Research Progress on the Extraction and Purification of Anthocyanins and Their Interactions with Proteins
Abstract
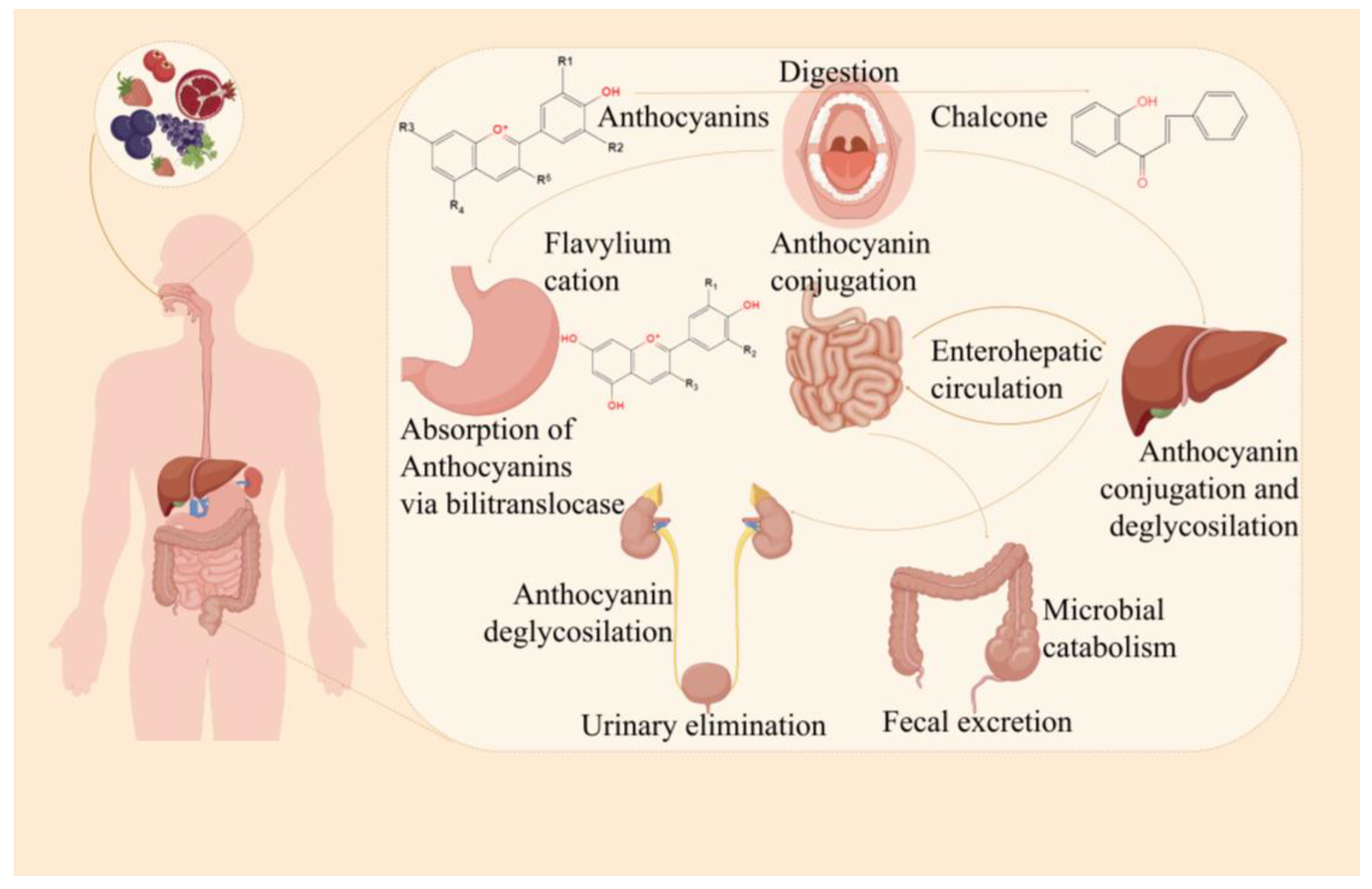
1. Introduction
2. Structure and Properties of Anthocyanins
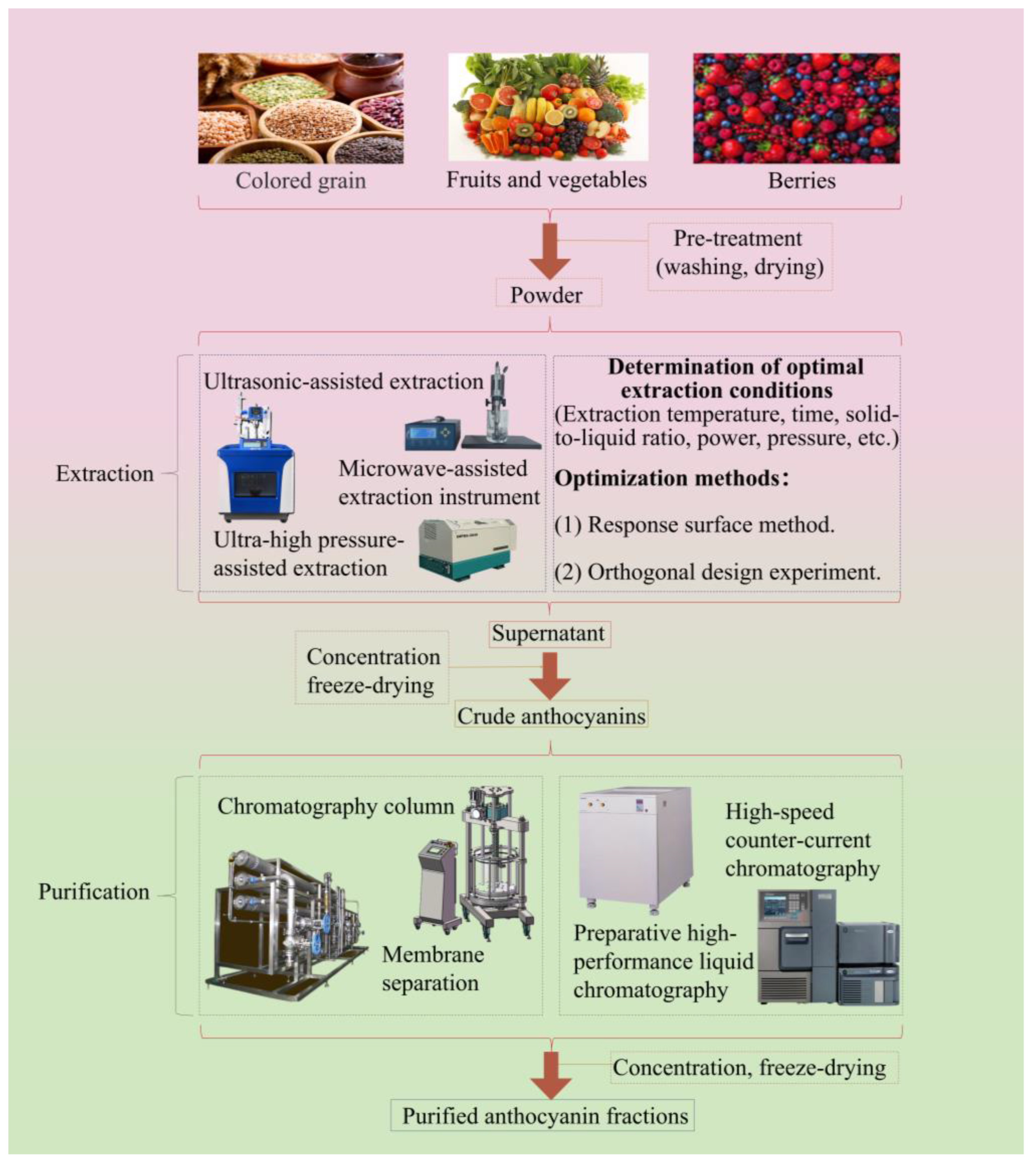
3. Anthocyanin Extraction Methods
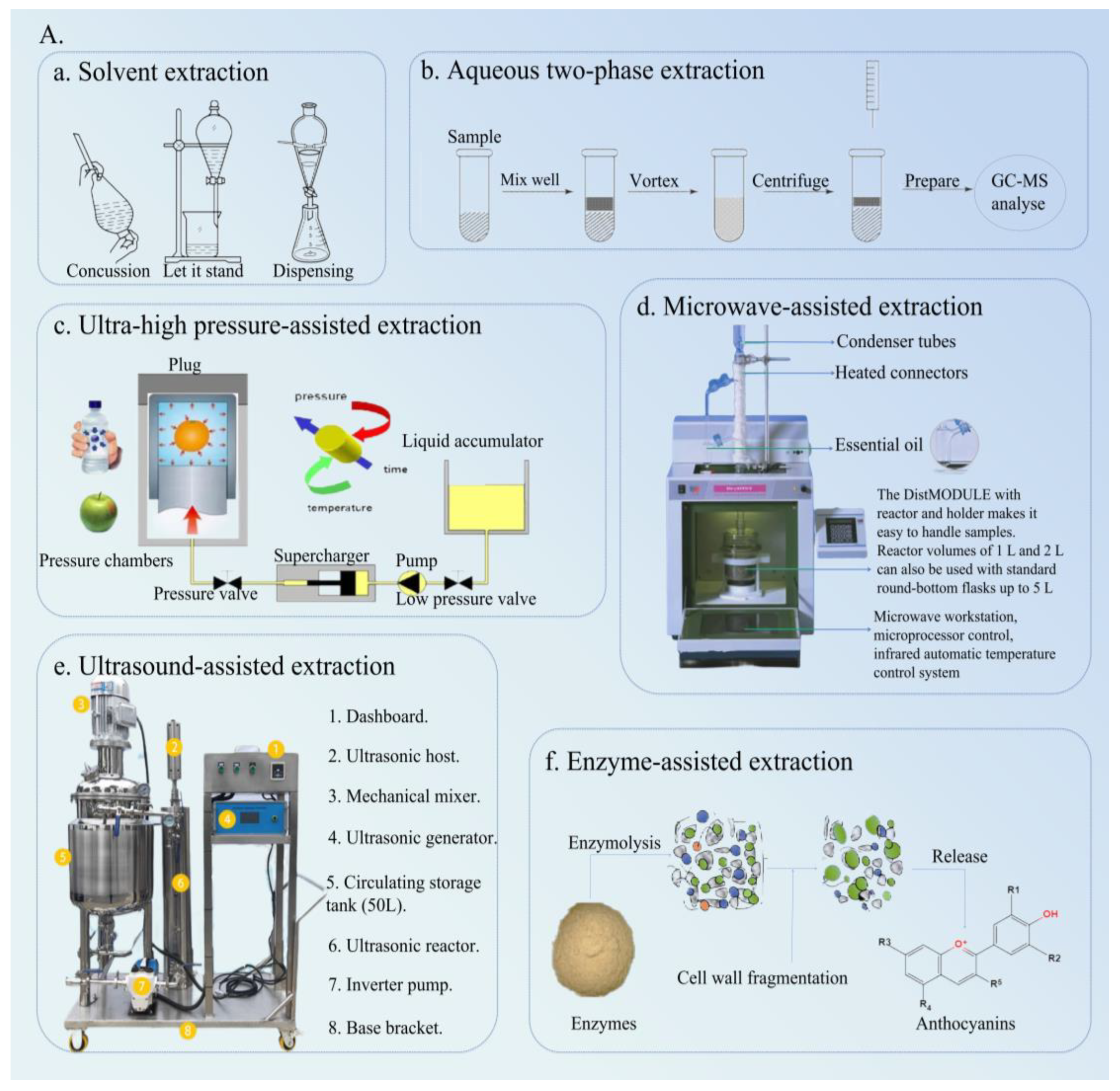
3.1. SEM
3.2. UAE
3.3. MAE
3.4. UHPAE
3.5. ATPE
3.6. EAE
3.7. Other Extraction Methods
| Source | Extraction Solvent | Extraction Method | Extraction Process | Yield | References |
|---|---|---|---|---|---|
| Lycium ruthenicum Murr | 60% ethanol | SEM | Extraction temperature 48 °C, extraction time 4 min, static extraction pressure 8 MPa, cycle two times | 19.89 mg/g | [17] |
| Purple potato (Ipomoea batatas L.) | 77% ethanol | UHPAE | Extraction temperature 67 °C, extraction time 25 min, solid-to-liquid ratio 1:58 g/mL | 200.13 mg/100 g | [18] |
| Mulberry (Morus alba L.) | ChCl-HL ratio 2:57 (v/v) | DESs | Extraction temperature 24.31 °C, extraction time 54.10 min | 76.60 mL/g | [19] |
| Aronia melanocarpa | Choline chloride-glycerol | UMAE | Extraction temperature 58–64 °C, extraction time 60–105 min, solid-to-liquid ratio 1:30–1:40 g/mL | 448.87 mg/g | [20] |
| Andean blackberry (Rubus glaucusBenth.) | 80% ethanol and 0.1% acidified hydrochloric acid | UAE | Extraction temperature 40 °C, ultrasound power 110 W, extraction time 15 min, solid-to-solvent ratio 1:10 g/mL | 162.00 mg/L | [24] |
| Rubia sylvatica Nakai | 30% ethanol | UAE | Extraction temperature 55 °C, pH 3.0, ultrasound power 400 W, liquid-to-solid ratio 20 mL/g, extraction time 20 min | 22.35 ± 0.89 mg /g | [25] |
| Blueberry wine pomace (Vaccinium ashei) | 70% ethanol, and 0.01% hydrochloric acid | UAE | Extraction temperature 61.03 °C, liquid-to-solid ratio 21.70 mL/g, extraction time 23.67 min, ultrasound power 400 w | 4.27 mg/g | [26] |
| Mulberry wine residues (Morus alba L.) | 80% ethanol | UAE | Extraction temperature 52 °C, ultrasound power 315 W, enzyme dosage 0.22%, extraction time 94 min | 5.98 mg/g | [27] |
| Purple sweet potato (Ipomea batatas L.) | 83% polyethylene glycol (200) | UAE | Extraction temperature 64 °C, extraction time 80 min | 83.78 mg/100 g | [28] |
| Red cabbage (Brassica oleracea L. var. capitata. f. rubra) | 50% ethanol | MAE | Extraction time 5 min, solid-to-liquid ratio 1:20 g/mL, microwave power 200 W | 241.20 mg/g | [30] |
| Adzuki bean (Vigna angularis) seed coat | 30% ethanol | MAE | Microwave power 463 W, solid-to-liquid ratio 1:30 g/mL, extraction time 42 min | 32.08 mg/100 g | [31] |
| Blackberry (Rubus fruticosus) | 52% ethanol | MAE | Microwave power 469 W, liquid-to-solid ratio 25:1 g/mL, microwave time 4 min | 2.18 ± 0.06 mg/g | [32] |
| Cranberry (Vaccinium macrocarpon Ait.) | 52% ethanol | MAE | Extraction temperature 50 °C, extraction time 8 s, solid-to-liquid ratio 1:28 g/mL | 3.06 ± 0.05 mg/g | [33] |
| Purple sweet potato (Ipomea batatas L.) | 30% ethanol | MAE | Solid-to-liquid ratio 1:3 g/mL, microwave power 320 W, extraction time 500 s | 31.16 mg/100 g | [34] |
| Purple corn (Zea mays L.) seeds | Anhydrous ethanol | UHPAE | Extraction pressure 270 MPa, ultra-high pressure time 1.9 min, ultrasound time 8.5 min | 49.68 mg/100 g | [35] |
| Mulberry (Morus alba L.) | 75% ethanol | UHPAE | Extraction pressure 430 MPa, liquid-to-solid ratio 12:1 mL/g | 1.97 ± 0.02 mg/g | [36] |
| Vacuum freeze-dried strawberry (Fragaria x ananassa Duch.) slices | Anhydrous ethanol | UHPAE | Extraction pressure 100 MPa, ultra-high pressure time 5 min, ultrasound time 25 min | 304.39 mg/kg | [38] |
