Infrared Spectroscopy of Neutral and Cationic Benzonitrile–Methanol Binary Clusters in Supersonic Jets
Abstract
1. Introduction
2. Results
2.1. Time-of-Flight Mass Spectrum
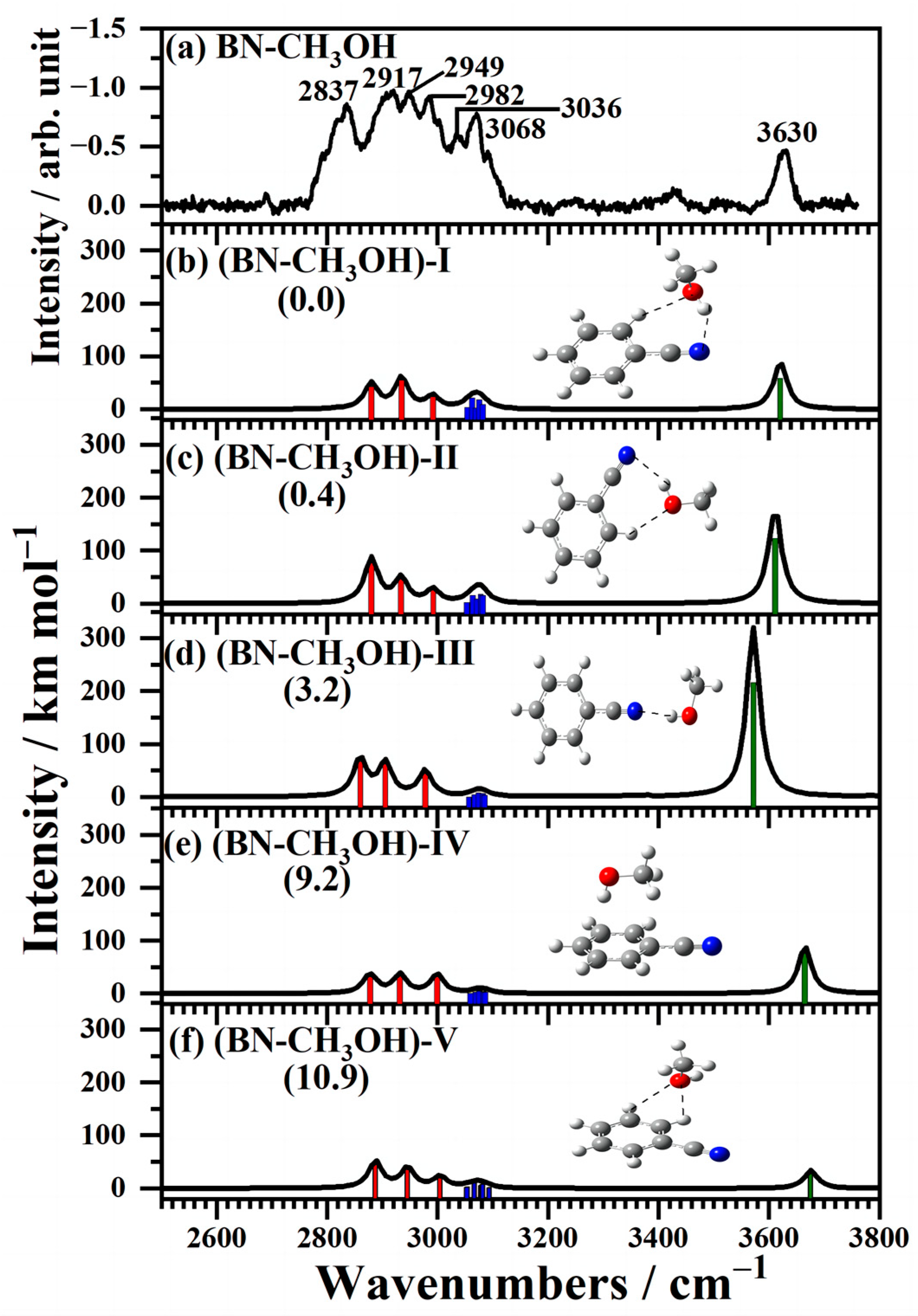
2.2. IR Spectra of Neutral BN–CH3OH Clusters
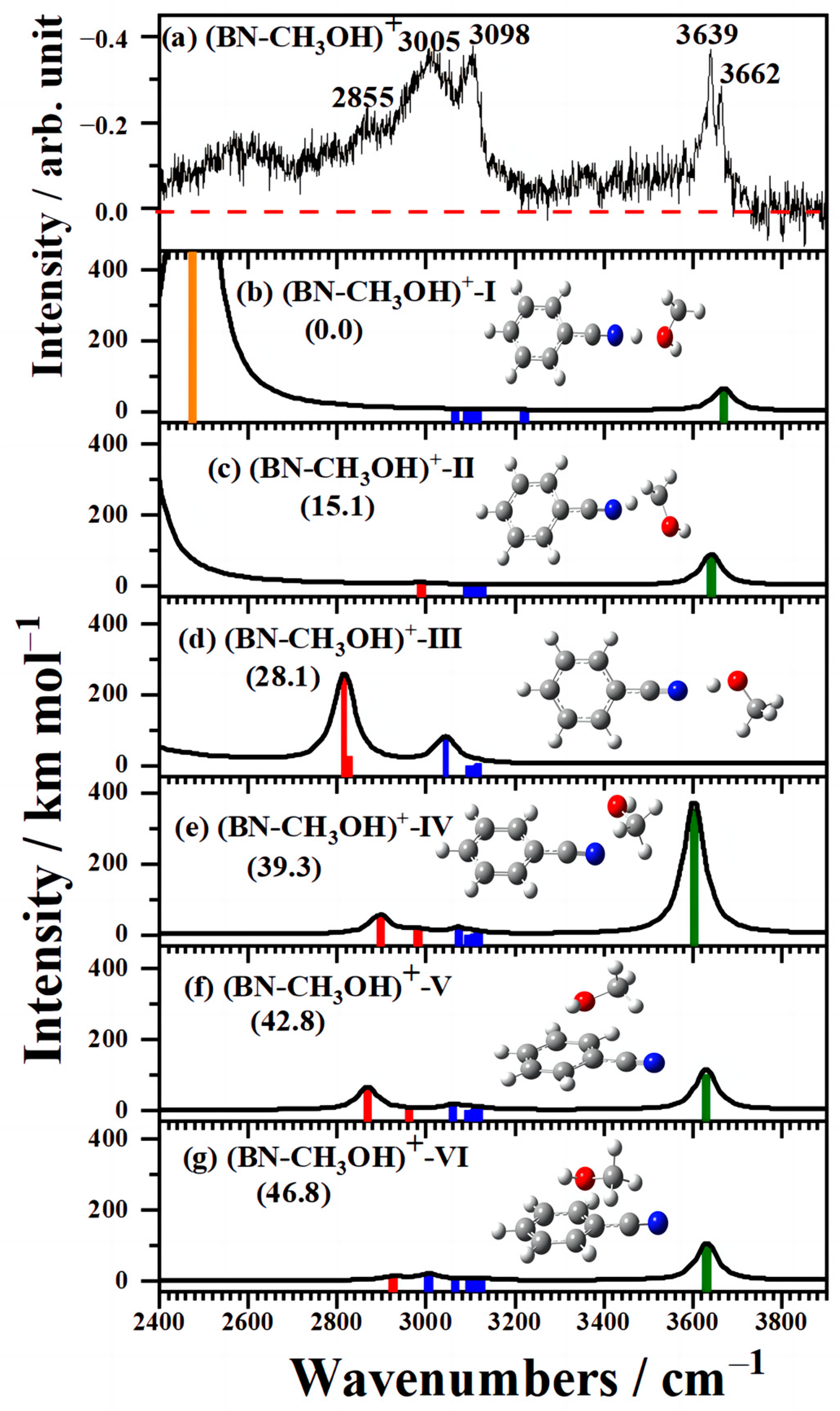
2.3. IR Spectra of Cationic (BN–CH3OH)+ Clusters
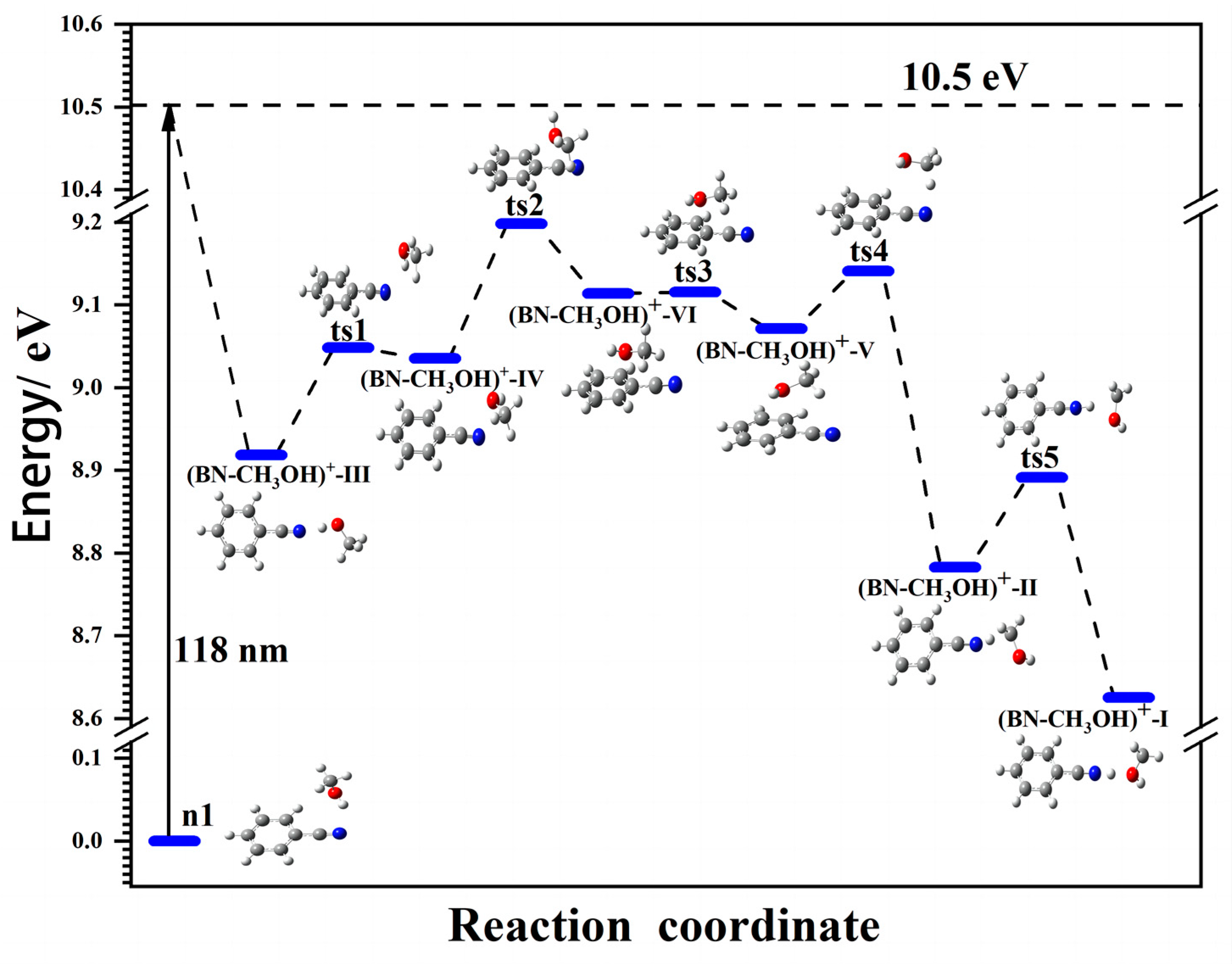
2.4. Energy Diagram for the Isomerization Reaction of (BN–CH3OH)+
3. Experimental and Computational Methods
3.1. Experimental Method
3.2. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willacy, K.; Allen, M.; Yung, Y. A new astrobiological model of the atmosphere of Titan. Astrophys. J. 2016, 829, 79. [Google Scholar] [CrossRef]
- Guélin, M.; Cernicharo, J. Organic molecules in interstellar space: Latest advances. Front. Astron. Space Sci. 2022, 9, 787567. [Google Scholar] [CrossRef]
- Herbst, E.; van Dishoeck, E.F. Complex organic interstellar molecules. Annu. Rev. Astron. Astrophys. 2009, 47, 427–480. [Google Scholar] [CrossRef]
- McGuire, B.A. 2018 Census of interstellar, circumstellar, extragalactic, protoplanetary disk, and exoplanetary molecules. Astrophys. J. Suppl. Ser. 2018, 239, 17. [Google Scholar] [CrossRef]
- Callahan, M.P.; Smith, K.E.; Cleaves, H.J.; Ruzicka, J.; Stern, J.C.; Glavin, D.P.; House, C.H.; Dworkin, J.P. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Natl. Acad. Sci. USA 2011, 108, 13995–13998. [Google Scholar] [CrossRef]
- Brigiano, F.S.; Jeanvoine, Y.; Largo, A.; Spezia, R. The formation of urea in space I. Ion–molecule, neutral-neutral, and radical gas phase reactions. Astron. Astrophys. 2018, 610, A26. [Google Scholar] [CrossRef]
- Rivilla, V.M.; Jimenez-Serra, I.; Martin-Pintado, J.; Briones, C.; Rodriguez-Almeida, L.F.; Rico-Villas, F.; Tercero, B.; Zeng, S.; Colzi, L.; de Vicente, P.; et al. Discovery in space of ethanolamine, the simplest phospholipid head group. Proc. Natl. Acad. Sci. USA 2021, 118, e2101314118. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.D.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef]
