chem. 2011, 7, 668–677. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef]
- Kazeminejad, Z.; Marzi, M.; Shiroudi, A.; Kouhpayeh, S.A.; Farjam, M.; Zarenezhad, E. Novel 1,2,4-triazoles as antifungal agents. BioMed Res. Int. 2022, 2022, 4584846. [Google Scholar] [CrossRef]
- Moghadam, E.S.; Mireskandari, K.; Abdel-Jalil, R.; Amini, M. An approach to pharmacological targets of pyrrole family from medicinal chemistry viewpoint. Mini Rev. Med. Chem. 2022, 22, 2486–2561. [Google Scholar] [PubMed]
- Stoe, C. X–AREA: Program for the Acquisition and Analysis of Data, Version 1.30; Stoe & Cie GmbH: Darmatadt, Germany, 2005. [Google Scholar]
- Stoe, C. X–RED: Program for Data Reduction and Absorption Correction, Version 1.28 b; Stoe & Cie GmbH: Darmatadt, Germany, 2005. [Google Scholar]
- Sheldrick, G. SHELXL-97. In Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Holbeck, S.L.; Camalier, R.; Crowell, J.A.; Govindharajulu, J.P.; Hollingshead, M.; Anderson, L.W.; Polley, E.; Rubinstein, L.; Srivastava, A.; Wilsker, D. The National Cancer Institute ALMANAC: A comprehensive screening resource for the detection of anticancer drug pairs with enhanced therapeutic activity NCI ALMANAC of approved cancer drug combinations. Cancer Res. 2017, 77, 3564–3576. [Google Scholar] [CrossRef]
- Uenver, Y.; Düğdü, E.; Sancak, K.; Er, M.; Karaoğlu, S.A. Synthesis and antimicrobial and antitumor activity of some new [1,2,4] triazole-5-one derivatives. Turk. J. Chem. 2009, 33, 135–147. [Google Scholar]
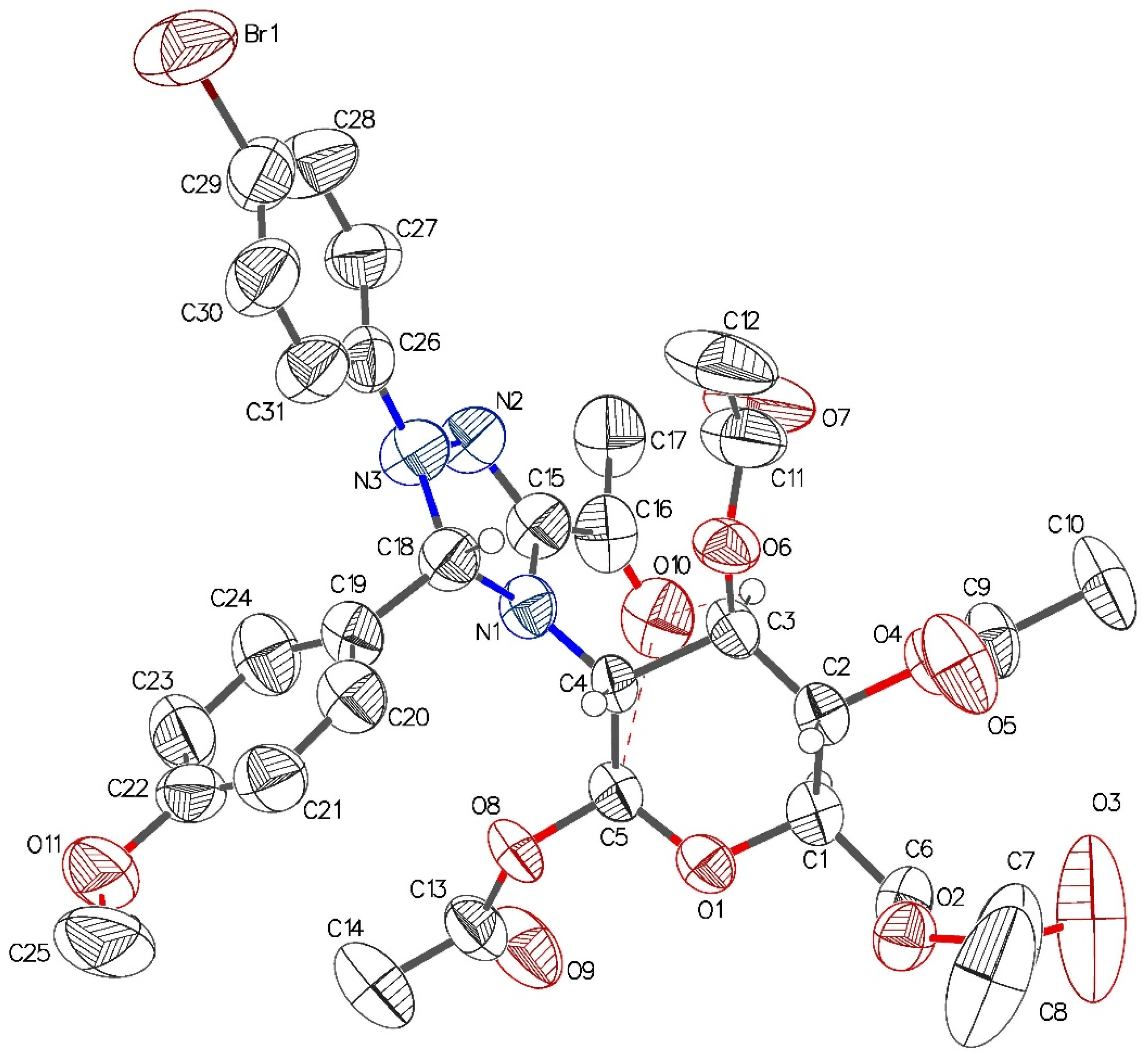


| Empirical formula | C31H34BrN3O11 |
| Molar mass (g/mol) | 704.52 |
| Temperature (K) | 298.15 |
| Crystal system | monoclinic |
| Space group | P21 |
| a (Å) | 11.850(2) |
| b (Å) | 10.170(2) |
| c (Å) | 16.090(3) |
| α (°) | 90 |
| β (°) | 104.40(3) |
| γ (°) | 90 |
| Volume (Å3) | 1878.2(7) |
| Z | 2 |
| ρcalc (g/cm3) | 1.246 |
| μ (mm–1) | 1.149 |
| F (000) | 728.0 |
