Enhanced Hydrogel Materials: Incorporating Vitamin C and Plant Extracts for Biomedical Applications
Abstract
1. Introduction
2. Results and Discussion
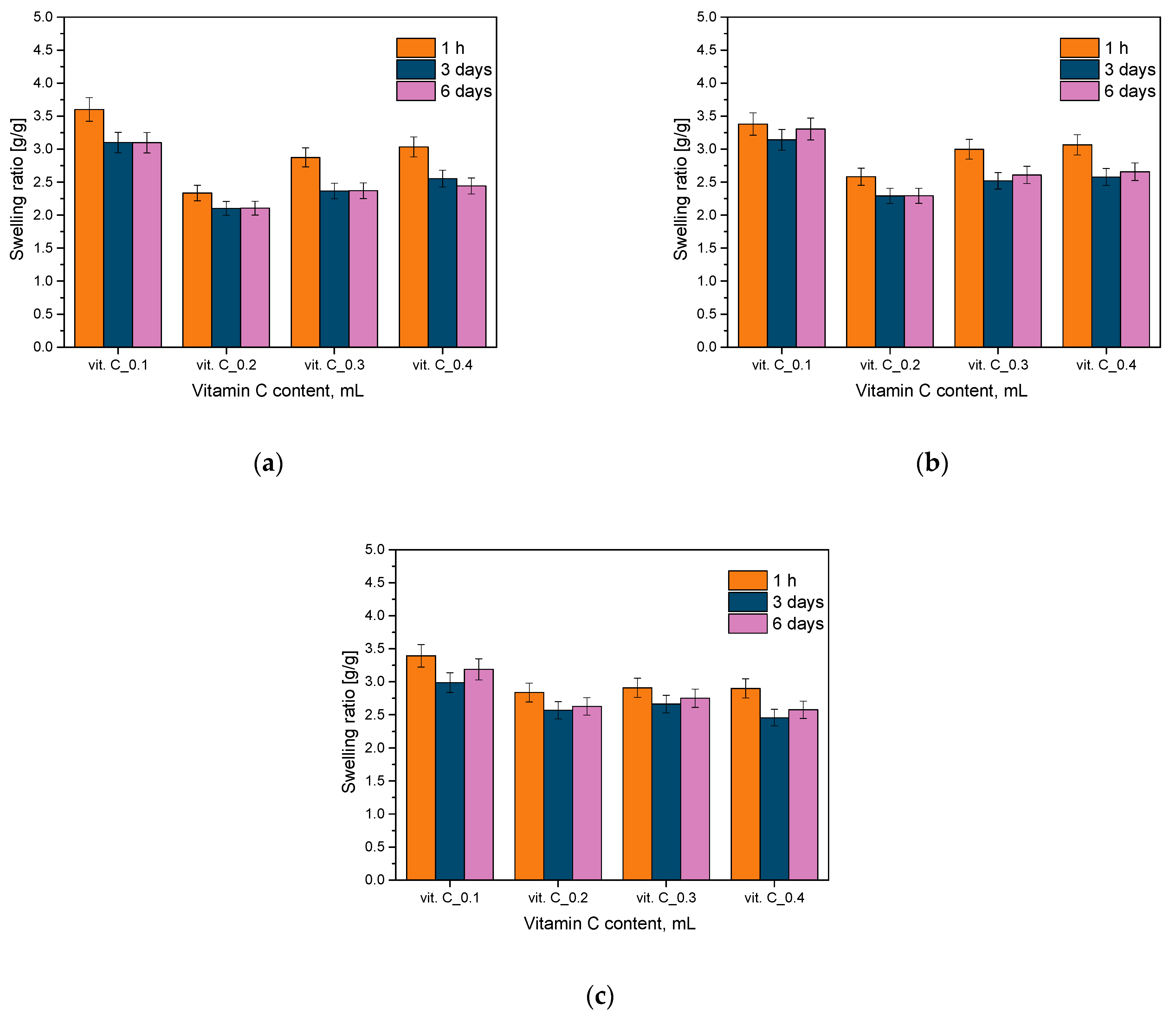
2.1. Analysis of Sorption Capacity
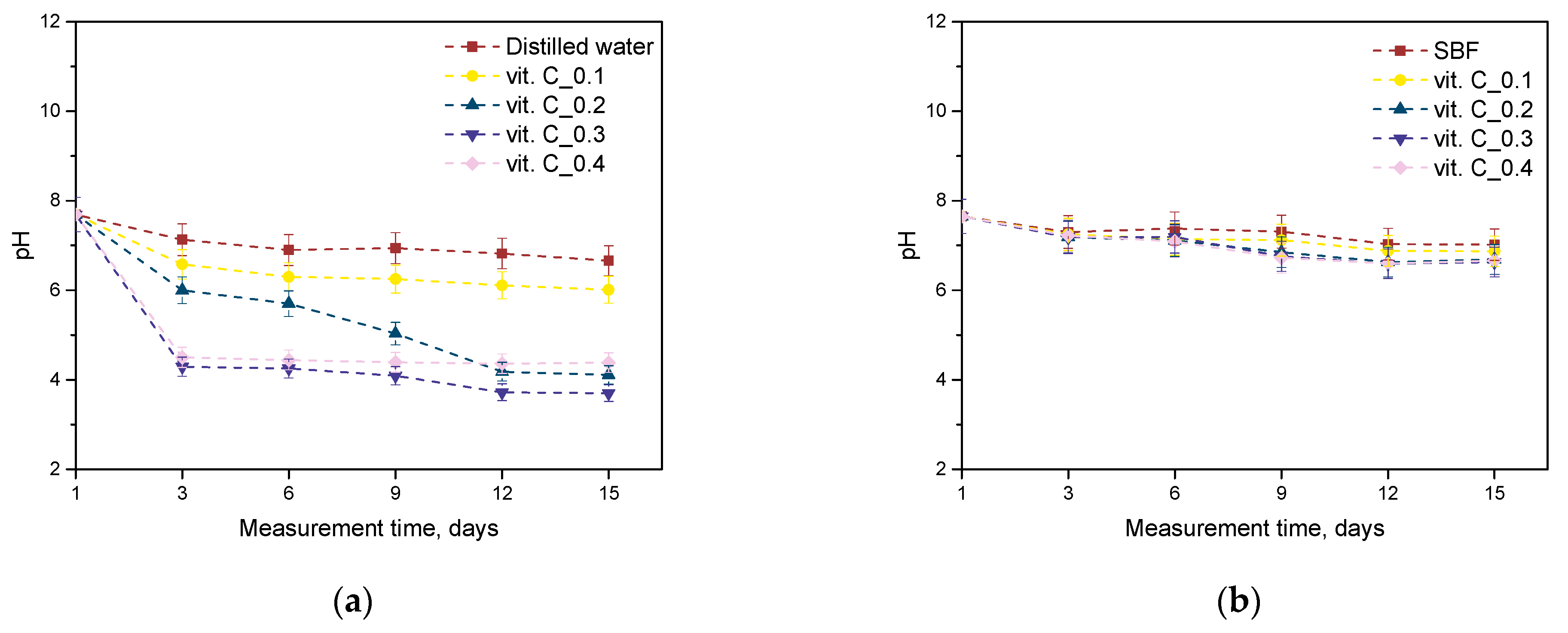
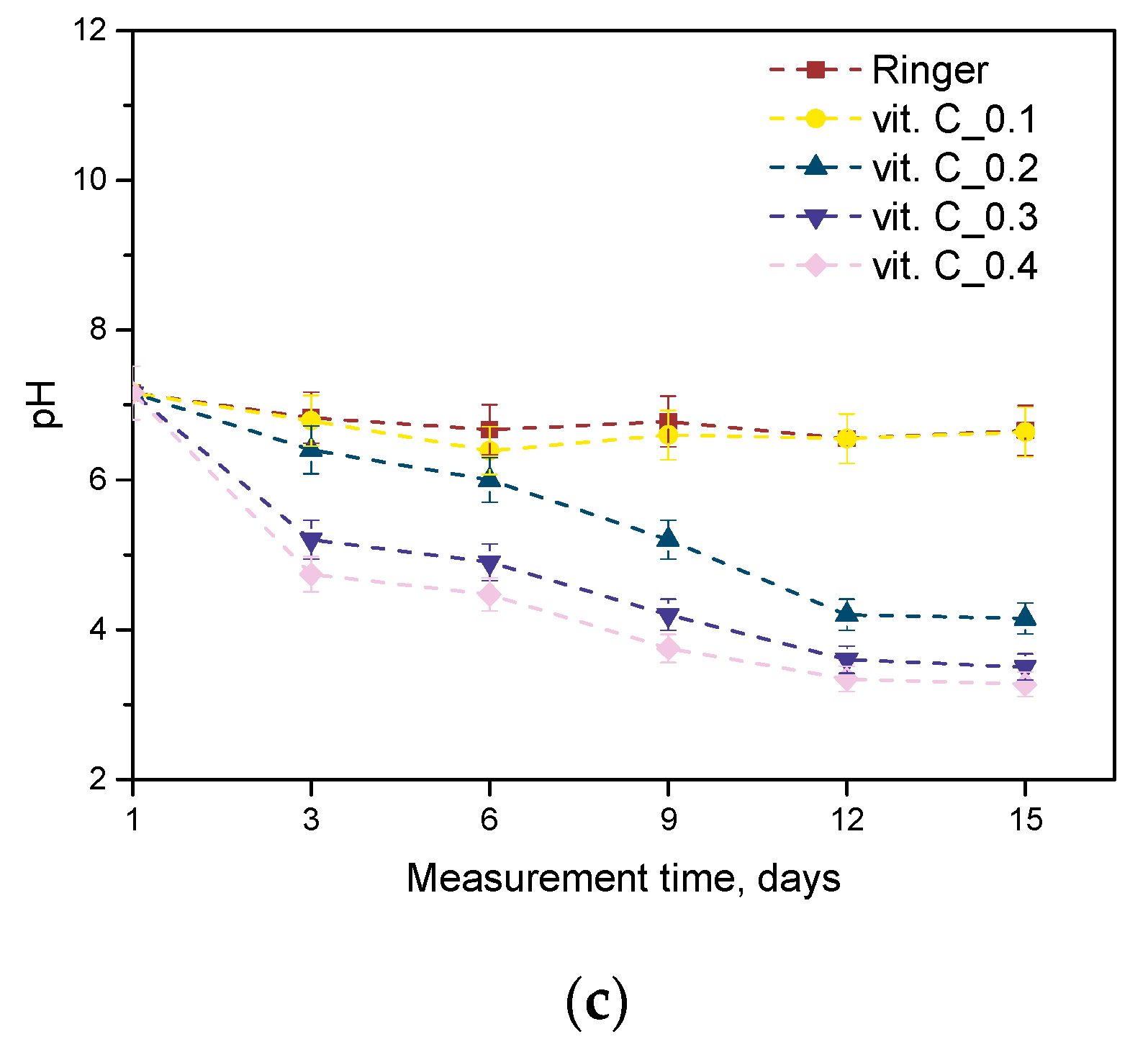
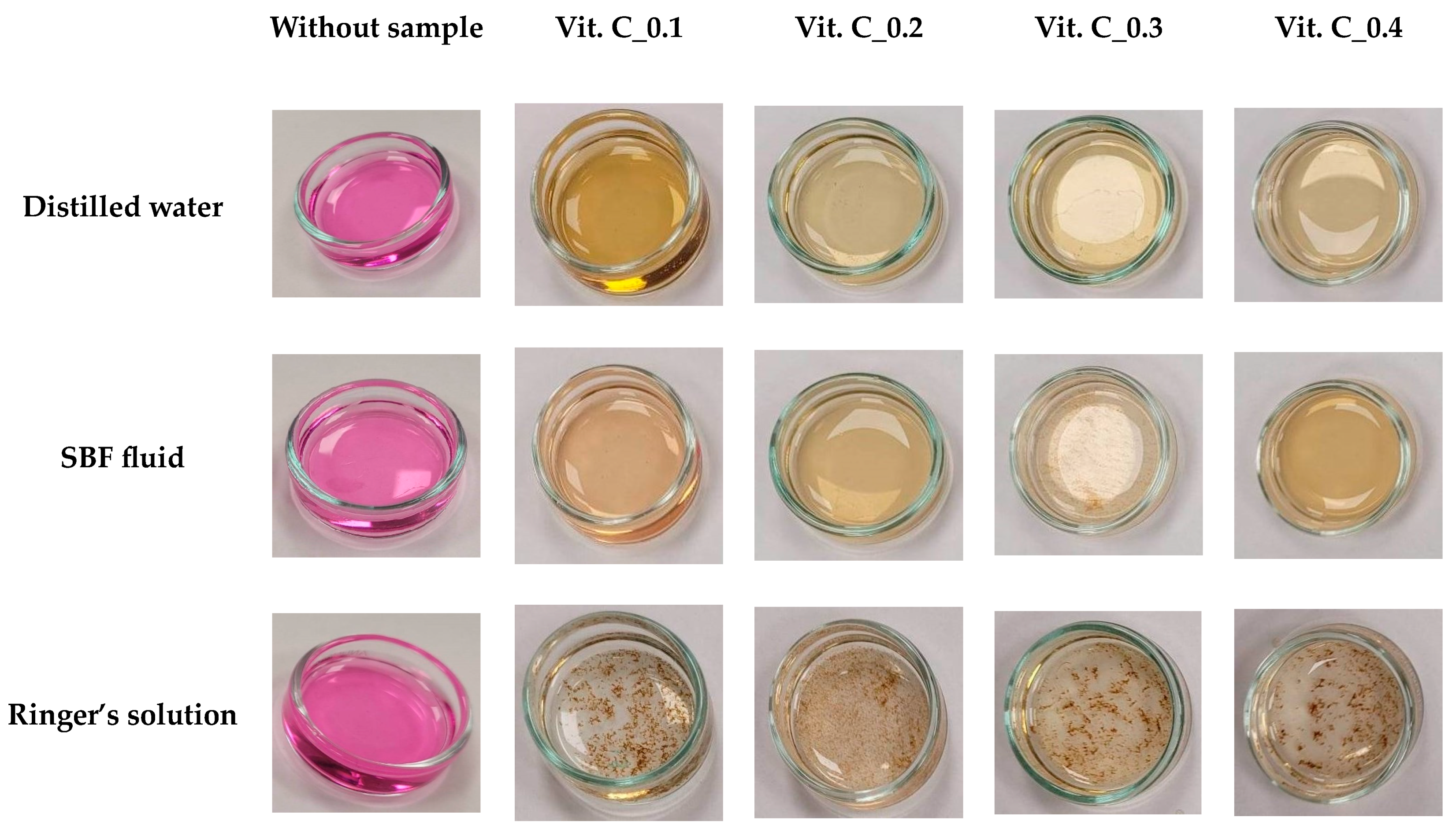
2.2. Results of the Incubation Study
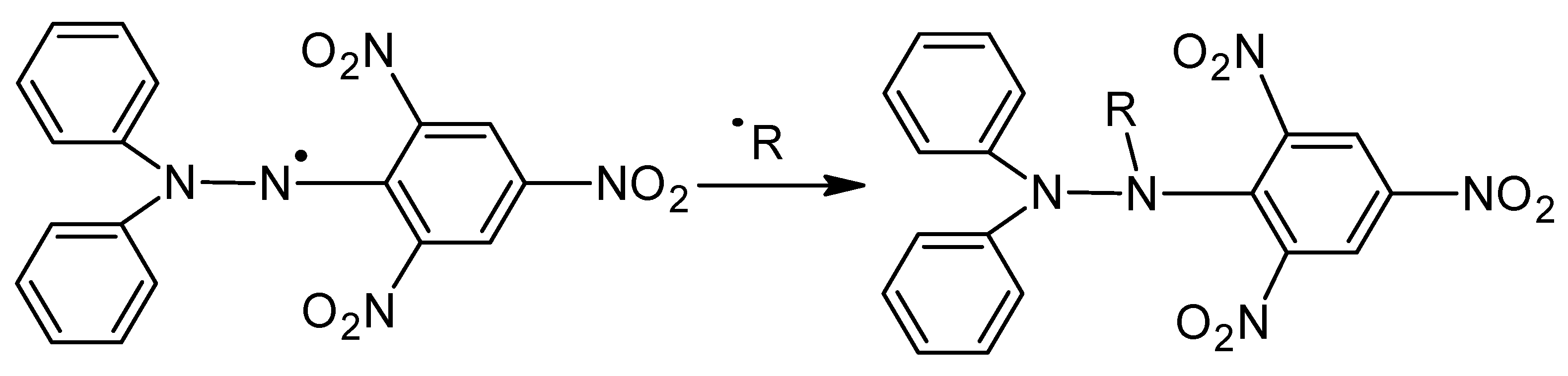
