Kaempferol Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis (NASH) by Suppressing Neutrophil-Mediated NLRP3-ASC/TMS1-Caspase 3 Signaling
Abstract
1. Introduction
2. Results
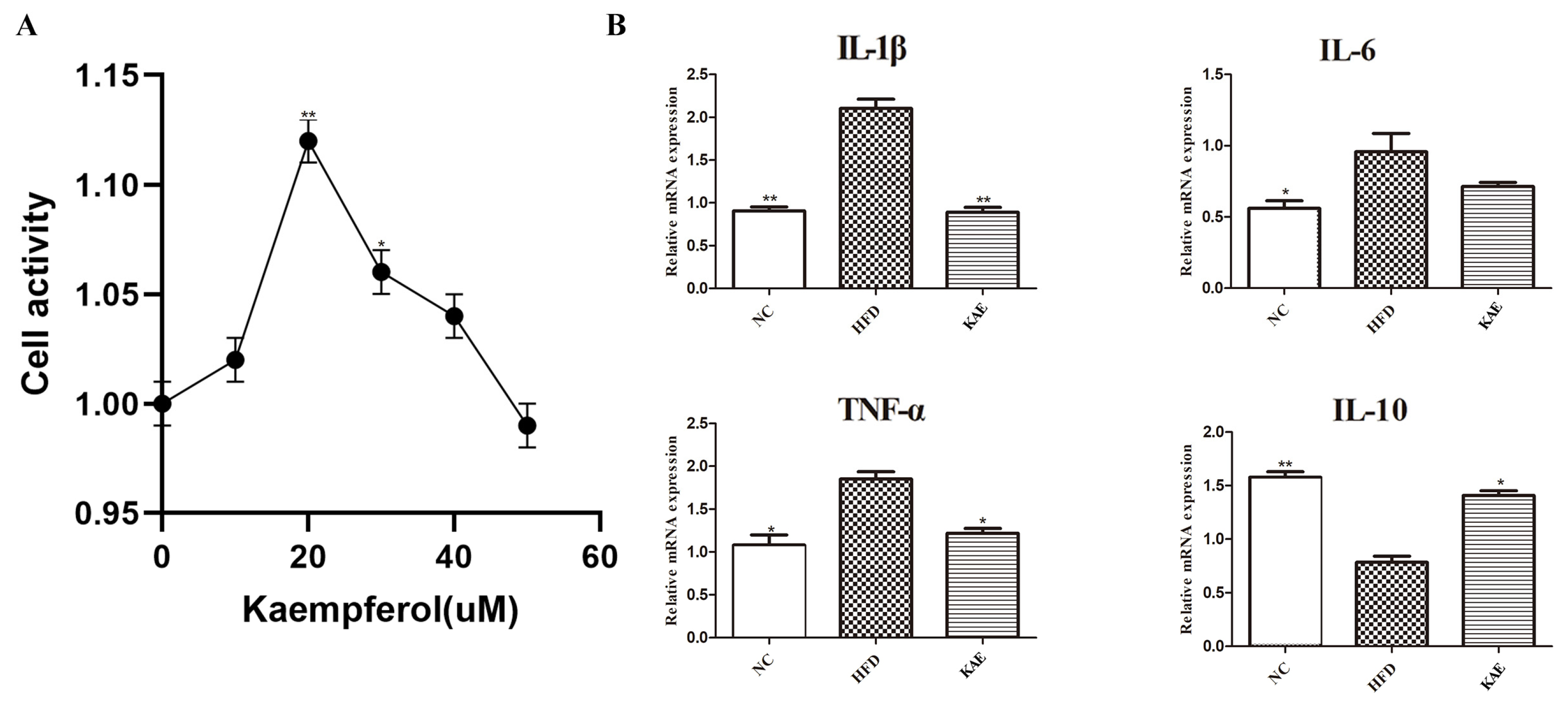
2.1. The Impact of Kaempferol on HepG2 Cells
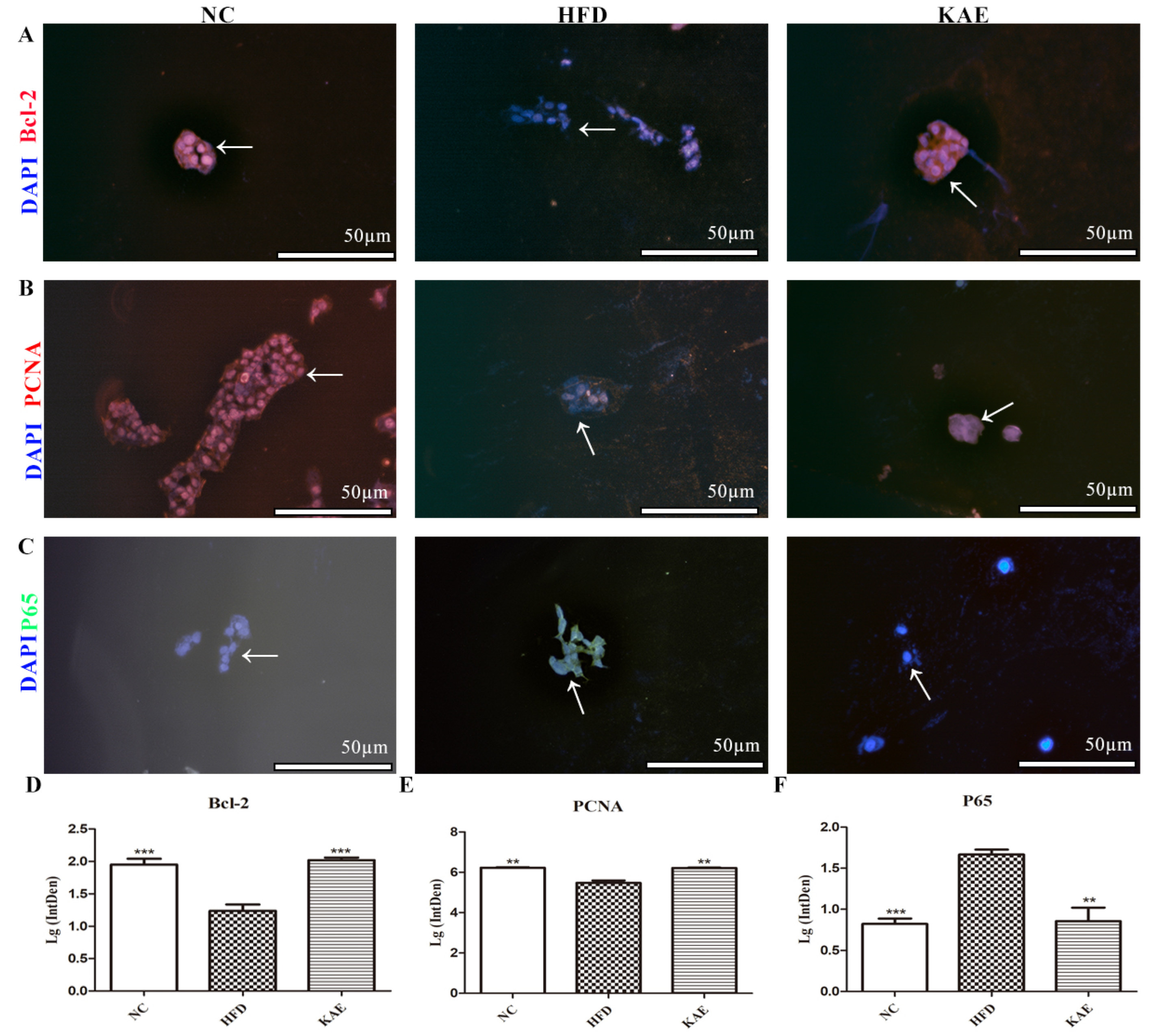
2.2. Immunofluorescence Assay for Assessing Proliferative Function in HepG2 Cell Line following Kaempferol Treatment
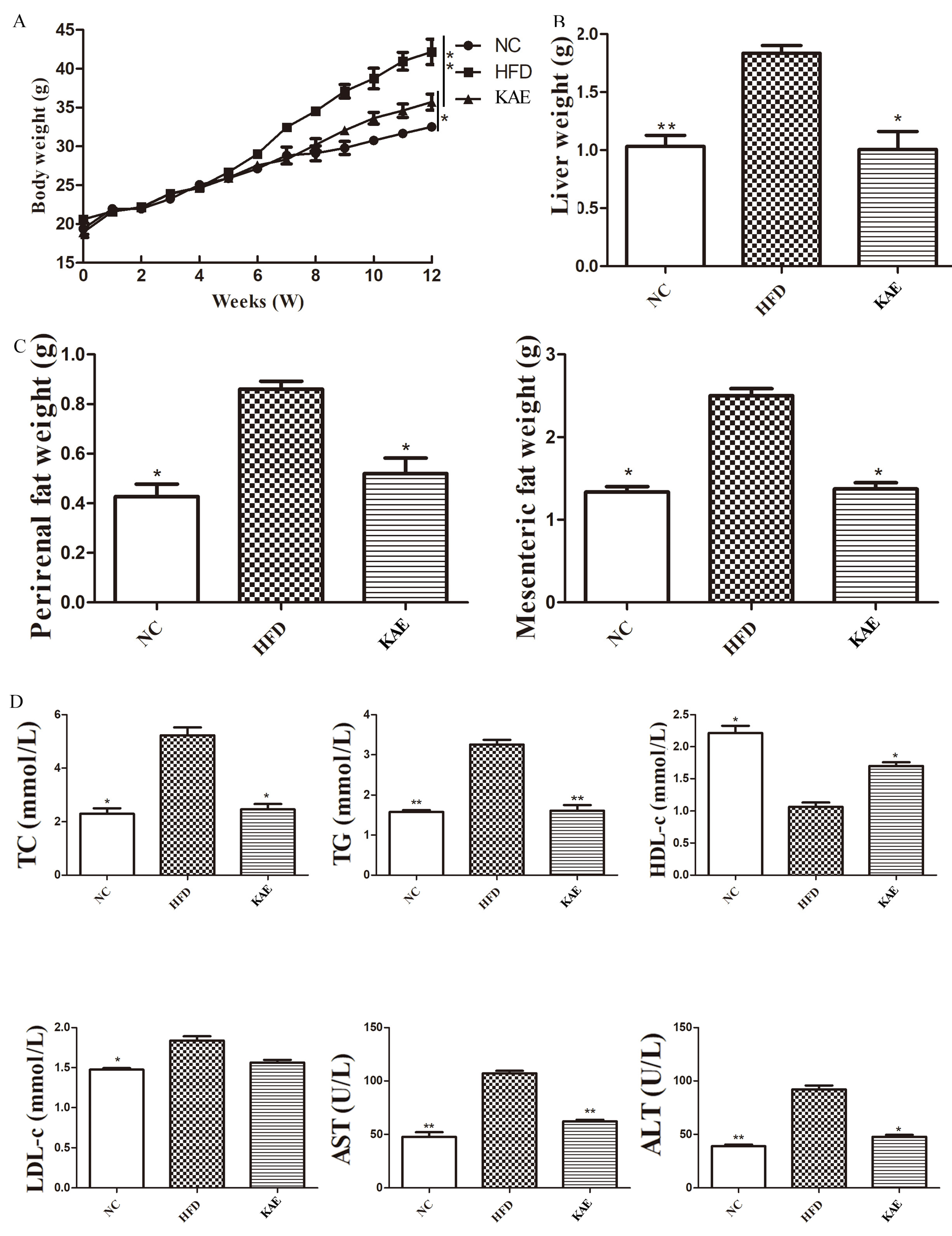
2.3. The Effect of Kaempferol on Lipid Metabolism in NASH Model Mice
