The TaCl5-Mediated Reaction of Dimethyl 2-Phenylcyclopropane-1,1-dicarboxylate with Aromatic Aldehydes as a Route to Substituted Tetrahydronaphthalenes
Abstract
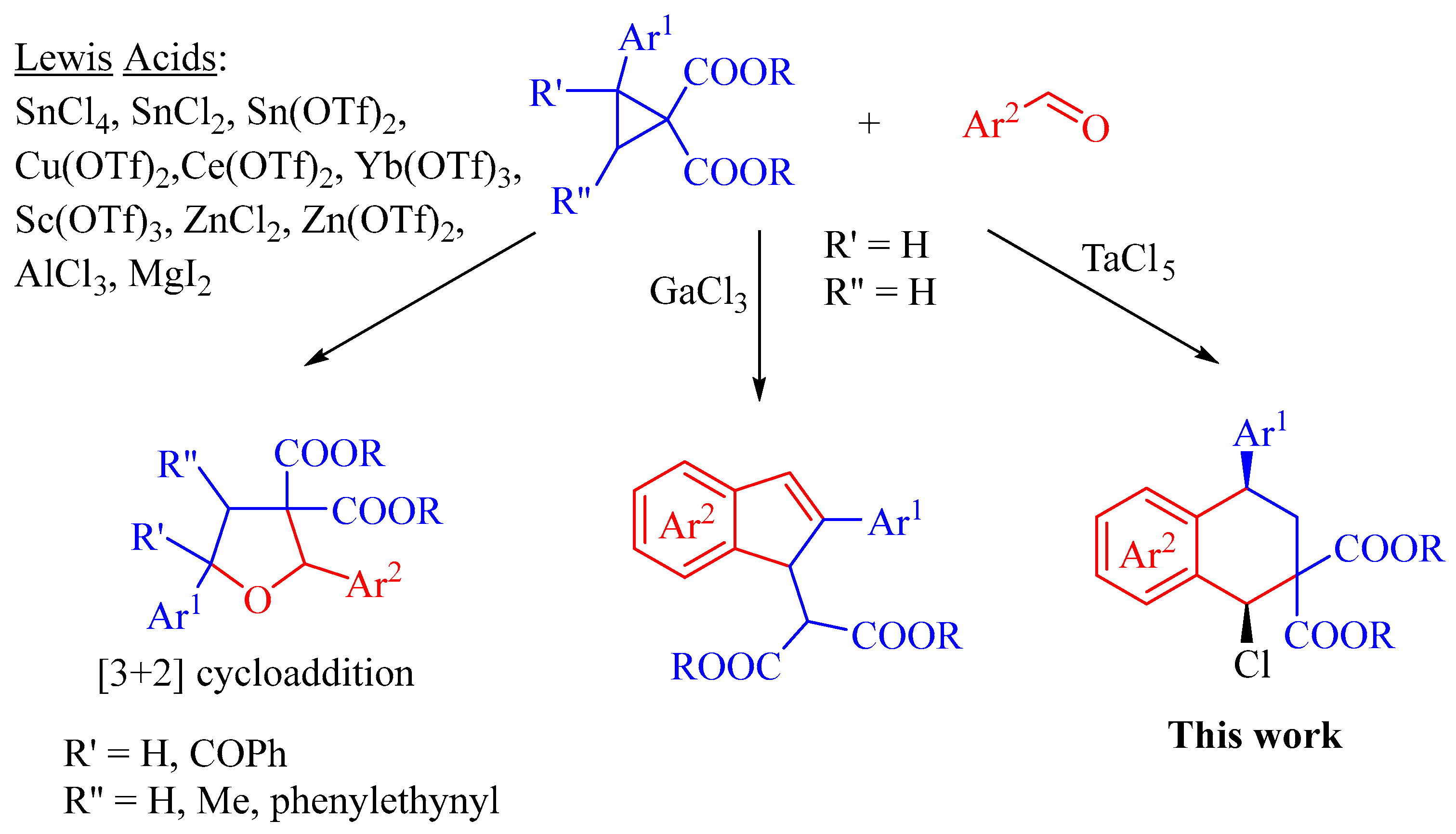
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Preparation of 4-Phenyl-3,4-dihydronaphtalene-2,2(1H)-dicarboxylate 2a–g via Reaction of Dimethyl 2-Phenylcyclopropane-1,1-dicarboxylate and Benzaldehyde Derivative
3.2.1. Dimethyl 1-Chloro-6-iodo-4-phenyl-3,4-dihydronaphtalene-2,2(1H)-dicarboxylate (2a) (Typical Procedure)
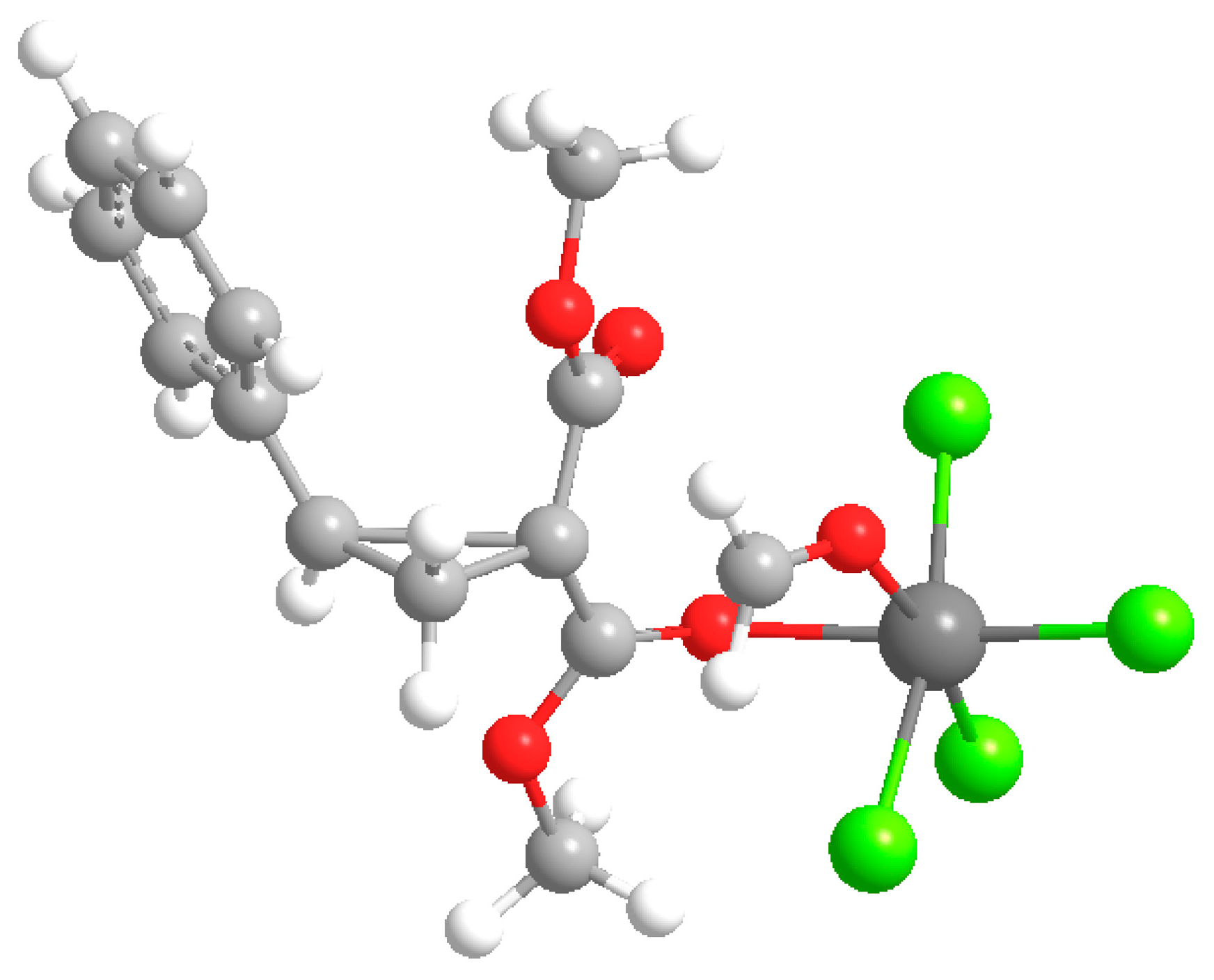
3.2.2. Crystal Data for Dimethyl 1-Chloro-6-iodo-4-phenyl-3,4-dihydronaphtalene-2,2(1H)-dicarboxylate (2a)
3.2.3. Dimethyl 6-Bromo-1-chloro-4-phenyl-3,4-dihydronaphtalene-2,2(1H)-dicarboxylate (2b)
3.2.4. Dimethyl 1,6-Dichloro-4-phenyl-3,4-dihydronaphtalene-2,2(1H)-dicarboxylate (2c)
3.2.5. Dimethyl 1-Chloro-6-fluoro-4-phenyl-3,4-dihydronaphtalene-2,2(1H)-dicarboxylate (2d)
3.2.6. Dimethyl 1-Chloro-6-methyl-4-phenyl-3,4-dihydronaphtalene-2,2(1H)-dicarboxylate (2e)
3.2.7. Dimethyl 1,7-Dichloro-4-phenyl-3,4-dihydronaphthalene-2,2(1H)-dicarboxylate (2f)
3.2.8. Dimethyl 1-Chloro-8-fluoro-4-phenyl-3,4-dihydronaphthalene-2,2(1H)-dicarboxylate (2g)
3.2.9. Dimethyl 2-(2-Chloro-2-phenylethyl)malonate (3) [21]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, T.F.; Kaschel, J.; Werz, D.B. A New Golden Age for Donor–Acceptor Cyclopropanes. Angew. Chem. Int. Ed. 2014, 53, 5504–5523. [Google Scholar] [CrossRef] [PubMed]