| Crystal size (mm) | 0.10 × 0.10 × 0.05 |
| Radiation | Mo-Kα (λ = 0.71073 Å) |
| 2Θ range for data collection (°) | 4.904–49.414 |
| Index ranges | −13 ≤ h ≤ 13, −11 ≤ k ≤ 11, −18 ≤ l ≤ 18 |
| Reflections collected | 12,658 |
| Independent reflections | 6077 [Rint = 0.0798, Rsigma = 0.0869] |
| Data/restraints/parameters | 6077/355/418 |
| Goodness of fit on F2 | 1.015 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0722, wR2 = 0.1646 |
| Final R indexes [all data] | R1 = 0.1433, wR2 = 0.2008 |
| Largest diff. peak/hole (e Å–3) | 0.24/−0.35 |
| D–H···A | D–H | H···A | D–A | D–H···A | Symmetry Codes |
|---|---|---|---|---|---|
| C(3)–H(3)···O(10) | 0.98 | 2.48 | 3.163(12) | 126.7 | |
| C(5)–H(5)···O(10) | 0.98 | 2.30 | 3.046(12) | 132.3 | |
| C(8)–H(8C)···O(10) | 0.96 | 2.66 | 3.48(2) | 143.1 | −x + 1, y + ½, −z + 1 |
| C(10)–H(10B)···O(9) | 0.96 | 2.59 | 3.404(14) | 142.2 | x + 1, y, z |
| C(20)–H(20)···O(3) | 0.93 | 2.59 | 3.481(16) | 160.3 | −x + 1, y + ½, −z + 1 |
| C(12)–H(12B)···Br(1) | 0.96 | 2.94 | 3.89(2) | 170.0 | −x + 1, y−½, −z |
| C(29)–Br(1) | 1.887(13) |
| C(16)–O(10) | 1.189(13) |
| C(15)–N(1) | 1.415(13) |
| C(15)–N(2) | 1.299(12) |
| N(2)–N(3) | 1.361(11) |
| C(18)–N(1) | 1.490(12) |
| C(18)–N(3) | 1.505(13) |
| C(18)–C(19) | 1.485(14) |
| C(7)–O(2) | 1.340(14) |
| C(7)–O(3) | 1.165(17) |
| C(9)–O(4) | 1.358(11) |
| C(9)–O(5) | 1.174(13) |
| C(11)–O(6) | 1.343(14) |
| C(11)–O(7) | 1.186(15) |
| C(13)–O(8) | 1.341(13) |
| C(13)–O(9) | 1.217(14) |
| Compound ID | NCI Code | Mean G% |
|---|---|---|
| 8a | 835808 | 92.73 |
| 8b | 835811 | 70.77 |
| 8c | 835812 | 58.86 |
| 8d | 835813 | 78.32 |
| 8e | 835815 | 73.52 |
| 8f | 835816 | 61.24 |
| Cancer Panel Type | Cancer Cell Type | G% | |
|---|---|---|---|
| 8c NCI Code 835812 | 8f NCI Code 835816 | ||
| Leukemia | HL-60(TB) | 14.42 | 34.14 |
| RPMI-8226 | 35.38 | 38.03 | |
| MOLT-4 | 41.79 | 44.34 | |
| CNS Cancer | SF-268 | 58.40 | 58.57 |
