Conyza canadensis from Jordan: Phytochemical Profiling, Antioxidant, and Antimicrobial Activity Evaluation
Abstract
1. Introduction
2. Results
2.1. GC/MS Analysis
2.2. Total Phenolics Content (TPC), Total Flavonoids Content (TFC), DPPH Radical Scavenging Activity, and Iron Chelating Activity
2.3. LC-MS/MS Analysis for Phenolic Compounds and Flavonoids
2.4. Antimicrobial Activity, Minimum Inhibitory Concentration (MIC), and Minimum Bactericidal Concentration (MBC) Determination
3. Discussion
3.1. CCHD-EOs Data Analysis
3.2. TPC, TFC, DPPH Scavenging and Iron Chelating Activity for CCM Extract
3.3. LC-MS/MS Analysis for Phenolic Compounds and Flavonoids
3.4. Antimicrobial Assay
4. Materials and Methods
4.1. Plant Material
4.2. Hydro-Distillation and Extraction of Essential Oils
4.3. GC-MS Analysis
4.4. Preparation of the Alcoholic Extract
4.5. Total Phenolic Content (TPC) and Total Flavonoids Content (TFC)
4.6. DPPH Free Radical Scavenging Activity
4.7. Iron Chelating Activity
4.8. LC-MS/MS Analysis of the CCM
4.9. Strains, Media and Materials for Antimicrobial Activity
4.10. Antifungal and Antibacterial Activity
4.11. Assay for MIC and MBC
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doğan, M.N.; Kaya-Altop, E.; Türkseven, S.G.; Serim, A.T. Determination of glyphosate-resistant Conyza spp. in orchards and vineyards in Turkey. Phytoparasitica 2022, 50, 567–578. [Google Scholar] [CrossRef]
- Qasem, J.R.; Alfalahi, A.O.; Alsubeie, M.S.; Almehemdi, A.F.; Synowiec, A. Genetic variations among Fleabane (Conyza bonariensis (L.) Cronquist) populations in Jordan and their susceptibility levels to contact herbicides. Agriculture 2023, 13, 435. [Google Scholar] [CrossRef]
- Mendes, R.R.; Takano, H.K.; Gonçalves Netto, A.; Picoli Junior, G.J.; Cavenaghi, A.L.; Silva, V.F.V.; Nicolai, M.; Christoffoleti, P.J.; Oliveira Junior, R.S.D.; Melo, M.S.C.D.; et al. Monitoring glyphosate- and chlorimuron- resistant Conyza spp. populations in Brazil. An. Acad. Bras. Ciências 2021, 93, e20190425. [Google Scholar] [CrossRef] [PubMed]
- Veres, K.; Csupor-Löffler, B.; Lázár, A.; Hohmann, J. Antifungal activity and composition of essential oils of Conyza canadensis herbs and roots. Sci. World J. 2012, 2012, 489646. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.; Küçükboyaci, N.; Demirci, B. Chemical composition and antimicrobial activity of the essential oil of Conyza canadensis (L.) Cronquist from Turkey. J. Essent. Oil Res. 2017, 29, 336–343. [Google Scholar] [CrossRef]
- Sharma, R.K.; Verma, N.; Jha, K.K.; Singh, N.K.; Kumar, B. Phytochemistry, pharmacological activity, traditional & medicinal uses of Erigeron species: A review. IJARI 2014, 2, 379–383. [Google Scholar]
- Mrabti, H.N.; Doudach, L.; Mekkaoui, M.; Khalil, Z.; Harraqui, K.; Fozia, F.; Mrabti, N.N.; El-Shazly, M.; Alotaibi, A.; Ullah, R.; et al. Profile of medicinal plants traditionally used for the treatment of skin burns. Evid. Based Complement. Altern. Med. 2022, 2022, 3436665. [Google Scholar] [CrossRef]
- Lateef, R.; Bhat, K.A.; Chandra, S.; Banday, G.A. Chemical composition, antimicrobial and antioxidant activities of the essential oil of Conyza canadensis growing wild in Kashmir valley. Am. J. Essent. Oils Nat. Prod. 2018, 6, 35–41. [Google Scholar]
- Aragão, L.W.; Fernandes, S.S.L.; Mallmann, V.; Facco, J.T.; Matos, M.d.F.C.; Cabral, M.R.P.; de Carvalho, J.E.; Ruiz, A.L.T.G.; Cardoso, C.A.L.; da Silva, R.C.d.L.; et al. Chemical composition and evaluation of antitumoral activity of leaf and root essential oils of Conyza canadensis (Asteraceae). Orbital Electron. J. Chem. 2019, 11, 284–291. [Google Scholar] [CrossRef]
