Promising Molecular Architectures for Two-Photon Probes in the Diagnosis of α-Synuclein Aggregates
Abstract
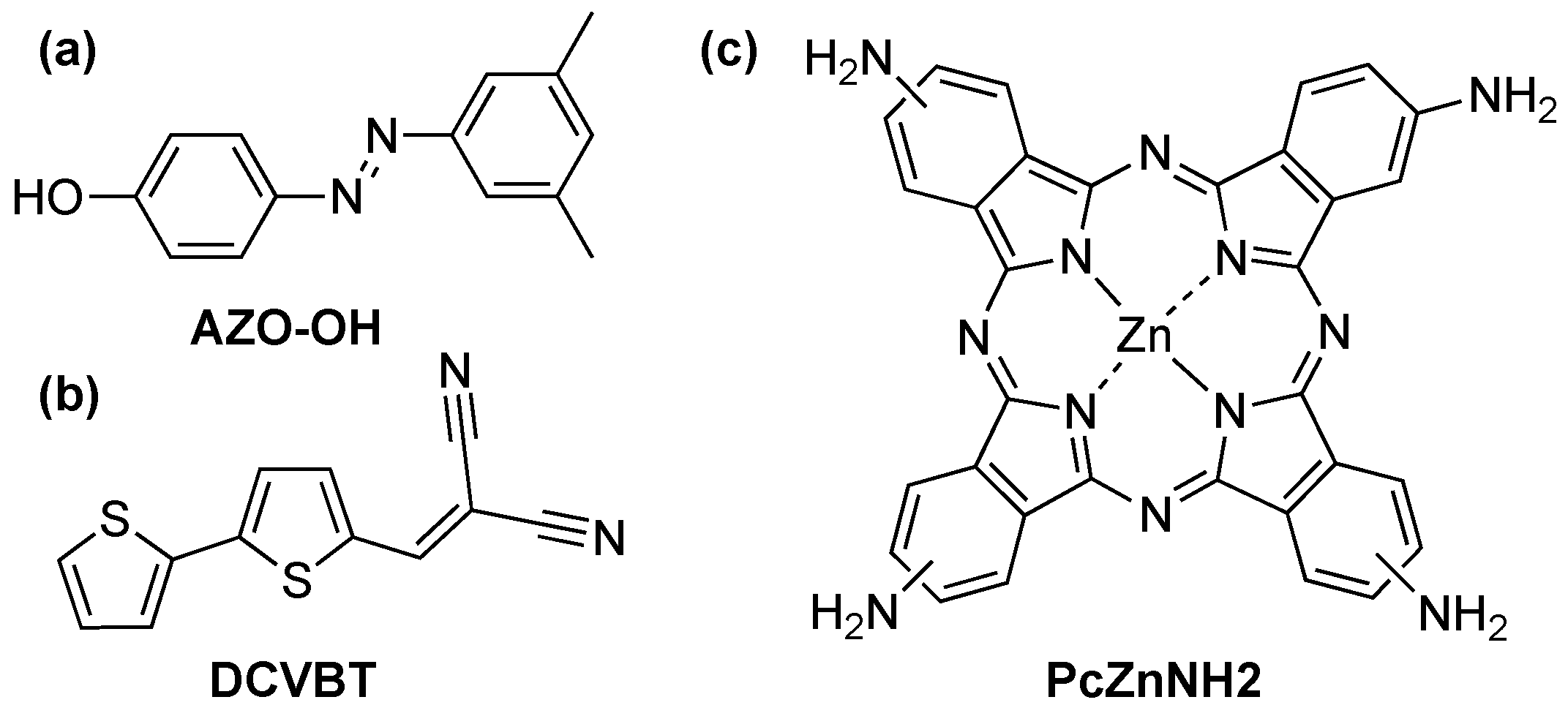
1. Introduction
2. Results and Discussion
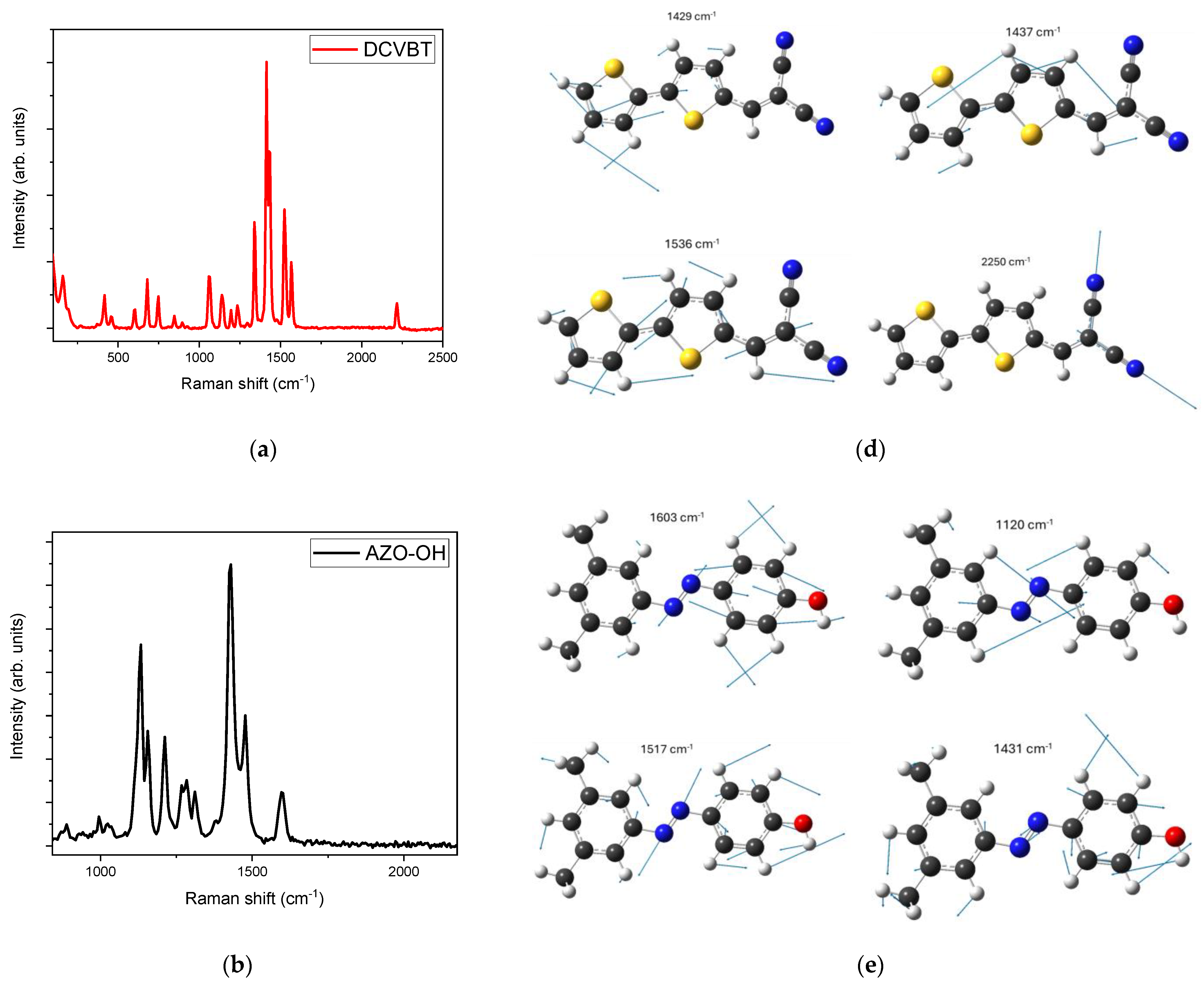
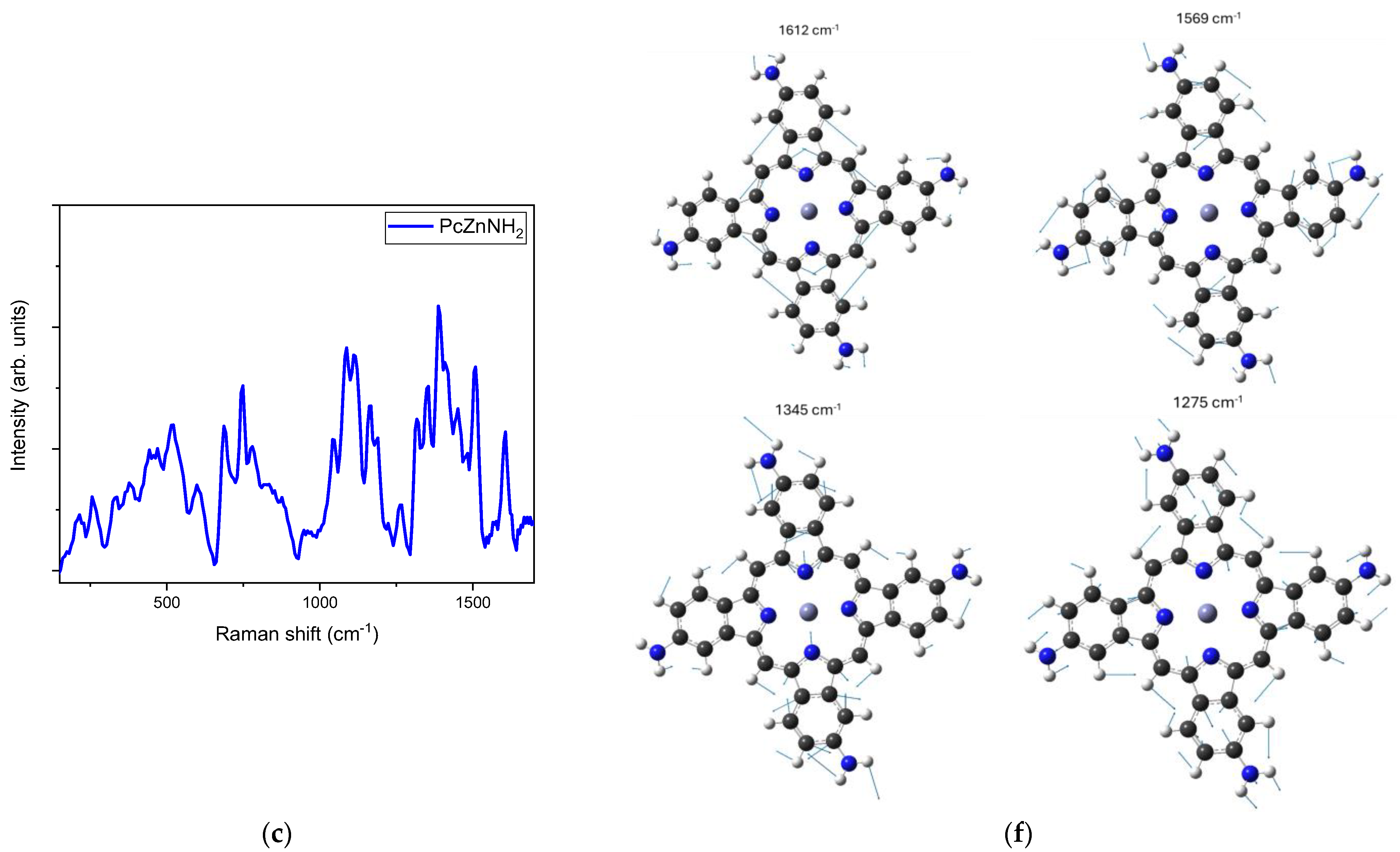
2.1. Raman Characterization
2.2. Interaction of the Probes with α-syn
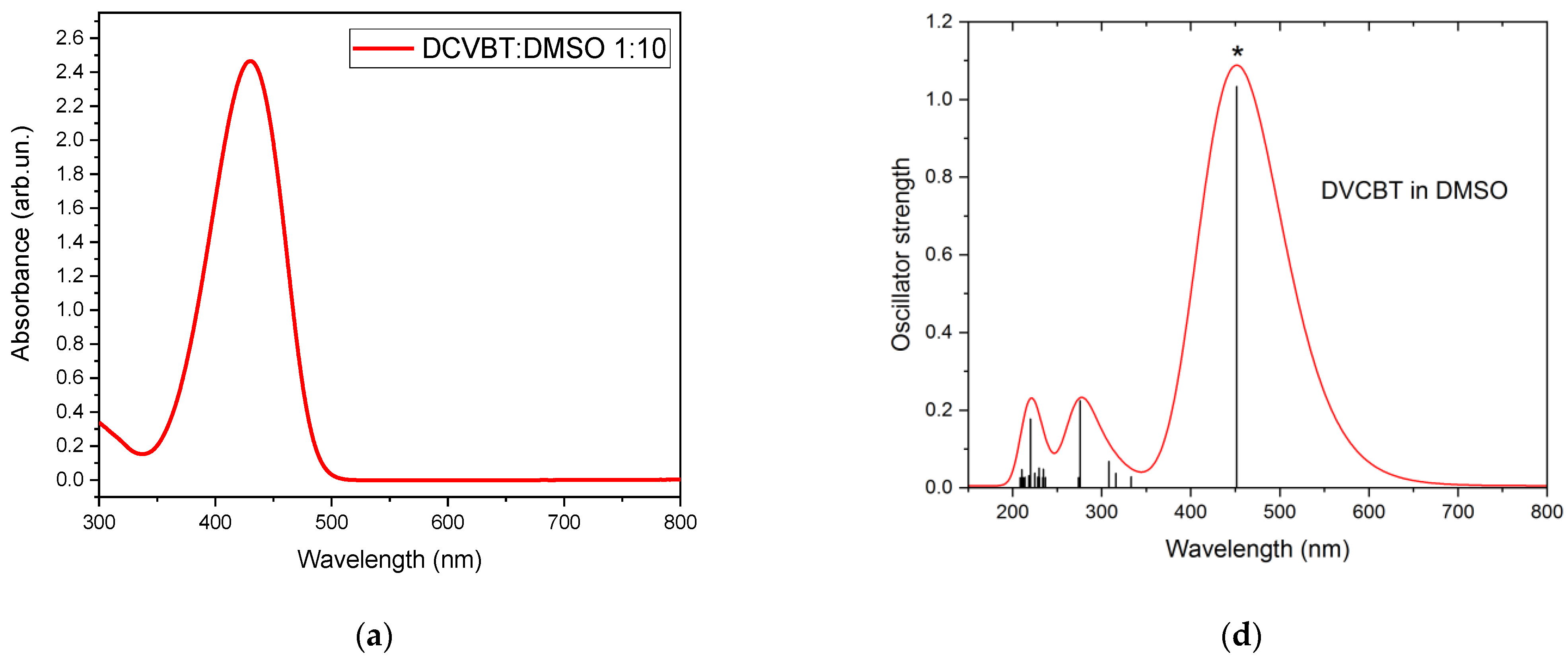
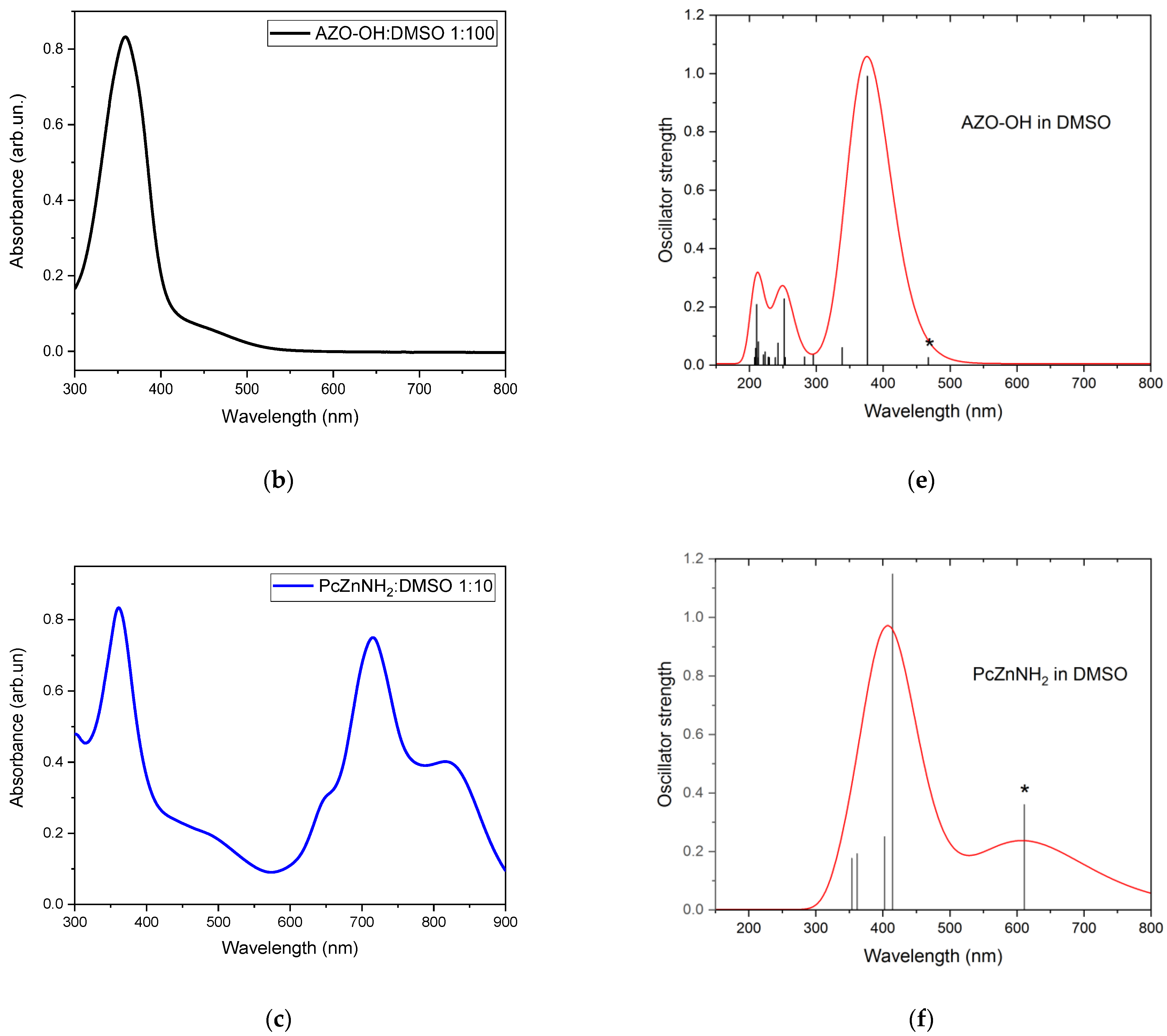
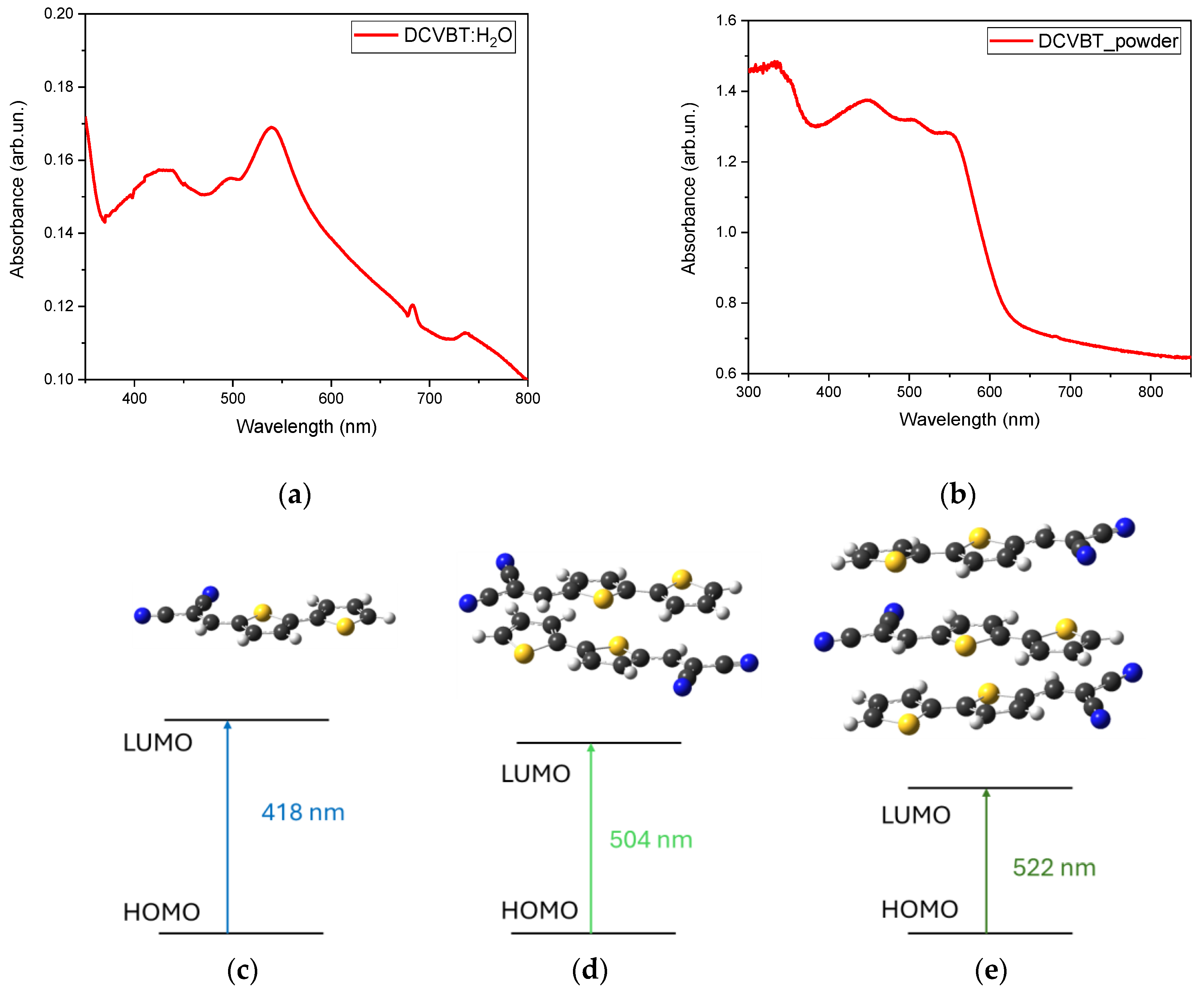
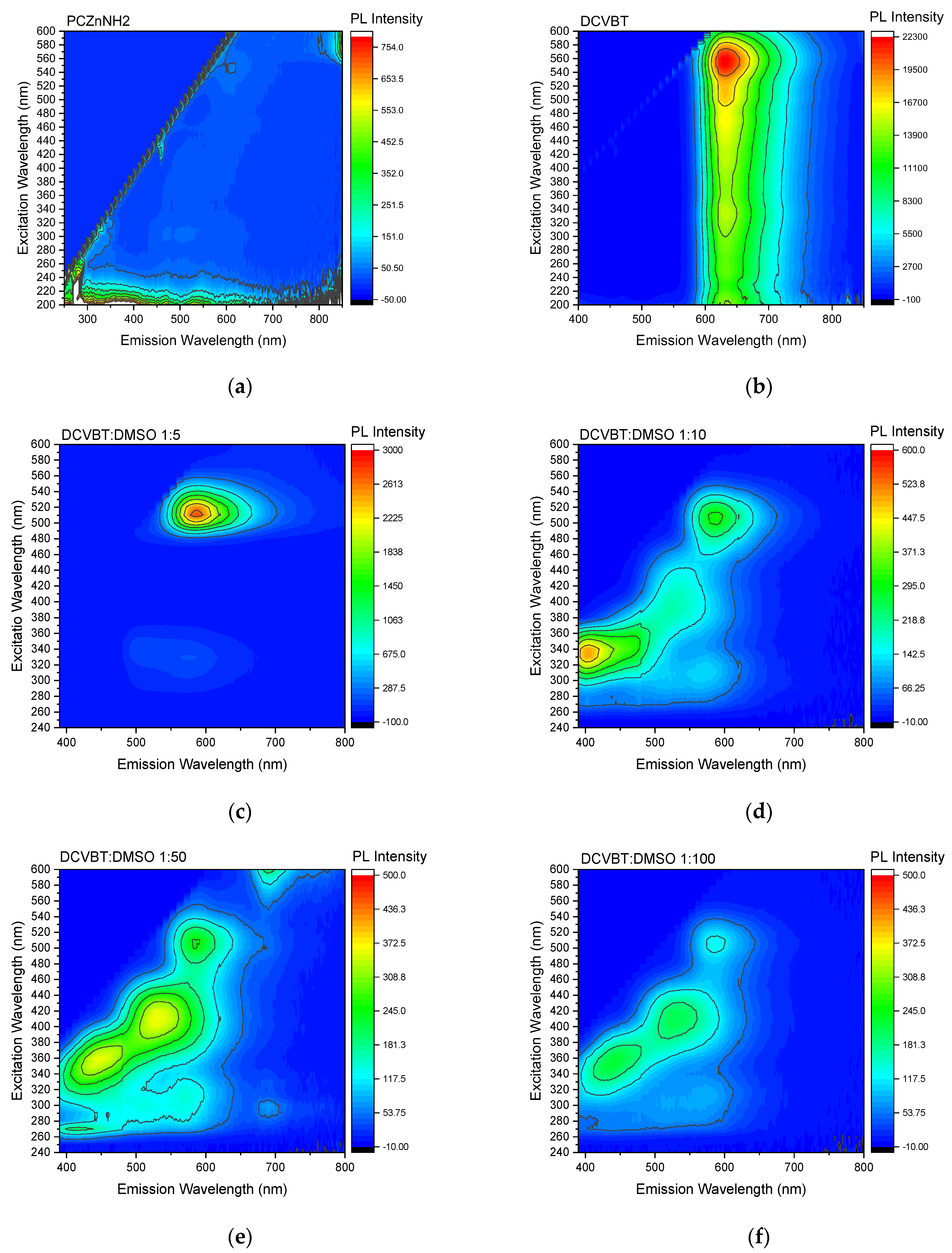
2.3. Steady-State Absorption and PLE Measurements
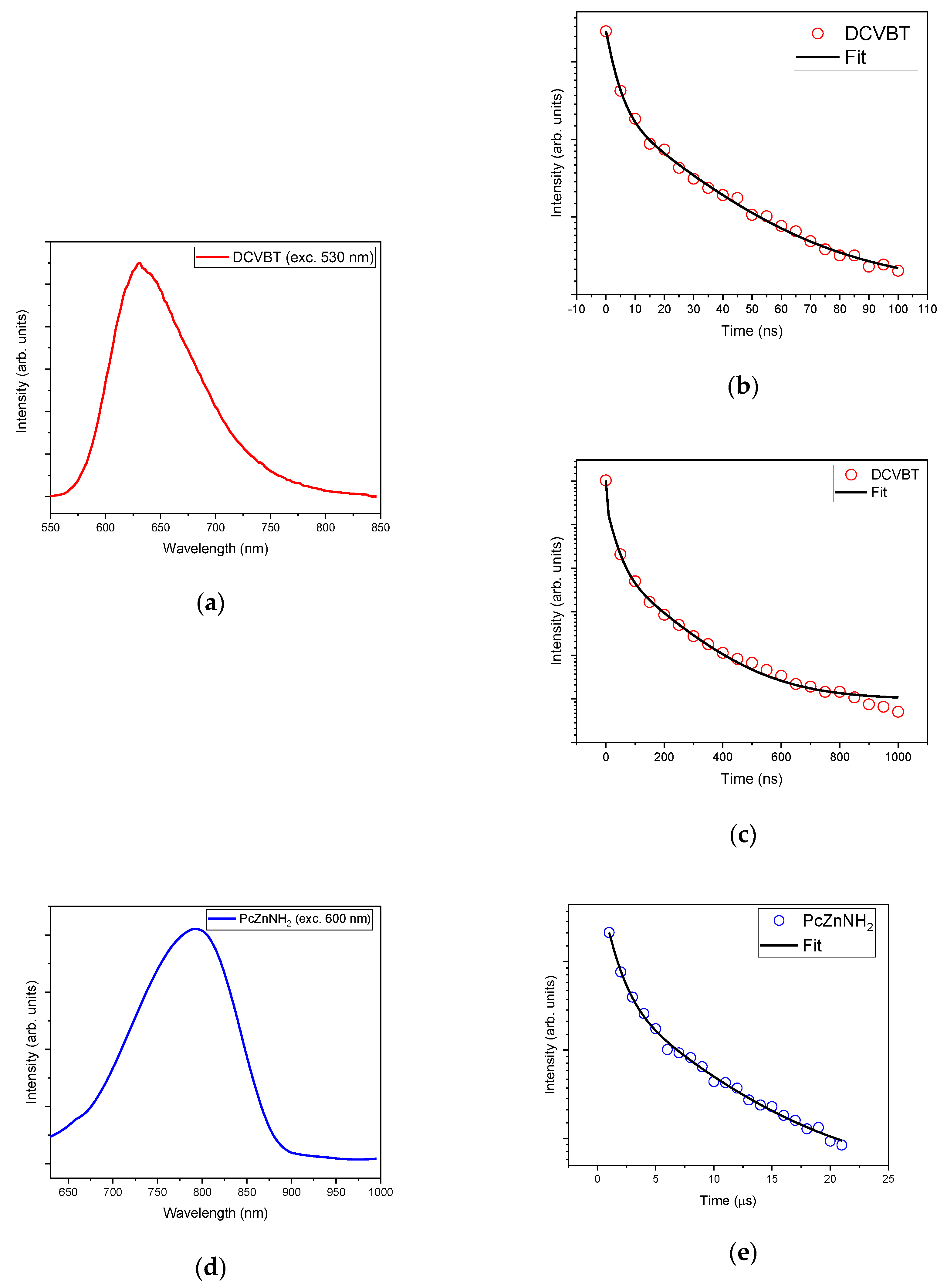
2.4. Time-Resolved Photoluminescence (TRPL)
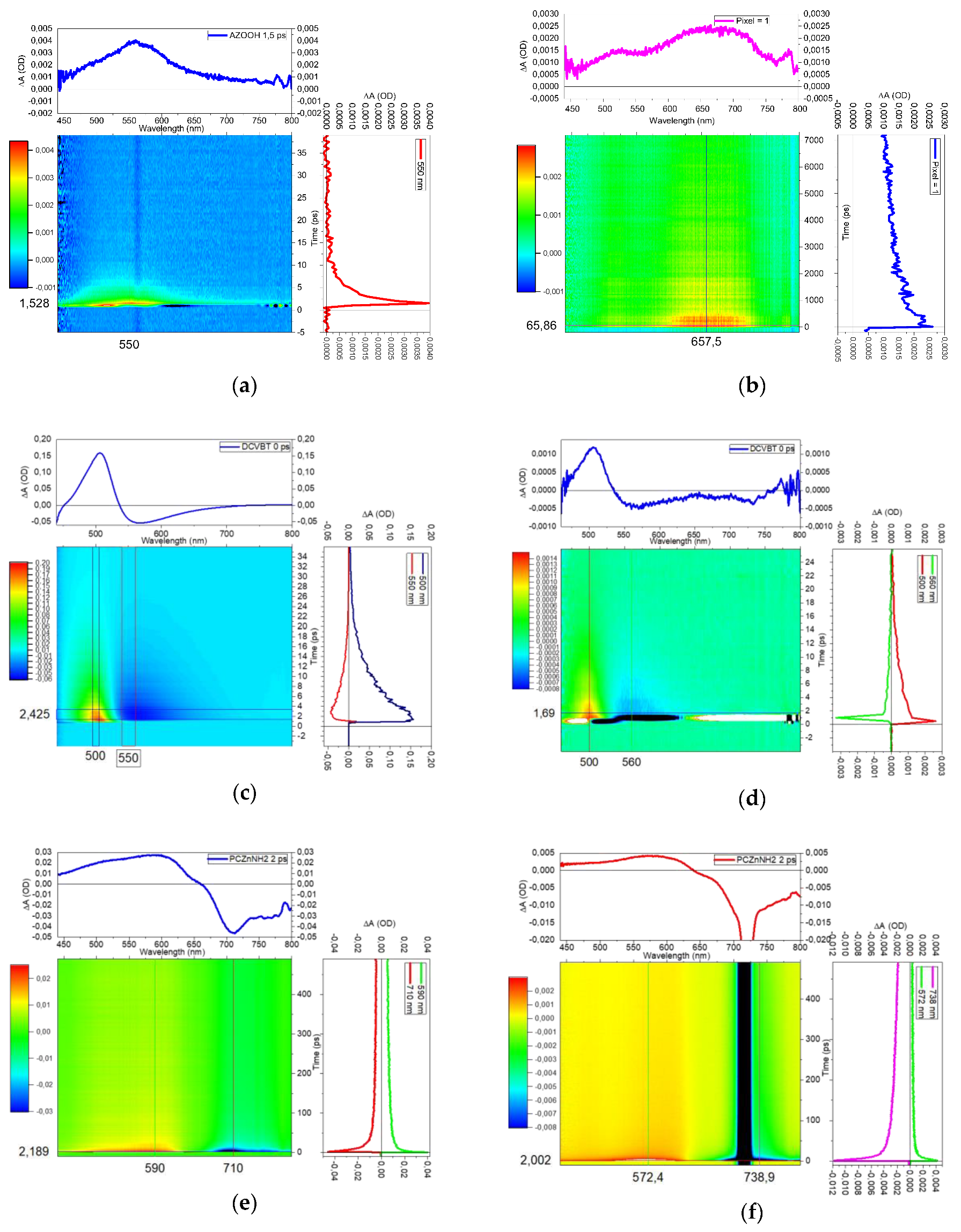
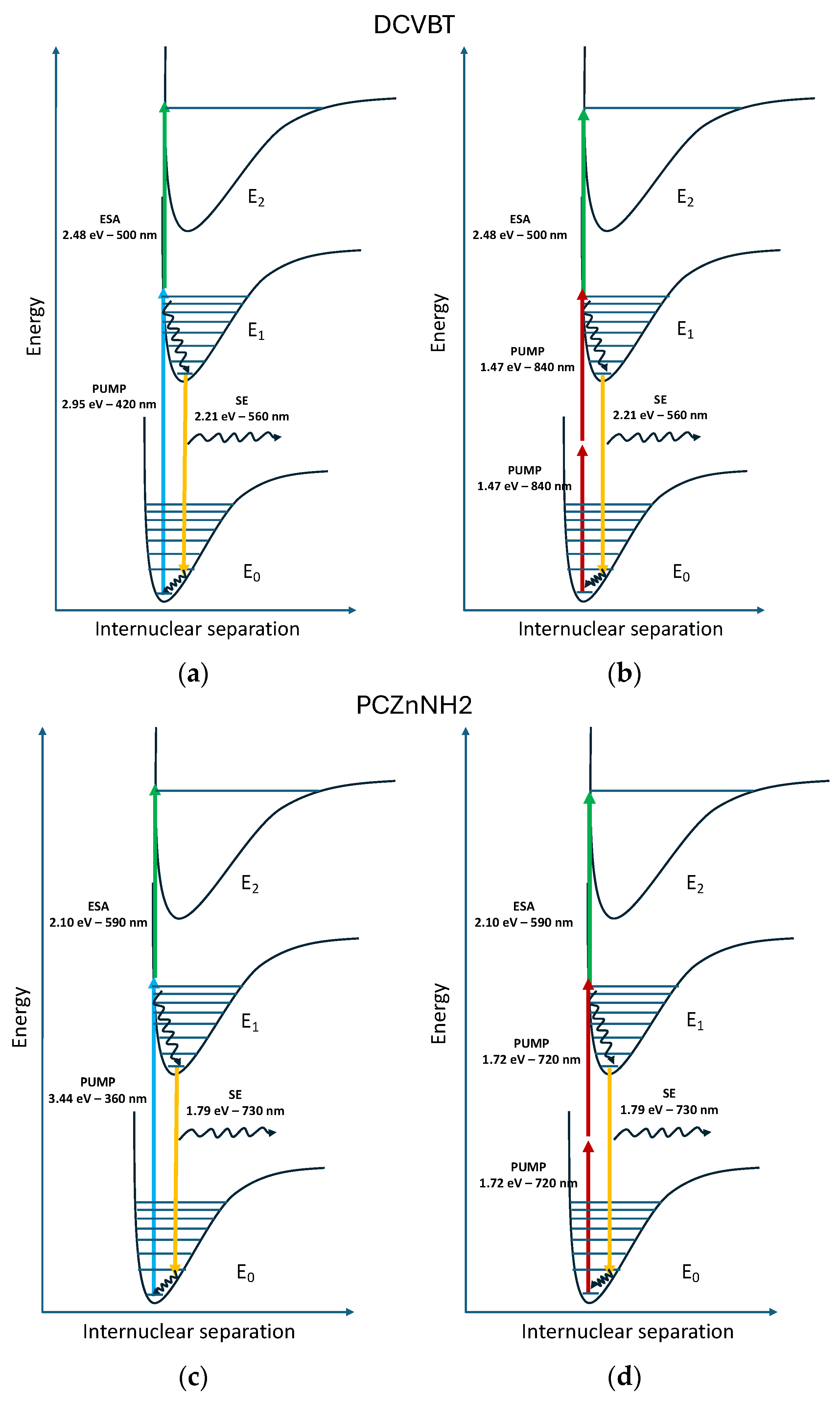
2.5. Transient Absorption Measurements
3. Materials and Methods
3.1. Synthesis of Studied Molecules
3.2. NMR
3.3. Raman
3.4. Steady-State Absorption, Photoluminescence Excitation (PLE), and Quantum Yield
3.5. Time-Resolved Photoluminescence (TRPL)
3.6. Transient Absorption (TA)
3.7. DFT Calculations
3.8. Docking Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Verwilst, P.; Kim, H.S.; Kim, S.; Kang, C.; Kim, J.S. Shedding light on tau protein aggregation: The progress in developing highly selective fluorophores. Chem. Soc. Rev. 2018, 47, 2249–2265. [Google Scholar] [CrossRef]
- Lashuel, H.A.; Overk, C.R.; Oueslati, A.; Masliah, E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat. Rev. Neurosci. 2013, 14, 38–48. [Google Scholar] [CrossRef]
