Structure Characterization and Dye Adsorption Properties of Modified Fiber from Wheat Bran
Abstract
1. Introduction
2. Results and Discussion
2.1. Basic Compositions and Total Dietary Fiber Contents of Wheat Bran
2.2. Monosaccharide Composition Analysis
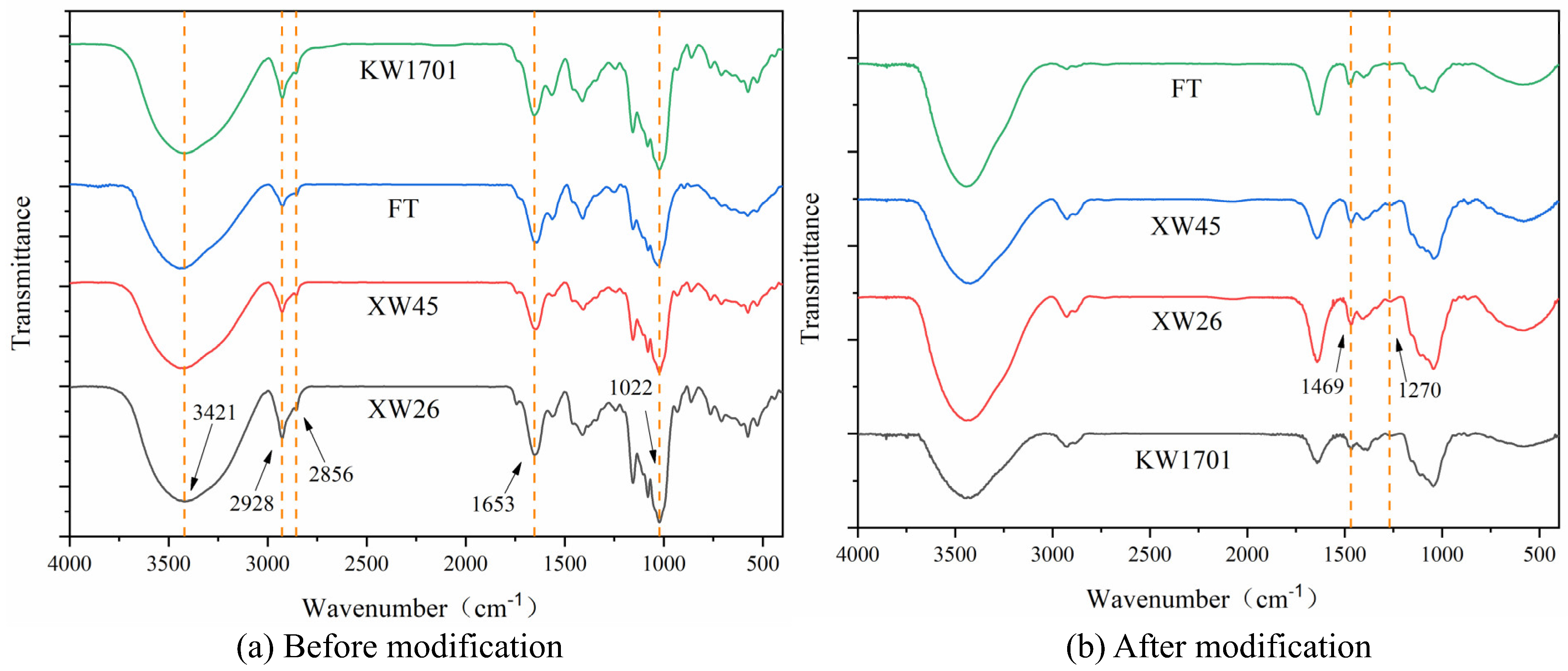
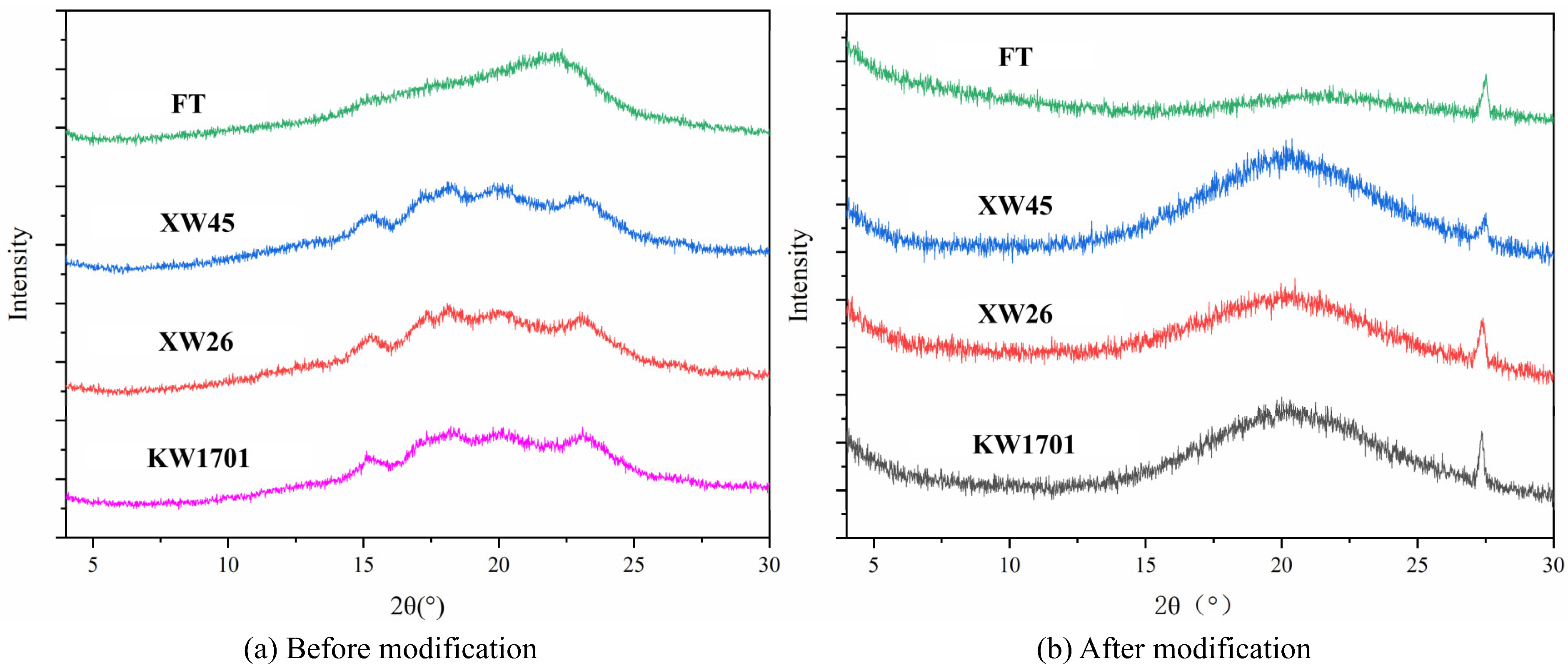
2.3. Structure Characterization
2.3.1. Optical Microscope and Scanning Electron Microscope Observation
2.3.2. Fourier-Transform Infrared Spectroscopy Analysis
2.3.3. X-ray Diffraction Analysis
2.4. Dye Adsorption Capacities of Wheat Bran Fibers before and after Modification
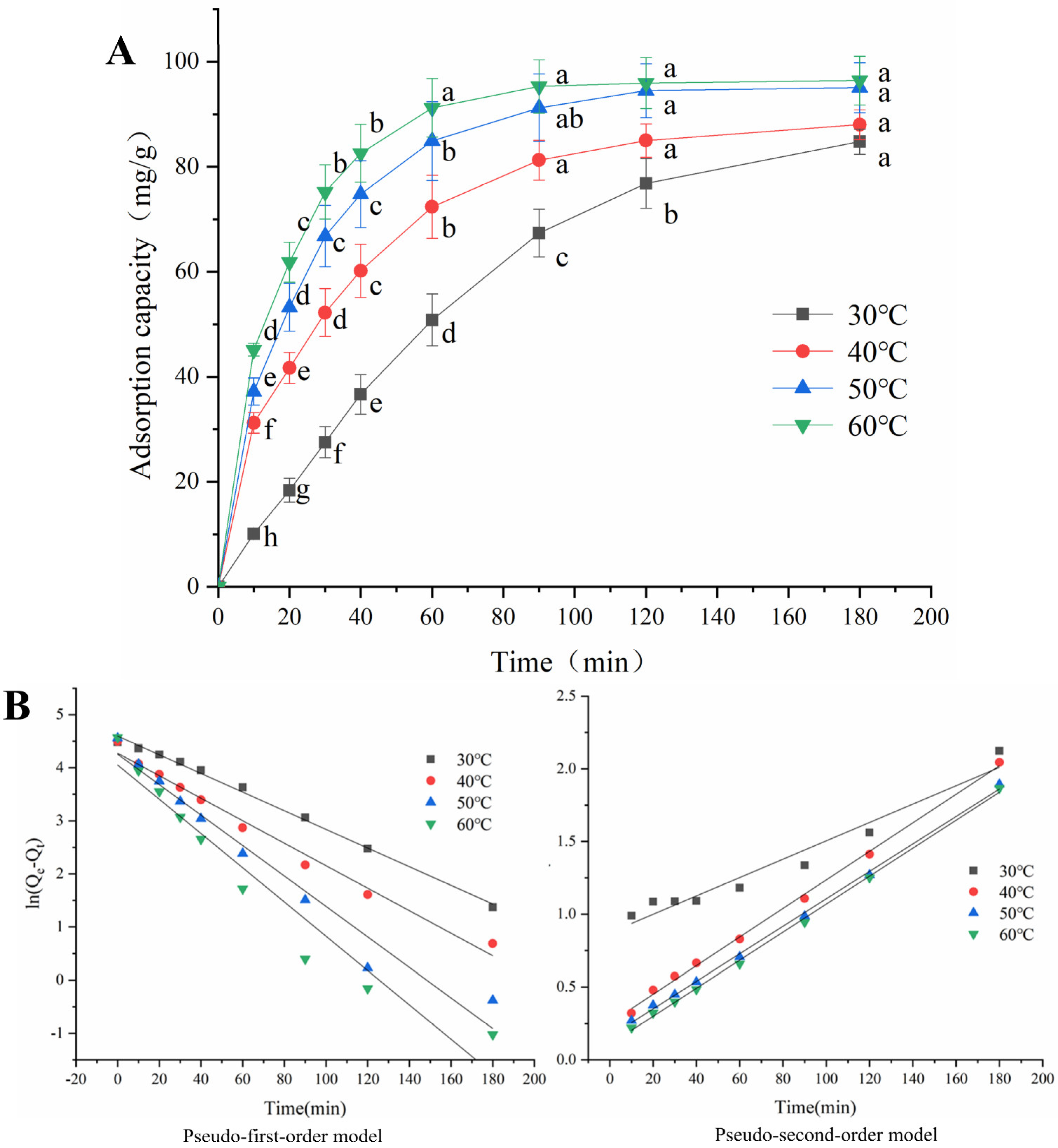
2.5. Adsorption Kinetics
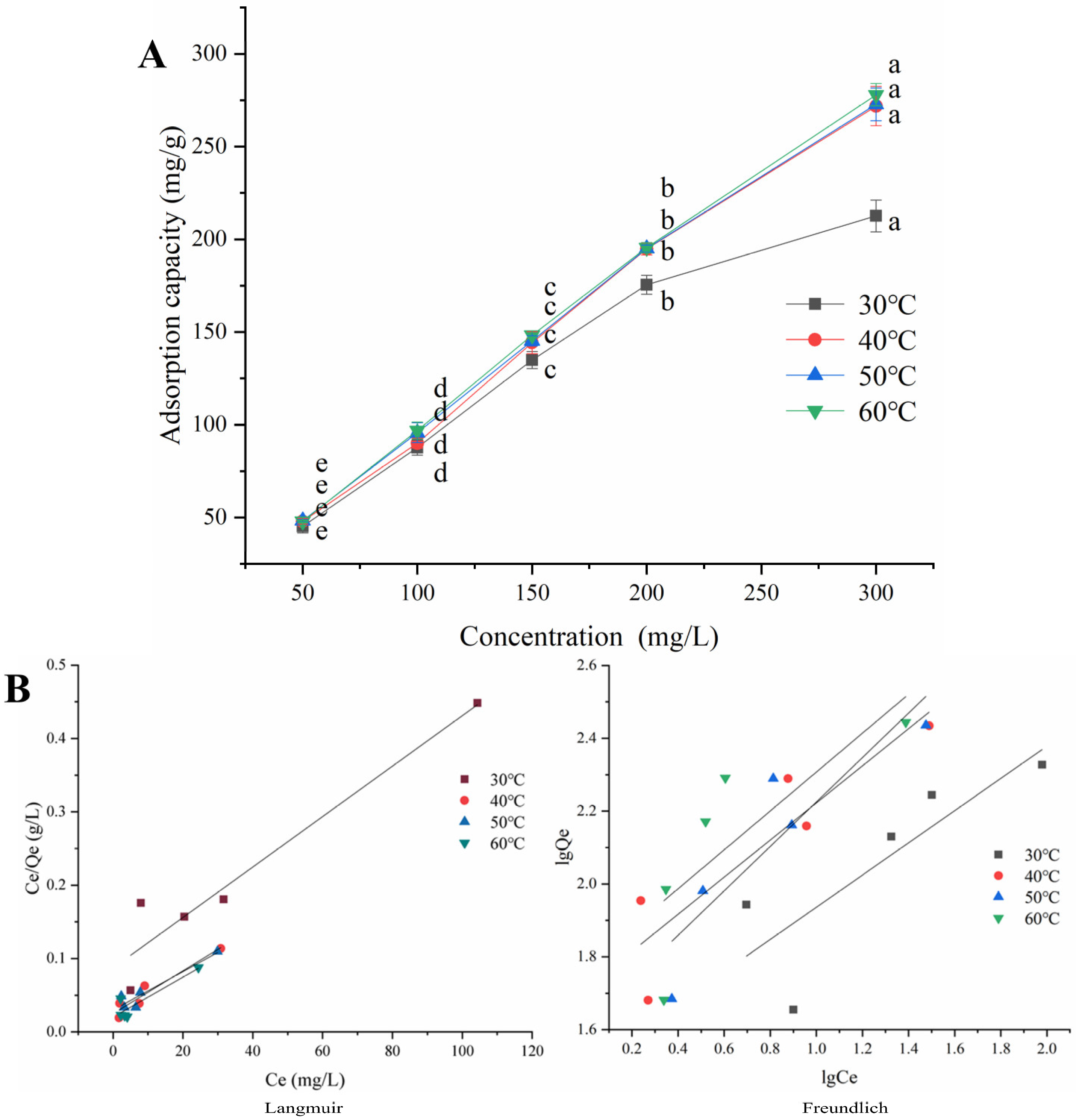
2.6. Adsorption Isotherms
2.7. Adsorption Thermodynamic Parameters
3. Materials and Methods
3.1. Materials and Reagents
3.2. The Basic Composition of Wheat Bran
3.3. Determination of Dietary Fiber Content
3.4. Preparation of Wheat Bran Fiber
3.5. Monosaccharide Composition Analysis
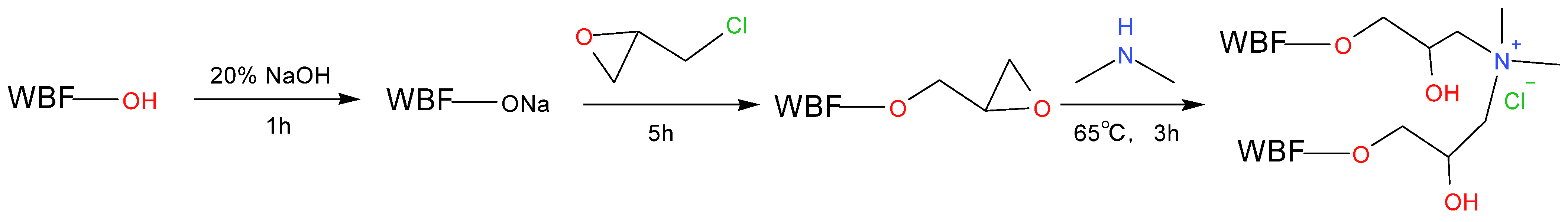
3.6. Modification Techniques of Fiber
3.7. Structure Characterization of Wheat Bran Fibers
3.7.1. Optical Microscopic Observation
3.7.2. Scanning Electron Microscopic Observation
3.7.3. X-ray Diffraction
3.7.4. Fourier-Transform Infrared (FTIR) Spectra Analysis
3.8. Dye Adsorption Properties of Fiber
3.9. Adsorption Kinetics
3.10. Adsorption Isotherms
3.11. Adsorption Thermodynamic Parameter
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prückler, M.; Siebenhandl-Ehn, S.; Apprich, S.; Höltinger, S.; Haas, C.; Schmid, E.; Kneifel, W. Wheat bran-based biorefinery 1: Composition of wheat bran and strategies of functionalization. LWT-Food Sci. Technol. 2014, 56, 211–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, H.; Guo, D.; Zhang, R.; Su, M.; Hou, Z.; Zhou, H.; Liang, R.; Xie, C.; You, M.; et al. Identifying changes in the wheat kernel proteome under heat stress using iTRAQ. Crop J. 2018, 6, 600–610. [Google Scholar] [CrossRef]
