Fe-Doped g-C3N4/Bi2MoO6 Heterostructured Composition with Improved Visible Photocatalytic Activity for Rhodamine B Degradation
Abstract
1. Introduction
2. Results and Discussion
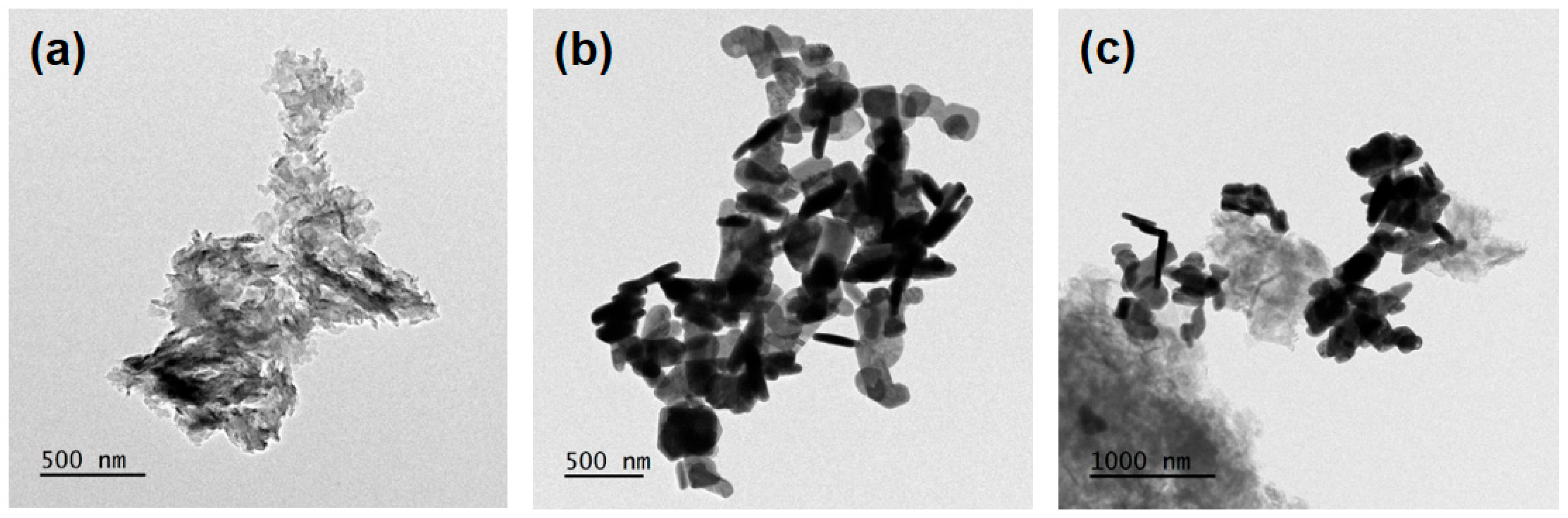
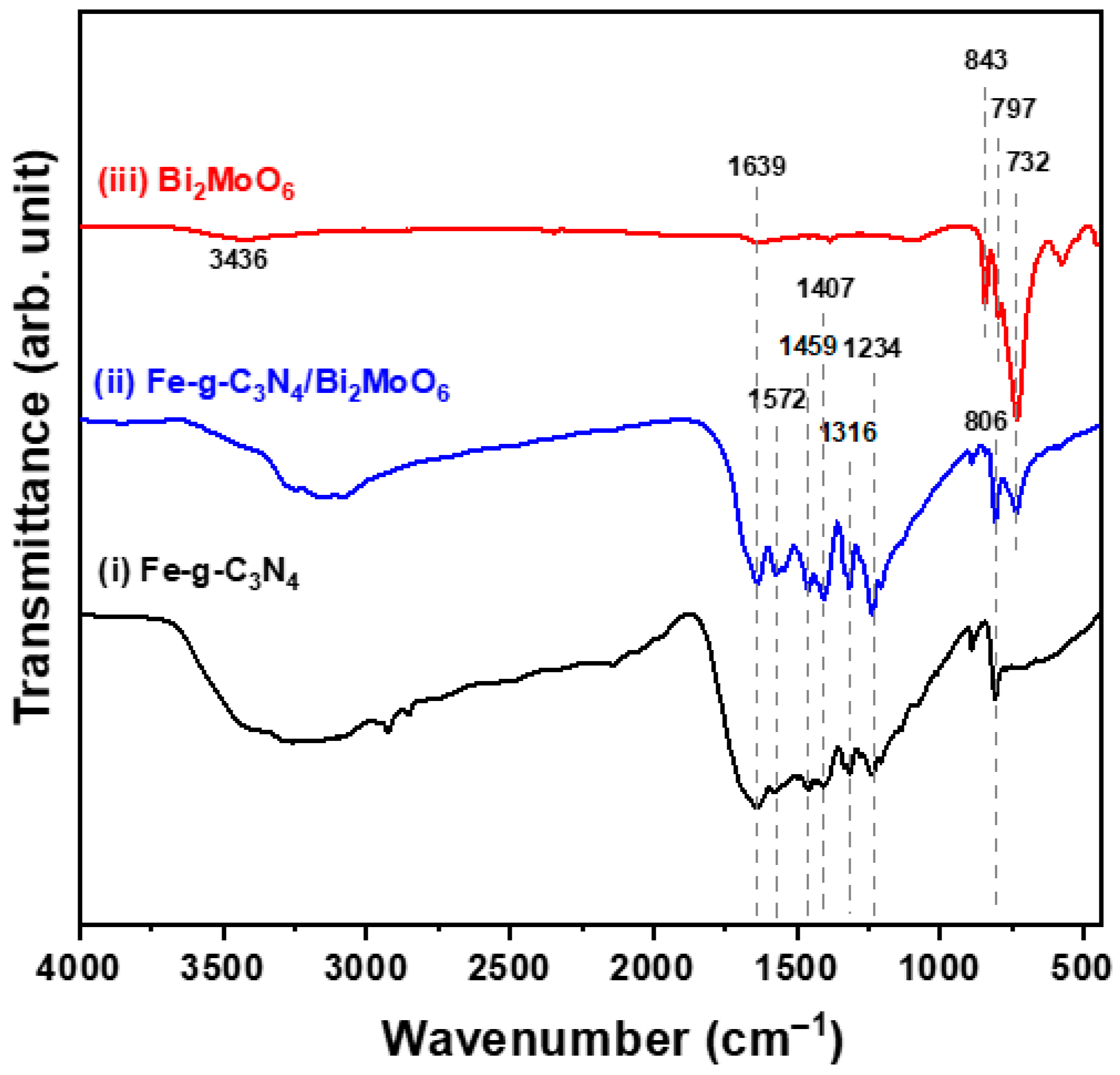
2.1. Physical Properties of Semiconducting Nano-Photocatalysts
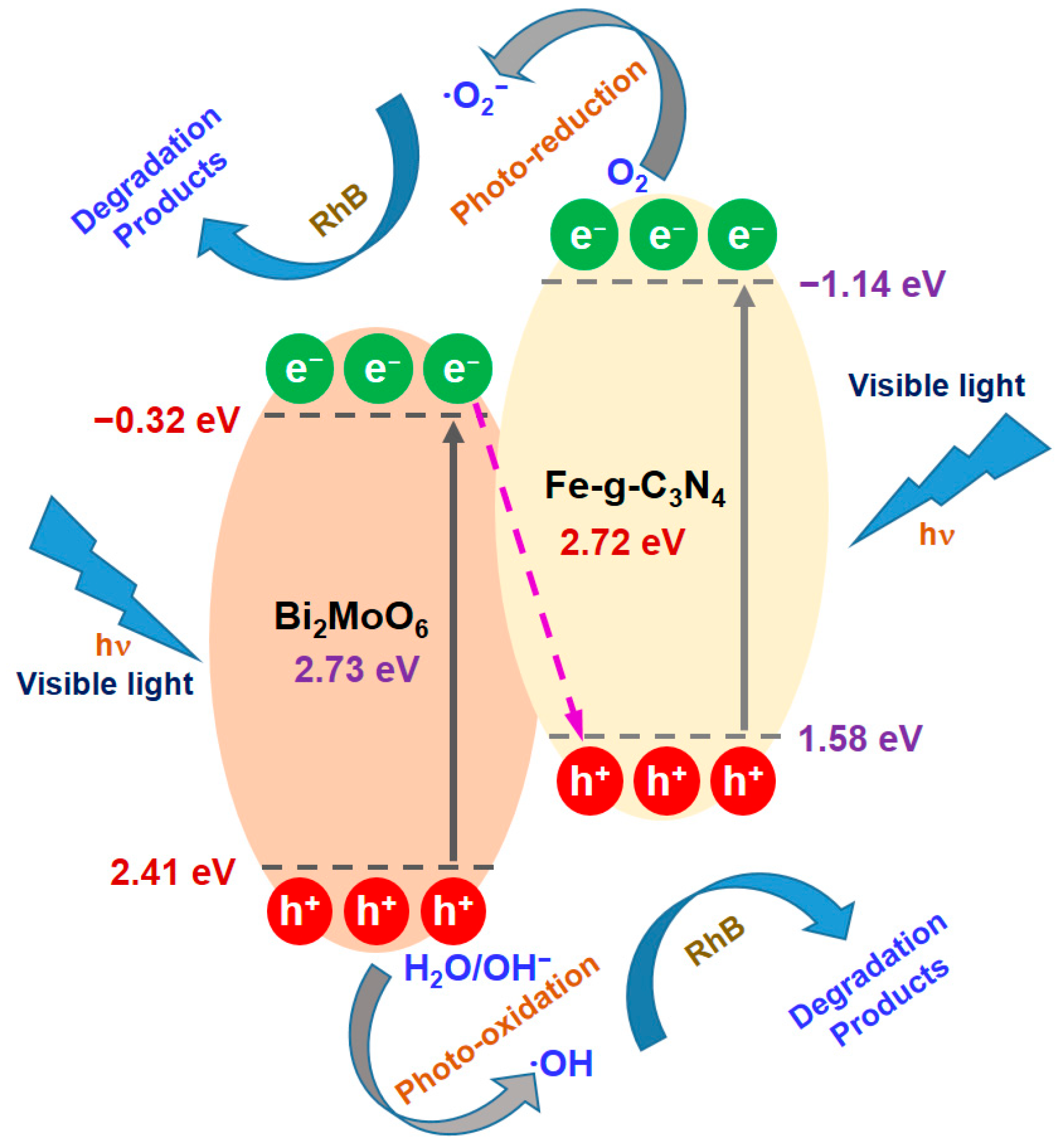
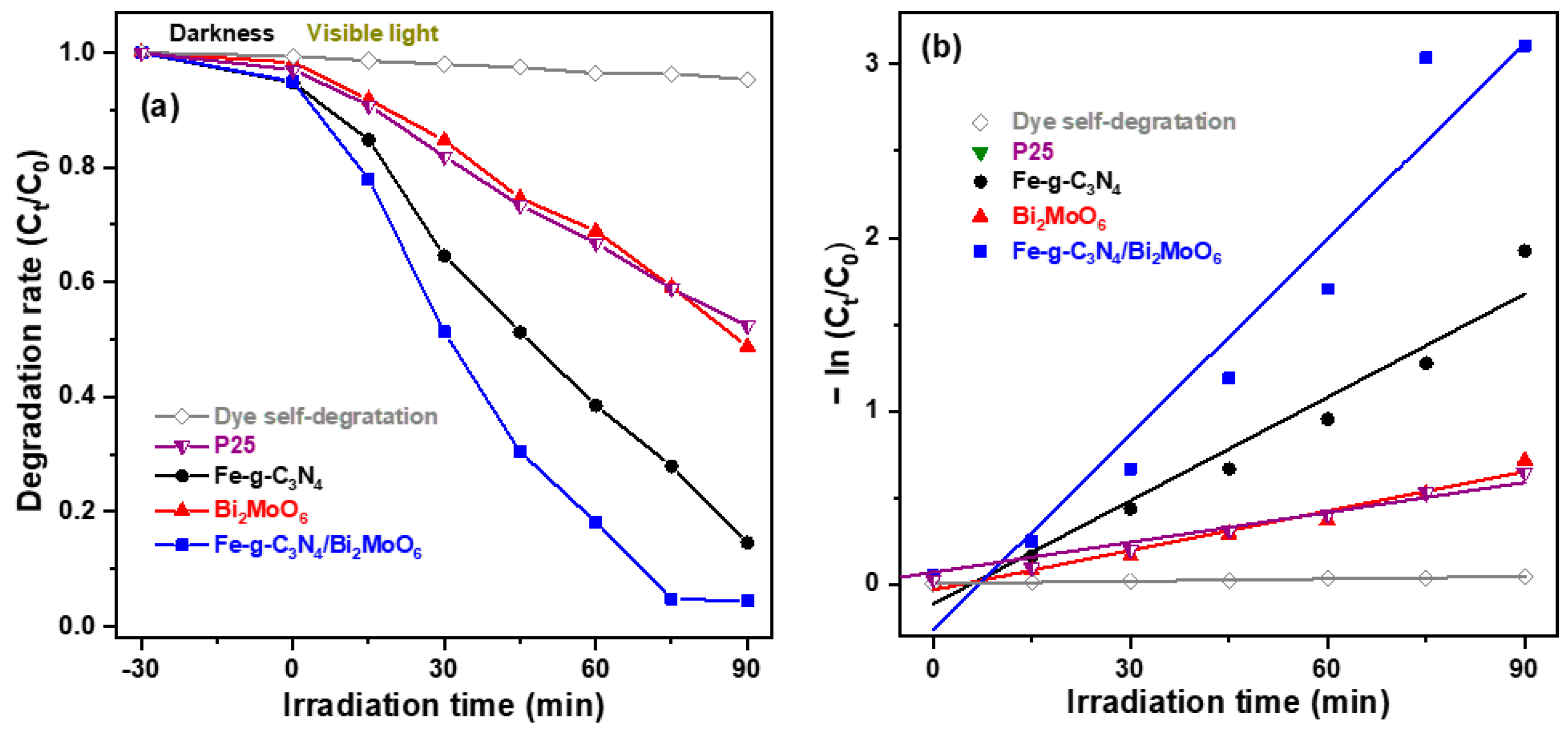
2.2. Photocatalytic Degradation Performance of Semiconducting Nano-Photocatalysts
3. Materials and Methods
3.1. Procedures for Preparing or Synthesizing Three Types of Visible Light Photocatalysts
3.2. Characterization of Physical Properties and Measurement of Photocatalytic Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Wang, C.; Wang, G. Photocatalytic advanced oxidation processes for water treatment: Recent advances and perspective. Chem. Asian J. 2020, 15, 3239–3253. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Tsay, C.Y.; Chung, C.Y.; Chen, C.Y.; Chang, Y.C.; Chang, C.J.; Wu, J.J. Enhanced Photocatalytic Performance of Visible-Light-Driven BiVO4 Nanoparticles through W and Mo Substituting. Catalysts 2023, 13, 475. [Google Scholar] [CrossRef]
- Yayapao, O.; Thongtem, T.; Phuruangrat, A.; Thongtem, S. Synthesis and characterization of highly efficient Gd doped ZnO photocatalyst irradiated with ultraviolet and visible radiations. Mater. Sci. Semicond. Process. 2015, 39, 786–792. [Google Scholar] [CrossRef]