2.4. The NLRP3-ASC/TMS1/Caspase-3 Pathway Initiated Liver Injury in NASH Mice
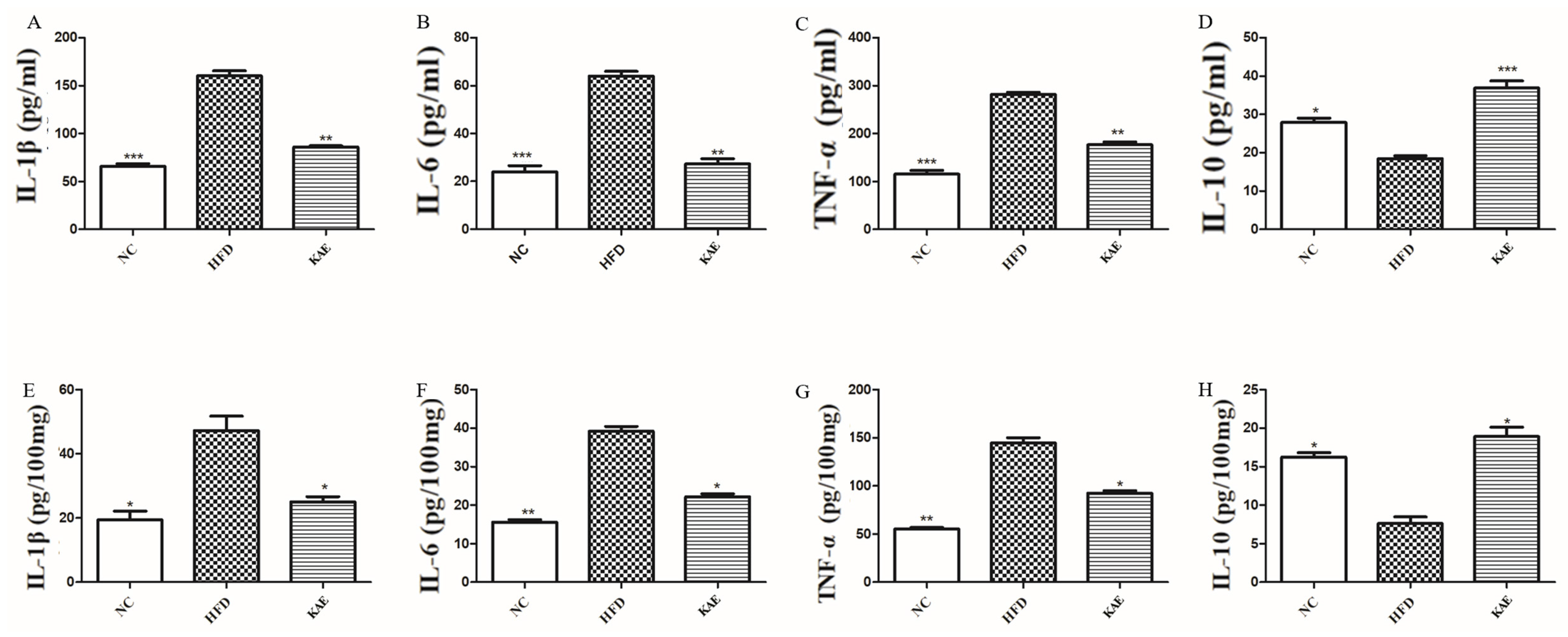
2.5. The Function of Kaempferol Supplements on Inflammation in a NASH Mouse Model
2.6. The Activation of MPO and NE Overexpression Caused Injury in NASH Mice
2.7. Effect of Kaempferol on Proliferation in Liver Tissue of NASH Model Mice
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Serum Inflammation Factor and Metabolism Assay
4.3. Histological Analysis
4.4. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR)
4.5. Cell Culture
4.6. Immunofluorescence Staining
4.7. Western Blot Analysis
- Dissect the tissue of interest with clean tools, on ice preferably, and as quickly as possible to prevent degradation by proteases;
- Place the tissue in round-bottom microcentrifuge tubes or Eppendorf tubes and immerse in liquid nitrogen to snap freeze. For a ~5 mg piece of tissue, add ~300 μL of ice-cold lysis buffer rapidly to the tube, rinse the blade twice with another 2 × 200 μL lysis buffer;
- Centrifuge for 10 min at 14,000× g at 4 °C in a microcentrifuge.
- Remove a small volume of lysate to perform a protein quantification assay;
- Determine how much protein to load and add an equal volume 2× Laemmli sample buffer;
- Boil each lysate in sample buffer at 100 °C for 5 min.
- Load equal amounts of protein into the wells of the SDS-PAGE gel, along with a molecular weight marker. Load 20–30 μg of total protein from tissue homogenate;
- Run the gel for 1–2 h at 100 V.
- The membrane can be either nitrocellulose or PVDF. Activate PVDF with methanol for 1 min and rinse with transfer buffer before preparing the stack;
- Run for 120 min at 250 mAh.