- Pietiäinen, S.; Moldin, A.; Ström, A.; Malmberg, C.; Langton, M. Effect of physicochemical properties, pre-processing, and extraction on the functionality of wheat bran arabinoxylans in breadmaking—A review. Food Chem. 2022, 383, 132584. [Google Scholar] [CrossRef]
- Neves, M.A.; Kimura, T.; Shimizu, N.; Shiiba, K. Production of alcohol by simultaneous saccharification and fermentation of low-grade wheat flour. Braz. Arch. Biol. Technol. 2006, 49, 481–490. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Essawy, A.A.; Ali, A.E.; Abdel-Mottaleb, M.S.A. Application of novel copolymer-TiO2 membranes for some textile dyes adsorptive removal from aqueous solution and photocatalytic decolorization. J. Hazard. Mater. 2008, 157, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Önal, Y. Kinetics of adsorption of dyes from aqueous solution using activated carbon prepared from waste apricot. J. Hazard. Mater. 2006, 137, 1719–1728. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- El-Shafie, A.S.; Rahman, E.; GadelHak, Y.; Mahmoud, R.; El-Azazy, M. Techno-economic assessment of waste mandarin biochar as a green adsorbent for binary dye wastewater effluents of methylene blue and basic fuchsin: Lab- and large-scale investigations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 306, 123621. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of Synthetic Azo Dyes of Textile Industry: A Sustainable Approach Using Microbial Enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131. [Google Scholar] [CrossRef]
