Carbon Dots Derived from Non-Biomass Waste: Methods, Applications, and Future Perspectives
Abstract
1. Introduction
2. Waste Precursors
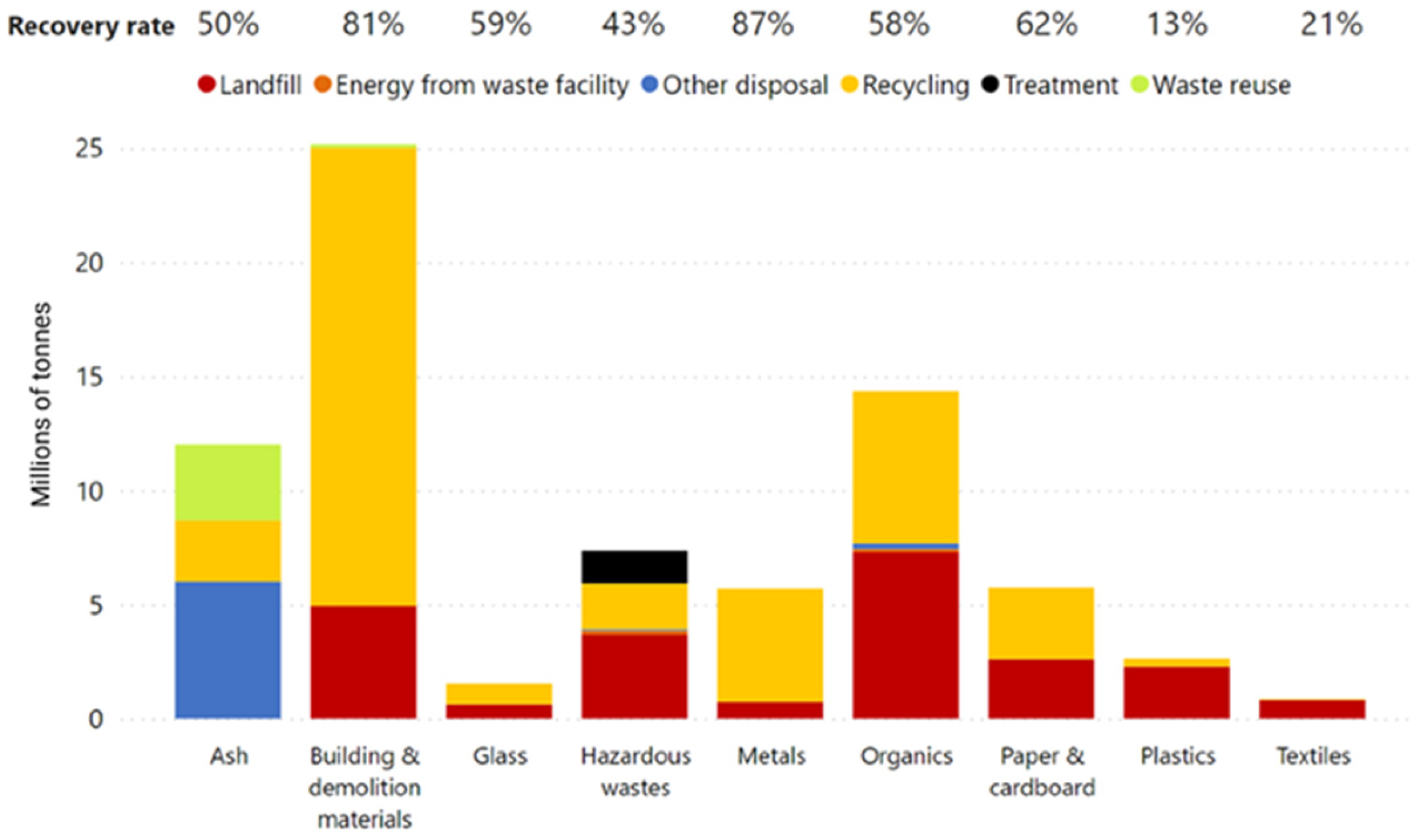
2.1. Ash
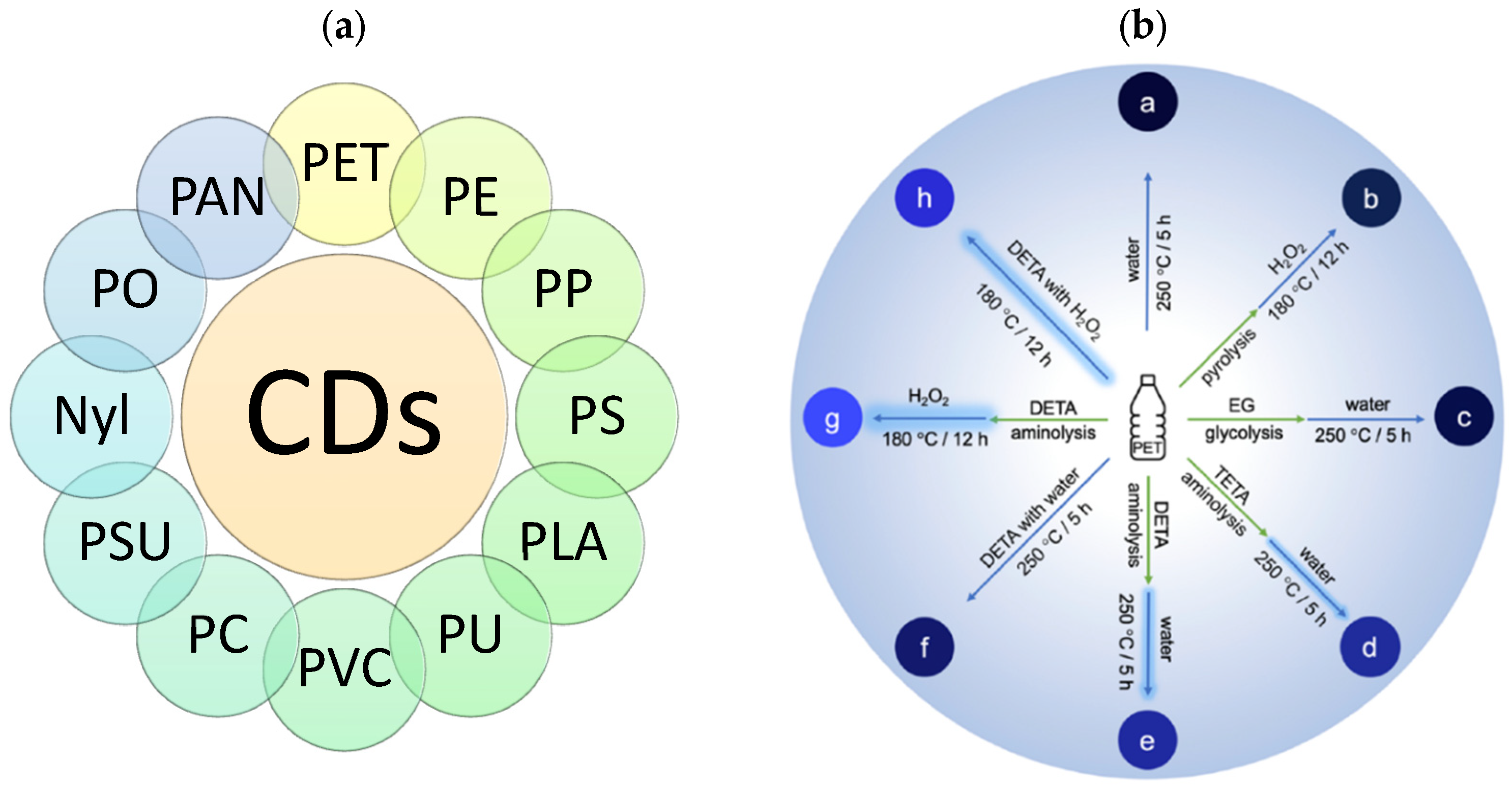
2.2. Waste Plastics
2.3. Waste Textiles and Wastepaper
| Method | Carbon Precursor | Conditions | QY (%) | Size (nm) | Ref. |
|---|---|---|---|---|---|
| Hydrothermal method | Wastepaper | 150–200 °C for 10 h | 10.80 | 150 °C: 4.0–12.0 180 °C: 3.0–7.0 200 °C: 2.0–5.0 | [79] |
| Kraft softwood pulp | 240 °C for 4 h | NA | Diameter: 2–6 Length: 40–60 | [118] | |
| Carbon paper | 180 °C for 8 h | ∼5.1 | 4.8 | [81] | |
| Wastepaper | 210 °C for 12 h | 10–27 | 2.6 to 4.4 | [82] | |
| Wastepaper | 220 °C for 15 h | 20 | 2–4 | [83] | |
| Degrease cotton (Human waste) | 200 °C for 13 h | 10.20 | 2–4 | [84] | |
| Waste PET textiles | 260 °C for 24 h | 97.30 | 1.6–4.6 | [85] | |
| Absorbent cotton | 200 °C for 15 h | NA | 1.4–5.6 | [86] | |
| Terylene waste | 260 °C for 18 h | 49.36 | 2.5–7.0 | [87] | |
| Waste silk cloth | 250 °C for 5 h | 19.10 | 2.2 ± 6.1 | [88] | |
| Eucalyptus fibers | 120 °C, 140 °C, 160 °C, and 180 °C for 24 h | NA | 1.5–4.0 | [89] | |
| Cellulose | 180 °C for 72 h | 21.7 | 4.2 | [119] | |
| Cellulose | 210 °C for 14 h | 32.3 | 5.45 | [120] | |
| Cellulose | 200 °C for 12 h | 2.9–18.3 | 2.11–8.72 | [121] | |
| Microcrystalline Cellulose | 240 °C for 12 h | 54 | 0.5–6.5 | [122] | |
| Burn | Wastepaper | Burn | 9.3 | 2–5 | [80] |
2.4. Other Wastes
3. Applications of CDs Derived from Non-Biomass Waste
3.1. Sensing
3.2. Information Encryption
3.3. LEDs
3.4. Solar Cells
3.5. Plant Growth Promotion
4. Conclusions
- (1)
- Expanding the range of non-biomass waste materials as carbon precursors for CDs synthesis.