4.8. Cell Counting Kit 8 Assay
4.9. Statistical Analysis
4.10. Ethical Statement
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, G.; Wang, J.; Xu, J.; Zhao, F.; Hu, M.; Xu, Z.; Yang, B.; Guo, J.; Sun, S.; et al. Pedunculoside attenuates pathological phenotypes of fibroblast-like synoviocytes and protects against collagen-induced arthritis. Scand. J. Rheumatol. 2019, 48, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shao, M.; Zhang, C.; Xiang, H.; Wang, J.; Wu, T.; Ji, G. Kaempferol attenuates nonalcoholic steatohepatitis by regulating serum and liver bile acid metabolism. Front. Pharmacol. 2022, 13, 946360. [Google Scholar] [CrossRef] [PubMed]
- Kibble, M.; Saarinen, N.; Tang, J.; Wennerberg, K.; Makela, S.; Aittokallio, T. Network pharmacology applications to map the unexplored target space and therapeutic potential of natural products. Nat. Prod. Rep. 2015, 32, 1249–1266. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.E.; Wong, V.W.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Tawfiq, R.A.; Nassar, N.N.; Hammam, O.A.; Allam, R.M.; Elmazar, M.M.; Abdallah, D.M.; Attia, Y.M. Obeticholic acid orchestrates the crosstalk between ileal autophagy and tight junctions in non-alcoholic steatohepatitis: Role of TLR4/TGF-beta1 axis. Chem. Biol. Interact. 2022, 361, 109953. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bhattacharjee, P.; Kar, A.; Mukherjee, P.K. LC-MS/MS analysis and network pharmacology of Trigonella foenum-graecum—A plant from Ayurveda against hyperlipidemia and hyperglycemia with combination synergy. Phytomedicine 2019, 60, 152944. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Ribeiro, M.; Szabo, G. Role of the Inflammasome in Liver Disease. Annu. Rev. Pathol. 2022, 17, 345–365. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- El-Kashef, D.H.; Zaghloul, R.A. Ameliorative effect of montelukast against carbon tetrachloride-induced hepatotoxicity: Targeting NLRP3 inflammasome pathway. Life Sci. 2022, 304, 120707. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Jiang, K.; Wen, Z.; Cao, X.; Wu, S. Linear ubiquitination of LKB1 activates AMPK pathway to inhibit NLRP3 inflammasome response and reduce chondrocyte pyroptosis in osteoarthritis. J. Orthop. Translat. 2023, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Messaoud-Nacer, Y.; Culerier, E.; Rose, S.; Maillet, I.; Rouxel, N.; Briault, S.; Ryffel, B.; Quesniaux, V.F.J.; Togbe, D. STING agonist diABZI induces PANoptosis and DNA mediated acute respiratory distress syndrome (ARDS). Cell Death Dis. 2022, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.Y.; Li, C.G.; Shu, J.X.; Xu, L.H.; Ouyang, D.Y.; Mai, F.Y.; Zeng, Q.Z.; Zhang, C.C.; Li, R.M.; He, X.H. ATP induces caspase-3/gasdermin E-mediated pyroptosis in NLRP3 pathway-blocked murine macrophages. Apoptosis 2019, 24, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.J.; Wang, Y.Q.; Shang, S.Q.; Xu, S.; Guo, M. TMT induces apoptosis and necroptosis in mouse kidneys through oxidative stress-induced activation of the NLRP3 inflammasome. Ecotoxicol. Environ. Saf. 2022, 230, 113167. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.F.A.; Wong-Brown, M.W.; Bowden, N.A. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019, 10, 177. [Google Scholar] [CrossRef] [PubMed]
- Yalniz, F.F.; Wierda, W.G. Targeting BCL2 in Chronic Lymphocytic Leukemia and Other Hematologic Malignancies. Drugs 2019, 79, 1287–1304. [Google Scholar] [CrossRef]
