Supramolecular Materials as Solid-Phase Microextraction Coatings in Environmental Analysis
Abstract
1. Introduction
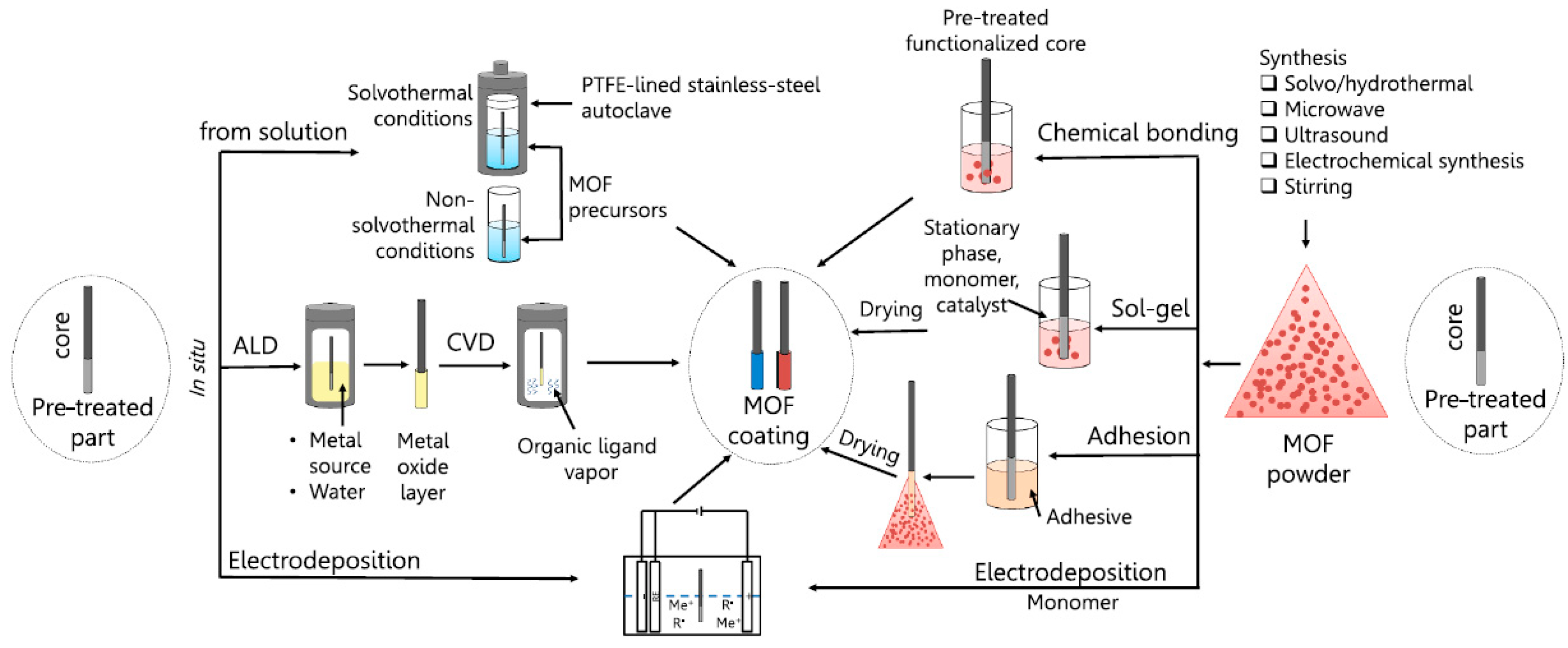
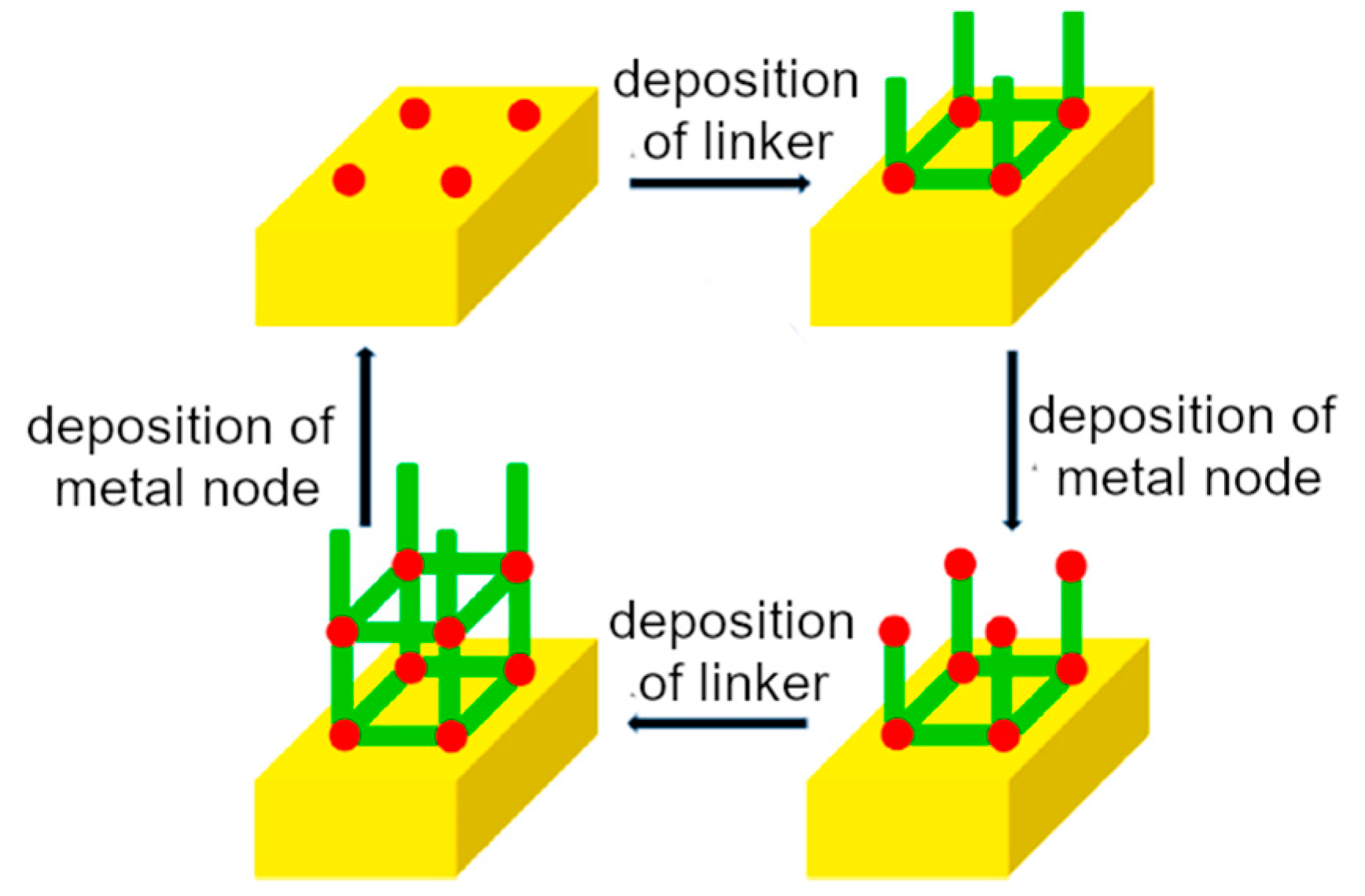
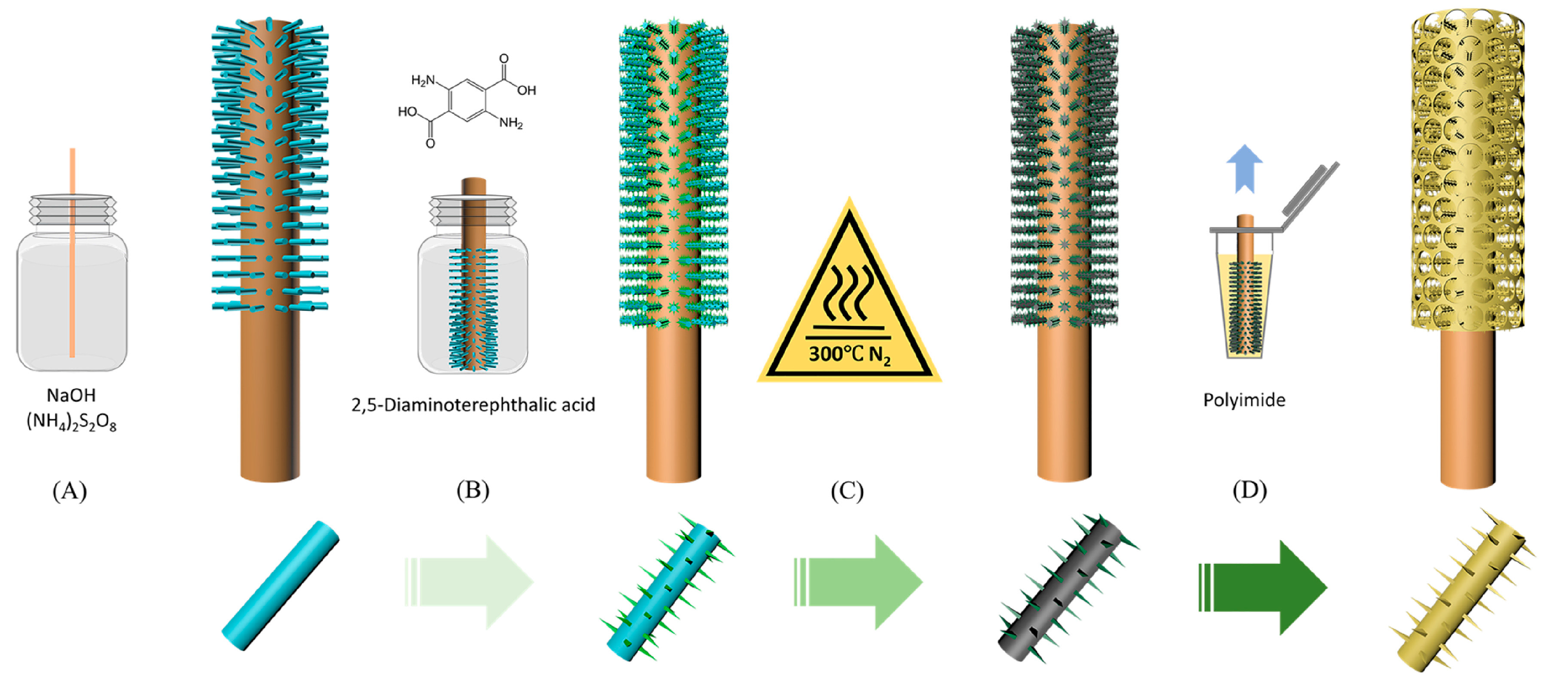
2. Supramolecular-Based Coatings: Deposition Methods
3. Metal–Organic Frameworks
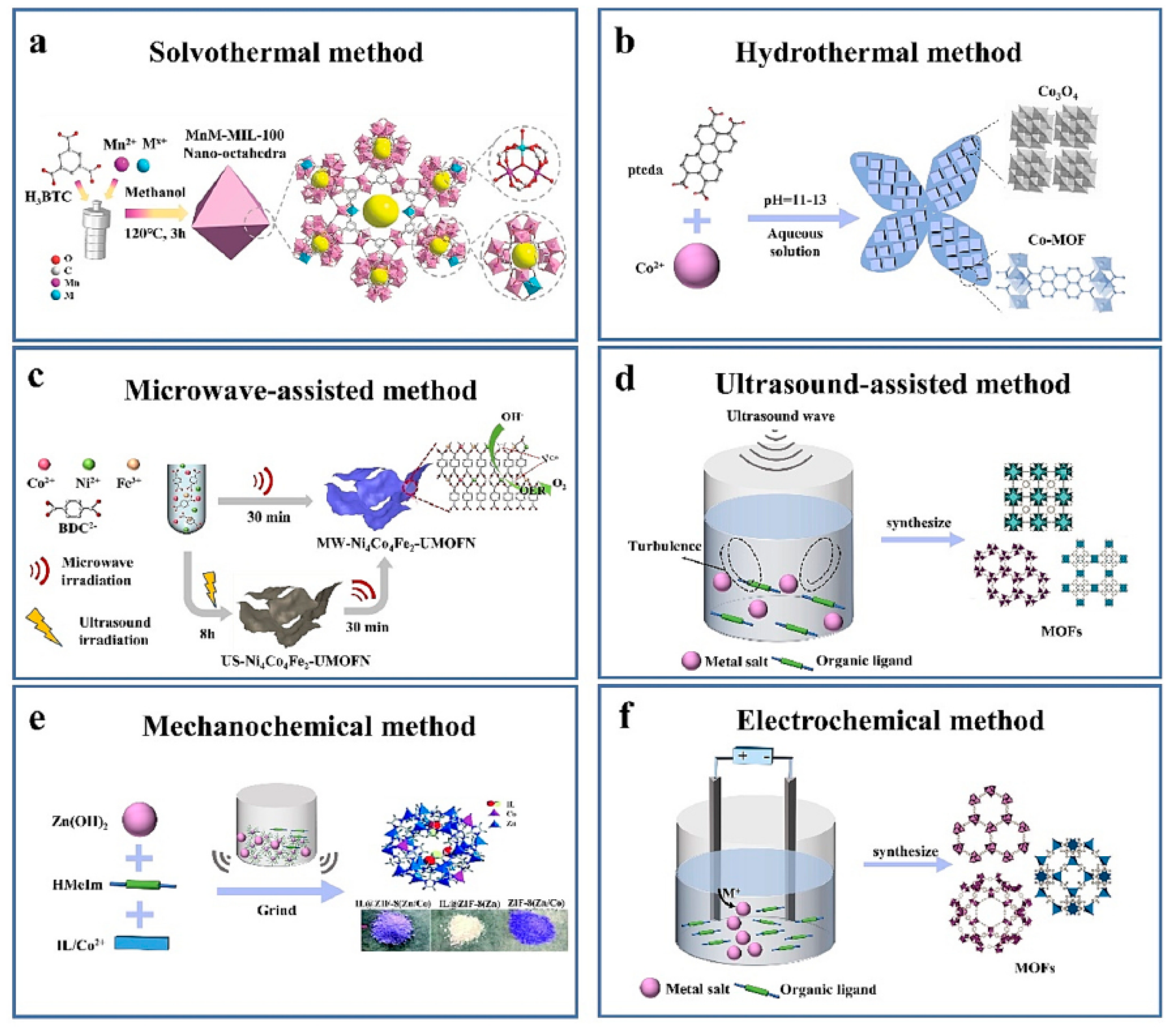
3.1. Synthetic Procedures
3.2. MOF Features for SPME Extraction
3.3. MOFs as SPME Coatings for Environmental Applications
3.3.1. Extraction of Benzene, Toluene, Ethylbenzene, and Xylenes
3.3.2. Extraction of Polycyclic Aromatic Hydrocarbons
3.3.3. Extraction of Organophosphorus and Organochlorine Pesticides
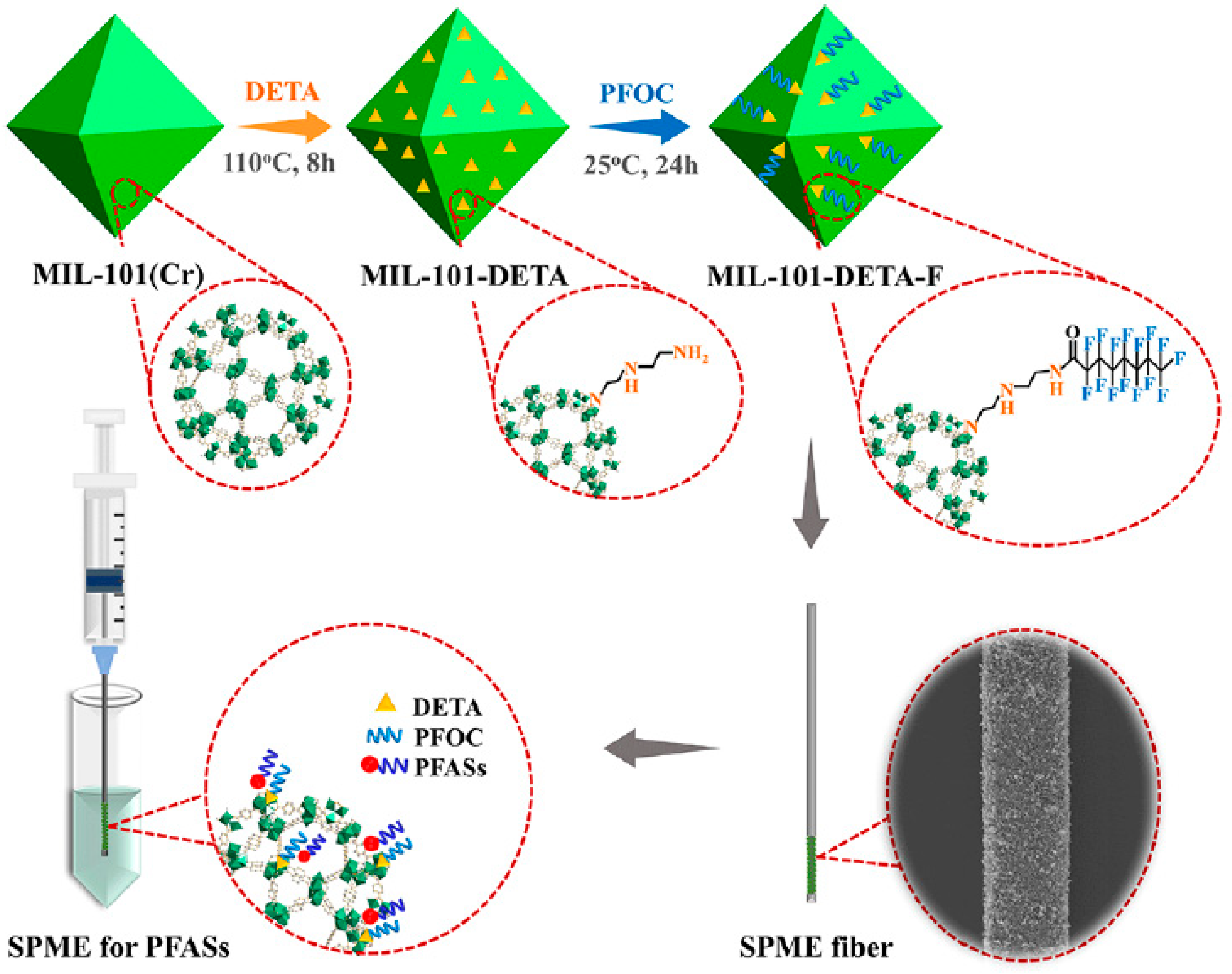
3.3.4. Extraction of Poly- and Perfluoroalkyl Substances
3.3.5. Extraction of Pharmaceutical and Personal Care Products
3.3.6. Extraction of Polychlorinated Biphenyls
3.3.7. Extraction of Other Compound Classes
| Analyte | Material | Deposition Method | Matrix | Extraction Mode | Platform | LOD (ng/L) | EFs | References |
|---|---|---|---|---|---|---|---|---|
| BTEX | SOM-ZIF-8 | physical adhesion | wastewater | HS | GC-FID | 1.0–12 | - | [48] |
| BTEX | NSZIF-8Si | physical adhesion | river water | HS | GC-MS | 0.02–0.21 | - | [49] |
| BTEX | MIL-101-NH2-derived urchin-like nanoporous carbon | physical adhesion | pond water and river water | HS | GC-MS | 0.08–0.36 | - | [50] |
| BTEX, styrene, and trimethylbenzene | PAN/MIL-53(Al)@MOF@SBA-15/4,4′-bipyridine hybrid nanocomposite | in situ electrodeposition | tap water, mineral water, well water, and wastewater | HS | GC-FID | 2.3–3.6 | 318–385 | [51] |
| BTEX + 14 VOCs | MOF-199 | in situ growth | air | DI | GC-MS | 0.03–0.09 a | - | [52] |
| 16 PAHs and 11 nitro-PAHs | ZIF-8 | sol–gel deposition | tap water, surface water, and wastewater | DI | GC-MS | 0.3–27.0 | - | [54] |
| 3 PAHs and 5 PPCPs | ZIF-8 | CVD deposition and in situ growth | wastewater | DI | GC-FID | 600–2000 | - | [55] |
| 8 PAHs | ZIF-8 | in situ electrodeposition | lake water | HS | GC-FID | 10–54 | - | [56] |
| 6 PAHs | PANI/ZnO nanorods/ZIF-8 | in situ growth | sewage water | EE-SPME/DI-SPME | GC-FID | 8.2–134 | - | [57] |
| 6 PAHs | Co@ZIF-67 | in situ growth and electrodeposition | snow, lake water, river water, and wastewater | DI | HPLC-UV | 5–42 | - | [58] |
| 7 PAHs | MAF-66 | physical adhesion | lake water and food | HS | GC-FID | 0.1–7.5 | 127–3108 | [59] |
| 16 PAHs | MAF-5 and MAF-6 | physical adhesion | wastewater and milk products | DI | HPLC-FLD | 6–540 | - | [60] |
| 8 PAHs | HKUST-1 membrane | in situ growth and physical adhesion | lake water | HS | GC-MS | 0.1–9.9 | - | [61] |
| 16 PAHs | PUM-210 | physical adhesion | contaminated water | DI | GC-MS | 0.50–3.7 | 300–14,950 | [62] |
| 10 PAHs | PI(Cu-DAT) | in situ growth and dip-coating | river water and fish muscle | HS/DI | GC-MS | 0.3–2.1, 4.0–18.9 | - | [63] |
| diazinon and chloropyrifos | PAN/Ni-MOF | post-synthetic electrodeposition | river water, farm water, groundwater, and beverages | HS | CD-IMS | 200–300 | - | [65] |
| ethion | ZIF-67 film | in situ electrodeposition | underground water and agricultural wastewaters | DI | SESI-IMS | 100 | - | [66] |
| 6 OCPs | NU-1000 | physical adhesion | river water and seawater | DI | GC-MS | 0.011–0.058 | 972–2275 | [67] |
| 8 chlorobenzenes | Ni@NiO/PCs | physical adhesion | tap water and river water | DI | GC-MS | 0.07–0.165 | - | [68] |
| PFOA | ZIF-8, UiO-66, MIL88-A, and Tb2(BDC)3 | in situ growth | contaminated tap water, rainwater, and seawater | DI | Direct-MS | 11 | - | [70] |
| 8 PFASs | NH2-ZIF-8 | physical adhesion | river water, seawater, and wastewater | DI | HPLC-MS/MS | 0.15–0.75 | - | [71] |
| 11 PFASs | NH2-UiO-66(Zr)-hp | physical adhesion | tap water, river water, and pond water | DI | HPLC-MS/MS | 0.035–0.616 | 6.5–48 | [72] |
| 9 PFASs | MIL-101-DETA-F | physical adhesion | tap water, river water, and wastewater | DI | UHPLC-MS/MS | 0.004–0.12 | 70–112 | [73] |
| Ibuprofen and diclofenac | Zr-MOF@GO | physical adhesion | river water | DI | GC-FID | 1–30 | - | [75] |
| 3 PPCPs | Polyfam/Co-MOF-74 composite nanofibers | post-synthetic electrodeposition | wastewater and biological fluids | Thin film-SPME | HPLC-UV | 30–200 | 24–37 | [76] |
| 3 PPCPs | MIL-101 | physical adhesion | municipal wastewater | HS | GC-MS | 4–60 | - | [77] |
| 6 methylsiloxanes and 7 musk fragrances | CIM-80(Al) | in situ growth | wastewater and seawater | HS | GC-MS | 100–3500 b | - | [78] |
| 5 UV filters | NH2-UiO-66(Zr) | physical adhesion | river water and pond water | HS | GC-MS | 0.2–2.1 | 865–3321 | [79] |
| 5 fluoroquinolones | ZIF-8 | in situ deposition | tap water, river water, and wastewater | IT | HPLC-FLD | 0.14–0.61 | 255–296 | [80] |
| 7 PCBs | ZIF-67 derived N-CNTCs | physical adhesion | river water | DI | GC-MS | 0.10–0.22 | - | [83] |
| 5 PCBs | ZIF-8 derived HCNBs | physical adhesion | river water, pond water, and rainwater | HS | GC-MS | 0.0017–0.0042 | - | [84] |
| 1-naphthol and 2-naphthol | HZ-PMOF | physical adhesion | urban water samples | HS | GC-MS/MS | 1.0 | - | [85] |
| methyl tert-butyl ether | PPy@MIL-101(Cr) | post-synthetic electrodeposition | soil | HS | GC-FID | 0.01 c | - | [86] |
| 5 odorous organic compounds | MOF-74-C | in situ growth | tap water, lake water, and wastewater | DI | GC-MS | 0.01–100 | 520–3000 | [88] |
| 6 substituted phenolic compounds | NH2-UiO-66(Zr) derived Zr/N-OMC | physical adhesion | river water and pond water | HS | GC-MS | 0.21–1.7 | - | [89] |
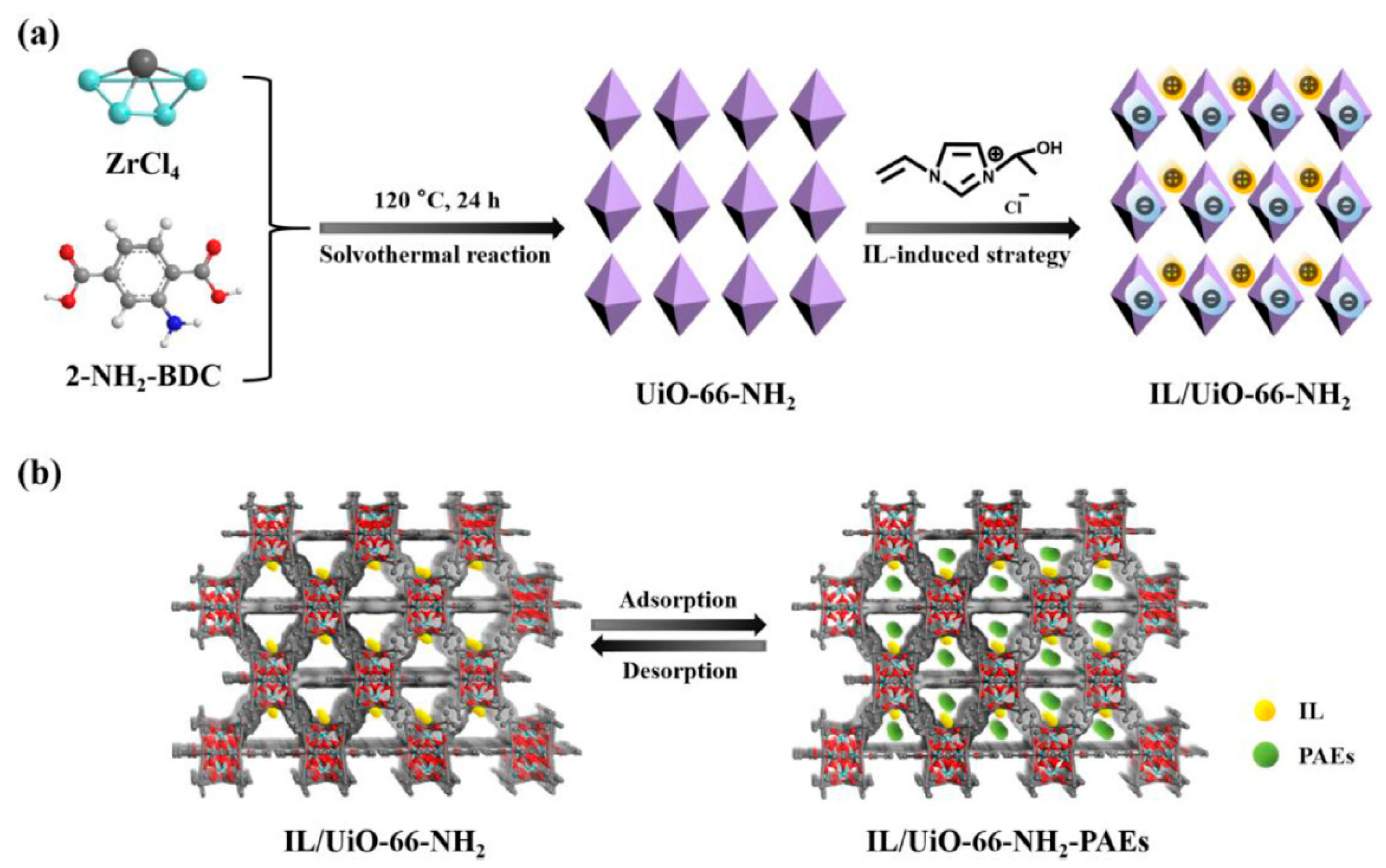
| 8 PAEs | IL/UiO-66-NH2 | in situ growth | river water, lake water, and bottled water | DI | GC-MS | 0.2–0.4 | - | [90] |
| Microcystin-LR | PAN/UiO@UiO2-N3-aptamer | post-synthetic electrodeposition | tap water, pond water, and river water | DI | LC-MS | 3 | - | [91] |
4. Covalent Organic Frameworks (COFs)
4.1. Synthetic Procedures
4.2. COF Features for SPME Extraction
4.3. COFs as SPME Coatings for Environmental Applications
4.3.1. Extraction of Polycyclic Aromatic Hydrocarbons and Nitroaromatic Compounds
4.3.2. Extraction of Phenols and Derivatives
4.3.3. Extraction of Polybrominated Diphenyl Ethers and Polyhalogenated Biphenyls
4.3.4. Extraction of Pesticides and Insecticides from Water Samples
4.3.5. Extraction of per- and Polyfluorinated Alkyl Substances
4.3.6. Extraction of Other Compound Classes by COF-Based SPME
| Analyte | Material | Deposition Method | Extraction Mode | Matrix | Platform | LOD (ng/L) | EFs | References |
|---|---|---|---|---|---|---|---|---|
| 5 PAHs | BTCH-PTA-COF | sol–gel deposition | HS | beverages and river water | GC-FID | 30–50 | 767–1411 | [101] |
| 5 PAHs | TFPA-TAPP-COF | in situ growth | HS | river water | GC-FID | 6–24 | - | [105] |
| 5 PAHs | porphyrin- COF | physical adhesion | HS | lake water and soil | GC-FID | 250–5000 | - | [106] |
| 6 PAHs | TAPB-TMC-COF | physical adhesion | HS | river water, pond water, and industrial wastewater | GC-MS | 0.29–0.94 | 819–2420 | [107] |
| 7 PAHs | imine- COF-SCU1 | in situ deposition | HS | soil | NTD-GC-FID | 0.01–0.05 a | - | [94] |
| 6 PAHs | Zn-MOF/COF | physical adhesion | HS | soil | GC-FID | 0.1–1c | - | [108] |
| 6 PAHs | Cu-MOF/COF | in situ deposition | HS | soil | GC-FID | 0.1–0.5c | - | [109] |