- Cooper, G.; Kimmich, N.; Belisle, W.; Sarinana, J.; Brabham, K.; Garrel, L. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 2001, 414, 879–883. [Google Scholar] [CrossRef]
- Bernstein, M.P.; Allamandola, L.J.; Sandford, S.A. Complex organics in laboratory simulations of interstellar/cometary ices. Adv. Space Res. 1997, 19, 991–998. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Cami, J. Cosmic carbon chemistry: From the interstellar medium to the early Earth. Cold Spring Harbor Perspect. Biol. 2010, 2, a002097. [Google Scholar] [CrossRef]
- Goncharuk, V.V.; Zui, O.V. Water and carbon dioxide as the main precursors of organic matter on Earth and in space. J. Water Chem. Technol. 2015, 37, 2–3. [Google Scholar] [CrossRef]
- Wołos, A.; Roszak, R.; Zadlo-Dobrowolska, A.; Beker, W.; Mikulak-Klucznik, B.; Spolnik, G.; Dygas, M.; Szymkuc, S.; Grzybowski, B.A. Synthetic connectivity, emergence, and self regeneration in the network of prebiotic chemistry. Science 2020, 369, 1584–1598. [Google Scholar] [CrossRef]
- Hamid, A.M.; Bera, P.P.; Lee, T.J.; Aziz, S.G.; Alyoubi, A.O.; El-Shall, M.S. Evidence for the formation of pyrimidine cations from the sequential reactions of hydrogen cyanide with the acetylene radical cation. J. Phys. Chem. Lett. 2014, 5, 3392–3398. [Google Scholar] [CrossRef]
- Eckhardt, A.K.; Linden, M.M.; Wende, R.C.; Bernhardt, B.; Schreiner, P.R. Gas-phase sugar formation using hydroxymethylene as the reactive formaldehyde isomer. Nat. Chem. 2018, 10, 1141–1147. [Google Scholar] [CrossRef]
- McGuire, B.A.; Burkhardt, A.M.; Kalenskii, S.; Shingledecker, C.N.; Remijan, A.J.; Herbst, E.; McCarthy, M.C. Detection of the aromatic molecule benzonitrile (c-C6H5CN) in the interstellar medium. Science 2018, 359, 202–205. [Google Scholar] [CrossRef]
- Zhou, X.; Tse, M.K.; Wu, D.D.; Mak, T.C.W.; Chan, K.S. Diverse reactivity of rhodium β-(tetraphenyl)tetraphenyl porphyrin chlorides with benzonitrile: Formation of Rh porphyrin arene and imine complexes. J. Organomet. Chem. 2020, 598, 80–86. [Google Scholar] [CrossRef]