- Siriwong, P.; Thongtem, T.; Phuruangrat, A.; Thongtem, S. Hydrothermal synthesis, characterization, and optical properties of wolframite ZnWO4 nanorodes. CrystEngComm 2011, 13, 1564–1569. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Wannaopo, S.; Sakhon, T.; Kuntalue, B.; Thongtem, T.; Thongtem, S. Characterization and photocatalytic properties of BiVO4 synthesized by combustion method. J. Mol. Struct. 2023, 1274, 134420. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Putdum, S.; Dumrongrojthanath, P.; Ekthammathat, N.; Thongtem, S.; Thongtem, T. Enhanced properties for visible-light-driven photocatalysis of Ag nanoparticle modified Bi2MoO6 nanoplates. Mater. Sci. Semicond. Process. 2015, 34, 175–181. [Google Scholar] [CrossRef]
- Guo, J.; Shi, L.; Zhao, J.; Wang, Y.; Tang, K.; Zhang, W.; Xie, C.; Yuan, X. Enhanced visible-light photocatalytic activity of Bi2MoO6 nanoplates with heterogeneous Bi2MoO6-x@ Bi2MoO6 core-shell structure. Appl. Catal. B Environ. 2018, 224, 692–704. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Y.; Hussain, M.I.; Zhou, W.; Chen, Y.; Wang, L.N. gC3N4: Properties, pore modifications, and photocatalytic applications. Nanomaterials 2022, 12, 121. [Google Scholar] [CrossRef]
- Lv, J.; Dai, K.; Zhang, J.; Geng, L.; Liang, C.; Liu, Q.; Zhu, G.; Chen, C. Facile synthesis of Z-scheme graphitic-C3N4/Bi2MoO6 nanocomposite for enhanced visible photocatlytic properties. Appl. Surf. Sci. 2015, 358, 313–318. [Google Scholar] [CrossRef]
- Bicalho, H.A.; Lopez, J.L.; Binatti, I.; Batista, P.F.R.; Ardisson, J.D.; Resende, R.R.; Lorençon, E. Facile synthesis of highly dispersed Fe(II)-doped g-C3N4 and its application in Fenton-like catalysis. Mol. Catal. 2017, 435, 156–165. [Google Scholar] [CrossRef]
