Anticancer Potential of Indole Phytoalexins and Their Analogues
Abstract
1. Introduction
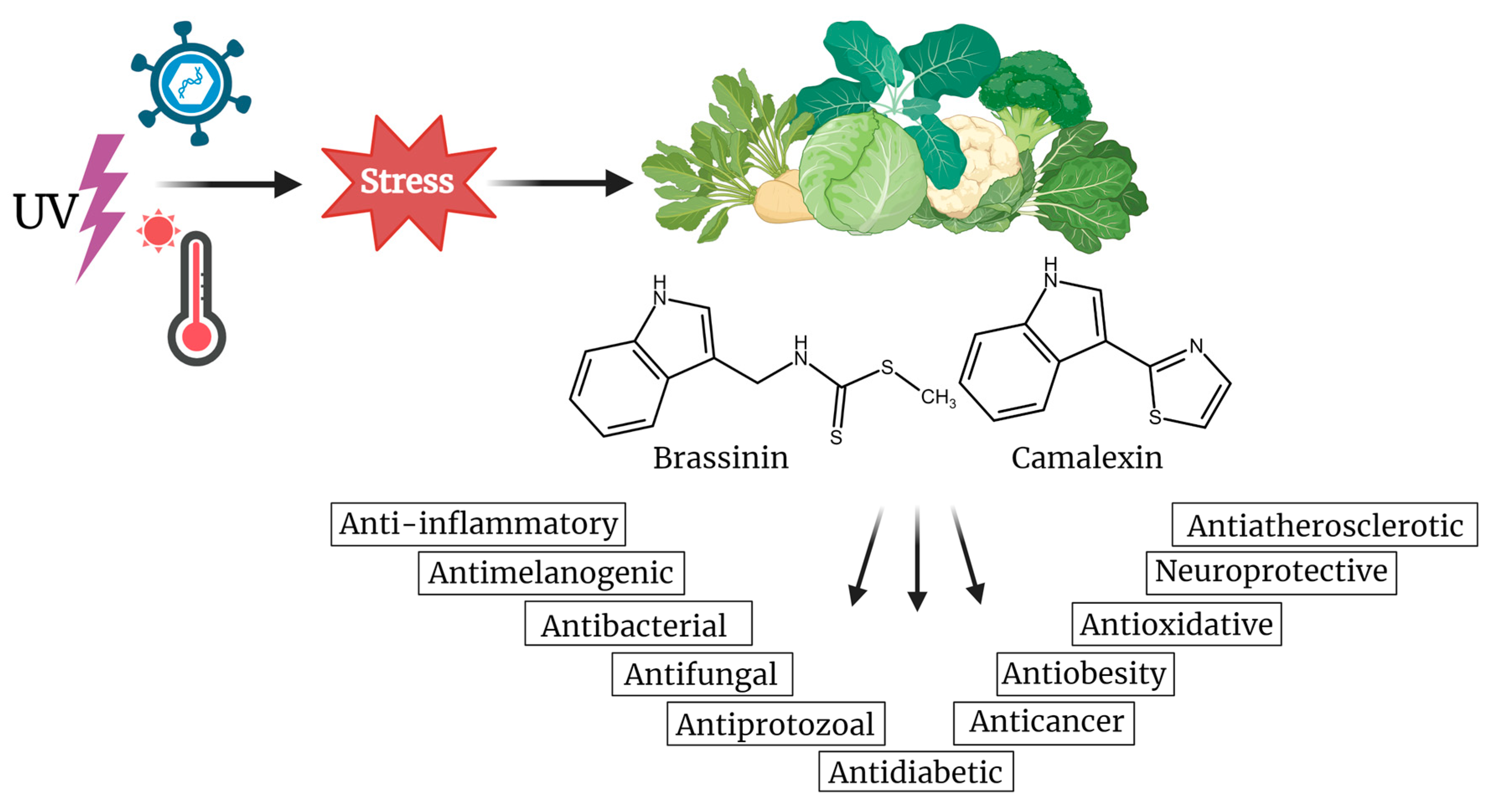
2. Origin and Structural Diversity of Indole Phytoalexins
3. Brassinin
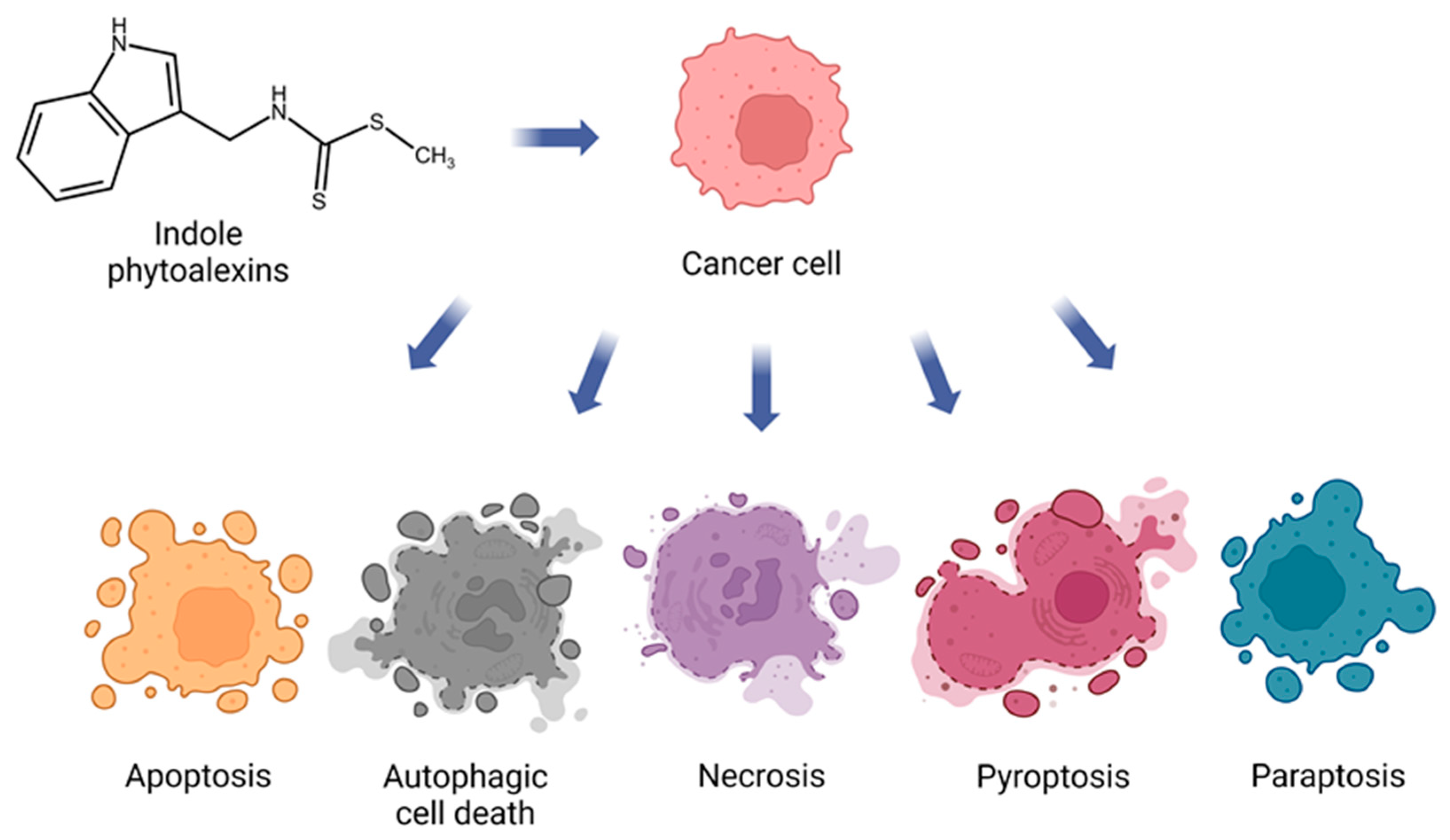
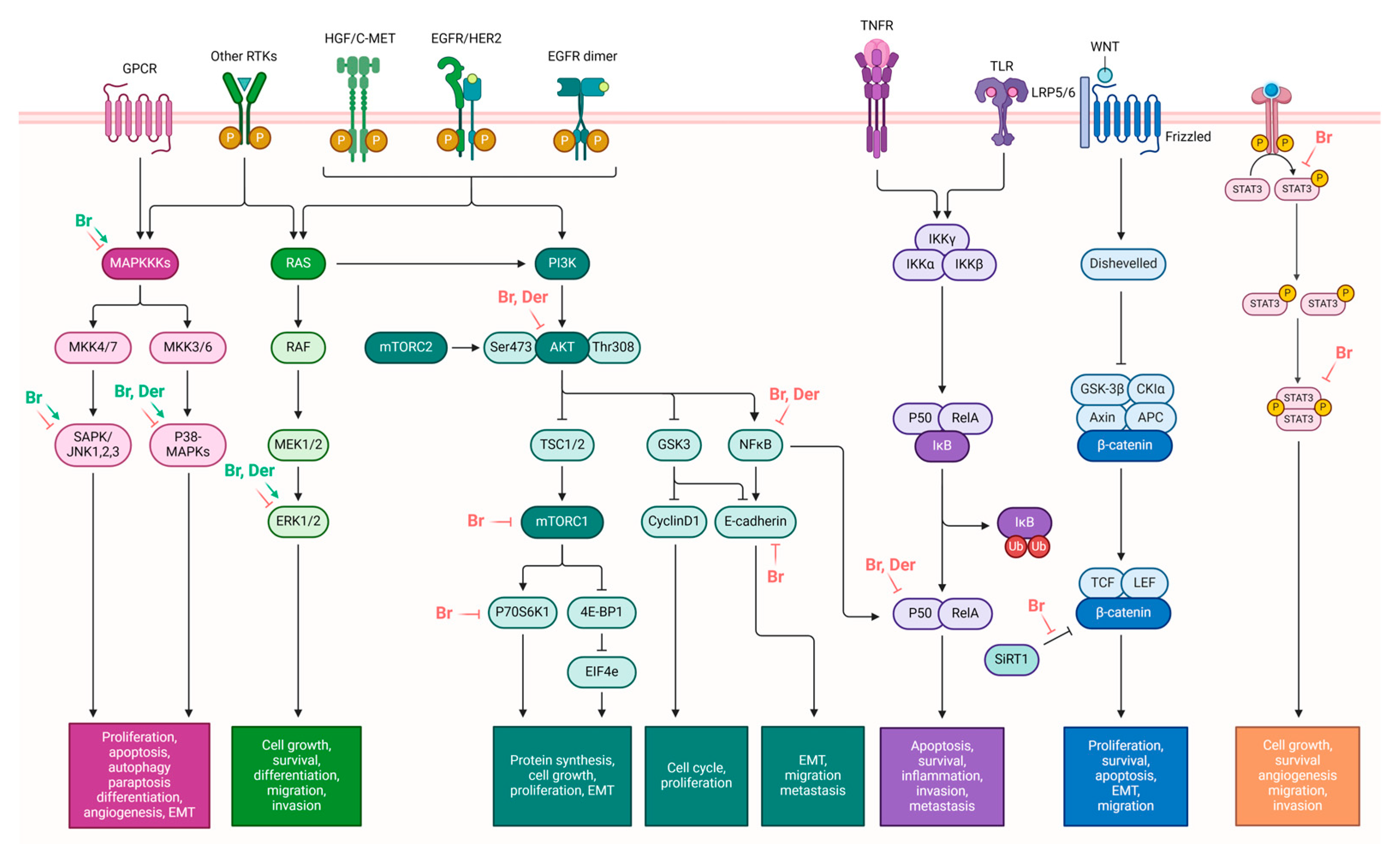
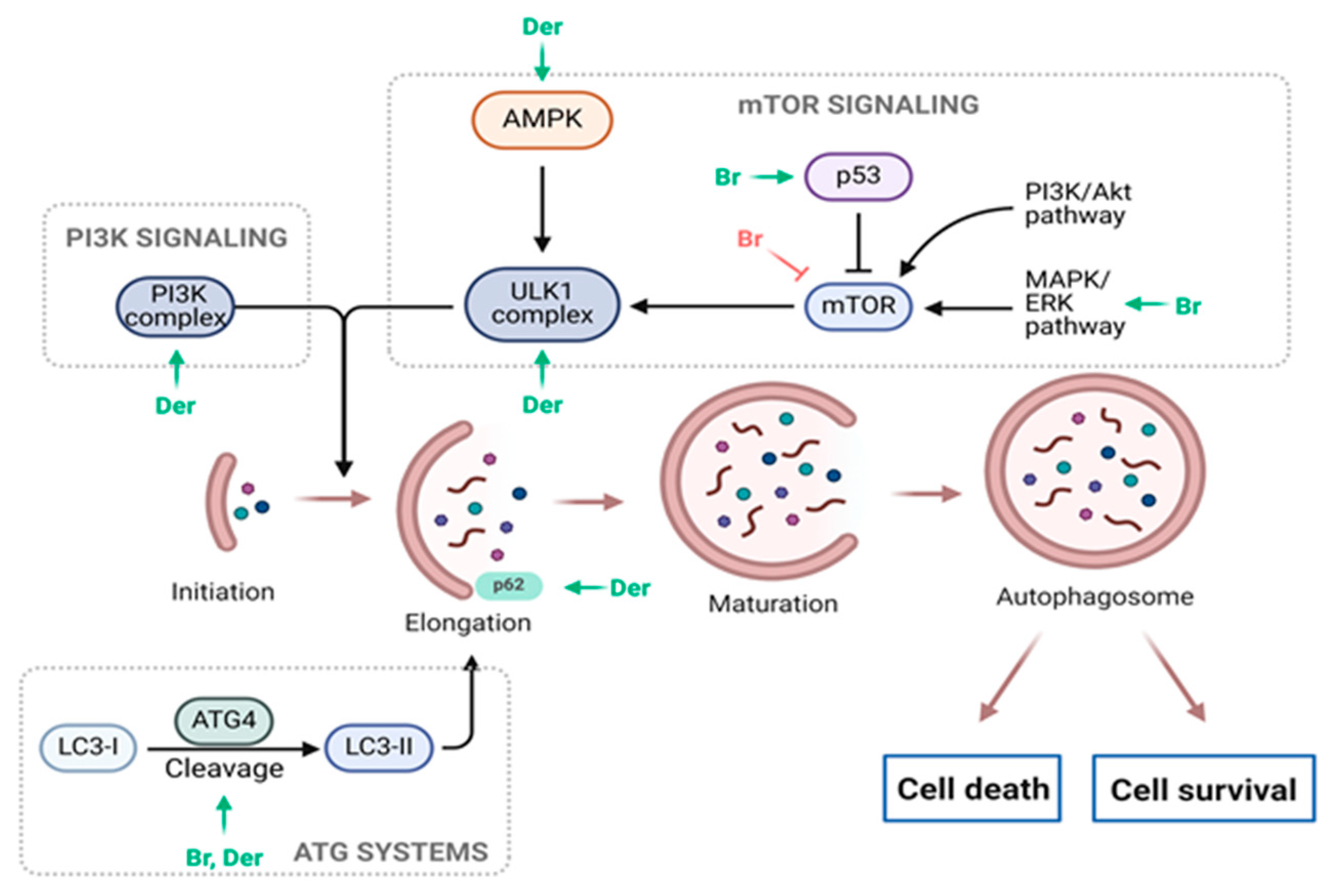
3.1. Anticancer Potential of Brassinin
3.2. Potential Impacts of Brassinin on Human Health
4. Camalexin
4.1. Anticancer Potential of Camalexin
4.2. Potential Impacts of Camalexin on Human Health
5. Antiproliferative Effects of Synthetic Analogues of Indole Phytoalexins
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Goel, A. Current understanding and future prospects on Berberine for anticancer therapy. Chem. Biol. Drug Des. 2023, 102, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Kalachaveedu, M.; Senthil, R.; Azhagiyamanavalan, S.; Ravi, R.; Meenakshisundaram, H.; Dharmarajan, A. Traditional medicine herbs as natural product matrices in cancer chemoprevention: A trans pharmacological perspective (scoping review). Phytother. Res. 2023, 37, 1539–1573. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Zheng, Q.A.; Sarma-Mamillapalle, V.K. The phytoalexins from Brassicaceae: Structure, biological activity, synthesis and biosynthesis. Nat. Prod. Commun. 2007, 2, 319–330. [Google Scholar] [CrossRef]
- Mezencev, R.; Galizzi, M.; Kutschy, P.; Docampo, R. Trypanosoma cruzi: Antiproliferative effect of indole phytoalexins on intracellular amastigotes. Exp. Parasitol. 2009, 122, 66–69. [Google Scholar] [CrossRef] [PubMed]
- N’guyen, G.Q.; Raulo, R.; Porquier, A.; Iacomi, B.; Pelletier, S.; Renou, J.-P.; Bataillé-Simoneau, N.; Campion, C.; Hamon, B.; Kwasiborski, A.; et al. Responses of the Necrotrophic Fungus Alternaria brassisicola to the Indolic Phytoalexin Brassinin. Front. Plant Sci. 2020, 11, 611643. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.H.; Trotel-Aziz, P.; Villaume, S.; Rabenoelina, F.; Clement, C.; Baillieul, F.; Aziz, A. Priming of camalexin accumulation in induced systemic resistance by beneficial bacteria against Botrytis cinerea and Pseudomonas syringae pv. tomato DC3000. J. Exp. Bot. 2022, 73, 3743–3757. [Google Scholar] [CrossRef] [PubMed]
- Kristofikova, Z.; Gazova, Z.; Siposova, K.; Bartos, A.; Ricny, J.; Kotoucova, J.; Sirova, J.; Ripova, D. Effects of Ferrofluid and Phytoalexin Spirobrassinin on Thioflavin-T-Based Fluorescence in Cerebrospinal Fluid of the Elderly and Multiple Sclerosis Patients. Neurochem. Res. 2014, 39, 1502–1510. [Google Scholar] [CrossRef]
- Mehta, R.G.; Liu, J.; Constantinou, A.; Hawthorne, M.; Pezzuto, J.M.; Moon, R.C.; Moriarty, R.M. Structure-activity relationships of brassinin in preventing the development of carcinogen-induced mammary lesions in organ culture. Anticancer Res. 1994, 14, 1209–1213. [Google Scholar]
- Izutani, Y.; Yogosawa, S.; Sowa, Y.; Sakai, T. [Corrigendum] Brassinin induces G1 phase arrest through increase of p21 and p27 by inhibition of the phosphatidylinositol 3-kinase signaling pathway in human colon cancer cells. Int. J. Oncol. 2016, 48, 1305. [Google Scholar] [PubMed]
- Park, J.H.; Kim, K.D.; Nam, D.; Shim, B.S.; Kim, S.; Ahn, K.S.; Choi, S.; Ahn, K.S. Brassinin induces apoptosis in PC-3 human prostate cancer cells through the suppression of PI3K/Akt/mTOR/S6K1 signaling cascades. Phytother. Res. 2014, 28, 423–431. [Google Scholar]