2.3. Results of Antioxidant Activity Analysis
2.4. Results of Infrared Spectroscopy Analysis
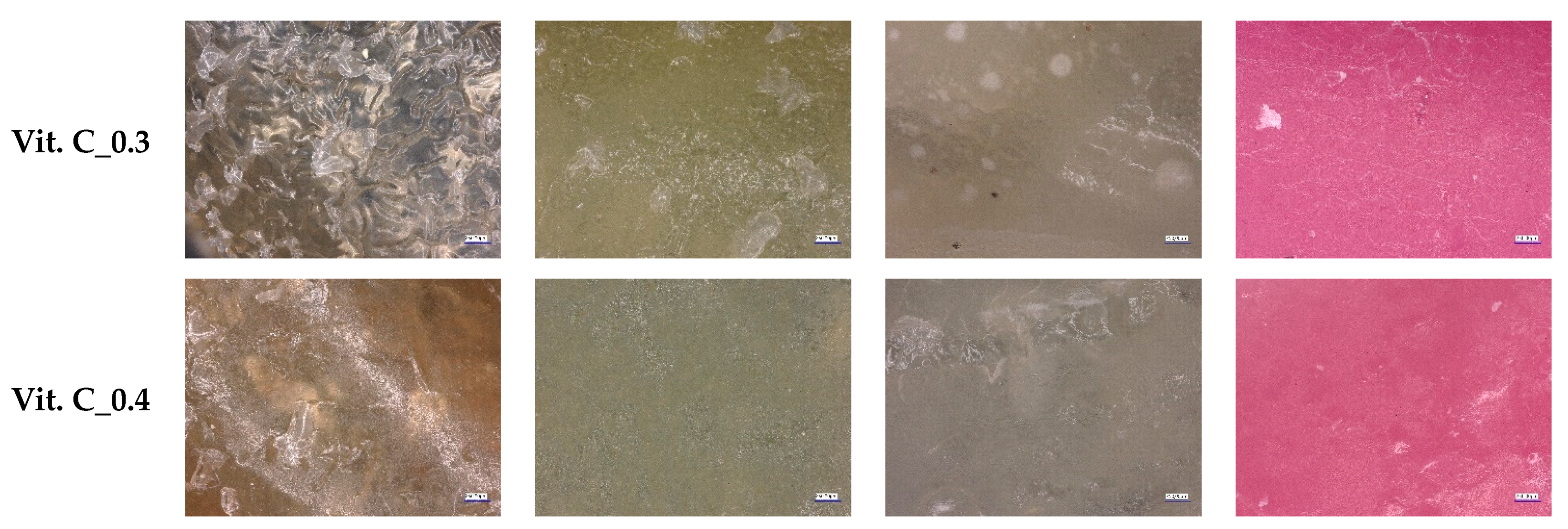
2.5. Optical Microscope Observations
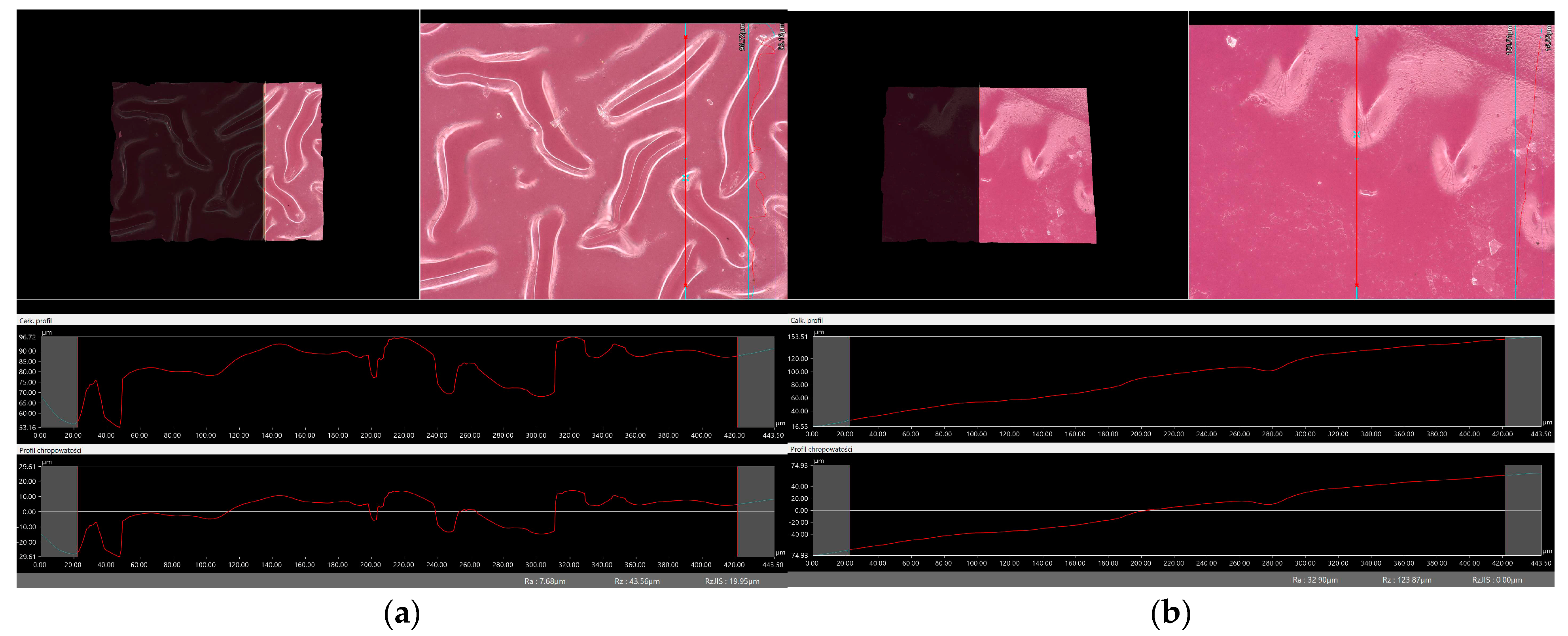
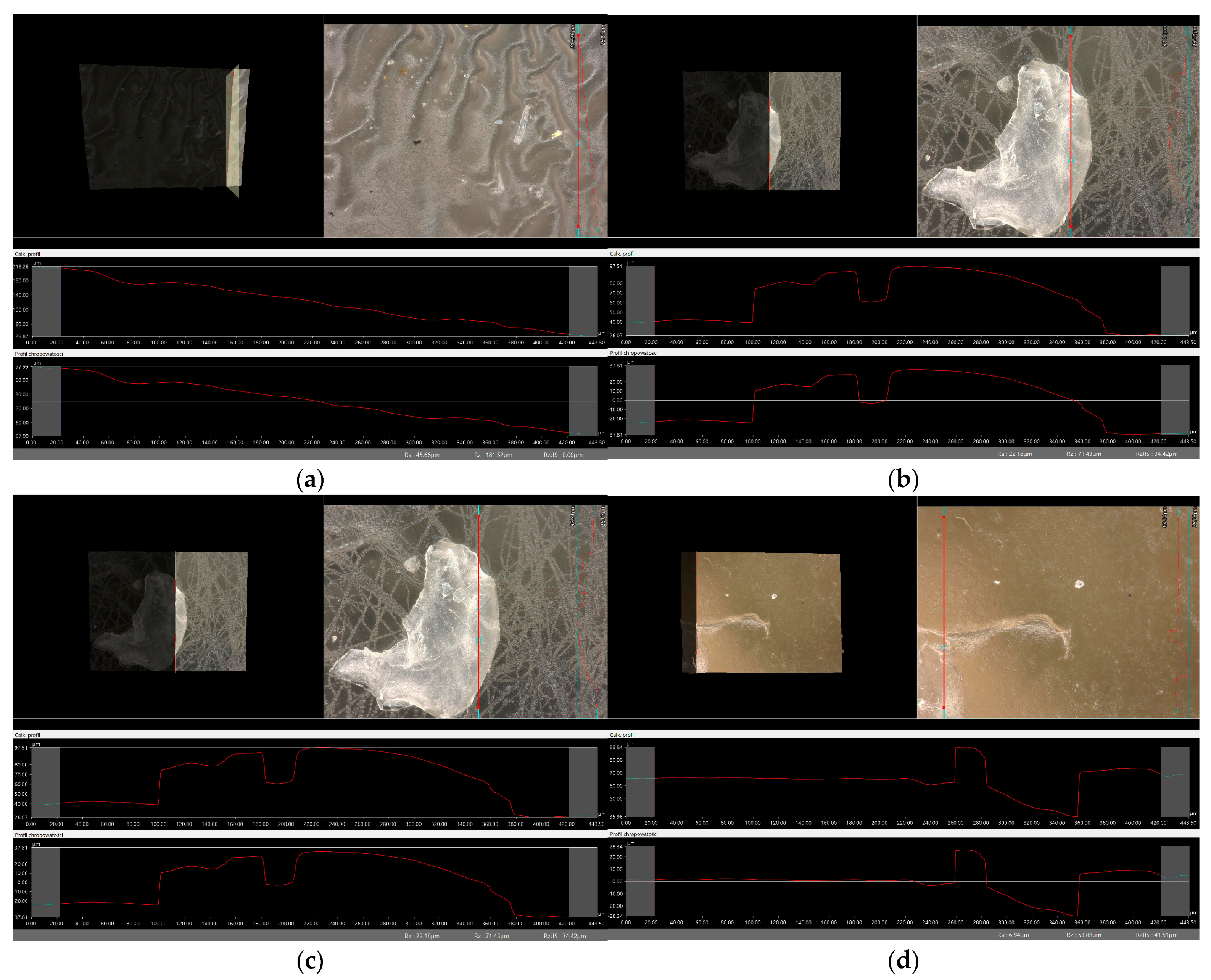
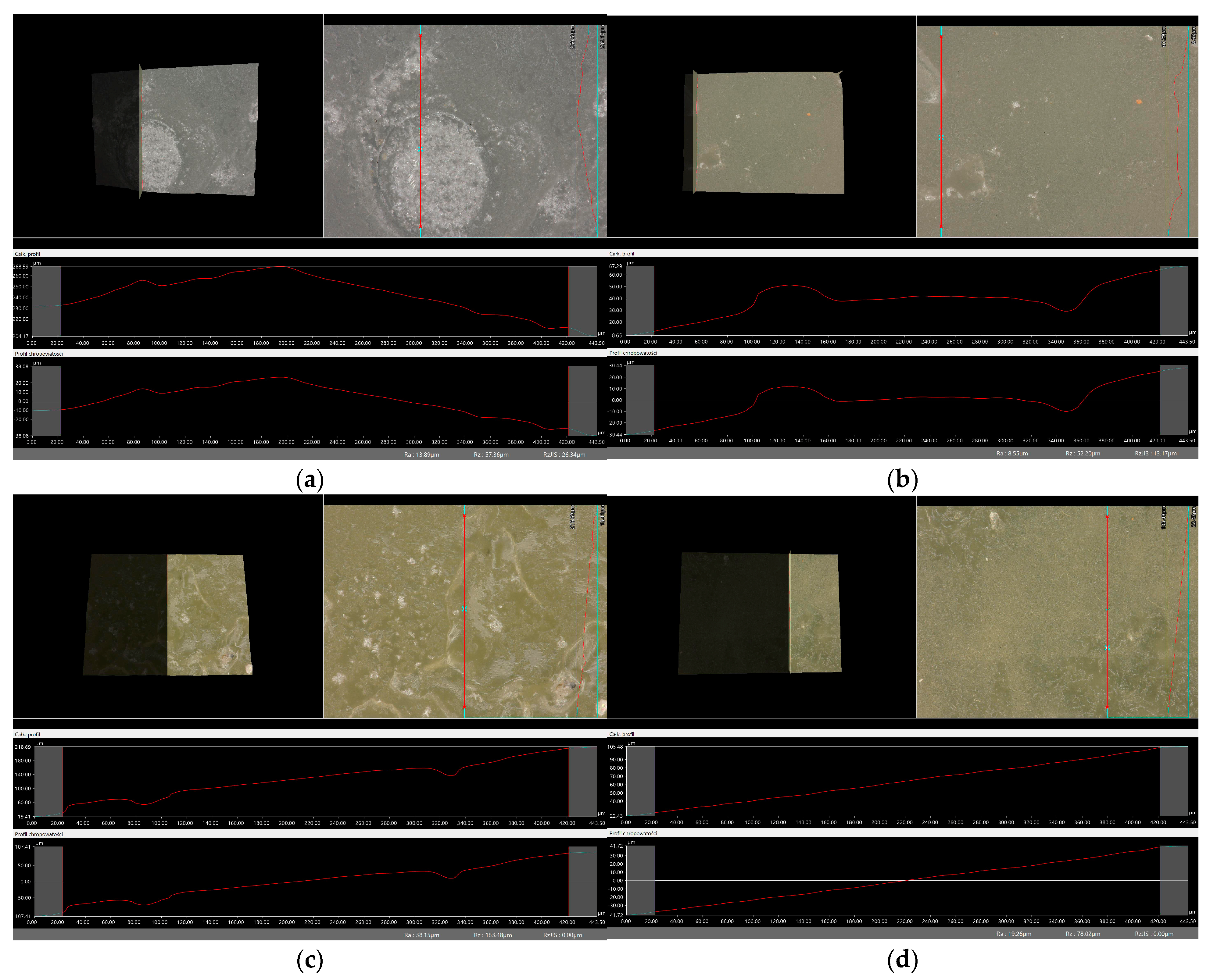
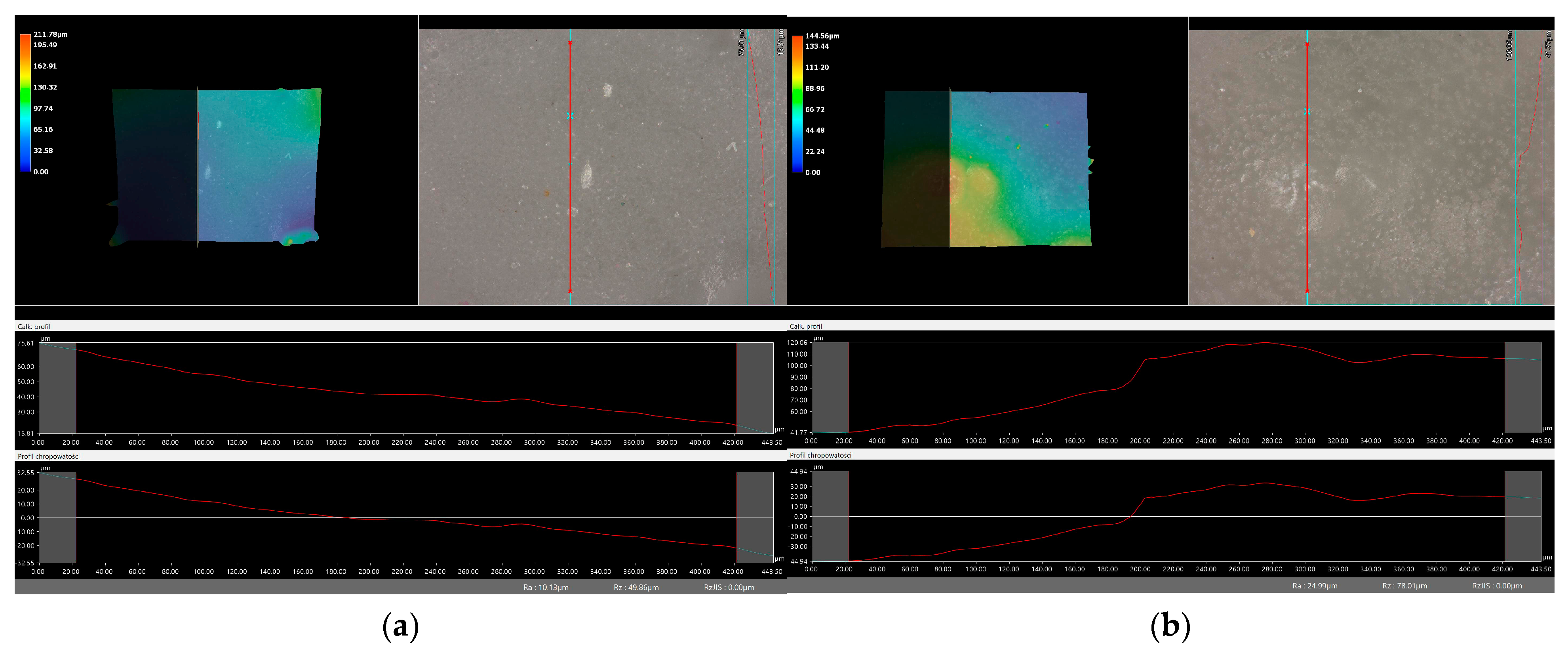
2.6. Digital Microscope Observations and Roughness Profile Analysis
2.7. Determination of Antioxidant Properties by the DPPH Radical Method
