Two-Dimensional GeC/MXY (M = Zr, Hf; X, Y = S, Se) Heterojunctions Used as Highly Efficient Overall Water-Splitting Photocatalysts
Abstract
1. Introduction
2. Results and Discussion
3. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Wu, D.; Zhang, X.; Zhao, X.; Zhou, Z. Computational screening of 2D materials and rational design of heterojunctions for water splitting photocatalysts. Small Methods 2018, 2, 1700359. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Moniz, S.J.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting—A critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef]
- Wang, W.; Xu, X.; Zhou, W.; Shao, Z. Recent progress in metal-organic frameworks for applications in electrocatalytic and photocatalytic water splitting. Adv. Sci. 2017, 4, 1600371. [Google Scholar] [CrossRef]
- Tran, M.N.; Moreau, M.; Addad, A.; Teurtrie, A.; Roland, T.; De Waele, V.; Dewitte, M.; Thomas, L.; Levêque, G.; Dong, C.; et al. Boosting gas-phase TiO2 photocatalysis with weak electric field strengths of volt/centimeter. ACS Appl. Mater. Interfaces 2024, 16, 14852–14863. [Google Scholar] [CrossRef]
- Song, X.; Wei, G.; Sun, J.; Peng, C.; Yin, J.; Zhang, X.; Jiang, Y.; Fei, H. Overall photocatalytic water splitting by an organolead iodide crystalline material. Nat. Catal. 2020, 3, 1027–1033. [Google Scholar] [CrossRef]
- Mao, J.; Ta, Q.T.H.; Tri, N.N.; Shou, L.; Seo, S.; Xu, W. 2D MoTe2 nanomesh with a large surface area and uniform pores for highly active hydrogen evolution catalysis. Appl. Mater. Today 2023, 35, 101939. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.F.; Wu, X.; Yang, J. Material design for photocatalytic water splitting from a theoretical perspective. Adv. Mater. 2018, 30, 1802106. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Chang, J.L.; Tang, W.; Xie, W.; Ang, Y.S. 2D materials and heterostructures for photocatalytic water-splitting: A theoretical perspective. J. Phys. D Appl. Phys. 2022, 55, 293002. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based heterostructured photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Wang, G.; Tang, W.; Xie, W.; Tang, Q.; Wang, Y.; Guo, H.; Gao, P.; Dang, S.; Chang, J. Type-II CdS/PtSSe heterostructures used as highly efficient water-splitting photocatalysts. Appl. Surf. Sci. 2022, 589, 152931. [Google Scholar] [CrossRef]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Moniz, S.J.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting–materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef]
- Li, H.; Tu, W.; Zhou, Y.; Zou, Z. Z-Scheme photocatalytic systems for promoting photocatalytic performance: Recent progress and future challenges. Adv. Sci. 2016, 3, 1500389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, L.; Zheng, Q.; Gao, P.; Zhao, J.; Yang, J. Direct Z-scheme water splitting photocatalyst based on two-dimensional Van Der Waals heterostructures. J. Phys. Chem. Lett. 2018, 9, 5419–5424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hisatomi, T.; Jia, Q.; Tokudome, H.; Zhong, M.; Wang, C.; Pan, Z.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Tang, W.; Xu, C.; He, J.; Zeng, Q.; Xie, W.; Gao, P.; Chang, J. Two-dimensional CdO/PtSSe heterojunctions used for Z-scheme photocatalytic water-splitting. Appl. Surf. Sci. 2022, 599, 153960. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xian, Q.; Zhao, Y. Direct Z-scheme TiO2–ZnIn2S4 nanoflowers for cocatalyst-free photocatalytic water splitting. Appl. Catal. B Environ. 2021, 291, 120126. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, X.; Chen, L.; Xiong, Y.; Xu, H. Van der Waals heterostructures comprised of ultrathin polymer nanosheets for efficient Z-scheme overall water splitting. Angew. Chem. Int. Ed. 2018, 130, 3512–3516. [Google Scholar] [CrossRef]