- Azeem, M.; Zaman, T.; Tahir, M.; Haris, A.; Iqbal, Z.; Binyameen, M.; Nazir, A.; Shad, S.A.; Majeed, S.; Mozūraitis, R. Chemical composition and repellent activity of native plants essential oils against dengue mosquito, Aedes aegypti. Ind. Crops Prod. 2019, 140, 111609. [Google Scholar] [CrossRef]
- Hoi, T.M.; Huong, L.T.; Chinh, H.V.; Hau, D.V.; Satyal, P.; Tai, T.A.; Dai, D.N.; Hung, N.H.; Hien, V.T.; Setzer, W.N. Essential oil compositions of three invasive Conyza species collected in Vietnam and their larvicidal activities against Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus. Molecules 2020, 25, 4576. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.G.; Haris, A.; Binyameen, M.; Nazir, A.; Mozūratis, R.; Azeem, M. Chemical composition, larvicidal and repellent activities of wild plant essential oils against Aedes aegypti. Biology 2023, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, S.C.N.; Cantrell, C.L.; Duke, C.O.; Wedge, D.E.; Nandula, V.K.; Moraes, R.M.; Cerdeira, N.L. Bioassay-directed isolation and identification of phytotoxic and fungitoxic acetylenes from Conyza canadensis. J. Agri. Food Chem. 2012, 60, 5893–5898. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.M.; Li, T.Y.; Zhao, D.Q.; Shao, S.; Bi, S.N. A new derivative of triterpene with anti-melanoma B16 activity from Conyza canadensis. CCL 2010, 21, 834–837. [Google Scholar] [CrossRef]
- Banday, J.A.; Farooq, S.; Qurishi, M.A.; Koul, S.; Razdan, T.K. Conyzagenin-A and B, two new epimeric lanostane triterpenoids from Conyza canadensis. Nat. Prod. Res. 2013, 27, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Ali Khan, S.; EL Hanafy, A.A.; Khan, A.; Anwar, Y.; Shah, Z.; Maher, S. Phytochemical investigation of Conyza canadensis (L.). Middle East. J. Sci. Res. 2016, 24, 1104–1111. [Google Scholar]
- Aamer, M.; Iqbal, S.; Jabeen, A.; Ahmad, M.S.; Asrar, M.; Yousuf, S.; Wahab, A.-T.; Choudhary, M.I.; Wang, Y. Bioassay-guided isolation of anti-inflammatory constituents from Erigeron canadensis. Chem. Nat. Compd. 2021, 57, 749–751. [Google Scholar] [CrossRef]
- Abood, M.; Kadhim, E.J. Phytochemical investigation of some active components in Iraqi Conyza canadensis (Syn. Erigeron canadensis). IJDDT 2021, 11, 669–675. [Google Scholar]
- Opiyo, S.A.; Njoroge, P.W.; Kuria, K.M. Chemical composition and biological activity of extracts from Conyza species. IOSR-JAC 2023, 16, 61–71. [Google Scholar] [CrossRef]
- Edziri, H.; Mastouri, M.; Ammar, S.; Mahjoub, M.A.; Brahim, S.; Kenani, A.; Zine, M.; Aouni, M. Antibacterial, antioxidant and cytotoxic activities of extracts of Conyza canadensis (L.) Cronquist growing in Tunisia. Med. Chem. Res. 2009, 18, 447–454. [Google Scholar] [CrossRef]
- Güneş, H.; OKtay, M.; Çelebį, F.; Tül, B. Screening of antimicrobial and anticancer potentials of some plant extracts from Mugla provience. JABS 2014, 8, 16–21. [Google Scholar]
- El-Akhal, J.; Oliveira, A.P.; Bencheikh, R.; Valentão, P.; Andrade, P.B.; Morato, M. Vasorelaxant mechanism of herbal extracts from Mentha suaveolens, Conyza canadensis, Teucrium polium and Salvia verbenaca in the Aorta of Wistar Rats. Molecules 2022, 27, 8752. [Google Scholar] [CrossRef] [PubMed]
- Paulino, B.N.; Silva, G.N.S.; Araújo, F.F.; Néri-Numa, I.A.; Pastore, G.M.; Bicas, J.L.; Molina, G. Beyond natural aromas: The bioactive and technological potential of monoterpene. Trends Food Sci. Technol. 2022, 128, 188–201. [Google Scholar] [CrossRef]
- Kumar, V.; Mathela, C.S.; Tewari, G.; Panwar, A.; Pandey, V. In vitro antimicrobial activity of essential oils and their acetylenic constituents. IJNPR 2017, 8, 63–68. [Google Scholar]
