A Novel Low-Density-Biomass-Carbon Composite Coated with Carpet-like and Dandelion-Shaped Rare-Earth-Doped Cobalt Ferrite for Enhanced Microwave Absorption
Abstract
1. Introduction
2. Results and Discussion
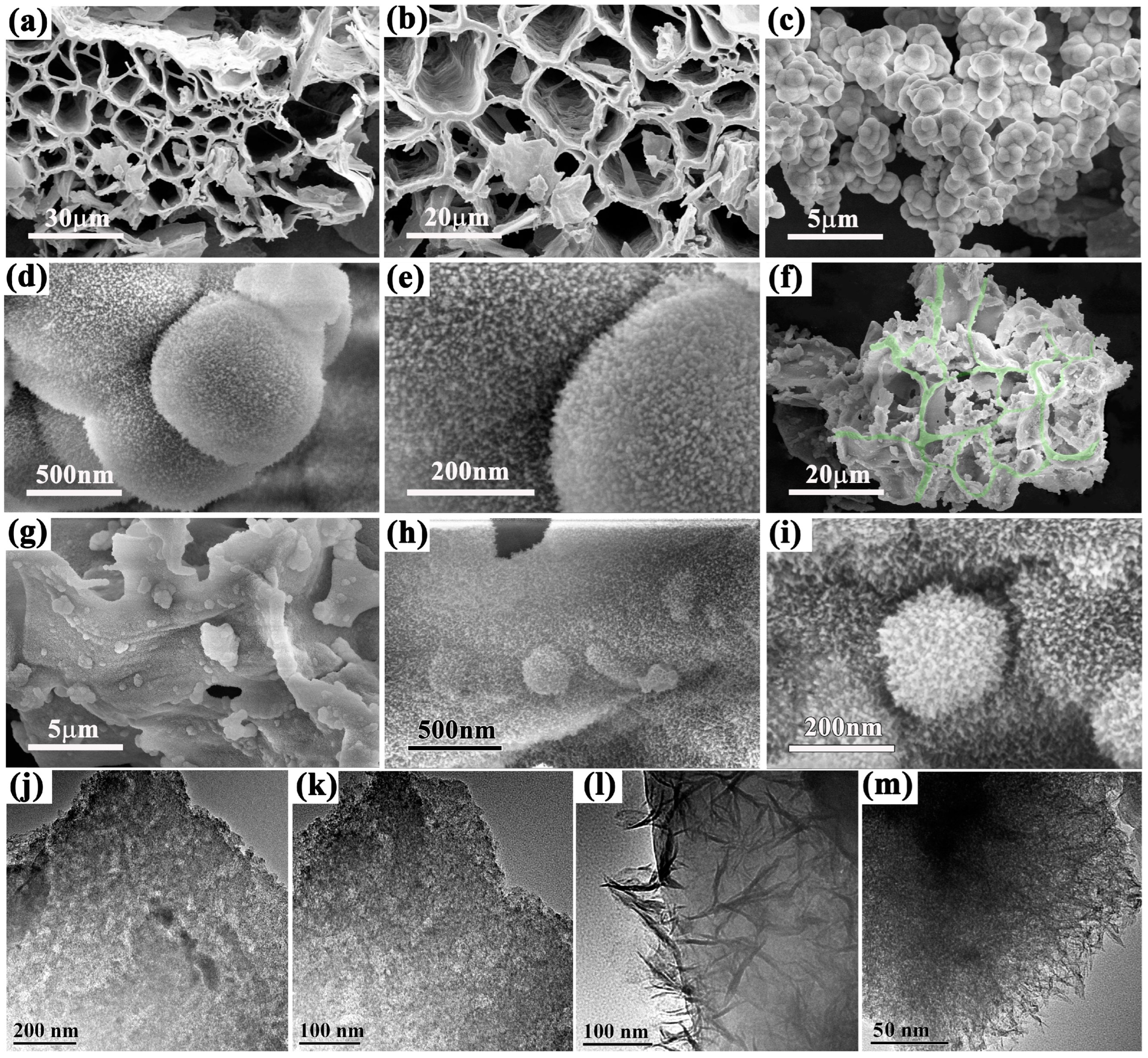
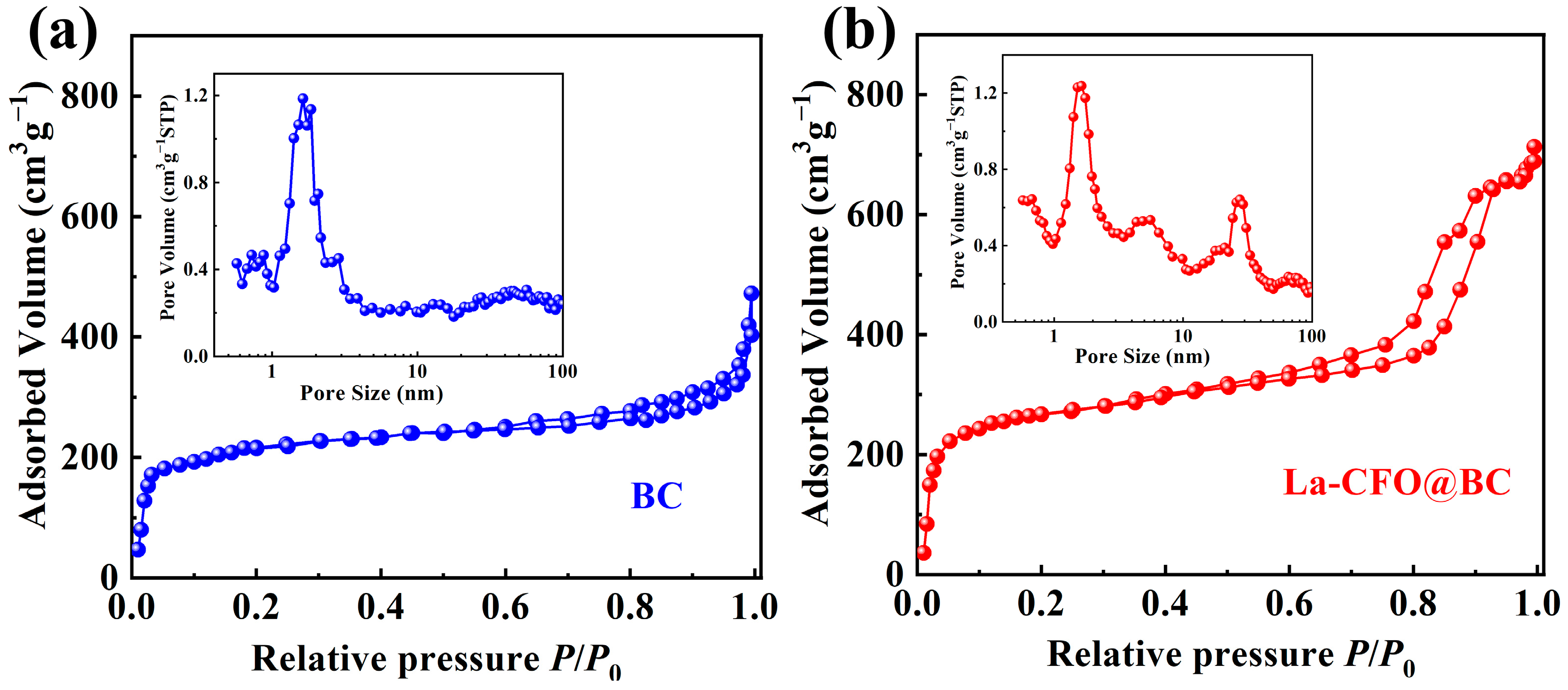
2.1. Configuration Analysis
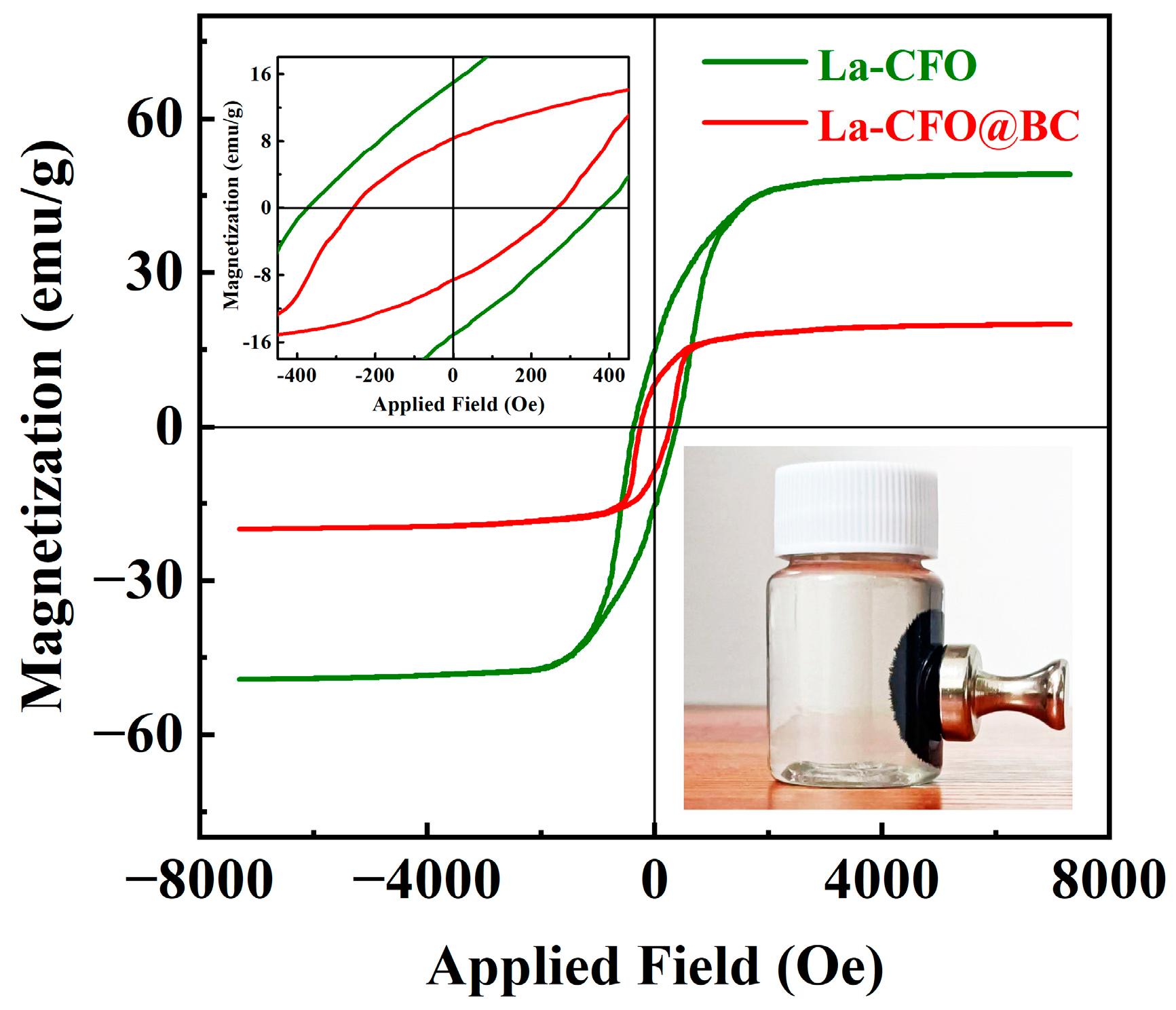
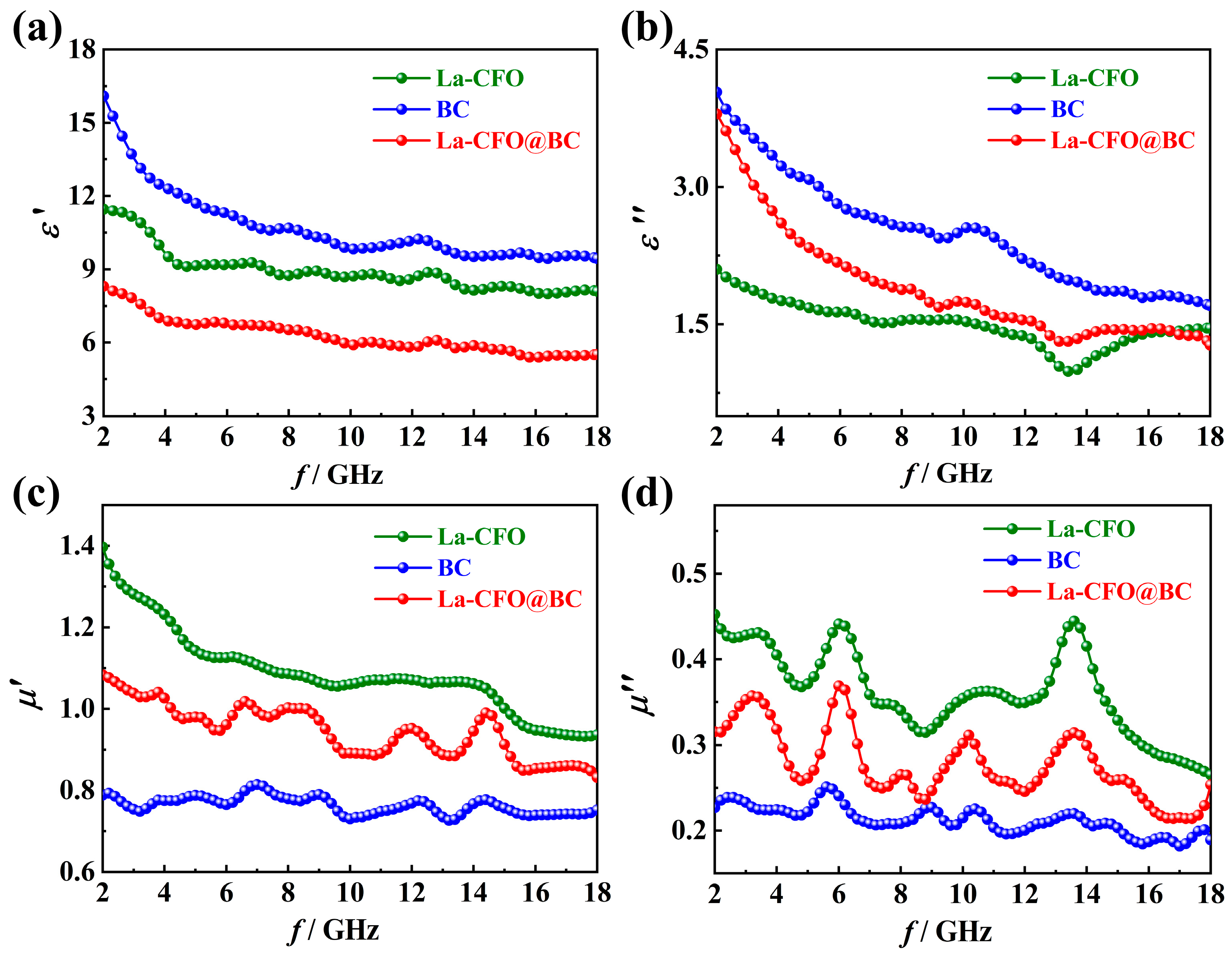
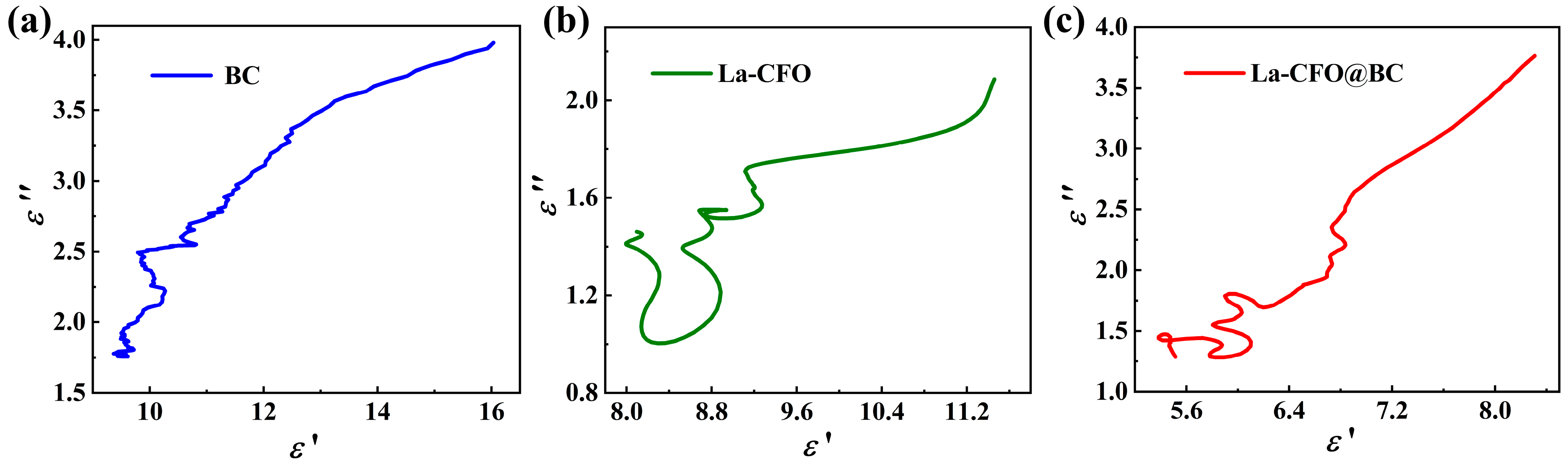
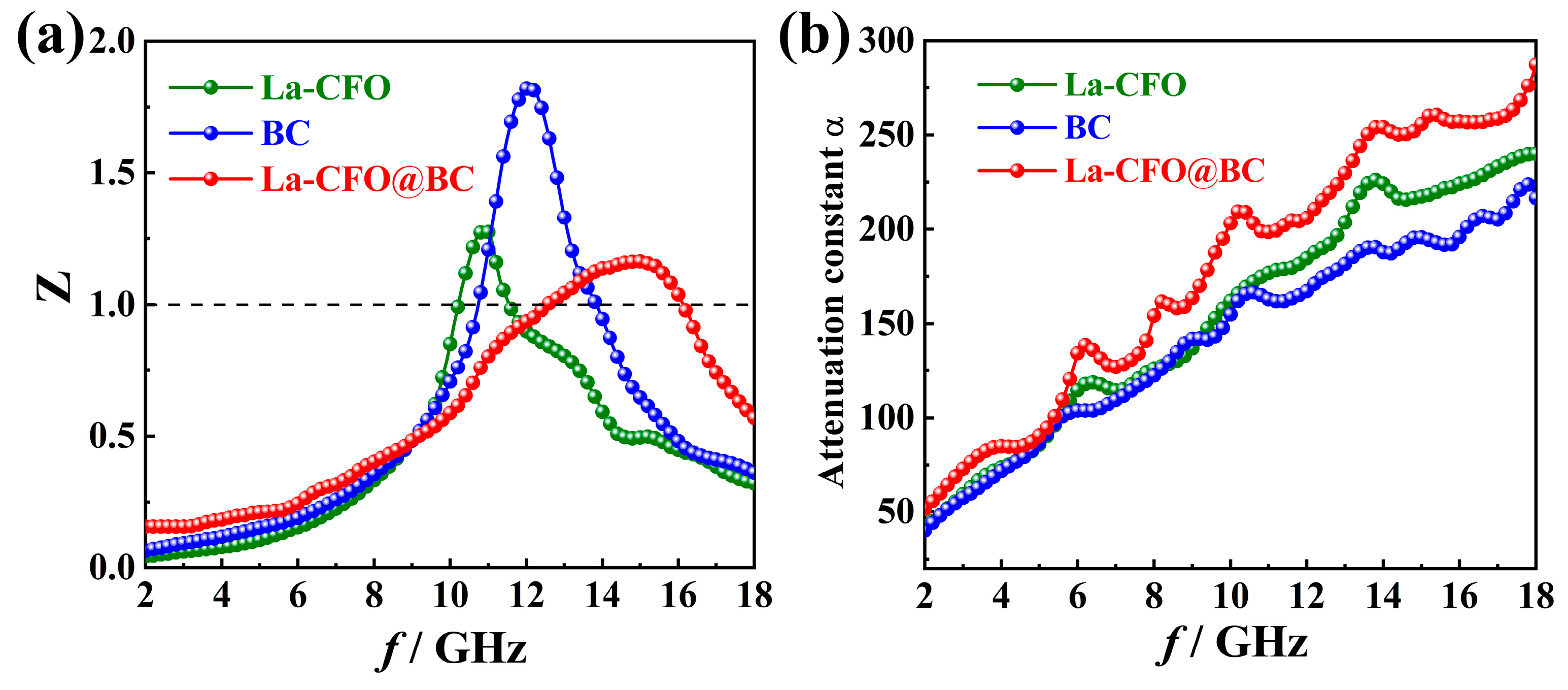
2.2. Magnetic Properties
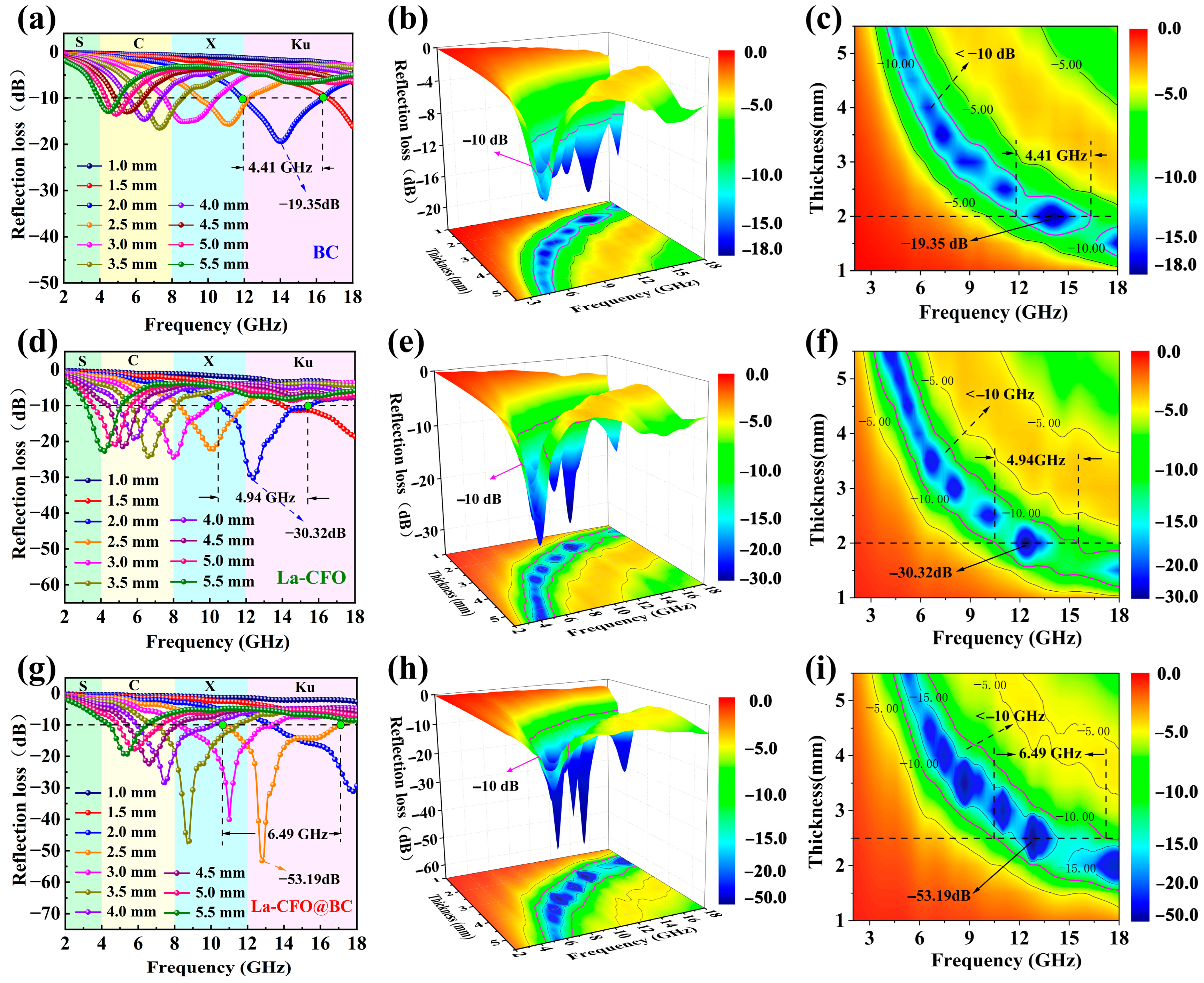
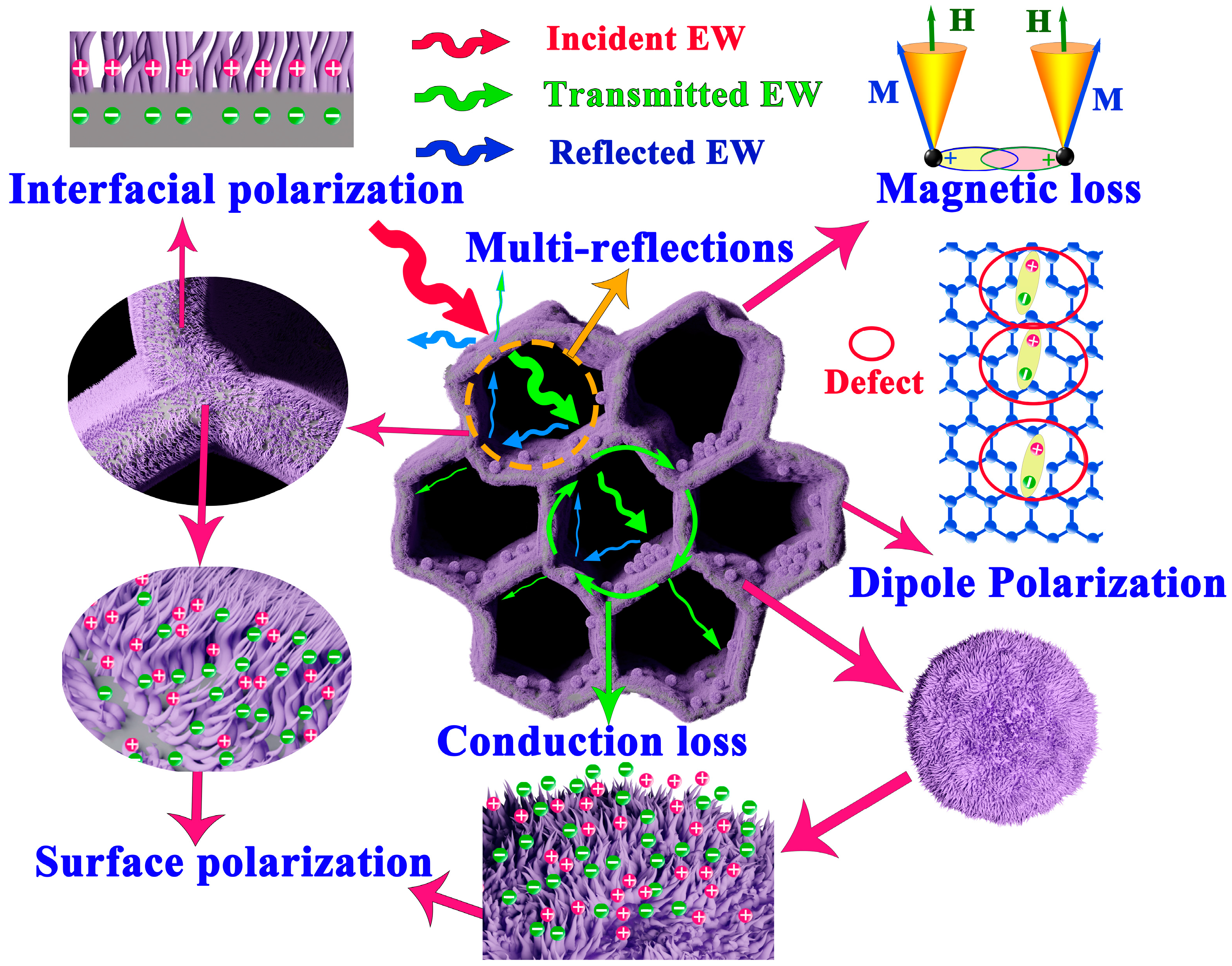
2.3. Microwave-Absorption Characteristics
3. Materials and Methods
3.1. Preparation of the Composites
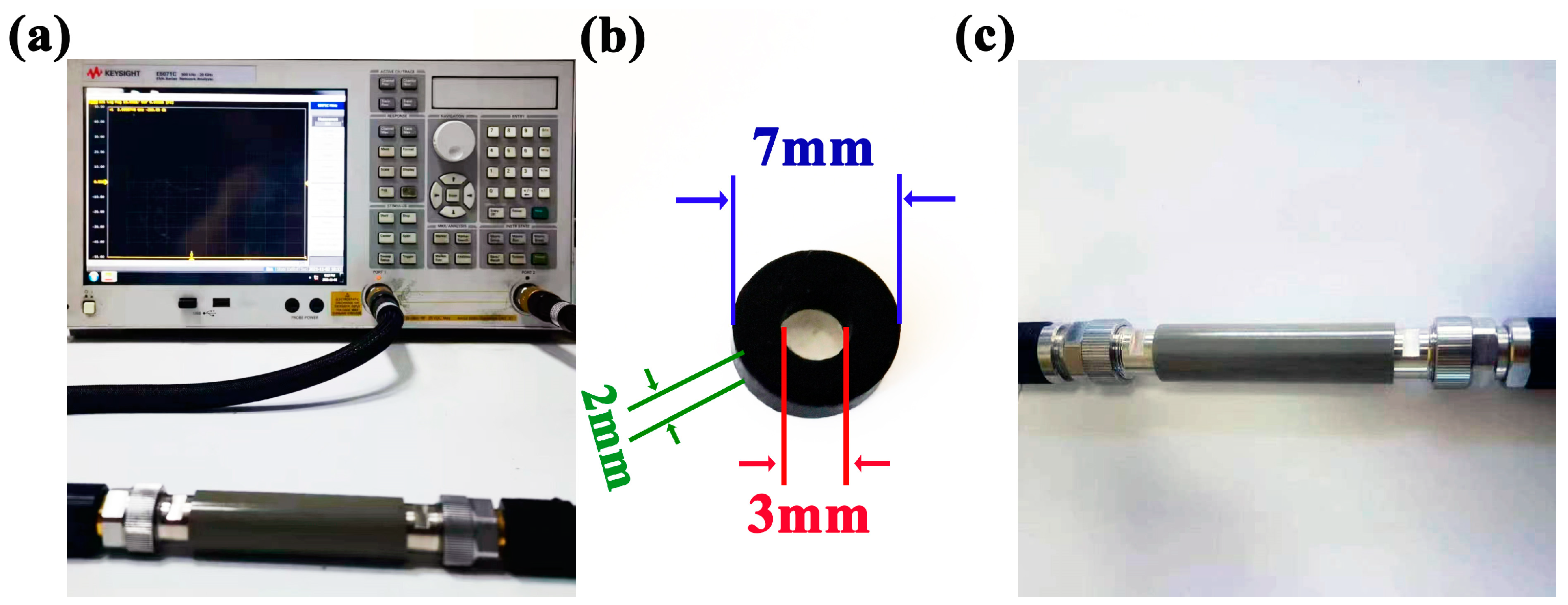
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, C.; Shao, C.; Songsong, W.; Ma, Y.; Liu, Y.; Ma, S.; Hu, X.; Cao, Z.; Ren, X.; Zhong, B. A review of 1D carbon-based materials assembly design for lightweight microwave absorption. Carbon 2023, 213, 118279. [Google Scholar] [CrossRef]
- Zhao, Z.; Qing, Y.; Kong, L.; Xu, H.; Fan, X.; Yun, J.; Zhang, L.; Wu, H. Advancements in microwave absorption motivated by interdisciplinary research. Adv. Mater. 2023, 36, 2304182. [Google Scholar] [CrossRef]
- Xiang, L.; Qi, X.; Rao, Y.; Wang, L.; Gong, X.; Chen, Y.; Peng, Q.; Zhong, W. A simple strategy to develop heterostructured carbon paper/Co nanoparticles composites with lightweight, tunable and broadband microwave absorption. Mater. Today Phys. 2023, 34, 101030. [Google Scholar] [CrossRef]
- Guan, H.; Wang, Q.; Wu, X.; Pang, J.; Jiang, Z.; Chen, G.; Dong, C.; Wang, L.; Gong, C. Biomass derived porous carbon (BPC) and their composites as lightweight and efficient microwave absorption materials. Compos. Part B 2021, 207, 108562. [Google Scholar] [CrossRef]
- Shi, Q.; Zhao, Y.; Li, M.; Li, B.; Hu, Z. 3D lamellar skeletal network of porous carbon derived from hull of water chestnut with excellent microwave absorption properties. J. Colloid Interface Sci. 2023, 641, 449–458. [Google Scholar] [CrossRef]
- Zhang, R.; Qiao, J.; Zhang, X.; Yang, Y.; Zheng, S.; Li, B.; Liu, W.; Liu, J.; Zeng, Z. Biomass-derived porous carbon for microwave absorption. Mater. Chem. Phys. 2022, 289, 126437. [Google Scholar] [CrossRef]