| Blueberry (Vaccinium corymbosum L.) | 57% ethanol | UHPAE | Extraction pressure 187 MPa, extraction time 6 min, solid-to-liquid ratio 1:29 g/mL | 5.16 ± 0.12 mg/g | [39] |
| Rose (Rosa hybrida) | 13.2% citric acid | UHPAE | Extraction pressure 400 MPa, extraction time 6 min, solid-to-liquid ratio 1:25 g/mL | 1089.42 mg/100 g | [40] |
| Roselle (Hibiscus sabdariffa L.) | 40% ethanol and 0.24 g/mL(NH4)2SO4 | ATPE | Liquid-to-solid ratio 40:1 mL/g, extraction temperature 40.5 °C, extraction time 24.5 min | 4.12 mg/g | [42] |
| Black soybean hull (Glycinemax (L.) Merr.) | 30% ethanol and 22% (NH4)2SO4 | ATPE | Solid-to-liquid ratio 1:56 g/mL, pH 3.0 | 2.81 mg/g | [43] |
| Aqueous grape (Vitis vinifera L.) pomace | 40% ethanol and 26% (NH4)2SO4 | ATPE | Solid-to-liquid ratio 1:38 g/mL, pH 3.0 | 3.05 ± 0.07 mg/g | [44] |
| Purple sweet potato (Ipomea batatas L.) | 25% ethanol and 22% (NH4)2SO4 | ATPE | Liquid-to-solid ratio 45:1 mL/g, pH 3.3 | 311.00 mg/100 g | [45] |
| Blueberry (Vaccinium corymbosum L.) | 24% ethanol and 18% (NH4)2SO4 | ATPE | Ultrasound power 300 W, solid-to-liquid ratio 1:30 g/mL, extraction time 60 min | 2.14 ± 0.05 mg/g | [46] |
| Lycium ruthenicum Murr | 80% ethanol | EAE | Extraction temperature 49 °C, extraction time 1 h, liquid-to-solid ratio 21:1 mL/g | 24.68 ± 0.03 mg/g | [49] |
| Grape (Vitis vinifera L.) skins | 60% ethanol | EAE | Extraction temperature 50 °C, ultrasound power 400 W, pectinase dosage 0.16%, extraction time 28 min | 3.01 ± 0.04 mg/g | [50] |
| Lycium ruthenicum Murr | Anhydrous ethanol | EAE | Extraction temperature 38 °C, extraction time 37 min | 19.51 ± 0.21 mg/g | [51] |
| Acanthopanax senticosus dried fruit | Methanol | EAE | Liquid-to-solid ratio 18:1 mL/g, pectinase 4.2%, digestion temperature 55 °C, digestion time 3.0 h | 6.00 mg/g | [52] |
| Vaccinium bracteatum Thunb. Fruit | 50% ethanol | EAE | Cellulase–pectinase ratio 2:1, pH 4.0, solid-to-liquid ratio 1:30 g/mL, digestion temperature 50 °C, digestion time 180 min | 136.08 mg/100 g | [53] |
| Purple-fleshed potato (Ipomea batatas L.) | 96% ethanol | PEFAE | Electric field strength 3.4 kV/cm, processing time 105 s, extraction temperature 40 °C, extraction time 480 min | 65.80 mg/100 g | [57] |
| Blueberry (Vaccinium corymbosum L.) | 5% ethanol | Subcritical liquid and supercritical CO2 extraction | Extraction temperature 40 °C, extraction pressure 20 MPa, solvent flow rate 10 mL/min | 19.60 mg/g | [59] |
| Purple sweet potato (Ipomea batatas L.) | Anhydrous ethanol | Microbial fermentation | Initial sugar content 150 g/L, fermentation time 4 d, extraction temperature 29.8 °C, pH 4.10, amount culture added 0.16% | 8.80 mg/g | [61] |
| Purple potato (Ipomea batatas L.) | 65% ethanol | UMAE | Ultrasonication time 24 min, liquid-to-solid ratio 21:1 mL/g, ultrasound power 210 W, microwave time 1 min, microwave power 500 W | 1.11 mg/g | [62] |
| Rhodomyrtus Tomentosa | 50% ethanol | Cellulase–microwave combined method | Cellulase addition 2.8%, liquid-to-solid ratio 21:1 mL/g, microwave power 494 W, microwave time 6.5 min | 56.98 mg/100 g | [63] |
| Blueberry (Vaccinium corymbosum L.) pomace | Choline chloride– 1, 4-butanediol (molar ratio of 1:3) | Deep eutectic solvent combined with ultrasound technology | Moisture content 29%, extraction temperature 63 °C, liquid-to-solid ratio 36:1 mL/g | 11.40 ± 0.14 mg/g | [64] |
4. Purification of Anthocyanins
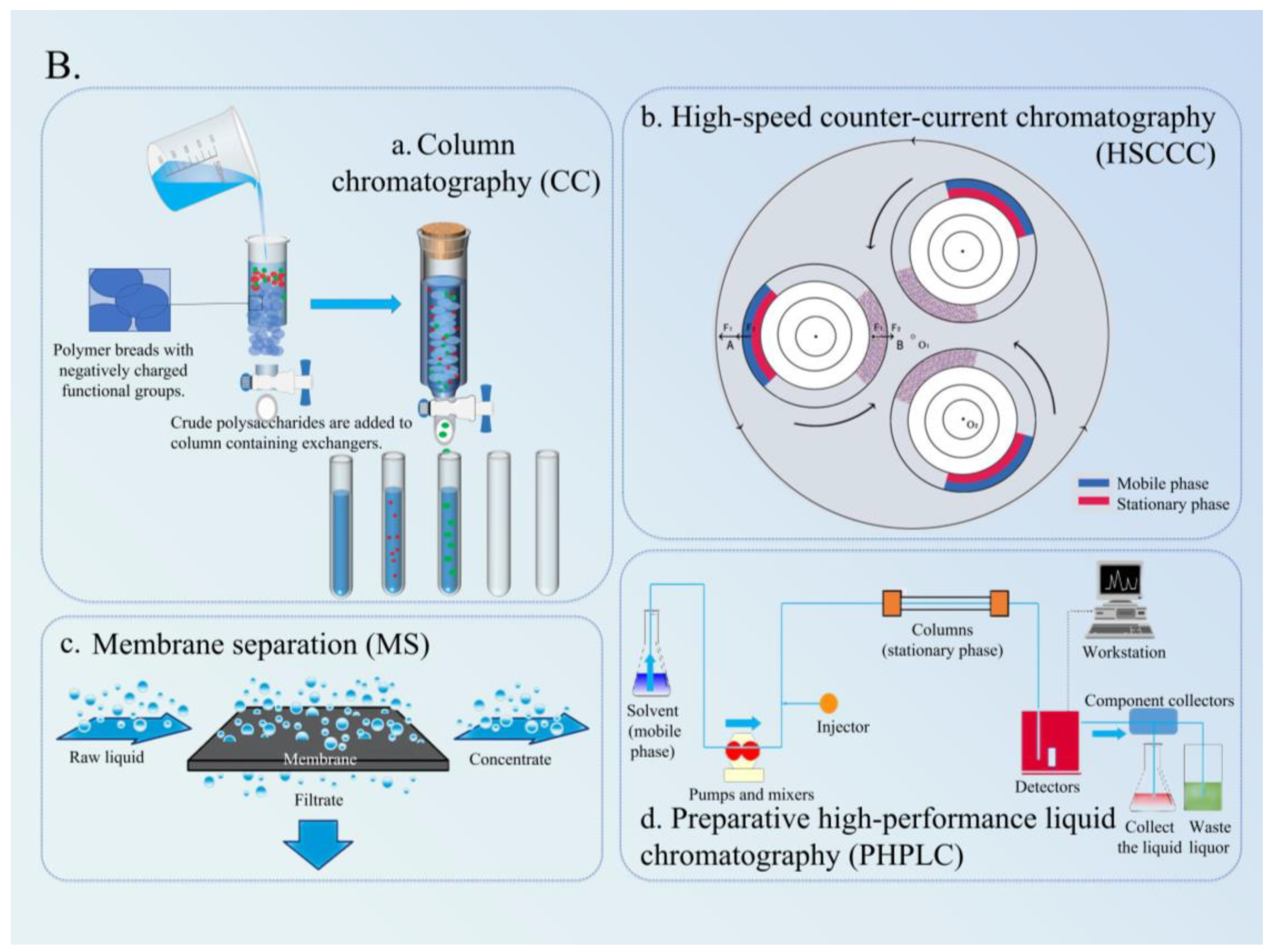
4.1. Column Chromatography
4.2. Membrane Separation
4.3. HSCCC
4.4. PHPLC
4.5. Combined Purification Method
5. Effects of Proteins on the Stability of Anthocyanins
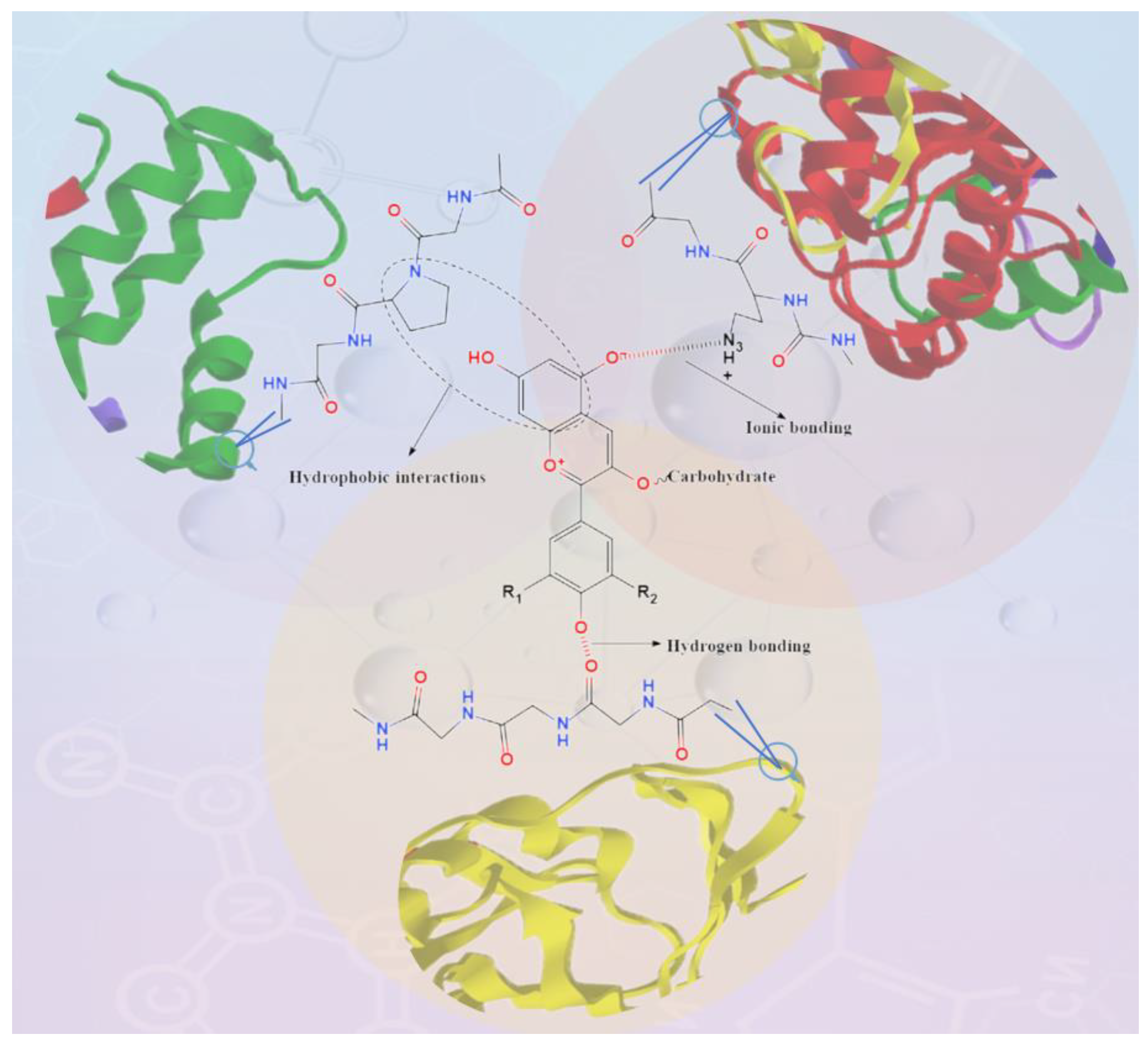
5.1. Protein Interacts with Anthocyanins