- Pandey, S.H.; Dhami, D.S.; Jha, A.; Shah, G.S.; Kumar, A.; Samant, M. Identification of trans-2-cis-8-matricaria-ester from the essential oil of Erigeron multiradiatus and evaluation of its antileishmanial potential by in vitro and in silico approaches. ACS Omega 2019, 4, 14640–14649. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.E.I.; Marei, G.I.K.; Rabea, E.I.; Taktak, N.E.M. Antimicrobial and antioxidant activities of hydrocarbon and oxygenated monoterpenes against some foodborne pathogens through in vitro and in silico studies. Pestic. Biochem. Phy. 2019, 158, 185–200. [Google Scholar] [CrossRef]
- El-Guiche, R.; Tahrouch, S.; Amri, O.; El Mehrach, K.; Hatimie, A. Antioxidant activity and total phenolic and flavonoid contents of 30 medicinal and aromatic plants located in the south of Morocco. IJNTR 2015, 1, 7–11. [Google Scholar]
- Polat, D.Ç.; İlgün, S.; Karatoprak, G.Ş.; Akkol, E.K.; Capasso, R. Phytochemical profiles, antioxidant, cytotoxic, and anti-inflammatory activities of traditional medicinal plants: Centaurea pichleri subsp. pichleri, Conyza canadensis, and Jasminum fruticans. Molecules 2022, 27, 8249. [Google Scholar] [CrossRef] [PubMed]
- Toah, E.T.; Payne, V.K.; Cedric, Y.; Nadia, N.A.C.; Joël, A.T.R. In vitro oocysticidal sporulation inhibition of Eimeria tenella and antioxidant efficacy of ethanolic and aqueous extracts of Conyza aegyptiaca. JASVM 2021, 6, 30–40. [Google Scholar] [CrossRef]
- Nisar, Z.; Shah, N.; Ishaq, M.; Amir, Z.; Khan, M.; Samie, M.; Khan, H.; Azeem, S.; Uddin, G.; Rauf, A. Phytochemical Analysis and Antioxidant Studies of Conyza bonarensis. Acad. J. Plant Sci. 2013, 6, 109–112. [Google Scholar] [CrossRef]
- Elshamy, A.I.; El Gendy, A.-E.N.; Farrag, A.-R.H.; Nassar, M.I. Antidiabetic and antioxidant activities of phenolic extracts of Conyza dioscoridis L. shoots. Int. J. Pharm. Pharm. Sci. 2015, 7, 65–72. [Google Scholar]
- Sagar, A.; Rana, J.; Prakash, V. Antibacterial and antioxidant activities of two medicinal plants: Erigeron alpinus L. and Gentianella moorcroftiana Wall. ex G. Don. Plant Arch. 2018, 18, 817–824. [Google Scholar]
- Sobhanifar, S.; Worrall, L.J.; Gruninger, R.J.; Wasney, G.A.; Blaukopf, M.; Baumann, L.; Lameignere, E.; Solomonson, M.; Brown, E.D.; Withers, S.G. Structure and mechanism of Staphylococcus aureus TarM, the wall teichoic acid α-glycosyltransferase. Proc. Natl. Acad. Sci. USA 2015, 112, E576–E585. [Google Scholar] [CrossRef] [PubMed]
- Silhavy, T.J.; Kahne, D.; Walker, S. The bacterial cell envelope. Cold Spring Harbor Perspect. Biol. 2010, 2, a000414. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; Ou-Yang, Y.-S.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2009, 18, 1049–1056. [Google Scholar] [CrossRef]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- El Zalabani, S.M.; Hetta, M.H.; Ismail, A.S. Genetic Profiling, Chemical Characterization and Biological Evaluation of Two Conyza Species Growing in Egypt. J. Appl. Pharm. Sc. 2012, 2, 054–061. [Google Scholar] [CrossRef]
- Tamokou, J.-D.-D.; Matsuete-Takongmo, G. Antibacterial and Wound Healing Properties of Selected Cameroonian Medicinal Plants. JIRMEPS 2023, 18, 34–51. [Google Scholar] [CrossRef]
- Al-Jaber, H.I.; Shakya, A.K.; Elagbar, Z.A. HPLC profiling of selected phenolic acids and flavonoids in Salvia eigii, Salvia hierosolymitana and Salvia viridis growing wild in Jordan and their in vitro antioxidant activity. PeerJ 2020, 8, e9769. [Google Scholar] [CrossRef]
- Odeh, A.A.; Al-Jaber, H.I.; Barhoumi, L.M.; Al-Fawares, O.l.; Shakya, A.K.; Al-Qudah, M.A.; Al-Sanabra, O.M. Phytochemical and bioactivity evaluation of secondary metabolites and essential oils of Sedum rubens growing wild in Jordan. Arab. J. Chem. 2023, 16, 104712. [Google Scholar] [CrossRef]