- Zeng, C.; Hu, Y.; Zhang, T.; Dong, F.; Zhang, Y.; Huang, H. A core–satellite structured Z-scheme catalyst Cd0.5Zn0.5S/BiVO4 for highly efficient and stable photocatalytic water splitting. J. Mater. Chem. A 2018, 6, 16932–16942. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.T.F.; et al. High efficiency photocatalytic water splitting using 2D α-Fe2O3/g-C3N4 Z-scheme catalysts. Adv. Energy Mater. 2017, 7, 1700025. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, Z.; Fujitsuka, M.; Majima, T. Z-scheme photocatalytic water splitting on a 2D heterostructure of black phosphorus/bismuth vanadate using visible light. Angew. Chem. Int. Ed. 2018, 57, 2160–2164. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Chen, D.; Yang, S.; Yang, L.X.; Wang, J.J.; Cao, D.; Tu, W.; Yu, Z.T.; Zou, Z.G. Constructing noble-metal-free Z-scheme photocatalytic overall water splitting systems using MoS2 nanosheet modified CdS as a H2 evolution photocatalyst. J. Mater. Chem. A 2017, 5, 21205–21213. [Google Scholar] [CrossRef]
- Li, L.; Guo, C.; Shen, J.; Ning, J.; Zhong, Y.; Hu, Y. Construction of sugar-gourd-shaped CdS/Co1−xS hollow hetero-nanostructure as an efficient Z-scheme photocatalyst for hydrogen generation. Chem. Eng. J. 2020, 400, 125925. [Google Scholar] [CrossRef]
- Wei, T.; Zhu, Y.N.; An, X.; Liu, L.M.; Cao, X.; Liu, H.; Qu, J. Defect modulation of Z-scheme TiO2/Cu2O photocatalysts for durable water splitting. ACS Catal. 2019, 9, 8346–8354. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, Y.; Dong, C.L.; Huang, Y.C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 2021, 6, 388–397. [Google Scholar] [CrossRef]
- Li, Z.; Hou, J.; Zhang, B.; Cao, S.; Wu, Y.; Gao, Z.; Nie, X.; Sun, L. Two-dimensional Janus heterostructures for superior Z-scheme photocatalytic water splitting. Nano Energy 2019, 59, 537–544. [Google Scholar] [CrossRef]
- Hong, X.; Kim, J.; Shi, S.F.; Zhang, Y.; Jin, C.; Sun, Y.; Tongay, S.; Wu, J.; Zhang, Y.; Wang, F. Ultrafast charge transfer in atomically thin MoS2/WS2 heterostructures. Nat. Nanotechnol. 2014, 9, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Rivera, P.; Schaibley, J.R.; Jones, A.M.; Ross, J.S.; Wu, S.; Aivazian, G.; Klement, P.; Seyler, K.; Clark, G.; Ghimire, N.J.; et al. Observation of long-lived interlayer excitons in monolayer MoSe2–WSe2 heterostructures. Nat. Commun. 2015, 6, 6242. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.H.; Zhang, C.; Shiu, H.W.; Chuu, C.P.; Chen, C.H.; Chang, C.Y.S.; Chen, C.H.; Chou, M.Y.; Shih, C.K.; Li, L.J. Determination of band alignment in the single-layer MoS2/WSe2 heterojunction. Nat. Commun. 2015, 6, 7666. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Prezhdo, O.V. Quantum coherence facilitates efficient charge separation at a MoS2/MoSe2 van der Waals junction. Nano Lett. 2016, 16, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Niu, X.; Zhang, Y.; Wang, J. Janus MoSSe/WSeTe heterostructures: A direct Z-scheme photocatalyst for hydrogen evolution. J. Mater. Chem. A 2019, 7, 21835–21842. [Google Scholar] [CrossRef]
- Niu, X.; Bai, X.; Zhou, Z.; Wang, J. Rational design and characterization of direct Z-scheme photocatalyst for overall water splitting from excited state dynamics simulations. ACS Catal. 2020, 10, 1976–1983. [Google Scholar] [CrossRef]