5.1.1. Natural Proteins
5.1.2. Modified Proteins
5.2. Effects of Proteins on the Bioavailability of Anthocyanins
| Anthocyanin | Proteins | Interaction Forces | Interaction Effect | Binding Energy | References |
|---|---|---|---|---|---|
| Mv3glc | Vitis vinifera grape seed 7S globulin | Hydrogen, alkyl, and π-alkyl | The color stability of anthocyanins ↑ | ________ | [98] |
| C3G | CM proteins (α-LA, β-LG, αs1-CA, and β-CA) | Hydrogen bonding | The stability and bioavailability of anthocyanins ↑ | ________ | [99] |
| Purple potato (Ipomea batatas L.) flour | CA and WP | Hydrogen bonding and van der Waals forces | The contents of α-helix and β-turn ↓; The contents of β-sheet and irregular coil ↑ | CA-Pt3G (−10.29 kJ·mol−1); CA-Pn3G (−10.44 kJ·mol−1), WP-Pt3G (−10.23 kJ·mol−1), and WP-Pn3G (−9.21 kJ·mol−1) | [100] |
| M3G | WPI | Hydrogen bonding and hydrophobic forces | The contents of α-helix ↓; the contents of β-sheets ↑ | −141.30 kcal/mol | [101] |
| M3G | BSA | Electrostatic interactions and hydrogen bonding | The contents of α-helix ↑; the contents of β-sheet, turn, and random coil ↓ | ________ | [102] |
| C3G | β-LG | Hydrophobic interactions | The antioxidant capacities of C3G ↑ | ________ | [103] |
| C3G | SPI | Hydrophobic interactions | The contents of α-helix and no regular curl ↓; the contents of β-folding and cornering ↑ | ________ | [106] |
| Grape (Vitis vinifera L.) skin | WP | Hydrogen bonding and hydrophobic interactions | The thermal, oxidation and, photo stability of anthocyanins ↑ | ________ | [107] |
| C3G | SPP | Hydrophobic interactions | The contents of α-helix ↓; the contents of β-sheet and β-turn ↑ | ________ | [108] |
| M3G | WPI | Hydrogen bonding | The stability of anthocyanins ↑ | ________ | [109] |
| C3G | OVA | Hydrogen bonding and van der Waals forces | The contents of α-helix ↑; the contents of β-turn and random coil ↓ | ________ | [121] |
| Black rice (Oryza sativa L.) | Rice (Oryza sativa L.) protein | Hydrophobic and hydrogen bonding | The contents of β-sheet ↑ | ________ | [122] |
| Black rice (Oryza sativa L.) | WPI | Hydrophobic interactions | The emulsifying properties of the WPI-AN ↑ | ________ | [123] |
| Black rice (Oryza sativa L.) | SPI | Hydrogen bonding | The contents of α-helix ↑; the contents of β-sheet ↓ | ________ | [124] |
| C3G | SPI | Hydrophobic interactions | The contents of α-helix and random coil ↓; the contents of β-sheet ↑ | ________ | [125] |
| Rose (Rosa hybrida) | WPI | Hydrogen bonding and van der Waals forces | ________ | [126] | |
| C3G | SP | Hydrogen bonding and hydrophobic interactions | The surface hydrophobicity and thermostability of soy protein ↓ | −6.8072 Kcal/mol | [127] |
| C3G | β-Lg and β-CA | Hydrophobic interactions | The contents of α-helix and β-sheet ↓ | ________ | [128] |
| C3G | α-CA | Hydrogen bonding and van der Waals forces | α-helix ↑; β-folding and cornering ↓; no regular curl ↑ | ________ | [129] |
| β-CA | Electrostatic gravity | α-helix ↑; β-folding and cornering ↑; no regular curl ↓ | |||
| WP | ________ | No significant changes | |||
| β-LG | α-helix ↓; β-folding and cornering ↑ |
6. Effects of Protein–Anthocyanin Interactions on Protein Properties
6.1. Effects of the Interaction between Proteins and Anthocyanins on the Properties of Proteins
6.1.1. The Effect of the Interaction between Anthocyanins and Proteins on the Solubility of Proteins
6.1.2. The Effects of the Interaction between Anthocyanins and Proteins on the Foamability and Emulsification of Proteins
6.1.3. The Effects of the Interaction between Anthocyanins and Proteins on Digestive Characteristics