- Song, W.; Zhao, Q.; Wang, Z. Magnetic biomass porous carbon@Co/CoO nanocomposite for highly efficient microwave absorption. Mater. Res. Bull. 2023, 167, 112371. [Google Scholar] [CrossRef]
- Huang, X.; Wang, Y.; Lou, Z.; Chen, Y.; Li, Y.; Lv, H. Porous, magnetic carbon derived from bamboo for microwave absorption. Carbon 2023, 209, 118005. [Google Scholar] [CrossRef]
- Chang, Q.; Xie, Z.; Long, C.; Feng, X.; Shi, B.; Wu, H. Magnetic hierarchically porous biomass carbon for realizing broadband and strong absorption of electromagnetic wave. Ceram. Int. 2023, 49, 27015–27023. [Google Scholar] [CrossRef]
- Shang, T.; Lu, Q.; Zhao, J.; Chao, L.; Qin, Y.; Ren, N.; Yun, Y.; Yun, G. Novel three-dimensional graphene-like networks loaded with Fe3O4 nanoparticles for efficient microwave absorption. Nanomaterials 2021, 11, 1444. [Google Scholar] [CrossRef]
- Wang, L.; Guan, H.; Hu, J.; Huang, Q.; Dong, C.; Qian, W.; Wang, Y. Jute-based porous biomass carbon composited by Fe3O4 nanoparticles as an excellent microwave absorber. J. Alloys Compd. 2019, 803, 1119–1126. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Zhou, H.; Wu, X.; Zhang, W.; Wang, Q.; Luo, C. Fabrication of biomass-derived carbon decorated with NiFe2O4 particles for broadband and strong microwave absorption. Powder Technol. 2019, 345, 370–378. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Xie, W.; Song, S.; Zhang, Y.; Dong, L. In-situ growth and graphitization synthesis of porous Fe3O4/carbon fiber composites derived from biomass as lightweight microwave absorber. ACS Sustain. Chem. Eng. 2019, 7, 5318–5328. [Google Scholar] [CrossRef]
- Khanahmadi, S.; Masoudpanah, S. Preparation and microwave absorption properties of CoFe2O4/NiCo2O4 composite powders. Ceram. Int. 2023, 50, 9779–9788. [Google Scholar] [CrossRef]
- Shang, T.; Lu, Q.; Chao, L.; Qin, Y.; Yun, Y.; Yun, G. Effects of ordered mesoporous structure and La-doping on the microwave absorbing properties of CoFe2O4. Appl. Surf. Sci. 2018, 434, 234–242. [Google Scholar] [CrossRef]
- Sahu, S.; Gurrala, R.C.; Dobbidi, P. Effect of rare earth substitution on electromagnetic and microwave absorption properties of nickel-cobalt nano ferrite. Ceram. Int. 2023, 49, 40875–40893. [Google Scholar] [CrossRef]