- De Nanteuil, F.; De Simone, F.; Frei, R.; Benfatti, F.; Serrano, E.; Waser, J. Cyclization and annulation reactions of nitrogen-substituted cyclopropanes and cyclobutanes. Chem. Commun. 2014, 50, 10912–10928. [Google Scholar] [CrossRef] [PubMed]
- Novikov, R.A.; Tomilov, Y.V. Dimerization of donor–acceptor cyclopropanes. Mendeleev Commun. 2015, 25, 1–10. [Google Scholar] [CrossRef]
- Grover, H.K.; Emmett, M.R.; Kerr, M.A. Carbocycles from donor–acceptor cyclopropanes. Org. Biomol. Chem. 2015, 13, 655–671. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Varshnaya, R.K.; Dey, R.; Banerjee, P. Donor–Acceptor Cyclopropanes as an Expedient Building Block Towards the Construction of Nitrogen-Containing Molecules: An Update. Adv. Synth. Catal. 2020, 362, 1447–1484. [Google Scholar] [CrossRef]
- Tang, P.; Wei, Y.Y.; Wen, L.; Ma, H.J.; Yang, Y.; Jiang, Y. MgI2-Catalyzed Nucleophilic Ring-Opening Reactions of Donor–Acceptor Cyclopropanes with Indoline-2-thiones. J. Org. Chem. 2022, 87, 10890–10901. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Shen, P.; Sun, D.; Zhai, H.; Zhao, Y. The Regioselective Functionalization Reaction of Unprotected Carbazoles with Donor–Acceptor Cyclopropanes. J. Org. Chem. 2021, 86, 9189–9199. [Google Scholar] [CrossRef]
- Mishra, M.; Verma, K.; Banerjee, S.; Punniyamurthy, T. Iron-catalyzed cascade CC/CO bond formation of 2, 4-dienals with donor-acceptor cyclopropanes: Access to functionalized hexahydrocyclopentapyrans. Chem. Commun. 2024, 60, 2788–2791. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, X.; Xiao, W.; Chang, W.; Li, J. Divergent Copper-salt-controlled Reactions of Donor-Acceptor Cyclopropanes and N-Fluorobenzene Sulfonimide: Access to the 1, 3-Haloamines and Aminoindanes. Chem.-A Eur. J. 2023, 29, e202202544. [Google Scholar] [CrossRef] [PubMed]