- Lee, J.H.; Kim, C.; Sethi, G.; Ahn, K.S. Brassinin inhibits STAT3 signaling pathway through modulation of PIAS-3 and SOCS-3 expression and sensitizes human lung cancer xenograft in nude mice to paclitaxel. Oncotarget 2015, 6, 6386–6405. [Google Scholar] [CrossRef] [PubMed]
- Pilatova, M.; Ivanova, L.; Kutschy, P.; Varinska, L.; Saxunova, L.; Repovska, M.; Sarissky, M.; Seliga, R.; Mirossay, L.; Mojzis, J. In vitro toxicity of camalexin derivatives in human cancer and non-cancer cells. Toxicol. In Vitro 2013, 27, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Chripkova, M.; Drutovic, D.; Pilatova, M.; Mikes, J.; Budovska, M.; Vaskova, J.; Broggini, M.; Mirossay, L.; Mojzis, J. Brassinin and its derivatives as potential anticancer agents. Toxicol. In Vitro 2014, 28, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; Wu, W.; Yao, S.; Han, X.; He, D.; He, J.; Zheng, G.; Zhao, Y.; Cai, Z.; et al. Camalexin Induces Apoptosis via the ROS-ER Stress-Mitochondrial Apoptosis Pathway in AML Cells. Oxid. Med. Cell. Longev. 2018, 2018, 7426950. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Ham, J.; Song, J.; Song, G.; Lim, W. Brassinin Inhibits Proliferation in Human Liver Cancer Cells via Mitochondrial Dysfunction. Cells 2021, 10, 332. [Google Scholar] [CrossRef]
- Chripkova, M.; Zigo, F.; Mojzis, J. Antiproliferative Effect of Indole Phytoalexins. Molecules 2016, 21, 1626. [Google Scholar] [CrossRef]
- Kristal, A.R.; Lampe, J.W. Brassica vegetables and prostate cancer risk: A review of the epidemiological evidence. Nutr. Cancer 2002, 42, 1–9. [Google Scholar] [CrossRef]
- Pedras, M.S.; Yaya, E.E.; Glawischnig, E. The phytoalexins from cultivated and wild crucifers: Chemistry and biology. Nat. Prod. Rep. 2011, 28, 1381–1405. [Google Scholar] [CrossRef]
- Pedras, M.S.; Yaya, E.E. Dissecting metabolic puzzles through isotope feeding: A novel amino acid in the biosynthetic pathway of the cruciferous phytoalexins rapalexin A and isocyalexin A. Org. Biomol. Chem. 2013, 11, 1149–1166. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; To, Q.H. Interrogation of biosynthetic pathways of the cruciferous phytoalexins nasturlexins with isotopically labelled compounds. Org. Biomol. Chem. 2018, 16, 3625–3638. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, P.D.; Landtved, J.P.; Larsen, S.H.; Lindegaard, N.; Wøhlk, S.; Jensen, K.R.; Pattison, D.I.; Burow, M.; Bak, S.; Crocoll, C.; et al. Phytoalexins of the crucifer Barbarea vulgaris: Structural profile and correlation with glucosinolate turnover. Phytochemistry 2023, 213, 113742. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P. Sulfur-containing secondary metabolites from Arabidopsis thaliana and other Brassicaceae with function in plant immunity. Chembiochem 2012, 13, 1846–1859. [Google Scholar] [CrossRef] [PubMed]
- Soledade, M.; Pedras, C.; Abdoli, A. Pathogen inactivation of cruciferous phytoalexins: Detoxification reactions, enzymes and inhibitors. Rsc Adv. 2017, 7, 23633–23646. [Google Scholar]
- Pedras, M.S.; Hossain, S. Interaction of cruciferous phytoanticipins with plant fungal pathogens: Indole glucosinolates are not metabolized but the corresponding desulfo-derivatives and nitriles are. Phytochemistry 2011, 72, 2308–2316. [Google Scholar] [CrossRef]
- Pedras, M.S.; Yaya, E.E. Tenualexin, other phytoalexins and indole glucosinolates from wild cruciferous species. Chem. Biodivers. 2014, 11, 910–918. [Google Scholar] [CrossRef]
