Recent Advances in Amphipathic Peptidomimetics as Antimicrobial Agents to Combat Drug Resistance
Abstract
1. Introduction
2. Small Molecule Peptidomimetics
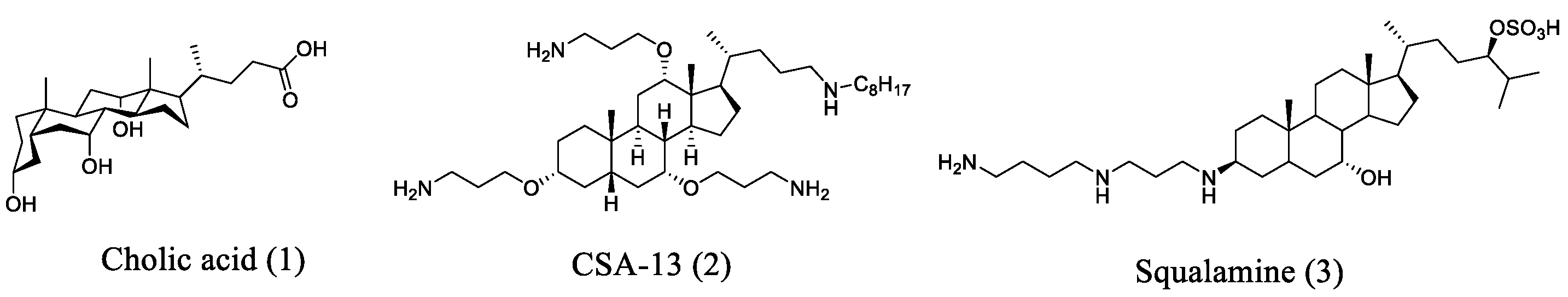
2.1. Cholic Acid-Based Ceragenin Derivatives
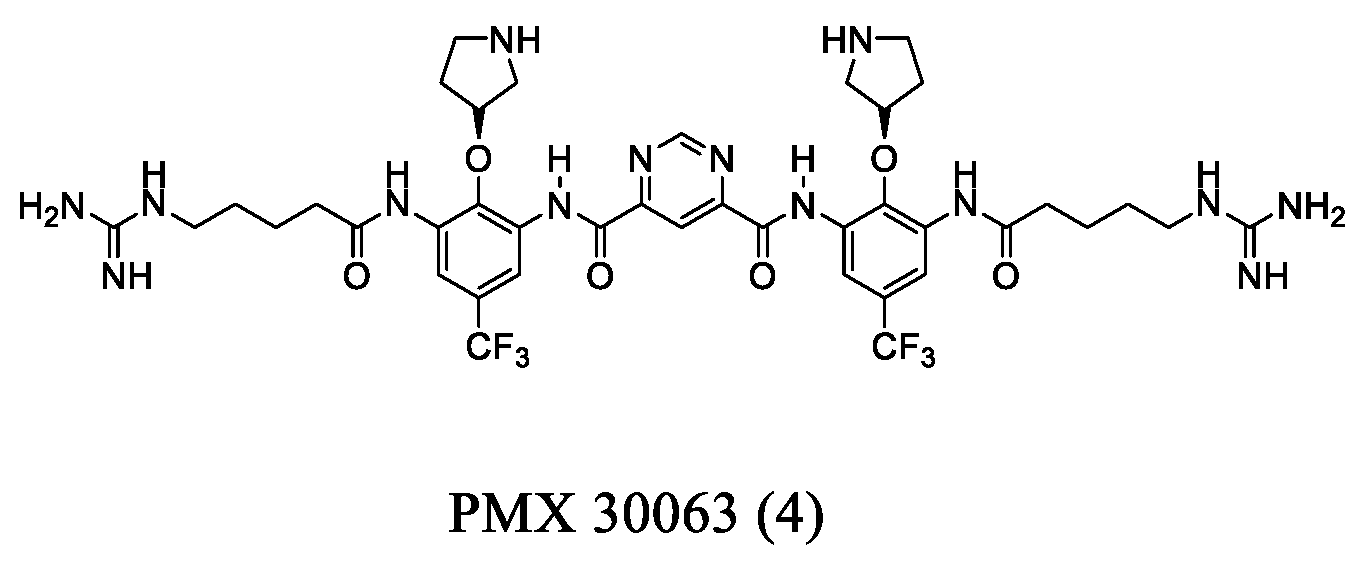
2.2. Arylamide-Based Small Molecules
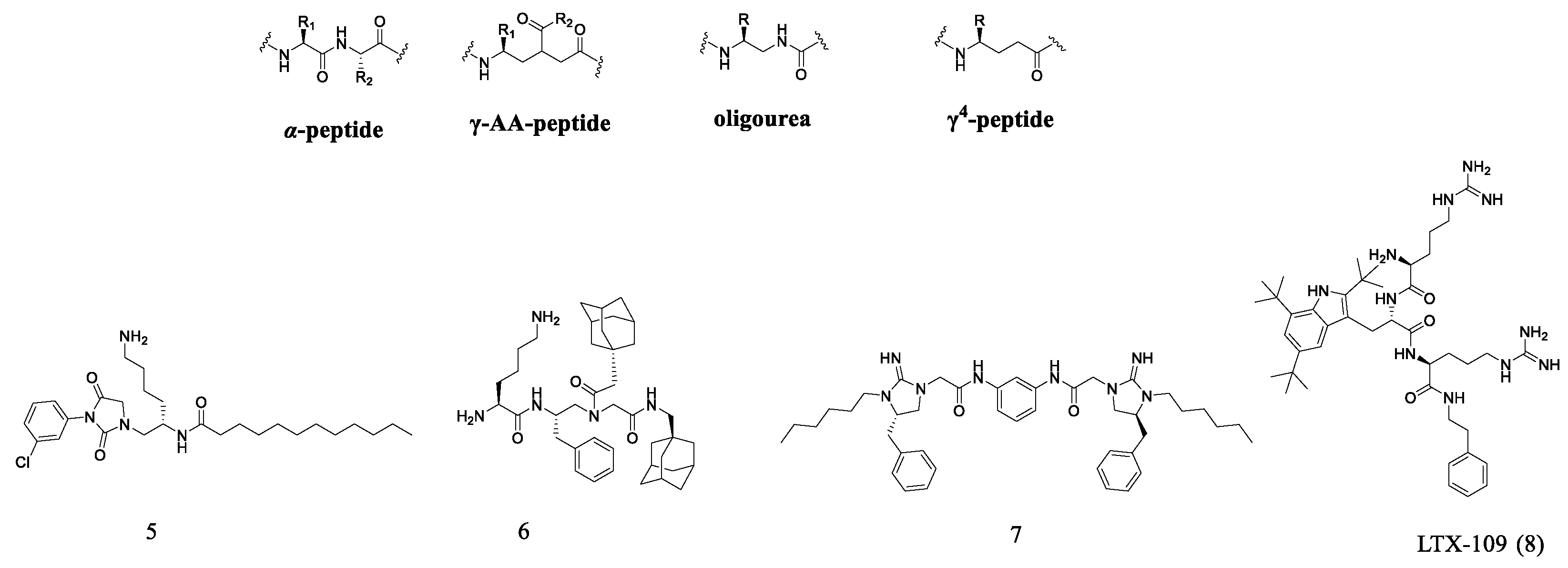
2.3. Oligopeptide Derivatives
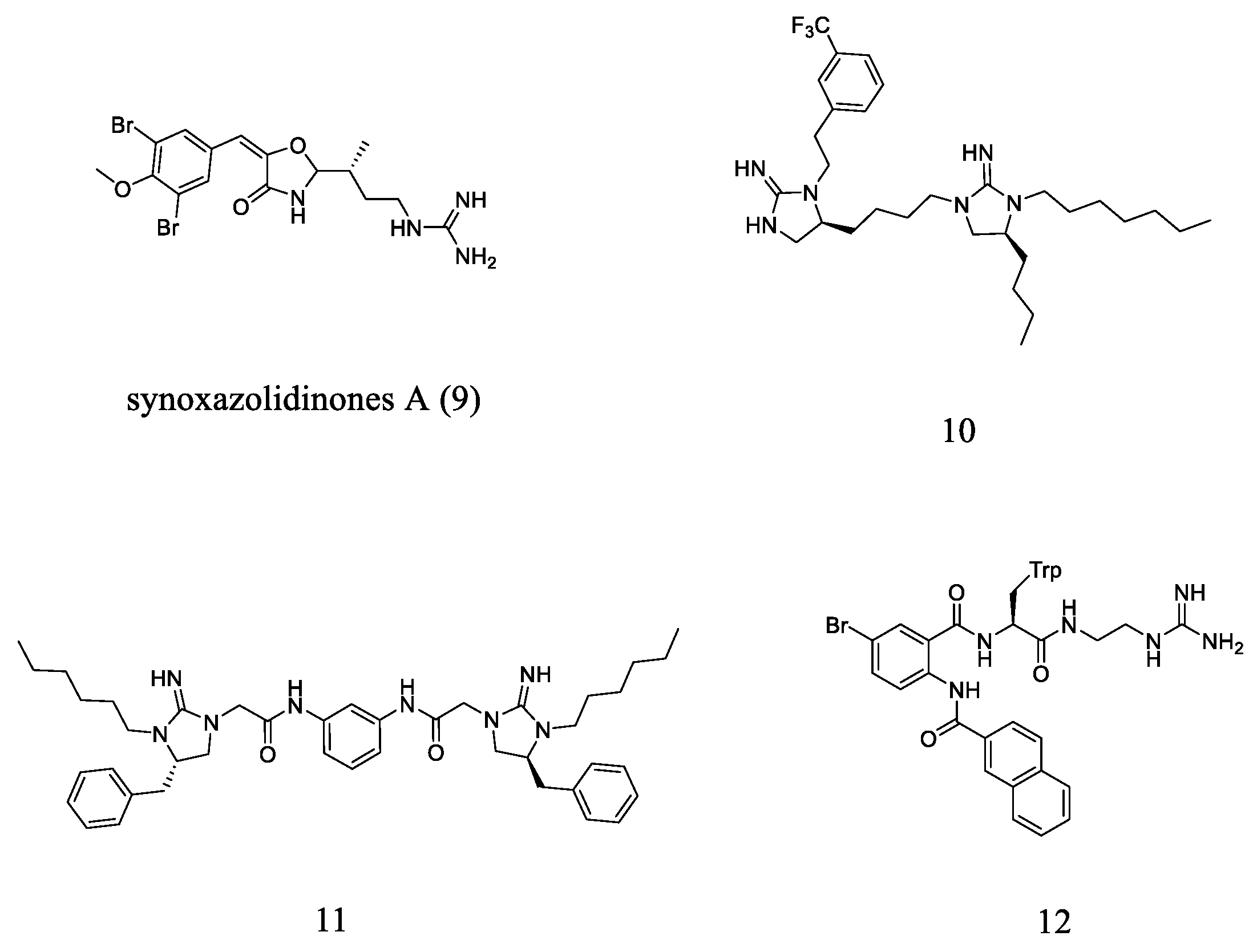
2.4. Guanidine-Based Small Molecules
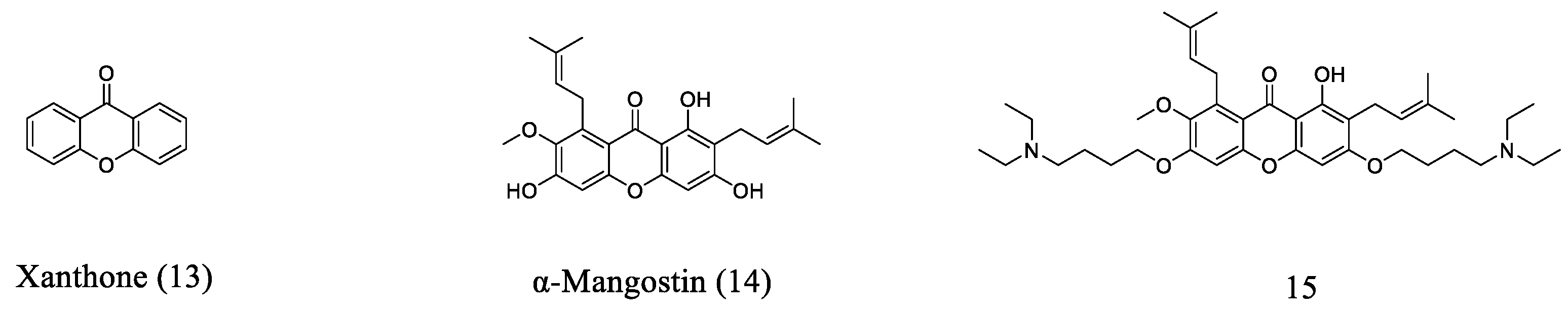
2.5. Xanthone-Based Small Molecules
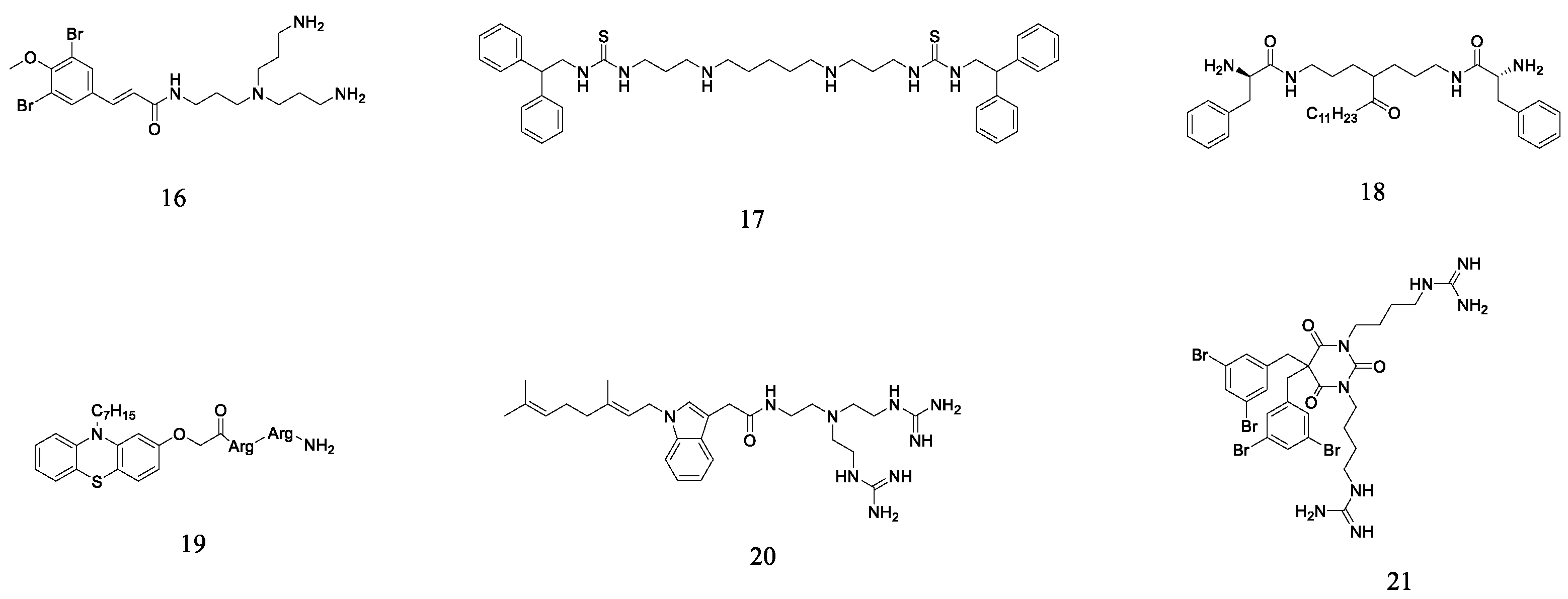
2.6. Other Small Molecule Peptidomimetics
3. AMP-Inspired Antimicrobial Cationic Oligomers and Polymers
3.1. β-Peptide Derivative Polymers
3.2. Poly(2-oxazoline)
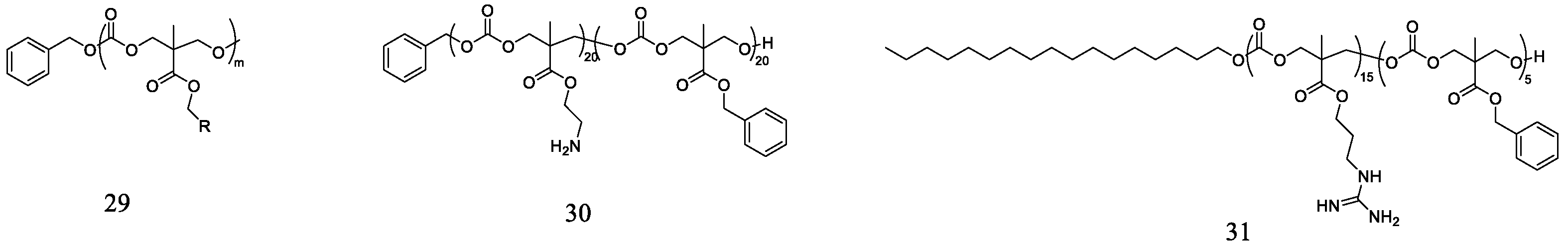
3.3. Polycarbonates
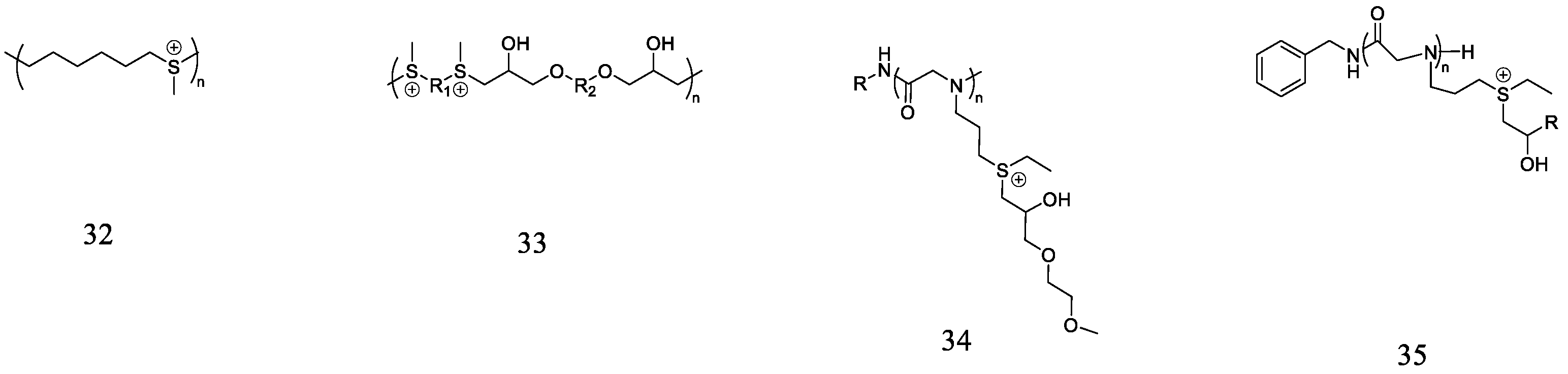
3.4. Polysulfoniums
3.5. Polyisocyanate Copolymers
4. Mechanism of Action
5. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Saiman, L. Infectious diseases of the fetus and newborn infant. JAMA 2012, 307, 1865–1866. [Google Scholar] [CrossRef]
- McKenney, J.; Bauman, S.; Neary, B.; Detels, R.; French, A.; Margolick, J. Prevalence, correlates, and outcomes of cryptococcal antigen positivity among patients with AIDS, United States, 1986–2012. Clin. Infect. Dis. 2015, 60, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Bee, G.C.W.; Lokken-Toyli, K.L.; Yeung, S.T.; Rodriguez, L.; Zangari, T.; Anderson, E.E.; Ghosh, S.; Rothlin, C.V.; Brodin, P.; Khanna, K.M.; et al. Age-dependent differences in efferocytosis determine the outcome of opsonophagocytic protection from invasive pathogens. Immunity 2023, 56, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Holá, V.; Imbert, C.; Kirketerp-Møller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef] [PubMed]
- Tarín-Pelló, A.; Suay-García, B.; Pérez-Gracia, M.-T. Antibiotic resistant bacteria: Current situation and treatment options to accelerate the development of a new antimicrobial arsenal. Expert Rev. Anti-Infect. Ther. 2022, 20, 1095–1108. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
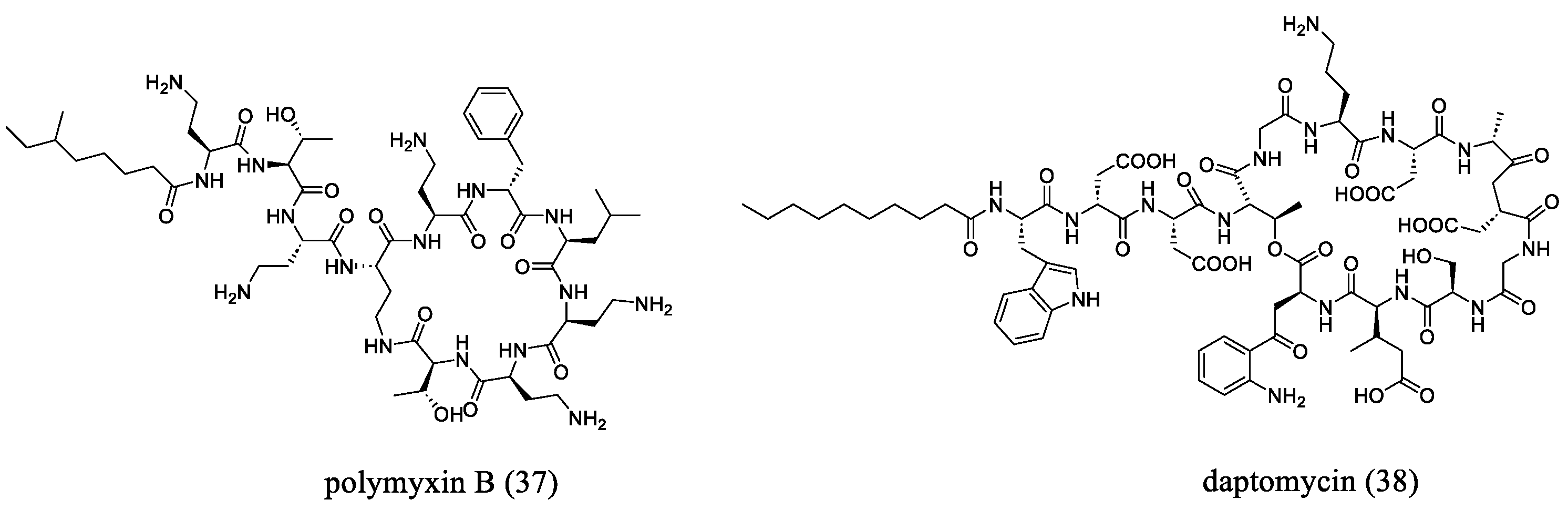
- Heidary, M.; Khosravi, A.D.; Khoshnood, S.; Nasiri, M.J.; Soleimani, S.; Goudarzi, M. Daptomycin. J. Antimicrob. Chemother. 2018, 73, 1–11. [Google Scholar] [CrossRef]
- Mookherjee, N.; Hancock, R.E.W. Cationic host defence peptides: Innate immune regulatory peptides as a novel approach for treating infections. Cell. Mol. Life Sci. 2007, 64, 922–933. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.W.; Diamond, G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000, 8, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Mookherjee, N.; Anderson, M.A.; Haagsman, H.P.; Davidson, D.J. Antimicrobial host defence peptides: Functions and clinical potential. Nat. Rev. Drug Discov. 2020, 19, 311–332. [Google Scholar] [CrossRef] [PubMed]
- Bechinger, B.; Gorr, S.U. Antimicrobial peptides: Mechanisms of action and resistance. J. Dent. Res. 2016, 96, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Marr, A.K.; Gooderham, W.J.; Hancock, R.E.W. Antibacterial peptides for therapeutic use: Obstacles and realistic outlook. Curr. Opin. Pharmacol. 2006, 6, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zheng, M.; Cai, J. Small molecules with membrane-active antibacterial activity. ACS Appl. Mater. Interfaces 2020, 12, 21292–21299. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Zhang, Z.; Yu, B.; Sun, H.; Leung, P.H.; Tao, X. Synthesis of polylactic acid oligomers for broad-spectrum antimicrobials. Polymers 2022, 14, 4399. [Google Scholar] [CrossRef] [PubMed]
- Teyssières, E.; Corre, J.-P.; Antunes, S.; Rougeot, C.; Dugave, C.; Jouvion, G.; Claudon, P.; Mikaty, G.; Douat, C.; Goossens, P.L.; et al. Proteolytically stable foldamer mimics of Host-Defense Peptides with protective activities in a murine model of bacterial infection. J. Med. Chem. 2016, 59, 8221–8232. [Google Scholar] [CrossRef]
- Zhang, T.; An, W.; Sun, J.; Duan, F.; Shao, Z.; Zhang, F.; Jiang, T.; Deng, X.; Boyer, C.; Gao, W. N-terminal lysozyme conjugation to a cationic polymer enhances antimicrobial activity and overcomes antimicrobial resistance. Nano Lett. 2022, 22, 8294–8303. [Google Scholar] [CrossRef]