- Hasan, H.S.; Shakya, A.K.; Al-Jaber, H.I.; Abu-Sal, H.E.; Barhoumi, L.M. Exploring Echinops polyceras Boiss. from Jordan: Essential oil composition, COX, protein denaturation inhibitory power and antimicrobial activity of the alcoholic extract. Molecules 2023, 28, 4238. [Google Scholar] [CrossRef]
- Halub, B.; Shakya, A.; Elagbar, Z.; Naik, R. GC-MS analysis and biological activity of essential oil of fruits, needles and bark of Pinus pinea grown wildly in Jordan. Acta Pol. Pharm. Drug Res. 2019, 76, 825–831. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2017. [Google Scholar]
- Bär, B.; Schultze, W. Composition of the essential oil of the flower heads of Matricaria perforata. Planta Med. 1996, 62, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaber, H.I. Variation in essential oil composition of Iris nigricans Dinsm. (Iridaceae) endemic to Jordan at different flowering stages. Arab. J. Chem. 2016, 9, S1190–S1196. [Google Scholar] [CrossRef]
- Al Jaber, H.I. Essential Oil Composition, Total Phenolic and Total Flavonoid content and in-vitro Antioxidant Properties of Hydro-alcoholic and Water Extracts of Salvia deserti Growing Wild in Jordan. JJC 2017, 12, 11–19. [Google Scholar]
- Al-Qudah, M.A.; Al-Jaber, H.I.; Abu Zarga, M.H.; Abu Orabi, S.T. Flavonoid and phenolic compounds from Salvia palaestina L. growing wild in Jordan and their antioxidant activities. Phytochemistry 2014, 99, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sudan, R.; Bhagat, M.; Gupta, S.; Singh, J.; Koul, A. Iron (FeII) chelation, ferric reducing antioxidant power, and immune modulating potential of Arisaema jacquemontii (Himalayan Cobra Lily). BioMed Res. Int. 2014, 2014, 179865. [Google Scholar] [CrossRef] [PubMed]
- Shadid, K.A.; Shakya, A.K.; Naik, R.R.; Al-Qaisi, T.S.; Oriquat, G.A.; Atoom, A.M.; Farah, H.S. Exploring the chemical constituents, antioxidant, xanthine oxidase and COX inhibitory activity of Commiphora gileadensis commonly grown wild in Saudi Arabia. Molecules 2023, 28, 2321. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests, 12th ed.; Approved Standard; CLSI Document Guideline M02-A12; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Mwafy, A.; Youssef, D.Y.; Mohamed, M.M. Antibacterial activity of zinc oxide nanoparticles against some multidrug-resistant strains of Escherichia coli and Staphylococcus aureus. Int. J. Vet. Sci. 2023, 12, 284–289. [Google Scholar] [CrossRef]
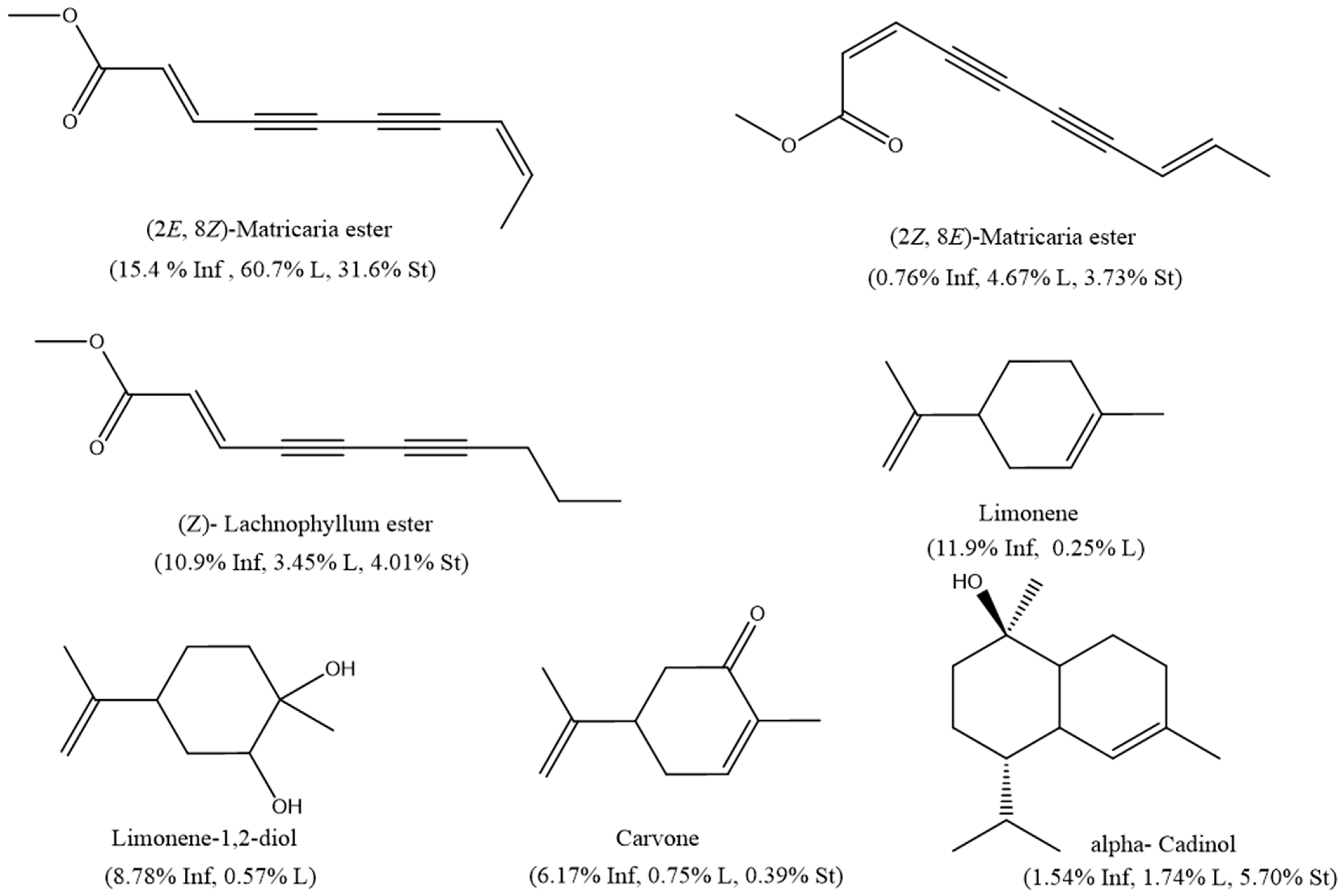
| No. | Calc. RI | Lit. RI | Compound Name | Class | Molecular Formula | % Composition | Method of Identification | ||
|---|---|---|---|---|---|---|---|---|---|
| Inf | L | St | |||||||
| 1 | 916 | 911 | Isobutyl isobutyrate | OAHCs | C8H16O2 | 0.23 | 0.41 | 0.39 | RI, MS |
| 2 | 919 | 923 | 2-Methyl-4-heptanone | OAHCs | C8H16O | - | 0.09 | - | RI, MS |
| 3 | 944 | 944 | 5-Mthyl-3-heptanone | OAHCs | C8H16O | - | 0.12 | - | RI, MS |
| 4 | 947 | 968 | p-Menthane | MHCs | C10H20 | 0.08 | 0.20 | 0.15 | RI, MS, NIST |
| 5 | 980 | 979 | β-Pinene | MHCs | C10H16 | 0.18 | - | - | RI, MS |
| 6 | 1007 | 1008 | iso-Sylvestrene | MHCs | C10H16 | 0.14 | - | - | RI, MS |
| 7 | 1026 | 1024 | p-Cymene | ArHCs | C10H14 | 0.16 | - | - | RI, NIST |
| 8 | 1031 | 1029 | Limonene | MHCs | C10H16 | 11.9 | 0.25 | - | RI, MS |
| 9 | 1125 | 1122 | trans-p-Mentha-2,8-dien-1-ol | OMHCs | C10H16O | 2.26 | 0.42 | - | RI, MS |
| 10 | 1134 | 1144 | Limona ketone | OMHCs | C9H14O | 0.46 | - | - | RI, MS, NIST |
| 11 | 1136 | 1142 | trans-Limonene oxide | OMHCs | C10H16O | 0.21 | - | - | RI, NIST |
| 12 | 1140 | 1137 | cis-p-Mentha-2,8-dien-1-ol | OMHCs | C10H16O | 2.84 | 0.42 | - | RI, MS |
| 13 | 1145 | 1139 | trans-Pinocarveol | OMHCs | C10H16O | 0.85 | - | - | RI, MS |