6.2. The Effect of the Interaction between Anthocyanins and Proteins on Protein Structure
7. Application of the Interaction between Anthocyanins and Proteins
7.1. Application of Anthocyanin–Protein Complexes in Liquid Systems
7.2. Application of Anthocyanin–Protein Complexes in Solid-State Systems
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez-Suarez, J.M.; Cuadrado, C.; Redondo, I.B.; Giampieri, F.; González-Paramas, A.M.; Santos-Buelga, C. Novel approaches in anthocyanin research–plant fortification and bioavailability issues. Trends Food Sci. Technol. 2021, 117, 92–105. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, L. Anthocyanin-dietary proteins interaction and its current applications in food industry. Food Rev. Int. 2023, 39, 3301–3313. [Google Scholar] [CrossRef]
- Zhu, B.; Zhong, Y.; Wang, D.; Deng, Y. Active and intelligent biodegradable packaging based on anthocyanins for preserving and monitoring protein-rich foods. Foods 2023, 12, 4491. [Google Scholar] [CrossRef]
- Jiao, X.; Li, B.; Zhang, Q.; Gao, N.; Zhang, X.; Meng, X. Effect of in vitro–simulated gastrointestinal digestion on the stability and antioxidant activity of blueberry polyphenols and their cellular antioxidant activity towards HepG2 cells. Int. J. Food Sci. Technol. 2018, 53, 61–71. [Google Scholar] [CrossRef]
- Pertuzatti, P.B.; Barcia, M.T.; Rebello, L.P.; Gómez-Alonso, S.; Duarte, R.T.; Duarte, M.C.; Godoy, H.T.; Hermosin-Gutierrez, I. Antimicrobial activity and differentiation of anthocyanin profiles of rabbiteye and highbush blueberries using HPLC–DAD–ESI–MSn and multivariate analysis. J. Funct. Foods 2016, 26, 506–516. [Google Scholar] [CrossRef]
- Eroglu Ozkan, E.; Seyhan, M.F.; Kurt Sirin, O.; Yilmaz-Ozden, T.; Ersoy, E.; Hatipoglu Cakmar, S.D.; Goren, A.C.; Yilmaz Aydogan, H.; Ozturk, O. Antiproliferative effects of turkish pomegranate (Punica granatum L.) extracts on MCF-7 human breast cancer cell lines with focus on antioxidant potential and bioactive compounds analyzed by LC-MS/MS. J. Food Biochem. 2021, 45, e13904. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Wang, T.; Cai, Z.; Cao, H.; Zhang, H.; Cao, Y.; Chen, B.; Yang, D. Statistics on the bioactive anthocyanin/proanthocyanin products in China online sales. Food Sci. Nutr. 2021, 9, 5428–5434. [Google Scholar] [CrossRef]
- Park, J.S.; Han, J.M.; Surendhiran, D.; Chun, B.S. Physicochemical and biofunctional properties of Sargassum thunbergii extracts obtained from subcritical water extraction and conventional solvent extraction. J. Supercrit. Fluids 2022, 182, 105535. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, X.; Huang, G. Ultrasound-assisted extraction and analysis of maidenhairtree polysaccharides. Ultrason. Sonochem. 2023, 95, 106395. [Google Scholar] [CrossRef]
- Cecilia, J.S.; Jesus, L.S.; Margret, B.; Mariana, N.V.; Miriam, R.W.; Eva, S.; Peter, W.; Antonio, S.C.; Alberto, F.G. Application and comparison of high-speed countercurrent chromatography and high-performance liquid chromatography in semi-preparative separation of decarboxymethyl oleuropein aglycone (3,4-DHPEA-EDA), a bioactive secoiridoid from extra-virgin olive oil. Eur. J. Lipid Sci. Technol. 2017, 119, 1500532. [Google Scholar]
- Zhang, L.; Yao, L.; Zhao, F.; Yu, A.; Zhou, Y.; Wen, Q.; Wang, J.; Zheng, T.; Chen, P. Protein and peptide-based nanotechnology for enhancing stability, bioactivity, and delivery of anthocyanins. Adv. Healthc. Mater. 2023, 12, e2300473. [Google Scholar] [CrossRef]
- GB2760-2014; National Food Safety Standard Standard for Uses of Food Additives. Ministry of Security of the People’s Republic of China: Beijing, China, 2014.