- Pan, Y.; Xia, Q.; Xiao, H. Cationic polymers with tailored structures for rendering polysaccharide-based materials antimicrobial: An overview. Polymers 2019, 11, 1283. [Google Scholar] [CrossRef]
- Humpola, M.V.; Spinelli, R.; Erben, M.; Perdomo, V.; Tonarelli, G.G.; Albericio, F.; Siano, A.S. D- and N-methyl amino acids for modulating the therapeutic properties of antimicrobial peptides and lipopeptides. Antibiotics 2023, 12, 821. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, M.; Muhammad, I.; Cui, Q.; Zhang, H.; Jia, Y.; Xu, Q.; Kong, L.; Ma, H. An antibacterial peptide with high resistance to trypsin obtained by substituting d-amino acids for trypsin cleavage sites. Antibiotics 2021, 10, 1465. [Google Scholar]
- Hacioglu, M.; Yilmaz, F.N.; Oyardi, O.; Bozkurt Guzel, C.; Inan, N.; Savage, P.B.; Dosler, S. Antimicrobial activity of Ceragenins against vancomycin-susceptible and -resistant Enterococcus spp. Pharmaceuticals 2023, 16, 1643. [Google Scholar] [CrossRef] [PubMed]
- Piktel, E.; Pogoda, K.; Roman, M.; Niemirowicz, K.; Tokajuk, G.; Wróblewska, M.; Szynaka, B.; Kwiatek, W.M.; Savage, P.B.; Bucki, R. Sporicidal activity of ceragenin CSA-13 against Bacillus subtilis. Sci. Rep. 2017, 7, 44452. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.-Z.; Feng, Y.; Pollard, J.; Chin, J.N.; Rybak, M.J.; Bucki, R.; Epand, R.F.; Epand, R.M.; Savage, P.B. Ceragenins: Cholic acid-based mimics of antimicrobial peptides. Acc. Chem. Res. 2008, 41, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Sofia, M.J.; Kakarla, R.; Kogan, N.A.; Wierichs, L.; Longley, C.B.; Bruker, K.; Axelrod, H.R.; Midha, S.; Babu, S.; et al. Cationic facial amphiphiles: A promising class of transfection agents. Proc. Natl. Acad. Sci. USA 1996, 93, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.B. Design, synthesis and characterization of cationic peptide and steroid antibiotics. Eur. J. Org. Chem. 2002, 2002, 759–768. [Google Scholar] [CrossRef]
- Li, C.; Peters, A.S.; Meredith, E.L.; Allman, G.W.; Savage, P.B. Design and synthesis of potent sensitizers of Gram-negative bacteria based on a cholic acid scaffolding. J. Am. Chem. Soc. 1998, 120, 2961–2962. [Google Scholar] [CrossRef]
- Chin, J.N.; Rybak, M.J.; Cheung, C.M.; Savage, P.B. Antimicrobial activities of ceragenins against clinical isolates of resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 1268–1273. [Google Scholar] [CrossRef]
- Ding, B.; Guan, Q.; Walsh, J.P.; Boswell, J.S.; Winter, T.W.; Winter, E.S.; Boyd, S.S.; Li, C.; Savage, P.B. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J. Med. Chem. 2002, 45, 663–669. [Google Scholar] [CrossRef]
- Guan, Q.; Li, C.; Schmidt, E.J.; Boswell, J.S.; Walsh, J.P.; Allman, G.W.; Savage, P.B. Preparation and characterization of cholic acid-derived antimicrobial agents with controlled stabilities. Org. Lett. 2000, 2, 2837–2840. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt-Guzel, C.; Savage, P.B.; Gerceker, A.A. In vitro Activities of the Novel Ceragenin CSA-13, alone or in combination with Colistin, Tobramycin, and Ciprofloxacin, against Pseudomonas aeruginosa strains isolated from cystic fibrosis patients. Chemotherapy 2012, 57, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.N.; Jones, R.N.; Sader, H.S.; Savage, P.B.; Rybak, M.J. Potential synergy activity of the novel ceragenin, CSA-13, against clinical isolates of Pseudomonas aeruginosa, including multidrug-resistant P. aeruginosa. J. Antimicrob. Chemother. 2008, 61, 365–370. [Google Scholar] [CrossRef]
- Nagant, C.; Feng, Y.; Lucas, B.; Braeckmans, K.; Savage, P.; Dehaye, J.P. Effect of a low concentration of a cationic steroid antibiotic (CSA-13) on the formation of a biofilm by Pseudomonas aeruginosa. J. Appl. Microbiol. 2011, 111, 763–772. [Google Scholar] [CrossRef]