- Jiang, X.; Gao, Q.; Xu, X.; Xu, G.; Li, D.; Cui, B.; Liu, D.; Qu, F. Design of a noble-metal-free direct Z-scheme photocatalyst for overall water splitting based on a SnC/SnSSe van der Waals heterostructure. Phys. Chem. Chem. Phys. 2021, 23, 21641–21651. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.F.; Li, X.; Yang, J. A rationally designed two-dimensional MoSe2/Ti2CO2 heterojunction for photocatalytic overall water splitting: Simultaneously suppressing electron–hole recombination and photocorrosion. Chem. Sci. 2021, 12, 2863–2869. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Dong, H.; Hou, T.; Li, Y. Monolayer graphitic germanium carbide (g-GeC): The promising cathode catalyst for fuel cell and lithium–oxygen battery applications. J. Mater. Chem. A 2018, 6, 2212–2218. [Google Scholar] [CrossRef]
- Şahin, H.; Cahangirov, S.; Topsakal, M.; Bekaroglu, E.; Akturk, E.; Senger, R.T.; Ciraci, S. Monolayer honeycomb structures of group-IV elements and III-V binary compounds: First-principles calculations. Phys. Rev. B 2009, 80, 155453. [Google Scholar] [CrossRef]
- Pan, L.; Liu, H.; Wen, Y.; Tan, X.; Lv, H.; Shi, J.; Tang, X. First-principles study of monolayer and bilayer honeycomb structures of group-IV elements and their binary compounds. Phys. Lett. A 2011, 375, 614–619. [Google Scholar] [CrossRef]
- Hao, A.; Yang, X.; Wang, X.; Zhu, Y.; Liu, X.; Liu, R. First-principles investigations on electronic, elastic and optical properties of XC (X = Si, Ge, and Sn) under high pressure. J. Appl. Phys. 2010, 108, 063531. [Google Scholar] [CrossRef]
- Peng, Q.; Liang, C.; Ji, W.; De, S. A first-principles study of the mechanical properties of g-GeC. Mech. Mater. 2013, 64, 135–141. [Google Scholar] [CrossRef]
- Ren, K.; Sun, M.; Luo, Y.; Wang, S.; Xu, Y.; Yu, J.; Tang, W. Electronic and optical properties of van der Waals vertical heterostructures based on two-dimensional transition metal dichalcogenides: First-principles calculations. Phys. Lett. A 2019, 383, 1487–1492. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Yan, L.; Luo, R. The deposition and optical properties of Ge1−xCx thin film and infrared multilayer antireflection coatings. Thin Solid Film. 2008, 516, 3189–3195. [Google Scholar] [CrossRef]
- Yuan, H.; Williams, R.S. Synthesis by laser ablation and characterization of pure germanium-carbon alloy thin films. Chem. Mater. 1993, 5, 479–485. [Google Scholar] [CrossRef]
- Xu, M.; Liang, T.; Shi, M.; Chen, H. Graphene-like two-dimensional materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lv, Y.; Ren, L.; Li, J.; Kong, L.; Zeng, Y.; Tao, Q.; Wu, R.; Ma, H.; Zhao, B.; et al. Efficient strain modulation of 2D materials via polymer encapsulation. Nat. Commun. 2020, 11, 1151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, M.; Zhang, C.; Dong, H.; Lin, W.; Zhuang, P.; Wen, Y.; Tian, B.; Cai, W.; Zhang, X. Fractal-theory-based control of the shape and quality of CVD-grown 2D materials. Adv. Mater. 2019, 31, 1902431. [Google Scholar] [CrossRef] [PubMed]
- Din, H.; Idrees, M.; Albar, A.; Shafiq, M.; Ahmad, I.; Nguyen, C.V.; Amin, B. Rashba spin splitting and photocatalytic properties of GeC-MSSe (M= Mo, W) van der Waals heterostructures. Phys. Rev. B 2019, 100, 165425. [Google Scholar] [CrossRef]