- Norbert, A.; John, S.S.; Shaji, S.; Jacob, M.V.; Philip, R.R. Green synthesized Cu-doped CeO2 nanoparticles for Congo red dye adsorption and antibacterial action. Nanotechnology 2024, 35, 265708. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Karthika, T.S.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Shao, X.; Wang, J.; Liu, Z.; Hu, N.; Liu, M.; Duan, C.; Zhang, R.; Quan, C. Cellulose based cation-exchange fiber as filtration material for the rapid removal of methylene blue from wastewater. Cellulose 2021, 28, 9355–9367. [Google Scholar] [CrossRef]
- Chakraborty, S.; De, S.; DasGupta, S.; Basu, J.K. Adsorption study for the removal of a basic dye: Experimental and modeling. Chemosphere 2005, 58, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Çiçek, F.; Özer, D.; Özer, A.; Özer, A. Low cost removal of reactive dyes using wheat bran. J. Hazard. Mater. 2007, 146, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Singh, B.; Singh, A. Comparison of dietary fibers obtained from seven Indian cereal grains. J. Cereal Sci. 2021, 102, 103331. [Google Scholar] [CrossRef]
- Yu, G.; Bei, J.; Zhao, J.; Li, Q.; Cheng, C. Modification of carrot (Daucus carota Linn. var. Sativa Hoffm.) pomace insoluble dietary fiber with complex enzyme method, ultrafine comminution, and high hydrostatic pressure. Food Chem. 2018, 257, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, A.M. Scanning Electron Microscopy: Principle and Applications in Nanomaterials Characterization. In Handbook of Materials Characterization; Sharma, S., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Chen, B.; Cai, Y.; Liu, T.; Huang, L.; Deng, X.; Zhao, Q.; Zhao, M. Improvements in physicochemical and emulsifying properties of insoluble soybean fiber by physical-chemical treatments. Food Hydrocoll. 2019, 93, 167–175. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Aboul-Enein, H.Y.; Fleschin, S. Recent Applications of Fourier Transform Infrared Spectrophotometry in Herbal Medicine Analysis. Appl. Spectrosc. Rev. 2011, 46, 251–260. [Google Scholar] [CrossRef]