- Nagant, C.; Tré-Hardy, M.; El-Ouaaliti, M.; Savage, P.; Devleeschouwer, M.; Dehaye, J.-P. Interaction between tobramycin and CSA-13 on clinical isolates of Pseudomonas aeruginosa in a model of young and mature biofilms. Appl. Microbiol. Biotechnol. 2010, 88, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Wnorowska, U.; Piktel, E.; Deptuła, P.; Wollny, T.; Król, G.; Głuszek, K.; Durnaś, B.; Pogoda, K.; Savage, P.B.; Bucki, R. Ceragenin CSA-13 displays high antibacterial efficiency in a mouse model of urinary tract infection. Sci. Rep. 2022, 12, 19164. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.E.; Snarr, J.; Chaudhary, V.; Jennings, J.D.; Shaw, H.; Christiansen, B.; Wright, J.; Jia, W.; Bishop, R.E.; Savage, P.B. In vitro evaluation of the potential for resistance development to ceragenin CSA-13. J. Antimicrob. Chemother. 2012, 67, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Alhanout, K.; Rolain, J.M.; Brunel, J.M. Squalamine as an example of a new potent antimicrobial agents class: A critical review. Curr. Med. Chem. 2010, 17, 3909–3917. [Google Scholar] [CrossRef] [PubMed]
- Vergoz, D.; Nilly, F.; Desriac, F.; Barreau, M.; Géry, A.; Lepetit, C.; Sichel, F.; Jeannot, K.; Giard, J.-C.; Garon, D.; et al. 6-Polyaminosteroid squalamine analogues display antibacterial activity against resistant pathogens. Int. J. Mol. Sci. 2023, 24, 8568. [Google Scholar] [CrossRef]
- Mammari, N.; Salles, E.; Beaussart, A.; El-Kirat-Chatel, S.; Varbanov, M. Squalamine and its aminosterol derivatives: Overview of biological effects and mechanisms of action of compounds with multiple therapeutic applications. Microorganisms 2022, 10, 1205. [Google Scholar] [CrossRef]
- El-Kirat-Chatel, S.; Varbanov, M.; Retourney, C.; Salles, E.; Risler, A.; Brunel, J.-M.; Beaussart, A. AFM reveals the interaction and nanoscale effects imposed by squalamine on Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2023, 226, 113324. [Google Scholar] [CrossRef] [PubMed]
- Tew, G.N.; Scott, R.W.; Klein, M.L.; DeGrado, W.F. De novo design of antimicrobial polymers, foldamers, and small molecules: From discovery to practical applications. Acc. Chem. Res. 2010, 43, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Isaacs, A.; Clements, D.; Liu, D.; Kim, H.; Scott, R.W.; Winkler, J.D.; DeGrado, W.F. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. Proc. Natl. Acad. Sci. USA 2009, 106, 6968–6973. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, R.P.; Romanowski, E.G.; Yates, K.A.; Mah, F.S. An independent evaluation of a novel peptide mimetic, Brilacidin (PMX30063), for ocular anti-infective. J. Ocul. Pharmacol. Ther. 2015, 32, 23–27. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, T.F.; de Castro, P.A.; Bastos, R.W.; Pinzan, C.F.; Souza, P.F.N.; Ackloo, S.; Hossain, M.A.; Drewry, D.H.; Alkhazraji, S.; Ibrahim, A.S.; et al. A host defense peptide mimetic, brilacidin, potentiates caspofungin antifungal activity against human pathogenic fungi. Nat. Commun. 2023, 14, 2052. [Google Scholar] [CrossRef] [PubMed]
- Yanmei, H.; Hyunil, J.; William, F.D.; Jun, W. Brilacidin, a COVID-19 Drug Candidate, demonstrates broad-spectrum antiviral activity against human coronaviruses OC43, 229E and NL63 through targeting both the virus and the host cell. bioRxiv 2021, 11, 467344. [Google Scholar]
- Shi, Y.; Teng, P.; Sang, P.; She, F.; Wei, L.; Cai, J. γ-AApeptides: Design, structure, and applications. Acc. Chem. Res. 2016, 49, 428–441. [Google Scholar] [CrossRef]
- Claudon, P.; Violette, A.; Lamour, K.; Decossas, M.; Fournel, S.; Heurtault, B.; Godet, J.; Mély, Y.; Jamart-Grégoire, B.; Averlant-Petit, M.-C.; et al. Consequences of isostructural main-chain modifications for the design of antimicrobial foldamers: Helical mimics of Host-Defense Peptides based on a heterogeneous amide/urea backbone. Angew. Chem. Int. Ed. 2010, 49, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, A.; Jadhav, S.V.; Gopi, H.N. α/γ4-Hybrid peptide helices: Synthesis, crystal conformations and analogy with the α-helix. Chem. Commun. 2012, 48, 7170–7172. [Google Scholar] [CrossRef]
- Jadhav, S.V.; Misra, R.; Singh, S.K.; Gopi, H.N. Efficient access to enantiopure γ4-amino acids with proteinogenic side-chains and structural investigation of γ4-Asn and γ4-Ser in hybrid peptide helices. Chem. A Eur. J. 2013, 19, 16256–16262. [Google Scholar] [CrossRef]