- Foster, J.; Pincock, A.L.; Pincock, J.A.; Thompson, K.A. Photochemical addition of 2,2,2-trifluoroethanol to benzonitrile and p-, m-, and o-methylbenzonitrile. J. Am. Chem. Soc. 1998, 120, 13354–13361. [Google Scholar] [CrossRef]
- Das, S.; Singha, P.K.; Singh, A.K.; Datta, A. The role of hydrogen bonding in the preferential solvation of 5-aminoquinoline in binary solvent mixtures. J. Phys. Chem. B 2021, 125, 12763–12773. [Google Scholar] [CrossRef]
- Liszt, H.S.; Pety, J.; Lucas, R. Limits on chemical complexity in diffuse clouds: Search for CH3OH and HC5N absorption. Astron. Astrophys. 2008, 486, 493–496. [Google Scholar] [CrossRef]
- Bisschop, S.E.; Jørgensen, J.K.; Bourke, T.L.; Bottinelli, S.; van Dishoeck, E.F. An interferometric study of the low-mass protostar IRAS 16293–2422: Small scale organic chemistry. Astron. Astrophys. 2008, 488, 959–968. [Google Scholar] [CrossRef]
- Fárník, M.; Fedor, J.; Kocisek, J.; Lengyel, J.; Pluharova, E.; Poterya, V.; Pysanenko, A. Pickup and reactions of molecules on clusters relevant for atmospheric and interstellar processes. Phys. Chem. Chem. Phys. 2021, 23, 3195–3213. [Google Scholar] [CrossRef]
- Balucani, N.; Leonori, F.; Petrucci, R.; Stazi, M.; Skouteris, D.; Rosi, M.; Casavecchia, P. Formation of nitriles and imines in the atmosphere of Titan: Combined crossed-beam and theoretical studies on the reaction dynamics of excited nitrogen atoms N(2D) with ethane. Faraday Discuss. 2010, 147, 189–216. [Google Scholar] [CrossRef]
- Balucani, N. Elementary reactions and their role in gas-phase prebiotic chemistry. Int. J. Mol. Sci. 2009, 10, 2304–2335. [Google Scholar] [CrossRef]
