Natural Product-Derived Phytochemicals for Influenza A Virus (H1N1) Prevention and Treatment
Abstract
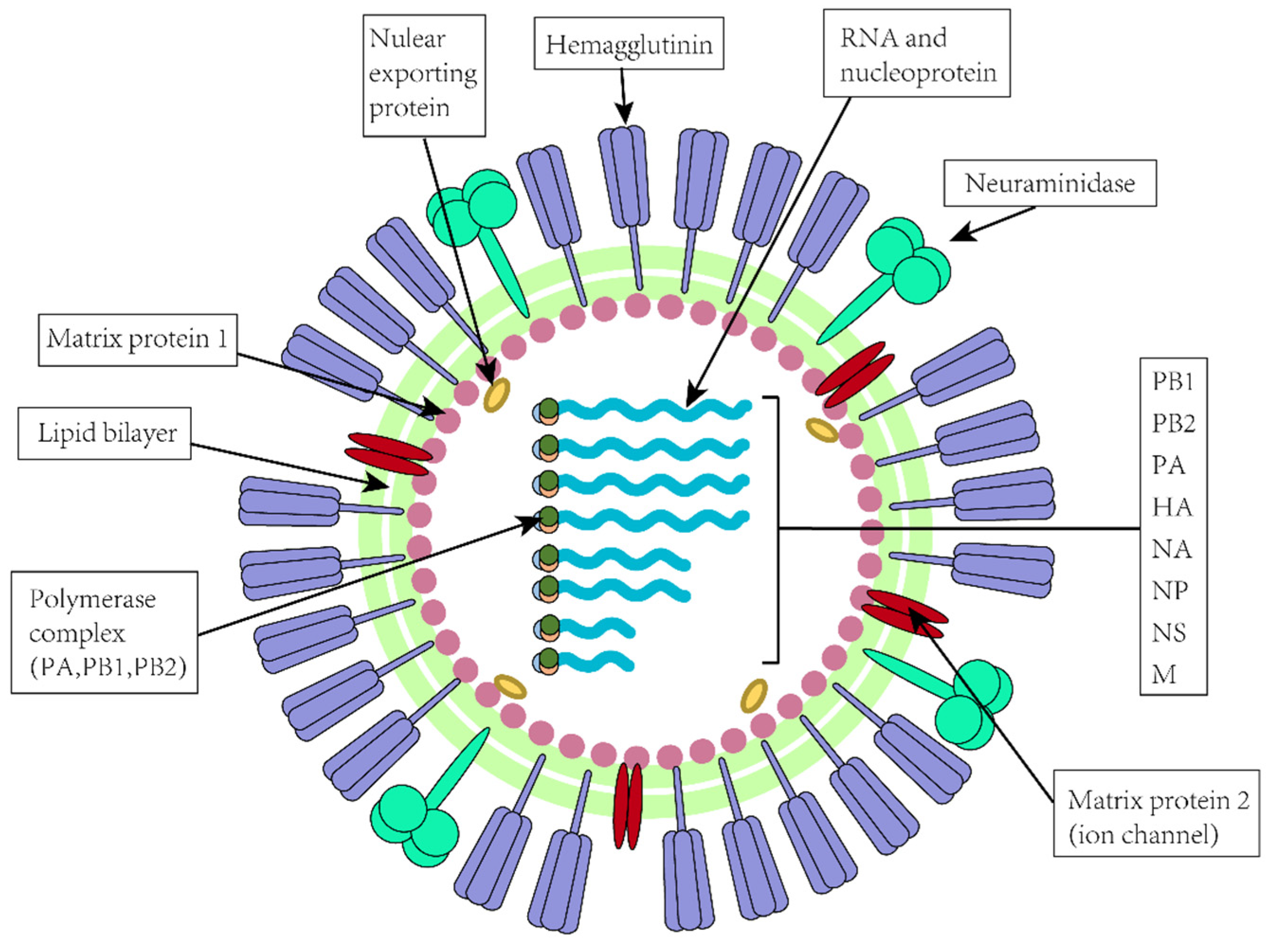
1. Introduction
2. Treatment Strategies against Influenza A (H1N1)
2.1. M2 Inhibitors
2.2. NA Inhibitors
2.3. Viral Polymerase Complex Inhibitor
2.4. NP Inhibitors
3. Natural Compounds That Exert Anti-Influenza A Effects
3.1. Marine Natural Products
3.2. Flavonoids
3.3. Alkaloids
3.4. Terpenoid Derivatives
3.5. Phenol Derivatives
3.6. Polysaccharides
3.7. Miscellaneous Compounds
3.8. Derivatives of Natural Products
4. Druggability
5. Conclusions and Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, G.J.; Vijaykrishna, D.; Bahl, J.; Lycett, S.J.; Worobey, M.; Pybus, O.G.; Ma, S.K.; Cheung, C.L.; Raghwani, J.; Bhatt, S.; et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature 2009, 459, 1122–1125. [Google Scholar] [CrossRef] [PubMed]
- Taubenberger, J.K.; Morens, D.M. 1918 Influenza: The mother of all pandemics. Emerg. Infect. Dis. 2006, 12, 15–22. [Google Scholar] [CrossRef]
- Sharon, B.; Schleiss, M.R. Pandemic Influenza A (H1N1). Pediatr. Res. 2009, 66, 599. [Google Scholar] [CrossRef] [PubMed]
- Enserink, M.; Cohen, J. Virus of the year. The novel H1N1 influenza. Science 2009, 326, 1607. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Jiang, N.; Shi, W.; Chi, X.; Liu, S.; Chen, J.L.; Wang, S. Development and Effects of Influenza Antiviral Drugs. Molecules 2021, 26, 810. [Google Scholar] [CrossRef]
- O’Hanlon, R.; Shaw, M.L. Baloxavir marboxil: The new influenza drug on the market. Curr. Opin. Virol. 2019, 35, 14–18. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Antiviral agents active against influenza A viruses. Nat. Rev. Drug Discov. 2006, 5, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Ntie-Kang, F.; Svozil, D. Volume 2 Advanced Concepts and Applications. In An Enumeration of Natural Products from Microbial, Marine and Terrestrial Sources; Ntie-Kang, F., Ed.; De Gruyter: Berlin, Germany, 2022; pp. 1–30. [Google Scholar]
- Abdal Dayem, A.; Choi, H.Y.; Kim, Y.B.; Cho, S.G. Antiviral effect of methylated flavonol isorhamnetin against influenza. PLoS ONE 2015, 10, e0121610. [Google Scholar] [CrossRef]
- Paules, C.; Subbarao, K. Influenza. Lancet 2017, 390, 697–708. [Google Scholar] [CrossRef]
- Pleschka, S. Overview of influenza viruses. Curr. Top. Microbiol. Immunol. 2013, 370, 1–20. [Google Scholar] [CrossRef]
- Durães-Carvalho, R.; Salemi, M. In-depth phylodynamics, evolutionary analysis and in silico predictions of universal epitopes of Influenza A subtypes and Influenza B viruses. Mol. Phylogenet. Evol. 2018, 121, 174–182. [Google Scholar] [CrossRef]
- Huang, S.S.H.; Banner, D.; Paquette, S.G.; Leon, A.J.; Kelvin, A.A.; Kelvin, D.J. Pathogenic influenza B virus in the ferret model establishes lower respiratory tract infection. J. Gen. Virol. 2014, 95, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, R.; Morick, D.; de Mutsert, G.; Osinga, N.; Bestebroer, T.; van der Vliet, S.; Smits, S.L.; Kuiken, T.; Rimmelzwaan, G.F.; Fouchier, R.A.; et al. Recurring influenza B virus infections in seals. Emerg. Infect. Dis. 2013, 19, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Goldhill, D.H.; Te Velthuis, A.J.W.; Fletcher, R.A.; Langat, P.; Zambon, M.; Lackenby, A.; Barclay, W.S. The mechanism of resistance to favipiravir in influenza. Proc. Natl. Acad. Sci. USA 2018, 115, 11613–11618. [Google Scholar] [CrossRef]
- Leneva, I.A.; Burtseva, E.I.; Yatsyshina, S.B.; Fedyakina, I.T.; Kirillova, E.S.; Selkova, E.P.; Osipova, E.; Maleev, V.V. Virus susceptibility and clinical effectiveness of anti-influenza drugs during the 2010–2011 influenza season in Russia. Int. J. Infect. Dis. 2016, 43, 77–84. [Google Scholar] [CrossRef]
- Haffizulla, J.; Hartman, A.; Hoppers, M.; Resnick, H.; Samudrala, S.; Ginocchio, C.; Bardin, M.; Rossignol, J.F. Effect of nitazoxanide in adults and adolescents with acute uncomplicated influenza: A double-blind, randomised, placebo-controlled, phase 2b/3 trial. Lancet Infect. Dis. 2014, 14, 609–618. [Google Scholar] [CrossRef]
- Pinto, L.H.; Holsinger, L.J.; Lamb, R.A. Influenza virus M2 protein has ion channel activity. Cell 1992, 69, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liu, T.; Zhang, J.; Zhan, P.; Liu, X. Influenza A virus polymerase: An attractive target for next-generation anti-influenza therapeutics. Drug Discov. Today 2018, 23, 503–518. [Google Scholar] [CrossRef]
- Kumar, B.; Asha, K.; Khanna, M.; Ronsard, L.; Meseko, C.A.; Sanicas, M. The emerging influenza virus threat: Status and new prospects for its therapy and control. Arch. Virol. 2018, 163, 831–844. [Google Scholar] [CrossRef]
- Bantia, S.; Arnold, C.S.; Parker, C.D.; Upshaw, R.; Chand, P. Anti-influenza virus activity of peramivir in mice with single intramuscular injection. Antivir. Res. 2006, 69, 39–45. [Google Scholar] [CrossRef]