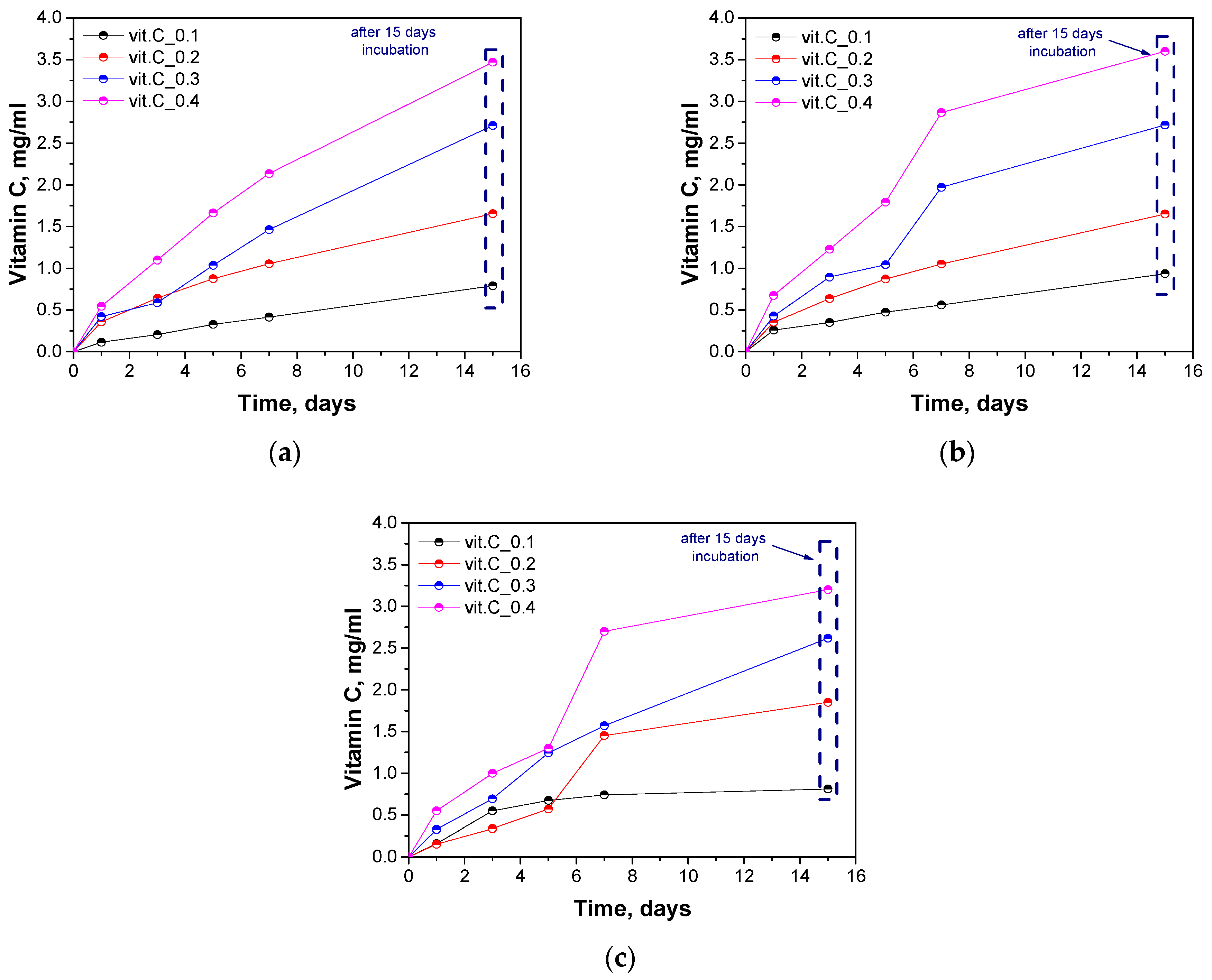
2.8. Determination of the Release of Vitamin C
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Hydrogel Materials
3.3. Sorption Capacity Analysis
- α—swelling ratio, g/g;
- mt—mass of swollen sample after time “t”, g;
- m0—mass of dry sample (before the study), g.
3.4. Incubation Studies in Simulated Body Fluids
3.5. Analysis of Antioxidant Properties
3.6. Characterization of the Chemical Structure of Hydrogel Materials via Fourier Transform Infrared (FT-IR) Spectroscopy
3.7. Observation Using a Digital Microscope and Determination of Surface Roughness
3.8. Determination of Antioxidant Properties by the DPPH Radical Method
- A0—absorbance of DPPH solution;
- As—absorbance of sample.
3.9. Determination of the Release of Vitamin C
4. Conclusions
- Studies of sorption capacity indicated that differences in the amount and nature of additives used in hydrogel materials have a significant impact on their absorption capacity, which is an important factor in assessing their suitability for medical applications.
- On the basis of the analysis of antioxidant properties and incubation studies, the possibility of releasing active substances with antioxidant activity was proved. A reduced pH value indicates the release of vitamin C and plant extracts from the polymer matrix; in turn, the reaction with potassium permanganate confirmed the antioxidant properties of the released substances.
- During incubation, no significant changes affecting the degradation of the hydrogel material were observed. Therefore, FT-IR analysis demonstrated the dynamic nature of the hydrogel materials and their compatibility with the incubation environment, highlighting their potential for biomedical applications.