- Tian, Y.; Chang, B.; Lu, J.; Fu, J.; Xi, F.; Dong, X. Hydrothermal Synthesis of Graphitic Carbon Nitride−Bi2WO6 Heterojunction with Enhanced Visible Light Photocatalytic Activities. Appl. Mater. Interfaces 2013, 5, 7079–7085. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Lyu, F.; Zhang, L.C.; Zeng, S.; Liang, S.X.; Li, Y.Y.; Lu, J. Pt nanoparticles decorated heterostructured g-C3N4/Bi2MoO6 microplates with highly enhanced photocatalytic activities under visible light. Sci. Rep. 2019, 9, 7636. [Google Scholar] [CrossRef] [PubMed]
- Van, M.N.; Mai, O.L.T.; Do, C.P.; Thi, H.L.; Manh, C.P.; Manh, H.N.; Thi, D.P.; Danh, B.D. Fe-Doped g-C3N4: High-Performance Photocatalysts in Rhodamine B Decomposition. Polymers 2020, 12, 1963. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Dong, X.; Zhai, S.; Wang, X.; Ma, H.; Zhang, X. Towards understanding the photocatalytic activity enhancement of ordered mesoporous Bi2MoO6 crystals prepared via a novel vacuum-assisted nanocasting method. RSC Adv. 2016, 6, 35709–35718. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, L.; Wang, H.; Huang, B.; Yuan, X.; Huang, J.; Zhang, J.; Zeng, G. Modulation of Bi2MoO6-Based Materials for Photocatalytic Water Splitting and Environmental Application: A Critical Review. Small 2019, 15, 1901008. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, W.; Zhai, Z.; Ren, K.; Wang, T.; Guan, H.; Shi, H. 2D/2D Bi2MoO6/g-C3N4 S-scheme heterojunction photocatalyst with enhanced visible-light activity by Au loading. J. Mater. Sci. Technol. 2020, 56, 216–226. [Google Scholar] [CrossRef]