- Jia, M.; Chen, J.; Liu, X.; Xie, M.; Nie, S.; Chen, Y.; Xie, J.; Yu, Q. Structural characteristics and functional properties of soluble dietary fiber from defatted rice bran obtained through Trichoderma viride fermentation. Food Hydrocoll. 2019, 94, 468–474. [Google Scholar] [CrossRef]
- Hua, M.; Sun, Y.; Shao, Z.; Lu, J.; Lu, Y.; Liu, Z. Functional soluble dietary fiber from ginseng residue: Polysaccharide characterization, structure, antioxidant, and enzyme inhibitory activity. J. Food Biochem. 2020, 44, e13524. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ye, R.; Chen, Y. Blasting extrusion processing: The increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 2015, 180, 106–115. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, C.; Li, J.; Hussain, S.; Yan, S.; Wang, Q. Effects of extrusion on structural and physicochemical properties of soluble dietary fiber from nodes of lotus root. LWT 2018, 93, 204–211. [Google Scholar] [CrossRef]
- Yang, B.; Wu, Q.; Song, X.; Yang, Q.; Kan, J. Physicochemical properties and bioactive function of Japanese grape (Hovenia dulcis) pomace insoluble dietary fibre modified by ball milling and complex enzyme treatment. Int. J. Food Sci. Technol. 2019, 54, 2363–2373. [Google Scholar] [CrossRef]
- Yang, X.; Dai, J.; Zhong, Y.; Wei, X.; Wu, M.; Zhang, Y.; Huang, A.; Wang, L.; Huang, Y.; Zhang, C.; et al. Characterization of insoluble dietary fiber from three food sources and their potential hypoglycemic and hypolipidemic effects. Food Funct. 2021, 12, 6576–6587. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Nguyen, T.V.; Chao, H.-P. Insight into the adsorption mechanism of cationic dye onto biosorbents derived from agricultural wastes. Chem. Eng. Commun. 2017, 204, 1020–1036. [Google Scholar] [CrossRef]