- Bright, R.A.; Medina, M.J.; Xu, X.; Perez-Oronoz, G.; Wallis, T.R.; Davis, X.M.; Povinelli, L.; Cox, N.J.; Klimov, A.I. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: A cause for concern. Lancet 2005, 366, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.A.; Shay, D.K.; Shu, B.; Cox, N.J.; Klimov, A.I. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA 2006, 295, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Palese, P.; Schulman, J.L.; Bodo, G.; Meindl, P. Inhibition of influenza and parainfluenza virus replication in tissue culture by 2-deoxy-2,3-dehydro-N-trifluoroacetylneuraminic acid (FANA). Virology 1974, 59, 490–498. [Google Scholar] [CrossRef] [PubMed]
- McClellan, K.; Perry, C.M. Oseltamivir: A review of its use in influenza. Drugs 2001, 61, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Anuwongcharoen, N.; Shoombuatong, W.; Tantimongcolwat, T.; Prachayasittikul, V.; Nantasenamat, C. Exploring the chemical space of influenza neuraminidase inhibitors. PeerJ 2016, 4, e1958. [Google Scholar] [CrossRef] [PubMed]
- Cheer, S.M.; Wagstaff, A.J. Zanamivir: An update of its use in influenza. Drugs 2002, 62, 71–106. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Pizzorno, A.; Abed, Y.; Boivin, G. Influenza virus resistance to neuraminidase inhibitors. Antivir. Res. 2013, 98, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Reece, P.A. Neuraminidase inhibitor resistance in influenza viruses. J. Med. Virol. 2007, 79, 1577–1586. [Google Scholar] [CrossRef]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef]
- Pflug, A.; Guilligay, D.; Reich, S.; Cusack, S. Structure of influenza A polymerase bound to the viral RNA promoter. Nature 2014, 516, 355–360. [Google Scholar] [CrossRef]
- Yuan, P.; Bartlam, M.; Lou, Z.; Chen, S.; Zhou, J.; He, X.; Lv, Z.; Ge, R.; Li, X.; Deng, T.; et al. Crystal structure of an avian influenza polymerase PAN reveals an endonuclease active site. Nature 2009, 458, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Tarbet, E.B.; Maekawa, M.; Furuta, Y.; Babu, Y.S.; Morrey, J.D.; Smee, D.F. Combinations of favipiravir and peramivir for the treatment of pandemic influenza A/California/04/2009 (H1N1) virus infections in mice. Antivir. Res. 2012, 94, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Byrn, R.A.; Jones, S.M.; Bennett, H.B.; Bral, C.; Clark, M.P.; Jacobs, M.D.; Kwong, A.D.; Ledeboer, M.W.; Leeman, J.R.; McNeil, C.F.; et al. Preclinical activity of VX-787, a first-in-class, orally bioavailable inhibitor of the influenza virus polymerase PB2 subunit. Antimicrob. Agents Chemother. 2015, 59, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Noshi, T.; Kitano, M.; Taniguchi, K.; Yamamoto, A.; Omoto, S.; Baba, K.; Hashimoto, T.; Ishida, K.; Kushima, Y.; Hattori, K.; et al. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antivir. Res. 2018, 160, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.J.; Read, E.K.; Dalton, R.M.; Medcalf, L.; Digard, P. Nuclear export of influenza A virus mRNAs requires ongoing RNA polymerase II activity. Traffic 2007, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cianci, C.; Gerritz, S.W.; Deminie, C.; Krystal, M. Influenza nucleoprotein: Promising target for antiviral chemotherapy. Antivir. Chem. Chemother. 2012, 23, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Dilly, S.; Fotso Fotso, A.; Lejal, N.; Zedda, G.; Chebbo, M.; Rahman, F.; Companys, S.; Bertrand, H.C.; Vidic, J.; Noiray, M.; et al. From Naproxen Repurposing to Naproxen Analogues and Their Antiviral Activity against Influenza A Virus. J. Med. Chem. 2018, 61, 7202–7217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Fan, W.; Zhang, S.; Jiao, P.; Shang, Y.; Cui, L.; Mahesutihan, M.; Li, J.; Wang, D.; Gao, G.F.; et al. Naproxen Exhibits Broad Anti-influenza Virus Activity in Mice by Impeding Viral Nucleoprotein Nuclear Export. Cell. Rep. 2019, 27, 1875–1885.e1875. [Google Scholar] [CrossRef] [PubMed]
- Kao, R.Y.; Yang, D.; Lau, L.S.; Tsui, W.H.; Hu, L.; Dai, J.; Chan, M.P.; Chan, C.M.; Wang, P.; Zheng, B.J.; et al. Identification of influenza A nucleoprotein as an antiviral target. Nat. Biotechnol. 2010, 28, 600–605. [Google Scholar] [CrossRef]
- Liu, C.L.; Hung, H.C.; Lo, S.C.; Chiang, C.H.; Chen, I.J.; Hsu, J.T.; Hou, M.H. Using mutagenesis to explore conserved residues in the RNA-binding groove of influenza A virus nucleoprotein for antiviral drug development. Sci. Rep. 2016, 6, 21662. [Google Scholar] [CrossRef]