- Guin, A.; Deswal, S.; Biju, A.T. Ring-Opening 1, 3-Carbothiolation of Donor–Acceptor Cyclopropanes Using Alkyl Halides and In Situ Generated Dithiocarbamates. J. Org. Chem. 2022, 87, 6504–6513. [Google Scholar] [CrossRef] [PubMed]
- Donor-Acceptor Cyclopropanes in Organic Synthesis; Banerjee, P., Biju, A.T., Eds.; Wiley-VCH GmbH: Weinheim, Germany, 2024; ISBN 978-3-527-83565-2. [Google Scholar]
- Pohlhaus, P.D.; Johnson, J.S. Enantiospecific Sn(II)- and Sn(IV)-catalyzed cycloadditions of aldehydes and donor–acceptor cyclopropanes. J. Am. Chem. Soc. 2005, 127, 16014–16015. [Google Scholar] [CrossRef] [PubMed]
- Pohlhaus, P.D.; Sanders, S.D.; Parsons, A.T.; Li, W.; Johnson, J.S. Scope and mechanism for lewis acid-catalyzed cycloadditions of aldehydes and donor− acceptor cyclopropanes: Evidence for a stereospecific intimate ion pair pathway. J. Am. Chem. Soc. 2008, 30, 8642–8650. [Google Scholar] [CrossRef]
- Parsons, A.T.; Campbell, M.J.; Johnson, J.S. Diastereoselective synthesis of tetrahydrofurans via palladium(0)-catalyzed [3+2] cycloaddition of vinylcyclopropanes and aldehydes. Org. Lett. 2008, 10, 2541–2544. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Shen, Y.; Li, K.; Sun, Y.; Hua, Y. AlCl3-Promoted Highly Regio-and Diastereoselective [3+2] Cycloadditions of Activated Cyclopropanes and Aromatic Aldehydes: Construction of 2, 5-Diaryl-3, 3, 4-trisubstituted Tetrahydrofurans. J. Org. Chem. 