- Jiang, X.; Xie, W.; Xu, X.; Gao, Q.; Li, D.; Cui, B.; Liu, D.; Qu, F. A bifunctional GeC/SnSSe heterostructure for highly efficient photocatalysts and photovoltaic devices. Nanoscale 2022, 14, 7292–7302. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, L.; Li, Y.; Zhao, W.; Kuang, A.; Li, Y.; Xia, L.; Li, Y.; Xiao, S. Biaxial strain tunable photocatalytic properties of 2D ZnO/GeC heterostructure. J. Phys. D Appl. Phys. 2020, 53, 015104. [Google Scholar] [CrossRef]
- Gao, X.; Shen, Y.; Ma, Y.; Wu, S.; Zhou, Z. ZnO/g-GeC van der Waals heterostructure: Novel photocatalyst for small molecule splitting. J. Mater. Chem. C 2019, 7, 4791–4799. [Google Scholar] [CrossRef]
- Cao, M.; Luan, L.; Wang, Z.; Zhang, Y.; Yang, Y.; Liu, J.; Tian, Y.; Wei, X.; Fan, J.; Xie, Y.; et al. Type-II GeC/ZnTe heterostructure with high-efficiency of photoelectrochemical water splitting. Appl. Phys. Lett. 2021, 119, 083101. [Google Scholar] [CrossRef]
- Huong, P.T.; Idrees, M.; Amin, B.; Hieu, N.N.; Phuc, H.V.; Hoa, L.T.; Nguyen, C.V. Electronic structure, optoelectronic properties and enhanced photocatalytic response of GaN–GeC van der Waals heterostructures: A first principles study. RSC Adv. 2020, 10, 24127–24133. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Hu, G.; Yuan, X.; Ren, J.; Zhao, X. Strain-tunable Zeeman splitting and optical properties of CrBr3/GeC van der Waals heterostructure. Results Phys. 2022, 37, 105559. [Google Scholar] [CrossRef]
- Lou, P.; Lee, J.Y. GeC/GaN vdW heterojunctions: A promising photocatalyst for overall water splitting and solar energy conversion. ACS Appl. Mater. Interf. 2020, 12, 14289–14297. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Shi, Y.; Yang, C.L. Two-dimensional MoSSe/g-GeC van der waals heterostructure as promising multifunctional system for solar energy conversion. Appl. Surf. Sci. 2021, 545, 148952. [Google Scholar] [CrossRef]
- Gao, X.; Shen, Y.; Liu, J.; Lv, L.; Zhou, M.; Zhou, Z.; Feng, Y.P.; Shen, L. Boost the large driving photovoltages for overall water splitting in direct Z-scheme heterojunctions by interfacial polarization. Catal. Sci. Technol. 2022, 12, 3614–3621. [Google Scholar] [CrossRef]
- Abdulsalam, M.; Joubert, D.P. Optical spectrum and excitons in bulk and monolayer MX2 (M = Zr, Hf; X = S, Se). Phys. Status Solidi B 2016, 253, 705–711. [Google Scholar] [CrossRef]
- Abdulsalam, M.; Rugut, E.; Joubert, D. Mechanical, thermal and thermoelectric properties of MX2 (M = Zr, Hf; X = S, Se). Mater. Today Commun. 2020, 25, 101434. [Google Scholar] [CrossRef]
- Bera, J.; Betal, A.; Sahu, S. Spin orbit coupling induced enhancement of thermoelectric performance of HfX2 (X = S, Se) and its Janus monolayer. J. Alloys Compd. 2021, 872, 159704. [Google Scholar] [CrossRef]
- Dimple; Jena, N.; Rawat, A.; Ahammed, R.; Mohanta, M.K.; De Sarkar, A. Emergence of high piezoelectricity along with robust electron mobility in Janus structures in semiconducting Group IVB dichalcogenide monolayers. J. Mater. Chem. A 2018, 6, 24885–24898. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Z. Mechanical and electronic properties of Janus monolayer transition metal dichalcogenides. J. Phys. Condens. Mat. 2018, 30, 215301. [Google Scholar] [CrossRef] [PubMed]
- Som, N.N.; Jha, P.K. Hydrogen evolution reaction of metal di-chalcogenides: ZrS2, ZrSe2 and Janus ZrSSe. Int. J. Hydrogen Energy 2020, 45, 23920–23927. [Google Scholar] [CrossRef]
- Hoat, D.; Naseri, M.; Hieu, N.N.; Ponce-Pérez, R.; Rivas-Silva, J.; Vu, T.V.; Cocoletzi, G.H. A comprehensive investigation on electronic structure, optical and thermoelectric properties of the HfSSe Janus monolayer. J. Phys. Chem. Solids 2020, 144, 109490. [Google Scholar] [CrossRef]