- Hu, Y.; Sneyd, H.; Dekant, R.; Wang, J. Influenza A Virus Nucleoprotein: A Highly Conserved Multi-Functional Viral Protein as a Hot Antiviral Drug Target. Curr. Top. Med. Chem. 2017, 17, 2271–2285. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.F.; Xu, L.; Wei, M.Y.; Wang, C.Y.; Gu, Y.C.; Shao, C.L. Recent progresses in marine microbial-derived antiviral natural products. Arch. Pharm. Res. 2020, 43, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Osmanova, N.; Schultze, W.; Ayoub, N. Azaphilones: A class of fungal metabolites with diverse biological activities. Phytochem. Rev. 2010, 9, 315–342. [Google Scholar] [CrossRef]
- Gao, J.M.; Yang, S.X.; Qin, J.C. Azaphilones: Chemistry and biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef]
- Eade, R.A.; Page, H.; Robertson, A.; Turner, K.; Whalley, W.B. 986. The chemistry of fungi. Part XXVIII. Sclerotiorin and its hydrogenation products. J. Chem. Soc. Resumed 1957, 4913–4924. [Google Scholar] [CrossRef]
- Seto, H.; Tanabe, M. Utilization of 13C-13C coupling in structural and biosynthetic studies. III. Ochrephilone—A new fungal metabolite. Tetrahedron Lett. 1974, 15, 651–654. [Google Scholar] [CrossRef]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-C.; Tseng, C.-P.; Yang, J.-M.; Ju, Y.-W.; Tseng, S.-N.; Chen, Y.-F.; Chao, Y.-S.; Hsieh, H.-P.; Shih, S.-R.; Hsu, J.T.A. Aurintricarboxylic acid inhibits influenza virus neuraminidase. Antivir. Res. 2009, 81, 123–131. [Google Scholar] [CrossRef]
- Jia, Q.; Du, Y.; Wang, C.; Wang, Y.; Zhu, T.; Zhu, W. Azaphilones from the Marine Sponge-Derived Fungus Penicillium sclerotiorum OUCMDZ-3839. Mar. Drugs 2019, 17, 260. [Google Scholar] [CrossRef]
- He, F.; Bao, J.; Zhang, X.Y.; Tu, Z.C.; Shi, Y.M.; Qi, S.H. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013, 76, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, Z.; Liu, P.; Wang, Y.; Xin, Z.; Zhu, W. New rubrolides from the marine-derived fungus Aspergillus terreus OUCMDZ-1925. J. Antibiot. 2014, 67, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.Q.; Lin, X.P.; Wang, Z.; Zhou, X.F.; Qin, X.C.; Kaliyaperumal, K.; Zhang, T.Y.; Tu, Z.C.; Liu, Y. Asteltoxins with Antiviral Activities from the Marine Sponge-Derived Fungus Aspergillus sp. SCSIO XWS02F40. Molecules 2015, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Z.; Zhu, G.; Wang, L.; Du, Y.; Wang, Y.; Zhu, W. Phenolic polyketides from the marine alga-derived Streptomyces sp. OUCMDZ-3434. Tetrahedron 2017, 73, 5451–5455. [Google Scholar] [CrossRef]
- Chen, D.; Lu, S.; Yang, G.; Pan, X.; Fan, S.; Xie, X.; Chen, Q.; Li, F.; Li, Z.; Wu, S.; et al. The seafood Musculus senhousei shows anti-influenza A virus activity by targeting virion envelope lipids. Biochem. Pharmacol. 2020, 177, 113982. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.K.; Tang, X.L.; Zhang, G.; Cheng, C.L.; Zhang, X.W.; Li, P.L.; Li, G.Q. Polyhydroxylated steroids from the South China Sea soft coral Sarcophyton sp. and their cytotoxic and antiviral activities. Mar. Drugs 2013, 11, 4788–4798. [Google Scholar] [CrossRef]
- Ha, T.K.; Dao, T.T.; Nguyen, N.H.; Kim, J.; Kim, E.; Cho, T.O.; Oh, W.K. Antiviral phenolics from the leaves of Cleistocalyx operculatus. Fitoterapia 2016, 110, 135–141. [Google Scholar] [CrossRef]
- Huh, J.; Ha, T.K.Q.; Kang, K.B.; Kim, K.H.; Oh, W.K.; Kim, J.; Sung, S.H. C-Methylated Flavonoid Glycosides from Pentarhizidium orientale Rhizomes and Their Inhibitory Effects on the H1N1 Influenza Virus. J. Nat. Prod. 2017, 80, 2818–2824. [Google Scholar] [CrossRef]
- Lee, I.K.; Hwang, B.S.; Kim, D.W.; Kim, J.Y.; Woo, E.E.; Lee, Y.J.; Choi, H.J.; Yun, B.S. Characterization of Neuraminidase Inhibitors in Korean Papaver rhoeas Bee Pollen Contributing to Anti-Influenza Activities In Vitro. Planta Med. 2016, 82, 524–529. [Google Scholar] [CrossRef]
- Jeong, H.J.; Ryu, Y.B.; Park, S.J.; Kim, J.H.; Kwon, H.J.; Kim, J.H.; Park, K.H.; Rho, M.C.; Lee, W.S. Neuraminidase inhibitory activities of flavonols isolated from Rhodiola rosea roots and their in vitro anti-influenza viral activities. Bioorg. Med. Chem. 2009, 17, 6816–6823. [Google Scholar] [CrossRef]
- Roschek, B., Jr.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Quy Ha, T.K.; Lee, C.; Li, W.; Oh, W.K.; Shim, S.H. Antiviral activities of compounds from aerial parts of Salvia plebeia R. Br. J. Ethnopharmacol. 2016, 192, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Jin, J.; Dou, J.; Guo, Q.; Ke, X.; Zhou, C.; Guo, M. Inhibitory Activity of Honeysuckle Extracts against Influenza A Virus In Vitro and In Vivo. Virol. Sin. 2021, 36, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, B.; Zhang, X.; Bing, F. Homonojirimycin, an alkaloid from dayflower inhibits the growth of influenza A virus in vitro. Acta Virol. 2013, 57, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kong, F.; Wei, J.; Wang, Y.; Wang, W.; Hong, K.; Zhu, W. Alkaloids from the mangrove-derived actinomycete Jishengella endophytica 161111. Mar. Drugs 2014, 12, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.K.; Tang, X.L.; Liu, Y.S.; Li, P.L.; Li, G.Q. Imidazole Alkaloids from the South China Sea Sponge Pericharax heteroraphis and Their Cytotoxic and Antiviral Activities. Molecules 2016, 21, 150. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.T.; Karimi, A.; Rafieian-Kopaei, M.; Fotouhi, F. In vitro antiviral effects of Peganum harmala seed extract and its total alkaloids against Influenza virus. Microb. Pathog. 2017, 110, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Leser, G.P.; Lamb, R.A. Repurposing Papaverine as an Antiviral Agent against Influenza Viruses and Paramyxoviruses. J. Virol. 2020, 94, e01888-19. [Google Scholar] [CrossRef] [PubMed]