- Mishra, A.; Jha, B. Isolation and characterization of extracellular polymeric substances from micro-algae Dunaliella salina under salt stress. Bioresour. Technol. 2009, 100, 3382–3386. [Google Scholar] [CrossRef] [PubMed]
- Ye, F. Synthesis and Property Evaluation of Quinoline Derivative Quaternary Ammonium Salt Corrosion Inhibitor. Master’s Thesis, China University of Petroleum, Qingdao, China, 2020. [Google Scholar] [CrossRef]
- Du, C. Synthesis and Characterization of Quaternary Ammonium Liquid Crystal Ionomer Containing Cholesterol Groups. Master’s Thesis, Northeastern University, Shenyang, China, 2014. [Google Scholar]
- Bhat, N.; Deshmukh, R. X-ray crystallographic studies of polymeric materials. Indian J. Pure Appl. Phys. 2002, 40, 361–366. [Google Scholar]
- Sang, J.; Li, L.; Wen, J.; Liu, H.; Wu, J.; Yu, Y.; Xu, Y.; Gu, Q.; Fu, M.; Lin, X. Chemical composition, structural and functional properties of insoluble dietary fiber obtained from the Shatian pomelo peel sponge layer using different modification methods. LWT 2022, 165, 113737. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, H.; Zheng, Y.; Wang, D.; Liu, Z.; Deng, Y.; Zhao, Y. Structure, physicochemical and bioactive properties of dietary fibers from Akebia trifoliata (Thunb.) Koidz. seeds using ultrasonication/shear emulsifying/microwave-assisted enzymatic extraction. Food Res. Int. 2020, 136, 109348. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, E.; Sun, S.; Shi, Z.; Yang, J.; Yang, H. The thermal properties of epichlorohydrin modified dendrocalamus sinicus and its products. J. Bamboo Res. 2021, 40, 49–54. [Google Scholar] [CrossRef]