- Kaur, H.; Yadav, S.; Srivastava, A.K.; Singh, N.; Rath, S.; Schneider, J.J.; Sinha, O.P.; Srivastava, R. High-yield synthesis and liquid-exfoliation of two-dimensional belt-like hafnium disulphide. Nano Res. 2018, 11, 343–353. [Google Scholar] [CrossRef]
- Yue, R.; Barton, A.T.; Zhu, H.; Azcatl, A.; Pena, L.F.; Wang, J.; Peng, X.; Lu, N.; Cheng, L.; Addou, R.; et al. HfSe2 thin films: 2D transition metal dichalcogenides grown by molecular beam epitaxy. ACS Nano 2015, 9, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, Y.; Wang, X.; Feng, Q.; Qiao, S.; Wen, W.; Chen, Y.; Cui, M.; Zhang, J.; Cai, C.; et al. Controlled synthesis of ZrS2 monolayer and few layers on hexagonal boron nitride. J. Am. Chem. Soc. 2015, 137, 7051–7054. [Google Scholar] [CrossRef] [PubMed]
- Tsipas, P.; Tsoutsou, D.; Marquez-Velasco, J.; Aretouli, K.; Xenogiannopoulou, E.; Vassalou, E.; Kordas, G.; Dimoulas, A. Epitaxial ZrSe2/MoSe2 semiconductor vd Waals heterostructures on wide band gap AlN substrates. Microelectron. Eng. 2015, 147, 269–272. [Google Scholar] [CrossRef]
- Zeng, Z.; Yin, Z.; Huang, X.; Li, H.; He, Q.; Lu, G.; Boey, F.; Zhang, H. Single-layer semiconducting nanosheets: High-yield preparation and device fabrication. Ang. Chem. Int. Ed. 2011, 123, 11289–11293. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, S.; Kholmanov, I.; Dong, L.; Er, D.; Chen, W.; Guo, H.; Jin, Z.; Shenoy, V.B.; Shi, L.; et al. Janus monolayer transition-metal dichalcogenides. ACS Nano 2017, 11, 8192–8198. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.Y.; Zhu, H.; Xiao, J.; Chuu, C.P.; Han, Y.; Chiu, M.H.; Cheng, C.C.; Yang, C.W.; Wei, K.H.; Yang, Y.; et al. Janus monolayers of transition metal dichalcogenides. Nat. Nanotechnol. 2017, 12, 744–749. [Google Scholar] [CrossRef]
- Lin, Y.C.; Liu, C.; Yu, Y.; Zarkadoula, E.; Yoon, M.; Puretzky, A.A.; Liang, L.; Kong, X.; Gu, Y.; Strasser, A.; et al. Low energy implantation into transition-metal dichalcogenide monolayers to form Janus structures. ACS Nano 2020, 14, 3896–3906. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Jain, M.; Bhattacharya, S. MoS2 and Janus (MoSSe) based 2D van der Waals heterostructures: Emerging direct Z-scheme photocatalysts. Nanoscale Adv. 2021, 3, 2837–2845. [Google Scholar] [CrossRef]
- Zhu, X.T.; Xu, Y.; Cao, Y.; Zou, D.F.; Sheng, W. Direct Z-scheme arsenene/HfS2 van der Waals heterojunction for overall photocatalytic water splitting: First-principles study. Appl. Surf. Sci. 2022, 574, 151650. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Wu, X.; Xu, N.; Zhang, Q. Two-dimensional arsenene/ZrS2 (HfS2) deterostructures as direct Z-Scheme photocatalysts for overall water splitting. J. Phys. Chem. C 2022, 126, 2587–2595. [Google Scholar] [CrossRef]
- Sun, R.; Yang, C.L.; Wang, M.S.; Ma, X.G. High solar-to-hydrogen efficiency photocatalytic hydrogen evolution reaction with the HfSe2/InSe heterostructure. J. Power Sources 2022, 547, 232008. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, Z.; Rao, D.; Wang, Y.; Shi, Q.; Liu, Y.; Wu, H.; Deng, K.; Liu, H.; Lu, R. Efficient band structure tuning, charge separation, and visible-light response in ZrS2-based van der Waals heterostructures. Energy Environ. Sci. 2016, 9, 841–849. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, X.; Zhao, S.; Wang, S.; Cui, J. Mechanism of photocatalytic water splitting of 2D WSeTe/XS2 (X = Hf, Sn, Zr) van der Waals heterojunctions under the interaction of vertical intrinsic electric and built-in electric field. Appl. Surf. Sci. 2022, 599, 154012. [Google Scholar] [CrossRef]