- Routray, K.L.; Saha, S.; Sanyal, D.; Behera, D. Role of rare-earth (Nd3+) ions on structural, dielectric, magnetic and Mossbauer properties of nano-sized CoFe2O4: Useful for high frequency application. Mater. Res. Express 2018, 6, 026107. [Google Scholar] [CrossRef]
- Raji, S.; Sharma, G.K.; Aranya, B.; Prabhakaran, K. Carbon composite foams from the wasted banana leaf for EMI shielding and thermal insulation. Carbon 2023, 213, 118259. [Google Scholar] [CrossRef]
- Ning, T.; Li, Q.; Ren, Q.; Wang, J.; Sun, Y.; Zhang, P.; Ma, W.; Yan, J.; Zhai, C.; Zhao, W. Kapok fibers-derived carbon microtubes as efficient electromagnetic wave absorption materials. Ceram. Int. 2023, 49, 29339–29347. [Google Scholar] [CrossRef]
- Wen, X.; Li, C.; Liu, H.; Fan, G.; Tang, Y.; Hao, C.; Ma, L.; Song, P. Green carbonization of waste coffee grounds into porous C/Fe hybrids for broadband and high-efficiency microwave absorption. J. Mater. Sci. Technol. 2024, 170, 1–10. [Google Scholar] [CrossRef]
- Che, J.; Zheng, H.; Lu, Z.; Yang, Z.; Gao, Y.; Wang, Y. Bimetallic sulfides embedded into porous carbon composites with tunable magneto-dielectric properties for lightweight biomass-reinforced microwave absorber. Ceram. Int. 2023, 49, 27094–27106. [Google Scholar] [CrossRef]
- Cao, A.; Shen, B.; Lin, Q.; Chen, S.; Huang, Z.; Ji, Z.; Zhang, Z. Influence of Stone-Wales defect on graphene friction: Pinning effect and wrinkle modification. Comput. Mater. Sci. 2020, 173, 109423. [Google Scholar] [CrossRef]
- Harrington, G.F.; Sun, L.; Yildiz, B.; Sasaki, K.; Perry, N.H.; Tuller, H.L. The interplay and impact of strain and defect association on the conductivity of rare-earth substituted ceria. Acta Mater. 2019, 166, 447–458. [Google Scholar] [CrossRef]
- Nepal, K.; Ugwumadu, C.; Gautam, A.; Kappagantula, K.; Drabold, D.A. Electronic conductivity in metal-graphene composites: The role of disordered carbon structures, defects, and impurities. J. Phys. Mater. 2024, 7, 025003. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Shi, Q.; Li, B.; Hu, Z.; Li, J.; Wan, X.; Yu, H. Co/C composites generated from biomass exhibit outstanding electromagnetic wave absorption. Diamond Relat. Mater. 2023, 138, 110191. [Google Scholar] [CrossRef]