- (2)
- Simplifying pre-treatment procedures by reducing the use of toxic chemicals, lowering temperatures, and decreasing pressure.
- (3)
- Exploring methods to enhance the properties of CDs, especially QY.
- (4)
- Developing techniques to synthesize CDs from mixed non-biomass waste sources.
- (5)
- Broadening the scope of CD applications from non-biomass waste.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parmesan, C.; Morecroft, M.D.; Trisurat, Y. Climate Change 2022: Impacts, Adaptation and Vulnerability. Ph.D. Thesis, GIEC, Geneva, Switzerland, 2022. [Google Scholar]
- Gautam, M.; Agrawal, M. Greenhouse gas emissions from municipal solid waste management: A review of global scenario. In Carbon Footprint Case Studies: Municipal Solid Waste Management, Sustainable Road Transport and Carbon Sequestration; Springer: Berlin/Heidelberg, Germany, 2021; pp. 123–160. [Google Scholar]
- Maria, C.; Góis, J.; Leitão, A. Challenges and perspectives of greenhouse gases emissions from municipal solid waste management in Angola. Energy Rep. 2020, 6, 364–369. [Google Scholar] [CrossRef]
- Yu, K.H.; Zhang, Y.; Li, D.; Montenegro-Marin, C.E.; Kumar, P.M. Environmental planning based on reduce, reuse, recycle and recover using artificial intelligence. Environ. Impact Assess. Rev. 2021, 86, 106492. [Google Scholar] [CrossRef]
- Zorpas, A.A. Strategy development in the framework of waste management. Sci. Total Environ. 2020, 716, 137088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham; Das, S. Impacts of plastic pollution on ecosystem services, sustainable development goals, and need to focus on circular economy and policy interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Shams, M.; Alam, I.; Mahbub, M.S. Plastic pollution during COVID-19: Plastic waste directives and its long-term impact on the environment. Environ. Adv. 2021, 5, 100119. [Google Scholar] [CrossRef] [PubMed]
- Moazzem, S.; Wang, L.; Daver, F.; Crossin, E. Environmental impact of discarded apparel landfilling and recycling. Resour. Conserv. Recycl. 2021, 166, 105338. [Google Scholar] [CrossRef]
- Moazzem, S.; Crossin, E.; Daver, F.; Wang, L. Environmental impact of apparel supply chain and textile products. Environ. Dev. Sustain. 2021, 24, 9757–9775. [Google Scholar] [CrossRef]
- Tomaras, J. Waste Management and Recycling. 2021. Available online: https://www.aph.gov.au/About_Parliament/Parliamentary_Departments/Parliamentary_Library/pubs/rp/BudgetReview202021/WasteManagementRecycling (accessed on 25 November 2023).
- Joe Pickin, C.W.; O’Farrell, K.; Stovell, L.; Nyunt, P.; Guazzo, S.; Lin, Y.; Caggiati-Shortell, G.; Chakma, P.; Edwards, C.; Lindley, B.; et al. National Waste Report 2022; The Department of Climate Change, Energy, the Environment and Water: Docklands, VIC, Australia, 2022.
- Tu, L.; Li, Q.; Qiu, S.; Li, M.; Shin, J.; Wu, P.; Singh, N.; Li, J.; Ding, Q.; Hu, C.; et al. Recent developments in carbon dots: A biomedical application perspective. R. Soc. Chem. J. 2023, 11, 338–353. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.-A.; Hien, N.T.; Hoang, N.M.; Dang, H.-L.T.; Van Quy, T.; Hanh, N.T.; Vu, N.H.; Dao, V.-D. Carbon dots in environmental treatment and protection applications. Desalination 2023, 548, 116285. [Google Scholar] [CrossRef]
- Shahraki, H.S.; Ahmad, A.; Bushra, R. Green carbon dots with multifaceted applications—Waste to wealth strategy. FlatChem 2022, 31, 100310. [Google Scholar] [CrossRef]
- Truskewycz, A.; Yin, H.; Halberg, N.; Lai, D.T.; Ball, A.S.; Truong, V.K.; Rybicka, A.M.; Cole, I. Carbon dot therapeutic platforms: Administration, distribution, metabolism, excretion, toxicity, and therapeutic potential. Small 2022, 18, 2106342. [Google Scholar] [CrossRef] [PubMed]
- Houshyar, S.; Yin, H.; Pope, L.; Zizhou, R.; Dekiwadia, C.; Hill-Yardin, E.L.; Yeung, J.M.; John, S.; Fox, K.; Tran, N. Smart suture with iodine contrasting nanoparticles for computed tomography. OpenNano 2023, 9, 100120. [Google Scholar] [CrossRef]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A review of carbon dots produced from biomass wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef] [PubMed]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis—A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Khairol Anuar, N.K.; Tan, H.L.; Lim, Y.P.; So’aib, M.S.; Abu Bakar, N.F. A review on multifunctional carbon-dots synthesized from biomass waste: Design/fabrication, characterization and applications. Front. Energy Res. 2021, 9, 67. [Google Scholar] [CrossRef]
- de Oliveira, B.P.; da Silva Abreu, F.O.M. Carbon quantum dots synthesis from waste and by-products: Perspectives and challenges. Mater. Lett. 2021, 282, 128764. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, M.; Bhandari, B.; Yang, C.-h. Food waste as a carbon source in carbon quantum dots technology and their applications in food safety detection. Trends Food Sci. Technol. 2020, 95, 86–96. [Google Scholar] [CrossRef]
- Lou, Y.; Hao, X.; Liao, L.; Zhang, K.; Chen, S.; Li, Z.; Ou, J.; Qin, A.; Li, Z. Recent advances of biomass carbon dots on syntheses, characterization, luminescence mechanism, and sensing applications. Nano Sel. 2021, 2, 1117–1145. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Lee, Y.R. Sustainable synthesis of multifunctional carbon dots using biomass and their applications: A mini-review. J. Environ. Chem. Eng. 2021, 9, 105802. [Google Scholar] [CrossRef]
- Lin, X.; Xiong, M.; Zhang, J.; He, C.; Ma, X.; Zhang, H.; Kuang, Y.; Yang, M.; Huang, Q. Carbon dots based on natural resources: Synthesis and applications in sensors. Microchem. J. 2021, 160, 105604. [Google Scholar] [CrossRef]
- Wareing, T.C.; Gentile, P.; Phan, A.N. Biomass-based carbon dots: Current development and future perspectives. ACS Nano 2021, 15, 15471–15501. [Google Scholar] [CrossRef] [PubMed]
- Radnia, F.; Mohajeri, N.; Zarghami, N. New insight into the engineering of green carbon dots: Possible applications in emerging cancer theranostics. Talanta 2020, 209, 120547. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.; Lee, N.Y. Green synthesis of carbon quantum dots and their environmental applications. Environ. Res. 2022, 212, 113283. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Hung, Y.-S.; Weng, Y.-M.; Chen, W.; Lai, Y.-S. Sustainable development of carbon nanodots technology: Natural products as a carbon source and applications to food safety. Trends Food Sci. Technol. 2019, 86, 144–152. [Google Scholar] [CrossRef]