- Bandaranayake, W.M. Bioactivities, bioactive compounds and chemical constituents of mangrove plants. Wetl. Ecol. Manag. 2002, 10, 421–452. [Google Scholar] [CrossRef]
- Gong, K.K.; Li, P.L.; Qiao, D.; Zhang, X.W.; Chu, M.J.; Qin, G.F.; Tang, X.L.; Li, G.Q. Cytotoxic and Antiviral Triterpenoids from the Mangrove Plant Sonneratia paracaseolaris. Molecules 2017, 22, 1319. [Google Scholar] [CrossRef]
- Zhu, Q.; Amen, Y.M.; Ohnuki, K.; Shimizu, K. Anti-influenza effects of Ganoderma lingzhi: An animal study. J. Funct. Foods 2017, 34, 224–228. [Google Scholar] [CrossRef]
- Yamamoto, T.; Izumi, N.; Ui, H.; Sueki, A.; Masuma, R.; Nonaka, K.; Hirose, T.; Sunazuka, T.; Nagai, T.; Yamada, H.; et al. Wickerols A and B: Novel anti-influenza virus diterpenes produced by Trichoderma atroviride FKI-3849. Tetrahedron 2012, 68, 9267–9271. [Google Scholar] [CrossRef]
- Yan, X.; Ouyang, H.; Wang, W.; Liu, J.; Li, T.; Wu, B.; Yan, X.; He, S. Antimicrobial Terpenoids from South China Sea Soft Coral Lemnalia sp. Mar. Drugs 2021, 19, 294. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Yau, L.-F.; Mai, Z.-T.; Li, R.-F.; Yang, Z.-F.; Zhu, G.-Y.; Jiang, Z.-H.; Wang, J.-R. Aculeatusane A: A new diterpenoid from the whole plants of Celastrus Aculeatus Merr. Phytochem. Lett. 2020, 40, 72–75. [Google Scholar] [CrossRef]
- Parhira, S.; Yang, Z.F.; Zhu, G.Y.; Chen, Q.L.; Zhou, B.X.; Wang, Y.T.; Liu, L.; Bai, L.P.; Jiang, Z.H. In vitro anti-influenza virus activities of a new lignan glycoside from the latex of Calotropis gigantea. PLoS ONE 2014, 9, e104544. [Google Scholar] [CrossRef]
- Yang, Z.F.; Bai, L.P.; Huang, W.B.; Li, X.Z.; Zhao, S.S.; Zhong, N.S.; Jiang, Z.H. Comparison of in vitro antiviral activity of tea polyphenols against influenza A and B viruses and structure-activity relationship analysis. Fitoterapia 2014, 93, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yim, J.H.; Kim, S.Y.; Kim, H.S.; Lee, W.G.; Kim, S.J.; Kang, P.S.; Lee, C.K. In vitro inhibition of influenza A virus infection by marine microalga-derived sulfated polysaccharide p-KG03. Antivir. Res. 2012, 93, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Wang, W.; Zhao, X.; Zhang, J.; Ewart, S.H. Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. J. Ocean. Univ. China 2012, 11, 205–212. [Google Scholar] [CrossRef]
- Yu, G.; Li, M.; Wang, W.; Liu, X.; Zhao, X.; Lv, Y.; Li, G.; Jiao, G.; Zhao, X. Structure and anti-influenza A (H1N1) virus activity of three polysaccharides from Eucheuma denticulatum. J. Ocean. Univ. China 2012, 11, 527–532. [Google Scholar] [CrossRef]
- Peng, M.H.; Dai, W.P.; Liu, S.J.; Yu, L.W.; Wu, Y.N.; Liu, R.; Chen, X.L.; Lai, X.P.; Li, X.; Zhao, Z.X.; et al. Bioactive glycosides from the roots of Ilex asprella. Pharm. Biol. 2016, 54, 2127–2134. [Google Scholar] [CrossRef]
- Lee, B.W.; Ha, T.K.Q.; Cho, H.M.; An, J.P.; Kim, S.K.; Kim, C.S.; Kim, E.; Oh, W.K. Antiviral activity of furanocoumarins isolated from Angelica dahurica against influenza a viruses H1N1 and H9N2. J. Ethnopharmacol. 2020, 259, 112945. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Yang, L.; Sun, W.; Si, L.; Xiao, S.; Wang, Q.; Dechoux, L.; Thorimbert, S.; Sollogoub, M.; Zhou, D.; et al. Design, synthesis and biological evaluation of gentiopicroside derivatives as potential antiviral inhibitors. Eur. J. Med. Chem. 2017, 130, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Quan, L.; Zhou, Y.; Cheng, Y.; Li, H.; Chen, X.; Li, R.; Liu, D. Exploring the anti-influenza virus activity of novel triptolide derivatives targeting nucleoproteins. Bioorg. Chem. 2022, 129, 106118. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Yarovaya, O.I.; Baranova, D.V.; Galochkina, A.V.; Shtro, A.A.; Kireeva, M.V.; Borisevich, S.S.; Gatilov, Y.V.; Zarubaev, V.V.; Salakhutdinov, N.F. Quaternary ammonium salts based on (-)-borneol as effective inhibitors of influenza virus. Arch. Virol. 2021, 166, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Xue, H.J.; Ye, W.C.; Fang, B.H.; Liu, Y.H.; Yuan, S.H.; Yu, P.; Wang, Y.Q. Activity of andrographolide and its derivatives against influenza virus in vivo and in vitro. Biol. Pharm. Bull. 2009, 32, 1385–1391. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fang, J.S.; Lian, W.W.; Pang, X.C.; Liu, A.L.; Du, G.H. In vitro antiviral effects and 3D QSAR study of resveratrol derivatives as potent inhibitors of influenza H1N1 neuraminidase. Chem. Biol. Drug Des. 2015, 85, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Huang, B.L.; Dai, S.Y.; Song, L.D.; Yu, H.F.; Yu, X.L.; Zhang, R.P. Synthesis of pterodontic acid derivatives and the study of their anti-influenza A virus (H1N1) activity. Fitoterapia 2021, 152, 104942. [Google Scholar] [CrossRef]