- Haug, B.E.; Stensen, W.; Stiberg, T.; Svendsen, J.S. Bulky nonproteinogenic amino acids permit the design of very small and effective cationic antibacterial peptides. J. Med. Chem. 2004, 47, 4159–4162. [Google Scholar] [CrossRef] [PubMed]
- Haug, B.E.; Stensen, W.; Kalaaji, M.; Rekdal, Ø.; Svendsen, J.S. Synthetic antimicrobial peptidomimetics with therapeutic potential. J. Med. Chem. 2008, 51, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Pawlak, J.; Johnson, L.; Bonilla, H.; Saravolatz, L.D.; Fakih, M.G.; Fugelli, A.; Olsen Wenche, M. In vitro activities of LTX-109, a synthetic antimicrobial peptide, against Methicillin-resistant, Vancomycin-intermediate, Vancomycin-resistant, Daptomycin-nonsusceptible, and Linezolid-nonsusceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 2012, 56, 4478–4482. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Janson, H.; Wold, H.; Fugelli, A.; Andersson, K.; Hâkangârd, C.; Olsson, P.; Olsen Wenche, M. LTX-109 is a novel agent for nasal decolonization of Methicillin-resistant and -sensitive Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 145–151. [Google Scholar] [CrossRef]
- Isaksson, J.; Brandsdal, B.O.; Engqvist, M.; Flaten, G.E.; Svendsen, J.S.M.; Stensen, W. A synthetic antimicrobial peptidomimetic (LTX 109): Stereochemical impact on membrane disruption. J. Med. Chem. 2011, 54, 5786–5795. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Pawlak, J.; Martin, H.; Saravolatz, S.; Johnson, L.; Wold, H.; Husbyn, M.; Olsen, W.M. Postantibiotic effect and postantibiotic sub-MIC effect of LTX-109 and mupirocin on Staphylococcus aureus blood isolates. Lett. Appl. Microbiol. 2017, 65, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Semenya, D.; Castagnolo, D. Antimicrobial drugs bearing guanidine moieties: A review. Eur. J. Med. Chem. 2021, 216, 113293. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.; Strøm, M.B.; Svenson, J.; Jaspars, M.; Milne, B.F.; Tørfoss, V.; Andersen, J.H.; Hansen, E.; Stensvåg, K.; Haug, T. Synoxazolidinones A and B: Novel bioactive alkaloids from the ascidian Synoicum pulmonaria. Org. Lett. 2010, 12, 4752–4755. [Google Scholar] [CrossRef] [PubMed]
- Andreev, K.; Bianchi, C.; Laursen, J.S.; Citterio, L.; Hein-Kristensen, L.; Gram, L.; Kuzmenko, I.; Olsen, C.A.; Gidalevitz, D. Guanidino groups greatly enhance the action of antimicrobial peptidomimetics against bacterial cytoplasmic membranes. Biochim. Biophys. Acta (BBA)-Biomembr. 2014, 1838, 2492–2502. [Google Scholar] [CrossRef]
- Fleeman, R.; LaVoi, T.M.; Santos, R.G.; Morales, A.; Nefzi, A.; Welmaker, G.S.; Medina-Franco, J.L.; Giulianotti, M.A.; Houghten, R.A.; Shaw, L.N. Combinatorial libraries as a tool for the discovery of novel, broad-spectrum antibacterial agents targeting the ESKAPE pathogens. J. Med. Chem. 2015, 58, 3340–3355. [Google Scholar] [CrossRef]
- Teng, P.; Nimmagadda, A.; Su, M.; Hong, Y.; Shen, N.; Li, C.; Tsai, L.-Y.; Cao, J.; Li, Q.; Cai, J. Novel bis-cyclic guanidines as potent membrane-active antibacterial agents with therapeutic potential. Chem. Commun. 2017, 53, 11948–11951. [Google Scholar] [CrossRef] [PubMed]
- Nizalapur, S.; Kimyon, O.; Yee, E.; Ho, K.; Berry, T.; Manefield, M.; Cranfield, C.G.; Willcox, M.; Black, D.S.; Kumar, N. Amphipathic guanidine-embedded glyoxamide-based peptidomimetics as novel antibacterial agents and biofilm disruptors. Org. Biomol. Chem. 2017, 15, 2033–2051. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, R.; Yasir, M.; Berry, T.; Cranfield, C.G.; Nizalapur, S.; Yee, E.; Kimyon, O.; Taunk, A.; Ho, K.K.K.; Cornell, B.; et al. Design and synthesis of short amphiphilic cationic peptidomimetics based on biphenyl backbone as antibacterial agents. Eur. J. Med. Chem. 2018, 143, 1702–1722. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, R.; Yasir, M.; Yee, E.; Willcox, M.; Black, D.S.; Kumar, N. Guanidine functionalized anthranilamides as effective antibacterials with biofilm disruption activity11Electronic supplementary information (ESI) available. Org. Biomol. Chem. 2018, 16, 5871–5888. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.X.; Loureiro, D.R.P.; Dias, A.L.; Reis, S.; Pinto, M.M.M.; Afonso, C.M.M. Bioactive marine Xanthones: A review. Mar. Drugs 2022, 20, 58. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, R.H.; El-Ghorab, M.H.D.; El-Barbary, A.M.; Zayed, F.M.; Goransson, U.; Larsson, S.; Verpoorte, R. Naturally occurring Xanthones; Latest investigations: Isolation, structure elucidation and chemosystematic significance. Curr. Med. Chem. 2009, 16, 2581–2626. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shen, J.; Zhu, K. Antibacterial activities of plant-derived xanthones. RSC Med. Chem. 2022, 13, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.-J.; Qiu, S.; Zou, H.; Lakshminarayanan, R.; Li, J.; Zhou, X.; Tang, C.; Saraswathi, P.; Verma, C.; Tan, D.T.H.; et al. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 834–844. [Google Scholar] [CrossRef]
- Zou, H.; Koh, J.-J.; Li, J.; Qiu, S.; Aung, T.T.; Lin, H.; Lakshminarayanan, R.; Dai, X.; Tang, C.; Lim, F.H.; et al. Design and synthesis of amphiphilic Xanthone-based, membrane-targeting antimicrobials with improved membrane selectivity. J. Med. Chem. 2013, 56, 2359–2373. [Google Scholar] [CrossRef]
- Xu, M.; Davis, R.A.; Feng, Y.; Sykes, M.L.; Shelper, T.; Avery, V.M.; Camp, D.; Quinn, R.J. Ianthelliformisamines A–C, Antibacterial Bromotyrosine-Derived Metabolites from the Marine Sponge Suberea ianthelliformis. J. Nat. Prod. 2012, 75, 1001–1005. [Google Scholar] [CrossRef]
- Pieri, C.; Borselli, D.; Di Giorgio, C.; De Méo, M.; Bolla, J.-M.; Vidal, N.; Combes, S.; Brunel, J.M. New Ianthelliformisamine derivatives as antibiotic enhancers against resistant Gram-negative bacteria. J. Med. Chem. 2014, 57, 4263–4272. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pachaiyappan, B.; Gruber, J.D.; Schmidt, M.G.; Zhang, Y.-M.; Woster, P.M. Antibacterial diamines targeting bacterial membranes. J. Med. Chem. 2016, 59, 3140–3151. [Google Scholar] [CrossRef] [PubMed]
- Konai, M.M.; Ghosh, C.; Yarlagadda, V.; Samaddar, S.; Haldar, J. Membrane active Phenylalanine conjugated lipophilic Norspermidine derivatives with selective antibacterial activity. J. Med. Chem. 2014, 57, 9409–9423. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Cai, Q.; Liang, W.; Zhong, K.; Liu, J.; Li, H.; Fang, S.; Zhong, R.; Liu, S.; Lin, S. Design of phenothiazine-based cationic amphiphilic derivatives incorporating arginine residues: Potential membrane-active broad-spectrum antimicrobials combating pathogenic bacteria in vitro and in vivo. Eur. J. Med. Chem. 2023, 260, 115733. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, H.; Liu, J.; Zhong, R.; Li, H.; Fang, S.; Liu, S.; Lin, S. Synthesis and biological evaluation of indole-based peptidomimetics as antibacterial agents against Gram-positive bacteria. Eur. J. Med. Chem. 2021, 226, 113813. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, M.H.; Engqvist, M.; Ausbacher, D.; Anderssen, T.; Langer, M.K.; Haug, T.; Morello, G.R.; Liikanen, L.E.; Blencke, H.-M.; Isaksson, J.; et al. Amphipathic barbiturates as mimics of antimicrobial peptides and the marine natural products Eusynstyelamides with activity against multi-resistant clinical isolates. J. Med. Chem. 2021, 64, 11395–11417. [Google Scholar] [CrossRef] [PubMed]
- Haney, E.F.; Mansour, S.C.; Hancock, R.E.W. Antimicrobial pptides: An introduction. In Antimicrobial Peptides: Methods and Protocols; Hansen, P.R., Ed.; Springer: New York, NY, USA, 2017; pp. 3–22. [Google Scholar]
- Jiang, Z.; Vasil, A.I.; Hale, J.D.; Hancock, R.E.W.; Vasil, M.L.; Hodges, R.S. Effects of net charge and the number of positively charged residues on the biological activity of amphipathic α-helical cationic antimicrobial peptides. Pept. Sci. 2008, 90, 369–383. [Google Scholar] [CrossRef]
- Judzewitsch, P.R.; Nguyen, T.-K.; Shanmugam, S.; Wong, E.H.H.; Boyer, C. Towards sequence-controlled antimicrobial polymers: Effect of polymer block order on antimicrobial activity. Angew. Chem. Int. Ed. 2018, 57, 4559–4564. [Google Scholar] [CrossRef] [PubMed]
- Wenn, B.; Junkers, T. Continuous microflow photoRAFT polymerization. Macromolecules 2016, 49, 6888–6895. [Google Scholar] [CrossRef]
- Ergene, C.; Yasuhara, K.; Palermo, E.F. Biomimetic antimicrobial polymers: Recent advances in molecular design. Polym. Chem. 2018, 9, 2407–2427. [Google Scholar] [CrossRef]