- Wang, C.; Li, Q.; Kong, X.; Zheng, H.; Wang, T.; Zhao, Y.; Li, G.; Xie, H.; Yang, J.; Wu, G.; et al. Observation of carbon-carbon coupling reaction in neutral transition-metal carbonyls. J. Phys. Chem. Lett. 2021, 12, 1012–1017. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Jiang, N.; Zhang, Z.; Xie, M.; Hu, Y. Structural rearrangement of the acrylonitrile (AN) cluster in the gas phase under VUV one-photon radiation explored by mass-selected infrared spectroscopy. Spectrochim. Acta Part A 2020, 226, 117620. [Google Scholar] [CrossRef]
- Xie, M.; Sun, X.; Li, W.; Guan, J.; Liang, Z.; Hu, Y. A facile route for the formation of complex nitrogen-containing prebiotic molecules in the interstellar medium. J. Phys. Chem. Lett. 2022, 13, 8207–8213. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Han, B.; Chen, Z.; Zhang, X.; He, G.; Chen, G. Construction of natural-product-like cyclophane-braced peptide macrocycles via sp3 C-H arylation. J. Am. Chem. Soc. 2019, 141, 9401–9407. [Google Scholar] [CrossRef]
- Salonen, L.M.; Ellermann, M.; Diederich, F. Aromatic rings in chemical and biological recognition: Energetics and structures. Angew. Chem. Int. Ed. 2011, 50, 4808–4842. [Google Scholar] [CrossRef]
- Dopfer, O. IR spectroscopy of microsolvated aromatic cluster ions: Ionization-induced switch in aromatic molecule–solvent recognition. Z. Phys. Chem. 2005, 219, 125–168. [Google Scholar] [CrossRef]
- Enomoto, S.; Miyazaki, M.; Fujii, A.; Mikami, N. Electronic and infrared spectroscopy of [benzene-(methanol)n]+ (n =1–6). J. Phys. Chem. A 2005, 109, 9471–9480. [Google Scholar] [CrossRef]
- Chatterjee, K.; Dopfer, O. Switching of binding site from nonpolar to polar ligands toward cationic benzonitrile revealed by infrared spectroscopy. J. Chem. Phys. 2018, 149, 174315. [Google Scholar] [CrossRef]
- Kwok, W.M.; George, M.W.; Grills, D.C.; Ma, C.S.; Matousek, P.; Parker, A.W.; Phillips, D.; Toner, W.T.; Towrie, M. Direct observation of a hydrogen-bonded charge-transfer state of 4-dimethylaminobenzonitrile in methanol by time-resolved IR spectroscopy. Angew. Chem. Int. Edit. 2003, 42, 1826–1830. [Google Scholar] [CrossRef]
- Choi, S.; Park, J.; Kwak, K.; Cho, M. Substituent effects on the vibrational properties of the CN stretch mode of aromatic nitriles: IR probes useful for time-resolved IR Spectroscopy. Chem. Asian J. 2021, 16, 2626–2632. [Google Scholar] [CrossRef]