- Xue, S.T.; He, W.Y.; Ma, L.L.; Wang, H.Q.; Wang, B.; Zheng, G.H.; Ji, X.Y.; Zhang, T.; Li, Y.H.; Jiang, J.D.; et al. Synthesis and anti-influenza virus activities of a novel class of gastrodin derivatives. Molecules 2013, 18, 3789–3805. [Google Scholar] [CrossRef] [PubMed]
- Tret’yakova, E.V.; Ma, X.; Kazakova, O.B.; Shtro, A.A.; Petukhova, G.D.; Klabukov, A.M.; Dyatlov, D.S.; Smirnova, A.A.; Xu, H.; Xiao, S. Synthesis and evaluation of diterpenic Mannich bases as antiviral agents against influenza A and SARS-CoV-2. Phytochem. Lett. 2022, 51, 91–96. [Google Scholar] [CrossRef]
- Chukicheva, I.Y.; Buravlev, E.V.; Dvornikova, I.A.; Fedorova, I.V.; Zarubaev, V.V.; Slita, A.V.; Esaulkova, Y.L.; Kutchin, A.V. Evaluation of antiviral activity of terpenophenols and some of their N- and O-derivatives. Russ. Chem. Bull. 2022, 71, 2473–2481. [Google Scholar] [CrossRef]
- Cui, M.Y.; Xiao, M.W.; Xu, L.J.; Chen, Y.; Liu, A.L.; Ye, J.; Hu, A.X. Bioassay of ferulic acid derivatives as influenza neuraminidase inhibitors. Arch. Pharm. 2020, 353, e1900174. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Chuyen, N.V. Anti-Hyperglycemic Activity of an Aqueous Extract from Flower Buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Biosci. Biotechnol. Biochem. 2007, 71, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.T.; Tung, B.T.; Nguyen, P.H.; Thuong, P.T.; Yoo, S.S.; Kim, E.H.; Kim, S.K.; Oh, W.K. C-Methylated Flavonoids from Cleistocalyx operculatus and Their Inhibitory Effects on Novel Influenza A (H1N1) Neuraminidase. J. Nat. Prod. 2010, 73, 1636–1642. [Google Scholar] [CrossRef]
- Min, B.S.; Thu, C.V.; Dat, N.T.; Dang, N.H.; Jang, H.S.; Hung, T.M. Antioxidative flavonoids from Cleistocalyx operculatus buds. Chem. Pharm. Bull. 2008, 56, 1725–1728. [Google Scholar] [CrossRef]
- Min, B.S.; Cuong, T.D.; Lee, J.S.; Shin, B.S.; Woo, M.H.; Hung, T.M. Cholinesterase inhibitors from Cleistocalyx operculatus buds. Arch. Pharm. Res. 2010, 33, 1665–1670. [Google Scholar] [CrossRef]
- Li, B.; Ni, Y.; Zhu, L.J.; Wu, F.B.; Yan, F.; Zhang, X.; Yao, X.S. Flavonoids from Matteuccia struthiopteris and Their Anti-influenza Virus (H1N1) Activity. J. Nat. Prod. 2015, 78, 987–995. [Google Scholar] [CrossRef]
- Basnet, P.; Kadota, S.; Shimizu, M.; Xu, H.X.; Namba, T. 2′-Hydroxymatteucinol, a new C-methyl flavanone derivative from Matteccia orientalis; potent hypoglycemic activity in streptozotocin (STZ)-induced diabetic rat. Chem. Pharm. Bull. 1993, 41, 1790–1795. [Google Scholar] [CrossRef]
- Pu, W.-L.; Zhang, M.-Y.; Bai, R.-Y.; Sun, L.-K.; Li, W.-H.; Yu, Y.-L.; Zhang, Y.; Song, L.; Wang, Z.-X.; Peng, Y.-F.; et al. Anti-inflammatory effects of Rhodiola rosea L.: A review. Biomed. Pharmacother. 2020, 121, 109552. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tang, M.; Yuan, S.; Fu, S.; Li, Y.; Li, Y.; Wang, Q.; Cao, Y.; Liu, L.; Zhang, Q. Rhodiola rosea: A Therapeutic Candidate on Cardiovascular Diseases. Oxidative Med. Cell. Longev. 2022, 2022, 1348795. [Google Scholar] [CrossRef]
- Amsterdam, J.D.; Panossian, A.G. Rhodiola rosea L. as a putative botanical antidepressant. Phytomedicine 2016, 23, 770–783. [Google Scholar] [CrossRef]
- Liu, A.L.; Wang, H.D.; Lee, S.M.; Wang, Y.T.; Du, G.H. Structure-activity relationship of flavonoids as influenza virus neuraminidase inhibitors and their in vitro anti-viral activities. Bioorg. Med. Chem. 2008, 16, 7141–7147. [Google Scholar] [CrossRef] [PubMed]
- de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Barak, V.; Halperin, T.; Kalickman, I. The effect of Sambucol, a black elderberry-based, natural product, on the production of human cytokines: I. Inflammatory cytokines. Eur. Cytokine Netw. 2001, 12, 290–296. [Google Scholar] [PubMed]
- Santin, J.R.; Benvenutti, L.; Broering, M.F.; Nunes, R.; Goldoni, F.C.; Patel, Y.B.K.; de Souza, J.A.; Kopp, M.A.T.; de Souza, P.; da Silva, R.C.V.; et al. Sambucus nigra: A traditional medicine effective in reducing inflammation in mice. J. Ethnopharmacol. 2022, 283, 114736. [Google Scholar] [CrossRef] [PubMed]
- Zakay-Rones, Z.; Thom, E.; Wollan, T.; Wadstein, J. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza A and B virus infections. J. Int. Med. Res. 2004, 32, 132–140. [Google Scholar] [CrossRef]