- Pedras, M.S.; To, Q.H. Unveiling the first indole-fused thiazepine: Structure, synthesis and biosynthesis of cyclonasturlexin, a remarkable cruciferous phytoalexin. Chem. Commun. 2016, 52, 5880–5883. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.; To, Q.H. Non-indolyl cruciferous phytoalexins: Nasturlexins and tridentatols, a striking convergent evolution of defenses in terrestrial plants and marine animals? Phytochemistry 2015, 113, 57–63. [Google Scholar] [CrossRef]
- Pedras, M.S.; Alavi, M.; To, Q.H. Expanding the nasturlexin family: Nasturlexins C and D and their sulfoxides are phytoalexins of the crucifers Barbarea vulgaris and B. verna. Phytochemistry 2015, 118, 131–138. [Google Scholar] [CrossRef]
- Takasugi, M.; Katsui, N.; Shirata, A. Isolation of three Novel Sulfur-Containing Phytoalexins from the Chinese-Cabbage Brassica campestris L. ssp pekinensis (Cruciferae). J. Chem. Soc. Chem. Commun. 1986, 14, 1077–1078. [Google Scholar] [CrossRef]
- Anarat-Cappillino, G.; Sattely, E.S. The chemical logic of plant natural product biosynthesis. Curr. Opin. Plant Biol. 2014, 19, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Kim, C.Y.; Hwang, J.; Suh, H.J.; Choi, H.S. Brassinin, a phytoalexin in cruciferous vegetables, suppresses obesity-induced inflammatory responses through the Nrf2-HO-1 signaling pathway in an adipocyte-macrophage co-culture system. Phytother. Res. 2019, 33, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Hwang, J.; Choi, H.S. Brassinin, a brassica-derived phytochemical, regulates monocyte-to-macrophage differentiation and inflammatory responses in human monocytes and murine macrophages. J. Pharm. Pharmacol. 2020, 72, 1245–1255. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Ryu, H.; Jeong, H.H.; Lee, B. Brassinin Abundant in Brassicaceae Suppresses Melanogenesis through Dual Mechanisms of Tyrosinase Inhibition. Foods 2022, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Alarfaj, A.A.; Hirad, A.H.; Natarajan, N.; Iyappan, P.; Al Ali, S.H.H. Brassinin Exhibits Anti-Diabetic Activity against Streptozotocin-induced Diabetes Mellitus in Experimental Rats. Indian J. Pharm. Educ. 2023, 57, S701–S709. [Google Scholar] [CrossRef]
- Han, B.H.; Yoon, J.J.; Choi, E.S.; Jeong, D.H.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Inhibitory effect of brassinin on TNF-alpha-induced vascular inflammation in human umbilical vein endothelial cells. Mol. Med. Rep. 2017, 16, 6890–6895. [Google Scholar] [CrossRef]
- Park, W.Y.; Park, J.E.; Jung, J.H. Apoptotic Effect of Brassinin via Inhibition of CNOT2 and Activation of p53 and Its Combination Effect with Doxorubicin. Appl. Sci. 2021, 11, 10036. [Google Scholar] [CrossRef]
- Yang, M.H.; Ha, I.J.; Lee, S.G.; Lee, J.; Um, J.Y.; Sethi, G.; Ahn, K.S. Brassinin Induces Apoptosis, Autophagy, and Paraptosis via MAPK Signaling Pathway Activation in Chronic Myelogenous Leukemia Cells. Biology 2023, 12, 307. [Google Scholar] [CrossRef]
- Yang, M.H.; Baek, S.H.; Ha, I.J.; Um, J.Y.; Ahn, K.S. Brassinin enhances the anticancer actions of paclitaxel by targeting multiple signaling pathways in colorectal cancer cells. Phytother. Res. 2021, 35, 3875–3885. [Google Scholar] [CrossRef]