- Opoku, F.; Akoto, O.; Oppong, S.O.B.; Adimado, A.A. Two-dimensional layered type-II MS2/BiOCl (M = Zr, Hf) van der Waals heterostructures: Promising photocatalysts for hydrogen generation. New J. Chem. 2021, 45, 20365–20373. [Google Scholar] [CrossRef]
- Ahmad, S.; Idrees, M.; Khan, F.; Nguyen, C.; Ahmad, I.; Amin, B. Strain engineering of Janus ZrSSe and HfSSe monolayers and ZrSSe/HfSSe van der Waals heterostructure. Chem. Phys. Lett. 2021, 776, 138689. [Google Scholar] [CrossRef]
- Fu, C.F.; Luo, Q.; Li, X.; Yang, J. Two-dimensional van der Waals nanocomposites as Z-scheme type photocatalysts for hydrogen production from overall water splitting. J. Mater. Chem. A 2016, 4, 18892–18898. [Google Scholar] [CrossRef]
- Gao, Y.; Fu, C.; Hu, W.; Yang, J. Designing direct Z-scheme heterojunctions enabled by edge-modified phosphorene nanoribbons for photocatalytic overall water splitting. J. Phys. Chem. Lett. 2021, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, J.; Wang, J.; Li, Q.; Yang, J. β-SnS/GaSe heterostructure: A promising solar-driven photocatalyst with low carrier recombination for overall water splitting. J. Mater. Chem. A 2022, 10, 3443–3453. [Google Scholar] [CrossRef]
- Xiong, R.; Shu, Y.; Yang, X.; Zhang, Y.; Wen, C.; Anpo, M.; Wu, B.; Sa, B. Direct Z-scheme WTe2/InSe van der Waals heterostructure for overall water splitting. Catal. Sci. Technol. 2022, 12, 3272–3280. [Google Scholar] [CrossRef]
- Dai, Z.N.; Cao, Y.; Yin, W.J.; Sheng, W.; Xu, Y. Z-scheme SnC/HfS2 van der Waals heterojunction increases photocatalytic overall water splitting. J. Phys. D Appl. Phys. 2022, 55, 315503. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Wang, G.; Chang, J.; Guo, S.D.; Wu, W.; Tang, W.; Guo, H.; Dang, S.; Wang, R.; Ang, Y.S. MoSSe/Hf(Zr)S2 heterostructures used for efficient Z-scheme photocatalytic water-splitting. Phys. Chem. Chem. Phys. 2022, 24, 25287–25297. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.; Mishchenko, A.; Carvalho, A.; Castro Neto, A. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef]
- Björkman, T.; Gulans, A.; Krasheninnikov, A.V.; Nieminen, R.M. van der Waals bonding in layered compounds from advanced density-functional first-principles calculations. Phys. Rev. Lett. 2012, 108, 235502. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, Z.; Huang, B.; Wang, X.; Niu, H.; Guo, Y.; Li, B.; Zheng, R.; Wu, H. Theoretical study on the photocatalytic properties of 2D InX (X = S, Se)/transition metal disulfide (MoS2 and WS2) van der Waals heterostructures. Nanoscale 2020, 12, 20025–20032. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, W.Q.; Hu, W.; Yang, K.; Zhou, B.X.; Pan, A.; Huang, G.F. Two-dimensional MoS2-graphene-based multilayer van der Waals heterostructures: Enhanced charge transfer and optical absorption, and electric-field tunable Dirac point and band gap. Chem. Mater. 2017, 29, 5504–5512. [Google Scholar] [CrossRef]
- Bafekry, A.; Obeid, M.; Nguyen, C.V.; Ghergherehchi, M.; Tagani, M.B. Graphene hetero-multilayer on layered platinum mineral jacutingaite (Pt2HgSe3): Van der Waals heterostructures with novel optoelectronic and thermoelectric performances. J. Mater. Chem. A 2020, 8, 13248–13260. [Google Scholar] [CrossRef]