2011, 76, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Slade, M.C.; Johnson, J.S. Cyclopropane–Aldehyde Annulations at Quaternary Donor Sites: Stereoselective Access to Highly Substituted Tetrahydrofurans. Org. Lett. 2011, 13, 1996–1999. [Google Scholar] [CrossRef] [PubMed]
- Haubenreisser, S.; Hensenne, P.; Schröder, S.; Niggemann, M. The alkynyl moiety as a donor for donor–acceptor cyclopropanes. Org. Lett. 2013, 15, 2262–2265. [Google Scholar] [CrossRef]
- Benfatti, F.; Nanteuil, F.; Waser, J. Iron-catalyzed [3+2] annulation of aminocyclopropanes with aldehydes: Stereoselective synthesis of aminotetrahydrofurans. Org. Lett. 2012, 14, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Hashimoto, Y.; Saigo, K. Ring-opening aldol-type reaction of 2,2-dialkoxycyclopropanecarboxylic esters with carbonyl compounds. 3. The diastereoselective synthesis of 2, 3, 4-trisubstituted gamma-lactones. J. Org. Chem. 1993, 58, 5226–5234. [Google Scholar] [CrossRef]
- Borisov, D.D.; Novikov, R.A.; Tomilov, Y.V. GaCl3-Mediated Reactions of Donor–Acceptor Cyclopropanes with Aromatic Aldehydes. Angew. Chem. Int. Ed. 2016, 55, 12233–12237. [Google Scholar] [CrossRef] [PubMed]
- Makar, S.; Saha, T.; Singh, S.K. Naphthalene, a versatile platform in medicinal chemistry: Sky-high perspective. Eur. J. Med. Chem. 2019, 161, 252–276. [Google Scholar] [CrossRef] [PubMed]
- Elrayess, A.A.; Elshihawy, H. Naphthalene: An overview. Rec. Pharm. Biomed. Sci. 2023, 7, 145–153. [Google Scholar] [CrossRef]