| 6 PAHs and BTEX | 2DTP/MIL-101-Cr | physical adhesion | HS | soil | VA-GC-FID | 2.1–5 a (BTEX), 0.07–1.6 a (PAHs) | - | [110] |
| 7 PAHs | TpBD-COF | in situ electrodeposition | DI | tap water and lake water | GC-FID | 1000–5000 | - | [111] |
| 8 PAHs | triazine- COF | physical adhesion | IT | tap water, river water, rainwater, and beverages | LC-UV | 4–10 | 1110–2763 | [112] |
| 8 PAHs | TpPa-1–1000 | physical adhesion | DI | soil | GC-MS | 3.1–8.6 a | - | [102] |
| 8 PAHs | g-C3N4@TpBD | sol–gel deposition | DI | pond water, river water, lake water, well water, rainwater, and snow | GC-MS | 20–50 | - | [103] |
| 8 PAHs, 4 estrogens and 4 bisphenols | TiO2NARs-CFs | in situ growth | IT | tap water, rainwater, and river water | LC-UV | 1–10 | 405–6784 | [104] |
| 5 nitroaromatic compounds | MA/PFC-1-HOF | physical adhesion | HS | lake water, river water, and domestic water | GC-MS | 4.3–20.8 | 393–1708 | [132] |
| 5 CPhs | TPB-DMTP-COF | physical adhesion | HS | underground, reservoir, and drinking water | GC-MS/MS | 0.0048–0.015 | 1741–4265 | [113] |
| 5 CPhs | CuPc-MCOF | physical adhesion | EE-SPME | seawater and seafood | GC-MS/MS | 0.8–5 | 339–988 | [114] |
| 2 CPhs, 2-nitrophenol, 2 dimethylphenols | TpBD COF | in situ growth | HS | water and soil | GC-MS | 0.39–0.72 | 11,080–58,762 | [115] |
| 6 CPhs | TpBD-Me2HNFs-12 | in situ growth | HS | river water | GC-FID | - | 452–2632 | [138] |
| BPA | COF-GO | physical adhesion | DI | river water and seawater | CFDI-MS | 22.2 | - | [97] |
| 5 PBDEs | TpPa-1 | physical adhesion | DI | ground water, drinking water, and pond water | GC-NCI-MS | 0.0058–0.022 | 2035–6859 | [116] |
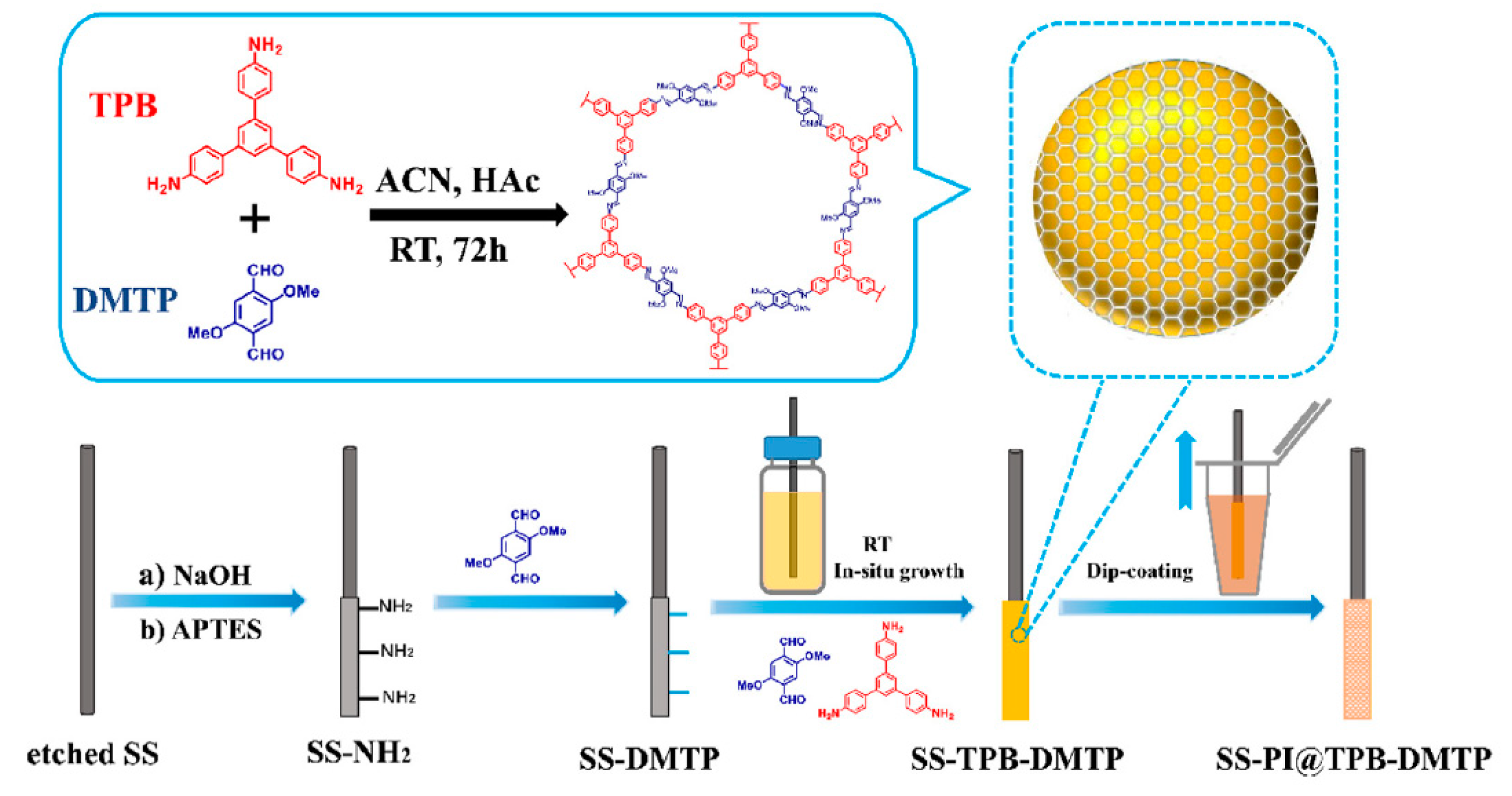
| 6 PBDEs | PI@TPB-DMTP | in situ growth | DI | lake water, river water, and wastewater | GC-NCI-MS | 0.0083–0.0190 | 1470–3555 | [100] |
| 6 PBDEs | COF-γ-PIL | physical adhesion | DI | lake water, river water, and seawater | GC-MS | 0.0021–0.014 | 913–3625 | [117] |
| 6 PBBs | TAPB-DMTP-DB COF | physical adhesion | HS | river water | GC-MS | 0.04–0.28 | 4400–11,360 | [118] |
| TBBPA | TpBD-COF | in situ deposition | DI | tap water, river water, seawater, and beverages | CFDI-MS | 0.92 | 185 | [119] |
| 4 TBBPA analoges | TpPaBD50-COF | in situ deposition | DI | river water and seawater | CFDI-MS | 0.5–12 | - | [120] |
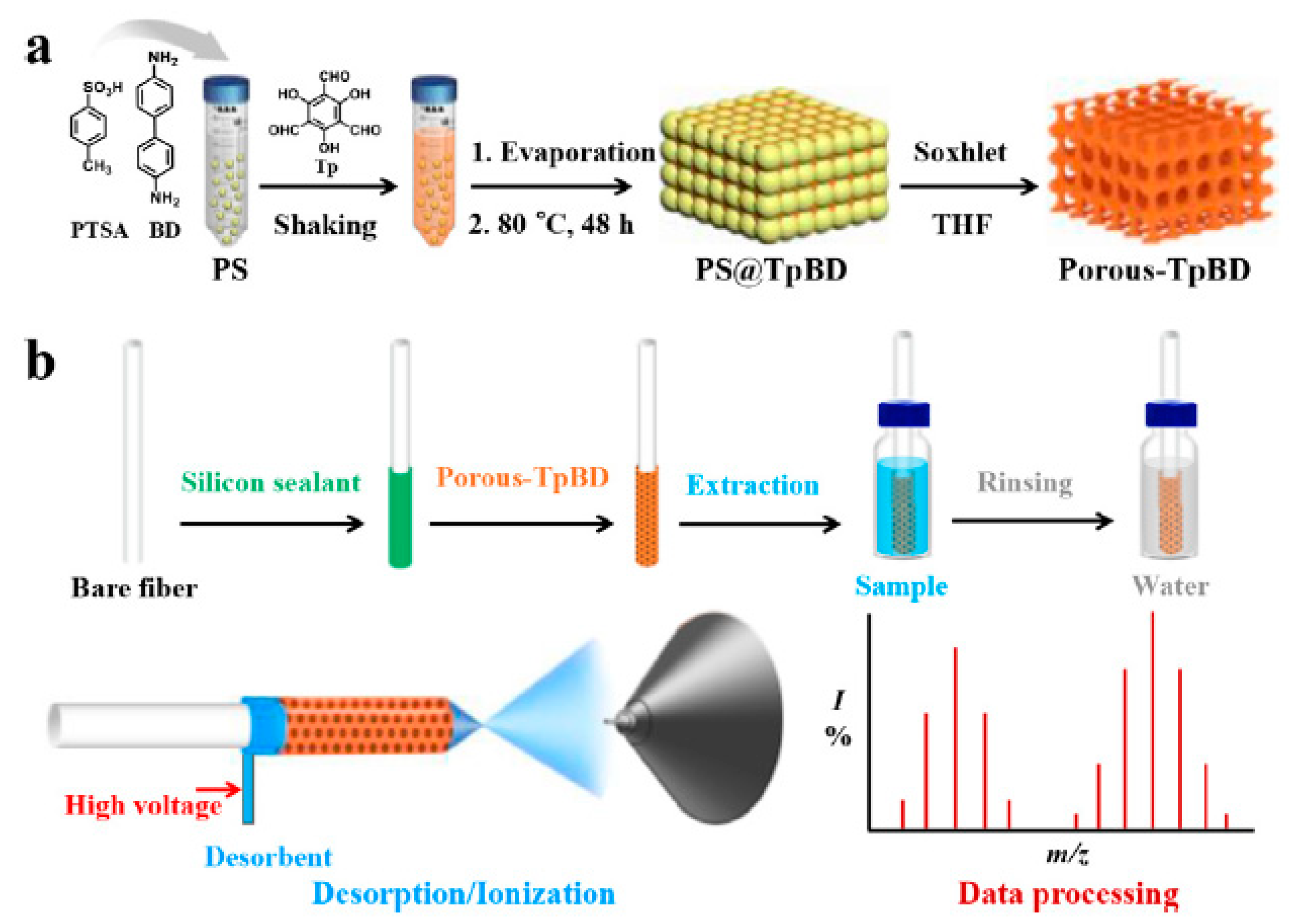
| 4 TBBPA analoges | porous-TpBD | in situ deposition | DI | river water and seawater | CFDI-MS | 0.1–1 | - | [98] |
| 6 PCBs | PAN-SiO2@TpPa | physical adhesion | HS | river, lake, and seawater | GC-ECD | 0.1–5 | 2602–5611 | [121] |
| 17 PCBs | chlorinated-TpPa-1 | in situ growth | HS | seawater, river water, and reservoir water | GC-MS | 0.0015–0.0088 | 699–4281 | [122] |
| 15 PCBs | 3D TpTAM-COF | physical adhesion | HS | river water and soil | GC-MS | 0.001–0.020 | 5308–10,305 | [99] |
| 5 OCPs | Tp-Azo-COF | physical adhesion | DI | tap and well water and beverages | GC-MS/MS | 0.002–0.08 | 1061–3693 | [123] |
| Trifluralin, chlorpyrifos | porous PTA/TAPPT COF | in situ deposition | DI | agriculture wastewater and vegetables | GC-CD-IMS | 130, 150 | 1950, 2123 | [124] |
| Triclosan, methyltriclosn | NiFe2O4@COF | physical adhesion | DI | tap water, river water, and barreled water | GC-ECD | 1–7 | 279–334 | [125] |
| 14 OCPs | COF-CN | physical adhesion | HS | river water | GC-MS/MS | 0.0010–13.54 | 540–5065 | [126] |
| Benzoylurea insecticide | COF-(CF3)2 | physical adhesion | DI | lake water, river water, pond water, wastewater and farmland water | UHPLC-MS/MS | 0.06–0.50 | 44–105 | [127] |
| 11 Pyrethroid insecticides | COFTDBA-TTL | physical adhesion | DI | river water | GC-MS | 0.170–1.68 | 2584–7199 | [128] |
| 8 PFASs | TH-COF | physical adhesion | DI | drinking water, underground water, and river water | UPLC-MS/MS | 0.0020–0.0045 | - | [129] |
| 14 PFASs | COF-NH-CO-F9 | physical adhesion | DI | tap water, river water, lake water, pond water, wastewater, and farmland water | UHPLC-MS/MS | 0.0035–0.18 | 66–160 | [130] |
| 14 PFASs | COF-F-1 | physical adhesion | DI | lake water and blood | NanoESI-MS | 0.02–0.8 | 105–4538 | [131] |
| 5 PAEs | TpTph-COF | in situ growth | DI | lake water and seawater | GC-MS/MS | 0.02–0.08 | 1140–3720 | [96] |
| 6 PAEs | porphyrin-based COF | in situ growth | EE-SPME | beverages, lake water, industrial wastewater, and oysters | GC-MS/MS | 50–2000 | 1329 (diethylhexyl phthalate) | [136] |
| 6 Synthetic musks | TpPa-1 | physical adhesion | DI | river water, tap water, and underground water | GC-MS/MS | 0.04–0.31 | 1214–12,487 | [137] |
5. Supramolecular Macrocycles
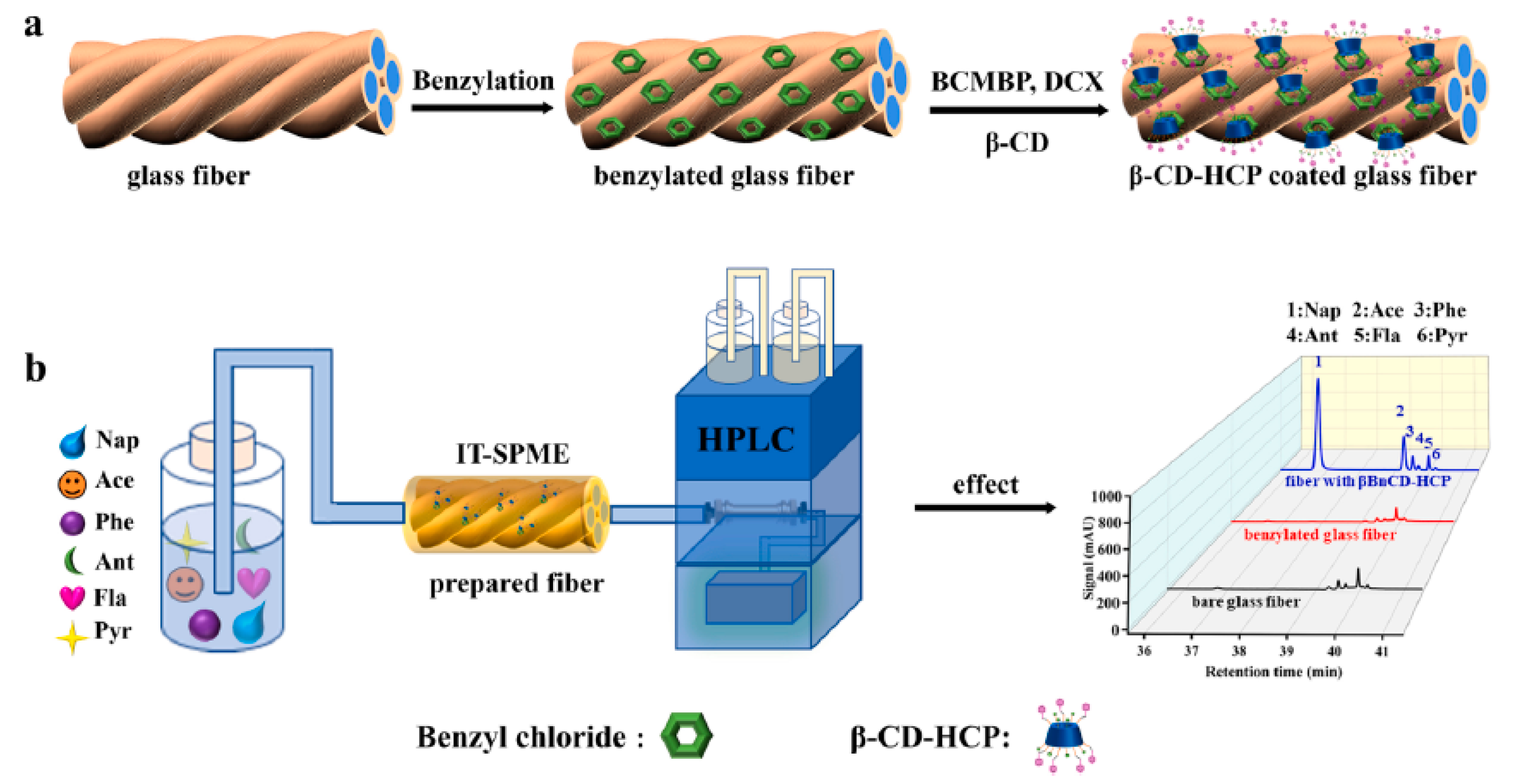
5.1. Cyclodextrins as SPME Coatings
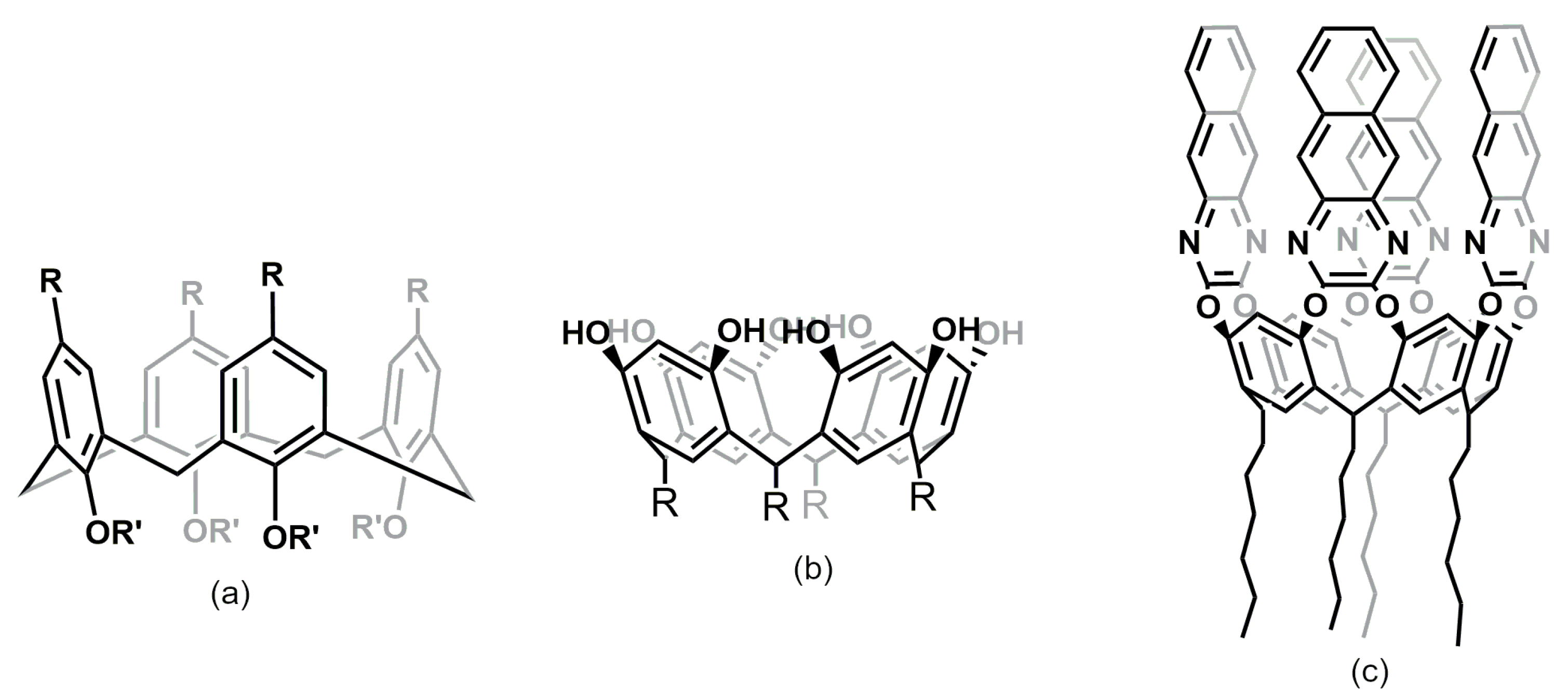
5.2. Calixarenes and Cavitands as SPME Coatings
| Analyte | Material | Deposition Method | Extraction Mode | Matrix | Platform | LOD (ng/L) | EFs | References |
|---|---|---|---|---|---|---|---|---|
| 6 PAHs | β-CD-crosslinked polymer | in situ growth | bottled water, lake water, tap water, and soil water | IT | LC-UV | 4–8 | 2130–2670 | [143] |
| 16 PAHs | γ-CD-MWCNTs-H2O2 | physical adhesion | snow | DI | GC-MS | 0.1–0.7 | 3770–113,300 | [145] |
| Fluoxetine and norfluoxetine | β-CD-MWCNTs | physical adhesion | Tap water, river water, and well water | DI | LC-UV | 300–400 | 144–151 | [144] |
| BTEX | γ-CD-MOF | physical adhesion | River water and pond water | HS | LC-MS | 0.13–0.29 | - | [146] |
| 1,2,4-trichlorobenzene, 1,2,3-trichlorobenzene, 1,2,3,4-tetrachlorobenzene | PU-PSU/Calix[4]arene nanofibers | in situ electrodeposition | Tap water, river water, sewage water, and wastewater | HS | GC-μECD | 0.1–1.0 | - | [148] |
| 16 PAHs | BenzoQxCav | physical adhesion | snow | DI | GC-MS | 0.03–0.30 | 10,260–125,500 | [150] |
6. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 2DTP/MIL-101-Cr | two-dimensional triazine structure/MIL101-Cr hybrid MOF/COF |
| ALD | atomic layer deposition |
| APTES | (3-aminopropyl) triethoxysilane |
| BD | benzidine |
| BDC | terephthalic acid |
| BenzoQxCav | tetrabenzoquinoxaline cavitand |
| BET | Brunauer–Emmett–Teller |
| BTCH-PTA-COF | hydrazone-based covalent organic framework |
| BTEX | Benzene, toluene, ethylbenzene and xylenes |
| CAR | carboxen |
| CD | cyclodextrin |
| CD-IMS | corona discharge-ion mobility spectrometry |
| COF | covalent organic framework |
| COF-CN | ultrastable crystalline quinoline-linked 2D COF |
| COF-GO | COF–graphene oxide composite material |
| COF-PIL | poly(1-vinyl-3-methylimidazolium bis ((trifluoromethyl) sulfonyl) imide) |
| CPhs | chlorophenols |
| Cu-DAT | copper-2,5-diaminoterephthalate crystals |
| CuPc-MCOF | copper-doped phthalocyanine metal covalent organic framework |
| CVD | chemical vapor deposition |
| CVR | chemical vapor reaction |
| DBA | 2,5-dimethoxybenzaldehyde |
| DETA | diethylenetriamine |
| DI | direct immersion |
| DMTP | 2,5-dimethoxyterephaldehyde |
| DVB | polydivinylbenzene |
| EE-SPME | electroenhanced-solid phase microextraction |
| EF | enrichment factor |
| g-C3N4 | graphitic carbon nitride |
| GC-FID | gas chromatography-flame ionization detection |
| GC-MS | Gas chromatography-mass spectrometry |
| GC-NCI-MS | gas chromatography-negative chemical ion-mass spectrometry |
| GC-μECD | gas chromatography-micro electron capture detector |
