High H2O-Assisted Proton Conduction in One Highly Stable Sr(II)-Organic Framework Constructed by Tetrazole-Based Imidazole Dicarboxylic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystal Structure
2.2. Infrared Spectral Analysis
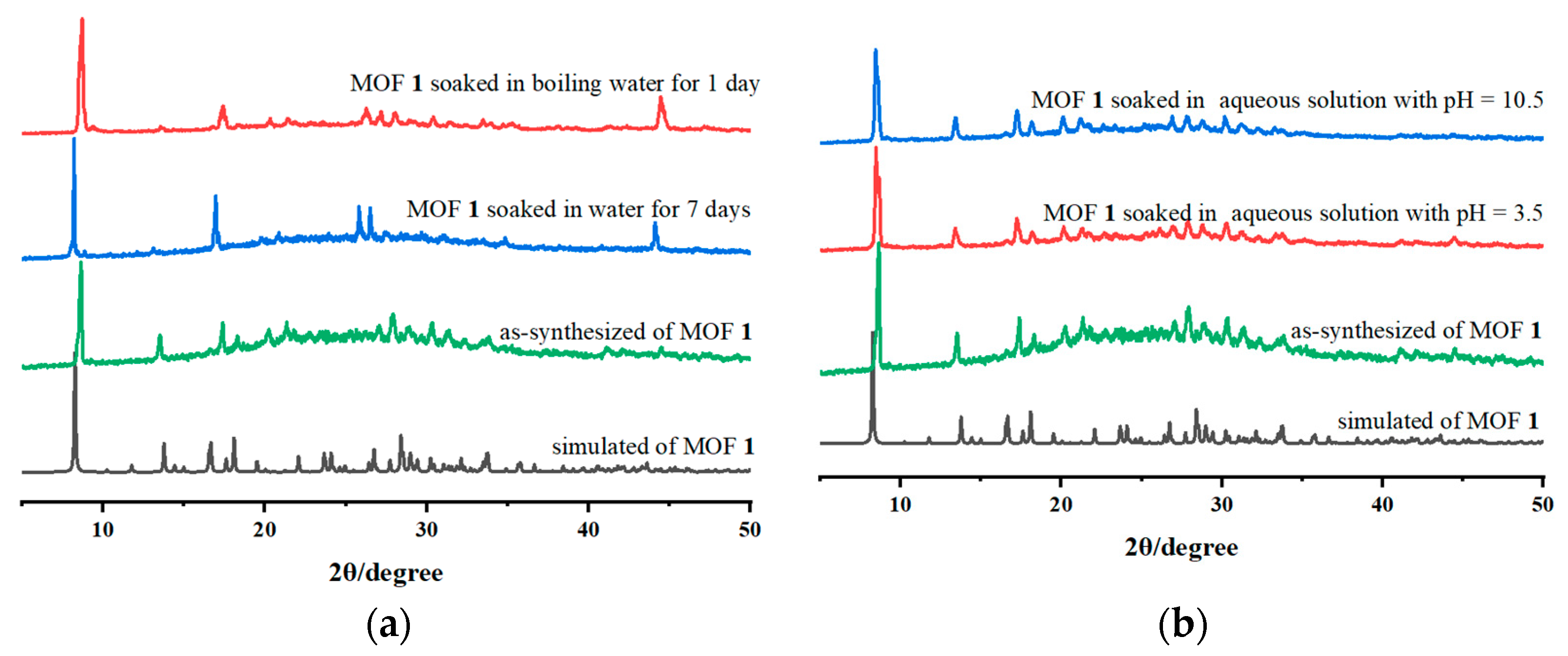
2.3. Stability Analysis
2.4. Porosity Analysis
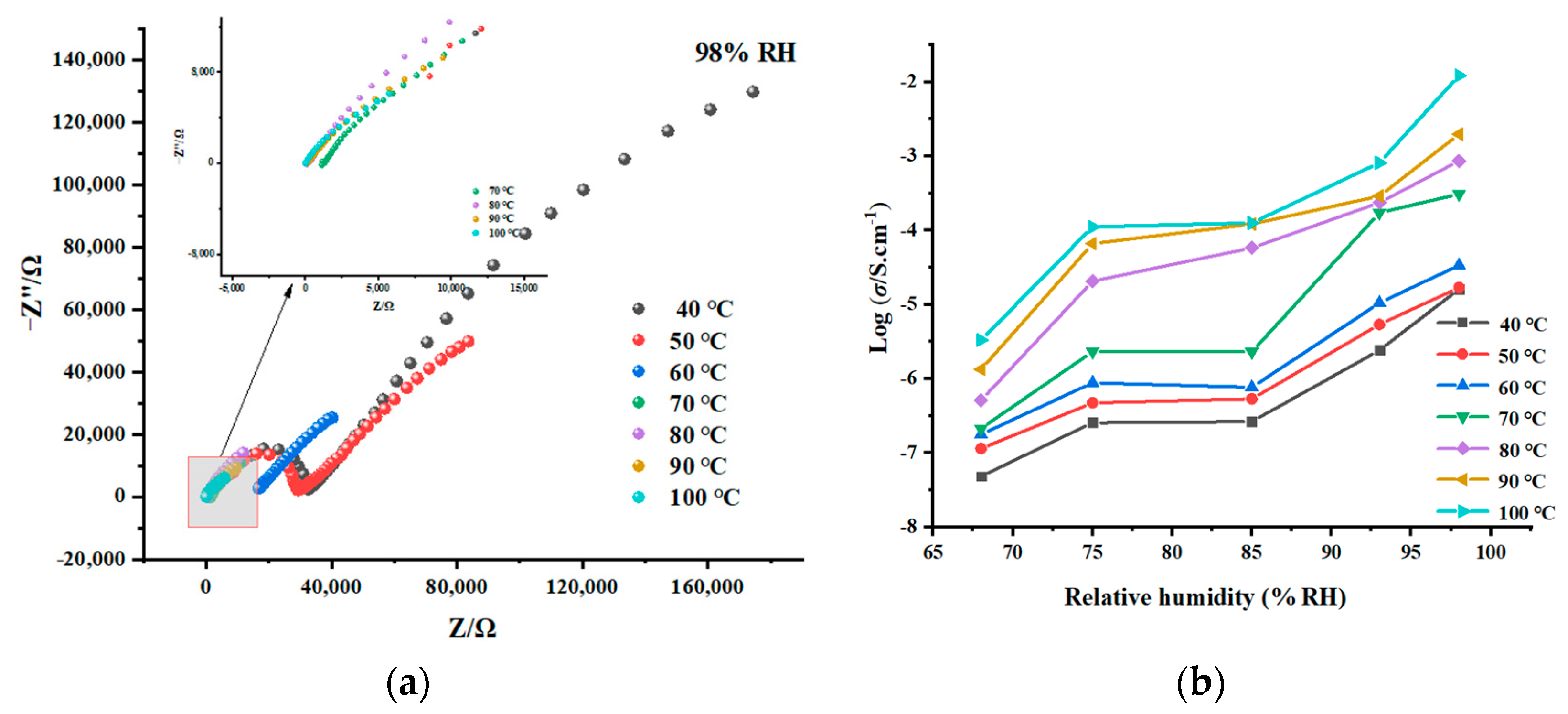
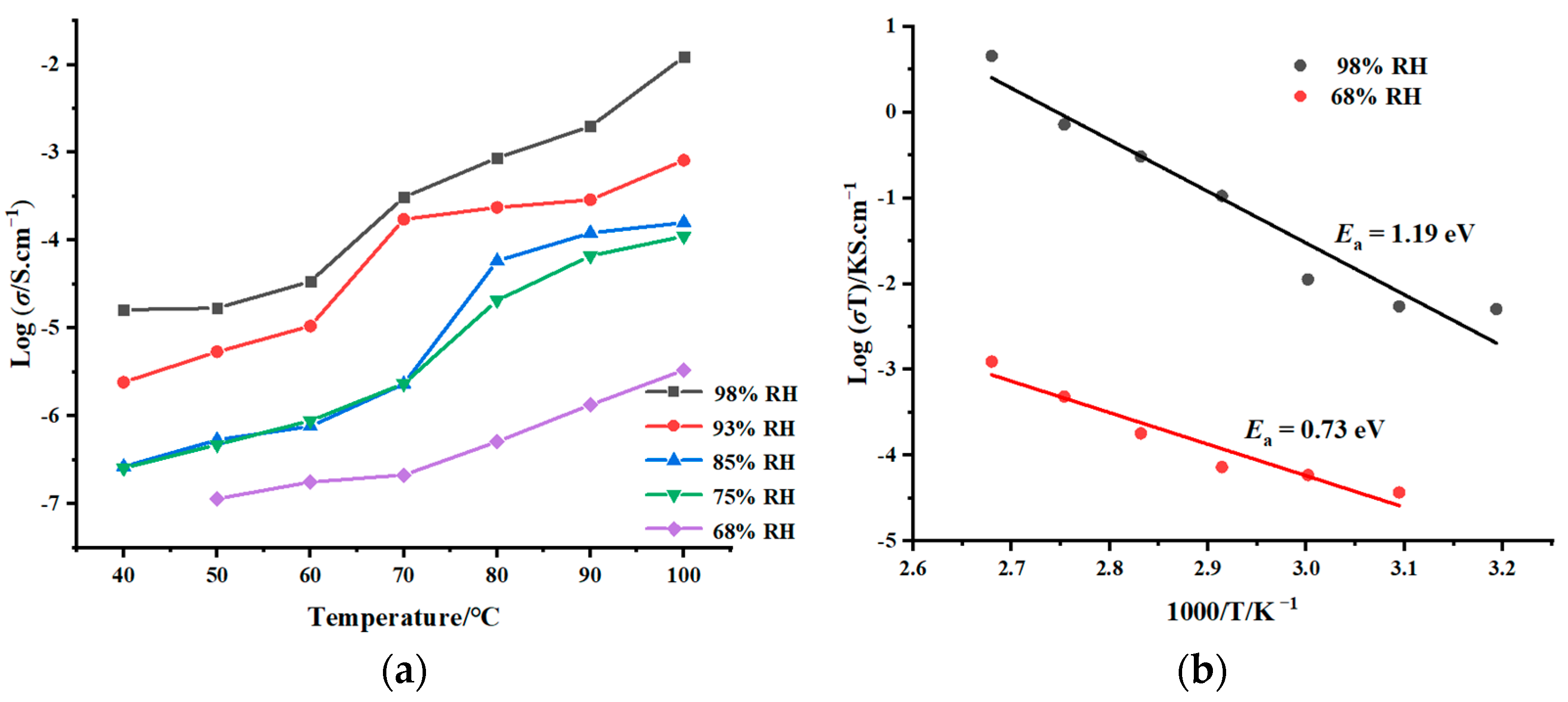
2.5. Proton-Conducting Properties
3. Materials and Methods
3.1. Materials
3.2. Determinations
3.3. Preparation of MOF 1
3.4. Crystal Structure Measurement
3.5. Stability Determinations
3.6. Proton-Conducting Determinations
3.7. The Simulation of Nyquist Plots
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asghar, M.R.; Xu, Q. A review of advancements in commercial and non-commercial Nafion-based proton exchange membranes for direct methanol fuel cells. J. Polym. Res. 2024, 31, 125. [Google Scholar] [CrossRef]
- Yang, G.; Lee, C.; Qiao, X.; Babu, S.K.; Martinez, U.; Spendelow, J.S. Advanced electrode structures for proton exchange membrane fuel cells: Current status and path forward. Electrochem. Energy Rev. 2024, 7, 9. [Google Scholar] [CrossRef]
- Yang, B.; Xiang, Z. Nanostructure engineering of cathode layers in proton exchange membrane fuel cells: From catalysts to membrane electrode assembly. ACS Nano 2024, 18, 11598–11630. [Google Scholar] [CrossRef]
- Yaldagard, M.; Arkas, M. Enhanced mass activity and durability of bimetallic Pt-Pd nanoparticles on sulfated-zirconia-doped graphene nanoplates for oxygen reduction reaction in proton exchange membrane fuel cell applications. Molecules 2024, 29, 2129. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolsky, Y.A.; Jannasch, P.; Lafitte, B.; Belomoina, N.M.; Rusanov, A.L.; Likhachev, D.Y. Achievements in the field of proton-conductive portion electrolyte membranes. Russ. J. Electrochem. 2007, 43, 489–501. [Google Scholar] [CrossRef]
- Hu, Y.; Li, X.; Yan, L.; Yue, B. Improving the overall characteristics of proton exchange membranes via nanophase separation technologies: A progress review. Fuel Cells 2017, 17, 3–17. [Google Scholar] [CrossRef]
- Prykhodko, Y.; Fatyeyeva, K.; Hespel, L.; Marais, S. Progress in hybrid composite Nafion®-based membranes for proton exchange fuel cell application. Chem. Eng. J. 2021, 409, 127329. [Google Scholar] [CrossRef]