- Kwon, H.H.; Ahn, C.H.; Lee, H.J.; Sim, D.Y.; Park, J.E.; Park, S.Y.; Kim, B.; Shim, B.S.; Kim, S.H. The Apoptotic and Anti-Warburg Effects of Brassinin in PC-3 Cells via Reactive Oxygen Species Production and the Inhibition of the c-Myc, SIRT1, and beta-Catenin Signaling Axis. Int. J. Mol. Sci. 2023, 24, 13912. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Becker, V.; Qiu, M.; Tang, T.; Ampofo, E.; Menger, M.D.; Laschke, M.W. Brassinin Promotes the Degradation of Tie2 and FGFR1 in Endothelial Cells and Inhibits Triple-Negative Breast Cancer Angiogenesis. Cancers 2022, 14, 3540. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Lee, J.H.; Ko, J.H.; Jung, S.H.; Sethi, G.; Ahn, K.S. Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Molecules 2019, 24, 1584. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.M.; Cao, X.B.; Li, S.X.; Zhang, F.; Guan, Y.F. Brassinin inhibits proliferation and induces cell cycle arrest and apoptosis in nasopharyngeal cancer C666-1 cells. Arab. J. Chem. 2022, 15, 104018. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, D.; Ko, H.M.; Jang, H.J. Inhibition of CNOT2 Induces Apoptosis via MID1IP1 in Colorectal Cancer Cells by Activating p53. Biomolecules 2021, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, J.H.; Hwang, J.; Park, J.E.; Kim, J.H.; Park, W.Y.; Suh, J.Y.; Kim, S.H. CNOT2 Is Critically Involved in Atorvastatin Induced Apoptotic and Autophagic Cell Death in Non-Small Cell Lung Cancers. Cancers 2019, 11, 1470. [Google Scholar] [CrossRef] [PubMed]
- Sohn, E.J.; Jung, D.B.; Lee, H.; Han, I.; Lee, J.; Lee, H.; Kim, S.H. CNOT2 promotes proliferation and angiogenesis via VEGF signaling in MDA-MB-231 breast cancer cells. Cancer Lett. 2018, 412, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Li, Y.; Jiang, W.Q.; Zhou, L.F. MAPK/JNK signalling: A potential autophagy regulation pathway. Biosci. Rep. 2015, 35, e00199. [Google Scholar] [CrossRef]
- Lee, D.; Kim, I.Y.; Saha, S.; Choi, K.S. Paraptosis in the anti-cancer arsenal of natural products. Pharmacol. Ther. 2016, 162, 120–133. [Google Scholar] [CrossRef]
- Fontana, F.; Moretti, R.M.; Raimondi, M.; Marzagalli, M.; Beretta, G.; Procacci, P.; Sartori, P.; Montagnani Marelli, M.; Limonta, P. delta-Tocotrienol induces apoptosis, involving endoplasmic reticulum stress and autophagy, and paraptosis in prostate cancer cells. Cell Prolif. 2019, 52, e12576. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.T.; Chen, M.F.; Yang, C.W. Curcumin Induces p53-Null Hepatoma Cell Line Hep3B Apoptosis through the AKT-PTEN-FOXO4 Pathway. Evid.-Based Complement. Altern. Med. 2017, 2017, 4063865. [Google Scholar] [CrossRef] [PubMed]
- Trepiana, J.; Meijide, S.; Navarro, R.; Hernandez, M.L.; Ruiz-Sanz, J.I.; Ruiz-Larrea, M.B. Influence of oxygen partial pressure on the characteristics of human hepatocarcinoma cells. Redox Biol. 2017, 12, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Liu, D.; Fueyo, J.; Gomez-Manzano, C. Tie2: A journey from normal angiogenesis to cancer and beyond. Histol. Histopathol. 2008, 23, 773–780. [Google Scholar] [PubMed]
- Lin, P.; Polverini, P.; Dewhirst, M.; Shan, S.; Rao, P.S.; Peters, K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J. Clin. Investig. 1997, 100, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Buxton, J.A.; Acheson, A.; Radziejewski, C.; Maisonpierre, P.C.; Yancopoulos, G.D.; Channon, K.M.; Hale, L.P.; Dewhirst, M.W.; George, S.E.; et al. Antiangiogenic gene therapy targeting the endothelium-specific receptor tyrosine kinase Tie2. Proc. Natl. Acad. Sci. USA 1998, 95, 8829–8834. [Google Scholar] [CrossRef] [PubMed]
- Siemeister, G.; Schirner, M.; Weindel, K.; Reusch, P.; Menrad, A.; Marme, D.; Martiny-Baron, G. Two independent mechanisms essential for tumor angiogenesis: Inhibition of human melanoma xenograft growth by interfering with either the vascular endothelial growth factor receptor pathway or the Tie-2 pathway. Cancer Res. 1999, 59, 3185–3191. [Google Scholar]