- There was no clear relationship between the roughness profile of hydrogel samples obtained after synthesis and hydrogels after incubation testing. This result may be due to various factors affecting the morphology and structure of the material, both during the synthesis and incubation processes. The incubation process can lead to structural and morphological changes in the material due to, among other things, changes in the pH of the incubation environment or the presence of chemicals. Differences in environmental conditions can lead to different reactions in the material. It is noteworthy, however, that the analysis showed no significant effects indicating the breakdown and degradation of the incubated polymer matrix.
- The findings from the study indicated that the samples demonstrated sorptive and antioxidant capabilities, making them suitable for further development as wound dressings. A significant advantage of these materials is their adaptability; they can be modified to achieve specific properties tailored to their intended applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qi, L.; Zhang, C.; Wang, B.; Yin, J.; Yan, S. Progress in Hydrogels for Skin Wound Repair. Macromol. Biosci. 2022, 22, e2100475. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.-C.; Chang, C.-C.; Chan, H.-P.; Chung, T.-W.; Shu, C.-W.; Chuang, K.-P.; Duh, T.-H.; Yang, M.-H.; Tyan, Y.-C. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Wu, J.; Kang, Y.; Sun, P.; Xiao, Z.; Zhao, D. Recent Advances of Magnetic Chitosan Hydrogel: Preparation, Properties and Applications. Int. J. Biol. Macromol. 2023, 247, 125722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Qiu, P.; Wang, Y.; Wang, Y.; Zhou, J.; Zhang, B.; Zhang, L.; Gou, D. Chitosan-Based Hydrogel Wound Dressing: From Mechanism to Applications, a Review. Int. J. Biol. Macromol. 2023, 244, 125250. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gupta, V.K.; Amiri, H.; Pan, J.; Aghbashlo, M.; Tabatabaei, M.; Rajaei, A. Recent Developments in Improving the Emulsifying Properties of Chitosan. Int. J. Biol. Macromol. 2023, 239, 124210. [Google Scholar] [CrossRef]
- Fakhri, E.; Eslami, H.; Maroufi, P.; Pakdel, F.; Taghizadeh, S.; Ganbarov, K.; Yousefi, M.; Tanomand, A.; Yousefi, B.; Mahmoudi, S.; et al. Chitosan Biomaterials Application in Dentistry. Int. J. Biol. Macromol. 2020, 162, 956–974. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial Properties of Chitosan and Chitosan Derivatives in the Treatment of Enteric Infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, K.; Gong, Y.; Zhu, W.; Zhu, J.; Pan, F.; Chao, Y.; Xiao, Z.; Liu, Y.; Wang, X.; et al. Vitamin C Supramolecular Hydrogel for Enhanced Cancer Immunotherapy. Biomaterials 2022, 287, 121673. [Google Scholar] [CrossRef]
- Rs, N.; Reddy, M.V.N.J.; Batra, S.; Srivastava, S.K.; Syal, K. Vitamin C and Its Therapeutic Potential in the Management of COVID19. Clin. Nutr. ESPEN 2022, 50, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Rezai, S.; Rahzani, K.; Hekmatpou, D.; Rostami, A. Effect of Oral Calendula Officinalis on Second-Degree Burn Wound Healing. Scars Burn. Heal. 2023, 9, 20595131221134052. [Google Scholar] [CrossRef] [PubMed]
- Zacarias, C.A.; de Mendonça Florenziano, R.F.; de Andrade, T.A.M.; de Aro, A.A.; do Amaral, M.E.C.; Dos Santos, G.M.T.; Esquisatto, M.A.M. Arnica montana L. Associated with Microcurrent Accelerates the Dermis Reorganisation of Skin Lesions. Int. J. Exp. Pathol. 2023, 104, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Givol, O.; Kornhaber, R.; Visentin, D.; Cleary, M.; Haik, J.; Harats, M. A Systematic Review of Calendula Officinalis Extract for Wound Healing. Wound Repair Regen. 2019, 27, 548–561. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Zaman, S.U.; Khan, B.A.; Amir, M.N.; Ebrahimzadeh, M.A. Calendula Extract: Effects on Mechanical Parameters of Human Skin. Acta Pol. Pharm. 2011, 68, 693–701. [Google Scholar] [PubMed]