- Kolokolov, D.I.; Lim, D.; Kitagawa, H. Characterization of proton dynamics for the understanding of conduction mechanism in proton conductive metal-organic frameworks. Chem. Rec. 2020, 20, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yang, Y.; Dou, B.; Li, Z.; Li, G. Proton conductive carboxylate-based metal-organic frameworks. Coord. Chem. Rev. 2020, 403, 213100. [Google Scholar] [CrossRef]
- Liu, R.; Wang, D.; Shi, J.; Li, G. Proton conductive metal sulfonate frameworks. Coord. Chem. Rev. 2021, 431, 213747. [Google Scholar] [CrossRef]
- Chen, X.; Li, G. Proton conductive Zr-based MOFs. Inorg. Chem. Front. 2020, 7, 3765–3784. [Google Scholar] [CrossRef]
- Lim, D.; Kitagawa, H. Proton transport in metal-organic frameworks. Chem. Rev. 2020, 120, 8416–8467. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, Y.; Zhuang, Q.; Li, G. Recent advances in MOFs-based proton exchange membranes. Coord. Chem. Rev. 2022, 471, 214740. [Google Scholar] [CrossRef]
- Sahoo, R.; Pal, S.C.; Das, M.C. Solid-state proton conduction driven by coordinated water molecules in metal-organic frameworks and coordination polymers. ACS Energy Lett. 2022, 7, 4490–4500. [Google Scholar] [CrossRef]
- Jhariat, P.; Kumari, P.; Panda, T. Structural features of proton-conducting metal organic and covalent organic frameworks. CrystEngComm 2020, 22, 6425–6443. [Google Scholar] [CrossRef]
- Sahoo, R.; Luo, S.; Pendyala, N.K.; Chand, S.; Fu, Z.; Das, M.C. Coordinated water molecule-induced solid-state superprotonic conduction by a highly scalable and pH-stable coordination polymer (CP). Mater. Chem. Front. 2023, 7, 3373–3381. [Google Scholar] [CrossRef]
- Bao, S.-S.; Shimizu, G.K.H.; Zheng, L.-M. Proton conductive metal phosphonate frameworks. Coord. Chem. Rev. 2019, 378, 577–594. [Google Scholar] [CrossRef]
- Han, B.-X.; Jiang, Y.-F.; Sun, X.-R.; Li, Z.-F.; Li, G. Proton conductive N-heterocyclic metal-organic frameworks. Coordin. Chem. Rev. 2021, 432, 213754. [Google Scholar] [CrossRef]