- Leng, L.; Liu, R.; Xu, S.; Mohamed, B.A.; Yang, Z.; Hu, Y.; Chen, J.; Zhao, S.; Wu, Z.; Peng, H. An overview of sulfur-functional groups in biochar from pyrolysis of biomass. J. Environ. Chem. Eng. 2022, 10, 107185. [Google Scholar] [CrossRef]
- Chun, Y.; Sheng, G.; Chiou, C.T.; Xing, B. Compositions and sorptive properties of crop residue-derived chars. Environ. Sci. Technol. 2004, 38, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.H.; Antonietti, M.; Titirici, M.M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhou, J.; Tao, J.; Gu, Y.; Yu, C.; Chen, P.; Yao, Z. Commonly neglected ester groups enhanced microwave absorption. Small 2023, 19, 2304536. [Google Scholar] [CrossRef]
- Zeng, S.; Li, K.; Xu, X.; Zhang, J.; Xue, Y. Efficiently catalytic degradation of tetracycline via persulfate activation with plant-based biochars: Insight into endogenous mineral self-template effect and pyrolysis catalysis. Chemosphere 2023, 337, 139309. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar surface functional groups as affected by biomass feedstock, biochar composition and pyrolysis temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Yadav, R.S.; Havlica, J.; Masilko, J.; Kalina, L.; Wasserbauer, J.; Hajdúchová, M.; Enev, V.; Kuřitka, I.; Kožáková, Z. Impact of Nd3+ in CoFe2O4 spinel ferrite nanoparticles on cation distribution, structural and magnetic properties. J. Magn. Magn. Mater. 2016, 399, 109–117. [Google Scholar] [CrossRef]
- Wang, W.P.; Yang, H.; Xian, T.; Jiang, J.L. XPS and magnetic properties of CoFe2O4 nanoparticles synthesized by a polyacrylamide gel route. Mater. Trans. 2012, 53, 1586–1589. [Google Scholar] [CrossRef]
- Chagas, C.A.; de Souza, E.F.; de Carvalho, M.C.; Martins, R.L.; Schmal, M. Cobalt ferrite nanoparticles for the preferential oxidation of CO. Appl. Catal. A 2016, 519, 139–145. [Google Scholar] [CrossRef]
- Benedet, M.; Rizzi, G.A.; Barreca, D.; Gasparotto, A.; Maccato, C. XPS analysis of graphitic carbon nitride functionalized with CoO and CoFe2O4. Surf. Sci. Spectra 2023, 30, 014004. [Google Scholar] [CrossRef]
- Siddiqui, H.; Shrivastava, M.; Parra, M.R.; Pandey, P.; Ayaz, S.; Qureshi, M. The effect of La3+ ion doping on the crystallographic, optical and electronic properties of CuO nanorods. Mater. Lett. 2018, 229, 225–228. [Google Scholar] [CrossRef]