| HCNBs | hollow carbon nanobubbles |
| HOF | hydrogen-bonded organic framework |
| HPLC-FLD | High-performance liquid chromatography-fluorescence detection |
| HS | headspace |
| HZ-PMOF | hollow zirconium-porphyrin-based MOF |
| IARC | International Agency for Research on Cancer |
| IL | ionic liquid |
| IS-VA-HS-SPME | in-syringe vacuum-assisted headspace solid-phase microextraction |
| IT | in-tube |
| LC-UV | liquid chromatography-UV detection |
| LOD | limit of detection |
| LOQs | limits of quantitation |
| MAF | metal azolate framework |
| MC | mechanochemical synthesis |
| MIL | Matériaux de l′Institut Lavoisier |
| MIL-101-DETA-F | amino and fluorine dual-functionalized MIL-101(Cr) |
| MOF | metal organic framework |
| MS/MS | tandem mass spectrometry |
| MWCNTs | multiwalled carbon nanotubes |
| nanoESI-MS | nanoelectrospray ionization-mass spectrometry |
| NAR | titania nanorod array |
| N-CNTCs | nitrogen-doped carbon nanotube cages |
| NH2-UiO-66(Zr)-hp | Amino-functionalized UiO-66(Zr)-capped nanocrystals |
| NH2-ZIF-8 | amino-functionalized ZIF-8 |
| Ni@NiO/PCs | lotus-like Ni@NiO embedded porous carbons |
| NSZIF-8Si | ZIF-8–superhydrophobic MOF composite material |
| NTD | needle trap device |
| OCP | organochlorine pesticide |
| OPP | organophosphorus pesticide |
| PA | polyacrylate |
| Pa | p-phenylenediamine |
| PAE | phthalic acid ester |
| PAHs | polycyclic aromatic hydrocarbons |
| PAN | polyacrylonitrile |
| PAN/Ni-MOF | polyacrylonitrile/nickel-based MOF |
| PAN/UiO@UiO2-N3-aptamer | UiO seeded azide aptamer-functionalized MOF |
| PANI | polyaniline |
| PBB | polybrominated biphenyl |
| PBDE | polybrominated diphenyl ether |
| PCB | polychlorinated biphenyl |
| PDA | poly(dopamine) |
| PDMS | polydimethylsiloxane |
| PES | polyethersulfone |
| PFASs | poly- and perfluoroalkyl substances |
| PFOA | perfluorooctanoic acid |
| PIL | poly(ionic liquid)s |
| PPCPs | pharmaceutical and personal care products |
| PPy | polypyrrole |
| PPy@MIL-101(Cr) | PPy/chromium-based MOF nanocomposite |
| PU-PSU/calix[4]arene | polyurethane–polysulfone/calix[4]arene |
| RSD | relative standard deviation |
| SESI-IMS | secondary electrospray ionization-ion mobility spectrometry |
| SOM | single-crystal ordered macroporous |
| SPME | solid-phase microextraction |
| SS | stainless steel |
| TAM | (p-aminophenyl)methane |
| TAPB | 1,3,5-tris(4-aminophenyl)benzene |
| TAPB-TMC-COF | 1,3,5-tris(4-aminophenyl)benzene and trimesoyl chloride COF |
| TBBPA | tetrabromobisphenol A |
| TFPA-TAPP-COF | tris(4-formyl phenyl)amine-etra(4-aminophenyl)porphyrin COF |
| TH-COF | dioxin-linked COF |
| TMC | trimesoyl chloride |
| Tp | 1,3,5-triformylphloroglucinol |
| Tp−Azo−COF | ketoenamine COF |
| TpPa-1–1000 | 1,3,5-triformylphloroglucinol—p-phenylenediamine N-doped porous carbons |
| TpTph-COF | 1,3,5-triformylphloroglucinol—4,4′-diamino-p-terphenyl COF |
| UHPLC-MS/MS | ultra high performance liquid chromatography |
| US EPA | United States Environmental Protection Agency |
| ZIF | Zeolitic Imidazolate Frameworks |
| Zr/N-OMC | zirconium and nitrogen co-doped ordered mesoporous carbon |
| Zr-MOF@GO | zirconium-based MOF and graphene oxide coating |
| Σ | total concentration |
References
- Belardi, R.P.; Pawliszyn, J.B. The Application of Chemically Modified Fused Silica Fibers in the Extraction of Organics from Water Matrix Samples and Their Rapid Transfer to Capillary Columns. Water Qual. Res. J. 1989, 24, 179–191. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacl, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ríos, G.A.; Mirabelli, M.F. Solid Phase Microextraction-Mass Spectrometry: Metanoia. TrAC-Trends Anal. Chem. 2019, 112, 201–211. [Google Scholar] [CrossRef]
- Riboni, N.; Fornari, F.; Bianchi, F.; Careri, M. Recent Advances in in Vivo Spme Sampling. Separations 2020, 7, 6. [Google Scholar] [CrossRef]
- Zheng, J.; Kuang, Y.; Zhou, S.; Gong, X.; Ouyang, G. Latest Improvements and Expanding Applications of Solid-Phase Microextraction. Anal. Chem. 2023, 95, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Murtada, K. Trends in Nanomaterial-Based Solid-Phase Microextraction with a Focus on Environmental Applications—A Review. Trends Environ. Anal. Chem. 2020, 25, e00077. [Google Scholar] [CrossRef]
- Lashgari, M.; Yamini, Y. An Overview of the Most Common Lab-Made Coating Materials in Solid Phase Microextraction. Talanta 2019, 191, 283–306. [Google Scholar] [CrossRef] [PubMed]
- Souza-Silva, É.A.; Reyes-Garcés, N.; Gómez-Ríos, G.A.; Boyaci, E.; Bojko, B.; Pawliszyn, J. A Critical Review of the State of the Art of Solid-Phase Microextraction of Complex Matrices III. Bioanalytical and Clinical Applications. TrAC-Trends Anal. Chem. 2015, 71, 249–264. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 Principles of Green Analytical Chemistry and the SIGNIFICANCE Mnemonic of Green Analytical Practices. TrAC-Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Zhou, W.; Wieczorek, M.N.; Javanmardi, H.; Pawliszyn, J. Direct Solid-Phase Microextraction-Mass Spectrometry Facilitates Rapid Analysis and Green Analytical Chemistry. TrAC-Trends Anal. Chem. 2023, 166, 117167. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; García-Jares, C.; Dagnac, T. Environmental Applications of Solid-Phase Microextraction. TrAC Trends Anal. Chem. 2019, 112, 1–12. [Google Scholar] [CrossRef]
- Qian, H.L.; Wang, Y.; Yan, X.P. Covalent Organic Frameworks for Environmental Analysis. TrAC-Trends Anal. Chem. 2022, 147, 116516. [Google Scholar] [CrossRef]
- Xu, C.H.; Chen, G.S.; Xiong, Z.H.; Fan, Y.X.; Wang, X.C.; Liu, Y. Applications of Solid-Phase Microextraction in Food Analysis. TrAC-Trends Anal. Chem. 2016, 80, 12–29. [Google Scholar] [CrossRef]
- Hu, K.; Chen, L.; Gao, S.; Liu, W.; Wei, B.; He, Q. Application Progress of Covalent Organic Frameworks (COFs) Materials in the Detection of Food Contaminants. Food Control 2024, 155, 110072. [Google Scholar] [CrossRef]
- Leszczyńska, D.; Hallmann, A.; Treder, N.; Bączek, T.; Roszkowska, A. Recent Advances in the Use of SPME for Drug Analysis in Clinical, Toxicological, and Forensic Medicine Studies. Talanta 2024, 270, 125613. [Google Scholar] [CrossRef] [PubMed]
- El-Deen, A.K.; Hussain, C.M. Magnetic Analytical Extractions of Forensic Samples: Latest Developments and Future Perspectives. Trends Environ. Anal. Chem. 2023, 39, e00209. [Google Scholar] [CrossRef]
- Gao, Y.; Sheng, K.; Bao, T.; Wang, S. Recent Applications of Organic Molecule-Based Framework Porous Materials in Solid-Phase Microextraction for Pharmaceutical Analysis. J. Pharm. Biomed. Anal. 2022, 221, 115040. [Google Scholar] [CrossRef]
- Hu, B.; Ouyang, G. In Situ Solid Phase Microextraction Sampling of Analytes from Living Human Objects for Mass Spectrometry Analysis. TrAC-Trends Anal. Chem. 2021, 143, 116368. [Google Scholar] [CrossRef]
- Roszkowska, A.; Miękus, N.; Bączek, T. Application of Solid-Phase Microextraction in Current Biomedical Research. J. Sep. Sci. 2019, 42, 285–302. [Google Scholar] [CrossRef]
- Kou, X.; Tong, L.; Huang, S.; Chen, G.; Zhu, F.; Ouyang, G. Recent Advances of Covalent Organic Frameworks and Their Application in Sample Preparation of Biological Analysis. TrAC-Trends Anal. Chem. 2021, 136, 116182. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Alam, M.N.; Pawliszyn, J. Review of Geometries and Coating Materials in Solid Phase Microextraction: Opportunities, Limitations, and Future Perspectives. Anal. Chim. Acta 2017, 984, 42–65. [Google Scholar] [CrossRef] [PubMed]