- Meng, W.; Bai, X.; Wang, B.; Liu, Z.; Lu, S.; Yang, B. Biomass-derived carbon dots and their applications. Energy Environ. Mater. 2019, 2, 172–192. [Google Scholar] [CrossRef]
- Arpita; Kumar, P.; Kataria, N.; Narwal, N.; Kumar, S.; Kumar, R.; Khoo, K.S.; Show, P.L. Plastic Waste-Derived Carbon Dots: Insights of Recycling Valuable Materials Towards Environmental Sustainability. Curr. Pollut. Rep. 2023, 9, 433–453. [Google Scholar] [CrossRef] [PubMed]
- Hallaji, Z.; Bagheri, Z.; Ranjbar, B. The role of fluorescent carbon dots in the fate of plastic waste. J. Environ. Chem. Eng. 2023, 11, 110322. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Huang, L.; Zheng, G.; Zhang, P.; Jin, Y.; Jiao, Z.; Sun, X. Study on the fluorescence properties of carbon dots prepared via combustion process. J. Lumin. 2019, 206, 608–612. [Google Scholar] [CrossRef]
- Chahal, S.; Macairan, J.-R.; Yousefi, N.; Tufenkji, N.; Naccache, R. Green synthesis of carbon dots and their applications. RSC Adv. 2021, 11, 25354–25363. [Google Scholar] [CrossRef]
- Hu, S.; Trinchi, A.; Atkin, P.; Cole, I. Tunable photoluminescence across the entire visible spectrum from carbon dots excited by white light. Angew. Chem. Int. Ed. 2015, 54, 2970–2974. [Google Scholar] [CrossRef]
- Kolanowska, A.; Dzido, G.; Krzywiecki, M.; Tomczyk, M.M.; Łukowiec, D.; Ruczka, S.; Boncel, S. Carbon quantum dots from amino acids revisited: Survey of renewable precursors toward high quantum-yield blue and green fluorescence. ACS Omega 2022, 7, 41165–41176. [Google Scholar] [CrossRef]
- Zhu, S.; Tang, S.; Zhang, J.; Yang, B. Control the size and surface chemistry of graphene for the rising fluorescent materials. Chem. Commun. 2012, 48, 4527–4539. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Sun, Z.; Zhang, H.; Sun, X.; Jiang, Y.; Bai, Z. The fluorescence mechanism of carbon dots, and methods for tuning their emission color: A review. Microchim. Acta 2019, 186, 583. [Google Scholar] [CrossRef] [PubMed]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, K.H.; Chen, P. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem. C 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Kaushik, J.; Saini, D.; Singh, R.; Dubey, P.; Sonkar, S.K. Surface adhered fluorescent carbon dots extracted from the harmful diesel soot for sensing Fe (III) and Hg (II) ions. New J. Chem. 2021, 45, 20164–20172. [Google Scholar]
- Mondal, N.K.; Singha, P.; Sen, K.; Mondal, A.; Debnath, P.; Mondal, A.; Mishra, D. Waste plastics acts as a good growth promoter: A laboratory-based study. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z. Sewage sludge in microwave oven: A sustainable synthetic approach toward carbon dots for fluorescent sensing of para-Nitrophenol. J. Hazard. Mater. 2020, 382, 121048. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, K.M.; Sonker, A.K.; Sonkar, S.K.; Sarkar, S. Pollutant soot of diesel engine exhaust transformed to carbon dots for multicoloured imaging of E. coli and sensing cholesterol. RSC Adv. 2014, 4, 30100–30107. [Google Scholar] [CrossRef]
- Alluqmani, S.M.; Alabdallah, N.M. Preparation and application of nanostructured carbon from oil fly ash for growth promotion and improvement of agricultural crops with different doses. Sci. Rep. 2022, 12, 17033. [Google Scholar] [CrossRef]
- Alluqmani, S.M.; Loulou, M.; Ouerfelli, J.; Alshahrie, A.; Salah, N. Annealing effect on structural and optical properties of nanostructured carbon of oil fly ash modified titania thin-film. Results Phys. 2021, 25, 104335. [Google Scholar] [CrossRef]
- Chaudhary, P.; Verma, A.; Mishra, A.; Yadav, D.; Pal, K.; Yadav, B.; Kumar, E.R.; Thapa, K.B.; Mishra, S.; Dwivedi, D. Preparation of carbon quantum dots using bike pollutant soot: Evaluation of structural, optical and moisture sensing properties. Phys. E Low-Dimens. Syst. Nanostructures 2022, 139, 115174. [Google Scholar] [CrossRef]
- Ganesan, K.; Hayagreevan, C.; Jeevagan, A.J.; Adinaveen, T.; Sophie, P.L.; Amalraj, M.; Bhuvaneshwari, D. Candle soot derived carbon dots as potential corrosion inhibitor for stainless steel in HCl medium. J. Appl. Electrochem. 2023, 54, 89–102. [Google Scholar] [CrossRef]
- Huang, H.; Cui, Y.; Liu, M.; Chen, J.; Wan, Q.; Wen, Y.; Deng, F.; Zhou, N.; Zhang, X.; Wei, Y. A one-step ultrasonic irradiation assisted strategy for the preparation of polymer-functionalized carbon quantum dots and their biological imaging. J. Colloid Interface Sci. 2018, 532, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, T.; Ma, Y. Nanosized carbon dots from organic matter and biomass. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2016, 31, 823–826. [Google Scholar] [CrossRef]
- Pankaj, A.; Tewari, K.; Singh, S.; Singh, S.P. Waste candle soot derived nitrogen doped carbon dots based fluorescent sensor probe: An efficient and inexpensive route to determine Hg (II) and Fe (III) from water. J. Environ. Chem. Eng. 2018, 6, 5561–5569. [Google Scholar] [CrossRef]
- Song, Y.; Lu, F.; Li, H.; Wang, H.; Zhang, M.; Liu, Y.; Kang, Z. Degradable carbon dots from cigarette smoking with broad-spectrum antimicrobial activities against drug-resistant bacteria. ACS Appl. Bio Mater. 2018, 1, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Thulasi, S.; Kathiravan, A.; Asha Jhonsi, M. Fluorescent carbon dots derived from vehicle exhaust soot and sensing of tartrazine in soft drinks. ACS Omega 2020, 5, 7025–7031. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, S.; Mariadoss, A.J.; Arunkumar, K.; Muthupandian, A. Fuel waste to fluorescent carbon dots and its multifarious applications. Sens. Actuators B Chem. 2019, 282, 972–983. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, H.; Long, Y.; Zhang, L.; Gao, M.; Bai, W. Microwave–hydrothermal synthesis of fluorescent carbon dots from graphite oxide. Carbon 2011, 49, 3134–3140. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, X.; Ruan, H.; Yin, K.; Li, H. Production of yellow-emitting carbon quantum dots from fullerene carbon soot. Sci. China Mater. 2017, 60, 141–150. [Google Scholar] [CrossRef]