- Saeed, U.; Lang, A.E.; Masellis, M. Neuroimaging Advances in Parkinson’s Disease and Atypical Parkinsonian Syndromes. Front. Neurol. 2020, 11, 572976. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Doré, V.; Burnham, S.C.; Masters, C.L.; Rowe, C.C. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat. Rev. Neurol. 2018, 14, 225–236. [Google Scholar] [CrossRef]
- Murugan, A.A.; Zaleśny, R.; Kongsted, J.; Nordberg, A.; Ågren, H. Promising two-photon probes for in vivo detection of β amyloid deposits. Chem. Commun. 2014, 50, 11694–11697. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liang, Z.; Zhou, B.; Li, X.; Lui, C.; Ip, N.Y.; Qu, J.Y. In Vivo Near-Infrared Two-Photon Imaging of Amyloid Plaques in Deep Brain of Alzheimer’s Disease Mouse Model. ACS Chem. Neurosci. 2018, 9, 3128–3136. [Google Scholar] [CrossRef]
- Zhang, Y.; Luan, N.; Li, K.; Leng, J.; Hu, W. Sensing performance investigations on two-photon fluorescent probes for detecting β-amyloid in Alzheimer’s disease. Sensors 2020, 20, 1760. [Google Scholar] [CrossRef]
- Wu, Q.; Lin, Y.; Gu, J.; Sigurdsson, E.M. Dynamic assessment of tau immunotherapies in the brains of live animals by two-photon imaging. EBioMedicine 2018, 35, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Fang, H.; Wang, X.; Xu, J.; Zhang, C.; Xu, L.; Li, L. A two-photon fluorescent probe for visualizing endoplasmic reticulum peroxynitrite in Parkinson’s disease models. Sens. Actuators B Chem. 2021, 328, 129003. [Google Scholar] [CrossRef]