- Zeng, J.; Zhong, J.B.; Li, J.Z.; Wang, S.H.; Hu, W. Improved photocatalytic activity of La3+-doped Bi2O3. Adv. Mater. Res. 2011, 239, 86–89. [Google Scholar] [CrossRef]
- Van Der Heide, P. Systematic x-ray photoelectron spectroscopic study of La1-xSrx-based perovskite-type oxides. Surf. Interface Anal. 2002, 33, 414–425. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Y.; Zhao, Y.; Zhou, X.; Zheng, H. La3+/La (OH)3 loaded magnetic cationic hydrogel composites for phosphate removal: Effect of lanthanum species and mechanistic study. Water Res. 2017, 126, 433–441. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, L.; Wu, H. Dielectric loss mechanism in electromagnetic wave absorbing materials. Adv. Sci. 2022, 9, 2105553. [Google Scholar] [CrossRef]
- Chang, Q.; Li, C.; Sui, J.; Waterhouse, G.I.; Zhang, Z.-M.; Yu, L.-m. Ni/Ni3ZnC0.7 modified alginate-derived carbon composites with porous structures for electromagnetic wave absorption. Carbon 2022, 200, 166–177. [Google Scholar] [CrossRef]
- Shen, Z.; Zu, Y.; Chen, Y.; Gong, J.; Sun, C. Microwave absorption performance of porous carbon particles modified by nickel with different morphologies. J. Mater. Sci. Technol. 2023, 137, 79–90. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Q.; Huang, L.; Yang, X.; Yuan, Y. Recycling Polyethylene into High-Value Porous Carbon Composites for Microwave Absorption. Adv. Eng. Mater. 2023, 25, 2300366. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, C.; Cui, B.; Xu, X.; Li, M.; Xu, Z.; Tan, H.; Wang, C.; Wang, Y. Lotus leaf derived NiS/carbon nanofibers/porous carbon heterogeneous structures for strong and broadband microwave absorption. Small 2023, 19, 2304918. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Yu, J.; Jiang, S.; Chen, Y. Biomass carbon materials with porous array structures derived from soybean dregs for effective electromagnetic wave absorption. Diamond Relat. Mater. 2022, 126, 109054. [Google Scholar] [CrossRef]
- Ren, L.; Wang, Y.; Chai, L.; Zhou, S.; He, Q.; Du, H.; Wu, G. Hierarchical porous carbon prepared using swelling-induced biomass structure-controllable method with excellent microwave absorption performance. Mater. Chem. Phys. 2022, 279, 125739. [Google Scholar] [CrossRef]
- Fang, J.; Li, P.; Liu, Y.; Min, Y. Cobalt magnetic particles and carbon composite microtubes as high-performance electromagnetic wave absorbers. J. Mater. Chem. C 2021, 9, 2474–2482. [Google Scholar] [CrossRef]