- Hong, W.T.; Moon, B.K.; Yang, H.K. Microwave irradiation and color converting film application of carbon dots originated from wasted toner powder. Mater. Res. Bull. 2022, 156, 111999. [Google Scholar] [CrossRef]
- Chan, K.; Zinchenko, A. Aminolysis-assisted hydrothermal conversion of waste PET plastic to N-doped carbon dots with markedly enhanced fluorescence. J. Environ. Chem. Eng. 2022, 10, 107749. [Google Scholar] [CrossRef]
- Wang, R.; Li, S.; Huang, H.; Liu, B.; Gao, L.; Qu, M.; Wei, Y.; Wei, J. Preparation of carbon dots from PET waste by one-step hydrothermal method and its application in light blocking films and LEDs. J. Fluoresc. 2023, 33, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Wang, R.; Zhang, M.; Dong, Z.; Zhang, A.; Qu, M.; Gao, L.; Wei, Y.; Wei, J. Solvothermal preparation of nitrogen-doped carbon dots with PET waste as precursor and their application in LEDs and water detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 289, 122178. [Google Scholar] [CrossRef] [PubMed]
- Lauria, A.; Lizundia, E. Luminescent carbon dots obtained from polymeric waste. J. Clean. Prod. 2020, 262, 121288. [Google Scholar] [CrossRef]
- Hu, Y.; Li, M.; Gao, Z.; Wang, L.; Zhang, J. Waste polyethylene terephthalate derived carbon dots for separable production of 5-hydroxymethylfurfural at low temperature. Catal. Lett. 2021, 151, 2436–2444. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Xu, Y. Nitrogen-doped carbon dots from polystyrene for three analytes sensing and their logic recognition. Inorg. Chem. Commun. 2023, 148, 110369. [Google Scholar] [CrossRef]
- Liang, L.; Wong, S.C.; Lisak, G. Effects of plastic-derived carbon dots on germination and growth of pea (Pisum sativum) via seed nano-priming. Chemosphere 2023, 316, 137868. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, M.; Elbeh, M.; Baban, N.S.; Pereira, L.; Matula, J.; Song, Y.-A.; Ramadi, K.B. High-yield, one-pot upcycling of polyethylene and polypropylene waste into blue-emissive carbon dots. Green Chem. 2023, 25, 1925–1937. [Google Scholar] [CrossRef]
- Kommula, B.; Banoo, M.; Roy, R.S.; Sil, S.; Sah, A.K.; Rawat, B.; Chakraborty, S.; Meena, P.; Kailasam, K.; Gautam, U.K. Landscaping sustainable conversion of waste plastics to carbon dots and enormous diversity in O2 harvesting, hypoxia, autophagy. Carbon 2023, 213, 118304. [Google Scholar] [CrossRef]
- Muro-Hidalgo, J.M.; Bazany-Rodríguez, I.J.; Hernández, J.G.; Pabello, V.M.L.; Thangarasu, P. Histamine Recognition by Carbon Dots from Plastic Waste and Development of Cellular Imaging: Experimental and Theoretical Studies. J. Fluoresc. 2023, 33, 2041–2059. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Chaudhary, G.R.; Chaudhary, S.; Umar, A. Rapid analysis of trace sulphite ion using fluorescent carbon dots produced from single use plastic cups. Eng. Sci. 2021, 17, 101–112. [Google Scholar] [CrossRef]
- Kumari, M.; Chaudhary, S. Modulating the physicochemical and biological properties of carbon dots synthesised from plastic waste for effective sensing of E. coli. Colloids Surf. B Biointerfaces 2020, 196, 111333. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Das, G. Environmentally benign synthesis of fluorescent carbon nanodots using waste PET bottles: Highly selective and sensitive detection of Pb2+ ions in aqueous medium. New J. Chem. 2021, 45, 8747–8754. [Google Scholar] [CrossRef]
- Song, H.; Liu, X.; Wang, B.; Tang, Z.; Lu, S. High production-yield solid-state carbon dots with tunable photoluminescence for white/multi-color light-emitting diodes. Sci. Bull. 2019, 64, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, V.; Siddaiah, B.; Raji, K.; Ramamurthy, P. Green synthesis of multifunctionalized, nitrogen-doped, highly fluorescent carbon dots from waste expanded polystyrene and its application in the fluorimetric detection of Au3+ ions in aqueous media. ACS Sustain. Chem. Eng. 2018, 6, 1627–1638. [Google Scholar] [CrossRef]
- Li, S.; Hu, J.; Aryee, A.A.; Sun, Y.; Li, Z. Three birds, one stone: Disinfecting and turning waste medical masks into valuable carbon dots for sodium hydrosulfite and Fe3+ detection enabled by a simple hydrothermal treatment. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 296, 122659. [Google Scholar] [CrossRef]
- Kumari, A.; Kumar, A.; Sahu, S.K.; Kumar, S. Synthesis of green fluorescent carbon quantum dots using waste polyolefins residue for Cu2+ ion sensing and live cell imaging. Sens. Actuators B Chem. 2018, 254, 197–205. [Google Scholar] [CrossRef]
- Perikala, M.; Bhardwaj, A. Waste to white light: A sustainable method for converting biohazardous waste to broadband white LEDs. RSC Adv. 2022, 12, 11443–11453. [Google Scholar] [CrossRef]
- Cruz, M.I.S.D.; Thongsai, N.; de Luna, M.D.G.; In, I.; Paoprasert, P. Preparation of highly photoluminescent carbon dots from polyurethane: Optimization using response surface methodology and selective detection of silver (I) ion. Colloids Surf. A Physicochem. Eng. Asp. 2019, 568, 184–194. [Google Scholar] [CrossRef]
- Shaw, V.; Mondal, A.; Mondal, A.; Koley, R.; Mondal, N.K. Effective utilization of waste plastics towards sustainable control of mosquito. J. Clean. Prod. 2023, 386, 135826. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z.; Yang, J.; Chen, H.; Han, L. Environmentally benign conversion of waste polyethylene terephthalate to fluorescent carbon dots for “on-off-on” sensing of ferric and pyrophosphate ions. J. Colloid Interface Sci. 2019, 538, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Chen, X.; Li, Q.; Zhang, A.; Ma, G.; Wei, Y.; Qu, M.; Gao, L.; Wei, J. Solvothermal preparation of nitrogen and phosphorus-doped carbon dots with PET waste as precursor and its application. Mater. Today Commun. 2023, 34, 104918. [Google Scholar] [CrossRef]
- Gu, W.; Dong, Z.; Zhang, A.; Ma, T.; Hu, Q.; Wei, J.; Wang, R. Functionalization of PET with carbon dots as copolymerizable flame retardants for the excellent smoke suppressants and mechanical properties. Polym. Degrad. Stab. 2022, 195, 109766. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, X.; Sheng, Y.; Shen, J.; Huang, P.; Guo, S.; Pan, J.; Liu, B.; Feng, B. Simple one-step synthesis of water-soluble fluorescent carbon dots from waste paper. New J. Chem. 2014, 38, 906–909. [Google Scholar] [CrossRef]