- Psakhye, I.; Kawasumi, R.; Abe, T.; Hirota, K.; Branzei, D. PCNA recruits cohesin loader Scc2 to ensure sister chromatid cohesion. Nat. Struct. Mol. Biol. 2023, 30, 1286–1294. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, H.M.; Rozenberg, H.; Daitchman, D.; Levy, Y. Does PCNA diffusion on DNA follow a rotation-coupled translation mechanism? Nat. Commun. 2020, 11, 5000. [Google Scholar] [CrossRef]
- Mondol, T.; Stodola, J.L.; Galletto, R.; Burgers, P.M. PCNA accelerates the nucleotide incorporation rate by DNA polymerase delta. Nucleic Acids Res. 2019, 47, 1977–1986. [Google Scholar] [CrossRef]
- Gu, L.; Lingeman, R.; Yakushijin, F.; Sun, E.; Cui, Q.; Chao, J.; Hu, W.; Li, H.; Hickey, R.J.; Stark, J.M.; et al. The Anticancer Activity of a First-in-class Small-molecule Targeting PCNA. Clin. Cancer Res. 2018, 24, 6053–6065. [Google Scholar] [CrossRef]
- Deng, Y.; Ma, J.; Weng, X.; Wang, Y.; Li, M.; Yang, T.; Dou, Z.; Yin, Z.; Shang, J. Kaempferol-3-O-Glucuronide Ameliorates Non-Alcoholic Steatohepatitis in High-Cholesterol-Diet-Induced Larval Zebrafish and HepG2 Cell Models via Regulating Oxidation Stress. Life 2021, 11, 445. [Google Scholar] [CrossRef]
- Alshehri, A.S.; El-Kott, A.F.; El-Gerbed, M.S.A.; El-Kenawy, A.E.; Albadrani, G.M.; Khalifa, H.S. Kaempferol prevents cadmium chloride-induced liver damage by upregulating Nrf2 and suppressing NF-kappaB and keap1. Environ. Sci. Pollut. Res. Int. 2022, 29, 13917–13929. [Google Scholar] [CrossRef] [PubMed]
- Kouhestani, S.; Jafari, A.; Babaei, P. Kaempferol attenuates cognitive deficit via regulating oxidative stress and neuroinflammation in an ovariectomized rat model of sporadic dementia. Neural Regen. Res. 2018, 13, 1827–1832. [Google Scholar] [CrossRef]
- Wang, H.; Chen, L.; Zhang, X.; Xu, L.; Xie, B.; Shi, H.; Duan, Z.; Zhang, H.; Ren, F. Kaempferol protects mice from d-GalN/LPS-induced acute liver failure by regulating the ER stress-Grp78-CHOP signaling pathway. Biomed. Pharmacother. 2019, 111, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Lee, B.; Kwon, M.; Choi, J.S.; Jeong, H.O.; Chung, H.Y.; Kim, H.R. Kaempferol Isolated from Nelumbo nucifera Inhibits Lipid Accumulation and Increases Fatty Acid Oxidation Signaling in Adipocytes. J. Med. Food 2015, 18, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Tavakol, S.; Ahmadi, Z.; Roomiani, S.; Mohammadinejad, R.; Samarghandian, S. Therapeutic effects of kaempferol affecting autophagy and endoplasmic reticulum stress. Phytother. Res. 2020, 34, 911–923. [Google Scholar] [CrossRef]
- Stefan, N.; Haring, H.U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef]
- Huby, T.; Gautier, E.L. Immune cell-mediated features of non-alcoholic steatohepatitis. Nat. Rev. Immunol. 2022, 22, 429–443. [Google Scholar] [CrossRef]
- Estes, C.; Anstee, Q.M.; Arias-Loste, M.T.; Bantel, H.; Bellentani, S.; Caballeria, J.; Colombo, M.; Craxi, A.; Crespo, J.; Day, C.P.; et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J. Hepatol. 2018, 69, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.G.; Kim, S.U.; Wong, V.W. New trends on obesity and NAFLD in Asia. J. Hepatol. 2017, 67, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shao, M.; Xiang, H.; Zheng, P.; Wu, T.; Ji, G. Integrative transcriptomics and metabolomics explore the mechanism of kaempferol on improving nonalcoholic steatohepatitis. Food Funct. 2020, 11, 10058–10069. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Shao, M.; Lu, Y.; Wang, J.; Wu, T.; Ji, G. Kaempferol Alleviates Steatosis and Inflammation During Early Non-Alcoholic Steatohepatitis Associated With Liver X Receptor alpha-Lysophosphatidylcholine Acyltransferase 3 Signaling Pathway. Front. Pharmacol. 2021, 12, 690736. [Google Scholar] [CrossRef] [PubMed]