- Liu, S.; Liang, J.; Wu, Q.; Hu, X. Synthesis, characterization and dye adsorption properties of modified jackfruit peel cellulose adsorbent. J. Yunnan Univ. (Nat. Sci. Ed.) 2022, 44, 328–337. [Google Scholar]
- Han, P.; Song, C.; Song, J.; Li, Y.; Han, R. Adsorption of Congo Red from Solution by Rice Husk in Batch Mode. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, H.; Ji, F.; Yuan, S. Removal of Congo Red from aqueous solution by cattail root. J. Hazard. Mater. 2010, 173, 292–297. [Google Scholar] [CrossRef]
- De Castro, L.E.N.; Battocchio, D.A.J.; Ribeiro, L.F.; Colpini, L.M.S. Development of Adsorbent Materials Using Residue from Coffee Industry and Application in Food dye Adsorption Processes. Braz. Arch. Biol. Technol. 2023, 66, e23210125. [Google Scholar] [CrossRef]
- Ronghua, L.; Manlin, L.; Zengqiang, Z.; Zhaofu, M.; Zhengliang, Y.; Xining, S. Adsorption of methylene blue from water by citric acid modified orange peel. J. Northwest AF Univ. 2009, 37, 173–187. [Google Scholar]
- Azzaz, A.A.; Jellali, S.; Bousselmi, L. Optimization of a cationic dye adsorption onto a chemically modified agriculture by-product using response surface methodology. In Proceedings of the 2016 7th International Renewable Energy Congress (IREC), Hammamet, Tunisia, 22–24 March 2016; pp. 1–5. [Google Scholar]
- Wu, J. Study on the Extraction of Cellulose Components from Pineapple Peel and Modification as Dye Adsorbent. Master’s Thesis, South China University of Technology, Guangzhou, China, 2010. [Google Scholar]
- GB 5009.3-2016; National Food Safety Standard Determination of Moisture Content in Foods. National Health and Family Planning Commission: Beijing, China, 2016.