- Zhang, C.F.; Yang, C.L.; Wang, M.S.; Ma, X.G. Z-Scheme photocatalytic solar-energy-to-hydrogen conversion driven by the HfS2/SiSe heterostructure. J. Mater. Chem. C 2022, 10, 5474–5481. [Google Scholar] [CrossRef]
- Wang, G.; Li, Z.; Wu, W.; Guo, H.; Chen, C.; Yuan, H.; Yang, S.A. A two-dimensional h-BN/C2N heterostructure as a promising metal-free photocatalyst for overall water-splitting. Phys. Chem. Chem. Phys. 2020, 22, 24446–24454. [Google Scholar] [CrossRef]
- Zhou, Z.; Yuan, S.; Wang, J. Theoretical progress on direct Z-scheme photocatalysis of two-dimensional heterostructures. Front. Phys. 2021, 16, 43203. [Google Scholar] [CrossRef]
- He, C.; Zhang, J.; Zhang, W.; Li, T. Type-II InSe/g-C3N4 heterostructure as a high-efficiency oxygen evolution reaction catalyst for photoelectrochemical water splitting. J. Phys. Chem. Lett. 2019, 10, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huang, W.Q.; Wang, L.L.; Tian, Z.A.; Hu, W.; Ma, Y.; Wang, X.; Pan, A.; Huang, G.F. Insights into enhanced visible-light photocatalytic hydrogen evolution of g-C3N4 and highly reduced graphene oxide composite: The role of oxygen. Chem. Mater. 2015, 27, 1612–1621. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, B.; Yu, J. A new understanding of the photocatalytic mechanism of the direct Z-scheme g-C3N4/TiO2 heterostructure. Phys. Chem. Chem. Phys. 2016, 18, 31175–31183. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Jiang, J.; Zhang, Q.; Xiong, Y. Steering charge kinetics in photocatalysis: Intersection of materials syntheses, characterization techniques and theoretical simulations. Chem. Phys. Rev. 2015, 44, 2893–2939. [Google Scholar]
- Fu, C.F.; Wu, X.; Yang, J. Theoretical design of two-dimensional visible light-driven photocatalysts for overall water splitting. Chem. Phys. Rev. 2022, 3, 011310. [Google Scholar] [CrossRef]
- Huang, Z.F.; Song, J.; Wang, X.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.J. Switching charge transfer of C3N4/W18O49 from type-II to Z-scheme by interfacial band bending for highly efficient photocatalytic hydrogen evolution. Nano Energy 2017, 40, 308–316. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band bending in semiconductors: Chemical and physical consequences at surfaces and interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Wang, J.; Zhao, M. Spontaneous full photocatalytic water splitting on 2D MoSe2/SnSe2 and WSe2/SnSe2 vdW heterostructures. Nanoscale 2019, 11, 14836–14843. [Google Scholar] [CrossRef]
- Tian, F.; Liu, C. DFT description on electronic structure and optical absorption properties of anionic S-doped anatase TiO2. J. Phys. Chem. B 2006, 110, 17866–17871. [Google Scholar] [CrossRef]
- Bengtsson, L. Dipole correction for surface supercell calculations. Phys. Rev. B 1999, 59, 12301. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Ashkenazi, J. Magnetism with generalized-gradient-approximation density functionals. Phys. Rev. B 1992, 46, 11570. [Google Scholar] [CrossRef] [PubMed]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Moellmann, J.; Grimme, S. DFT-D3 study of some molecular crystals. J. Phys. Chem. C 2014, 118, 7615–7621. [Google Scholar] [CrossRef]
- Kümmel, S.; Kronik, L. Orbital-dependent density functionals: Theory and applications. Rev. Mod. Phys. 2008, 80, 3. [Google Scholar] [CrossRef]
- Heyd, J.; Scuseria, G.E.; Ernzerhof, M. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207–8215. [Google Scholar] [CrossRef]



| Systems | a (Å) | a (Å) (Refs.) | (Å) | (Å) (Refs.) | (eV) | (eV) (Refs.) | (D) | (eV) |