- Delińska, K.; Rakowska, P.W.; Kloskowski, A. Porous Material-Based Sorbent Coatings in Solid-Phase Microextraction Technique: Recent Trends and Future Perspectives. TrAC-Trends Anal. Chem. 2021, 143, 116386. [Google Scholar] [CrossRef]
- Godage, N.H.; Gionfriddo, E. A Critical Outlook on Recent Developments and Applications of Matrix Compatible Coatings for Solid Phase Microextraction. TrAC-Trends Anal. Chem. 2019, 111, 220–228. [Google Scholar] [CrossRef]
- Zheng, J.; Huang, J.; Yang, Q.; Ni, C.; Xie, X.; Shi, Y.; Sun, J.; Zhu, F.; Ouyang, G. Fabrications of Novel Solid Phase Microextraction Fiber Coatings Based on New Materials for High Enrichment Capability. TrAC Trends Anal. Chem. 2018, 108, 135–153. [Google Scholar] [CrossRef]
- Peng, S.; Huang, X.; Huang, Y.; Huang, Y.; Zheng, J.; Zhu, F.; Xu, J.; Ouyang, G. Novel Solid-Phase Microextraction Fiber Coatings: A Review. J. Sep. Sci. 2022, 45, 282–304. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Huang, Y.; Ruan, Q.; Chen, C.; Liu, S.; Ouyang, G. Recent Developments on Solid Phase Microextraction (SPME) Coatings for in Vivo Analysis. Green Anal. Chem. 2023, 6, 100069. [Google Scholar] [CrossRef]
- Gong, X.; Lin, S.; Huang, X.; Peng, S.; Shen, M.; Ouyang, S.; Zheng, J.; Xu, J.; Ouyang, G. Applications of in Vivo SPME Based on Mass Spectrometry for Environmental Pollutants Analysis and Non-Target Metabolomics: A Review. Green Anal. Chem. 2022, 1, 100004. [Google Scholar] [CrossRef]
- Feng, X.; Kuang, Y.; Gan, L.; Zhou, S.; Zheng, J.; Ouyang, G. Solid Phase Microextraction for the Bioanalysis of Emerging Organic Pollutants. TrAC Trends Anal. Chem. 2024, 177, 117786. [Google Scholar] [CrossRef]
- Omarova, A.; Bakaikina, N.V.; Muratuly, A.; Kazemian, H.; Baimatova, N. A Review on Preparation Methods and Applications of Metal–Organic Framework-Based Solid-Phase Microextraction Coatings. Microchem. J. 2022, 175, 107147. [Google Scholar] [CrossRef]
- Guo, W.; Tao, H.; Tao, H.; Shuai, Q.; Huang, L. Recent Progress of Covalent Organic Frameworks as Attractive Materials for Solid-Phase Microextraction: A Review. Anal. Chim. Acta 2023, 1287, 341953. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Are Metal-Organic Frameworks Able to Provide a New Generation of Solid-Phase Microextraction Coatings?—A Review. Anal. Chim. Acta 2016, 939, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Torabi, E.; Mirzaei, M.; Bazargan, M.; Amiri, A. A Critical Review of Covalent Organic Frameworks-Based Sorbents in Extraction Methods. Anal. Chim. Acta 2022, 1224, 340207. [Google Scholar] [CrossRef]
- Choy, K.L. Chemical Vapour Deposition of Coatings. Prog. Mater. Sci. 2003, 48, 57–170. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, L.; Ding, Y.; Xiao, H.; Shi, Q.; Li, X.; Li, A.; Fang, G. Atomic Layer Deposition Meets Metal–Organic Frameworks. Prog. Mater. Sci. 2023, 138, 101159. [Google Scholar] [CrossRef]
- Bianchi, F.; Mattarozzi, M.; Careri, M.; Mangia, A.; Musci, M.; Grasselli, F.; Bussolati, S.; Basini, G. An SPME-GC-MS Method Using an Octadecyl Silica Fibre for the Determination of the Potential Angiogenesis Modulators 17β-Estradiol and 2-Methoxyestradiol in Culture Media. Anal. Bioanal. Chem. 2010, 396, 2639–2645. [Google Scholar] [CrossRef]
- Amiri, A. Solid-Phase Microextraction-Based Sol–Gel Technique. TrAC Trends Anal. Chem. 2016, 75, 57–74. [Google Scholar] [CrossRef]
- Aziz-Zanjani, M.O.; Mehdinia, A. Electrochemically Prepared Solid-Phase Microextraction Coatings-A Review. Anal. Chim. Acta 2013, 781, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yan, S.; Yan, X.; Lv, Y. Recent Advances in Metal-Organic Frameworks: Synthesis, Application and Toxicity. Sci. Total Environ. 2023, 902, 165944. [Google Scholar] [CrossRef] [PubMed]
- Głowniak, S.; Szczęśniak, B.; Choma, J.; Jaroniec, M. Mechanochemistry: Toward Green Synthesis of Metal–Organic Frameworks. Mater. Today 2021, 46, 109–124. [Google Scholar] [CrossRef]
- Cui, X.Y.; Gu, Z.Y.; Jiang, D.Q.; Li, Y.; Wang, H.F.; Yan, X.P. In Situ Hydrothermal Growth of Metal-Organic Framework 199 Films on Stainless Steel Fibers for Solid-Phase Microextraction of Gaseous Benzene Homologues. Anal. Chem. 2009, 81, 9771–9777. [Google Scholar] [CrossRef]
- Jiang, H.; Li, J.; Hu, X.; Shen, J.; Sun, X.; Han, W.; Wang, L. Ordered Mesoporous Silica Film as a Novel Fiber Coating for Solid-Phase Microextraction. Talanta 2017, 174, 307–313. [Google Scholar] [CrossRef]
- Shamsipur, M.; Gholivand, M.B.; Shamizadeh, M.; Hashemi, P. Preparation and Evaluation of a Novel Solid-Phase Microextraction Fiber Based on Functionalized Nanoporous Silica Coating for Extraction of Polycyclic Aromatic Hydrocarbons From Water Samples Followed by GC–MS Detection. Chromatographia 2015, 78, 795–803. [Google Scholar] [CrossRef]
- Shirey, R.E. SPME Commercial Devices and Fibre Coatings. In Handbook of Solid Phase Microextraction; Pawliszyn, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 99–133. ISBN 9780124160170. [Google Scholar]
- Liu, X.; Qian, B.; Zhang, D.; Yu, M.; Chang, Z.; Bu, X. Recent Progress in Host–Guest Metal–Organic Frameworks: Construction and Emergent Properties. Coord. Chem. Rev. 2023, 476, 214921. [Google Scholar] [CrossRef]
- Trzciński, J.W.; Pinalli, R.; Riboni, N.; Pedrini, A.; Bianchi, F.; Zampolli, S.; Elmi, I.; Massera, C.; Ugozzoli, F.; Dalcanale, E. In Search of the Ultimate Benzene Sensor: The EtQxBox Solution. ACS Sens. 2017, 2, 590–598. [Google Scholar] [CrossRef]
- Riboni, N.; Trzcinski, J.W.; Bianchi, F.; Massera, C.; Pinalli, R.; Sidisky, L.; Dalcanale, E.; Careri, M. Conformationally Blocked Quinoxaline Cavitand as Solid-Phase Microextraction Coating for the Selective Detection of BTEX in Air. Anal. Chim. Acta 2016, 905, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.; Gu, Z.Y.; Wang, H.F.; Yan, X.P. Metal-Organic-Framework-Based Tandem Molecular Sieves as a Dual Platform for Selective Microextraction and High-Resolution Gas Chromatographic Separation of n -Alkanes in Complex Matrixes. Anal. Chem. 2011, 83, 7094–7101. [Google Scholar] [CrossRef] [PubMed]
- Maya, F.; Ghani, M. Ordered Macro/Micro-Porous Metal-Organic Framework of Type ZIF-8 in a Steel Fiber as a Sorbent for Solid-Phase Microextraction of BTEX. Microchim. Acta 2019, 186, 425. [Google Scholar] [CrossRef]
- Wei, S.; Kou, X.; Liu, Y.; Zhu, F.; Xu, J.; Ouyang, G. Facile Construction of Superhydrophobic Hybrids of Metal-Organic Framework Grown on Nanosheet for High-Performance Extraction of Benzene Homologues. Talanta 2020, 211, 120706. [Google Scholar] [CrossRef]
- Hu, Q.; Liu, S.; Chen, X.; Xu, J.; Zhu, F.; Ouyang, G. Enhancing Enrichment Ability of a Nanoporous Carbon Based Solid-Phase Microextraction Device by a Morphological Modulation Strategy. Anal. Chim. Acta 2019, 1047, 1–8. [Google Scholar] [CrossRef]
- Kardani, F.; Mirzajani, R. Electrospun Polyacrylonitrile/MIL-53(Al) MOF@ SBA-15/4, 4′-Bipyridine Nanofibers for Headspace Solid-Phase Microextraction of Benzene Homologues in Environmental Water Samples with GC-FID Detection. Microchem. J. 2022, 180, 107591. [Google Scholar] [CrossRef]