- Imanishi, N.; Tuji, Y.; Katada, Y.; Maruhashi, M.; Konosu, S.; Mantani, N.; Terasawa, K.; Ochiai, H. Additional inhibitory effect of tea extract on the growth of influenza A and B viruses in MDCK cells. Microbiol. Immunol. 2002, 46, 491–494. [Google Scholar] [CrossRef]
- Nagai, T.; Moriguchi, R.; Suzuki, Y.; Tomimori, T.; Yamada, H. Mode of action of the anti-influenza virus activity of plant flavonoid, 5,7,4′-trihydroxy-8-methoxyflavone, from the roots of Scutellaria baicalensis. Antivir. Res. 1995, 26, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Knox, Y.M.; Hayashi, K.; Suzutani, T.; Ogasawara, M.; Yoshida, I.; Shiina, R.; Tsukui, A.; Terahara, N.; Azuma, M. Activity of anthocyanins from fruit extract of Ribes nigrum L. against influenza A and B viruses. Acta Virol. 2001, 45, 209–215. [Google Scholar]
- Song, J.M.; Lee, K.H.; Seong, B.L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Serkedjieva, J.; Manolova, N.; Zgórniak-Nowosielska, I.; Zawilińska, B.; Grzybek, J. Antiviral activity of the infusion (SHS-174) from flowers of Sambucus nigra L., aerial parts of Hypericum perforatum L., and roots of Saponaria officinalis L. against influenza and herpes simplex viruses. Phytother. Res. 1990, 4, 97–100. [Google Scholar] [CrossRef]
- Cao, Y.X.; Ji, P.; Wu, F.L.; Dong, J.Q.; Li, C.C.; Ma, T.; Yang, H.C.; Wei, Y.M.; Hua, Y.L. Lonicerae japonicae caulis: A review of its research progress of active metabolites and pharmacological effects. Front. Pharmacol. 2023, 14, 1277283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, X.; Liu, J.; Dong, L.; Chen, Q.; Liu, J.; Kong, H.; Zhang, Q.; Qi, X.; Hou, D.; et al. Honeysuckle-encoded atypical microRNA2911 directly targets influenza A viruses. Cell Res. 2015, 25, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Afifi, F.U.; Kasabri, V.; Al-Jaber, H.I.; Abu-Irmaileh, B.E.; Al-Qudah, M.A.; Abazaa, I.F. Composition and Biological Activity of Volatile Oil from Salviajudaica and S. multicaulis from Jordan. Nat. Prod. Commun. 2016, 11, 535–538. [Google Scholar] [PubMed]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [PubMed]
- Krol, A.; Kokotkiewicz, A.; Luczkiewicz, M. White Sage (Salvia apiana)—A Ritual and Medicinal Plant of the Chaparral: Plant Characteristics in Comparison with Other Salvia Species. Planta Med. 2022, 88, 604–627. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Huang, B.; Yu, K.; Shi, F.; Liu, T.; Xu, W. Caffeic acid derivatives: A new type of influenza neuraminidase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 3556–3560. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.; Hanada, A.; Matsubara, C.; Horio, Y.; Sumitani, H.; Ogata, T.; Isegawa, Y. Anti-influenza A virus activity of flavonoids in vitro: A structure-activity relationship. J. Nat. Med. 2023, 77, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Abookleesh, F.L.; Al-Anzi, B.S.; Ullah, A. Potential Antiviral Action of Alkaloids. Molecules 2022, 27, 903. [Google Scholar] [CrossRef]
- Youn, J.Y.; Park, H.Y.; Cho, K.H. Anti-hyperglycemic activity of Commelina communis L.: Inhibition of alpha-glucosidase. Diabetes Res. Clin. Pract. 2004, 66 (Suppl. 1), S149–S155. [Google Scholar] [CrossRef]
- Yang, Q.; Ye, G.; Zhao, W.-M. Chemical Constituents of Commelina communis Linn. Biochem. Syst. Ecol. 2007, 35, 621–623. [Google Scholar] [CrossRef]
- Hong, K.; Gao, A.H.; Xie, Q.Y.; Gao, H.; Zhuang, L.; Lin, H.P.; Yu, H.P.; Li, J.; Yao, X.S.; Goodfellow, M.; et al. Actinomycetes for marine drug discovery isolated from mangrove soils and plants in China. Mar. Drugs 2009, 7, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.Y.; Wang, C.; Wang, R.; Qu, Z.; Lin, H.P.; Goodfellow, M.; Hong, K. Jishengella endophytica gen. nov., sp. nov., a new member of the family Micromonosporaceae. Int. J. Syst. Evol. Microbiol. 2011, 61, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; Kehraus, S.; König, G.M.; Woerheide, G.; Wright, A.D. New and biologically active imidazole alkaloids from two sponges of the genus Leucetta. J. Nat. Prod. 2002, 65, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Plubrukarn, A.; Smith, D.W.; Cramer, R.E.; Davidson, B.S. (2E,9E)-pyronaamidine 9-(N-methylimine), a new imidazole alkaloid from the northern Mariana islands sponge Leucetta sp. cf. chagosensis. J. Nat. Prod. 1997, 60, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kawabata, T.; Kato, H.; Ohta, T.; Rotinsulu, H.; Mangindaan, R.E.; van Soest, R.W.; Ukai, K.; Kobayashi, H.; Namikoshi, M. Naamidines H and I, cytotoxic imidazole alkaloids from the Indonesian marine sponge Leucetta chagosensis. J. Nat. Prod. 2007, 70, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Ralifo, P.; Crews, P. A new structural theme in the imidazole-containing alkaloids from a calcareous Leucetta sponge. J. Org. Chem. 2004, 69, 9025–9029. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Schmitz, F.J.; Tanner, R.S.; Kelly-Borges, M. New imidazole alkaloids and zinc complexes from the micronesian sponge Leucetta cf. chagosensis. J. Nat. Prod. 1998, 61, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.W.; Mong, S.; Hemling, M.E.; Freyer, A.J.; Offen, P.H.; DeBrosse, C.W.; Sarau, H.M.; Westley, J.W. New leukotriene B4 receptor antagonist: Leucettamine A and related imidazole alkaloids from the marine sponge Leucetta microraphis. J. Nat. Prod. 1993, 56, 116–121. [Google Scholar] [CrossRef]