- Hachiya, M.; Matsuda, Y.; Suhara, K.; Mikami, N.; Fujii, A. Infrared predissociation spectroscopy of cluster cations of protic molecules, (NH3)n+, n = 2–4 and (CH3OH)n+, n = 2,3. J. Chem. Phys. 2008, 129, 094306. [Google Scholar] [CrossRef]
- Ishikawa, S.; Ebata, T.; Mikami, N. Structures and the vibrational relaxations of size-selected benzonitrile–(H2O)n=1–3 and-(CH3OH)n=1–3 clusters studied by fluorescence detected Raman and infrared spectroscopies. J. Chem. Phys. 1999, 110, 9504–9515. [Google Scholar] [CrossRef]
- Borst, D.R.; Pratt, D.W.; Schafer, M. Molecular recognition in the gas phase. Dipole-bound complexes of benzonitrile with water, ammonia, methanol, acetonitrile, and benzonitrile itself. Phys. Chem. Chem. Phys. 2007, 9, 4563–4571. [Google Scholar] [CrossRef]
- Yang, D.; Qi, R. Time-dependent density functional theory study on electronic excited-state hydrogen bonding of benzonitrile in methanol solution. J. Clust. Sci. 2014, 25, 1019–1028. [Google Scholar] [CrossRef]
- Sun, F.; Xie, M.; Zhang, Y.; Song, W.; Sun, X.; Hu, Y. Spectroscopic evidence of the C-N covalent bond formed between two interstellar molecules (ISM): Acrylonitrile and ammonia. Phys. Chem. Chem. Phys. 2021, 23, 9672–9678. [Google Scholar] [CrossRef]
- Hansen, P.E. A spectroscopic overview of intramolecular hydrogen bonds of NH···O, S, N type. Molecules 2021, 26, 2409. [Google Scholar] [CrossRef]
- Ohta, K.; Matsuda, Y.; Mikami, N.; Fujii, A. Intermolecular proton-transfer in acetic acid clusters induced by vacuum-ultraviolet photoionization. J. Chem. Phys. 2009, 131, 184304. [Google Scholar] [CrossRef]
- Bene, J.; Jordan, M. A comparative study of anharmonicity and matrix effects on the complexes XH:NH3, X=F, Cl, and Br. J. Chem. Phys. 1998, 108, 3205–3212. [Google Scholar] [CrossRef]