- Ye, Y.; Gong, L.; Xiang, S.; Zhang, Z.; Chen, B. Metal-organic frameworks as a versatile platform for proton conductors. Adv. Mater. 2020, 32, 1907090. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.-L.; Xue, M.; Liu, Y.-Y.; Yu, Y.-H.; Liu, Y.-R.; Li, G. Proton conductive metal–organic frameworks based on main-group metals. Coordin. Chem. Rev. 2022, 452, 214301. [Google Scholar] [CrossRef]
- Ren, H.-M.; Wang, H.-W.; Jiang, Y.-F.; Tao, Z.-X.; Mu, C.-Y.; Li, G. Proton conductive lanthanide-based metal-organic frameworks: Synthesis strategies, structural features and recent progress. Top. Curr. Chem. 2022, 380, 9. [Google Scholar] [CrossRef]
- Tian, Y.; Liang, G.; Fan, T.; Shang, J.; Shang, S.; Ma, Y.; Matsuda, R.; Liu, M.; Wang, M.; Li, L.; et al. Grafting free carboxylic acid groups onto the pore surface of 3D porous coordination polymers for high proton conductivity. Chem. Mat. 2019, 31, 8494–8503. [Google Scholar] [CrossRef]
- Zou, L.; Yao, S.; Zhao, J.; Li, D.; Li, G.; Huo, Q.; Liu, Y. Enhancing proton conductivity in a 3D metal-organic framework by the cooperation of guest [Me2NH2]+ cations, water molecules, and host carboxylates. Cryst. Growth Des. 2017, 17, 3556–3561. [Google Scholar] [CrossRef]
- Qin, Y.; Xue, M.; Dou, B.; Sun, Z.; Li, G. High protonic conduction in two metal-organic frameworks containing high-density carboxylic groups. New J. Chem. 2020, 44, 2741–2748. [Google Scholar] [CrossRef]
- Elahi, S.M.; Chand, S.; Deng, W.; Pal, A.; Das, M.C. Polycarboxylate-templated coordination polymers: Role of templates for superprotonic conductivities of up to 10−1 S cm−1. Angew. Chem. Int. Ed. Engl. 2018, 57, 6662–6666. [Google Scholar] [CrossRef]
- Zhuang, Q.; Kang, L.; Zhang, B.; Li, Z.; Li, G. Remarkable water-mediated proton conductivity of two porous zirconium (IV)/hafnium(IV) metal-organic frameworks bearing porphyrinlcarboxylate ligands. J. Colloid. Interface Sci. 2024, 657, 482–490. [Google Scholar] [CrossRef]
- Liu, X.; Huang, S.; Lian, Y.; Dong, X.; Zang, S. Electrostatic attraction induces cationic covalent-organic framework to pack inorganic acid ions for promoting proton conduction. Chem. Commun. 2022, 58, 6084–6087. [Google Scholar] [CrossRef]