- Copp, B.R.; Fairchild, C.R.; Cornell, L.; Casazza, A.M.; Robinson, S.; Ireland, C.M. Naamidine A is an antagonist of the epidermal growth factor receptor and an in vivo active antitumor agent. J. Med. Chem. 1998, 41, 3909–3911. [Google Scholar] [CrossRef]
- Akee, R.K.; Carroll, T.R.; Yoshida, W.Y.; Scheuer, P.J.; Stout, T.J.; Clardy, J. Two imidazole alkaloids from a sponge. J. Org. Chem. 1990, 55, 1944–1946. [Google Scholar] [CrossRef]
- Crews, P.; Clark, D.P.; Tenney, K. Variation in the Alkaloids among Indo-Pacific Leucetta Sponges. J. Nat. Prod. 2003, 66, 177–182. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, Z.T.; Branford-White, C.J.; Xu, H.; Wang, C.H. Classification and differentiation of the genus Peganum indigenous to China based on chloroplast trnL-F and psbA-trnH sequences and seed coat morphology. Plant Biol. 2011, 13, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Mina, C.N.; Farzaei, M.H.; Gholamreza, A. Medicinal properties of Peganum harmala L. in traditional Iranian medicine and modern phytotherapy: A review. J. Tradit. Chin. Med. 2015, 35, 104–109. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Mikaili, P.; Aghajanshakeri, S.; Asghari, M.H.; Shayegh, J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn. Rev. 2013, 7, 199–212. [Google Scholar] [CrossRef]
- Liu, H.M.; Tu, Y.K. The efficacy of papaverine administration by different routes for the treatment of experimental acute cerebral vasospasm. J. Clin. Neurosci. 2002, 9, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Chappie, T.A.; Humphrey, J.M.; Allen, M.P.; Estep, K.G.; Fox, C.B.; Lebel, L.A.; Liras, S.; Marr, E.S.; Menniti, F.S.; Pandit, J.; et al. Discovery of a series of 6,7-dimethoxy-4-pyrrolidylquinazoline PDE10A inhibitors. J. Med. Chem. 2007, 50, 182–185. [Google Scholar] [CrossRef]
- Yildiz, N.; Gokkaya, N.K.; Koseoglu, F.; Gokkaya, S.; Comert, D. Efficacies of papaverine and sildenafil in the treatment of erectile dysfunction in early-stage paraplegic men. Int. J. Rehabil. Res. 2011, 34, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, Y.; Sheng, W.; Sun, J.; Qin, G. Chemical constituents of Isatis indigotica. Planta Med. 1997, 63, 55–57. [Google Scholar] [CrossRef]
- Guo, Q.; Li, D.; Xu, C.; Zhu, C.; Guo, Y.; Yu, H.; Wang, X.; Shi, J. Indole alkaloid glycosides with a 1′-(phenyl)ethyl unit from Isatis indigotica leaves. Acta Pharm. Sin. B 2020, 10, 895–902. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in Pharmacological Activities of Terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Patra, A.; Chaudhuri, S.K.; Panda, S.K. Betulin-3-Caffeate from Quercus suber, 13C-nmr Spectra of Some Lupenes. J. Nat. Prod. 1988, 51, 217–220. [Google Scholar] [CrossRef]
- Bishop, K.S.; Kao, C.H.; Xu, Y.; Glucina, M.P.; Paterson, R.R.; Ferguson, L.R. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry 2015, 114, 56–65. [Google Scholar] [CrossRef]
- Cao, Y.; Wu, S.-H.; Dai, Y.-C. Species clarification of the prize medicinal Ganoderma mushroom “Lingzhi”. Fungal Divers. 2012, 56, 49–62. [Google Scholar] [CrossRef]
- Paterson, R.R. Ganoderma—A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S.; Guo, J.J.; Bao, J.L.; Li, X.W.; Chen, X.P.; Lu, J.J.; Wang, Y.T. Anti-cancer properties of triterpenoids isolated from Ganoderma lucidum—A review. Expert Opin. Investig. Drugs 2013, 22, 981–992. [Google Scholar] [CrossRef]
- Fatmawati, S.; Shimizu, K.; Kondo, R. Ganoderol B: A potent α-glucosidase inhibitor isolated from the fruiting body of Ganoderma lucidum. Phytomedicine 2011, 18, 1053–1055. [Google Scholar] [CrossRef]
- Liang, L.F.; Chen, W.T.; Mollo, E.; Yao, L.G.; Wang, H.Y.; Xiao, W.; Guo, Y.W. Sarcophytrols G–L, Novel Minor Metabolic Components from South China Sea Soft Coral Sarcophyton trocheliophorum Marenzeller. Chem. Biodivers. 2017, 14, e1700079. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, X.; Liu, H.; Luo, X.; Sung, P.J.; Li, P.; Li, G. Clavukoellians G-K, New Nardosinane and Aristolane Sesquiterpenoids with Angiogenesis Promoting Activity from the Marine Soft Coral Lemnalia sp. Mar. Drugs 2020, 18, 171. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Sun, J.; Chen, J.; Zhang, H.; Guo, Y.W.; Wang, H. Terpenoids from Marine Soft Coral of the Genus Lemnalia: Chemistry and Biological Activities. Mar. Drugs 2018, 16, 320. [Google Scholar] [CrossRef]