- Tanaka, S.; Sugimachi, K.; Yamashita Yi, Y.; Ohga, T.; Shirabe, K.; Shimada, M.; Wands, J.R.; Sugimachi, K. Tie2 vascular endothelial receptor expression and function in hepatocellular carcinoma. Hepatology 2002, 35, 861–867. [Google Scholar] [CrossRef]
- Li, X.L.; Martinez-Ledesma, E.; Zhang, C.; Gao, F.; Zheng, S.Y.; Ding, J.; Wu, S.F.; Nguyen, N.; Clifford, S.C.; Wen, P.Y.; et al. Tie2-FGFR1 Interaction Induces Adaptive PI3K Inhibitor Resistance by Upregulating Aurora A/PLK1/CDK1 Signaling in Glioblastoma. Cancer Res. 2019, 79, 5088–5101. [Google Scholar] [CrossRef]
- Baek, S.H.; Ko, J.H.; Lee, J.H.; Kim, C.; Lee, H.; Nam, D.; Lee, J.; Lee, S.G.; Yang, W.M.; Um, J.Y.; et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-beta-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell Physiol. 2017, 232, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Bakar-Ates, F.; Ozkan, E. The combined treatment of brassinin and imatinib synergistically downregulated the expression of MMP-9 in SW480 colon cancer cells. Phytother. Res. 2019, 33, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Vachtenheim, J.; Borovansky, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Moon, K.M.; Lee, B.S.; Yang, J.H.; Park, K.I.; Cho, W.K.; Ma, J.Y. Swertiajaponin inhibits skin pigmentation by dual mechanisms to suppress tyrosinase. Oncotarget 2017, 8, 95530–95541. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.A.; Lee, I.K. The role of Nrf2: Adipocyte differentiation, obesity, and insulin resistance. Oxid. Med. Cell. Longev. 2013, 2013, 184598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, S.; Jiang, X.; Wang, Y.H.; Li, F.; Wang, Y.G.; Zheng, Y.; Cai, L. The role of the Nrf2/Keap1 pathway in obesity and metabolic syndrome. Rev. Endocr. Metab. Disord. 2015, 16, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Victor, V.M.; Rocha, M.; Solá, E.; Bañuls, C.; Garcia-Malpartida, K.; Hernández-Mijares, A. Oxidative Stress, Endothelial Dysfunction and Atherosclerosis. Curr. Pharm. Design 2009, 15, 2988–3002. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.R. TNF-mediated inflammatory disease. J. Pathol. 2008, 214, 149–160. [Google Scholar] [CrossRef]
- Mollazadeh, M.; Mohammadi-Khanaposhtani, M.; Valizadeh, Y.; Zonouzi, A.; Faramarzi, M.A.; Kiani, M.; Biglar, M.; Larijani, B.; Hamedifar, H.; Mahdavi, M.; et al. Novel Coumarin Containing Dithiocarbamate Derivatives as Potent alpha-Glucosidase Inhibitors for Management of Type 2 Diabetes. Med. Chem. 2021, 17, 264–272. [Google Scholar] [CrossRef]
- Deshpande, T.A.; Martínez-Málaga, J.; Priefer, R. Dithiocarbamates as potential PTP1B inhibitors for diabetes management. Results Chem. 2022, 4, 100372. [Google Scholar] [CrossRef]
- Glawischnig, E. Camalexin. Phytochemistry 2007, 68, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Mezencev, R.; Updegrove, T.; Kutschy, P.; Repovska, M.; McDonald, J.F. Camalexin induces apoptosis in T-leukemia Jurkat cells by increased concentration of reactive oxygen species and activation of caspase-8 and caspase-9. J. Nat. Med. 2011, 65, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.A.; Neal, C.L.; Chetram, M.; Vo, B.; Mezencev, R.; Hinton, C.; Odero-Marah, V.A. The phytoalexin camalexin mediates cytotoxicity towards aggressive prostate cancer cells via reactive oxygen species. J. Nat. Med. 2013, 67, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Taga, C.; Ozawa, M.; Kanno, Y.; Sanada, N.; Kizu, R. Camalexin, an indole phytoalexin, inhibits cell proliferation, migration, and mammosphere formation in breast cancer cells via the aryl hydrocarbon