| 14 | 1167 | 1164 | Pinocarvone | OMHCs | C10H14O | 0.36 | - | - | RI, MS |
| 15 | 1172 | 1168 | 3-Thujanol | OMHCs | C10H18O | 0.55 | - | - | RI, MS |
| 16 | 1182 | 1180 | (E)-Isocitral | OMHCs | C10H16O | 0.50 | - | - | RI, MS |
| 17 | 1191 | 1189 | trans-p-Mentha-1(7),8-dien-2-ol | OMHCs | C10H16O | 0.95 | - | - | RI, MS |
| 18 | 1195 | 1182 | Isomenthol | OMHCs | C10H20O | - | - | 0.34 | RI, MS |
| 19 | 1199 | 1188 | α-Terpineol | OMHCs | C10H18O | - | 0.43 | 0.51 | RI, MS |
| 20 | 1199 | 1192 | cis-Dihydrocarvone | OMHCs | C10H16O | 1.06 | - | - | RI, MS |
| 21 | 1202 | 1193 | Dihydrocarveol | OMHCs | C10H18O | 0.99 | - | - | RI, MS |
| 22 | 1203 | 1197 | Verbanol | OMHCs | C10H18O | 0.97 | - | - | RI, MS |
| 23 | 1209 | 1200 | trans-Dihydrocarvone | OMHCs | C10H16O | 0.34 | - | - | RI, MS |
| 24 | 1222 | 1216 | trans-Carveol | OMHCs | C10H16O | 5.04 | 0.61 | - | RI, MS |
| 25 | 1235 | 1229 | cis-Carveol | OMHCs | C10H16O | 1.58 | 0.16 | - | RI, MS |
| 26 | 1239 | 1230 | cis-p-Mentha-1(7),8-dien-2-ol | OMHCs | C10H16O | 0.19 | - | - | RI, MS |
| 27 | 1243 | 1237 | Pulegone | OMHCs | C10H16O | - | - | 3.43 | RI, MS |
| 28 | 1249 | 1243 | Carvone | OMHCs | C10H14O | 6.17 | 0.75 | 0.39 | RI, MS |
| 29 | 1274 | 1258 | Carvenone | OMHCs | C10H16O | 0.48 | - | - | RI, MS |
| 30 | 1281 | 1275 | p-Mentha-1-en-7-al | OMHCs | C10H16O | 0.50 | - | - | RI, MS |
| 31 | 1285 | 1289 | Limonene-10-ol | OMHCs | C10H16O | 0.24 | - | - | RI, MS |
| 32 | 1292 | 1294 | Limonene diepoxide | OMHCs | C10H16O2 | 1.16 | - | - | RI, MS, NIST |
| 33 | 1304 | 1295 | Perilla alcohol | OMHCs | C10H16O | 0.38 | - | - | RI, MS, NIST |
| 34 | 1334 | 1342 | trans-Carvyl acetate | OMHCs | C12H18O2 | 0.29 | - | - | RI, MS |
| 35 | 1345 | 1343 | Piperitenone | OMHCs | C10H14O | - | - | 1.63 | RI, MS |
| 36 | 1351 | 1343 | Limonene-1,2-diol | OMHCs | C12H18O2 | 8.78 | 0.57 | - | RI, MS, NIST |
| 37 | 1358 | 1358 | 2,6-Dimethyl-2,7-octadiene-1,6-diol | OMHCs | C10H18O2 | 0.80 | - | - | RI, MS, NIST |
| 38 | 1365 | 1368 | Piperitenone oxide | OMHCs | C10H14O2 | - | - | 2.84 | RI, MS |
| 39 | 1377 | 1370 | n-Undecanol | OAHCs | C11H24O | - | - | 0.19 | RI, MS |
| 40 | 1389 | 1381 | β-Patchoulene | SHCs | C15H24 | 0.33 | - | 0.28 | RI, MS |
| 41 | 1393 | 1390 | β-Elemene | SHCs | C15H24 | 0.18 | - | - | RI, MS |
| 42 | 1397 | 1392 | (Z)-Jasmone | OMHCs | C11H16O | - | 0.83 | 1.01 | RI, MS |
| 43 | 1410 | 1409 | Citronellyl oxy-acetaldehyde | OMHCs | C12H22O2 | 0.66 | - | - | RI, MS |
| 44 | 1436 | 1434 | trans-α-Bergamotene | SHCs | C15H24 | 4.27 | 1.23 | 2.82 | RI, MS |
| 45 | 1449 | 1436 | Neryl acetone | OMHCs | C13H22O | 1.16 | 1.86 | 1.73 | RI, MS |
| 46 | 1484 | 1480 | ar-Curcumene | SHCs | C15H22 | 1.14 | 0.78 | 2.18 | RI, MS |
| 47 | 1489 | 1459 | Sesquisabinene | SHCs | C15H24 | 1.01 | 0.35 | 0.39 | RI, MS |
| 48 | 1507 | 1497 | Methyl p-tert butylphenyl acetate | ArHCs | C13H18O2 | - | 0.12 | - | RI, MS |
| 49 | 1510 | 1505 | β-Bisabolene | SHCs | C15H24 | 0.13 | - | - | RI, MS |
| 50 | 1516 | 1511 | (Z)-Lachnophyllum ester | PAcs | C11H12O2 | 10.9 | 3.45 | 4.01 | RI, MS |
| 51 | 1525 | 1527 | (2Z,8E)-Matricaria ester | PAcs | C11H10O2 | 0.76 | 4.67 | 3.73 | NIST |
| 52 | 1530 | 1540 | (2E,8Z)-Matricaria ester | PAcs | C11H10O2 | 15.4 | 60.7 | 31.6 | NIST |
| 53 | 1543 | 1548 | (E)-Allyl cinnamate | ArHCs | C12H12O2 | - | 0.11 | - | RI, MS |
| 54 | 1549 | 1544 | cis-Sesquisabinene hydrate | OSHCs | C15H26O | - | 0.23 | - | RI, MS |
| 55 | 1560 | 1563 | epi-Longipinanol | OSHCs | C15H26O | 0.21 | 0.25 | 0.64 | RI, MS |
| 56 | 1563 | 1563 | (E)-Nerolidol | OSHCs | C15H26O | 1.33 | 3.67 | 3.71 | RI, MS |