- Omarova, A.; Baimatova, N.; Kazemian, H. MOF-199-Based Coatings as SPME Fiber for Measurement of Volatile Organic Compounds in Air Samples: Optimization of in Situ Deposition Parameters. Microchem. J. 2023, 185, 108263. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Priority Pollutant List; United States Environmental Protection Agency: Washington, DC, USA, 2014.
- Kong, J.; Zhu, F.; Huang, W.; He, H.; Hu, J.; Sun, C.; Xian, Q.; Yang, S. Sol–Gel Based Metal-Organic Framework Zeolite Imidazolate Framework-8 Fibers for Solid-Phase Microextraction of Nitro Polycyclic Aromatic Hydrocarbons and Polycyclic Aromatic Hydrocarbons in Water Samples. J. Chromatogr. A 2019, 1603, 92–101. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Gutiérrez-Serpa, A.; Cruz, A.J.; Ameloot, R.; Ayala, J.H.; Afonso, A.M.; Pasán, J.; Rodríguez-Hermida, S.; Pino, V. Solid-Phase Microextraction Coatings Based on the Metal-Organic Framework ZIF-8: Ensuring Stable and Reusable Fibers. Talanta 2020, 215, 120910. [Google Scholar] [CrossRef]
- Lian, C.; Feng, X.; Tian, M.; Tian, Y.; Zhang, Y. Electrodeposition of Zeolitic Imidazolate Framework Coating on Stainless Steel Wire for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons in Water Samples. Microchem. J. 2022, 175, 107146. [Google Scholar] [CrossRef]
- Zeng, J.; Li, Y.; Zheng, X.; Li, Z.; Zeng, T.; Duan, W.; Li, Q.; Shang, X.; Dong, B. Controllable Transformation of Aligned ZnO Nanorods to ZIF-8 as Solid-Phase Microextraction Coatings with Tunable Porosity, Polarity, and Conductivity. Anal. Chem. 2019, 91, 5091–5097. [Google Scholar] [CrossRef]
- Du, J.; Zhang, R.; Wang, F.; Wang, X.; Du, X. Template-Directed Fabrication of Zeolitic Imidazolate Framework-67-Derived Coating Materials on Nickel/Titanium Alloy Fiber Substrate for Selective Solid-Phase Microextraction. J. Chromatogr. A 2020, 1618, 460855. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Guo, C.; Li, Y. Metal Azolate Framework-66-Coated Fiber for Headspace Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. J. Chromatogr. A 2019, 1584, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Du, T.; Kou, H.; Du, X.; Lu, X. Zn(II)-Imidazole Derived Metal Azolate Framework as an Effective Adsorbent for Double Coated Solid-Phase Microextraction of Sixteen Polycyclic Aromatic Hydrocarbons. Talanta 2020, 214, 120866. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Huang, L.; Xiao, H.; Shuai, Q.; Hu, S. In Situ Self-Transformation Metal into Metal-Organic Framework Membrane for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons. Talanta 2019, 202, 145–151. [Google Scholar] [CrossRef]
- Bianchi, F.; Pankajakshan, A.; Fornari, F.; Mandal, S.; Pelagatti, P.; Bacchi, A.; Mazzeo, P.P.; Careri, M. A Zinc Mixed-Ligand Microporous Metal-Organic Framework as Solid-Phase Microextraction Coating for Priority Polycyclic Aromatic Hydrocarbons from Water Samples. Microchem. J. 2020, 154, 104646. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, T.; Wang, F.; Zhu, F.; Ouyang, G. Sheathed in Situ Heteroepitaxial Growth Metal-Organic Framework Probe for Detection of Polycyclic Aromatic Hydrocarbons in River Water and Living Fish. Sci. Total Environ. 2020, 729, 138971. [Google Scholar] [CrossRef] [PubMed]
- Campanale, C.; Massarelli, C.; Losacco, D.; Bisaccia, D.; Triozzi, M.; Uricchio, V.F. The Monitoring of Pesticides in Water Matrices and the Analytical Criticalities: A Review. TrAC-Trends Anal. Chem. 2021, 144, 116423. [Google Scholar] [CrossRef]
- Amini, S.; Ebrahimzadeh, H.; Seidi, S.; Jalilian, N. Preparation of Polyacrylonitrile/Ni-MOF Electrospun Nanofiber as an Efficient Fiber Coating Material for Headspace Solid-Phase Microextraction of Diazinon and Chlorpyrifos Followed by CD-IMS Analysis. Food Chem. 2021, 350, 129242. [Google Scholar] [CrossRef]
- Mohammadi, V.; Jafari, M.T.; Saraji, M. Flexible/Self-Supported Zeolitic Imidazolate Framework-67 Film as an Adsorbent for Thin-Film Microextraction. Microchem. J. 2019, 146, 98–105. [Google Scholar] [CrossRef]
- Gong, X.; Xu, L.; Huang, S.; Kou, X.; Lin, S.; Chen, G.; Ouyang, G. Application of the NU-1000 Coated SPME Fiber on Analysis of Trace Organochlorine Pesticides in Water. Anal. Chim. Acta 2022, 1218, 339982. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Dong, P.; Liu, H.; Li, H.; Chen, C.; Feng, S.; Fan, J. Lotus-like Ni@NiO Nanoparticles Embedded Porous Carbon Derived from MOF-74/Cellulose Nanocrystal Hybrids as Solid Phase Microextraction Coating for Ultrasensitive Determination of Chlorobenzenes from Water. J. Hazard. Mater. 2022, 429, 128384. [Google Scholar] [CrossRef]
- Zarębska, M.; Bajkacz, S. Poly– and Perfluoroalkyl Substances (PFAS)—Recent Advances in the Aquatic Environment Analysis. TrAC Trends Anal. Chem. 2023, 163, 117062. [Google Scholar] [CrossRef]
- Suwannakot, P.; Lisi, F.; Ahmed, E.; Liang, K.; Babarao, R.; Gooding, J.J.; Donald, W.A. Metal-Organic Framework-Enhanced Solid-Phase Microextraction Mass Spectrometry for the Direct and Rapid Detection of Perfluorooctanoic Acid in Environmental Water Samples. Anal. Chem. 2020, 92, 6900–6908. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Xu, L.; Kou, X.; Zheng, J.; Kuang, Y.; Zhou, S.; Huang, S.; Zheng, Y.; Ke, W.; Chen, G.; et al. Amino-Functionalized Metal–Organic Frameworks for Efficient Solid-Phase Microextraction of Perfluoroalkyl Acids in Environmental Water. Microchem. J. 2022, 179, 107661. [Google Scholar] [CrossRef]
- Ouyang, S.; Liu, G.; Peng, S.; Zheng, J.; Ye, Y.X.; Zheng, J.; Tong, Y.; Hu, Y.; Zhou, N.; Gong, X.; et al. Superficially Capped Amino Metal-Organic Framework for Efficient Solid-Phase Microextraction of Perfluorinated Alkyl Substances. J. Chromatogr. A 2022, 1669, 462959. [Google Scholar] [CrossRef]
- Jia, Y.; Qian, J.; Pan, B. Dual-Functionalized MIL-101(Cr) for the Selective Enrichment and Ultrasensitive Analysis of Trace Per- And Poly-Fluoroalkyl Substances. Anal. Chem. 2021, 93, 11116–11122. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Adhikary, S.; Bhattacharya, S.; Dutta, S.; Chatterjee, S.; Banerjee, D.; Ganguly, A.; Rajak, P. Pharmaceuticals and Personal Care Products as Emerging Environmental Contaminants: Prevalence, Toxicity, and Remedial Approaches. ACS Chem. Health Saf. 2023, 30, 362–388. [Google Scholar] [CrossRef]
- Liu, H.; Fan, H.; Dang, S.; Li, M.; A, G.; Yu, H. A Zr-MOF@GO-Coated Fiber with High Specific Surface Areas for Efficient, Green, Long-Life Solid-Phase Microextraction of Nonsteroidal Anti-Inflammatory Drugs in Water. Chromatographia 2020, 83, 1065–1073. [Google Scholar] [CrossRef]