- GB 5009.4-2016; National Food Safety Standard Determination of Ash in Foods. National Health and Family Planning Commission: Beijing, China, 2016.
- GB 5009.5-2016; National Food Safety Standard Determination of Protein in Foods. National Health and Family Planning Commission: Beijing, China, 2016.
- GB 5009.6-2016; National Food Safety Standard Determination of Fat in Foods. National Health and Family Planning Commission: Beijing, China, 2016.
- Wang, J.; Wang, Y.; Yang, G. Research on the physicochemical characteristics and laxative effects of insoluble dietary fiber from bananas. Sci. Technol. Food Ind. 2017, 38, 337–341. [Google Scholar] [CrossRef]
- Zhu, M.; Huang, R.; Wen, P.; Song, Y.; He, B.; Tan, J.; Hao, H.; Wang, H. Structural characterization and immunological activity of pectin polysaccharide from kiwano (Cucumis metuliferus) peels. Carbohydr. Polym. 2021, 254, 117371. [Google Scholar] [CrossRef]
- Li, J. Study on the Extraction of Dietary Fiber from Tea Residue and Its Modification as Dye Adsorbent. Master’s Thesis, South China University of Technology, Guangzhou, China, 2012. [Google Scholar]
- Tang, C.; Yang, J.; Zhang, F.; Kan, J.; Wu, L.; Zheng, J. Insight into the physicochemical, structural, and in vitro hypoglycemic properties of bamboo shoot dietary fibre: Comparison of physical modification methods. Int. J. Food Sci. Technol. 2022, 57, 4998–5010. [Google Scholar] [CrossRef]
- Huang, H.; Chen, J.; Chen, Y.; Xie, J.; Liu, S.; Sun, N.; Hu, X.; Yu, Q. Modification of tea residue dietary fiber by high-temperature cooking assisted enzymatic method: Structural, physicochemical and functional properties. LWT 2021, 145, 111314. [Google Scholar] [CrossRef]
- Roy, A.; Adhikari, B.; Majumder, S.B. Equilibrium, Kinetic, and Thermodynamic Studies of Azo Dye Adsorption from Aqueous Solution by Chemically Modified Lignocellulosic Jute Fiber. Ind. Eng. Chem. Res. 2013, 52, 6502–6512. [Google Scholar] [CrossRef]
- Rudzinski, W.; Plazinski, W. Kinetics of Dyes Adsorption at the Solid−Solution Interfaces: A Theoretical Description Based on the Two-Step Kinetic Model. Environ. Sci. Technol. 2008, 42, 2470–2475. [Google Scholar] [CrossRef]
- Duran, C.; Ozdes, D.; Gundogdu, A.; Senturk, H.B. Kinetics and Isotherm Analysis of Basic Dyes Adsorption onto Almond Shell (Prunus dulcis) as a Low Cost Adsorbent. J. Chem. Eng. Data 2011, 56, 2136–2147. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Li, F.; Luo, S.; Li, Q.; Zhou, S. Preparation of Waste Coffee-grounds Carbon and Study on Phenol Adsorption Ability. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2022, 37, 38–46. [Google Scholar] [CrossRef]
- Sultana, S.; Islam, K.; Hasan, A.; Khan, H.J.; Khan, M.A.R.; Deb, A.; Raihan, A.; Rahman, W. Adsorption of crystal violet dye by coconut husk powder: Isotherm, kinetics and thermodynamics perspectives. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100651. [Google Scholar] [CrossRef]
- Chan, L.; Cheung, W.; Allen, S.; McKay, G. Error Analysis of Adsorption Isotherm Models for Acid Dyes onto Bamboo Derived Activated Carbon. Chin. J. Chem. Eng. 2012, 20, 535–542. [Google Scholar] [CrossRef]