| 57 | 1574 | 1564 | Geranyl butanoate | OMHCs | C14H24O2 | - | 0.11 | 0.48 | RI, MS |
| 58 | 1577 | 1568 | (Z)-3-Hexen-1-yl-benzoate | ArHCs | C13H16O2 | - | 0.10 | - | NIST |
| 59 | 1580 | 1597 | (2E,8E)-Matricaria ester | PAcs | C11H10O2 | - | - | 0.43 | NIST |
| 60 | 1583 | 1583 | ar-Turmerol | ArHCs | C15H22O | - | - | 0.49 | RI, MS |
| 61 | 1585 | 1579 | trans-Sesquisabinene hydrate | OSHCs | C15H26O | - | 0.81 | - | RI, MS |
| 62 | 1586 | 1578 | Spathulenol | OSHCs | C15H24O | - | - | 3.08 | RI, MS |
| 63 | 1587 | 1569 | Longipinanol | OSHCs | C15H26O | 0.43 | - | - | RI, MS |
| 64 | 1589 | 1595 | Cubeban-11-ol | OSHCs | C15H26O | 0.31 | - | - | RI, MS |
| 65 | 1590 | 1592 | Viridiflorol | OSHCs | C15H26O | - | 0.09 | - | RI, MS |
| 66 | 1593 | 1583 | Caryophyllene oxide | OSHCs | C15H24O | 0.86 | 0.41 | 3.08 | RI, MS |
| 67 | 1596 | 1586 | Presilphiperfolan-8-ol | OSHCs | C15H26O | - | - | 0.19 | RI, MS |
| 68 | 1603 | 1596 | Fokienol | OSHCs | C15H24O | - | 0.53 | 0.62 | RI, MS |
| 69 | 1603 | 1602 | Ledol | OSHCs | C15H26O | 0.29 | - | - | RI, MS |
| 70 | 1612 | 1619 | 2,(7Z)-Bisaboladien-4-ol | OSHCs | C15H26O | 0.33 | 0.83 | 0.85 | RI, MS |
| 71 | 1618 | 1607 | Geranyl isovalerate | OSHCs | C15H26O | 0.97 | 1.22 | - | RI, MS |
| 72 | 1625 | 1628 | Isospathulenol | OSHCs | C15H24O | - | 0.08 | - | NIST |
| 73 | 1635 | 1645 | Cubenol | OSHCs | C15H26O | - | - | 0.80 | RI, MS |
| 74 | 1637 | 1637 | β-Acorenol | OSHCs | C15H26O | - | 0.49 | - | RI, MS |
| 75 | 1643 | 1648 | Khusilal | OSHCs | C14H18O | - | - | 2.93 | RI, MS |
| 76 | 1650 | 1640 | tau-Cadinol | OSHCs | C15H26O | - | 1.73 | 2.91 | RI, MS |
| 77 | 1657 | 1653 | Himachalol | OSHCs | C15H26O | - | 0.64 | 1.72 | RI, MS |
| 78 | 1665 | 1654 | α-Cadinol | OSHCs | C15H26O | 1.54 | 1.74 | 5.70 | RI, MS |
| 79 | 1669 | 1658 | α-Bisabolol oxide B | OSHCs | C15H26O2 | 0.21 | 0.38 | 0.82 | RI, MS |
| 80 | 1674 | 1679 | (Z)-Methyl epi-jasmonate | OMHCs | C13H20O3 | - | - | 0.49 | RI, MS |
| 81 | 1678 | 1684 | epi-α-Bisabolol | OSHCs | C15H26O | - | 0.10 | 0.34 | RI, MS |
| 82 | 1681 | 1689 | 2,3-Dihydrofarnesol | OSHCs | C15H28O | 0.31 | 0.48 | 1.38 | RI, MS |
| 83 | 1692 | 1685 | α-Bisabolol | OSHCs | C15H26O | 0.30 | 1.22 | 1.07 | RI, MS |
| 84 | 1696 | 1698 | (2Z,6Z)-Farnesol | OSHCs | C15H26O | - | - | 0.34 | RI, MS |
| 85 | 1695 | 1702 | Sesquicineol-2-one | OSHCs | C15H24O2 | 0.88 | - | - | RI, MS |
| 86 | 1701 | 1700 | Amorpha-4,9-diene-2-ol | OSHCs | C15H24O | - | 0.19 | - | RI, MS |
| 87 | 1717 | 1713 | Cedroxyde | OSHCs | C15H24O | - | 1.32 | 0.34 | RI, MS |
| 88 | 1737 | 1729 | γ-(Z)-Curcumen-12-ol | OSHCs | C15H24O | 0.29 | - | 0.34 | RI, MS |
| 89 | 1745 | 1740 | Oplopanone | OSHCs | C15H26O2 | 0.51 | - | 0.20 | RI, MS |
| 90 | 1752 | 1753 | Xanthorrhizol | OSHCs | C15H22O | - | - | 0.79 | RI, MS |
| 91 | 1780 | 1760 | Benzyl Benzoate | ArHCs | C14H12O2 | 0.69 | 0.36 | 0.74 | RI, MS |
| 92 | 1808 | 1807 | 2-Ethylhexyl salicylate | ArHCs | C15H22O3 | - | - | 0.32 | RI, MS |
| 93 | 1829 | 1820 | Isolongifolol acetate | OSHCs | C17H28O2 | 0.30 | 0.12 | 0.57 | RI, MS |
| 94 | 1836 | 1826 | (E)-Nerolidyl isobutyrate | OSHCs | C19H32O2 | 0.34 | 0.17 | 0.51 | RI, MS |
| 95 | 1880 | 1887 | Oplopanonyl acetate | OSHCs | C17H28O3 | - | - | 0.37 | RI, MS |
| 96 | 1895 | 1913 | (5Z,9E)-Farnesyl acetate | OSHCs | C18H30O4 | - | - | 0.61 | RI, MS |
| 97 | 1892 | 1875 | n-Hexadecanol | OAHCs | C16H34O | - | 0.29 | - | RI, MS |
| 98 | 1897 | 1890 | 8-Isobutyryloxy isobornyl isobutanoate | OMHCs | C18H30O4 | - | 0.93 | 0 | RI, MS |
| 99 | 1920 | 1914 | 11,12-Dihydroxy valencene | OSHCs | C15H26O2 | 0.51 | - | 0.66 | RI, MS |
| 100 | 1921 | 1930 | Musk ambrette | OAHCs | C16H28O2 | - | 0.18 | - | RI, MS |