- Kang, H.; Shu, W.; Yu, J.; Wang, Y.; Zhang, X.; Zhang, R.; Jing, J.; Zhang, X. Endoplasmic Reticulum-Targeted Two-Photon Fluorescent Probe for the Detection of Nitroxyl in a Parkinson’s Disease Model. Anal. Chem. 2023, 95, 6295–6302. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, J.; Li, P.; Tang, B.; James, T.D. Two-photon small-molecule fluorescence-based agents for sensing, imaging, and therapy within biological systems. Chem. Soc. Rev. 2021, 50, 702–734. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Chen, H.C.; Lu, S.; Hai, J.; Guo, W.; Jing, Y.H.; Wang, B. Simultaneous Sensing of H2S and ATP with a Two-Photon Fluorescent Probe in Alzheimer’s Disease: Toward Understanding Why H2S Regulates Glutamate-Induced ATP Dysregulation. Anal. Chem. 2022, 94, 11573–11581. [Google Scholar] [CrossRef] [PubMed]
- Hanczyc, P.; Słota, P.; Radzewicz, C.; Fita, P. Two-photon excited lasing for detection of amyloids in brain tissue. J. Photochem. Photobiol. B Biol. 2022, 228, 112392. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ryan, P.; Rudrawar, S.; Quinn, R.J.; Zhang, H.; Mellick, G.D. Advances in the development of imaging probes and aggregation inhibitors for alpha-synuclein. Acta Pharmacol. Sin. 2020, 41, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Tarnowicz-Staniak, N.; Deiana, M.; Pokładek, Z.; Samoć, M.; Matczyszyn, K. Two-photon absorption and two-photon-induced isomerization of azobenzene compounds. RSC Adv. 2020, 10, 40489–40507. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y. Two-Photon Fluorescent Probes for Amyloid-β Plaques Imaging In Vivo. Molecules 2023, 28, 6184. [Google Scholar] [CrossRef] [PubMed]