- Ma, T.; Wu, J.; Mi, Y.; Chen, Q.; Ma, D.; Chai, C. Novel Z-scheme g-C3N4/C@Bi2MoO6 composite with enhanced visible-light photocatalytic activity for β-naphthol degradation. Sep. Purif. Technol. 2017, 183, 54–65. [Google Scholar] [CrossRef]
- Stelo, F.; Kublik, N.; Ullah, S.; Wdnder, H. Recent advances in Bi2MoO6 based Z-scheme heterojunctions for photocatalytic degradation of pollutants. J. Alloys Compd. 2020, 829, 154591. [Google Scholar] [CrossRef]
- Li, L.; Mao, M.; She, X.; Yi, M.; He, M.; Pan, L.; Chen, Z.; Xu, H.; Li, H. Direct Z-scheme photocatalyst for efficient water pollutant degradation: A case study of 2D g-C3N4/BiVO4. Mater. Chem. Phys. 2020, 241, 122308. [Google Scholar] [CrossRef]
- Pinchujit, S.; Phuruangrat, A.; Wannaopo, S.; Sakhon, T.; Kuntalue, B.; Thongtem, T.; Thongtem, S. Synthesis and characterization of heterostructure Pt/BiWO6 nanocomposites with enhanced photodegradation efficiency induced by visible radiation. Solid State Sci. 2022, 134, 107064. [Google Scholar] [CrossRef]
- Li, P.; Hu, Y.; Lu, D.; Wu, J.; Lv, Y. Study on g-C3N4/BiVO4 Binary Composite Photocatalytic Materials. Micromachines 2023, 14, 639. [Google Scholar] [CrossRef]
- Liu, C.; Dai, H.; Tan, C.; Pan, Q.; Hu, F.; Peng, X. Photo-Fenton degradation of tetracycline over Z-scheme Fe-g-C3N4/BiVO4 heterojunctions: Mechanism insight, degradation pathways and DFT calculation. Appl. Catal. B Environ. 2022, 310, 121326. [Google Scholar] [CrossRef]
- Chen, F.; Niu, C.; Yang, Q.; Li, X.; Zeng, G. Facile synthesis of visible-light-active BiOI modified Bi2MoO6 photocatalysts with highly enhanced photocatalytic activity. Ceram Int. 2016, 42, 2515. [Google Scholar] [CrossRef]