- Wei, J.; Shen, J.; Zhang, X.; Guo, S.; Pan, J.; Hou, X.; Zhang, H.; Wang, L.; Feng, B. Simple one-step synthesis of water-soluble fluorescent carbon dots derived from paper ash. RSC Adv. 2013, 3, 13119–13122. [Google Scholar] [CrossRef]
- Devi, S.; Gupta, R.K.; Paul, A.K.; Tyagi, S. Waste carbon paper derivatized Carbon Quantum Dots/(3-Aminopropyl) triethoxysilane based fluorescent probe for trinitrotoluene detection. Mater. Res. Express 2018, 6, 025605. [Google Scholar] [CrossRef]
- Park, S.J.; Park, J.Y.; Chung, J.W.; Yang, H.K.; Moon, B.K.; Yi, S.S. Color tunable carbon quantum dots from wasted paper by different solvents for anti-counterfeiting and fluorescent flexible film. Chem. Eng. J. 2020, 383, 123200. [Google Scholar] [CrossRef]
- Lin, B.; Yan, Y.; Guo, M.; Cao, Y.; Yu, Y.; Zhang, T.; Huang, Y.; Wu, D. Modification-free carbon dots as turn-on fluorescence probe for detection of organophosphorus pesticides. Food Chem. 2018, 245, 1176–1182. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, F.; Li, X.; Wu, H.; Xu, J.; Niu, X.; Pan, J.; Zhang, T.; Yang, D. A facile one-pot synthesis of fluorescent carbon dots from degrease cotton for the selective determination of chromium ions in water and soil samples. J. Lumin. 2017, 188, 230–237. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, G.; Zhang, A.; Gu, W.; Wei, J.; Wang, R. Preparation of carbon dots with ultrahigh fluorescence quantum yield based on PET waste. ACS Omega 2022, 7, 38037–38044. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, S.; Zhang, M.; Zhong, W.; Xiao, Z.; Luo, Y. Modulation doping of absorbent cotton derived carbon dots for quantum dot-sensitized solar cells. Phys. Chem. Chem. Phys. 2019, 21, 26133–26145. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, R.; Xie, W.; Ma, G.; Zhang, A.; Liu, B.; Huang, H.; Gao, L.; Qu, M.; Wei, Y. Solvent-thermal preparation of sulfur and nitrogen-doped carbon dots with PET waste as precursor and application in light-blocking film. J. Nanoparticle Res. 2023, 25, 18. [Google Scholar] [CrossRef]
- Vadivel, R.; Nirmala, M.; Raji, K.; Siddaiah, B.; Ramamurthy, P. Synthesis of highly luminescent carbon dots from postconsumer waste silk cloth and investigation of its electron transfer dynamics with methyl viologen dichloride. J. Indian Chem. Soc. 2021, 98, 100181. [Google Scholar] [CrossRef]
- Chen, X.; Song, Z.; Li, S.; Thang, N.T.; Gao, X.; Gong, X.; Guo, M. Facile one-pot synthesis of self-assembled nitrogen-doped carbon dots/cellulose nanofibril hydrogel with enhanced fluorescence and mechanical properties. Green Chem. 2020, 22, 3296–3308. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, Y.; Zhang, T.; Ma, J.; Li, H.; Zhou, J.; Wang, Z.; Sun, R. Preparation of carbon dots from waste cellulose diacetate as a sensor for tetracycline detection and fluorescence ink. Int. J. Biol. Macromol. 2020, 164, 4289–4298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, Z.; Niu, J.; Du, Z.; Federica, C.; Zhu, Z.; Yang, K.; Li, Y.; Zhao, B.; Pedersen, T.H. Systematical analysis of sludge treatment and disposal technologies for carbon footprint reduction. J. Environ. Sci. 2023, 128, 224–249. [Google Scholar] [CrossRef]
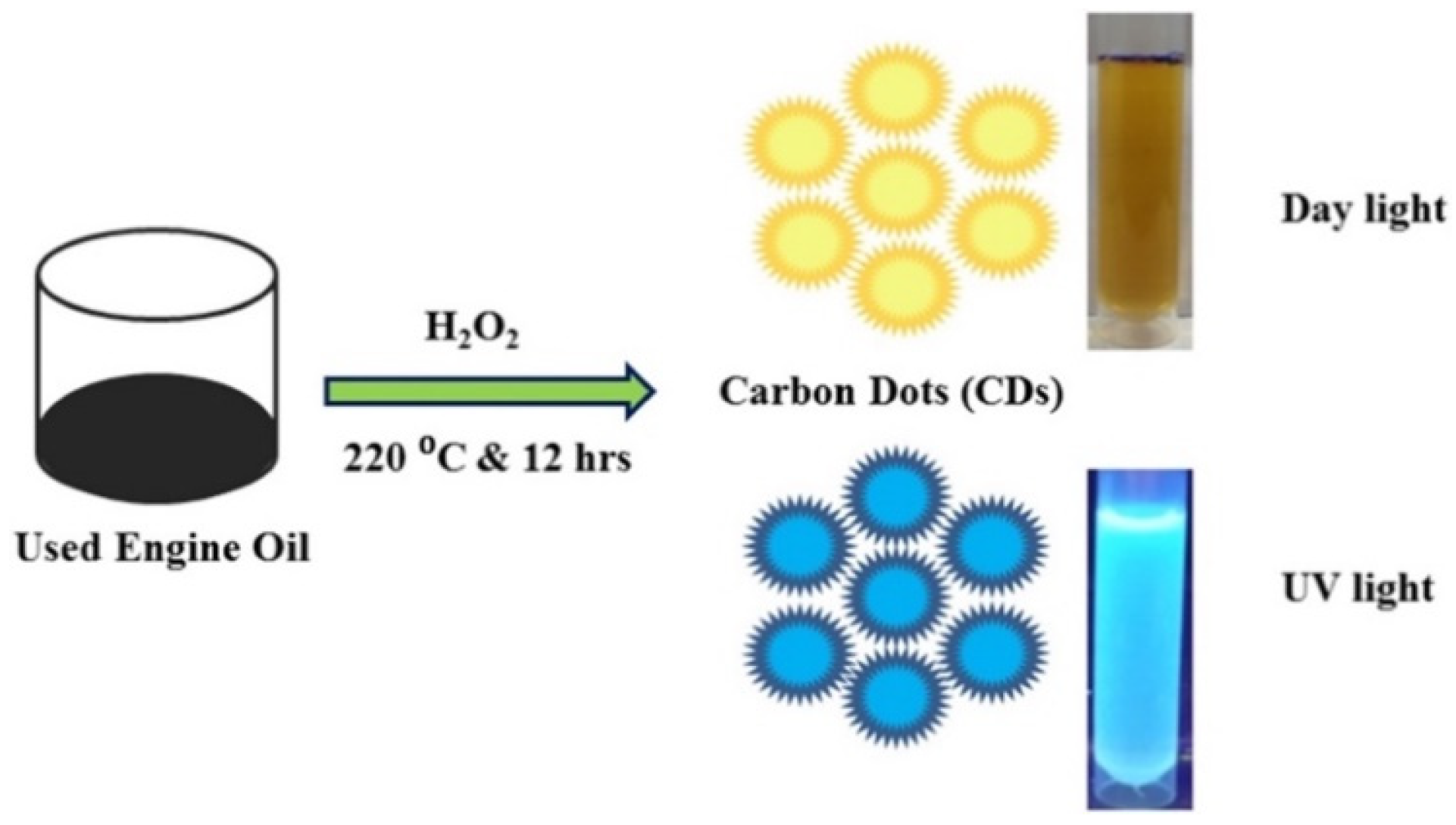
- Kalanidhi, K.; Nagaraaj, P. A green approach for synthesis of highly fluorescent carbon dots from waste engine oil: A strategy for waste to value added products. Diam. Relat. Mater. 2022, 121, 108724. [Google Scholar]
- Zhang, Y.; Wang, L.; Chen, L.; Ma, B.; Zhang, Y.; Ni, W.; Tsang, D.C. Treatment of municipal solid waste incineration fly ash: State-of-the-art technologies and future perspectives. J. Hazard. Mater. 2021, 411, 125132. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Gao, S.; Geng, Y.; Wu, C. A study on the chemical state of carbon present in fine ash from gasification. Asia-Pac. J. Chem. Eng. 2019, 14, e2336. [Google Scholar] [CrossRef]
- Das, T.; Saikia, B.K.; Dekaboruah, H.; Bordoloi, M.; Neog, D.; Bora, J.J.; Lahkar, J.; Narzary, B.; Roy, S.; Ramaiah, D. Blue-fluorescent and biocompatible carbon dots derived from abundant low-quality coals. J. Photochem. Photobiol. B Biol. 2019, 195, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.M.; Chirayil, G.T. Facile synthesis of preformed mixed nano-carbon structure from low rank coal. Manag. Syst. Prod. Eng. 2018, 36, 14–20. [Google Scholar]
- Saikia, M.; Hower, J.C.; Das, T.; Dutta, T.; Saikia, B.K. Feasibility study of preparation of carbon quantum dots from Pennsylvania anthracite and Kentucky bituminous coals. Fuel 2019, 243, 433–440. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Meng, F.; Wang, B.; Cheng, Y.; Zhu, C. N-doped carbon dots synthesized by rapid microwave irradiation as highly fluorescent probes for Pb2+ detection. New J. Chem. 2015, 39, 3357–3360. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, Y.; Gao, X.; Cai, C.; Lin, H. Facile, quick, and gram-scale synthesis of ultralong-lifetime room-temperature-phosphorescent carbon dots by microwave irradiation. Angew. Chem. Int. Ed. 2018, 57, 6216–6220. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros, T.V.; Manioudakis, J.; Noun, F.; Macairan, J.-R.; Victoria, F.; Naccache, R. Microwave-assisted synthesis of carbon dots and their applications. J. Mater. Chem. C 2019, 7, 7175–7195. [Google Scholar] [CrossRef]
- Marinello, S.; Lolli, F.; Gamberini, R. Roadway tunnels: A critical review of air pollutant concentrations and vehicular emissions. Transp. Res. Part D Transp. Environ. 2020, 86, 102478. [Google Scholar] [CrossRef]
- Kumar, P.G.; Lekhana, P.; Tejaswi, M.; Chandrakala, S. Effects of vehicular emissions on the urban environment-a state of the art. Mater. Today Proc. 2021, 45, 6314–6320. [Google Scholar] [CrossRef]
- Gu, M.; Pan, Y.; Walters, W.W.; Sun, Q.; Song, L.; Wang, Y.; Xue, Y.; Fang, Y. Vehicular emissions enhanced ammonia concentrations in winter mornings: Insights from diurnal nitrogen isotopic signatures. Environ. Sci. Technol. 2022, 56, 1578–1585. [Google Scholar] [CrossRef]
- Milku Augustine, K.; Attiogbe, F.; Derkyi, N.; Atepor, L. A Review of Policies and Legislations of Vehicular Exhaust Emissions in Ghana and Their Enforcement. Aerosol Sci. Eng. 2023, 7, 169–181. [Google Scholar] [CrossRef]