- Dong, X.; Wang, R.; Wang, J.; Zang, S.; Mak, T.C.W. Highly selective fe3+ sensing and proton conduction in a water-stable sulfonate-carboxylate tb-organic-framework. J. Mater. Chem. A 2015, 3, 641–647. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Liu, S.; Han, Z.; Tang, Q.; Li, F.; Zang, S. Synergy between isomorphous acid and basic metal-organic frameworks for anhydrous proton conduction of low-cost hybrid membranes at high temperatures. ACS Appl. Mater. Interfaces 2018, 10, 38209–38216. [Google Scholar] [CrossRef]
- Meilin, W.; Xiaoxiang, W.; Jingjing, S.; Xianying, D. A 3D POM-MOF composite based on Ni(II) ion and 2,2’-bipyridyl-3,3’-dicarboxylic acid: Crystal structure and proton conductivity. J. Solid State Chem. 2013, 202, 200–206. [Google Scholar]
- Qi, X.; Wu, W.; Qin, L.; Zhang, R.; Zhu, X.; Zhang, X.; Lun, H.; Li, Y.M. Three keggin poms-based coordination polymers constructed by linear N-heterocyclic ligand for proton conduction, photocatalytic activity and magnetic property. J. Solid State Chem. 2022, 312, 123167. [Google Scholar] [CrossRef]
- Niu, X.; Yu, Y.; Mu, C.; Xie, X.; Liu, Y.; Liu, Z.; Li, L.; Li, G.; Li, J. High proton conduction in two highly water-stable lanthanide coordination polymers from a triazole multicarboxylate ligand. Inorg. Chem. 2021, 60, 13242–13251. [Google Scholar] [CrossRef]
- Jia, H.; Han, B.; Li, G. Two proton-conductive metal-organic frameworks constructed by pyridyl imidazole dicarboxylate ligands. J. Solid State Chem. 2023, 327, 124268. [Google Scholar] [CrossRef]
- Panda, T.; Kundu, T.; Banerjee, R. Self-assembled one-dimensional functionalized metal-organic nanotubes (MONTs) for proton conduction. Chem. Commun. 2012, 48, 5464–5466. [Google Scholar] [CrossRef]
- Sun, Z.; Yu, S.; Zhao, L.; Wang, J.; Li, Z.; Li, G. A highly stable two-dimensional copper(ii) organic framework for proton conduction and ammonia impedance sensing. Chem.-Eur. J. 2018, 24, 10829–10839. [Google Scholar] [CrossRef]
- Liang, X.; Li, B.; Wang, M.; Wang, J.; Liu, R.; Li, G. Effective approach to promoting the proton conductivity of metal-organic frameworks by exposure to aqua-ammonia vapor. Acs Appl. Mater. Interfaces 2017, 9, 25082–25086. [Google Scholar] [CrossRef]
- Chen, W.; Wang, J.; Zhao, L.; Dai, W.; Li, Z.; Li, G. Enhancing proton conductivity of a highly water stable 3d sr(ii) metal-organic framework by exposure to aqua-ammonia vapor. J. Alloys Compd. 2018, 750, 895–901. [Google Scholar] [CrossRef]