- Novikov, R.A.; Balakirev, D.O.; Timofeev, V.P.; Tomilov, Y.V. Complexes of Donor–Acceptor Cyclopropanes with Tin, Titanium, and Gallium Chlorides—Mechanism Studies. Organometallics 2012, 31, 8627–8638. [Google Scholar] [CrossRef]
- Marchetti, F.; Pampaloni, G. Interaction of niobium and tantalum pentahalides with O-donors: Coordination chemistry and activation reactions. Chem. Commun. 2012, 48, 635–653. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.C.; Zhou, L.; Kirillov, A.M.; Fang, R.; Yang, L. Computational study on GaCl3-mediated reactions of donor–acceptor cyclopropanes with aromatic aldehydes: Mechanism and role of GaCl3 and aldehydes. Org. Chem. Front. 2018, 5, 1702–1712. [Google Scholar] [CrossRef]
- Gabdullin, A.M.; Kadikova, R.N.; Mozgovoj, O.S.; Ramazanov, I.R. TaCl5-Catalyzed Amidation of Carboxylic Acids with Amines. ChemistrySelect 2023, 8, e202204298. [Google Scholar] [CrossRef]
- Gabdullin, A.M.; Kadikova, R.N.; Yulbarisov, A.B.; Mozgovoi, O.S.; Ramazanov, I.R. TaCl5 in the synthesis of amides from saturated monobasic carboxylic acids and functionally substituted primary aromatic amines. Russ. Chem. Bull. 2023, 72, 2350–2356. [Google Scholar] [CrossRef]
- Hayashi, R.; Cook, G.R. Remarkably mild and efficient intramolecular Friedel− Crafts cyclization catalyzed by In (III). Org. Lett. 2007, 9, 1311–1314. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Monot, J.; El-Hellani, A.; Michelet, B.; Guillot, R.; Bour, C.; Gandon, V. Cationic Gallium (III) Halide Complexes: A New Generation of π-Lewis Acids. Chem.-A Eur. J. 2012, 18, 10239–10243. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Ueda, M.; Yamasaki, N.; Fujii, A.; Sasaki, I.; Igawa, K.; Kasai, Y.; Imagawa, H.; Nishizawa, M. Aryl–Allene Cyclization via a Hg(OTf)2-Catalytic Pathway. Org. Lett. 2016, 18, 2864–2867. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, X.; Yao, R.; Li, C.; Xu, Z.; Zhang, L.; Han, G.; Hou, J.; Liu, Y.; Song, Y. Iron-Catalyzed Alkene Trifluoromethylation in Tandem with Phenol Dearomatizing Spirocyclization: Regioselective Construction of Trifluoromethylated Spirocarbocycles. Adv. Synth. Catal. 2022, 364, 637–642. [Google Scholar] [CrossRef]
- Lu, Z.; Hu, C.; Guo, J.; Li, J.; Cui, Y.; Jia, Y. Water-controlled regioselectivity of Pd-catalyzed domino reaction involving a C–H activation process: Rapid synthesis of diverse carbo-and heterocyclic skeletons. Org. Lett. 2010, 12, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zuo, F.; Wang, B.; Xian, X.; Tang, J.; Zhang, H.; Zhang, Z.; Ke, Q.; Chen, W. Highly efficient catalyst for 1,1,2-trichloroethane dehydrochlorination via BN3 frustrated Lewis acid-base pairs. Nano Res. 2024, 17, 4773–4781. [Google Scholar] [CrossRef]
- Fringuelli, F.; Germani, R.; Pizzo, F.; Savelli, G. One-pot two-steps synthesis of 1,2-diol. Synth. Commun. 1989, 19, 1939–1943. [Google Scholar] [CrossRef]
- Doppiu, A.; Salzer, A. A New Route to Cationic Half-Sandwich Ruthenium (II) Complexes with Chiral Cyclopentadienylphosphane Ligands. Eur. J. Inorg. Chem. 2004, 2004, 2244–2252. [Google Scholar] [CrossRef]
- Sapeta, K.; Kerr, M.A. The cycloaddition of nitrones with homochiral cyclopropanes. J. Org. Chem. 2007, 72, 8597–8599. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1.; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