| 101 | 1927 | 1921 | Hexadecanoic acid, methyl ester | FA | C17H34O2 | - | 0.24 | - | RI, MS |
| 102 | 1966 | 1947 | Isophytol | ODHCs | C20H40O | - | - | 0.93 | RI, MS |
| 103 | 2096 | 2097 | Linoleic acid, methyl ester | FA | C19H34O2 | - | 0.07 | - | NIST |
| 104 | 2102 | 2108 | Linolenic acid, methyl ester | FA | C19H32O2 | - | 0.30 | - | NIST |
| 105 | 2113 | 2100 | Heneicosane | AHCs | C21H44 | - | 0.74 | 0.49 | RI, MS |
| 106 | 2144 | 2133 | Linoleic acid | FA | C18H32O2 | 0.15 | 0.21 | 0.20 | RI, MS |
| 107 | 2148 | 2149 | Abienol | ODHS | C20H34O | - | - | 0.35 | RI, MS |
| 108 | 2503 | 2500 | n-Pentacosane | AHCs | C25H52 | 0.74 | 0.09 | - | RI, MS |
| 109 | 2704 | 2700 | Heptacosane | AHCs | C27H56 | 0.29 | - | - | RI, MS |
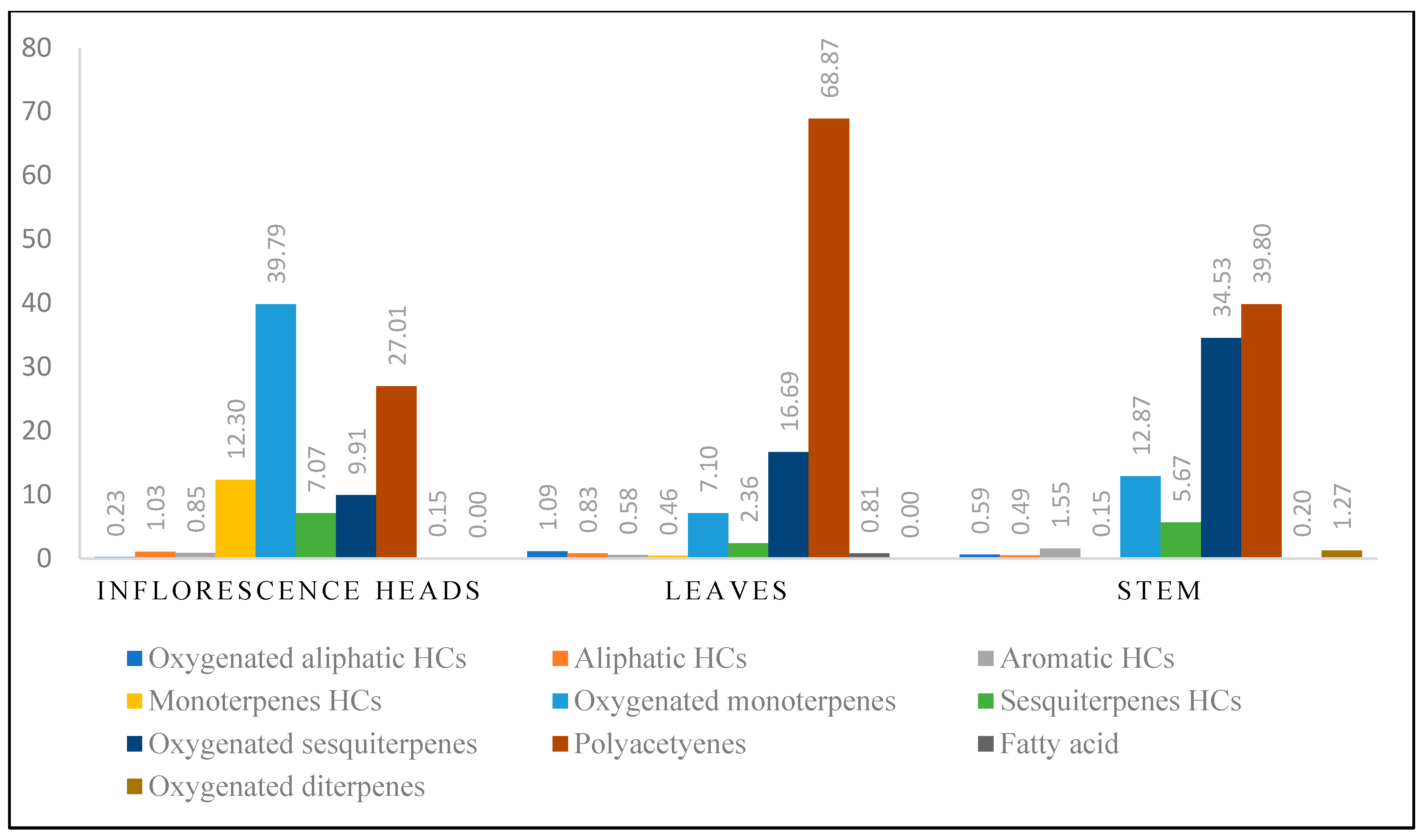
| Oxygenated aliphatic hydrocarbons (OAHCs) | 0.23 | 1.09 | 0.59 | ||||||
| Aliphatic hydrocarbons (AHCs) | 1.03 | 0.83 | 0.49 | ||||||
| Aromatic hydrocarbons (ArHCs) | 0.85 | 0.58 | 1.55 | ||||||
| Monoterpenes hydrocarbons (MHCs) | 12.30 | 0.46 | 0.15 | ||||||
| Oxygenated monoterpenes (OMHCs) | 39.79 | 7.10 | 12.87 | ||||||
| Sesquiterpenes hydrocarbons (SHCs) | 7.07 | 2.36 | 5.67 | ||||||
| Oxygenated sesquiterpenes (OSHCs) | 9.91 | 16.69 | 34.53 | ||||||
| Polyacetylenes (PAcs) | 27.00 | 68.90 | 39.80 | ||||||
| Fatty acid (FA) | 0.15 | 0.81 | 0.20 | ||||||
| Oxygenated diterpenes (ODHCs) | - | - | 1.28 | ||||||
| Total Identified compounds | 98.34 | 98.83 | 97.13 | ||||||
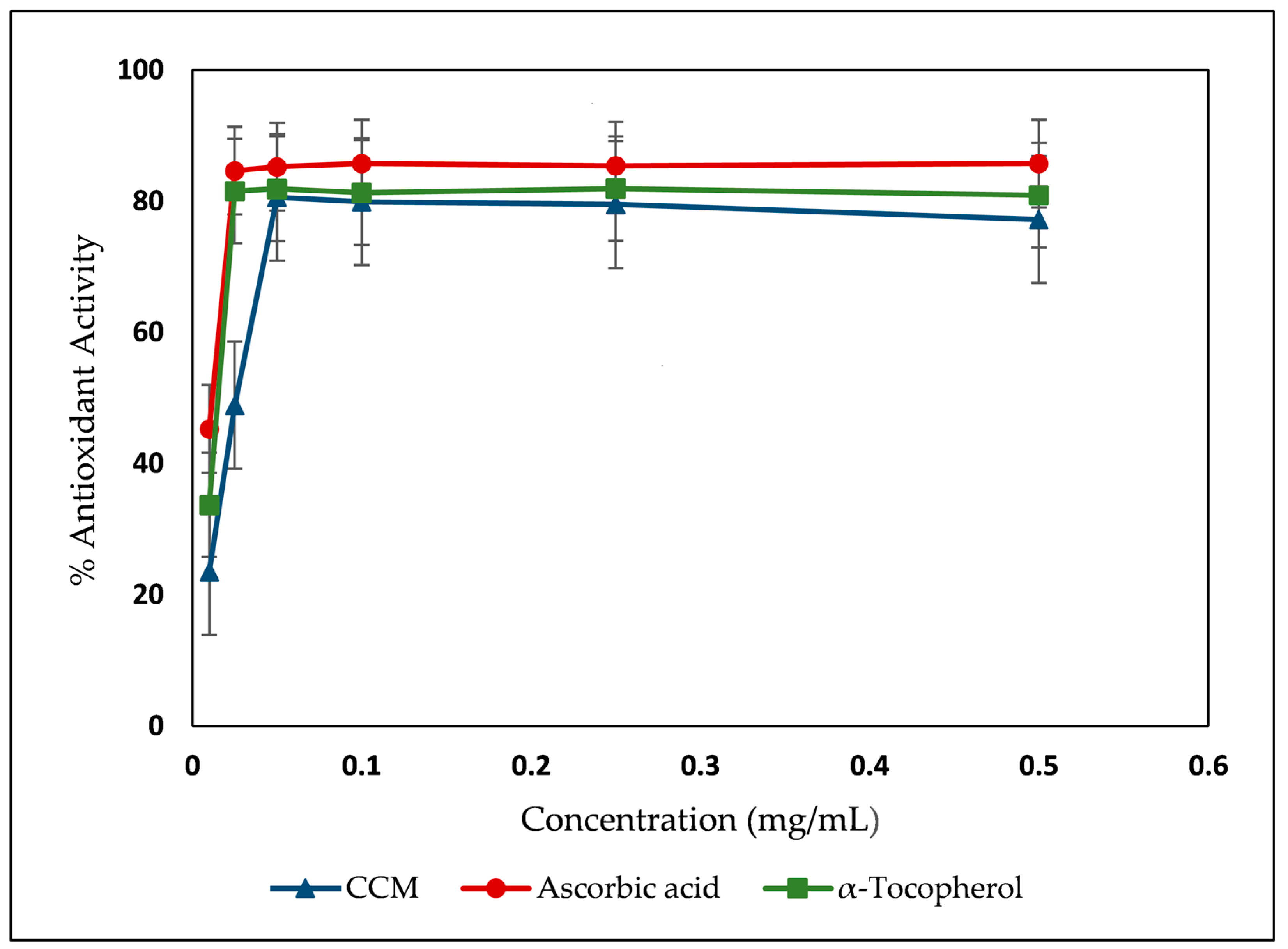
| Sample | Yield | TPC | TFC | DPPH IC50 (μg/mL) | Iron Chelating Activity IC50 (µg/mL) |
|---|---|---|---|---|---|
| CCM | 7.63% | 95.59 ± 0.40 | 467.0 ± 10.5 | 23.75 ± 0.86 | 5396.08 ± 15.05 |
| Ascorbic acid | - | - | - | 1.79 ± 0.12 | - |
| α-Tocopherol | - | - | - | 5.00 ± 0.24 | - |
| EDTA | - | - | - | - | 20.15 ± 0.09 |
| No. | Compound Name | Molecular Formula | Structural Formula | Rt (min) | Concentration (mg Compound /kg Plant) |
|---|---|---|---|---|---|
| Phenolics | |||||
| 1. | Vanillic acid | C8H8O4 | 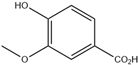 | 3.217 | 18.57 |
| 2. | Syringic acid | C9H10O5 | 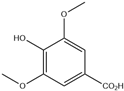 | 3.598 | 20.168 |
| 3. | p-Coumaric acid | C9H8O3 | 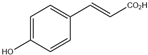 | 4.449 | 2.821 |
| 4. | Ferulic acid | C10H10O4 | 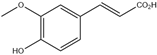 | 4.800 | 40.413 |
| 5. | Resveratrol | C14H12O3 | 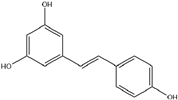 | 5.561 | 15.174 |
| 6. | Rosmarinic acid | C18H16O8 | 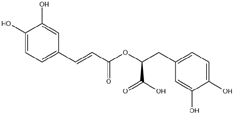 | 5.863 | 1441.125 |
| 7. | Salvianolic acid B | C36H30O16 | 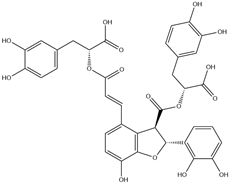 | 6.151 | 0.789 |
| 8. | Salvianolic acid A | C26H22O10 | 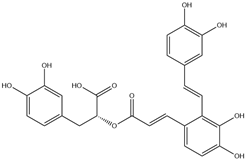 | 6.948 | 21.312 |
| 9. | Chlorogenic acid | C16H18O9 | 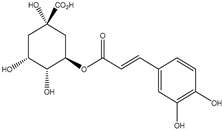 | 7.560 | t* |
| 10. | Caffeic acid phenethyl ester | C17H16O4 | 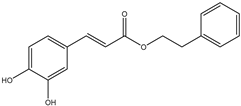 | 9.215 | 231.8 |
| 11. | Gallic acid | C7H6O5 | 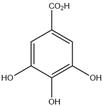 | 9.467 | t* |
| 12. | Carnosic acid | C20H28O4 | 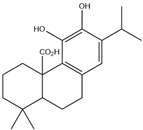 | 20.693 | t* |
| Flavonoids | |||||
| 1. | Catechin | C15H14O6 | 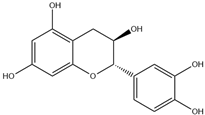 | 2.005 | 9.455 |
| 2. | Hesperidin | C28H34O15 | 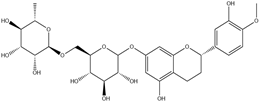 | 5.599 | 16.47 |
| 3. | Rutin | C27H30O16 | 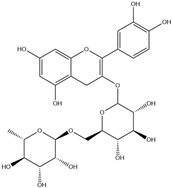 | 5.856 | t* |
| 4. | Apigenin-7-O-glucoside | C21H20O10 | 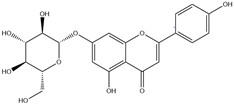 | 6.172 | 46.13 |
| 5. | Hesperetin | C16H14O6 | 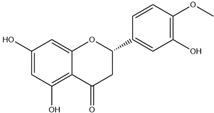 | 6.992 | 6.0612 |
| 6. | Quercetin | C15H10O7 | 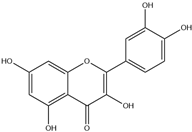 | 7.194 | 8.693 |