- Kuroda, K.; Caputo, G.A.; DeGrado, W.F. The role of hydrophobicity in the antimicrobial and hemolytic activities of polymethacrylate derivatives. Chem.-A Eur. J. 2009, 15, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, P.; Xie, J.; Zhang, S.; Xiao, X.; Qiao, Z.; Shao, N.; Zhou, M.; Zhang, W.; Dai, C.; et al. Host defense peptide mimicking poly-β-peptides with fast, potent and broad spectrum antibacterial activities. Biomater. Sci. 2019, 7, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Etayash, H.; Qian, Y.; Pletzer, D.; Zhang, Q.; Xie, J.; Cui, R.; Dai, C.; Ma, P.; Qi, F.; Liu, R.; et al. Host defense peptide-mimicking amphiphilic β-peptide polymer (Bu:DM) exhibiting anti-biofilm, immunomodulatory, and in vivo anti-infective activity. J. Med. Chem. 2020, 63, 12921–12928. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Deng, S.; Cong, Z.; Zhang, H.; Lu, Z.; Shao, N.; Bhatti, S.A.; Zhou, C.; Cheng, J.; Gellman, S.H.; et al. Secondary amine pendant β-peptide polymers displaying potent antibacterial activity and promising therapeutic potential in treating MRSA-induced wound infections and keratitis. J. Am. Chem. Soc. 2022, 144, 1690–1699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shi, C.; Cong, Z.; Chen, Q.; Bi, Y.; Zhang, J.; Ma, K.; Liu, S.; Gu, J.; Chen, M.; et al. Microbial metabolite inspired β-peptide polymers displaying potent and selective antifungal activity. Adv. Sci. 2022, 9, 2104871. [Google Scholar] [CrossRef] [PubMed]
- Mowery, B.P.; Lee, S.E.; Kissounko, D.A.; Epand, R.F.; Epand, R.M.; Weisblum, B.; Stahl, S.S.; Gellman, S.H. Mimicry of antimicrobial Host-Defense Peptides by random copolymers. J. Am. Chem. Soc. 2007, 129, 15474–15476. [Google Scholar] [CrossRef] [PubMed]
- Mowery, B.P.; Lindner, A.H.; Weisblum, B.; Stahl, S.S.; Gellman, S.H. Structure−activity relationships among random Nylon-3 copolymers that mimic antibacterial Host-Defense Peptides. J. Am. Chem. Soc. 2009, 131, 9735–9745. [Google Scholar] [CrossRef]
- Lienkamp, K.; Madkour, A.E.; Musante, A.; Nelson, C.F.; Nüsslein, K.; Tew, G.N. Antimicrobial polymers prepared by ROMP with unprecedented selectivity: A molecular construction kit approach. J. Am. Chem. Soc. 2008, 130, 9836–9843. [Google Scholar] [CrossRef] [PubMed]
- Lienkamp, K.; Tew, G.N. Synthetic mimics of antimicrobial peptides—A versatile ring-opening metathesis polymerization based platform for the synthesis of selective antibacterial and cell-penetrating polymers. Chem.-A Eur. J. 2009, 15, 11784–11800. [Google Scholar] [CrossRef]
- Zhou, M.; Qian, Y.; Xie, J.; Zhang, W.; Jiang, W.; Xiao, X.; Chen, S.; Dai, C.; Cong, Z.; Ji, Z.; et al. Poly(2-oxazoline)-based functional peptide mimics: Eradicating MRSA infections and persisters while alleviating antimicrobial resistance. Angew. Chem. Int. Ed. 2020, 59, 6412–6419. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, M.; Chen, S.; Xie, J.; Chen, M.; Zhang, H.; Wu, Y.; Chen, X.; Liu, R. Peptide-mimicking poly(2-oxazoline)s possessing potent antifungal activity and BBB penetrating property to treat invasive infections and meningitis. J. Am. Chem. Soc. 2023, 145, 25753–25765. [Google Scholar] [CrossRef] [PubMed]
- Ng, V.W.L.; Tan, J.P.K.; Leong, J.; Voo, Z.X.; Hedrick, J.L.; Yang, Y.Y. Antimicrobial polycarbonates: Investigating the impact of nitrogen-containing heterocycles as quaternizing agents. Macromolecules 2014, 47, 1285–1291. [Google Scholar] [CrossRef]
- Nimmagadda, A.; Liu, X.; Teng, P.; Su, M.; Li, Y.; Qiao, Q.; Khadka, N.K.; Sun, X.; Pan, J.; Xu, H.; et al. Polycarbonates with potent and selective antimicrobial activity toward Gram-positive bacteria. Biomacromolecules 2017, 18, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Chakraborty, S.; Gao, R.; Wang, S.; Gu, M.; Shen, N.; Wei, L.; Cao, C.; Sun, X.; Cai, J. Antimicrobial guanidinylate polycarbonates show oral in vivo efficacy against Clostridioides Difficile. Adv. Healthc. Mater. 2024, e2303295. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.; Zhao, J.; Zhu, Z.; Rao, J. Main-chain sulfonium-containing homopolymers with negligible hemolytic toxicity for eradication of bacterial and fungal biofilms. ACS Macro Lett. 2021, 10, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, J.; Zhang, J.; Zhu, Z.; Rao, J. Broad-spectrum bactericidal activity and remarkable selectivity of main-chain sulfonium-containing polymers with alternating sequences. ACS Macro Lett. 2021, 10, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, M.; Lin, M.; Yang, X.; Sun, J. A convenient approach for antibacterial polypeptoids featuring sulfonium and oligo(ethylene glycol) subunits. Biomater. Sci. 2020, 8, 6969–6977. [Google Scholar] [CrossRef]