- Chand, S.; Pal, S.C.; Pal, A.; Ye, Y.; Lin, Q.; Zhang, Z.; Xiang, S.; Das, M.C. Metalo hydrogen-bonded organic frameworks (mhofs) as new class of crystalline materials for protonic conduction. Chem.-Eur. J. 2019, 25, 1691–1695. [Google Scholar] [CrossRef]
- Han, B.; Chen, H.; Zhao, Y.; Liu, Y.; Li, G. Water-mediated proton conduction in two stable fluorophenyl imidazole dicarboxylate-based cadmium(ii) complexes. Transit. Met. Chem. 2020, 45, 267–278. [Google Scholar] [CrossRef]
- Zhang, F.; Dong, L.; Qin, J.; Guan, W.; Liu, J.; Li, S.; Lu, M.; Lan, Y.; Su, Z.; Zhou, H. Effect of imidazole arrangements on proton-conductivity in metal-organic frameworks. J. Am. Chem. Soc. 2017, 139, 6183–6189. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Z.; Ju, M.; Xu, J.; Wang, Z. Enhancing proton conductivity of proton exchange membranes via anchoring imidazole-loaded mil-101-nh2 onto sulfonated poly (arylene ether ketone sulfone) by chemical bonding. Int. J. Energy Res. 2022, 46, 23480–23492. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Xiao, S.; Li, Z.; Li, G. High protonic conductivity of three highly stable nanoscale hafnium(IV) metal-organic frameworks and their imidazole-loaded products. Inorg. Chem. 2022, 61, 4938–4947. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Li, X.; Liu, R.; Zhao, X.; Hao, H.; Yan, H.; Zhu, H.; Zhou, H.; Zhong, D. Pore size effects on high-efficiency proton conduction in three stable 3d al-based MOFs modified with imidazole. Inorg. Chem. Front. 2023, 10, 7359–7368. [Google Scholar] [CrossRef]
- Feng, J.; Yu, S.; Guo, K.; Li, J.; Li, G. Water-mediated proton conduction for a highly stable strontium-organic framework from imidazole multi-carboxylate ligand. Polyhedron 2019, 169, 1–7. [Google Scholar] [CrossRef]
- Cai, S.L.; He, Z.H.; Wu, W.H.; Liu, F.X.; Huang, X.L.; Zheng, S.R.; Zhang, W.G. A series of alkaline earth metal coordination polymers constructed from two newly designed imidazole-based dicarboxylate ligands containing pyridinylmethyl groups. CrystEngComm 2017, 19, 3003–3016. [Google Scholar] [CrossRef]
- Liu, Q.-Q.; Liu, S.-S.; Liu, X.-F.; Xu, X.-J.; Dong, X.-Y.; Zhang, H.-J.; Zang, S.-Q. Superprotonic conductivity of UiO-66 with missing-linker defects in aqua-ammonia vapor. Inorg. Chem. 2022, 61, 3406–3411. [Google Scholar] [CrossRef]