receptor. J. Nat. Med. 2022, 76, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Saito, N.; Zhao, S.; Terai, K.; Hiruta, N.; Park, Y.; Bujo, H.; Nemoto, K.; Kanno, Y. Heregulin-induced cell migration is promoted by aryl hydrocarbon receptor in HER2-overexpressing breast cancer cells. Exp. Cell Res. 2018, 366, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Kanno, Y.; Saito, N.; Terai, K.; Sanada, N.; Kizu, R.; Hiruta, N.; Park, Y.; Bujo, H.; Nemoto, K. Aryl hydrocarbon receptor counteracts pharmacological efficacy of doxorubicin via enhanced AKR1C3 expression in triple negative breast cancer cells. Biochem. Biophys. Res. Commun. 2019, 516, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, K.; Kobayashi, Y.; Ohtake, F.; Ikuta, T.; Matsushima, Y.; Mimura, J.; Pettersson, S.; Pollenz, R.S.; Sakaki, T.; Hirokawa, T.; et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 13481–13486. [Google Scholar] [CrossRef]
- Saito, N.; Kanno, Y.; Yamashita, N.; Degawa, M.; Yoshinari, K.; Nemoto, K. The Differential Selectivity of Aryl Hydrocarbon Receptor (AHR) Agonists towards AHR-Dependent Suppression of Mammosphere Formation and Gene Transcription in Human Breast Cancer Cells. Biol. Pharm. Bull. 2021, 44, 571–578. [Google Scholar] [CrossRef]
- Almasry, M.; Jemaa, M.; Mischitelli, M.; Lang, F.; Faggio, C. Camalexin-Induced Cell Membrane Scrambling and Cell Shrinkage in Human Erythrocytes. Cell Physiol. Biochem. 2017, 41, 731–741. [Google Scholar] [CrossRef]
- Sieroslawska, A.; Rymuszka, A. Combined effects of two phytoalexins, brassinin and camalexin, on the cells of colorectal origin. Toxicon 2023, 234, 107283. [Google Scholar] [CrossRef]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Manasa, K.; Chitra, V. Evaluation of in-vitro antioxidant activity of camalexin- A novel anti- parkinson’s agent. Res. J. Pharm. Technol. 2020, 13, 578–582. [Google Scholar] [CrossRef]
- Lang, E.; Lang, F. Mechanisms and pathophysiological significance of eryptosis, the suicidal erythrocyte death. Semin. Cell Dev. Biol. 2015, 39, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, O.; Kalíková, K.; Gondová, T.; Budovská, M.; Salayová, A.; Tesarová, E. Fast enantioseparation of indole phytoalexins in additive free supercritical fluid chromatography. J. Chromatogr. A 2019, 1596, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Mezencev, R.; Kutschy, P.; Salayova, A.; Curillova, Z.; Mojzis, J.; Pilatova, M.; McDonald, J. Anticancer properties of 2-piperidyl analogues of the natural indole phytoalexin 1-methoxyspirobrassinol. Chemotherapy 2008, 54, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Kutschy, P.; Sykora, A.; Curillová, Z.; Repovská, M.; Pilátová, M.; Mojzis, J.; Mezencev, R.; Pazdera, P.; Hromjáková, T. Glyoxyl Analogs of Indole Phytoalexins: Synthesis and Anticancer Activity. Collect. Czechoslov. Chem. C 2010, 75, 887–903. [Google Scholar] [CrossRef]
- Budovská, M.; Pilátová, M.; Varinská, L.; Mojzis, J.; Mezencev, R. The synthesis and anticancer activity of analogs of the indole phytoalexins brassinin, 1-methoxyspirobrassinol methyl ether and cyclobrassinin. Bioorgan. Med. Chem. 2013, 21, 6623–6633. [Google Scholar] [CrossRef] [PubMed]
- Tischlerova, V.; Kello, M.; Budovska, M.; Mojzis, J. Indole phytoalexin derivatives induce mitochondrial-mediated apoptosis in human colorectal carcinoma cells. World J. Gastroenterol. 2017, 23, 4341–4353. [Google Scholar] [CrossRef]