- Maryanti, R.; Nandiyanto, A.B.D.; Manullang, T.I.B.; Hufad, A.; Sunardi, S. Adsorption of Dye on Carbon Microparticles: Physicochemical Properties during Adsorption, Adsorption Isotherm and Education for Students with Special Needs. Sains Malays. 2020, 49, 2977–2988. [Google Scholar] [CrossRef]


| Wheat Bran Source | Carbohydrates (g/100 g) | Protein (%) | Fat (g/100 g) | Ash (%) | Moisture (%) | Energy (KJ/100 g) | TDF (g/100 g) |
|---|---|---|---|---|---|---|---|
| FT | 58.78 ± 0.06 c | 19.48 ± 0.08 a | 1.91 ± 0.02 a | 6.52 ± 0.03 a | 13.31 ± 0.02 d | 1401 ± 0.5 d | 58.49 ± 0.40 a |
| XW 26 | 64.10 ± 0.07 a | 17.63 ± 0.03 b | 1.48 ± 0.03 b | 2.89 ± 0.06 c | 13.91 ± 0.03 b | 1444 ± 0.2 b | 29.67 ± 0.57 b |
| XW 45 | 64.27 ± 0.33 a | 16.98 ± 0.19 c | 1.88 ± 0.01 a | 3.10 ± 0.01 b | 13.77 ± 0.12 c | 1451 ± 2.1 a | 27.77 ± 0.65 c |
| KW 1701 | 62.99 ± 0.03 b | 17.02 ± 0.11 c | 1.88 ± 0.02 a | 3.13 ± 0.03 b | 14.99 ± 0.08 a | 1430 ± 1.8 c | 30.57 ± 0.61 b |
| Wheat Bran Source | Rha (%) | Ara (%) | Gal (%) | Glc (%) | Xyl (%) |
|---|---|---|---|---|---|
| FT | 0.29 | 24.62 | 1.84 | 25.01 | 47.60 |
| XW 26 | 0.00 | 10.94 | 0.96 | 64.67 | 23.42 |
| XW 45 | 0.00 | 8.48 | 1.00 | 72.89 | 17.63 |
| KW 1701 | 0.00 | 11.42 | 1.42 | 65.00 | 22.16 |
| T (°C) | (exp) (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | Langmuir Isotherm | Freundlich Isotherm | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (calc) (mg/g) | (min−1) | R2 | (calc) (mg/g) | (g·mg−1 ·min−1) | R2 | (mg/g) | (L/mg) | R2 | R2 | ||||
| 30 | 88.70 | 99.27 | 0.0176 | 0.9959 | 158.73 | 4.547 × 10−5 | 0.9534 | 294.1 | 3.904 × 10−2 | 0.9263 | 31.19 | 2.262 | 0.6954 |
| 40 | 90.01 | 71.90 | 0.0212 | 0.9861 | 102.04 | 3.780 × 10−4 | 0.9978 | 344.8 | 1.165 × 10−1 | 0.9265 | 51.64 | 1.963 | 0.8182 |
| 50 | 95.74 | 70.60 | 0.0287 | 0.9669 | 106.38 | 5.471 × 10−4 | 0.9983 | 384.6 | 8.754 × 10−2 | 0.9028 | 41.21 | 1.639 | 0.8038 |
| 60 | 96.80 | 57.58 | 0.0323 | 0.9438 | 104.17 | 8.621 × 10−4 | 0.9985 | 370.4 | 1.343 × 10−1 | 0.8360 | 59.31 | 1.872 | 0.6183 |
| T/K | /(KJ·mol−1) | /(J·mol−1·K−1) | /(KJ·mol−1) |
|---|---|---|---|
| 303 | 55.10 | 198.61 | −5.085 |
| 313 | −7.071 | ||
| 323 | −9.057 | ||
| 333 | −11.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quan, W.; Wang, J.; Huang, J.; Zhang, D. Structure Characterization and Dye Adsorption Properties of Modified Fiber from Wheat Bran. Molecules 2024, 29, 2581. https://doi.org/10.3390/molecules29112581
Quan W, Wang J, Huang J, Zhang D. Structure Characterization and Dye Adsorption Properties of Modified Fiber from Wheat Bran. Molecules. 2024; 29(11):2581. https://doi.org/10.3390/molecules29112581
Chicago/Turabian StyleQuan, Wenbin, Juan Wang, Jihong Huang, and Dale Zhang. 2024. "Structure Characterization and Dye Adsorption Properties of Modified Fiber from Wheat Bran" Molecules 29, no. 11: 2581. https://doi.org/10.3390/molecules29112581
APA StyleQuan, W., Wang, J., Huang, J., & Zhang, D. (2024). Structure Characterization and Dye Adsorption Properties of Modified Fiber from Wheat Bran. Molecules, 29(11), 2581. https://doi.org/10.3390/molecules29112581