- Su, Q.; Sun, J.; Wang, J.; Yang, Z.; Cheng, W.; Zhang, S. Urea-derived graphitic carbon nitride as an efficient heterogeneous catalyst for CO2 conversion into cyclic carbonates. Catal. Sci. Tech. 2014, 4, 1556–1562. [Google Scholar] [CrossRef]
- Wu, P.; Wang, J.; Zhao, J.; Guo, L.; Osterloh, F.E. Structure defects in g-C3N4 limit visible light driven hydrogen evolution and photovoltage. J. Mater. Chem. A 2014, 2, 20338–20344. [Google Scholar] [CrossRef]
- Shanmugam, V.; Muppudathi, A.L.; Jayavel, S.; Jeyaperumal, K.S. Construction of high efficient g-C3N4 nanosheets combined with Bi2MoO6-Ag photocatalysts for visible-light-driven photocatalytic activity and inactivation of bacterias. Arab. J. Chem. 2020, 13, 2439–2455. [Google Scholar] [CrossRef]
- Wu, S.; Yi, B.; Lan, D. Fabrication of Bi2MoO6/g-C3N4 visible-light driven photocatatalyst for enhanced tetracycline degradation. J. Photochem. Photobiol. A Chem. 2023, 444, 115013. [Google Scholar] [CrossRef]
- Xue, M.; Meng, F.; Ma, Y.; Zhou, S. Growing of ultra-thin Bi2MoO6 nanoflowers on Co/N-doped graphitic carbon nanoshells as attractive custom supports: Excellent photocatalytic degradation activity for pollutants. Appl. Surf. Sci. 2023, 613, 156100. [Google Scholar] [CrossRef]
- Wang, D.; Ren, B.; Chen, S.; Liu, S.; Feng, W.; Sun, Y. Novel BiVO4/TiO2 composites with Z- scheme heterojunction for photocatalytic degradation. Mater. Lett. 2023, 330, 133229. [Google Scholar] [CrossRef]