- Luo, H.-B.; Wang, M.; Zhang, J.; Tian, Z.-F.; Zou, Y.; Ren, X.-M. Open-framework chalcogenide showing both intrinsic anhydrous and water-assisted high proton conductivity. ACS Appl. Mater. Interfaces 2018, 10, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Chen, S.; Xu, K.; Kang, L.; Li, Z.; Li, G. Syntheses and High Proton Conductivities of Two 3D Zr(IV)/Hf(IV)-MOFs from Furandicarboxylic Acid. Inorg. Chem. 2023, 62, 11570–11580. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yao, Z.; Ye, Y.; Liu, C.; Lin, Q.; Chen, S.; Xiang, S.; Zhang, Z. Enhancement of Intrinsic Proton Conductivity and Aniline Sensitivity by Introducing Dye Molecules into the MOF Channel. ACS Appl. Mater. Interfaces 2019, 11, 16490–16495. [Google Scholar] [CrossRef]
- Pal, S.C.; Das, M.C. Superprotonic conductivity of MOFs and other crystalline platforms beyond 10−1 S cm−1. Adv. Funct. Mater. 2021, 31, 2101584. [Google Scholar] [CrossRef]
- Yu, J.-W.; Yu, H.-J.; Yao, Z.-Y.; Li, Z.-H.; Ren, Q.; Luo, H.-B.; Zou, Y.; Wang, L.; Ren, X.-M. A water-stable open-framework zirconium(IV) phosphate and its water-assisted high proton conductivity. CrystEngComm 2021, 23, 6093–6097. [Google Scholar] [CrossRef]
- Ye, Y.X.; Guo, W.G.; Wang, L.H.; Li, Z.Y.; Song, Z.J.; Chen, J.; Zhang, Z.J.; Xiang, S.C.; Chen, B.L. Straightforward loading of imidazole molecules into metal-organic framework for high proton conduction. J. Am. Chem. Soc. 2017, 139, 15604–15607. [Google Scholar] [CrossRef] [PubMed]
- Kreuer, K.-D.; Rabenau, A.; Weppner, W. Vehicle mechanism, a new model for the interpretation of the conductivity of fast proton conductors. Angew. Chem. Int. Ed. 1982, 21, 208–209. [Google Scholar] [CrossRef]
- Meng, X.R.; Wu, X.J.; Li, D.W.; Hou, H.W.; Fan, Y.T. Influence of The anion on the coordination mode of an unsymmetrical n-heterocyclic ligand in Cd(II) complexes: From discrete molecule to one- and two-dimensional structures. Polyhedron 2010, 29, 2619–2628. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]

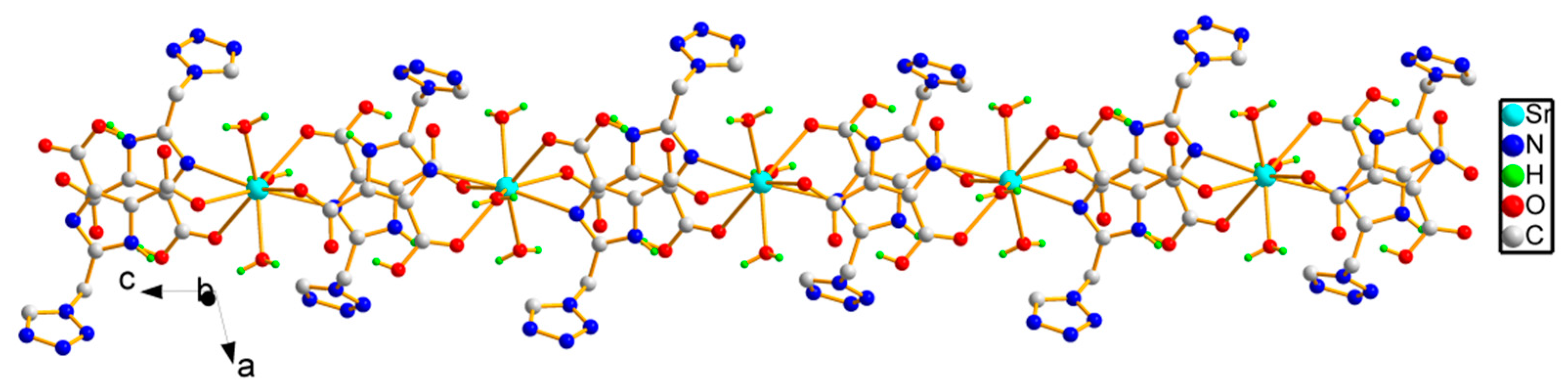
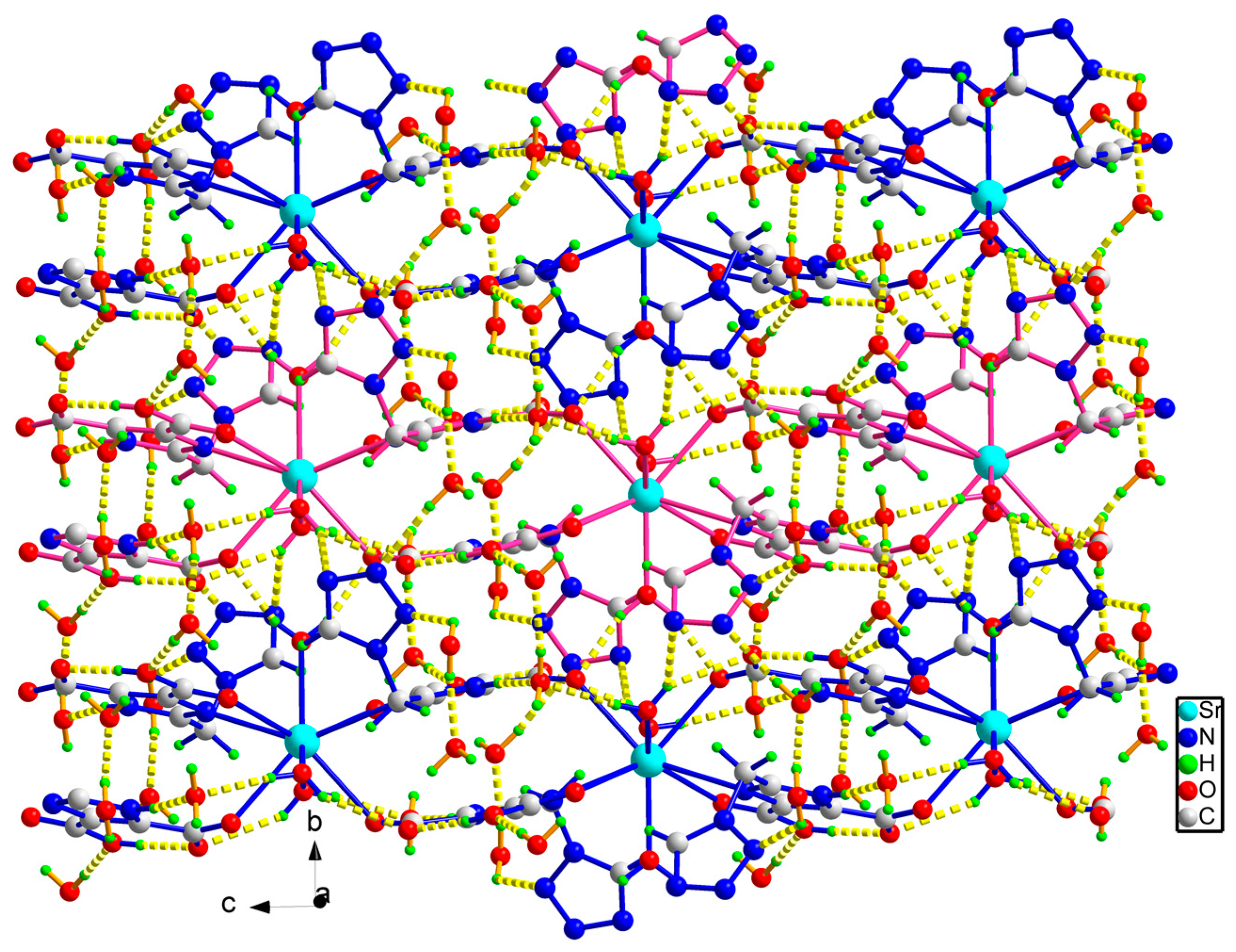
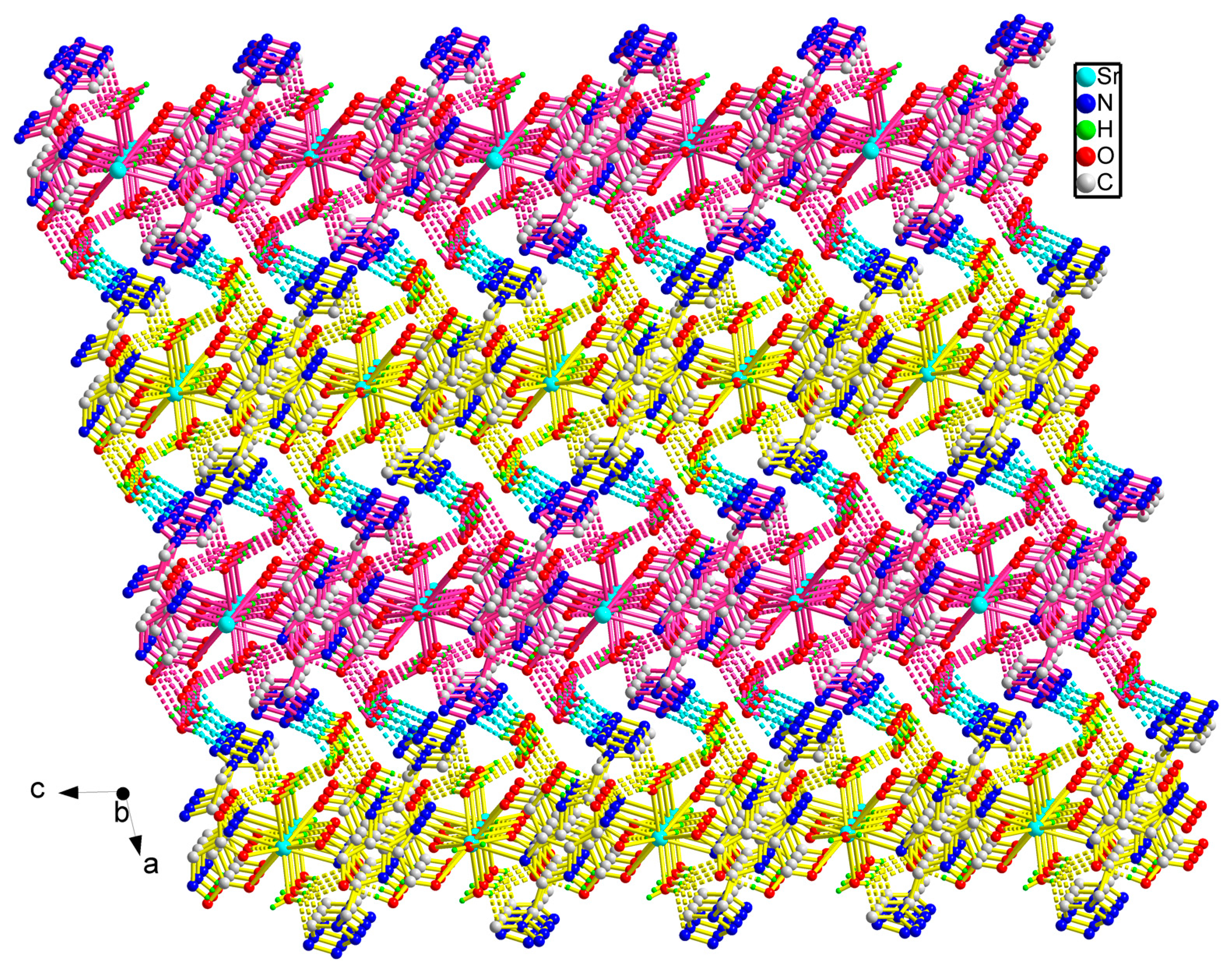
| Sr(1)-O(6) | 2.545(2) | Sr(1)-O(5) | 2.5634(14) |