- Gerard, V.; Ay, E.; Morlet-Savary, F.; Graff, B.; Galopin, C.; Ogren, T.; Mutilangi, W.; Lalevee, J. Thermal and photochemical stability of anthocyanins from black carrot, grape juice, and purple sweet potato in model beverages in the presence of ascorbic acid. J. Agric. Food Chem. 2019, 67, 5647–5660. [Google Scholar] [CrossRef]
- Zeng, Y.Y.; Guo, D.; Li, X.S.; Cai, D.B.; Sun, J.X.; Bai, W.B. Progress in research on the structure and properties of pyranoanthocyanins. Food Sci. 2022, 43, 199–209. [Google Scholar]
- Wu, H.; Oliveira, G.; Lila, M.A. Protein-binding approaches for improving bioaccessibility and bioavailability of anthocyanins. Compr. Rev. Food Sci. Food Saf. 2023, 22, 333–354. [Google Scholar] [CrossRef]
- Robert, P.; Fredes, C. The encapsulation of anthocyanins from berry–type fruits. Trends in foods. Molecules 2015, 20, 5875–5888. [Google Scholar] [CrossRef]
- Wang, Q.X.; Li, J.Y.; Cheng, Y.; Bai, J.; Luo, Y.J.; Gao, W.J. High–efficiency solvent extraction of anthocyanins from Lycium ruthenicum Murr and its color stability. Nat. Prod. Res. Dev. 2020, 32, 103–109. [Google Scholar]
- Zhang, X.S.; Qi, M.N.; An, S.M.; Jin, H. Optimization extraction of total anthocyanidins from purple potatoes by pressure solvent method. Sci. Technol. Food Ind. 2015, 36, 278–282. [Google Scholar]
- Bi, Y.; Chi, X.; Zhang, R.; Lu, Y.; Wang, Z.; Dong, Q.; Jiang, L. Highly efficient extraction of mulberry anthocyanins in deep eutectic solvents: Insights of degradation kinetics and stability evaluation. Innov. Food Sci. Emerg. Technol. 2020, 66, 102512. [Google Scholar] [CrossRef]
- Lin, S.; Meng, X.; Tan, C.; Tong, Y.; Wan, M.; Wang, M.; Ma, Y. Composition and antioxidant activity of anthocyanins from Aronia melanocarpa extracted using an ultrasonic–microwave–assisted natural deep eutectic solvent extraction method. Ultrason. Sonochem. 2022, 89, 106102. [Google Scholar] [CrossRef]
- Xie, F.X.; Qiu, Z.M.; Tu, S.H.; Xie, X.L. Comparison between ethanol and ultrasonic extraction method used in extraction of gardenia yellow. J. Nanchang Univ. 2005, 83, 278–281. [Google Scholar]
- Feng, F.; Ge, Y.J.; Dai, R.; Xie, H. Research progress of ultrasonic assisted extraction technology. Food Ind. 2022, 43, 239–243. [Google Scholar]
- Fan, Y.G.; Cao, L.H. Research progress on extraction methods of anthocyanins. Anhui Chem. Ind. 2022, 48, 6–10. [Google Scholar]
- Gravier-Rodriguez, G.; Jurado-Basante, S.; Arias-Contreras, A.N.; Gamarra Castillo, O.J.; Porras, A.; Sanchez-Camargo, A.D. Ultrasound-assisted extraction of anthocyanins from Andean blackberry and their use as an indicator in sustainable smart biofilms developed with cocoa bean shells as natural fiber-filled PLA composite materials. Food Packag. Shelf Life 2023, 40, 101165. [Google Scholar] [CrossRef]
- Chen, X.Q.; Li, Z.H.; Wang, Z.J.; Liu, L.L.; Sun, T.T.; Ma, J.Z.; Zhang, Y. Ultrasound-assisted extraction of total anthocyanins from Rubia sylvatica Nakai fruit and radical scavenging activity of the extract. Ind. Crops Prod. 2020, 150, 112420. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Yue, P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, G.; Khan, M.A.; Yan, Z.; Beta, T. Ultrasonic–assisted enzymatic extraction and identification of anthocyanin components from mulberry wine residues. Food Chem. 2020, 323, 126714. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Q.; Belwal, T.; Li, L.; Aalim, H.; Wu, Q.; Luo, Z. Ultrasonic impact on viscosity and extraction efficiency of polyethylene glycol: A greener approach for anthocyanins recovery from purple sweet potato. Food Chem. 2019, 283, 59–67. [Google Scholar] [CrossRef]
- Zhang, X.X.; Chai, Z.; Feng, J.; Cui, L.; Li, C.Y.; Li, Y.; Huang, W.Y. Extraction and biological activity of Arctium lappa L. polysaccharides. Food Ferment. Ind. 2021, 47, 280–288. [Google Scholar]
- Yigit, U.; Turabi Yolacaner, E.; Hamzalioglu, A.; Gokmen, V. Optimization of microwave-assisted extraction of anthocyanins in red cabbage by response surface methodology. J. Food Process. Preserv. 2022, 46, e16120. [Google Scholar] [CrossRef]
- Jin, L.M.; Bai, J.; Sui, S.Y.; Hu, Y.G.; Niu, G.C. Optimization of microwave–assisted extraction of anthocyanins from adzuki bean seed coat by response surface methodology and its stability. Sci. Technol. Food Ind. 2021, 42, 187–194. [Google Scholar]
- Wen, Y.; Chen, H.; Zhou, X.; Deng, Q.; Zhao, Y.; Zhao, C. Optimization of the microwave–assisted extraction and antioxidant activities of anthocyanins from blackberry using a response surface methodology. R. Soc. Chem. Adv. 2015, 5, 19686–19695. [Google Scholar] [CrossRef]
- Xue, H.; Tan, J.; Fan, L.; Li, Q.; Cai, X. Optimization microwave-assisted extraction of anthocyanins from cranberry using response surface methodology coupled with genetic algorithm and kinetics model analysis. J. Food Process Eng. 2021, 44, e13688. [Google Scholar] [CrossRef]
- Liu, W.; Yang, C.; Zhou, C.; Wen, Z.; Dong, X. An improved microwave-assisted extraction of anthocyanins from purple sweet potato in favor of subsequent comprehensive utilization of pomace. Food Bioprod. Process. 2019, 115, 1–9. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y. Study on extraction of anthocyanins from purple corn seeds by ultra-high pressure assisted ultrasonic method. J. Shenyang Agric. Univ. 2022, 53, 590–599. [Google Scholar]
- Li, P.; Ma, J.; Zhang, H.Z.; Wang, Y.; Wang, Y.; Ma, Y.H.; Yuan, Y.S. Study on ultra-high pressure-assisted extraction of anthocyanins from mulberry and lts antioxidant activity. Food Res. Dev. 2021, 42, 109–115. [Google Scholar]
- Mohdaly, A.A.A.; Roby, M.H.; Sultan, S.A.R.; Groß, E.; Smetanska, I. Potential of low cost agro-industrial wastes as a natural antioxidant on carcinogenic acrylamide formation in potato fried chips. Molecules 2022, 27, 7516. [Google Scholar] [CrossRef]
- Zhang, L.H.; Qiao, Y.; Wang, C.; An, K.J.; Liao, L. Effect of ultra high pressure combined with ultrasonic pretreatment on the quality and antioxidant activity of vacuum freeze–dried strawberry slices. J. Chin. Inst. Food Sci. Technol. 2020, 20, 157–167. [Google Scholar]
- Wang, X.; Han, Q.Y.; Xue, H.K.; Yu, G.P. Research on optimization of ultra-high pressure extraction process of anthocyanin from blueberry by response surface methodology and its activity. China Condiments 2020, 45, 147–153. [Google Scholar]
- Zhang, W.; Yan, C.; Zhang, X.; Jin, Y.Q.; Duan, M.Y.; Wu, Y.C. Study on ultra-high pressure extraction and stability of anthocyanins from rose. China Condiments 2018, 43, 151–157. [Google Scholar]
- Hu, J.M.; Xu, S.Q.; Huang, Q.; Zhou, L. Simultaneous extraction of flavonoids and pectin from navel orange peel byethanol/dipotassium hydrogen phosphate aqueous two-phase system. J. Food Saf. Qual. 2021, 12, 8883–8890. [Google Scholar]
- Liu, X.L.; Niu, T.T.; Xie, Y.W.; Kang, Q.M.; Huang, Y.Y.; Chen, W.M. Extraction of anthocyanins by aqueous two-phase from roselle and its antioxidant activity. China Food Addit. 2022, 33, 85–91. [Google Scholar]
- Zhai, S.; Zhang, H.Y.; Yu, R.M.; Zhang, H. Optimization of anthocyanin by aqueous two phase extraction from black soybean hull by response surface methodology. Food Res. Dev. 2017, 38, 24–31. [Google Scholar]
- Xu, D.N.; Yang, H.L.; Fan, L.L.; Pan, Y.L. Optimization of extraction process and component analysis of anthocyanins from grape pomace in aqueous two-phase extraction with genetic algorithm. Sci. Technol. Food Ind. 2021, 42, 168–174. [Google Scholar]
- Liu, X.; Mu, T.; Sun, H.; Zhang, M.; Chen, J. Optimisation of aqueous two-phase extraction of anthocyanins from purple sweet potatoes by response surface methodology. Food Chem. 2013, 141, 3034–3041. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Tian, Y.H.; Lu, H.X. Optimization of ultrasound assisted aqueous two-phase extraction of anthocyanins from blueberry and its antitumor activity. China Food Addit. 2023, 34, 18–26. [Google Scholar]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme–assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, Q.X.; Li, J.Y. Process optimization for enzymatic assisted extraction of anthocyanins from Lycium ruthenicum Murr. Food Res. Dev. 2018, 39, 35–40. [Google Scholar]
- Tan, J.; Li, Q.; Xue, H.; Tang, J. Ultrasound-assisted enzymatic extraction of anthocyanins from grape skins: Optimization, identification, and antitumor activity. J. Food Sci. 2020, 85, 3731–3744. [Google Scholar] [CrossRef]