- Smith, A.G.; Miles, V.N.; Holmes, D.T.; Chen, X.; Lei, W. Clinical Trials, Potential Mechanisms, and Adverse Effects of Arnica as an Adjunct Medication for Pain Management. Medicines 2021, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Žitek, T.; Postružnik, V.; Knez, Ž.; Golle, A.; Dariš, B.; Marevci, M.K. Arnica montana L. Supercritical Extraction Optimization for Antibiotic and Anticancer Activity. Front. Bioeng. Biotechnol. 2022, 10, 897185. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.-H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an Antioxidant: Evaluation of Its Role in Disease Prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Duca, A.; Sturza, A.; Moacă, E.-A.; Negrea, M.; Lalescu, V.-D.; Lungeanu, D.; Dehelean, C.-A.; Muntean, D.-M.; Alexa, E. Identification of Resveratrol as Bioactive Compound of Propolis from Western Romania and Characterization of Phenolic Profile and Antioxidant Activity of Ethanolic Extracts. Molecules 2019, 24, 3368. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, M.; Tariq, S.; Ahmad, A.; Bashir, S.; Bibi, S.; Aslam Dogar, N.; Batool, D.; Ishaq, S. Spectrophotometric Determination of Vitamin C in Underground Vegetables and Kinetic Modelling to Probe the Effect of Temperature and PH on Degradation of Vitamin C. Pak. J. Bot. 2022, 54, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
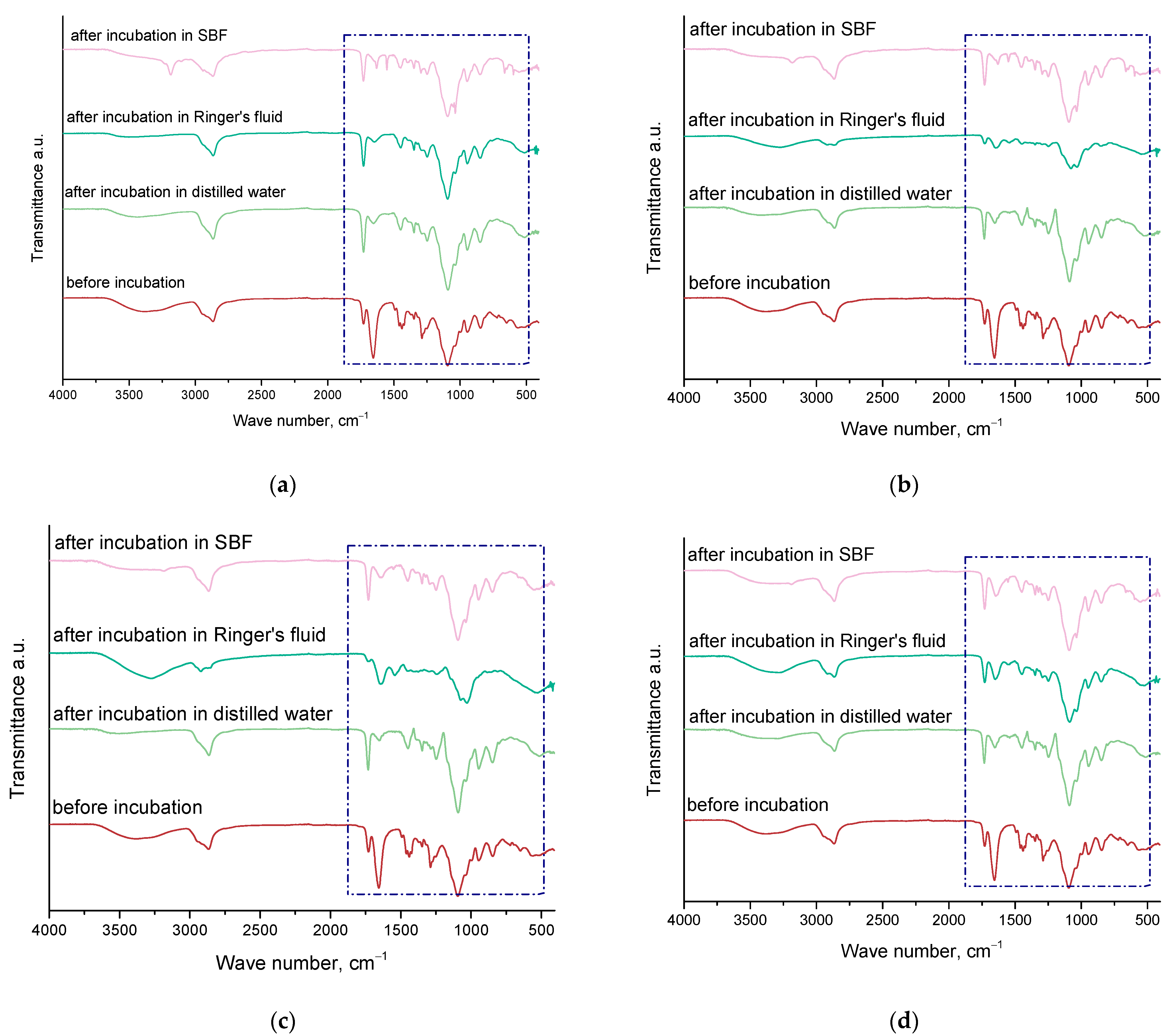



| Wave Number [cm−1] | Vibration Type | Characteristic Binding |
|---|---|---|
| 3182–3503 | Tensile | O-H |
| 2863–2942 | Stretching symmetric and asymmetric | C-H |
| 1724–1741 | Tensile | C=O |
| 1629–1655 | Tensile | C=C |
| 1421–1455 | Deformation (bending) | C-H |
| 1035–1096 | Tensile | C-O |
| Sample Number | Ra, µm Unincubated Sample | Ra, µm SBF Liquid | Ra, µm Ringer’s Solution | Ra, µm Distilled Water |
|---|---|---|---|---|
| vit. C_0.1 | 7.68 | 13.89 | 10.13 | 45.66 |
| vit. C_0.2 | 32.90 | 8.55 | 24.99 | 22.18 |
| vit. C_0.3 | 19.53 | 38.15 | 12.63 | 32.78 |
| vit. C_0.4 | 60.94 | 19.26 | 38.60 | 6.94 |
| Incubation Fluid | vit. C_0.1 | vit. C_0.2 | vit. C_0.3 | vit. C_0.4 |
|---|---|---|---|---|
| Distilled water | 88.38 ± 0.16 | 91.69 ± 0.74 | 93.48 ± 0.86 | 97.03 ± 0.41 |
| SBF liquid | 84.14 ± 0.37 | 86.96 ± 0.80 | 8.39 ± 0.22 | 95.34 ± 0.28 |
| Ringer’s solution | 85.75 ± 0.49 | 87.04 ± 0.54 | 90.11 ± 0.78 | 98.45 ± 0.63 |
| Base Solution * | PEGDA 700 g/mol, [mL] | Photoinitiator, [µL] | Vitamin C, [g] | Calendula Officinalis, [mL] | Arnica Montana, [mL] | Sample Name |
|---|---|---|---|---|---|---|
| 10 | 2.0 | 50 | 0.1 | 1.5 | 1.5 | vit.C_0.1 |
| 0.2 | vit.C_0.2 | |||||
| 0.3 | vit.C_0.3 | |||||
| 0.4 | vit.C_0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kędzierska, M.; Sala, K.; Bańkosz, M.; Grzela, K.; Potemski, P.; Miernik, K.; Tyliszczak, B. Enhanced Hydrogel Materials: Incorporating Vitamin C and Plant Extracts for Biomedical Applications. Molecules 2024, 29, 2633. https://doi.org/10.3390/molecules29112633
Kędzierska M, Sala K, Bańkosz M, Grzela K, Potemski P, Miernik K, Tyliszczak B. Enhanced Hydrogel Materials: Incorporating Vitamin C and Plant Extracts for Biomedical Applications. Molecules. 2024; 29(11):2633. https://doi.org/10.3390/molecules29112633
Chicago/Turabian StyleKędzierska, Magdalena, Katarzyna Sala, Magdalena Bańkosz, Klaudyna Grzela, Piotr Potemski, Krzysztof Miernik, and Bożena Tyliszczak. 2024. "Enhanced Hydrogel Materials: Incorporating Vitamin C and Plant Extracts for Biomedical Applications" Molecules 29, no. 11: 2633. https://doi.org/10.3390/molecules29112633
APA StyleKędzierska, M., Sala, K., Bańkosz, M., Grzela, K., Potemski, P., Miernik, K., & Tyliszczak, B. (2024). Enhanced Hydrogel Materials: Incorporating Vitamin C and Plant Extracts for Biomedical Applications. Molecules, 29(11), 2633. https://doi.org/10.3390/molecules29112633