| Photocatalyst | Average Crystallite Size (nm) | Average Particle Size (nm) | SBET (m2/g) | Pore Volume (cm3/g) | Optical Bandgap (eV) | Photodegradation Efficiency (%) | Reaction Rate Constant (min−1) |
|---|---|---|---|---|---|---|---|
| Fe-g-C3N4 | 4.3 | 1860 | 32.90 | 0.243 | 2.74 | 85.40 | 0.0199 |
| Bi2MoO6 | 38.6 | 328 | 7.42 | 0.013 | 2.73 | 51.21 | 0.0076 |
| Fe-g-C3N4/Bi2MoO6 | 40.5 | 1455 | 31.33 | 0.234 | 2.72 | 95.53 | 0.0376 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsay, C.-Y.; Chung, C.-Y.; Chang, C.-J.; Chang, Y.-C.; Chen, C.-Y.; Wu, S.-Y. Fe-Doped g-C3N4/Bi2MoO6 Heterostructured Composition with Improved Visible Photocatalytic Activity for Rhodamine B Degradation. Molecules 2024, 29, 2631. https://doi.org/10.3390/molecules29112631
Tsay C-Y, Chung C-Y, Chang C-J, Chang Y-C, Chen C-Y, Wu S-Y. Fe-Doped g-C3N4/Bi2MoO6 Heterostructured Composition with Improved Visible Photocatalytic Activity for Rhodamine B Degradation. Molecules. 2024; 29(11):2631. https://doi.org/10.3390/molecules29112631
Chicago/Turabian StyleTsay, Chien-Yie, Ching-Yu Chung, Chi-Jung Chang, Yu-Cheng Chang, Chin-Yi Chen, and Shu-Yii Wu. 2024. "Fe-Doped g-C3N4/Bi2MoO6 Heterostructured Composition with Improved Visible Photocatalytic Activity for Rhodamine B Degradation" Molecules 29, no. 11: 2631. https://doi.org/10.3390/molecules29112631
APA StyleTsay, C.-Y., Chung, C.-Y., Chang, C.-J., Chang, Y.-C., Chen, C.-Y., & Wu, S.-Y. (2024). Fe-Doped g-C3N4/Bi2MoO6 Heterostructured Composition with Improved Visible Photocatalytic Activity for Rhodamine B Degradation. Molecules, 29(11), 2631. https://doi.org/10.3390/molecules29112631