| 7. | Luteolin | C15H10O6 | 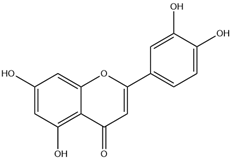 | 7.265 | 8.235 |
| 8. | Apigenin | C15H10O5 | 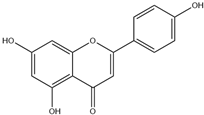 | 8.097 | 21.01 |
| 9. | 3-O-Methylquercetin | C16H12O7 | 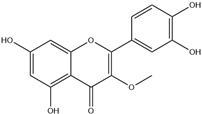 | 8.031 | 36.64 |
| Bacteria | Diameter of Inhibition Zone (mm) | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|---|
| S. aureus | 40.0 ± 0.0 | 3.125 | 6.25 |
| Compound Name | Pakistan [10] | Kashmir Valley (India) [8] | Pakistan [12] | China [11] | Turkey [5] | Hungary [4] | Brazil [9] | Present Study | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AP | AP | AP | AP | AP | Rt | AP | Rt | L | Rt | Inf | L | St | |
| β-Pinene | 2.6 | 11.83 | - | 8.8 | 9.7 | 2.3 | 2.8 | 1.3 | 0.3 | - | 0.18 | - | - |
| Limonene | 41.3 | 23.78 | 28.4 | 41.5 | 28.1 | 0.9 | 79.2 | 1.0 | 38.0 | - | 11.9 | 0.25 | - |
| trans-β-Ocimene | 8.2 | 16.02 | 5.0 | - | 0.8 | - | 0.9 | - | - | - | - | - | - |
| Carvone | - | - | - | 3.8 | 0.5 | - | - | - | 1.2 | - | 6.17 | 0.75 | 0.39 |
| trans-α-Bergamotene | 2.7 | 2.07 | 3.6 | - | 0.8 | - | 2.9 | trace | - | - | 4.27 | 1.23 | 2.82 |
| cis-Lachnophyllum ester | 6.5 | 21.25 | 16.3 | 5.5 | 2.9 | 86.5 | - | - | - | 91.6 | 10.9 | 3.45 | 4.01 |
| 2Z,8Z-Matricaria ester | - | - | - | - | - | 3.9 | 2.1 | 88.2 | - | 6.7 | - | - | - |
| 2E,8Z-Matricaria ester | - | - | - | - | - | 0.5 | 0.3 | 1.9 | - | - | 15.4 | 60.7 | 31.6 |
| Germacrene D | 10.3 | 0.31 | 4.6 | - | 2.1 | - | - | - | - | - | - | - | - |
| Spathulenol | - | 0.18 | - | - | 16.3 | 2.0 | 0.3 | - | 10.7 | - | - | - | 3.08 |
| Caryophyllene oxide | - | 0.23 | - | 1.1 | 3.3 | 0.6 | - | - | 22.3 | - | 0.86 | 0.41 | 3.08 |
| (E)-β-Farnesence | - | 7.84 | 2.5 | - | 0.2 | - | - | - | - | - | - | - | - |
| ar-Curcumene | - | 2.99 | - | - | 0.3 | - | - | - | - | - | 1.14 | 0.78 | 2.18 |
| Conyza spp. | Location | DPPH, IC50 (µg/mL) | Reference |
|---|---|---|---|
| C. canadensis | Jordan | 23.75 ± 0.86 | Current study |
| C. canadensis | Morocco | 88.19 | [27] |
| C. candensis | Tunisia | 120.0 ± 0.5 | [20] |
| C. aegyptiaca | Cameroon | 26.01 ± 1.09 | [29] |
| C bonariensis | Pakistan | 44.55 | [30] |
| C. dioscoridis | Egypt | 266.60 | [31] |
| E. alpinus | India | 38.75 | [32] |
| Conyza Species. | Origin (Part) | MIC (µg/mL) | Reference |
|---|---|---|---|
| C. canadensis | Jordan, Arial part | 3.125 | Current study |
| C. canadensis | Tunisia, Aerial part | 500 | [20] |
| C. dioscoridis L. | Egypt, Aerial part | >800 | [38] |
| C. bonariensis | Egypt, Aerial part | >800 | [38] |
| E. floribundus | Cameroon, Leaves | 512 | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barhoumi, L.M.; Shakya, A.K.; Al-Fawares, O.; Al-Jaber, H.I. Conyza canadensis from Jordan: Phytochemical Profiling, Antioxidant, and Antimicrobial Activity Evaluation. Molecules 2024, 29, 2403. https://doi.org/10.3390/molecules29102403
Barhoumi LM, Shakya AK, Al-Fawares O, Al-Jaber HI. Conyza canadensis from Jordan: Phytochemical Profiling, Antioxidant, and Antimicrobial Activity Evaluation. Molecules. 2024; 29(10):2403. https://doi.org/10.3390/molecules29102403
Chicago/Turabian StyleBarhoumi, Lina M., Ashok K. Shakya, O’la Al-Fawares, and Hala I. Al-Jaber. 2024. "Conyza canadensis from Jordan: Phytochemical Profiling, Antioxidant, and Antimicrobial Activity Evaluation" Molecules 29, no. 10: 2403. https://doi.org/10.3390/molecules29102403
APA StyleBarhoumi, L. M., Shakya, A. K., Al-Fawares, O., & Al-Jaber, H. I. (2024). Conyza canadensis from Jordan: Phytochemical Profiling, Antioxidant, and Antimicrobial Activity Evaluation. Molecules, 29(10), 2403. https://doi.org/10.3390/molecules29102403