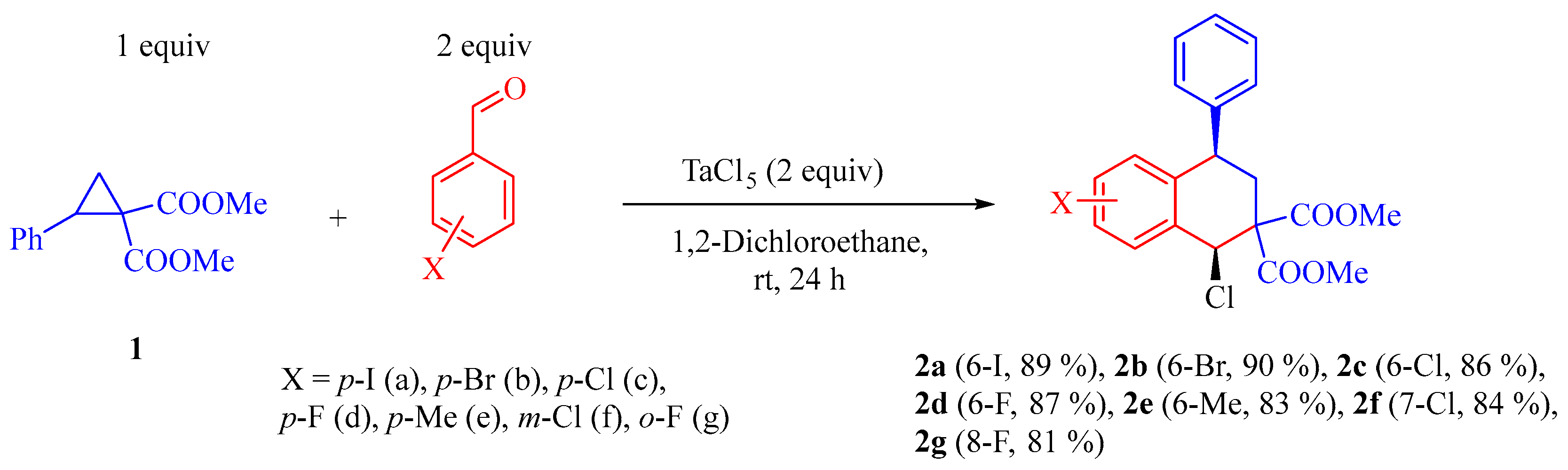

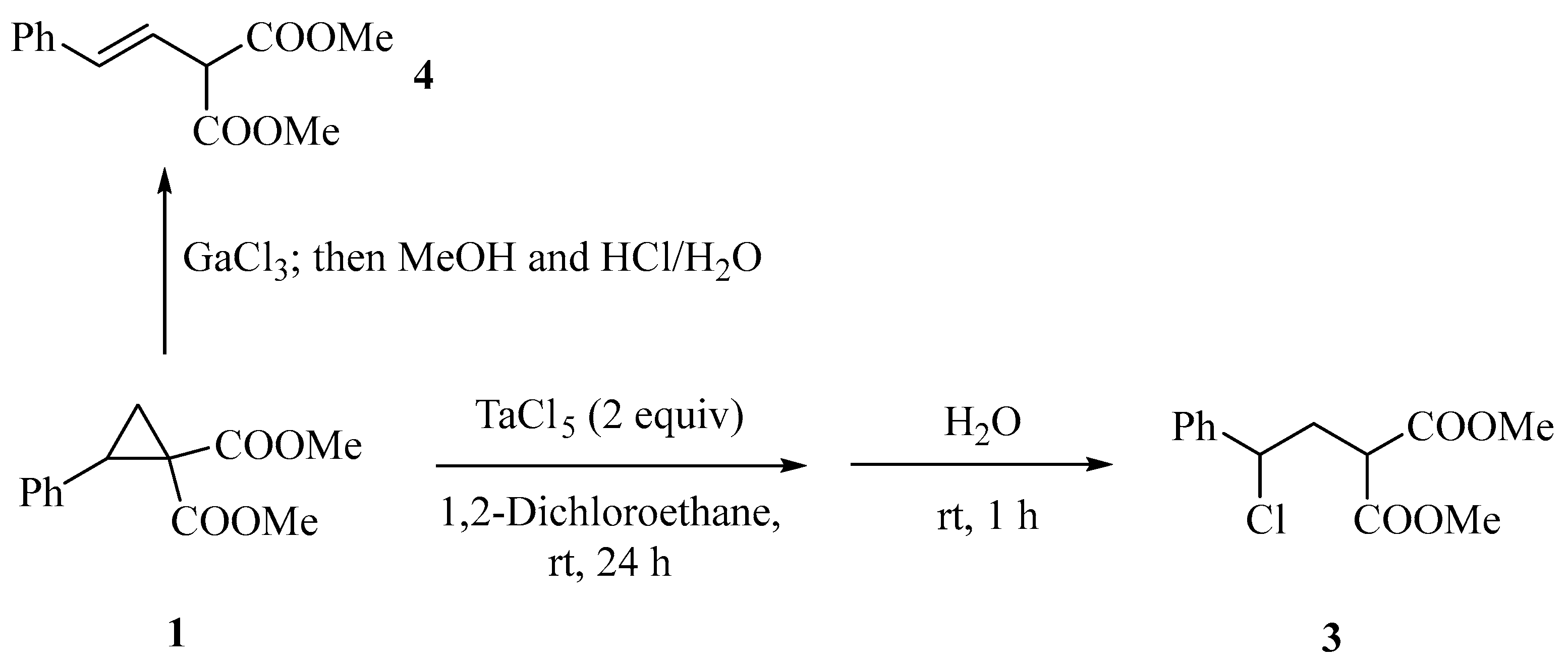


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zosim, T.P.; Kadikova, R.N.; Novikov, R.A.; Korlyukov, A.A.; Mozgovoj, O.S.; Ramazanov, I.R. The TaCl5-Mediated Reaction of Dimethyl 2-Phenylcyclopropane-1,1-dicarboxylate with Aromatic Aldehydes as a Route to Substituted Tetrahydronaphthalenes. Molecules 2024, 29, 2715. https://doi.org/10.3390/molecules29122715
Zosim TP, Kadikova RN, Novikov RA, Korlyukov AA, Mozgovoj OS, Ramazanov IR. The TaCl5-Mediated Reaction of Dimethyl 2-Phenylcyclopropane-1,1-dicarboxylate with Aromatic Aldehydes as a Route to Substituted Tetrahydronaphthalenes. Molecules. 2024; 29(12):2715. https://doi.org/10.3390/molecules29122715
Chicago/Turabian StyleZosim, Tat’yana P., Rita N. Kadikova, Roman A. Novikov, Alexander A. Korlyukov, Oleg S. Mozgovoj, and Ilfir R. Ramazanov. 2024. "The TaCl5-Mediated Reaction of Dimethyl 2-Phenylcyclopropane-1,1-dicarboxylate with Aromatic Aldehydes as a Route to Substituted Tetrahydronaphthalenes" Molecules 29, no. 12: 2715. https://doi.org/10.3390/molecules29122715
APA StyleZosim, T. P., Kadikova, R. N., Novikov, R. A., Korlyukov, A. A., Mozgovoj, O. S., & Ramazanov, I. R. (2024). The TaCl5-Mediated Reaction of Dimethyl 2-Phenylcyclopropane-1,1-dicarboxylate with Aromatic Aldehydes as a Route to Substituted Tetrahydronaphthalenes. Molecules, 29(12), 2715. https://doi.org/10.3390/molecules29122715