- Gulia, S.; Tiwari, R.; Mendiratta, S.; Kaur, S.; Goyal, S.; Kumar, R. Review of scientific technology-based solutions for vehicular pollution control. Clean Technol. Environ. Policy 2020, 22, 1955–1966. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Frontiers in carbon dots: Design, properties and applications. Mater. Chem. Front. 2019, 3, 2571–2601. [Google Scholar] [CrossRef]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Gu, J.-D. Biodegradability of plastics: The issues, recent advances, and future perspectives. Environ. Sci. Pollut. Res. 2021, 28, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Sharuddin, S.D.A.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on pyrolysis of plastic wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Maqsood, T.; Dai, J.; Zhang, Y.; Guang, M.; Li, B. Pyrolysis of plastic species: A review of resources and products. J. Anal. Appl. Pyrolysis 2021, 159, 105295. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Kitano, K.; Takeda, S.; Tsurue, T. Influence of mineral and chemical composition of coal ashes on their fusibility. Fuel Process. Technol. 1995, 45, 27–51. [Google Scholar] [CrossRef]
- Naik, V.M.; Bhosale, S.V.; Kolekar, G.B. A brief review on the synthesis, characterisation and analytical applications of nitrogen doped carbon dots. Anal. Methods 2022, 14, 877–891. [Google Scholar] [CrossRef] [PubMed]
- Dhenadhayalan, N.; Lin, K.C.; Saleh, T.A. Recent advances in functionalized carbon dots toward the design of efficient materials for sensing and catalysis applications. Small 2020, 16, 1905767. [Google Scholar] [CrossRef]
- National Clothing Product Stewardship Schemes. Available online: https://ausfashioncouncil.com/wp-content/uploads/2023/05/AFC-NCPSS-Data-Report.pdf (accessed on 30 November 2023).
- Wang, C.; Xia, K.; Zhang, Y.; Kaplan, D.L. Silk-based advanced materials for soft electronics. Acc. Chem. Res. 2019, 52, 2916–2927. [Google Scholar] [CrossRef]
- He, H.; Zhang, Y.; Wang, P.; Hu, D. Preparation of sponge-cake-like N-doped porous carbon materials derived from silk fibroin by chemical activation. Microporous Mesoporous Mater. 2021, 317, 110998. [Google Scholar] [CrossRef]
- Munusamy, S.; Mandlimath, T.R.; Swetha, P.; Al-Sehemi, A.G.; Pannipara, M.; Koppala, S.; Shanmugam, P.; Boonyuen, S.; Pothu, R.; Boddula, R. Nitrogen-doped carbon dots: Recent developments in its fluorescent sensor applications. Environ. Res. 2023, 231, 116046. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Li, Y.; Ma, C.; Huang, Z.; Wang, C.; Li, J.; Chen, Z.; Liu, S. One-step hydrothermal synthesis of fluorescent nanocrystalline cellulose/carbon dot hydrogels. Carbohydr. Polym. 2017, 175, 7–17. [Google Scholar] [CrossRef]
- Shen, P.; Gao, J.; Cong, J.; Liu, Z.; Li, C.; Yao, J. Synthesis of cellulose-based carbon dots for bioimaging. ChemistrySelect 2016, 1, 1314–1317. [Google Scholar] [CrossRef]
- Liao, X.; Chen, C.; Zhou, R.; Huang, Q.; Liang, Q.; Huang, Z.; Zhang, Y.; Hu, H.; Liang, Y. Comparison of N-doped carbon dots synthesized from the main components of plants including cellulose, lignin, and xylose: Characterized, fluorescence mechanism, and potential applications. Dye Pigment. 2020, 183, 108725. [Google Scholar] [CrossRef]
- Huang, H.; Ge, H.; Ren, Z.; Huang, Z.; Xu, M.; Wang, X. Controllable synthesis of biocompatible fluorescent carbon dots from cellulose hydrogel for the specific detection of Hg2+. Front. Bioeng. Biotechnol. 2021, 9, 617097. [Google Scholar] [CrossRef]
- Wu, P.; Li, W.; Wu, Q.; Liu, Y.; Liu, S. Hydrothermal synthesis of nitrogen-doped carbon quantum dots from microcrystalline cellulose for the detection of Fe3+ ions in an acidic environment. RSC Adv. 2017, 7, 44144–44153. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Yan, M.; Ma, J.; Wang, J.; Liu, C.; Bao, Y.; Jin, H.; Fan, Q. Turning waste into treasure: Multicolor carbon dots synthesized from waste leather scrap and their application in anti-counterfeiting. ACS Sustain. Chem. Eng. 2023, 11, 5082–5092. [Google Scholar] [CrossRef]
- Craciun, A.; Diac, A.; Focsan, M.; Socaci, C.; Magyari, K.; Maniu, D.; Mihalache, I.; Veca, L.; Astilean, S.; Terec, A. Surface passivation of carbon nanoparticles with p-phenylenediamine towards photoluminescent carbon dots. RSC Adv. 2016, 6, 56944–56951. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, M.; Sharma, V.K. Nitrogen-doped graphene and graphene quantum dots: A review onsynthesis and applications in energy, sensors and environment. Adv. Colloid Interface Sci. 2018, 259, 44–64. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, C.; Sui, L.; Ran, G.; Song, Q. Phosphoric acid densified red emissive carbon dots with a well-defined structure and narrow band fluorescence for intracellular reactive oxygen species detection and scavenging. J. Mater. Chem. C 2023, 11, 2984–2994. [Google Scholar] [CrossRef]
- Goswami, J.; Rohman, S.S.; Guha, A.K.; Basyach, P.; Sonowal, K.; Borah, S.P.; Saikia, L.; Hazarika, P. Phosphoric acid assisted synthesis of fluorescent carbon dots from waste biomass for detection of Cr (VI) in aqueous media. Mater. Chem. Phys. 2022, 286, 126133. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, B.; Zhang, X.; Guo, L.; Zhang, S. Synthesized carbon dots with high N and S content as excellent corrosion inhibitors for copper in sulfuric acid solution. J. Mol. Liq. 2021, 338, 116702. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Tan, B.; Guo, L.; Li, H. Solvothermal synthesis of functionalized carbon dots from amino acid as an eco-friendly corrosion inhibitor for copper in sulfuric acid solution. J. Colloid Interface Sci. 2021, 604, 1–14. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, D.; Feng, P.; Hao, B.; Guo, Y.; Wang, S. Municipal sewage sludge incineration and its air pollution control. J. Clean. Prod. 2021, 295, 126456. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; dos Santos Lins, P.V.; de Magalhães Oliveira, L.M.; Sepulveda, P.; Ighalo, J.O.; Rajapaksha, A.U.; Meili, L. Sewage sludge-derived biochar for the adsorptive removal of wastewater pollutants: A critical review. Environ. Pollut. 2022, 293, 118581. [Google Scholar] [CrossRef]
- Chen, X.; Jeyaseelan, S.; Graham, N. Physical and chemical properties study of the activated carbon made from sewage sludge. Waste Manag. 2002, 22, 755–760. [Google Scholar] [CrossRef]
- Ding, A.; Zhang, R.; Ngo, H.H.; He, X.; Ma, J.; Nan, J.; Li, G. Life cycle assessment of sewage sludge treatment and disposal based on nutrient and energy recovery: A review. Sci. Total Environ. 2021, 769, 144451. [Google Scholar] [CrossRef]