- Murugan, N.A.; Zaleśny, R. Computational Investigations into Two-Photon Fibril Imaging Using the DANIR-2c Probe. J. Phys. Chem. B 2023, 127, 3119–3125. [Google Scholar] [CrossRef] [PubMed]
- Klingstedt, T.; Ghetti, B.; Holton, J.L.; Ling, H.; Nilsson, K.P.R.; Goedert, M. Luminescent conjugated oligothiophenes distinguish between α-synuclein assemblies of Parkinson’s disease and multiple system atrophy. Acta Neuropathol. Commun. 2019, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Kovalska, V.; Cherepanov, V.; Losytskyy, M.; Chernii, S.; Senenko, A.; Chernii, V.; Tretyakova, I.; Yarmoluk, S.; Volkov, S. Anti-fibrillogenic properties of phthalocyanines: Effect of the out-of-plane ligands. Bioorg. Med. Chem. 2014, 22, 6918–6923. [Google Scholar] [CrossRef]
- Lee, E.N.; Cho, H.J.; Lee, C.H.; Lee, D.; Chung, K.C.; Paik, S.R. Phthalocyanine Tetrasulfonates Affect the Amyloid Formation and Cytotoxicity of α-Synuclein. Biochemistry 2004, 43, 3704–3715. [Google Scholar] [CrossRef] [PubMed]
- Valiente-Gabioud, A.A.; Miotto, M.C.; Chesta, M.E.; Lombardo, V.; Binolfi, A.; Fernández, C.O. Phthalocyanines as Molecular Scaffolds to Block Disease-Associated Protein Aggregation. Acc. Chem. Res. 2016, 49, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Lo, P.C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Delgado, M.C.; Hernández, V.; Casado, J.; López Navarrete, J.T.; Raimundo, J.M.; Blanchard, P.; Roncali, J. Vibrational study of push-pull chromophores for second-order non-linear optics derived from rigidified thiophene π-conjugating spacers. J. Mol. Struct. 2003, 651–653, 151–158. [Google Scholar] [CrossRef]