- Khodayari, P.; Jalilian, N.; Ebrahimzadeh, H.; Amini, S. Trace-Level Monitoring of Anti-Cancer Drug Residues in Wastewater and Biological Samples by Thin-Film Solid-Phase Micro-Extraction Using Electrospun Polyfam/Co-MOF-74 Composite Nanofibers Prior to Liquid Chromatography Analysis. J. Chromatogr. A 2021, 1655, 462484. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, R.; Li, W.; Muir, D.C.G.; Zeng, E.Y. Development of a Solid-Phase Microextraction Method for Fast Analysis of Cyclic Volatile Methylsiloxanes in Water. Chemosphere 2020, 250, 126304. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, P.; Pacheco-Fernández, I.; Bernardo, F.; Homem, V.; Pasán, J.; Ayala, J.H.; Ratola, N.; Pino, V. Headspace Solid-Phase Microextraction Based on the Metal-Organic Framework CIM-80(Al) Coating to Determine Volatile Methylsiloxanes and Musk Fragrances in Water Samples Using Gas Chromatography and Mass Spectrometry. Talanta 2021, 232, 122440. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liu, H.; Tong, Y.; Xu, L.; Ye, Y.X.; Wen, C.; Zhou, N.; Xu, J.; Ouyang, G. Headspace Solid-Phase Microextraction of Semi-Volatile Ultraviolet Filters Based on a Superhydrophobic Metal-Organic Framework Stable in High-Temperature Steam. Talanta 2020, 219, 121175. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Liao, Y.; Huang, X.; Ye, Z.; Yuan, D. Metal-Organic Framework-Monolith Composite-Based in-Tube Solid Phase Microextraction on-Line Coupled to High-Performance Liquid Chromatography-Fluorescence Detection for the Highly Sensitive Monitoring of Fluoroquinolones in Water and Food Samples. Talanta 2019, 199, 499–506. [Google Scholar] [CrossRef]
- Jalili, V.; Ghanbari Kakavandi, M.; Ghiasvand, A.; Barkhordari, A. Microextraction Techniques for Sampling and Determination of Polychlorinated Biphenyls: A Comprehensive Review. Microchem. J. 2022, 179, 107442. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, C.; Feng, Z.; Chen, H.; Tong, P.; Wu, X.; Zhang, L. Metal-Organic Framework-Coated Stainless Steel Fiber for Solid-Phase Microextraction of Polychlorinated Biphenyls. J. Chromatogr. A 2018, 1570, 10–18. [Google Scholar] [CrossRef]
- Guo, Y.; He, X.; Huang, C.; Chen, H.; Lu, Q.; Zhang, L. Metal–Organic Framework-Derived Nitrogen-Doped Carbon Nanotube Cages as Efficient Adsorbents for Solid-Phase Microextraction of Polychlorinated Biphenyls. Anal. Chim. Acta 2020, 1095, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, S.; Liu, Y.; Wei, S.; Chen, G.; Gong, Z.; Ouyang, G. Hollow Carbon Nanobubbles-Coated Solid-Phase Microextraction Fibers for the Sensitive Detection of Organic Pollutants. Anal. Chim. Acta 2020, 1097, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, J.; Li, Q.; Wu, Y.; Liu, Y.; Ding, Q.; Chen, H.; Zhang, W.; Zhang, L. Hollow Zirconium-Porphyrin-Based Metal-Organic Framework for Efficient Solid-Phase Microextraction of Naphthols. Anal. Chim. Acta 2022, 1200, 339586. [Google Scholar] [CrossRef] [PubMed]
- Darabi, J.; Ghiasvand, A. Chromium-Based Polypyrrole/MIL-101 Nanocomposite as an Effective Sorbent for Headspace Microextraction of Methyl Tert-Butyl Ether in Soil Samples. Molecules 2020, 25, 644. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Careri, M.; Marengo, E.; Musci, M. Use of Experimental Design for the Purge-and-Trap-Gas Chromatography-Mass Spectrometry Determination of Methyl Tert.-Butyl Ether, Tert.-Butyl Alcohol and BTEX in Groundwater at Trace Level. J. Chromatogr. A 2002, 975, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; He, Y.; Qu, X.; Xu, Z.; Zheng, S.; Zhu, D.; Fu, H. In Situ Fabricated Porous Carbon Coating Derived from Metal-Organic Frameworks for Highly Selective Solid-Phase Microextraction. Anal. Chim. Acta 2019, 1078, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Huang, J.; Xie, X.; Shi, Y.; Zheng, J.; Ouyang, G. Simple Fabrication of Zirconium and Nitrogen Co-Doped Ordered Mesoporous Carbon for Enhanced Adsorption Performance towards Polar Pollutants. Anal. Chim. Acta 2019, 1070, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Mu, M.; Qin, M.; Zhu, J.; Tian, X.; Lou, X.; Zhou, Q.; Lu, M. Confinement Effect of Ionic Liquid: Improve of the Extraction Performance of Parent Metal Organic Framework for Phthalates. J. Chromatogr. A 2023, 1703, 464101. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; She, Z.; Lin, C.; Lin, X.; Xie, Z. Aptamer-Functionalized Metal-Organic Framework-Coated Nanofibers with Multi-Affinity Sites for Highly Sensitive, Selective Recognition of Ultra-Trace Microcystin-LR. Talanta 2022, 236, 122880. [Google Scholar] [CrossRef]
- Cóté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Feng, J.; Feng, J.; Ji, X.; Li, C.; Han, S.; Sun, H.; Sun, M. Recent Advances of Covalent Organic Frameworks for Solid-Phase Microextraction. TrAC-Trends Anal. Chem. 2021, 137, 116208. [Google Scholar] [CrossRef]
- Chegeni, S.; Nouriasl, K.; Ghiasvand, A. A Novel Needle Trap Device Containing an Imine-Based Covalent Organic Framework for Sampling of PAHs in Soil. Microchem. J. 2023, 193, 109034. [Google Scholar] [CrossRef]
- Jagirani, M.S.; Gumus, Z.P.; Soylak, M. Covalent Organic Frameworks, a Renewable and Emergent Source for the Separation and Pre-Concentration of the Traces of Targeted Species. Microchem. J. 2023, 191, 108820. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, Y.; Zhang, W.; Ma, W.; Wang, J.; Chen, H.; Ding, Q.; Zhang, L. Rapidly Covalent Immobilization of β-Ketoenamine-Linked Covalent Organic Framework on Fibers for Efficient Solid-Phase Microextraction of Phthalic Acid Esters. Talanta 2022, 243, 123380. [Google Scholar] [CrossRef]
- Gao, W.; Cheng, J.; Yuan, X.; Tian, Y. Covalent Organic Framework-Graphene Oxide Composite: A Superior Adsorption Material for Solid Phase Microextraction of Bisphenol A. Talanta 2021, 222, 121501. [Google Scholar] [CrossRef]
- Gao, W.; Li, M.; Fa, Y.; Zhao, Z.; Cai, Y.; Liang, X.; Yu, Y.; Jiang, G. Porous Covalent Organic Frameworks-Improved Solid Phase Microextraction Ambient Mass Spectrometry for Ultrasensitive Analysis of Tetrabromobisphenol-A Analogs. Chin. Chem. Lett. 2022, 33, 3849–3852. [Google Scholar] [CrossRef]
- Lu, F.; Wu, M.; Lin, C.; Lin, X.; Xie, Z. Efficient and Selective Solid-Phase Microextraction of Polychlorinated Biphenyls by Using a Three-Dimensional Covalent Organic Framework as Functional Coating. J. Chromatogr. A 2022, 1681, 463419. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Shao, Y.; Yue, Z.; Hu, Q.; Zheng, J.; Yuan, H.; Yu, A.; Zhang, W.; Zhang, S.; Ouyang, G. Sheathed In-Situ Room-Temperature Growth Covalent Organic Framework Solid-Phase Microextraction Fiber for Detecting Ultratrace Polybrominated Diphenyl Ethers from Environmental Samples. Anal. Chim. Acta 2021, 1176, 338772. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Deng, X.; Yang, N.; Qin, M.; Zhang, X.; Yao, X.; An, C.; Zhou, P.; Lu, X. A Durable Hydrophobicity Hydrazone Covalent Organic Framework Coating for Solid Phase Microextraction of Polycyclic Aromatic Hydrocarbons in Food and Environmental Sample. Chem. Eng. J. 2024, 481, 148562. [Google Scholar] [CrossRef]
- Yan, Q.; Huang, L.; Mao, N.; Shuai, Q. Covalent Organic Framework Derived Porous Carbon as Effective Coating for Solid Phase Microextraction of Polycyclic Aromatic Hydrocarbons Prior to Gas-Chromatography Mass Spectrometry Analysis. Talanta Open 2021, 4, 100060. [Google Scholar] [CrossRef]
- Zang, X.; Pang, Y.; Li, H.; Chang, Q.; Zhang, S.; Wang, C.; Wang, Z. Solid Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Water Samples by a Fiber Coated with Covalent Organic Framework Modified Graphitic Carbon Nitride. J. Chromatogr. A 2020, 1628, 461428. [Google Scholar] [CrossRef]
- Sun, M.; Feng, J.; Feng, J.; Sun, H.; Feng, Y.; Ji, X.; Li, C.; Han, S.; Sun, M. Biochar Nanosphere- and Covalent Organic Framework Nanosphere-Functionalized Titanium Dioxide Nanorod Arrays on Carbon Fibers for Solid-Phase Microextraction of Organic Pollutants. Chem. Eng. J. 2022, 433, 133645. [Google Scholar] [CrossRef]