- Wu, Z.; Yao, C.; Meng, Z.; Deng, Y.; Wang, Y.; Liu, J.; Wang, Y.; Zhou, H. Biomass-Derived Crocodile Skin-Like Porous Carbon for High-Performance Microwave Absorption. Adv. Sustain. Syst. 2022, 6, 2100454. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, Q.; Kara, U.I.; Mamtani, R.S.; Zhou, X.; Bian, H.; Yang, Z.; Li, Y.; Lv, H.; Adera, S. Biomass-derived carbon heterostructures enable environmentally adaptive wideband electromagnetic wave absorbers. Nano-Micro Lett. 2022, 14, 11. [Google Scholar] [CrossRef]
- Zhao, H.; Jin, C.; Lu, P.; Xiao, Z.; Cheng, Y. Anchoring well-dispersed magnetic nanoparticles on biomass-derived 2D porous carbon nanosheets for lightweight and efficient microwave absorption. Composites Part A 2022, 154, 106773. [Google Scholar] [CrossRef]
- Liang, N.; Yin, Z.; Guo, J.; Fang, W.; Wang, Q.; Tian, G.; Zhang, D.; Yue, H.; Feng, S. Yolk-shell structure synergistic defect engineering for boosting electromagnetic wave absorption in Co9S8@Humins-derived carbon. J. Mater. Sci. Technol. 2023, 164, 140–149. [Google Scholar] [CrossRef]
- Mao, F.; Long, L.; Zeng, G.; Chen, H.; Li, Y.; Zhou, W. Achieving excellent electromagnetic wave absorption property by constructing VO2 coated biomass carbon heterostructures. Diamond Relat. Mater. 2022, 130, 109422. [Google Scholar] [CrossRef]
- Cheng, Y.; Cao, J.; Li, Y.; Li, Z.; Zhao, H.; Ji, G.; Du, Y. The outside-in approach to construct Fe3O4 nanocrystals/mesoporous carbon hollow spheres core-shell hybrids toward microwave absorption. ACS Sustain. Chem. Eng. 2018, 6, 1427–1435. [Google Scholar] [CrossRef]
- Xie, A.; Sheng, D.; Liu, W.; Chen, Y.; Cheng, S. Enhancing electromagnetic absorption performance of Molybdate@Carbon by metal ion substitution. J. Mater. Sci. Technol. 2023, 163, 92–100. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, L.; Zhao, W.; Wang, T.; Yuan, L.; Guo, Y.; Xie, Y.; Cheng, T.; Meng, A.; Li, Z. Boosted electromagnetic wave absorption performance from synergistic induced polarization of SiCNWs@MnO2@PPy heterostructures. Nano Res. 2023, 16, 3558–3569. [Google Scholar] [CrossRef]