- Shen, M.; Liu, K.; Liang, Y.; Liu, G.; Sang, J.; Li, C. Extraction optimization and purification of anthocyanins from Lycium ruthenicum Murr. and evaluation of tyrosinase inhibitory activity of the anthocyanins. J. Food Sci. 2020, 85, 696–706. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Li, H.M.; Wang, X.Y.; Yang, T.Y.; Ma, H.; Zhu, D.; Niu, G.C. Optimization of enzymatic extraction and composition analysis of anthocyanins from Acanthopanax senticosus dried fruit. J. Food Saf. Qual. 2021, 12, 7006–7013. [Google Scholar]
- Kang, B.B.; Liu, L.Y.; Wang, X.; Ding, L.J.; Lin, H.T.; Chen, T.W. Complex enzyme extraction and antioxidant activity of anthocyanins from vaccinium bracteatum Thunb. fruits. Sci. Technol. Food Ind. 2020, 41, 214–220. [Google Scholar]
- Wang, T.; Guo, N.; Wang, S.X.; Kou, P.; Zhao, C.J.; Fu, Y.J. Ultrasound-negative pressure cavitation extraction of phenolic compounds from blueberry leaves and evaluation of its DPPH radical scavenging activity. Food Bioprod. Process. 2018, 108, 69–80. [Google Scholar] [CrossRef]
- Lu, Y.G.; Dong, Q. Development of pulsed electric field on extraction of natural products in. Food Mach. 2012, 28, 243–246. [Google Scholar]
- Qi, M.Y.; Liu, Q.Y.; Shi, S.S.; Xian, Y.H.; Yuan, Y. Recent Progress in the application of high–voltage electric field technologyin food sterilization. Food Sci. 2022, 43, 284–292. [Google Scholar]
- Puertolas, E.; Cregenzan, O.; Luengo, E.; Alvarez, I.; Raso, J. Pulsed-electric-field-assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 2013, 136, 1330–1336. [Google Scholar] [CrossRef]
- Tian, M.X.; Li, Y.D.; Hu, W.Z.; Wang, Y.Y.; Jiang, A.L.; Liu, C.H. Optimization of supercritical CO2 extraction of blueberry anthocyanins using response surface methodology. Sci. Technol. Food Ind. 2016, 37, 208–212. [Google Scholar]
- Paes, J.; Dotta, R.; Barbero, G.F.; Martínez, J. Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium myrtillus L.) residues using supercritical CO2 and pressurized liquids. J. Supercrit. Fluids 2014, 95, 8–16. [Google Scholar] [CrossRef]
- Liu, Y.F.; Lin, N.Y.; Cheng, J.; Ma, L.K.; Chen, N.Y.; Feng, H.K.; Wang, Q. Advances in preparation of polysaccharides by microbial fermentation. Food Ferment. Ind. 2021, 47, 281–287. [Google Scholar]
- Zhang, J.; Yang, Y.; Lv, R.; Zhan, K.; Chang4, X.; Zhang, C. Sugar reduction process of purple sweet potato concentrated juice by microbial fermentation for improved performance of natural pigments. Biochem. Eng. J. 2023, 191, 108781. [Google Scholar] [CrossRef]
- Yu, L.; Jiang, Y.Y.; Zhang, P. Optimization on ultrasonic–microwave extraction of anthocyanins from purple potato and their stability. Packag. Food Mach. 2023, 41, 40–45. [Google Scholar]
- Liao, C.Y.; Chen, S.; Zhou, L.; Deng, D.M. Optimization of extraction process of anthocyanins from Rhodomyrtus tomentosa by the cellulase-microwave combined method. Food Sci. Technol. 2021, 46, 198–204. [Google Scholar]
- Zhang, X.J.; Liu, Z.T.; Chen, X.Q.; Zhang, T.T.; Zhang, Y. Deep eutectic solvent combined with ultrasound technology: A promising integrated extraction strategy for anthocyanins and polyphenols from blueberry pomace. Food Chem. 2023, 422, 136224. [Google Scholar] [CrossRef]
- Yang, D.; Li, M.M.; Wang, W.J.; Zheng, G.D.; Yin, Z.P.; Chen, J.G. Separation and purification of anthocyanins from roselle by macroporous resins. LWT—Food Sci. Technol. 2022, 161, 113371. [Google Scholar] [CrossRef]
- Xu, H.R.; Li, L.Q.; Liu, Y.; Wang, Y.Y.; Yao, S.C.; Shen, M.Y.; Ruan, J.L. Purification study of purple solanum tuberosum anthocyanins by AB–8 macroporous. Chin. Pharm. J. 2017, 52, 2141–2145. [Google Scholar]
- Guo, Q.; Zhang, N.; Liu, H.; Wang, P.; Wang, Z. Study on purification of anthocyanins from blackcurrant marc by macroporous resin and primary identification. China Condiment 2010, 7, 100–113. [Google Scholar]
- Tian, Y.; Liimatainen, J.; Puganen, A.; Alakomi, H.L.; Sinkkonen, J.; Yang, B. Sephadex LH–20 fractionation and bioactivities of phenolic compounds from extracts of Finnish berry plants. Food Res. Int. 2018, 113, 115–130. [Google Scholar] [CrossRef]
- Xue, H.K.; Shen, L.Y.; Wang, X.R. Isolation and purification of anthocyanin from blueberry using macroporous resin combined Sephadex LH-20 Techniques. Food Sci. Technol. Res. 2019, 25, 29–38. [Google Scholar] [CrossRef]
- Wang, P.; Yu, M. Study on purification of anthocyanins from blackcurrant marc by macroporous resin. J. Harbin Univ. Commer. 2008, 28, 113–118. [Google Scholar]
- He, S.; Lou, Q.; Shi, J.; Sun, H.; Zhang, M.; Li, Q. Water extraction of anthocyanins from black rice and purification using membrane separation and resin adsorption. J. Food Process. Preserv. 2017, 41, e13091. [Google Scholar] [CrossRef]
- He, H.Y.; Chen, W.Y.; Yang, A.P.; Jiang, C.Y.; Zhang, R. Effect of blackcurrant polyphenols on lowering blood pressure. Food Ferment. Ind. 2020, 46, 97–103. [Google Scholar]
- Chandrasekhar, J.; Raghavarao, K. Separation and concentration of anthocyanins from jamun: An integrated process. Chem. Eng. Commun. 2015, 202, 1368–1379. [Google Scholar] [CrossRef]
- Cisse, M.; Vaillant, F.; Pallet, D. Selecting ultrafiltration and nanofiltration membranes to concentrate anthocyanins from roselle extract (Hibiscus sabdariffa L.). Food Res. Int. 2011, 44, 2607–2614. [Google Scholar]
- Munoz, P.; Perez, K.; Cassano, A.; Ruby-Figueroa, R. Recovery of anthocyanins and monosaccharides from grape marc extract by nanofiltration membranes. Molecules 2021, 26, 2003. [Google Scholar] [CrossRef]
- Chen, T.; Yang, X.; Wang, N.; Li, H.; Zhao, J.; Li, Y. Separation of six compounds including two n-butyrophenone isomers and two stibene isomers from Rheum tanguticum Maxim by recycling high speed counter-current chromatography and preparative high-performance liquid chromatography. J. Sep. Sci. 2018, 41, 3660–3668. [Google Scholar] [CrossRef]
- Du, Q.; Jerz, G.; Winterhalter, P. Isolation of two anthocyanin sambubiosides from bilberry (Vaccinium myrtillus) by high–speed counter-current chromatography. J. Chromatogr. A 2004, 1045, 59–63. [Google Scholar] [CrossRef]
- Li, Y.Y.; Li, L.X.; Cui, Y.; Sun, B.S. Isolation of anthocyanins from red grape skins by high-speed countercurrent chromatography. China Brew. 2017, 36, 157–161. [Google Scholar]
- Xue, H.K.; Li, P.C.; Zhong, X.; Liu, C.H.; Li, Q. Separation and purification of anthocyanins from mulberry fruit by high speed counter-current chromatography and their antioxidant activity. Food Sci. 2020, 41, 96–104. [Google Scholar]
- Chen, L.; Xin, X.; Feng, H.; Li, S.; Cao, Q.; Wang, X.; Vriesekoop, F. Isolation and identification of anthocyanin component in the fruits of Acanthopanax sessiliflorus (rupr. & maxim.) seem. by means of high speed counter current chromatography and evaluation of its antioxidant activity. Molecules 2020, 25, 1781. [Google Scholar] [CrossRef]
- Xue, H.; Zhu, X.; Tan, J.; Fan, L.; Li, Q.; Tang, J.; Cai, X. Counter-current fractionation-assisted bioassay-guided separation of active compound from blueberry and the interaction between the active compound and α-glucosidase. Foods 2021, 10, 509. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Li, X.N.; Zhang, C.P.; Yan, J.Z. Application of high performance preparative chromatography to the separation of natural products. Prog. Pharm. Sci. 2010, 34, 337–343. [Google Scholar]
- Wang, W.Q.; Deng, J.H.; Shi, X.B.; Liu, Y.H. Separation, purification and structural identification of anthocyanins in the skin of grape spinosa. Trans. Chin. Soc. Agric. Eng. 2016, 32, 296–301. [Google Scholar]
- Liu, J.B.; Chen, J.J.; Wang, E.L.; Liu, Y.J. Separation of anthocyanin monomers from blueberry fruits through chromatographic techniques. Food Sci. 2017, 38, 206–213. [Google Scholar]
- Wang, E.L.; Chen, J.J.; Liu, Y.J.; Liu, J.B. Preparation of anthocyanin aglycones from blueberries using combination of different chromatographic techniques. Food Sci. 2018, 39, 227–234. [Google Scholar]
- Grigoras, C.G.; Destandau, E.; Zubrzycki, S.; Elfakir, C. Sweet cherries anthocyanins: An environmental friendly extraction and purification method. Sep. Purif. Technol. 2012, 100, 51–58. [Google Scholar] [CrossRef]