- Tian, Y.; Hou, Y.; Yu, Q.; Wang, X.; Tian, M. Layer-by-Layer Self-Assembly of a Novel Covalent Organic Frameworks Microextraction Coating for Analyzing Polycyclic Aromatic Hydrocarbons from Aqueous Solutions via Gas Chromatography. J. Sep. Sci. 2020, 43, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Wu, F.; Luo, X.; Zhang, J. Porphyrin-Based Covalent Organic Framework Coated Stainless Steel Fiber for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons in Water and Soil Samples. Microchem. J. 2021, 168, 106364. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Wang, W.; Zhang, S.; Wang, C.; Zhou, J.; Wang, Z. Solid Phase Microextraction of Polycyclic Aromatic Hydrocarbons by Using an Etched Stainless-Steel Fiber Coated with a Covalent Organic Framework. Microchim. Acta 2019, 186, 145. [Google Scholar] [CrossRef]
- Koonani, S.; Ghiasvand, A. A Highly Porous Fiber Coating Based on a Zn-MOF/COF Hybrid Material for Solid-Phase Microextraction of PAHs in Soil. Talanta 2024, 267, 125236. [Google Scholar] [CrossRef] [PubMed]
- Nouriasl, K.; Ghiasvand, A. A Copper-Based MOF/COF Hybrid as an Innovative Fiber Coating for SPME Sampling of Polycyclic Aromatic Hydrocarbons from Environmental Matrices. Talanta Open 2023, 8, 100262. [Google Scholar] [CrossRef]
- Maleki, S.; Hashemi, P.; Adeli, M. A Simple and Portable Vacuum Assisted Headspace Solid Phase Microextraction Device Coupled to Gas Chromatography Based on Covalent Organic Framework/Metal Organic Framework Hybrid for Simultaneous Analysis of Volatile and Semi-Volatile Compounds in Soil. J. Chromatogr. A 2023, 1705, 464195. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, M.; Shen, X.; Zhu, H.; Li, B. The Preparation of Covalent Bonding COF-TpBD Coating in Arrayed Nanopores of Stainless Steel Fiber for Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons in Water. Int. J. Environ. Res. Public Health 2023, 20, 1393. [Google Scholar] [CrossRef]
- Feng, J.; Feng, J.; Han, S.; Ji, X.; Li, C.; Sun, M. Triazine-Based Covalent Porous Organic Polymer for the Online in-Tube Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons Prior to High-Performance Liquid Chromatography-Diode Array Detection. J. Chromatogr. A 2021, 1641, 462004. [Google Scholar] [CrossRef]
- Liu, L.; Meng, W.K.; Li, L.; Xu, G.J.; Wang, X.; Chen, L.Z.; Wang, M.L.; Lin, J.M.; Zhao, R.S. Facile Room-Temperature Synthesis of a Spherical Mesoporous Covalent Organic Framework for Ultrasensitive Solid-Phase Microextraction of Phenols Prior to Gas Chromatography-Tandem Mass Spectrometry. Chem. Eng. J. 2019, 369, 920–927. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Chen, H.; Ding, Q.; Xu, J.; Yu, Q.; Fang, M.; Zhang, L. Piperazine-Linked Metal Covalent Organic Framework-Coated Fibers for Efficient Electro-Enhanced Solid-Phase Microextraction of Chlorophenols. J. Chromatogr. A 2023, 1692, 463847. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Tao, H.; Shuai, Q.; Huang, L. Architectural Engineering Inspired in Situ Growth of Covalent Organic Frameworks as Outstanding Fiber Coating for Solid-Phase Microextraction of Phenols. Microchem. J. 2023, 189, 108564. [Google Scholar] [CrossRef]
- Liu, L.; Meng, W.K.; Zhou, Y.S.; Wang, X.; Xu, G.J.; Wang, M.L.; Lin, J.M.; Zhao, R.S. Β-Ketoenamine-Linked Covalent Organic Framework Coating for Ultra-High-Performance Solid-Phase Microextraction of Polybrominated Diphenyl Ethers from Environmental Samples. Chem. Eng. J. 2019, 356, 926–933. [Google Scholar] [CrossRef]
- Su, L.; Zheng, X.; Tang, J.; Wang, Q.; Zhang, L.; Wu, X. Poly(Ionic Liquid)s Threaded into Covalent Organic Framework for Synergistic Capture of Polybrominated Diphenyl Ethers. J. Hazard. Mater. 2024, 461, 132657. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Kuang, Y.; Shi, Y.; Hu, Y.; Chen, L.; Zheng, J.; Ouyang, G. Modulated Covalent Organic Frameworks with Higher Specific Surface Area for the Ultrasensitive Detection of Polybrominated Biphenyls. Chem. Eng. J. 2023, 453, 139743. [Google Scholar] [CrossRef]
- Gao, W.; Tian, Y.; Liu, H.; Cai, Y.; Liu, A.; Yu, Y.L.; Zhao, Z.; Jiang, G. Ultrasensitive Determination of Tetrabromobisphenol A by Covalent Organic Framework Based Solid Phase Microextraction Coupled with Constant Flow Desorption Ionization Mass Spectrometry. Anal. Chem. 2019, 91, 772–775. [Google Scholar] [CrossRef]
- Gao, W.; Li, G.; Liu, H.; Tian, Y.; Li, W.T.; Fa, Y.; Cai, Y.; Zhao, Z.; Yu, Y.L.; Qu, G.; et al. Covalent Organic Frameworks with Tunable Pore Sizes Enhanced Solid-Phase Microextraction Direct Ionization Mass Spectrometry for Ultrasensitive and Rapid Analysis of Tetrabromobisphenol A Derivatives. Sci. Total Environ. 2021, 764, 144388. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Ma, J.; Yu, Z.; Liu, S.; Yang, G.; Liu, Y.; Lin, C.; Ye, X.; Shi, Y.; Liu, M. Fabrication of Covalent Organic Frameworks Modified Nanofibrous Membrane for Efficiently Enriching and Detecting the Trace Polychlorinated Biphenyls in Water. Water Res. 2023, 235, 119892. [Google Scholar] [CrossRef]
- Su, L.; Zhang, N.; Tang, J.; Zhang, L.; Wu, X. In-Situ Fabrication of a Chlorine-Functionalized Covalent Organic Framework Coating for Solid-Phase Microextraction of Polychlorinated Biphenyls in Surface Water. Anal. Chim. Acta 2021, 1186, 339120. [Google Scholar] [CrossRef]
- Xin, J.; Xu, G.; Zhou, Y.; Wang, X.; Wang, M.; Lian, Y.; Zhao, R.S. Ketoenamine Covalent Organic Framework Coating for Efficient Solid-Phase Microextraction of Trace Organochlorine Pesticides. J. Agric. Food Chem. 2021, 69, 8008–8016. [Google Scholar] [CrossRef] [PubMed]
- Tabibi, A.; Jafari, M.T. High Efficient Solid-Phase Microextraction Based on a Covalent Organic Framework for Determination of Trifluralin and Chlorpyrifos in Water and Food Samples by GC-CD-IMS. Food Chem. 2022, 373, 131527. [Google Scholar] [CrossRef]
- Li, Y.; Dong, G.; Li, J.; Xiang, J.; Yuan, J.; Wang, H.; Wang, X. A Solid-Phase Microextraction Fiber Coating Based on Magnetic Covalent Organic Framework for Highly Efficient Extraction of Triclosan and Methyltriclosan in Environmental Water and Human Urine Samples. Ecotoxicol. Environ. Saf. 2021, 219, 112319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y.; Jia, X.; Zhang, Q.; Mao, J.; Feng, Y.; Yin, D.; Zhao, W.; Zhang, Y.; Ouyang, G.; et al. An Ultrastable 2D Covalent Organic Framework Coating for Headspace Solid-Phase Microextraction of Organochlorine Pesticides in Environmental Water. J. Hazard. Mater. 2023, 452, 131228. [Google Scholar] [CrossRef]
- Song, C.; Luo, Y.; Zheng, J.; Zhang, W.; Yu, A.; Zhang, S.; Ouyang, G. Facile and Large-Scale Synthesis of Trifluoromethyl-Grafted Covalent Organic Framework for Efficient Microextraction and Ultrasensitive Determination of Benzoylurea Insecticides. Chem. Eng. J. 2023, 462, 142220. [Google Scholar] [CrossRef]
- Han, J.; Yu, Y.; Wen, H.; Chen, T.; Chen, Y.; Chen, G.; Qiu, J.; Zhu, F.; Ouyang, G. Sea-Urchin-like Covalent Organic Framework as Solid-Phase Microextraction Fiber Coating for Sensitive Detection of Trace Pyrethroid Insecticides in Water. Sci. Total Environ. 2024, 912, 169129. [Google Scholar] [CrossRef]