| Sr(1)-O(5)#1 | 2.5634(14) | Sr(1)-O(1)#1 | 2.6712(12) |
| Sr(1)-O(1) | 2.6713(12) | Sr(1)-O(4)#2 | 2.7606(12) |
| Sr(1)-O(4)#3 | 2.7606(12) | Sr(1)-N(1)#1 | 2.8083(12) |
| Sr(1)-N(1) | 2.8084(12) | ||
| O(6)-Sr(1)-O(5) | 113.95(4) | O(6)-Sr(1)-O(5)#1 | 113.95(4) |
| O(5)-Sr(1)-O(5)#1 | 132.09(8) | O(6)-Sr(1)-O(1)#1 | 69.00(3) |
| O(5)-Sr(1)-O(1)#1 | 135.51(4) | O(5)#1-Sr(1)-O(1)#1 | 65.02(4) |
| O(6)-Sr(1)-O(1) | 69.00(3) | O(5)-Sr(1)-O(1) | 65.02(4) |
| O(5)#1-Sr(1)-O(1) | 135.51(4) | O(1)#1-Sr(1)-O(1) | 138.00(6) |
| O(6)-Sr(1)-O(4)#2 | 138.55(2) | O(5)-Sr(1)-O(4)#2 | 71.62(4) |
| O(5)#1-Sr(1)-O(4)#2 | 72.94(5) | O(1)#1-Sr(1)-O(4)#2 | 137.49(4) |
| O(5)-Sr(1)-O(4)#3 | 72.94(5) | O(5)#1-Sr(1)-O(4)#3 | 71.62(4) |
| O(1)#1-Sr(1)-O(4)#3 | 78.46(4) | O(1)-Sr(1)-O(4)#3 | 137.50(4) |
| O(4)#2-Sr(1)-O(4)#3 | 82.90(5) | O(6)-Sr(1)-N(1)#1 | 71.68(2) |
| O(5)-Sr(1)-N(1)#1 | 77.50(4) | O(5)#1-Sr(1)-N(1)#1 | 118.14(4) |
| O(4)#2-Sr(1)-N(1)#1 | 143.88(4) | O(4)#3-Sr(1)-N(1)#1 | 70.32(3) |
| O(6)-Sr(1)-N(1) | 71.68(3) | O(5)-Sr(1)-N(1) | 118.14(4) |
| O(1)-Sr(1)-N(1) | 60.93(3) | O(4)#2-Sr(1)-N(1) | 70.32(3) |
| O(4)#3-Sr(1)-N(1) | 143.88(3) | N(1)#1-Sr(1)-N(1) | 143.37(5) |
| D-H⋯A | d(D-H) (Å) | d(H⋯A) (Å) | d(D⋯A) (Å) | (D-H⋯A)(°) |
|---|---|---|---|---|
| N(2)-H(2)⋯O(7)#4 | 0.86 | 1.96 | 2.8100(18) | 167.3 |
| O(2)-H(2A)⋯O(3) | 0.82 | 1.64 | 2.4596(17) | 176.4 |
| O(5)-H(5A)⋯O(7)#5 | 0.85 | 2.11 | 2.961(2) | 173.9 |
| O(5)-H(5B)⋯N(6)#6 | 0.81 | 2.50 | 3.073(2) | 128.2 |
| O(5)-H(5B)⋯O(3)#3 | 0.81 | 2.36 | 3.0559(19) | 144.7 |
| O(6)-H(6C)⋯O(4)#7 | 0.85 | 1.98 | 2.8050(19) | 162.6 |
| C(6)-H(6A)⋯O(8)#8 | 0.97 | 2.47 | 3.374(2) | 155.1 |
| O(7)-H(7A)⋯O(8) | 0.85 | 1.95 | 2.794(2) | 173.1 |
| O(7)-H(7B)⋯N(4)#9 | 0.85 | 2.51 | 3.121(2) | 129.4 |
| O(8)-H(8A)⋯O(2)#10 | 0.85 | 2.06 | 2.8959(19) | 169.6 |
| O(8)-H(8B)⋯N(5) | 0.85 | 2.31 | 3.144(3) | 167.5 |
| T (°C) | 68% RH | 75% RH | 85% RH | 93% RH | 98% RH |
|---|---|---|---|---|---|
| 40 | 4.84 × 10−8 | 2.55 × 10−7 | 2.64 × 10−7 | 2.42 × 10−6 | 1.62 × 10−5 |
| 50 | 1.14 × 10−7 | 4.73 × 10−7 | 5.34 × 10−7 | 5.38 × 10−6 | 1.69 × 10−5 |
| 60 | 1.77 × 10−7 | 8.85 × 10−7 | 7.66 × 10−7 | 1.05 × 10−5 | 3.39 × 10−5 |
| 70 | 2.12 × 10−7 | 2.33 × 10−6 | 2.31 × 10−6 | 1.73 × 10−4 | 3.08 × 10−4 |