- Chorfa, N.; Savard, S.; Belkacemi, K. An efficient method for high–purity anthocyanin isomers isolation from wild blueberries and their radical scavenging activity. Food Chem. 2016, 197, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Peng, W.X.; Mao, H.Q.; Shen, H.L.; Zhou, X.; Zhang, Y.Q. Purification of 5,7,8,4′-tetrahydroxyisoflavone from fermentation of fructus sophorae by pre-HPLC. Biol. Chem. Eng. 2022, 8, 81–84. [Google Scholar]
- Xue, H.K.; Han, Q.Y.; Tan, J.Q.; Wang, Y.; Wang, X. Purification and thermal degradation kinetics of anthocyanins from blackcurrant. Fine Chem. 2019, 36, 721–729. [Google Scholar]
- Yu, Z.Y.; Zhao, J.H.; Li, X.G.; Xu, Y.Q.; Tang, X.; Yang, Y. Isolation and purification of anthocyanin from blueberry by sequentia medium pressure column chromatography on macroporous resin and Sephadex LH-20. Food Sci. 2018, 39, 118–123. [Google Scholar]
- Chen, L.; Xin, X.L.; Yuan, Q.P. Analysis of compositions of anthocyanins in wild mulberry. Sci. Technol. Food Ind. 2012, 33, 307–310. [Google Scholar]
- Ma, Z.; Guo, A.; Jing, P. Advances in dietary proteins binding with co-existed anthocyanins in foods: Driving forces, structure-affinity relationship, and functional and nutritional properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 10792–10813. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; He, Y.; Fan, K. Recent advances in stability improvement of anthocyanins by efficient methods and its application in food intelligent packaging: A review. Food Biosci. 2023, 56, 103164. [Google Scholar] [CrossRef]
- Moeiniafshari, A.A.; Zarrabi, A.; Bordbar, A.K. Exploring the interaction of naringenin with bovine beta-casein nanoparticles using spectroscopy. Food Hydrocoll. 2015, 51, 1–6. [Google Scholar] [CrossRef]
- Tang, L.; Li, S.; Bi, H.; Gao, X. Interaction of cyanidin-3-O-glucoside with three proteins. Food Chem. 2016, 196, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.; Tang, S.; Li, Z.; Chou, S.; Shu, C.; Chen, Y.; Chen, W.; Yang, S.; Yang, Y.; Tian, J.; et al. An updated review on the stability of anthocyanins regarding the interaction with food proteins and polysaccharides. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4378–4401. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Zhang, T.; Jiang, L. Soy protein: Molecular structure revisited and recent advances in processing technologies. Annu. Rev. Food Sci. Technol. 2021, 12, 119–147. [Google Scholar] [CrossRef] [PubMed]
- Chamizo–Gonzalez, F.; Gordillo, B.; Heredia, F.J. Elucidation of the 3D structure of grape seed 7S globulin and its interaction with malvidin 3–glucoside: A molecular modeling approach. Food Chem. 2021, 347, 129014. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Li, J.; Zhao, L.; Tuersuntuoheti, T.; Mehmood, A.; Zhou, N.; Hao, S.; Wang, C.; Guo, Y.; Lin, W. A molecular docking and molecular dynamics simulation study on the interaction between cyanidin-3-O-glucoside and major proteins in cow’s milk. J. Food Biochem. 2021, 45, e13570. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Yang, C.; Zhang, J.; Yu, Y.; Wang, Z. Study on the interaction mechanism of purple potato anthocyanins with casein and whey protein. Food Hydrocoll. 2020, 111, 106223. [Google Scholar] [CrossRef]
- Zang, Z.; Chou, S.; Tian, J.; Lang, Y.; Shen, Y.; Ran, X.; Gao, N.; Li, B. Effect of whey protein isolate on the stability and antioxidant capacity of blueberry anthocyanins: A mechanistic and in vitro simulation study. Food Chem. 2021, 336, 127700. [Google Scholar] [CrossRef]
- Lang, Y.; Li, E.; Meng, X.; Tian, J.; Ran, X.; Zhang, Y.; Zang, Z.; Wang, W.; Li, B. Protective effects of bovine serum albumin on blueberry anthocyanins under illumination conditions and their mechanism analysis. Food Res. Int. 2019, 122, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Hao, L.; Tan, Y.; Yang, Y.; Liu, L.; Li, C.; Du, P. Noncovalent interaction of cyanidin-3-O-glucoside with whey protein isolate and β-lactoglobulin: Focus on fluorescence quenching and antioxidant properties. LWT—Food Sci. Technol. 2021, 137, 110386. [Google Scholar] [CrossRef]
- Li, C.; Yun, D.; Wang, Z.; Xu, F.; Tang, C.; Liu, J. Development of shrimp freshness indicating films by embedding anthocyanins-rich rhododendron Simsii flower extract in locust bean gum/polyvinyl alcohol matrix. Materials 2022, 15, 7557. [Google Scholar] [CrossRef]
- Qin, X.; Yuan, D.; Wang, Q.; Hu, Z.; Wu, Y.; Cai, J.; Huang, Q.; Li, S.; Liu, G. Maillard–reacted whey protein isolates enhance thermal stability of anthocyanins over a wide ph range. J. Agric. Food Chem. 2018, 66, 9556–9564. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, C.; Gao, X.; Chen, Y.; Kumar Santhanam, R.; Wang, C.; Xu, L.; Chen, H. Interaction characterization of preheated soy protein isolate with cyanidin-3-O-glucoside and their effects on the stability of black soybean seed coat anthocyanins extracts. Food Chem. 2019, 271, 266–273. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xu, M.; Zeng, M.; Qin, F.; Chen, J. Preheated milk proteins improve the stability of grape skin anthocyanins extracts. Food Chem. 2016, 210, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Attaribo, T.; Jiang, X.; Huang, G.; Zhang, B.; Xin, X.; Zhang, Y.; Zhang, N.; Gui, Z. Studies on the interactional characterization of preheated silkworm pupae protein (SPP) with anthocyanins (C3G) and their effect on anthocyanin stability. Food Chem. 2020, 326, 126904. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.; Tian, J.; Chou, S.; Lang, Y.; Tang, S.; Yang, S.; Yang, Y.; Jin, Z.; Chen, W.; Liu, X.; et al. Investigation on the interaction mechanisms for stability of preheated whey protein isolate with anthocyanins from blueberry. Int. J. Biol. Macromol. 2024, 255, 127880. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Giusti, M.M. The effect of whey protein concentration and preheating temperature on the color and stability of purple corn, grape and black carrot anthocyanins in the presence of ascorbic acid. Food Res. Int. 2021, 144, 110350. [Google Scholar] [CrossRef]
- Han, F.; Yang, P.; Wang, H.; Fernandes, I.; Mateus, N.; Liu, Y. Digestion and absorption of red grape and wine anthocyanins through the gastrointestinal tract. Trends Food Sci. Technol. 2019, 83, 211–224. [Google Scholar] [CrossRef]
- Kamonpatana, K.; Giusti, M.M.; Chitchumroonchokchai, C.; MorenoCruz, M.; Riedl, K.M.; Kumar, P.; Failla, M.L. Susceptibility of anthocyanins to ex vivo degradation in human saliva. Food Chem. 2012, 135, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Gui, H.; Sun, L.; Liu, R.; Si, X.; Li, D.; Wang, Y.; Shu, C.; Sun, X.; Jiang, Q.; Qiao, Y.; et al. Current knowledge of anthocyanin metabolism in the digestive tract: Absorption, distribution, degradation, and interconversion. Crit. Rev. Food Sci. Nutr. 2023, 63, 5953–5966. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.R.; Murador, D.C.; Mesquita, L.M.; Rosso, V.V. Bioavailability of anthocyanins: Gaps in knowledge, challenges and future research. J. Food Compos. Anal. 2017, 68, 31–40. [Google Scholar] [CrossRef]
- Lila, M.A.; Burton-Freeman, B.; Grace, M.; Kalt, W. Unraveling anthocyanin bioavailability for human health. Annu. Rev. Food Sci. Technol. 2016, 7, 375–393. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; He, W.; Qie, X.; Chen, Y.; Zeng, M.; Qin, F.; Chen, J.; He, Z. Effects of β-cyclodextrin, whey protein, and soy protein on the thermal and storage stability of anthocyanins obtained from purple–fleshed sweet potatoes. Food Chem. 2020, 320, 126655. [Google Scholar] [CrossRef] [PubMed]
- Miyagusuku-Cruzado, G.; Jimenez-Flores, R.; Giusti, M.M. Whey protein addition and its increased light absorption and tinctorial strength of model solutions colored with anthocyanins. J. Dairy Sci. 2021, 104, 6449–6462. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; He, Y.; Wen, L.; Nan, H.; Zheng, F.; Liu, H.; Lu, S.; Wu, M.; Zhang, H. A review of the interaction between anthocyanins and proteins. Food Sci. Technol. Int. 2021, 27, 470–482. [Google Scholar] [CrossRef]
- Zang, Z.; Chou, S.; Geng, L.; Si, X.; Ding, Y.; Lang, Y.; Cui, H.; Gao, N.; Chen, Y.; Wang, M.; et al. Interactions of blueberry anthocyanins with whey protein isolate and bovine serum protein: Color stability, antioxidant activity, in vitro simulation, and protein functionality. LWT—Food Sci. Technol. 2021, 152, 112269. [Google Scholar] [CrossRef]