- Jia, T.; Hao, Y.; Qi, X.; Rao, Y.; Wang, L.; Ding, J.; Qu, Y.; Zhong, W. Interface engineering and impedance matching strategy to develop core@shell urchin-like NiO/Ni@carbon nanotubes nanocomposites for microwave absorption. J. Mater. Sci. Technol. 2024, 176, 1–12. [Google Scholar] [CrossRef]
- He, X.; Xiong, Z.; Lei, C.; Shen, Z.; Ni, A.; Xie, Y.; Liu, C. Excellent microwave absorption performance of LaFeO3/Fe3O4/C perovskite composites with optimized structure and impedance matching. Carbon 2023, 213, 118200. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, X.; Yu, R.; Stucky, G.D. Electromagnetic microwave absorption theory and recent achievements in microwave absorbers. Carbon 2020, 168, 606–623. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, L.; Han, Y.; Liu, P.; Xu, H.; Yu, G.; Wang, Y.; Wen, T.; Ju, W.; Gu, J. Hierarchical construction of CNT networks in aramid papers for high-efficiency microwave absorption. Nano Res. 2023, 16, 7801–7809. [Google Scholar] [CrossRef]
- Micheli, D.; Pastore, R.; Gradoni, G.; Marchetti, M. Tunable nanostructured composite with built-in metallic wire-grid electrode. AIP Adv. 2013, 3, 112132. [Google Scholar] [CrossRef]
- Li, Z.; Liang, J.; Wei, Z.; Cao, X.; Shan, J.; Li, C.; Chen, X.; Zhou, D.; Xing, R.; Luo, C. Lightweight foam-like nitrogen-doped carbon nanotube complex achieving highly efficient electromagnetic wave absorption. J. Mater. Sci. Technol. 2024, 168, 114–123. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, H.; Cheng, Y.; Bai, X.; Wen, B.; Lin, Y. Constructing a nitrogen-doped carbon and nickel composite derived from a mixed ligand nickel-based a metal–organic framework toward adjustable microwave absorption. Nanoscale 2021, 13, 9204–9216. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xing, X.; Zhu, H.; Li, J.; Liu, T. State of the art and prospects of Fe3O4/carbon microwave absorbing composites from the dimension and structure perspective. Adv. Colloid Interface Sci. 2023, 318, 102960. [Google Scholar] [CrossRef]
- Yu, L.; Zhu, Q.; Guo, Z.; Cheng, Y.; Jia, Z.; Wu, G. Unique electromagnetic wave absorber for three-dimensional framework engineering with copious heterostructures. J. Mater. Sci. Technol. 2024, 170, 129–139. [Google Scholar] [CrossRef]



| Energy Position (eV) | |||||||
|---|---|---|---|---|---|---|---|