- Budovska, M.; Balaz, M.; Mezencev, R.; Tischlerova, V.; Zigova, M.; Mojzis, J. Design, synthesis and anticancer activity of trifluoromethylphenylamino substituted spiroindoles. J. Fluor. Chem. 2018, 216, 24–32. [Google Scholar] [CrossRef]
- Budovska, M.; Tischlerova, V.; Mojzis, J.; Kozlov, O.; Gondova, T. An alternative approach to the synthesis of anticancer molecule spirobrassinin and its 2′-amino analogues. Monatshefte Fuer Chem. Mon. 2020, 151, 63–77. [Google Scholar] [CrossRef]
- Budovská, M.; Selesová, I.; Tischlerová, V.; Michalková, R.; Mojzis, J. Design, synthesis, and biological evaluation of novel 5-bromo derivatives of indole phytoalexins. Monatshefte Fuer Chem. Mon. 2020, 151, 1737–1758. [Google Scholar] [CrossRef]
- Budovska, M.; Krochtova, K.; Kuba, M.; Tischlerova, V.; Mojzis, J. Synthesis and cytotoxicity evaluation of novel 5-fluorinated indoles. J. Fluor. Chem. 2021, 250. [Google Scholar] [CrossRef]
- Marianna, B.; Radka, M.; Martin, K.; Janka, V.; Jan, M. Design, Synthesis and Antiproliferative Evaluation of Bis-Indole Derivatives with a Phenyl Linker: Focus on Autophagy. Molecules 2022, 28, 251. [Google Scholar] [CrossRef] [PubMed]
- Zigová, M.; Miškufová, V.; Budovská, M.; Michalková, R.; Mojžiš, J. Exploring the Antiproliferative and Modulatory Effects of 1-Methoxyisobrassinin on Ovarian Cancer Cells: Insights into Cell Cycle Regulation, Apoptosis, Autophagy, and Its Interactions with NAC. Molecules 2024, 29, 1773. [Google Scholar] [CrossRef]
- Budovska, M.; Krochtova, K.; Michalkova, R.; Mojzis, J. Aminoanalogues of isobrassinin, erucalexin and isocyclobrassinin: Synthesis and evaluation of the antiproliferative and cytotoxic properties. Tetrahedron 2022, 120, 132898. [Google Scholar] [CrossRef]
- Csomos, P.; Fodor, L.; Zupko, I.; Csampai, A.; Sohar, P. Synthesis and in vitro antiproliferative effect of isomeric analogs of cyclobrassinin phytoalexin possessing the 1,3-thiazino[5,6-b]indole-4-one skeleton. Arkivoc 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Solarova, Z.; Kello, M.; Varinska, L.; Budovska, M.; Solar, P. Inhibition of heat shock protein (Hsp) 90 potentiates the antiproliferative and pro-apoptotic effects of 2-(4′fluoro-phenylamino)-4H-1,3-thiazine[6,5-b]indole in A2780cis cells. Biomed. Pharmacother. 2017, 85, 463–471. [Google Scholar] [CrossRef]
- Solar, P.; Sytkowski, A.J. Differentially expressed genes associated with cisplatin resistance in human ovarian adenocarcinoma cell line A2780. Cancer Lett. 2011, 309, 11–18. [Google Scholar] [CrossRef]
- Solarova, Z.; Mojzis, J.; Solar, P. Hsp90 inhibitor as a sensitizer of cancer cells to different therapies (review). Int. J. Oncol. 2015, 46, 907–926. [Google Scholar]
- Li, Y.; Zhang, T.; Schwartz, S.J.; Sun, D. Sulforaphane potentiates the efficacy of 17-allylamino 17-demethoxygeldanamycin against pancreatic cancer through enhanced abrogation of Hsp90 chaperone function. Nutr. Cancer 2011, 63, 1151–1159. [Google Scholar] [CrossRef] [PubMed]

| Brassinin | Anticancer Potential | Molecular Target | Relevant Reference(s) |
| The modulation of pathways involved in cell survival, proliferation, and growth | ↓ JAK-STAT3 pathway ↑ MAPK pathway ↓ PI3K/Akt/mTOR pathway | [39,40,43] | |
| Induction of cancer cell death | ↑ p53, ↑ p21 ↑ caspase-8, caspase-9, and caspase-3 ↑ PARP cleavage ↑ Sub-G1 phase distribution in cells, ↑ G0/G1 ratio, ↓ S, G2/M ↑ Bax expression, ↓ Bcl-2 expression ↑ Phosphorylation of JNK, ERK, and p38 ↑ ROS production ↑ Mitochondrial damage ↑ ER stress-related proteins (ATF4 and CHOP) ↑ Cytoplasmic vacuolation ↑ GSH/GSSG ratio disruption ↓ Expression of CNOT2 ↑ Expression of LC3 ↓ Alix expression | [17,38,39,41,43,44] | |