- Ji, W.; Guo, Y.S.; Xie, H.M.; Wang, X.; Jiang, X.; Guo, D.S. Rapid Microwave Synthesis of Dioxin-Linked Covalent Organic Framework for Efficient Micro-Extraction of Perfluorinated Alkyl Substances from Water. J. Hazard. Mater. 2020, 397, 122793. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Zheng, J.; Zhang, Q.; Yuan, H.; Yu, A.; Zhang, W.; Zhang, S.; Ouyang, G. Multifunctionalized Covalent Organic Frameworks for Broad-Spectrum Extraction and Ultrasensitive Analysis of Per- and Polyfluoroalkyl Substances. Anal. Chem. 2023, 95, 7770–7778. [Google Scholar] [CrossRef]
- Hou, Y.J.; Deng, J.; He, K.; Chen, C.; Yang, Y. Covalent Organic Frameworks-Based Solid-Phase Microextraction Probe for Rapid and Ultrasensitive Analysis of Trace Per- A Nd Polyfluoroalkyl Substances Using Mass Spectrometry. Anal. Chem. 2020, 92, 10213–10217. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Kuang, Y.; Zhou, S.; Chen, L.; Zhou, N.; Zheng, J.; Ouyang, G. Melamine-Participant Hydrogen-Bonded Organic Frameworks with Strong Hydrogen Bonds and Hierarchical Micropores Driving Extraction of Nitroaromatic Compounds. Anal. Chim. Acta 2023, 1277, 341652. [Google Scholar] [CrossRef]
- Śmiełowska, M.; Zabiegała, B. Current Trends in Analytical Strategies for the Determination of Polybrominated Diphenyl Ethers (PBDEs) in Samples with Different Matrix Compositions–Part 2: New Approaches to PBDEs Determination. TrAC-Trends Anal. Chem. 2020, 132, 115889. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Yusoff, A.R.b.M.; Wirzal, M.D.H.; Barek, J.; Afridi, H.I.; Üstündag, Z. Methods for the Determination of Endocrine-Disrupting Phthalate Esters. Crit. Rev. Anal. Chem. 2016, 46, 146–159. [Google Scholar] [CrossRef]
- Lorre, E.; Riboni, N.; Bianchi, F.; Orlandini, S.; Furlanetto, S.; Careri, M.; Zilius, M. Quality by Design in the Optimization of the Ultrasonic Assisted Solvent Extraction for the GC-MS Determination of Plasticizers in Sediments and Shells. Talanta Open 2023, 8, 100258. [Google Scholar] [CrossRef]
- Chen, H.; Wang, J.; Zhang, W.; Guo, Y.; Ding, Q.; Zhang, L. In Situ Rapid Electrochemical Fabrication of Porphyrin-Based Covalent Organic Frameworks: Novel Fibers for Electro-Enhanced Solid-Phase Microextraction. ACS Appl. Mater. Interfaces 2023, 15, 12453–12461. [Google Scholar] [CrossRef]
- Wen, L.; Wu, P.; Wang, L.-L.; Chen, L.-Z.; Wang, M.-L.; Wang, X.; Lin, J.-M.; Zhao, R.-S. Solid-Phase Microextraction Using a β-Ketoenamine-Linked Covalent Organic Framework Coating for Efficient Enrichment of Synthetic Musks in Water Samples. Anal. Methods 2020, 12, 2434–2442. [Google Scholar] [CrossRef]
- Fang, Y.; Zhou, F.; Zhang, Q.; Deng, C.; Wu, M.; Shen, H.; Tang, Y.; Wang, Y. Hierarchical Covalent Organic Framework Hollow Nanofibers-Bonded Stainless Steel Fiber for Efficient Solid Phase Microextraction. Talanta 2024, 267, 125223. [Google Scholar] [CrossRef] [PubMed]
- Bertani, F.; Riboni, N.; Bianchi, F.; Brancatelli, G.; Sterner, E.S.; Pinalli, R.; Geremia, S.; Swager, T.M.; Dalcanale, E. Triptycene-Roofed Quinoxaline Cavitands for the Supramolecular Detection of BTEX in Air. Chem.-A Eur. J. 2016, 22, 3312–3319. [Google Scholar] [CrossRef]
- Bianchi, F.; Mattarozzi, M.; Betti, P.; Bisceglie, F.; Careri, M.; Mangia, A.; Sidisky, L.; Ongarato, S.; Dalcanale, E. Innovative Cavitand-Based Sol-Gel Coatings for the Environmental Monitoring of Benzene and Chlorobenzenes via Solid-Phase Microextraction. Anal. Chem. 2008, 80, 6423–6430. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, Y.; Zhao, B.; Jia, Q. Supramolecular Adsorbents in Extraction and Separation Techniques—A Review. Anal. Chim. Acta 2020, 1122, 97–113. [Google Scholar] [CrossRef]
- Gentili, A. Cyclodextrin-Based Sorbents for Solid Phase Extraction. J. Chromatogr. A 2020, 1609, 460654. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Li, C.X.; Chen, X.; Lu, Z.; Zhang, M.; Zhu, X.; Yu, Y. In-Situ Preparation of β-Cyclodextrin-Crosslinked Polymer Coated Glass Fiber for in-Tube Solid-Phase Microextraction of Polycyclic Aromatic Hydrocarbons from Water Samples. Microchem. J. 2023, 194, 109285. [Google Scholar] [CrossRef]
- Ghorbani, M.; Esmaelnia, M.; Aghamohammadhasan, M.; Akhlaghi, H.; Seyedin, O.; Azari, Z.A. Preconcentration and Determination Of Fluoxetine and Norfluoxetine in Biological and Water Samples with β-Cyclodextrin Multi-Walled Carbon Nanotubes as a Suitable Hollow Fiber Solid Phase Microextraction Sorbent and High Performance Liquid Chromatography. J. Anal. Chem. 2019, 74, 540–549. [Google Scholar] [CrossRef]
- Riboni, N.; Bianchi, F.; Scaccaglia, M.; Bisceglie, F.; Secchi, A.; Massera, C.; Luches, P.; Careri, M. A Novel Multiwalled Carbon Nanotube–Cyclodextrin Nanocomposite for Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry Determination of Polycyclic Aromatic Hydrocarbons in Snow Samples. Microchim. Acta 2023, 190, 212. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Pu, W.; Yu, L.D.; Tong, Y.J.; Liu, X.; Wang, S.; Fu, Q.; Yang, H.; Chen, G.; Zhu, F.; et al. PDMS-Coated ΓCD-MOF Solid-Phase Microextraction Fiber for BTEX Analysis with Boosted Performances. Anal. Chim. Acta 2022, 1189, 339259. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.; Irico, A. Gas-Phase Interactions of Calixarene- and Resorcinarene-Cavitands with Molecular Guests Studied by Mass Spectrometry. Int. J. Mass Spectrom. 2002, 214, 23–36. [Google Scholar] [CrossRef]
- Najarzadekan, H.; Kamboh, M.A.; Sereshti, H.; Ahmad, I.; Sridewi, N.; Shahabuddin, S.; Rashidi Nodeh, H. Headspace Extraction of Chlorobenzenes from Water Using Electrospun Nanofibers Fabricated with Calix[4]Arene-Doped Polyurethane–Polysulfone. Polymers 2022, 14, 3760. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Q.; Zhou, L.; Sun, H.; Yao, X.; Hu, X. State-of-the-Art and Recent Progress in Resorcinarene-Based Cavitand R. Chin. Chem. Lett. 2023, 34, 108559. [Google Scholar] [CrossRef]
- Riboni, N.; Amorini, M.; Bianchi, F.; Pedrini, A.; Pinalli, R.; Dalcanale, E.; Careri, M. Ultra-Sensitive Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry Determination of Polycyclic Aromatic Hydrocarbons in Snow Samples Using a Deep Cavity BenzoQxCavitand. Chemosphere 2022, 303, 135144. [Google Scholar] [CrossRef] [PubMed]
- Amorini, M.; Riboni, N.; Pesenti, L.; Dini, V.A.; Pedrini, A.; Massera, C.; Gualandi, C.; Bianchi, F.; Pinalli, R.; Dalcanale, E. Reusable Cavitand-Based Electrospun Membranes for the Removal of Polycyclic Aromatic Hydrocarbons from Water. Small 2021, 18, 2104946. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riboni, N.; Ribezzi, E.; Bianchi, F.; Careri, M. Supramolecular Materials as Solid-Phase Microextraction Coatings in Environmental Analysis. Molecules 2024, 29, 2802. https://doi.org/10.3390/molecules29122802
Riboni N, Ribezzi E, Bianchi F, Careri M. Supramolecular Materials as Solid-Phase Microextraction Coatings in Environmental Analysis. Molecules. 2024; 29(12):2802. https://doi.org/10.3390/molecules29122802
Chicago/Turabian StyleRiboni, Nicolò, Erika Ribezzi, Federica Bianchi, and Maria Careri. 2024. "Supramolecular Materials as Solid-Phase Microextraction Coatings in Environmental Analysis" Molecules 29, no. 12: 2802. https://doi.org/10.3390/molecules29122802
APA StyleRiboni, N., Ribezzi, E., Bianchi, F., & Careri, M. (2024). Supramolecular Materials as Solid-Phase Microextraction Coatings in Environmental Analysis. Molecules, 29(12), 2802. https://doi.org/10.3390/molecules29122802