- Zu, F.; Yan, F.; Bai, Z.; Xu, J.; Wang, Y.; Huang, Y.; Zhou, X. The quenching of the fluorescence of carbon dots: A review on mechanisms and applications. Microchim. Acta 2017, 184, 1899–1914. [Google Scholar] [CrossRef]
- Bhat, S.A.; Hassan, T.; Majid, S. Heavy metal toxicity and their harmful effects on living organisms–a review. Int. J. Med. Sci. Diagn. Res. 2019, 3, 106–122. [Google Scholar]
- Wu, Y.; Chen, X.; Wu, W. Multiple Stimuli-Response Polychromatic Carbon Dots for Advanced Information Encryption and Safety. Small 2023, 19, 2206709. [Google Scholar] [CrossRef]
- Ji, C.; Xu, W.; Han, Q.; Zhao, T.; Deng, J.; Peng, Z. Light of Carbon: Recent Advancements of Carbon Dots for LEDs. Nano Energy 2023, 114, 108623. [Google Scholar] [CrossRef]
- Olabi, A.; Abdelkareem, M.A. Renewable energy and climate change. Renew. Sustain. Energy Rev. 2022, 158, 112111. [Google Scholar] [CrossRef]
- Hu, J.; Jia, W.; Wu, X.; Zhang, H.; Wang, Y.; Liu, J.; Yang, Y.; Tao, S.; Wang, X. Carbon dots can strongly promote photosynthesis in lettuce (Lactuca sativa L.). Environ. Sci. Nano 2022, 9, 1530–1540. [Google Scholar] [CrossRef]
- Guo, B.; Liu, G.; Wei, H.; Qiu, J.; Zhuang, J.; Zhang, X.; Zheng, M.; Li, W.; Zhang, H.; Hu, C. The role of fluorescent carbon dots in crops: Mechanism and applications. SmartMat 2022, 3, 208–225. [Google Scholar] [CrossRef]


| Title | Foci | Ref. |
|---|---|---|
| A Review of Carbon Dots Produced from Biomass Wastes |
| [17] |
| Recent Trends in the use of Green Sources for Carbon Dot Synthesis—A short review | Synthesis of CDs from green sources including biomass waste and non-biomass waste. | [18] |
| A Review on Multifunctional Carbon-Dots Synthesized from Biomass Waste: Design/Fabrication, Characterization and Applications |
| [19] |
| Carbon Quantum Dots Synthesis from Waste and By-Products: Perspectives and Challenges | Potentials, advantages, and challenges in synthesizing CDs from waste after comparing the quantum yield. | [20] |
| Food Waste as a Carbon Source in Carbon Quantum Dots Technology and their Applications in Food Safety Detection |
| [21] |
| Green Carbon Dots with Multifaceted Applications—Waste to Wealth Strategy | Synthesis routes, fluorescent properties and mechanisms, and applications of CDs from wastes with a focus on hydrothermal approach. | [14] |
| Recent Advances of Biomass Carbon Dots on Syntheses, Characterization, Luminescence Mechanism, and Sensing Applications |
| [22] |
| Sustainable Synthesis of Multifunctional Carbon Dots using Biomass and their Applications: A mini review |
| [23] |
| Carbon Dots based on Natural Resources: Synthesis and Applications in Sensors | Synthesis of CDs from biomass resources and their sensing applications. | [24] |
| Biomass-Based Carbon Dots: Current Development and Future Perspectives | Advantages and disadvantages on synthesis, properties, and applications of CDs from biomass waste and chemicals. | [25] |
| New Insight into the Engineering of Green Carbon Dots: Possible Applications in Emerging Cancer Theragnostic | Synthesis, physicochemical properties, and possible applications of CDs from natural sources. | [26] |
| Green Synthesis of Carbon Quantum Dots and their Environmental Applications |
| [27] |
| Sustainable Development of Carbon Nanodots Technology: Natural Products as a Carbon Source and Applications to Food Safety |
| [28] |
| Biomass-Derived Carbon Dots and Their Applications |
| [29] |
| Plastic Waste-Derived Carbon Dots: Insights of Recycling Valuable Materials Towards Environmental Sustainability | Synthesis routes, characterizations, and potential applications of CDs from plastic waste. | [30] |
| The Role of Fluorescent Carbon Dots in the Fate of Plastic Waste |
| [31] |
| Method | Carbon Precursor | Conditions | Size (nm) | QY (%) | Ref. |
|---|---|---|---|---|---|
| Ball Milling | Oil fly ash | 25 Hz and 400 rpm for 45 h in the air | <35 | NA | [43] |
| Oil fly ash | 25 Hz for 45 h in acetic acid | <100 | NA | [44] | |
| Burn | Cigarette Smoking | In the air | 4.5–7.0 | NA | [50] |
| Ethanol, n-Butanol, Domestic candle, and Benzene | In the air | 2.0–4.0 | NA | [32] | |
| Chemical Oxidation | Pollutant diesel soot | 10 h | 20–50 | 1.9 | [42] |
| Vehicle exhaust waste soot | 100 °C for 12 h | 2.2−4.6 | 3 | [51] | |
| Candle soot | 140 °C for 12 h | 112 | 0.5 | [53] | |
| Waste candle soot | 110 °C for 6 h | 2.0–5.0 | NA | [46] | |
| Fullerene carbon soot | 80–120 °C for 12–36 h | 2.0–3.0 | 3–5 | [54] | |
| Kerosene fuel soot | 100 °C for 12 h | 1.0–7.0 | NA | [52] | |
| Candle soot | 80 °C for 6 h | 2.0–5.0 | NA | [49] | |
| Candles | 20 h | 10–45 | NA | [48] | |
| Coal-T20 | Ice-cold condition for 6 h | 2.0–5.0 | 3 | [95] | |
| Coal-NK | Ice-cold condition for 6 h | 10–30 | 4 | [95] | |
| Coal- T60 | Ice-cold condition for 6 h | 1.0–6.0 | 8 | [95] | |
| Coal-NG | Ice-cold condition for 6 h | 1.0–4.0 | 14 | [95] | |
| Gondwana coal, Damodar Coal, Tertiary Indian coal | 2 h | 4.8–14.0 | NA | [96] | |
| Pennsylvania anthracite, and Kentucky bituminous coals | Ice-cold condition for 5–6 h | 2.0–12.0 | 4–53 | [97] | |
| Soxhlet-Purification | Diesel soot | In acetone | 20–30 | ~8 | [39] |
| Hydrothermal Method | Bike Pollutant Soot | 160 °C for 10 h | 1–10 | NA | [45] |
| Microwave pyrolysis | Red toner powder | 350 W for 30 s | 1–4 | 9.2 for internal and 8.4 for external efficiency | [55] |
| Method | Carbon Precursor | Conditions | QY (%) | Size (nm) | Ref. |
|---|---|---|---|---|---|
| Hydrothermal approach | Waste PET bottles | 180 °C for 12 h in diethylenetriamine (DETA) with H2O2 | 9.1 | 3.9–12.9 | [56] |
| Waste PET bottles | 260 °C for 12 h in ammonia water | 87.36 | 1.1–3.1 | [57] | |
| Waste PET bottles | 260 °C for 36 h | 48.16 | 1.6–2.9 | [58] | |
| PLA polymeric waste | 240 °C for 4 h in ultrapure water | NA | 2.99 ± 0.57 | [59] | |
| PS plastics | 180 °C for 8 h with HNO3 and ethylenediamine | NA | 2.66–5.18 | [61] | |
| Waste PET bottles | 110 °C for 15 h in H2O2 | NA | 1.3–4.0 | [62] | |
| PE plastic bags PP surgical masks | 180 °C for 12 h in HNO3 | 14 16 | 1.0–8.0 | [63] | |
| Waste PET bottles | 200 °C for 8 h in deionized water | 31.81 | 3.0–10.0 | [65] | |