- Billes, F.; Mohammed-Ziegler, I.; Mikosch, H.; Tyihák, E. Vibrational spectroscopy of resveratrol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 68, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Basova, T.; Berezin, A.; Nadolinny, V.; Peisert, H.; Chassé, T.; Banimuslem, H.; Hassan, A. Formation of ordered films of axially bridged aluminum phthalocyanine [(tBu)4PcAl]2O via magnetic field-induced reaction. J. Chem. Phys. 2013, 139, 204710. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.; Dozova, N.; McCaffrey, J.G.; FitzGerald, S.; Shafizadeh, N.; Crépin, C. Infra-red and Raman spectroscopy of free-base and zinc phthalocyanines isolated in matrices. Phys. Chem. Chem. Phys. 2010, 12, 10406–10422. [Google Scholar] [CrossRef]
- McNutt, A.T.; Francoeur, P.; Aggarwal, R.; Masuda, T.; Meli, R.; Ragoza, M.; Sunseri, J.; Koes, D.R. GNINA 1.0: Molecular docking with deep learning. J. Cheminform. 2021, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Basciu, A.; Callea, L.; Motta, S.; Bonvin, A.M.J.J.; Bonati, L.; Vargiu, A.V. Chapter Two—No dance, no partner! A tale of receptor flexibility in docking and virtual screening. Annu. Rep. Med. Chem. 2022, 59, 43–97. [Google Scholar]
- Theillet, F.X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H.M.; Stuiver, M.; Verzini, S.; Lorenz, D.; Van Rossum, M.; Goldfarb, D.; et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 2016, 530, 45–50. [Google Scholar] [CrossRef]