| Anti-Warburg effect | ↓ PK2, ↓ Glut1, ↓HK2, ↓LDH, ↓c-Myc, ↓SIRT1, ↓ β-catenin | [41] | |
| Inhibition of angiogenesis | ↓ Tie2, ↓ FGFR1 | [42] | |
| EMT modulation | ↓ TGF-β-induced fibronectin, ↓ vimentin, ↓ N-cadherin, ↓ MMP-9, ↓ MMP-2, ↓Twist, ↓ Snail, ↑ TGF-β-induced occludin, ↑ N-cadherin | [43,62] | |
| Camalexin | Modulation of AhR Pathway | ↑ Expression of AhR target genes ↑ Nuclear translocation of AhR ↑ AhR-mediated transcription ↓ Mammosphere formation in AhR-expressing breast cancer cells | [74] |
| Induction of cancer cell death | ↑ Caspase-3 and caspase-9 activity ↑ ER stress markers (phos-PERK, phos-eIF2α, ATF4, CHOP) ↓ Mcl-1 levels ↑ ROS levels ↑ SOD and CAT activity ↑ GSSG levels, ↓ GSH levels ↑ caspase 1 (pyroptosis) | [16,80] | |
| Suppression of tumor growth in NB4 and HL-60 tumor xenograft models | ↓ tumor volume, ↑ caspase-3 and caspase-9 | [16] |
| Group of Analogs | The Most Effective Analog | Cancer Cell Line | IC50 (μM) | Reference |
|---|---|---|---|---|
| 2′-aminoanalogues of 1-methoxyspirobrassinol methyl etether | (±)-trans-1,2-dimethoxy-2′-(3,5-bis-trifluoromethylphenylamino)spiro{indoline-3,5′ [4′,5′]dihydrothiazol}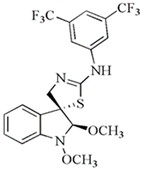 | HCT-116 | 32.2 | [89] |
| 2′-aminoanalogues of spirobrassinin | (±)-2′-[4-(Trifluoromethyl)phenylamino]spiro[indoline-3,5′-[4′,5′]dihydrothiazole]-2-one | Jurkat | 26.1 | [91] |
| 2′-aminoanalogues of cyclobrassinin | 2-(4′-fluorphenylamino)-4H-1,3-tiazino[6,5-b]indol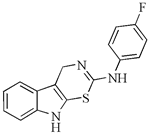 | A2780cis | 30 | [98] |
| 2′-aminoanalogues of 5-fluorospirobrassinin | (±)-5-Fluoro-2′-(3,4-dichlorophenylamino)spiro{indoline-3,5′-[4′,5′]dihydrothiazole}-2-one | Jurkat | 29.1 | [93] |
| 2,2′-diaminoanalogues of 1-methoxyspirobrassinol methyl ether | trans-(±)-1-Methoxy-2-[3,5-bis(trifluoromethyl)phenylamino]-2′-[3,5-bis(trifluoromethyl)phenylamino]spiro{indoline-3,5′-[4′,5′]dihydrothiazole}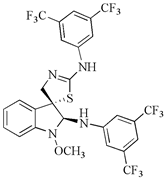 | CEM | 1 | [90] |
| Aminoanalogues of 1-methoxyisobrassinin | N-[(1-methoxyindol-2-yl)methyl]-N′-[3,5-bis(trifluoromethyl)phenyl]thiourea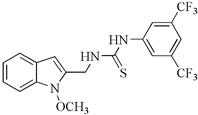 | Jurkat | 7.6 | [96] |
| Aminoanalogues of 5-bromo-1-Boc-brassinin | N-[[5-Bromo-1-(tert-butoxycarbonyl)indol-3-yl]methyl]-N’-[4-(trifluoromethyl)phenyl]thiourea | Jurkat | 5.1 | [92] |
| Bis-indole urea analogues with a phenyl linker | N,N′-(1,4-phenylene)bis{N′-[1-(tert-butoxycarbonyl)indol-3-yl]methyl (urea)}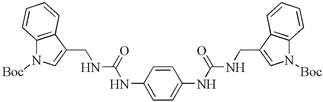 | A549 | 8.7 | [94] |
| Regioisomer of 1-methoxybrassinin | 1-methoxyisobrassinin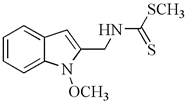 | A2780 A2780cis | 3.62 7.00 | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zigová, M.; Michalková, R.; Mojžiš, J. Anticancer Potential of Indole Phytoalexins and Their Analogues. Molecules 2024, 29, 2388. https://doi.org/10.3390/molecules29102388
Zigová M, Michalková R, Mojžiš J. Anticancer Potential of Indole Phytoalexins and Their Analogues. Molecules. 2024; 29(10):2388. https://doi.org/10.3390/molecules29102388
Chicago/Turabian StyleZigová, Martina, Radka Michalková, and Ján Mojžiš. 2024. "Anticancer Potential of Indole Phytoalexins and Their Analogues" Molecules 29, no. 10: 2388. https://doi.org/10.3390/molecules29102388
APA StyleZigová, M., Michalková, R., & Mojžiš, J. (2024). Anticancer Potential of Indole Phytoalexins and Their Analogues. Molecules, 29(10), 2388. https://doi.org/10.3390/molecules29102388