| Plastic polybags Cups Bottles | 300 °C for 2 h of thermal calcination and 200 °C for 5 h of hydrothermal treatment in deionized water | 60–69 | NA | [67] | |
| Waste PET bottles | 350 °C for 2 h in air and hydrothermal treatment at 180 °C for 12 h in H2O2 solution. | 5.2 | 3.0–10.0 | [68] | |
| Waste expanded PS Foam | 200 °C for 5 h in HNO3 | W-CDs *: 5.2, Y-CDs *: 3.4% O-CDs *: 3.1% | W-CDs *: 4.5, Y-CDs *: 3.5, O-CDs *: 2.3 | [69] | |
| Waste medical masks | 200 °C for 10 h in deionized water | NA | 1.0–6.0 | [71] | |
| Waste polyolefins | 120 °C for 12 h in HNO3 and H2SO4 | 4.84 | 1.5–3.5 | [72] | |
| Waste PET bottles | 180 °C for 12 h in H2O2 | 5.2 | 3.0–10.0 | [76] | |
| Waste PET bottles | 260 °C for 24 h | 14.2 | 1.8–4.6 | [77] | |
| Pyrolysis method | Waste plastic cups | 350 °C for 2 h | 59 | <10 | [66] |
| Waste PET bottles | 800 °C for 1h | NA | 2000–8000 | [40] | |
| PU foam | 200, 250, and 300 °C for 2, 4, and 6 h | 33 | 5.0–8.0 | [74] | |
| HDPE/LDPE, PET, PS, PVC, PP | 800 °C for 1 h | NA | NA | [75] |
| Carbon Precursor | Pre-Treatment | Method | Analyte | Limits of Detection (LOD) | Linear Range | Ref. |
|---|---|---|---|---|---|---|
| Harmful diesel soot | Magnetically purified | Soxhlet-purification with acetone | Fe3+ and Hg2+ | Fe3+: ~352 nM Hg2+: ~898 nM | NA | [39] |
| Candle soot | HNO3 and ethanol treatment | Stirring at 80 °C for 6 h with ethylene diamine and sodium lauryl sulphate (SDS) | Hg2+ and Fe3+ | Fe3+: 10 nM Hg2+: 50 nM | Fe3+: 20–50 μM Hg2+: 20–50 μM | [49] |
| PS plastics | Nitration | Solvothermal treatment at 180 °C for 8 h | Hg2+, Fe3+, and GSH | NA | Fe3+: 0.25–10 μM Hg2+: 0.5–20 μM GSH: 1–50 μM | [61] |
| Waste medical masks | NA | Hydrothermal treatment at 200 °C for 10 h | Na2S2O4 and Fe3+ | Na2S2O4: 19.44 μM Fe3+: 0.11 μM | Na2S2O4: 0.1–5 mM Fe3+: 1–300 μM | [71] |
| Waste engine oil | Filtration process by filter paper | Hydrothermal treatment at 200 °C for 12 h | Fe3+ | 0.055 μM | 0.6–3.3 μM | [92] |
| Waste PET bottles | Pyrolysis at 350 °C for 2 h in air | Hydrothermal treatment at 180 °C for 12 h | Fe3+ and pyrophosphate ions | Fe3+: 0.21 μM pyrophosphate: 0.86 μM | Fe3+: 0.5–400 μM pyrophosphate: 2–600 μM | [76] |
| Waste PET bottles | Shredding and air oxidation at 350 °C for 2 h. | Hydrothermal treatment at 170 °C for 8 h | Pb2+ | 21 nM | 0–2 μM | [68] |
| Waste expanded PS | NA | One-step solvothermal method at 150 °C for 8 h | Au3+ | 53 nM | 0–18 μM | [70] |
| White PU foam | Crushed | Pyrolysis at 200, 250, and 300 °C for 2, 4, and 6 h in H2SO4 | Ag+ | 2.8 μM | NA | [74] |
| Waste PO | Pyrolysis by ultrasonic and chemical oxidation approach at 700 W for 2 h. | Hydrothermal method at 120 °C for 12 h | Cu2+ | 6.33 nM | 1–8.0 μM | [72] |
| Degrease cotton | NA | One-pot hydrothermal method at 200 °C for 13 h | Cr4+ | 0.12 μg/mL | 1–6 mmol/L | [84] |
| Waste plastic cups | NA | Simple thermal calcination at 350 °C for 2 h | Sulphite anion | 0.34 μM | 0.001–50 μm | [66] |
| Sewage sludge | Dried and grounded into fine powder | Microwave-assisted heating with 700 W for 30 min. | Para-Nitrophenol | 0.069 μM | 0.2–20 μM | [41] |
| Cigarette filters | Cut and dried in an oven at 80 °C for 1 h | One-pot hydrothermal method at 240 °C for 15 h | Tetracycline | 0.06 μM | 0–80 μM | [90] |
| Carbon paper | Burn | Hydrothermal route at 180 °C for 8 h | Trinitrotoluene | 32.7 nM | 4.4 nM–26.4 μM | [81] |
| Vehicle exhaust waste soot | NA | One-pot acid reflexion method with nitric acid at 100 °C for 12 h | Tartrazine | 26 nM | 0.1 to 0.5 μM | [51] |
| Wastepaper | NA | Hydrothermal method at 220 °C for 15 h | Organophosphorus pesticides | 3 ng/mL | 0.01–1.0 μg/mL | [83] |
| PET waste bottles | Microwave alcoholysis with 540 W for 20 min followed by crushing into powder | Solvothermal method at 260 °C for 36 h | Water in organic solvent | 0.00001% | NA | [58] |
| Pollutant diesel soot | Purified via Soxhlet extraction method with different organic solvents | Chemical oxidation method refluxed 10 h | Cholesterol and E. coli | NA | NA | [42] |
| Single-use plastic waste such as plastic polybags, cups, and bottles | Calcination at 300 °C for 2 h. | Hydrothermal treatment at 200 °C for 5 h, | E. coli | 108 CFU/mL | NA | [67] |
| Waste PET bottles | Microwave alcoholysis with 540 W for 20 min followed by crashing into powder | Solvothermal method at 260 °C for 24 h | pH | NA | NA | [77] |
| Bike pollutant soot | Ground for 1 h and sieved using 15 mm sieving paper | Hydrothermal treatment at 160 °C for 10 h | Humidity | NA | NA | [45] |
| Carbon Precursor | Method | Emission Peak (nm) | Light Color | Size (nm) | QY (%) | Ref. |
|---|---|---|---|---|---|---|
| Waste PET bottles | Hydrothermal | 485 | Colorless to brown | 2.0 | 87.36 | [57] |
| Waste PET bottles | Solvothermal | 360 470 | Yellow light warm light | 2.3 | 48.16 | [58] |
| Waste expanded PS | Solvothermal | 470 530 630 | White Yellow Orange | 4.5 3.5 2.3 | 5.2 3.4 3.1 | [69] |
| PPE plastic waste, used disposable gloves, face shields, syringes, and food storage containers and bottles | Pyrolytic | 436 495 | White light | NA | 41 | [73] |
| Waste PET bottles | Solvothermal | 460 | White light | 2.8 | 14.2 | [77] |
| Waste PET textiles | Hydrothermal | 485 | Blue-green light | 2.8 | 97.3 | [85] |
| Wasted toner powder | Microwave irradiation | 557 | Yellow light | 2.1 | 9.2 for internal and 8.4 for external efficiency | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Yin, H.; Cole, I.; Houshyar, S.; Wang, L. Carbon Dots Derived from Non-Biomass Waste: Methods, Applications, and Future Perspectives. Molecules 2024, 29, 2441. https://doi.org/10.3390/molecules29112441
Chen W, Yin H, Cole I, Houshyar S, Wang L. Carbon Dots Derived from Non-Biomass Waste: Methods, Applications, and Future Perspectives. Molecules. 2024; 29(11):2441. https://doi.org/10.3390/molecules29112441
Chicago/Turabian StyleChen, Wenjing, Hong Yin, Ivan Cole, Shadi Houshyar, and Lijing Wang. 2024. "Carbon Dots Derived from Non-Biomass Waste: Methods, Applications, and Future Perspectives" Molecules 29, no. 11: 2441. https://doi.org/10.3390/molecules29112441
APA StyleChen, W., Yin, H., Cole, I., Houshyar, S., & Wang, L. (2024). Carbon Dots Derived from Non-Biomass Waste: Methods, Applications, and Future Perspectives. Molecules, 29(11), 2441. https://doi.org/10.3390/molecules29112441