- Chen, J.; Zaer, S.; Drori, P.; Zamel, J.; Joron, K.; Kalisman, N.; Lerner, E.; Dokholyan, N.V. The structural heterogeneity of α-synuclein is governed by several distinct subpopulations with interconversion times slower than milliseconds. Structure 2021, 29, 1048–1064.e6. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. Sartorius products. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.S.C.; de Souza, F.A.L.; Ceolin, G.A.; Jorge, F.E.; de Berrêdo, R.C.; Campos, C.T. Augmented Gaussian basis sets for the elements K, Sc-Kr, Rb, and Y-Xe: Application in HF, MP2, and DFT calculations of molecular electric properties. Comput. Theor. Chem. 2013, 1013, 62–69. [Google Scholar] [CrossRef]
- Mir, Y.; Van Lier, J.E.; Allard, J.F.; Morris, D.; Houde, D. Two-photon absorption cross section of excited phthalocyanines by a femtosecond Ti-sapphire laser. Photochem. Photobiol. Sci. 2009, 8, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Maciejewski, J.; Sobczuk, A.; Claveau, A.; Nicolai, A.; Petraglia, R.; Cervini, L.; Baudat, E.; Miéville, P.; Fazzi, D.; Corminboeuf, C.; et al. Photochromic Torsional Switch (PTS): A light-driven actuator for the dynamic tuning of π-conjugation extension. Chem. Sci. 2016, 8, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Shen, T.L.; Weng, W.H.; Huang, Y.C.; Huang, C.I.; Su, W.F.; Rwei, S.P.; Ho, K.C.; Wang, L. Molecular design of interfacial modifiers for polymer-inorganic hybrid solar cells. Adv. Energy Mater. 2012, 2, 245–252. [Google Scholar] [CrossRef]