- Bene, J.; Szczepaniak, K.; Chabrier, P.; Person, W.B. Resolving discrepancies between theory and experiment: IR Spectrum of the proton-shared HBr:Pyridine complex. J. Phys. Chem. A 1997, 101, 4481–4483. [Google Scholar] [CrossRef]
- Ohno, K.; Maeda, S. Global reaction route mapping on potential energy surfaces of formaldehyde, formic acid, and their metal-substituted analogues. J. Phys. Chem. A 2006, 110, 8933–8941. [Google Scholar] [CrossRef]
- Maeda, S.; Ohno, K. Global mapping of equilibrium and transition structures on potential energy surfaces by the scaled hypersphere search method: Applications to ab initio surfaces of formaldehyde and propyne molecules. J. Phys. Chem. A 2005, 109, 5742–5753. [Google Scholar] [CrossRef]
- Ohno, K.; Maeda, S. A scaled hypersphere search method for the topography of reaction pathways on the potential energy surface. Chem. Phys. Lett. 2004, 384, 277–282. [Google Scholar] [CrossRef]
- Gauld, J.W.; Radom, L. Effects of neutral bases on the isomerization of conventional radical cations CH3X•+ to their distonic isomers • CH2X+ H (X = F, OH, NH2): Proton-transport catalysis and other mechanisms. J. Am. Chem. Soc. 1997, 119, 9831–9839. [Google Scholar] [CrossRef]
- Mosley, J.D.; Young, J.W.; Huang, M.; McCoy, A.B.; Duncan, M.A. Infrared spectroscopy of the methanol cation and its methylene-oxonium isomer. J. Chem. Phys. 2015, 142, 114301. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, Y.; Jiang, K.; Yang, H.; Pan, Y.; Sun, C. Gas phase retro-Michael reaction resulting from dissociative protonation: Fragmentation of protonated warfarin in mass spectrometry. J. Mass Spectrom. 2012, 47, 1059–1064. [Google Scholar] [CrossRef]
- Hu, Y.; Guan, J.; Bernstein, E.R. Mass-selected IR-VUV (118 nm) spectroscopic studies of radicals, aliphatic molecules, and their clusters. Mass Spectrom. Rev. 2013, 32, 484–501. [Google Scholar] [CrossRef]
- Zhan, H.; Hu, Y.; Wang, P.; Chen, J. Molecular structures of gas-phase neutral morpholine and its monohydrated complexes: Experimental and theoretical approaches. RSC Adv. 2017, 7, 6179–6186. [Google Scholar] [CrossRef]