| Spectrum | Fe 2 p1/2 | Fe 2p3/2 | Co 2p1/2 | Co 2p3/2 | La 3d3/2 | La 3d5/2 | |
| Assignment | Os | 724.81 | 710.85 | 796.28 | 780.33 | 850.86 | 834.11 |
| Ts | 726.20 | 712.21 | 798.16 | 782.21 | 852.12 | 835.37 | |
| Feedstock | Samples | RLmin Value (dB) | EAB (GHz) | Thickness at EAB (mm) | Ref. |
|---|---|---|---|---|---|
| Soybean dregs | FK-SDC | −18.50 | 4.80 | 3.50 | [45] |
| Shaddock peel | G-800 | −29.50 | 5.80 | 1.70 | [46] |
| Kapokfibers | CMT-900 | −30.75 | 6.78 | 2.06 | [19] |
| Cotton fibers | PCMT/Co | −36.8 | 6.7 | 1.4 | [47] |
| Wheat flour | PCP@Ni-chain | −38.42 | 5.2 | 2.0 | [42] |
| Alginate | Ni/Ni3ZnC0.7/C-0.1 | −40.2 | 5.4 | 2.0 | [41] |
| Rice husk | BHPC | −47.46 | 3.4 | 2.8 | [48] |
| Bamboo | GC-8 | −51.00 | 4.2 | 1.66 | [49] |
| Shaddock peels | Fe3O4/PCS-2 | −50.3 | 4.1 | 1.6 | [50] |
| Humins | CS@C-700 | −51.4 | 5.2 | 1.84 | [51] |
| Coffee grounds | WCG-20–750 | −52.86 | 6.40 | 3.0 | [20] |
| Corn stalks | La-CFO@BC | −53.19 | 6.49 | 2.5 | This work |
| Chestnut needles | VO2–50 | −54.0 | 2.5 | 2.87 | [52] |
| Pine needles | PNC | −56.30 | 3.44 | 1.4 | [53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shang, T.; Zhu, H.; Shang, Y.; Wu, R.; Zhao, X. A Novel Low-Density-Biomass-Carbon Composite Coated with Carpet-like and Dandelion-Shaped Rare-Earth-Doped Cobalt Ferrite for Enhanced Microwave Absorption. Molecules 2024, 29, 2620. https://doi.org/10.3390/molecules29112620
Shang T, Zhu H, Shang Y, Wu R, Zhao X. A Novel Low-Density-Biomass-Carbon Composite Coated with Carpet-like and Dandelion-Shaped Rare-Earth-Doped Cobalt Ferrite for Enhanced Microwave Absorption. Molecules. 2024; 29(11):2620. https://doi.org/10.3390/molecules29112620
Chicago/Turabian StyleShang, Tao, Hongwei Zhu, Yichun Shang, Ruixia Wu, and Xuebing Zhao. 2024. "A Novel Low-Density-Biomass-Carbon Composite Coated with Carpet-like and Dandelion-Shaped Rare-Earth-Doped Cobalt Ferrite for Enhanced Microwave Absorption" Molecules 29, no. 11: 2620. https://doi.org/10.3390/molecules29112620
APA StyleShang, T., Zhu, H., Shang, Y., Wu, R., & Zhao, X. (2024). A Novel Low-Density-Biomass-Carbon Composite Coated with Carpet-like and Dandelion-Shaped Rare-Earth-Doped Cobalt Ferrite for Enhanced Microwave Absorption. Molecules, 29(11), 2620. https://doi.org/10.3390/molecules29112620