- Sun, J.; Li, M.; Lin, M.; Zhang, B.; Chen, X. High antibacterial activity and selectivity of the versatile polysulfoniums that combat drug resistance. Adv. Mater. 2021, 33, 2104402. [Google Scholar] [CrossRef] [PubMed]
- Bak, I.G.; Chae, C.-G.; Lee, J.-S. Synthetic control of helical polyisocyanates by living anionic polymerization toward peptide mimicry. Macromolecules 2022, 55, 1923–1945. [Google Scholar] [CrossRef]
- Bak, I.G.; Chae, C.-G.; Choi, J.; Song, W.-Y.; Seo, J.; Lee, E.; Lee, J.-S. Synthesis of alternating polyisocyanate copolymers by anionic polymerization for mimicking amphiphilic helical peptides. Angew. Chem. Int. Ed. 2022, 61, e202212398. [Google Scholar] [CrossRef]
- Zasloff, M. Magainins, a class of antimicrobial peptides from Xenopus skin: Isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 1987, 84, 5449–5453. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Why and how are peptide–lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta (BBA)-Biomembr. 1999, 1462, 1–10. [Google Scholar] [CrossRef]
- Yang, L.; Weiss, T.M.; Lehrer, R.I.; Huang, H.W. Crystallization of antimicrobial pores in membranes: Magainin and Protegrin. Biophys. J. 2000, 79, 2002–2009. [Google Scholar] [CrossRef] [PubMed]
- Savini, F.; Loffredo, M.R.; Troiano, C.; Bobone, S.; Malanovic, N.; Eichmann, T.O.; Caprio, L.; Canale, V.C.; Park, Y.; Mangoni, M.L.; et al. Binding of an antimicrobial peptide to bacterial cells: Interaction with different species, strains and cellular components. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183291. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, T.-L.; Huang, H.W. A pore model or the carpet model? The mode of action of AMPs on E. coli spheroplasts. Biophys. J. 2016, 110, 28a. [Google Scholar] [CrossRef]
- Ding, L.; Chi, E.Y.; Chemburu, S.; Ji, E.; Schanze, K.S.; Lopez, G.P.; Whitten, D.G. Insight into the mechanism of antimicrobial poly(phenylene ethynylene) polyelectrolytes: Interactions with phosphatidylglycerol lipid membranes. Langmuir 2009, 25, 13742–13751. [Google Scholar] [CrossRef]
- Eren, T.; Som, A.; Rennie, J.R.; Nelson, C.F.; Urgina, Y.; Nüsslein, K.; Coughlin, E.B.; Tew, G.N. Antibacterial and hemolytic activities of quaternary pyridinium functionalized polynorbornenes. Macromol. Chem. Phys. 2008, 209, 516–524. [Google Scholar] [CrossRef]
- Baul, U.; Kuroda, K.; Vemparala, S. Interaction of multiple biomimetic antimicrobial polymers with model bacterial membranes. J. Chem. Phys. 2014, 141, 084902. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.; Vemparala, S.; Pophristic, V.; Kuroda, K.; DeGrado, W.F.; McCammon, J.A.; Klein, M.L. Characterization of nonbiological antimicrobial polymers in aqueous solution and at water−lipid interfaces from all-atom molecular dynamics. J. Am. Chem. Soc. 2006, 128, 1778–1779. [Google Scholar] [CrossRef]
- Kim, H.; Yoo, Y.D.; Lee, G.Y. Identification of bacterial membrane selectivity of Romo1-derived antimicrobial peptide AMPR-22 via molecular dynamics. Int. J. Mol. Sci. 2022, 23, 7404. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, Z.; Zhen, W.; Zhang, L.; Min, X.; Wang, Y.; Liu, F.; Su, M. Design and synthesis of broad-spectrum antimicrobial amphiphilic peptidomimetics to combat drug-resistance. Bioorganic Chem. 2023, 140, 106766. [Google Scholar] [CrossRef] [PubMed]