| SF-295 | 29.43 | 36.96 | |
| Renal Cancer | 786-0 | 68.84 | 58.46 |
| A498 | 59.58 | 131.61 | |
| ACHN | 52.23 | 50.09 | |
| UO-31 | 32.19 | 27.59 | |
| Breast Cancer | MCF7 | 43.90 | 34.59 |
| T-47D | 26.78 | 34.57 | |
| MDA-MB-468 | 49.38 | 26.20 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Maqbali, A.S.; Al Rasbi, N.K.; Zoghaib, W.M.; Sivakumar, N.; Robertson, C.C.; Shongwe, M.S.; Grzegorzek, N.; Abdel-Jalil, R.J. Stereoselective Asymmetric Syntheses of Molecules with a 4,5-Dihydro-1H-[1,2,4]-Triazoline Core Possessing an Acetylated Carbohydrate Appendage: Crystal Structure, Spectroscopy, and Pharmacology. Molecules 2024, 29, 2839. https://doi.org/10.3390/molecules29122839
Al Maqbali AS, Al Rasbi NK, Zoghaib WM, Sivakumar N, Robertson CC, Shongwe MS, Grzegorzek N, Abdel-Jalil RJ. Stereoselective Asymmetric Syntheses of Molecules with a 4,5-Dihydro-1H-[1,2,4]-Triazoline Core Possessing an Acetylated Carbohydrate Appendage: Crystal Structure, Spectroscopy, and Pharmacology. Molecules. 2024; 29(12):2839. https://doi.org/10.3390/molecules29122839
Chicago/Turabian StyleAl Maqbali, Anwaar S., Nawal K. Al Rasbi, Wajdi M. Zoghaib, Nallusamy Sivakumar, Craig C. Robertson, Musa S. Shongwe, Norbert Grzegorzek, and Raid J. Abdel-Jalil. 2024. "Stereoselective Asymmetric Syntheses of Molecules with a 4,5-Dihydro-1H-[1,2,4]-Triazoline Core Possessing an Acetylated Carbohydrate Appendage: Crystal Structure, Spectroscopy, and Pharmacology" Molecules 29, no. 12: 2839. https://doi.org/10.3390/molecules29122839
APA StyleAl Maqbali, A. S., Al Rasbi, N. K., Zoghaib, W. M., Sivakumar, N., Robertson, C. C., Shongwe, M. S., Grzegorzek, N., & Abdel-Jalil, R. J. (2024). Stereoselective Asymmetric Syntheses of Molecules with a 4,5-Dihydro-1H-[1,2,4]-Triazoline Core Possessing an Acetylated Carbohydrate Appendage: Crystal Structure, Spectroscopy, and Pharmacology. Molecules, 29(12), 2839. https://doi.org/10.3390/molecules29122839