- Khanasa, T.; Jantasing, N.; Morada, S.; Leesakul, N.; Tarsang, R.; Namuangruk, S.; Kaewin, T.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promarak, V. Synthesis and characterization of 2D-D-π-A-type organic dyes bearing bis(3,6-di-tert-butylcarbazol-9-ylphenyl)aniline as donor moiety for dye-sensitized solar cells. Eur. J. Org. Chem. 2013, 2013, 2608–2620. [Google Scholar] [CrossRef]
- Bader, M.M.; Pham, P.T.T.; Elandaloussi, E.H. Dicyanovinyl-substituted oligothiophenes. Cryst. Growth Des. 2010, 10, 5027–5030. [Google Scholar] [CrossRef]
- Alzeer, J.; Roth, P.J.C.; Luedtke, N.W. An efficient two-step synthesis of metal-free phthalocyanines using a Zn(ii) template. Chem. Commun. 2009, 15, 1970–1971. [Google Scholar] [CrossRef]
- Tirado-Rives, J.; Jorgensen, W.L. Performance of B3LYP density functional methods for a large set of organic molecules. J. Chem. Theory Comput. 2008, 4, 297–306. [Google Scholar] [CrossRef]
- Da Chai, J.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Cappai, A.; Melis, C.; Stagi, L.; Ricci, P.C.; Mocci, F.; Carbonaro, C.M. Insight into the Molecular Model in Carbon Dots through Experimental and Theoretical Analysis of Citrazinic Acid in Aqueous Solution. J. Phys. Chem. C 2021, 125, 4836–4845. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to Isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Mocci, F.; Olla, C.; Cappai, A.; Corpino, R.; Ricci, P.C.; Chiriu, D.; Salis, M.; Carbonaro, C.M. Formation of citrazinic acid ions and their contribution to optical and magnetic features of carbon nanodots: A combined experimental and computational approach. Materials 2021, 14, 770. [Google Scholar] [CrossRef]