- Zhang, Z.; Nie, W.; Sun, F.; Zhang, Y.; Xie, M.; Hu, Y. Conformational landscapes and infrared spectra of gas-phase interstellar molecular clusters [(C3H3N)(CH3OH)n, n = 1–4]. J. Phys. Chem. A 2020, 124, 2398–2407. [Google Scholar] [CrossRef]
- Song, W.; Hu, Y.; Jin, S.; Li, Y. Dissociative photoionization of heterocyclic molecule-morpholine under VUV synchrotron radiation. Chin. J. Chem. Phys. 2019, 32, 259–266. [Google Scholar] [CrossRef]
- Matsuda, Y.; Mikami, N.; Fujii, A. Vibrational spectroscopy of size-selected neutral and cationic clusters combined with vacuum ultraviolet one-photon ionization detection. Phys. Chem. Chem. Phys. 2009, 11, 1279–1290. [Google Scholar] [CrossRef]
- Rahuman, M.H.; Muthu, S.; Raajaraman, B.R.; Raja, M.; Umamahesvari, H. Investigations on 2-(4-Cyanophenylamino) acetic acid by FT-IR, FT-Raman, NMR and UV-Vis spectroscopy, DFT (NBO, HOMO-LUMO, MEP and Fukui function) and molecular docking studies. Heliyon 2020, 6, e04976. [Google Scholar] [CrossRef]
- Weinberg, J.; Lerner, D.A. Theoretical study of 5-HTP. potential new drug resulting from the complexation of 5-HTP with ATP. J. Comput. Chem. 2013, 1, 1–4. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Hu, Y. Infrared Spectroscopy of Neutral and Cationic Benzonitrile–Methanol Binary Clusters in Supersonic Jets. Molecules 2024, 29, 2744. https://doi.org/10.3390/molecules29122744
Xiong X, Hu Y. Infrared Spectroscopy of Neutral and Cationic Benzonitrile–Methanol Binary Clusters in Supersonic Jets. Molecules. 2024; 29(12):2744. https://doi.org/10.3390/molecules29122744
Chicago/Turabian StyleXiong, Xianming, and Yongjun Hu. 2024. "Infrared Spectroscopy of Neutral and Cationic Benzonitrile–Methanol Binary Clusters in Supersonic Jets" Molecules 29, no. 12: 2744. https://doi.org/10.3390/molecules29122744
APA StyleXiong, X., & Hu, Y. (2024). Infrared Spectroscopy of Neutral and Cationic Benzonitrile–Methanol Binary Clusters in Supersonic Jets. Molecules, 29(12), 2744. https://doi.org/10.3390/molecules29122744