|---|---|---|---|---|---|---|---|---|
| GeC | 3.235 | 3.26 [54,55], 3.233 [56] | 1.868 | 1.882 [54], 1.887 [56] | 2.87 | 3.01 [54], 2.90 [55] | 0 | 0 |
| 3.263 [57] | 2.88 [56], 2.782 [57] | |||||||
| 2.85 [58] | ||||||||
| ZrS | 3.685 | 3.70 [64,65], 3.69 [67] | 2.574 | 2.58 [67], 2.570 [93] | 2.02 | 1.99 [67], 1.96 [93] | 0 | 0 |
| 3.669 [93] | ||||||||
| ZrSe | 3.800 | 3.82 [64,65], 3.75 [67] | 2.706 | 2.69 [67], 2.702 [93] | 1.19 | 1.07 [67], 1.14 [93] | 0 | 0 |
| 3.786 [93] | ||||||||
| ZrSSe | 3.743 | 3.73 [67] | 2.568 (2.713) | 2.55 (2.72) [67] | 1.46 | 1.37 [67] | 0.043 | 0.135 |
| HfS | 3.645 | 3.66 [64,65], 3.65 [66] | 2.552 | 2.55 [66], 2.56 [67] | 2.13 | 2.03 [67], 2.07 [93] | 0 | 0 |
| 3.65 [67], 3.628 [93] | 2.548 [93] | |||||||
| HfSe | 3.768 | 3.82 [64], 3.78 [65,66] | 2.685 | 2.69 [66], 2.68 [67] | 1.33 | 1.16 [67], 1.26 [93] | 0 | 0 |
| 3.72 [67], 3.751 [93] | 2.681 [93] | |||||||
| HfSSe | 3.705 | 3.71 [66], 3.68 [67] | 2.550 (2.687) | 2.55 (2.69) [66], 2.54 (2.69) [67] | 1.56 | 1.45 [67] | 0.035 | 0.110 |
| Systems | a (Å) | (Å) | (meV/Å) | (D) | (eV) | (e) |
|---|---|---|---|---|---|---|
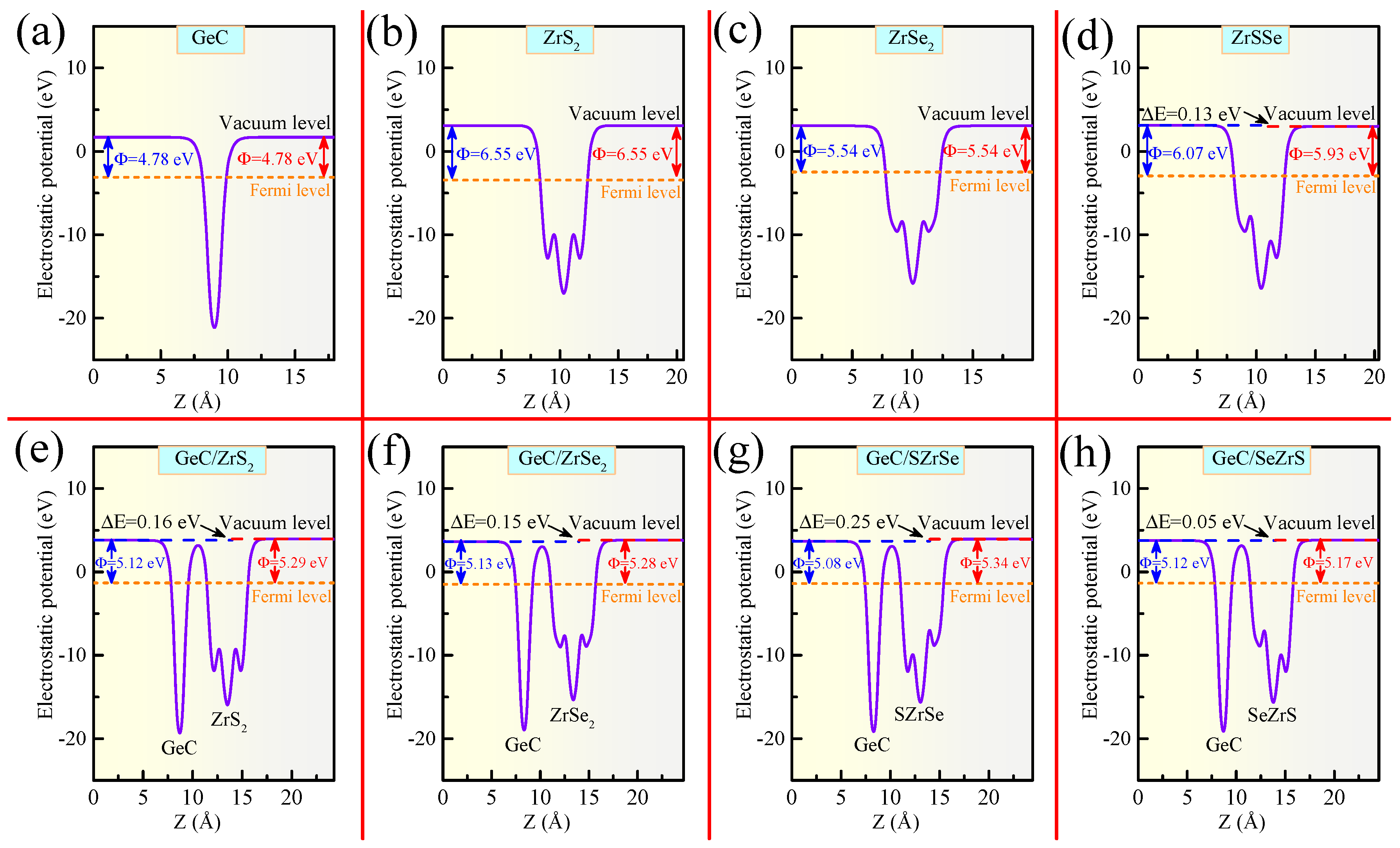
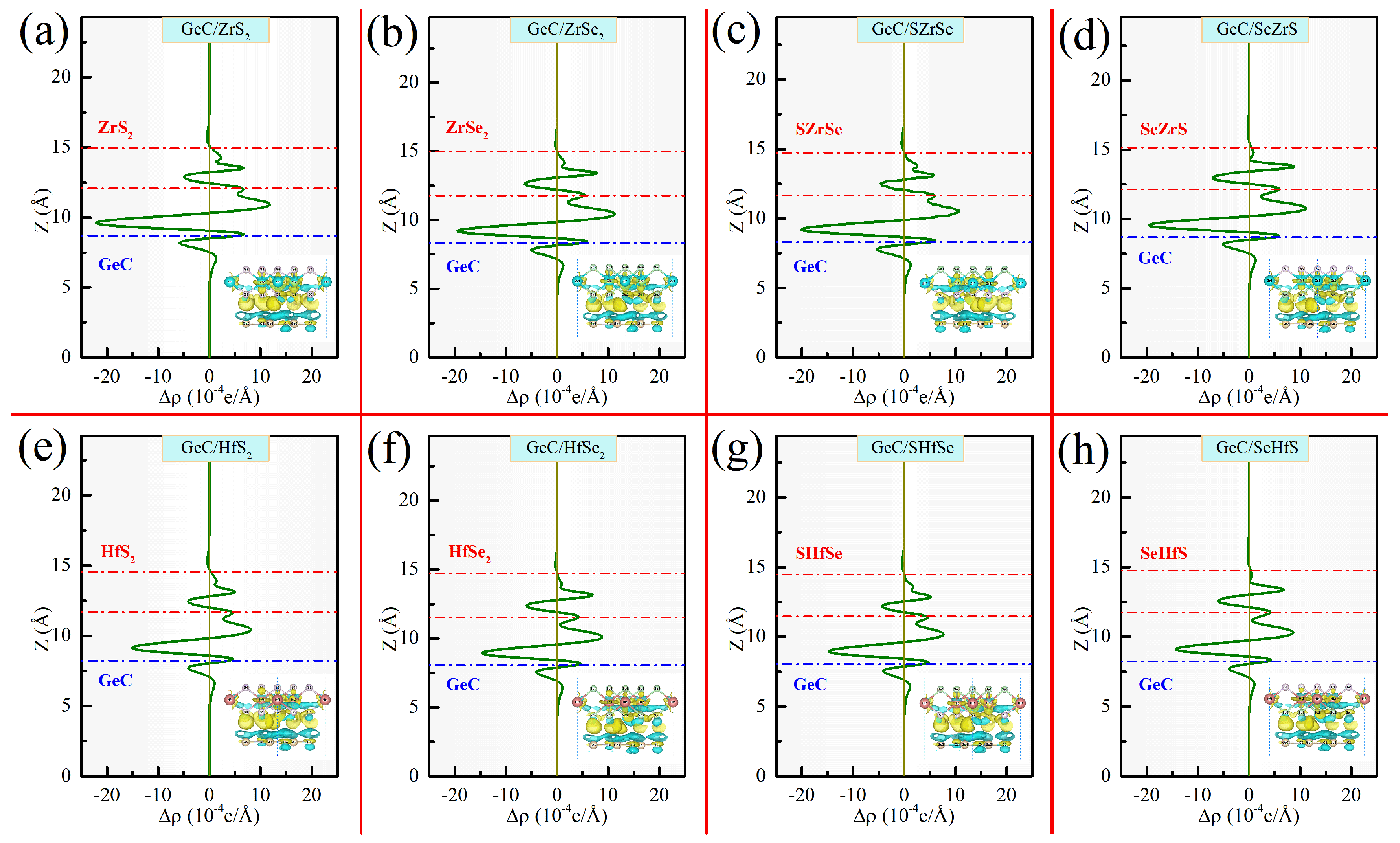
| GeC/ZrS | 6.426 | 3.367 | −18.5 | 0.16 | 0.16 | 0.11 |
| GeC/ZrSe | 6.527 | 3.446 | −28.1 | 0.15 | 0.15 | 0.09 |
| GeC/SZrSe | 6.477 | 3.376 | −28.8 | 0.24 | 0.25 | 0.11 |
| GeC/SeZrS | 6.477 | 3.436 | −29.4 | 0.04 | 0.05 | 0.09 |
| GeC/HfS | 6.392 | 3.468 | −25.2 | 0.08 | 0.09 | 0.08 |
| GeC/HfSe | 6.499 | 3.484 | −28.5 | 0.10 | 0.10 | 0.07 |
| GeC/SZrSe | 6.444 | 3.421 | −27.8 | 0.19 | 0.20 | 0.08 |
| GeC/SeZrS | 6.444 | 3.519 | −28.5 | 0.01 | 0.02 | 0.07 |
| Systems | (eV) | CBO (eV) | VBO (eV) | (V) | (V) |
|---|---|---|---|---|---|
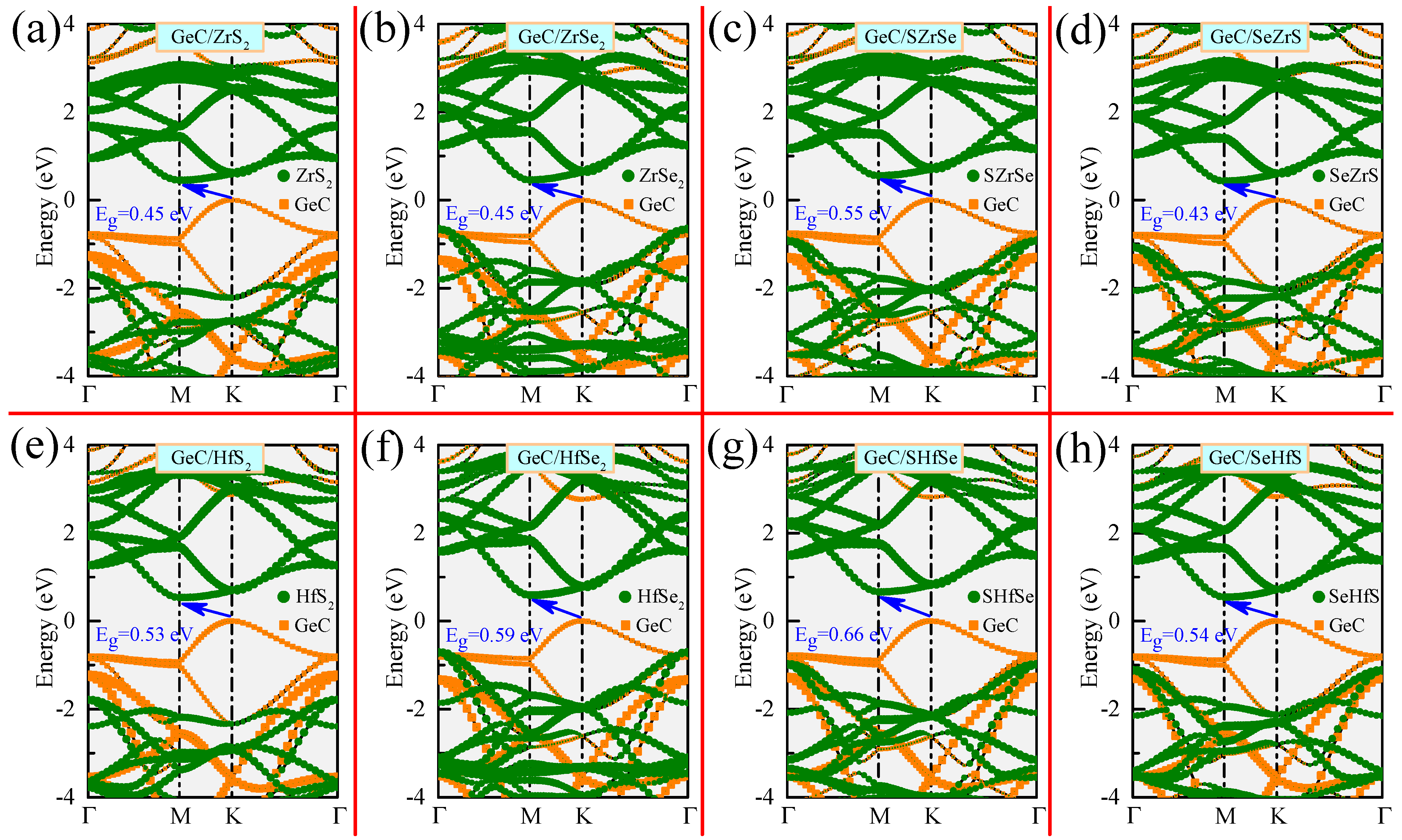
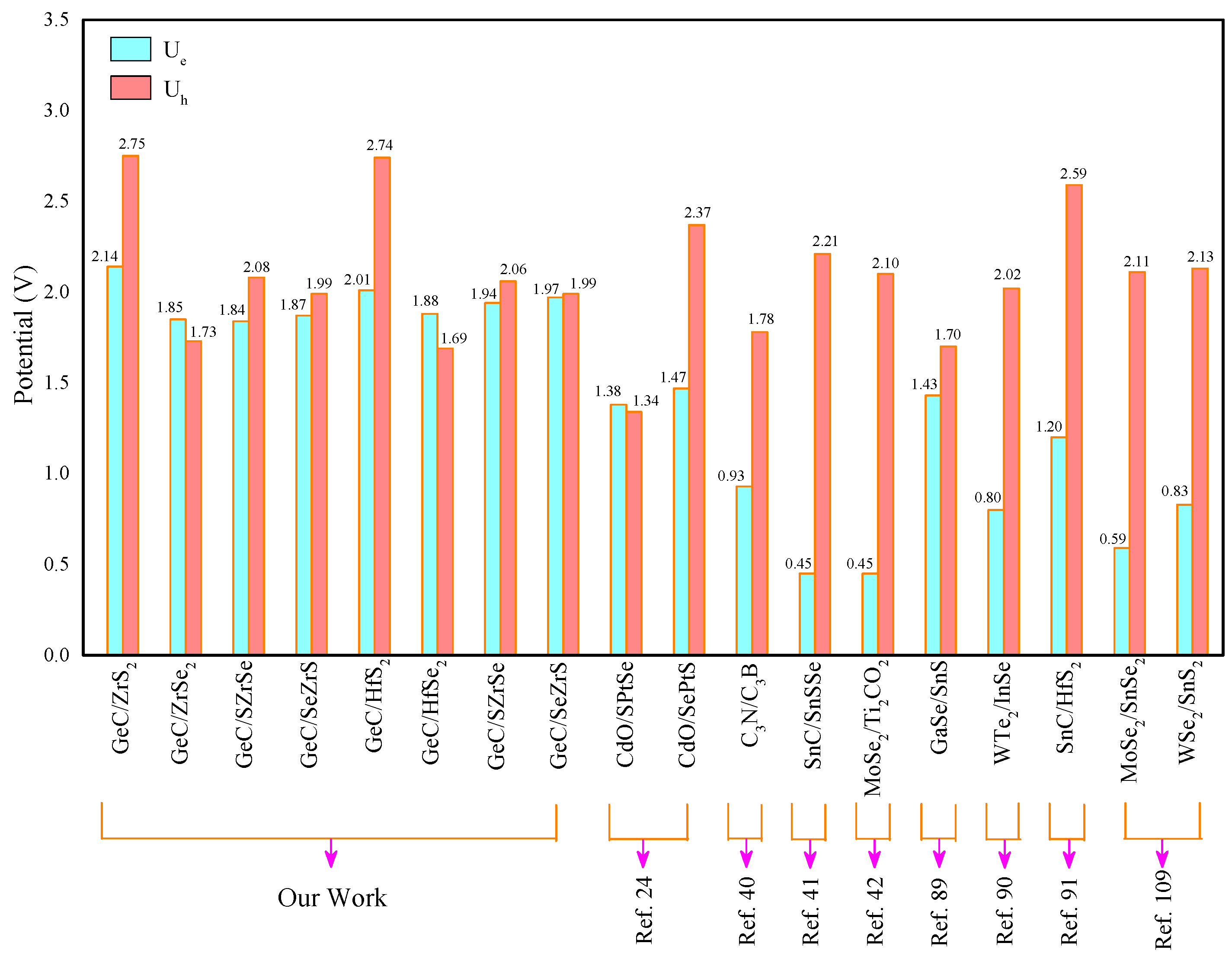
| GeC/ZrS | 0.45 | 2.59 | 1.70 | 2.14 | 2.75 |
| GeC/ZrSe | 0.45 | 2.30 | 0.68 | 1.85 | 1.73 |
| GeC/SZrSe | 0.55 | 2.19 | 0.92 | 1.84 | 2.08 |
| GeC/SeZrS | 0.43 | 2.32 | 1.07 | 1.87 | 1.99 |
| GeC/HfS | 0.53 | 2.34 | 1.79 | 2.01 | 2.74 |
| GeC/HfSe | 0.59 | 2.18 | 0.71 | 1.88 | 1.69 |
| GeC/SHfSe | 0.66 | 2.15 | 0.99 | 1.94 | 2.06 |
| GeC/SeHfS | 0.54 | 2.28 | 1.12 | 1.97 | 1.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Xie, W.; Guo, S.; Chang, J.; Chen, Y.; Long, X.; Zhou, L.; Ang, Y.S.; Yuan, H. Two-Dimensional GeC/MXY (M = Zr, Hf; X, Y = S, Se) Heterojunctions Used as Highly Efficient Overall Water-Splitting Photocatalysts. Molecules 2024, 29, 2793. https://doi.org/10.3390/molecules29122793
Wang G, Xie W, Guo S, Chang J, Chen Y, Long X, Zhou L, Ang YS, Yuan H. Two-Dimensional GeC/MXY (M = Zr, Hf; X, Y = S, Se) Heterojunctions Used as Highly Efficient Overall Water-Splitting Photocatalysts. Molecules. 2024; 29(12):2793. https://doi.org/10.3390/molecules29122793
Chicago/Turabian StyleWang, Guangzhao, Wenjie Xie, Sandong Guo, Junli Chang, Ying Chen, Xiaojiang Long, Liujiang Zhou, Yee Sin Ang, and Hongkuan Yuan. 2024. "Two-Dimensional GeC/MXY (M = Zr, Hf; X, Y = S, Se) Heterojunctions Used as Highly Efficient Overall Water-Splitting Photocatalysts" Molecules 29, no. 12: 2793. https://doi.org/10.3390/molecules29122793
APA StyleWang, G., Xie, W., Guo, S., Chang, J., Chen, Y., Long, X., Zhou, L., Ang, Y. S., & Yuan, H. (2024). Two-Dimensional GeC/MXY (M = Zr, Hf; X, Y = S, Se) Heterojunctions Used as Highly Efficient Overall Water-Splitting Photocatalysts. Molecules, 29(12), 2793. https://doi.org/10.3390/molecules29122793