| 80 | 5.12 × 10−7 | 2.07 × 10−5 | 5.81 × 10−5 | 2.37 × 10−4 | 8.66 × 10−4 |
| 90 | 1.34 × 10−6 | 6.64 × 10−5 | 1.21 × 10−4 | 2.90 × 10−4 | 1.98 × 10−3 |
| 100 | 3.02 × 10−6 | 1.11 × 10−4 | 1.26 × 10−4 | 8.13 × 10−4 | 1.22 × 10−2 |
| Compound | 1 |
|---|---|
| Empirical formula | C14H24N12O15Sr |
| Formula weight | 688.07 |
| Temperature, K | 300(2) K |
| Crystal size, mm3 | 0.22 × 0.21 × 0.16 |
| Crystal system | Monoclinic |
| Space group | C2/c |
| a, Å | 21.8700(10) |
| b, Å | 6.7421(2) |
| c, Å | 17.6471(7) |
| α, deg | 90 |
| β, deg | 101.865(2) |
| γ, deg | 90 |
| Volume, Å3 | 2546.47(17) |
| Z | 4 |
| Calculated density, g cm−3 | 1.795 |
| Absorption coefficient, mm−1 | 2.215 |
| F(000), e | 1400 |
| θ range for data collection, deg | 2.709–27.505 |
| Radiation | Mo Kα |
| Index ranges | −28 ≤ h ≤ 2 8, −8 ≤ k ≤ 8, −22 ≤ l ≤ 22 |
| Reflections collected/unique | 59,724/2920 |
| Rint | 0.0477 |
| Data/restraints/parameters | 2920/0/192 |
| Final indices R1/wR2 [I > 2σ(I)] | 0.0228/0.0594 |
| Final indices R1/wR2 (all data) | 0.0255/0.0608 |
| Goodness-of-fit on (F2) | 1.030 |
| Δρfin (max/min), e·Å−3 | 0.495/−0.333 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, J.; Li, Y.; Xie, L.; Tong, J.; Li, G. High H2O-Assisted Proton Conduction in One Highly Stable Sr(II)-Organic Framework Constructed by Tetrazole-Based Imidazole Dicarboxylic Acid. Molecules 2024, 29, 2656. https://doi.org/10.3390/molecules29112656
Feng J, Li Y, Xie L, Tong J, Li G. High H2O-Assisted Proton Conduction in One Highly Stable Sr(II)-Organic Framework Constructed by Tetrazole-Based Imidazole Dicarboxylic Acid. Molecules. 2024; 29(11):2656. https://doi.org/10.3390/molecules29112656
Chicago/Turabian StyleFeng, Junyang, Ying Li, Lixia Xie, Jinzhao Tong, and Gang Li. 2024. "High H2O-Assisted Proton Conduction in One Highly Stable Sr(II)-Organic Framework Constructed by Tetrazole-Based Imidazole Dicarboxylic Acid" Molecules 29, no. 11: 2656. https://doi.org/10.3390/molecules29112656
APA StyleFeng, J., Li, Y., Xie, L., Tong, J., & Li, G. (2024). High H2O-Assisted Proton Conduction in One Highly Stable Sr(II)-Organic Framework Constructed by Tetrazole-Based Imidazole Dicarboxylic Acid. Molecules, 29(11), 2656. https://doi.org/10.3390/molecules29112656