- Hochheiser, I.V.; Behrmann, H.; Hagelueken, G.; Rodriguez-Alcazar, J.F.; Kopp, A.; Latz, E.; Behrmann, E.; Geyer, M. Directionality of PYD filament growth determined by the transition of NLRP3 nucleation seeds to ASC elongation. Sci. Adv. 2022, 8, eabn7583. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z.J. PtdIns4P on dispersed trans-Golgi network mediates NLRP3 inflammasome activation. Nature 2018, 564, 71–76. [Google Scholar] [CrossRef]
- Schmacke, N.A.; O’Duill, F.; Gaidt, M.M.; Szymanska, I.; Kamper, J.M.; Schmid-Burgk, J.L.; Madler, S.C.; Mackens-Kiani, T.; Kozaki, T.; Chauhan, D.; et al. IKKbeta primes inflammasome formation by recruiting NLRP3 to the trans-Golgi network. Immunity 2022, 55, 2271–2284. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Wang, S.N.; Shi, Y.J.; Chen, J.; Ding, S.Q.; Tang, J.; Shen, L.; Wang, R.; Ding, H.; Hu, J.G.; et al. CRID3, a blocker of apoptosis associated speck like protein containing a card, ameliorates murine spinal cord injury by improving local immune microenvironment. J. Neuroinflamm. 2020, 17, 255. [Google Scholar] [CrossRef] [PubMed]
- Christgen, S.; Place, D.E.; Kanneganti, T.D. Toward targeting inflammasomes: Insights into their regulation and activation. Cell Res. 2020, 30, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kanneganti, T.D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016, 213, 617–629. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- de Rivero Vaccari, J.P.; Dietrich, W.D.; Keane, R.W. Therapeutics targeting the inflammasome after central nervous system injury. Transl. Res. 2016, 167, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wu, H. Structural Mechanisms of NLRP3 Inflammasome Assembly and Activation. Annu. Rev. Immunol. 2023, 41, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Hornung, V. The NLRP3 Inflammasome Renders Cell Death Pro-inflammatory. J. Mol. Biol. 2018, 430, 133–141. [Google Scholar] [CrossRef]
- Alonso Bellido, I.M.; Posada-Perez, M.; Hernandez-Rasco, F.; Vazquez-Reyes, S.; Cabanillas, M.; Herrera, A.J.; Bachiller, S.; Soldan-Hidalgo, J.; Espinosa-Oliva, A.M.; Joseph, B.; et al. Microglial Caspase-3 is essential for modulating hippocampal neurogenesis. Brain Behav. Immun. 2023, 112, 206–219. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, R.; Tsakem Lenou, E.; Basrur, V.; Kontoyiannis, D.L.; Ioakeimidis, F.; Mosialos, G.; Theiss, A.L.; Flavell, R.A.; Venuprasad, K. Deubiquitination of NLRP6 inflammasome by Cyld critically regulates intestinal inflammation. Nat. Immunol. 2020, 21, 626–635. [Google Scholar] [CrossRef]
- Shen, C.; Li, R.; Negro, R.; Cheng, J.; Vora, S.M.; Fu, T.M.; Wang, A.; He, K.; Andreeva, L.; Gao, P.; et al. Phase separation drives RNA virus-induced activation of the NLRP6 inflammasome. Cell 2021, 184, 5759–5774.e5720. [Google Scholar] [CrossRef] [PubMed]
- Franciszkiewicz, K.; Boissonnas, A.; Boutet, M.; Combadiere, C.; Mami-Chouaib, F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012, 72, 6325–6332. [Google Scholar] [CrossRef] [PubMed]
- van Schouwenburg, P.A.; Rispens, T.; Wolbink, G.J. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat. Rev. Rheumatol. 2013, 9, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, H.; Umemura, A.; Taniguchi, K.; Font-Burgada, J.; Dhar, D.; Ogata, H.; Zhong, Z.; Valasek, M.A.; Seki, E.; Hidalgo, J.; et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell 2014, 26, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gehart, H.; Artegiani, B.; LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; Chuva de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-Term Expansion of Functional Mouse and Human Hepatocytes as 3D Organoids. Cell 2018, 175, 1591–1606.e1519. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Zhang, S.; Zhu, M.; Lu, T.; Chen, K.; Wen, Z.; Wang, S.; Xiao, G.; Luo, D.; Jia, Y.; et al. Mice With Increased Numbers of Polyploid Hepatocytes Maintain Regenerative Capacity But Develop Fewer Hepatocellular Carcinomas Following Chronic Liver Injury. Gastroenterology 2020, 158, 1698–1712 e1614. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, B.; Chen, Z.; Deng, M.; Wang, Y.; Wang, J.; Chen, L.; Zhang, Z.; Xiao, X.; Chen, C.; et al. Identification of key pathways and genes in nonalcoholic fatty liver disease using bioinformatics analysis. Arch. Med. Sci. 2020, 16, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tian, S.; Wu, Z.; Xu, X.; Lei, L.; Li, Y.; Wang, B.; Huang, Y. Pharmacokinetic herb-disease-drug interactions: Effect of ginkgo biloba extract on the pharmacokinetics of pitavastatin, a substrate of Oatp1b2, in rats with non-alcoholic fatty liver disease. J. Ethnopharmacol. 2021, 280, 114469. [Google Scholar] [CrossRef]
- Kojima, S.; Kao, M.H.; Doupe, A.J.; Brainard, M.S. The Avian Basal Ganglia Are a Source of Rapid Behavioral Variation That Enables Vocal Motor Exploration. J. Neurosci. 2018, 38, 9635–9647. [Google Scholar] [CrossRef]
- Yadav, P.S.; Papaioannou, G.; Kobelski, M.M.; Demay, M.B. Phosphate-induced activation of VEGFR2 leads to caspase-9-mediated apoptosis of hypertrophic chondrocytes. iScience 2023, 26, 107548. [Google Scholar] [CrossRef]
- Han, X.; Zhao, S.; Song, H.; Xu, T.; Fang, Q.; Hu, G.; Sun, L. Kaempferol alleviates LD-mitochondrial damage by promoting autophagy: Implications in Parkinson’s disease. Redox Biol. 2021, 41, 101911. [Google Scholar] [CrossRef] [PubMed]
- Wen, E.; Cao, Y.; He, S.; Zhang, Y.; You, L.; Wang, T.; Wang, Z.; He, J.; Feng, Y. The mitochondria-targeted Kaempferol nanoparticle ameliorates severe acute pancreatitis. J. Nanobiotechnol. 2024, 22, 148. [Google Scholar] [CrossRef] [PubMed]



| High-Fat Diet with 60% Fat Energy | Nature Control Diet | |
|---|---|---|
| Fat | 60% | 10% |
| Protein | 20% | 20% |
| Carbohydrate | 20% | 70% |
| NM Accession Code | 5′-3′ | 3′-5′ | Amplicon Length | |
|---|---|---|---|---|
| IL-6 | NM_012589.2 | GCCTTCTTGGGACTGATGCT | TGTGACTCCAGCTTATCTCTTGG | 199 |
| IL-1β | NM_008361.4 | TGCCACCTTTTGACAGTGATG | TTCTTGTGACCCTGAGCGAC | 94 |
| IL-10 | NM_012854.2 | ACTACCAAAGCCACAAGGCA | ACACCTTGGTCTGGAGCTTATTA | 185 |
| IL-4 | NM_201270.1 | TCACAGCAACGAAGAACACCA | CAGGCATCGAAAAGCCCGAA | 172 |
| β-actin | NM_004882 | AAGGAGCCCCACGAGAAAAAT | ACCGAACTTGCATTGATTCCAG | 169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Li, D.; Gao, G. Kaempferol Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis (NASH) by Suppressing Neutrophil-Mediated NLRP3-ASC/TMS1-Caspase 3 Signaling. Molecules 2024, 29, 2630. https://doi.org/10.3390/molecules29112630
Yang H, Li D, Gao G. Kaempferol Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis (NASH) by Suppressing Neutrophil-Mediated NLRP3-ASC/TMS1-Caspase 3 Signaling. Molecules. 2024; 29(11):2630. https://doi.org/10.3390/molecules29112630
Chicago/Turabian StyleYang, He, Dandan Li, and Guolan Gao. 2024. "Kaempferol Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis (NASH) by Suppressing Neutrophil-Mediated NLRP3-ASC/TMS1-Caspase 3 Signaling" Molecules 29, no. 11: 2630. https://doi.org/10.3390/molecules29112630
APA StyleYang, H., Li, D., & Gao, G. (2024). Kaempferol Alleviates Hepatic Injury in Nonalcoholic Steatohepatitis (NASH) by Suppressing Neutrophil-Mediated NLRP3-ASC/TMS1-Caspase 3 Signaling. Molecules, 29(11), 2630. https://doi.org/10.3390/molecules29112630