- Ribnicky, D.; Roopchand, D.E.; Oren, A.; Grace, M.H.; Poulev, A.; Lila, M.A.; Havenaar, R.; Raskin, I. Effects of a high fat meal matrix and protein complexation on the bioaccessibility of blueberry anthocyanins using the TNO gastrointestinal model (TIM-1). Food Chem. 2014, 142, 349–357. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; He, Y.; Xu, Y.; Li, L.; Luo, Z. Interaction and binding mechanism of cyanidin-3-O-glucoside to ovalbumin in varying pH conditions: A spectroscopic and molecular docking study. Food Chem. 2020, 320, 126616. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Chen, Z.; Zhang, X.; Zhu, Z. Functional properties and structural changes of rice proteins with anthocyanins complexation. Food Chem. 2020, 331, 127336. [Google Scholar] [CrossRef]
- Cui, Q.; Dong, Y.; Zhang, A.; Wang, X.; Zhao, X.H. Multiple spectra analysis and calculation of the interaction between anthocyanins and whey protein isolate. Food Biosci. 2021, 44, 101353. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Qi, B.; Sui, X.; Jiang, L. Complexation of thermally-denatured soybean protein isolate with anthocyanins and its effect on the protein structure and in vitro digestibility. Food Res. Int. 2018, 106, 619–625. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Nawaz, A.; Li, K.; Li, C. Microencapsulated mulberry anthocyanins promote the in vitro-digestibility of whey proteins in glycated energy-ball models. Food Chem. 2021, 345, 128805. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Zhang, J.; Zhang, L. Interaction of preheated whey protein isolate with rose anthocyanin extracts in beverage model system: Influence on color stability, astringency and mechanism. Food Chem. 2023, 412, 135507. [Google Scholar] [CrossRef]
- Mansour, M.; Elmorsy, M.A.; Elkhedir, A.; Wu, T.; Xu, X.Y. Binding interaction between soy protein nanogel and red raspberry anthocyanin in acidic media: Spectroscopic characterization and molecular docking analysis. J. Mol. Struct. 2024, 1305, 137681. [Google Scholar] [CrossRef]
- He, W.; Mu, H.; Liu, Z.; Lu, M.; Hang, F.; Chen, J.; Zeng, M.; Qin, F.; He, Z. Effect of preheat treatment of milk proteins on their interactions with cyanidin-3-O-glucoside. Food Res. Int. 2018, 107, 394–405. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Hao, S.; Liu, G.R.; Wang, C.T.; Zhu, J.J. Study on interactions between cyanidin-3-glucoside and four kinds of milk proteins. Sci. Technol. Food Ind. 2018, 39, 11–18. [Google Scholar]
- Sui, X.; Sun, H.; Qi, B.; Zhang, M.; Li, Y.; Jiang, L. Functional and conformational changes to soy proteins accompanying anthocyanins: Focus on covalent and non-covalent interactions. Food Chem. 2018, 245, 871–878. [Google Scholar] [CrossRef]
- Wu, H.B.; Zhang, Q.; Qiu, S.; Li, D.M.; Zhang, Y.Y.; Jiang, L. Physicochemical properties of soymilk prepared from component deficient soybean under homogenization or heating conditions. Trans. Chin. Soc. Agric. Mach. 2021, 52, 374–385. [Google Scholar]
- Ozdal, T.; Çapanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Wu, S.; Fitzpatrick, J.; Cronin, K.; Miao, S. The effect of pH on the wetting and dissolution of milk protein isolate powder. J. Food Eng. 2019, 240, 114–119. [Google Scholar] [CrossRef]
- Wu, H.; Liu, H.N.; Di, Q.R.; Xiayidan, M.M.D.; Liu, X.L.; Zhou, J.Z. Effects of blackberry anthocyanins(BANs) coupled with high power pulse microwave(HPPM) on physiochemical properties of soybean protein lsolate(SPl) and application of HPPM treated SPl–BANs complex in cake. Food Sci. 2023, 44, 60–66. [Google Scholar]
- Zhao, H.J.; Lv, X.L.; Wang, M.S.; Wang, Y. Studies on the interaction between casein and black rice anthocyanin by spectroscopic methodology. China Food Addit. 2017, 12, 121–127. [Google Scholar]
- Guo, Y.; Jiang, T.; Li, S.Y.; Zhu, Z.; He, J.R. Preparation and structure identification of anthocyanins-protein complexes from purple sweet potato. Food Sci. 2016, 37, 109–113. [Google Scholar]
- Sui, X.; Dong, X.; Zhou, W. Combined effect of pH and high temperature on the stability and antioxidant capacity of two anthocyanins in aqueous solution. Food Chem. 2014, 163, 163–170. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Li, L.; Qi, B.; Ju, M.; Xu, Y.; Zhang, Y.; Sui, X. Covalent conjugates of anthocyanins to soy protein: Unravelling their structure features and in vitro gastrointestinal digestion fate. Food Res. Int. 2019, 120, 603–609. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Chen, H.; Tian, W.; Cui, W.M.; Zhao, G.M.; Shen, M.Y. Research progress on influencing factors of meat protein digestibility. Food Sci. 2022, 43, 349–357. [Google Scholar]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; Mcclements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Ma, Z.; Cheng, J.; Jiao, S.; Jing, P. Interaction of mulberry anthocyanins with soybean protein isolate: Effect on the stability of anthocyanins and protein in vitro digestion characteristics. Int. J. Food Sci. Technol. 2022, 57, 2267–2276. [Google Scholar] [CrossRef]
- Khalifa, I.; Lorenzo, J.M.; Bangar, S.P.; Morsy, O.M.; Nawaz, A.; Walayat, N.; Sobhy, R. Effect of the non-covalent and covalent interactions between proteins and mono- or di-glucoside anthocyanins on β-lactoglobulin-digestibility. Food Hydrocoll. 2022, 133, 107952. [Google Scholar] [CrossRef]
- Yi, J.; Qiu, M.; Liu, N.; Tian, L.; Zhu, X.; Decker, E.A.; McClements, D.J. Inhibition of lipid and protein oxidation in whey-protein-stabilized emulsions using a natural antioxidant: Black rice anthocyanins. J. Agric. Food Chem. 2020, 68, 10149–10156. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Z.; Zhao, J.; Liu, Y. The effect of non-covalent interaction of chlorogenic acid with whey protein and casein on physicochemical and radical-scavenging activity of in vitro protein digests. Food Chem. 2018, 268, 334–341. [Google Scholar] [CrossRef]
- Perez-Gregorio, M.R.; Simal-Gandara, J. A Critical Review of the characterization of polyphenol–protein interactions and of their potential use for improving food quality. Curr. Pharm. Des. 2017, 23, 2742–2753. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.D.C.G.; Viana, K.W.C.; Mendonca, A.C.; Neves, N.D.A.; Carvalho, A.F.D.; Minim, V.P.R.; Stringheta, P.C. Protein beverages containing anthocyanins of jabuticaba. Food Sci. Technol. 2018, 39, 112–119. [Google Scholar] [CrossRef]
- Chung, C.; Rojanasasithara, T.; Mutilangi, W.; McClements, D.J. Enhanced stability of anthocyanin–based color in model beverage systems through whey protein isolate complexation. Food Res. Int. 2015, 76, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.H.; Zhong, R.M.; Sun, J.; Guan, W.C.; Ling, W.H. Preparation of yogurt mixed with anthocyanin extract from black rice. Food Res. Dev. 2011, 32, 70–71. [Google Scholar]
- Jayaprakash, G.; Bains, A.; Chawla, P.; Fogarasi, M.; Fogarasi, S. A narrative review on rice proteins: Current scenario and food industrial application. Polymers 2022, 14, 3003. [Google Scholar] [CrossRef]
- Milincic, D.D.; Kostic, A.Z.; Gasic, U.M.; Levic, S.; Stanojevic, S.P.; Barac, M.B.; Pesic, M.B. Skimmed goat’s milk powder enriched with grape pomace seed extract: Phenolics and protein characterization and antioxidant properties. Biomolecules 2021, 11, 965. [Google Scholar] [CrossRef]
- Meng, Z.Y.; Luo, Z.Y.; Duan, H.Y.; Wang, C. Stability and anthocyanin bioavailability of red bayberry juice protein powder. Food Ferment. Ind. 2023, 49, 197–204. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, H.; Zha, M.; Tang, Y.; Zhao, J.; Du, X.; Wang, Y. Research Progress on the Extraction and Purification of Anthocyanins and Their Interactions with Proteins. Molecules 2024, 29, 2815. https://doi.org/10.3390/molecules29122815
Xue H, Zha M, Tang Y, Zhao J, Du X, Wang Y. Research Progress on the Extraction and Purification of Anthocyanins and Their Interactions with Proteins. Molecules. 2024; 29(12):2815. https://doi.org/10.3390/molecules29122815
Chicago/Turabian StyleXue, Hongkun, Min Zha, Yingqi Tang, Jianduo Zhao, Xiaopeng Du, and Yu Wang. 2024. "Research Progress on the Extraction and Purification of Anthocyanins and Their Interactions with Proteins" Molecules 29, no. 12: 2815. https://doi.org/10.3390/molecules29122815
APA StyleXue, H., Zha, M., Tang, Y., Zhao, J., Du, X., & Wang, Y. (2024). Research Progress on the Extraction and Purification of Anthocyanins and Their Interactions with Proteins. Molecules, 29(12), 2815. https://doi.org/10.3390/molecules29122815