- Nederberg, F.; Zhang, Y.; Tan, J.P.K.; Xu, K.; Wang, H.; Yang, C.; Gao, S.; Guo, X.D.; Fukushima, K.; Li, L.; et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 2011, 3, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chin, W.; Dong, H.; Xu, L.; Zhong, G.; Huang, Y.; Li, L.; Xu, K.; Wu, M.; Hedrick, J.L.; et al. Biodegradable antimicrobial polycarbonates with in vivo efficacy against multidrug-resistant MRSA systemic infection. Adv. Healthc. Mater. 2015, 4, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Zuttion, F.; Colom, A.; Matile, S.; Farago, D.; Pompeo, F.; Kokavecz, J.; Galinier, A.; Sturgis, J.; Casuso, I. High-speed atomic force microscopy highlights new molecular mechanism of daptomycin action. Nat. Commun. 2020, 11, 6312. [Google Scholar] [CrossRef]
- Chen, E.H.L.; Wang, C.-H.; Liao, Y.-T.; Chan, F.-Y.; Kanaoka, Y.; Uchihashi, T.; Kato, K.; Lai, L.; Chang, Y.-W.; Ho, M.-C.; et al. Visualizing the membrane disruption action of antimicrobial peptides by cryo-electron tomography. Nat. Commun. 2023, 14, 5464. [Google Scholar] [CrossRef] [PubMed]
- Melcrová, A.; Maity, S.; Melcr, J.; de Kok, N.A.W.; Gabler, M.; van der Eyden, J.; Stensen, W.; Svendsen, J.S.M.; Driessen, A.J.M.; Marrink, S.J.; et al. Lateral membrane organization as target of an antimicrobial peptidomimetic compound. Nat. Commun. 2023, 14, 4038. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Abraham, T.; Rapp, J.; Vastey, F.; Saad, N.; Balmir, E. Daptomycin: Evaluation of a high-dose treatment strategy. Int. J. Antimicrob. Agents 2011, 38, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Farbman, L.; Leibovici, L.; Paul, M. Colistin: New lessons on an old antibiotic. Clin. Microbiol. Infect. 2012, 18, 18–29. [Google Scholar] [CrossRef]
- Brown, K.L.; Poon, G.F.T.; Birkenhead, D.; Pena, O.M.; Falsafi, R.; Dahlgren, C.; Karlsson, A.; Bylund, J.; Hancock, R.E.W.; Johnson, P. Host defense peptide LL-37 selectively reduces proinflammatory macrophage responses. J. Immunol. 2011, 186, 5497–5505. [Google Scholar] [CrossRef]
- Guha, S.; Ferrie, R.P.; Ghimire, J.; Ventura, C.R.; Wu, E.; Sun, L.; Kim, S.Y.; Wiedman, G.R.; Hristova, K.; Wimley, W.C. Applications and evolution of melittin, the quintessential membrane active peptide. Biochem. Pharmacol. 2021, 193, 114769. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Yang, Z.-R.; Qin, H.; Ma, T.; Tang, J.; Xia, J.; Zhou, Z.; Jiang, H.; Zhu, J. Optimized charge/hydrophobicity balance of antimicrobial peptides against polymicrobial abdominal infections. Macromol. Biosci. 2024, 24, 2300451. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Liu, H.; Zhu, Y.; Zheng, H. Rational design of stapled antimicrobial peptides. Amino Acids 2023, 55, 421–442. [Google Scholar] [CrossRef] [PubMed]





| Compound | Source | Indication | Status | Clinical Trial Identifiers |
|---|---|---|---|---|
| PMX-30063 (4) | Synthetic | Oral mucositis in patients with head and neck cancer | Phase II complete; FDA fast track designation | NCT02324335, NCT01211470 |
| Acute bacterial skin and skin structure infections | Phase II complete; phase III planned; FDA fast track designation | NCT02052388 | ||
| LTX-109 (8) | Synthetic | Atopic dermatitis, skin infection | Phase II complete | NCT01223222, 2010-021438-68 |
| Impetigo | Phase II complete | NCT01803035 | ||
| Nasal infections by methicillin-resistant/ methicillin-sensitive Staphylococcus aureus | Phase I/II complete | NCT01158235, 2010-019254-40 | ||
| LL-37 | Human cathelicidin subunit | Venous leg ulcers | Phase IIb current | 2018-000536-10 [121] |
| Melittin | Honeybee venom | Anti-Gram+ and Gram−, antifungal, anti-MRSA | Phase I current | Not listed [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, M.; Su, Y. Recent Advances in Amphipathic Peptidomimetics as Antimicrobial Agents to Combat Drug Resistance. Molecules 2024, 29, 2492. https://doi.org/10.3390/molecules29112492
Su M, Su Y. Recent Advances in Amphipathic Peptidomimetics as Antimicrobial Agents to Combat Drug Resistance. Molecules. 2024; 29(11):2492. https://doi.org/10.3390/molecules29112492
Chicago/Turabian StyleSu, Ma, and Yongxiang Su. 2024. "Recent Advances in Amphipathic Peptidomimetics as Antimicrobial Agents to Combat Drug Resistance" Molecules 29, no. 11: 2492. https://doi.org/10.3390/molecules29112492
APA StyleSu, M., & Su, Y. (2024). Recent Advances in Amphipathic Peptidomimetics as Antimicrobial Agents to Combat Drug Resistance. Molecules, 29(11), 2492. https://doi.org/10.3390/molecules29112492