- Vidović, M.; Rikalovic, M.G. Alpha-Synuclein Aggregation Pathway in Parkinson’s Disease: Current Status and Novel Therapeutic Approaches. Cells 2022, 11, 1732. [Google Scholar] [CrossRef]
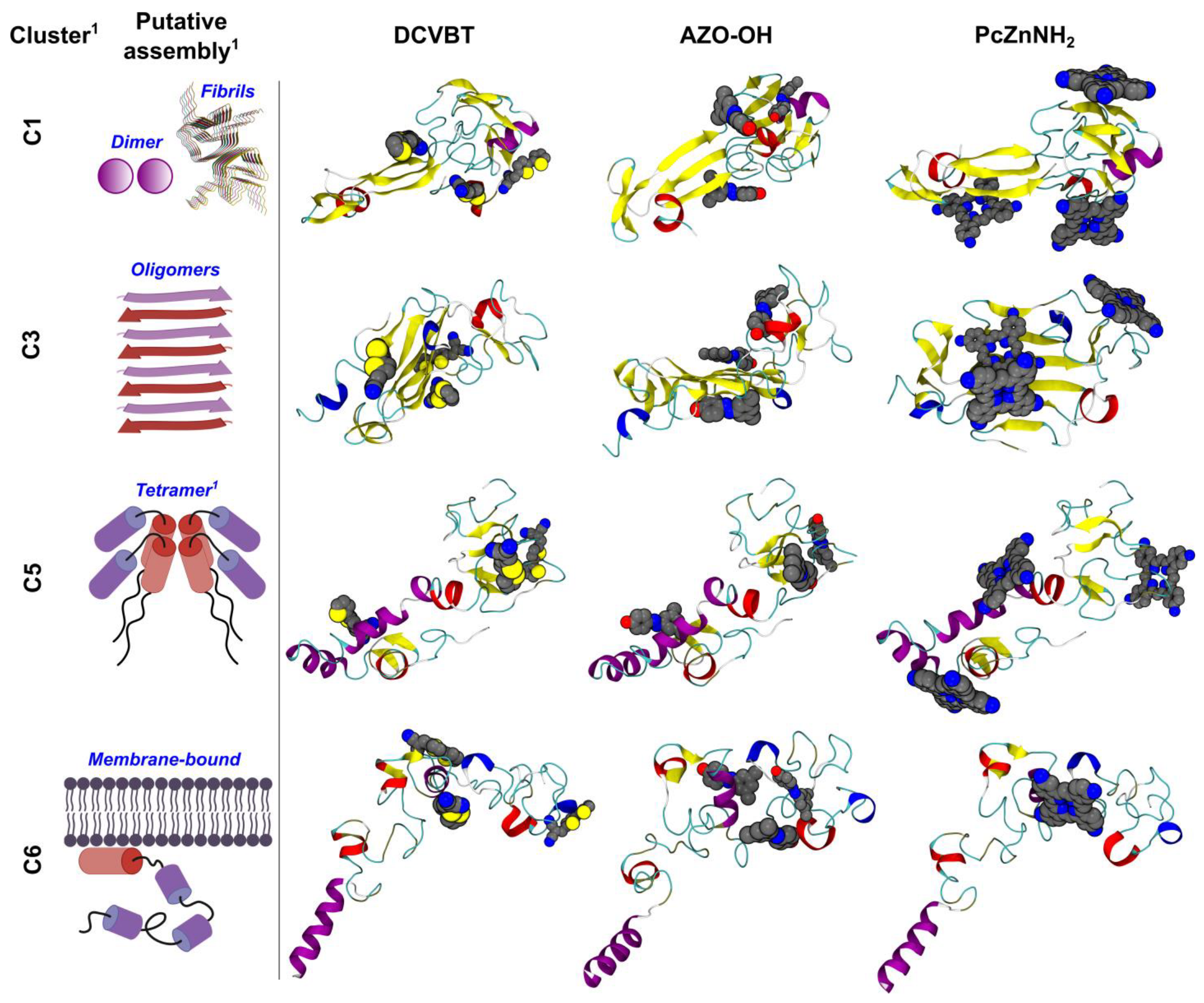
| Cluster * | DCVBT | AZO-OH | PcZnNH2 |
|---|---|---|---|
| C1 | −4.6 | −5.7 | −9.3 |
| C3 | −4.7 | −6.6 | −9.4 |
| C5 | −4.9 | −5.3 | −9.1 |
| C6 | −4.7 | −5.9 | −9.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcu, S.; Corpino, R.; Carbonaro, C.M.; Ricci, P.C.; Vargiu, A.V.; Sanna, A.L.; Sforazzini, G.; Chiriu, D. Promising Molecular Architectures for Two-Photon Probes in the Diagnosis of α-Synuclein Aggregates. Molecules 2024, 29, 2817. https://doi.org/10.3390/molecules29122817
Porcu S, Corpino R, Carbonaro CM, Ricci PC, Vargiu AV, Sanna AL, Sforazzini G, Chiriu D. Promising Molecular Architectures for Two-Photon Probes in the Diagnosis of α-Synuclein Aggregates. Molecules. 2024; 29(12):2817. https://doi.org/10.3390/molecules29122817
Chicago/Turabian StylePorcu, Stefania, Riccardo Corpino, Carlo Maria Carbonaro, Pier Carlo Ricci, Attilio Vittorio Vargiu, Anna Laura Sanna, Giuseppe Sforazzini, and Daniele Chiriu. 2024. "Promising Molecular Architectures for Two-Photon Probes in the Diagnosis of α-Synuclein Aggregates" Molecules 29, no. 12: 2817. https://doi.org/10.3390/molecules29122817
APA StylePorcu, S., Corpino, R., Carbonaro, C. M., Ricci, P. C., Vargiu, A. V., Sanna, A. L., Sforazzini, G., & Chiriu, D. (2024). Promising Molecular Architectures for Two-Photon Probes in the Diagnosis of α-Synuclein Aggregates. Molecules, 29(12), 2817. https://doi.org/10.3390/molecules29122817