- Tang, W.-H.; Bai, S.-T.; Tong, L.; Duan, W.-J.; Su, J.-W.; Chen, J.-X.; Xie, Y. Chemical constituents from Celastrus aculeatus Merr. Biochem. Syst. Ecol. 2014, 54, 78–82. [Google Scholar] [CrossRef]
- Tong, L.; Moudgil, K.D. Celastrus aculeatus Merr. suppresses the induction and progression of autoimmune arthritis by modulating immune response to heat-shock protein 65. Arthritis Res. Ther. 2007, 9, R70. [Google Scholar] [CrossRef] [PubMed]
- Dang, Z.; Jung, K.; Zhu, L.; Xie, H.; Lee, K.-H.; Chen, C.-H.; Huang, L. Phenolic Diterpenoid Derivatives as Anti-Influenza A Virus Agents. ACS Med. Chem. Lett. 2015, 6, 355–358. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of Phenolics by Focusing on Suppression of Chronic Diseases: A Review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Pari, K.; Rao, P.J.; Devakumar, C.; Rastogi, J.N. A Novel Insect Antifeedant Nonprotein Amino Acid from Calotropis gigantea. J. Nat. Prod. 1998, 61, 102–104. [Google Scholar] [CrossRef]
- Lhinhatrakool, T.; Sutthivaiyakit, S. 19-Nor- and 18,20-epoxy-cardenolides from the leaves of Calotropis gigantea. J. Nat. Prod. 2006, 69, 1249–1251. [Google Scholar] [CrossRef]
- Silva, M.C.C.; da Silva, A.B.; Teixeira, F.M.; de Sousa, P.C.P.; Rondon, R.M.M.; Honório, J.E.R.; Sampaio, L.R.L.; Oliveira, S.L.; Holonda, A.N.M.; de Vasconcelos, S.M.M. Therapeutic and biological activities of Calotropis procera (Ait.) R. Br. Asian Pacific Journal of Tropical Medicine 2010, 3, 332–336. [Google Scholar] [CrossRef]
- Parhira, S.; Zhu, G.Y.; Jiang, R.W.; Liu, L.; Bai, L.P.; Jiang, Z.H. 2′-Epi-uscharin from the latex of Calotropis gigantea with HIF-1 inhibitory activity. Sci. Rep. 2014, 4, 4748. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, W.J.; Ehrhardt, C.; Pleschka, S.; Berberich-Siebelt, F.; Wolff, T.; Walczak, H.; Planz, O.; Ludwig, S. NF-kappaB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. J. Biol. Chem. 2004, 279, 30931–30937. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, S.; Dai, J.; Wang, L.; Xu, Y.; Peng, X.; Xie, X.; Peng, C. Molecular mechanisms and applications of tea polyphenols: A narrative review. J. Food Biochem. 2021, 45, e13910. [Google Scholar] [CrossRef]
- Talarico, L.B.; Pujol, C.A.; Zibetti, R.G.; Faría, P.C.; Noseda, M.D.; Duarte, M.E.; Damonte, E.B. The antiviral activity of sulfated polysaccharides against dengue virus is dependent on virus serotype and host cell. Antivir. Res. 2005, 66, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int. J. Biol. Macromol. 1995, 17, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Balzarini, J.; Shigeta, S.; De Clercq, E. Differential inhibitory effects of sulfated polysaccharides and polymers on the replication of various myxoviruses and retroviruses, depending on the composition of the target amino acid sequences of the viral envelope glycoproteins. Antimicrob. Agents Chemother. 1991, 35, 2515–2520. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.H.; Lee, H.K. Axenic culture of Gyrodinium impudicum strain KG03, a marine red-tide microalga that produces exopolysaccharide. J. Microbiol. 2004, 42, 305–314. [Google Scholar] [PubMed]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef]
- Talarico, L.B.; Damonte, E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Witvrouw, M.; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Bade, R.; Chan, H.F.; Reynisson, J. Characteristics of known drug space. Natural products, their derivatives and synthetic drugs. Eur. J. Med. Chem. 2010, 45, 5646–5652. [Google Scholar] [CrossRef]
- Wang, Y.M.; Xu, M.; Wang, D.; Yang, C.R.; Zeng, Y.; Zhang, Y.J. Anti-inflammatory compounds of “Qin-Jiao”, the roots of Gentiana dahurica (Gentianaceae). J. Ethnopharmacol. 2013, 147, 341–348. [Google Scholar] [CrossRef]
- Jin, H.; Li, D.; Lin, M.H.; Li, L.; Harrich, D. Tat-Based Therapies as an Adjuvant for an HIV-1 Functional Cure. Viruses 2020, 12, 415. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Yarovaya, O.I.; Shernyukov, A.V.; Gatilov, Y.V.; Razumova, Y.V.; Zarubaev, V.V.; Tretiak, T.S.; Pokrovsky, A.G.; Kiselev, O.I.; Salakhutdinov, N.F. Discovery of a new class of antiviral compounds: Camphor imine derivatives. Eur. J. Med. Chem. 2015, 105, 263–273. [Google Scholar] [CrossRef]
- Sokolova, A.S.; Yarovaya, О.I.; Baev, D.S.; Shernyukov, А.V.; Shtro, A.A.; Zarubaev, V.V.; Salakhutdinov, N.F. Aliphatic and alicyclic camphor imines as effective inhibitors of influenza virus H1N1. Eur. J. Med. Chem. 2017, 127, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Sokolova, A.S.; Yarovaya, O.C.; Korchagina, D.V.; Zarubaev, V.V.; Tretiak, T.S.; Anfimov, P.M.; Kiselev, O.I.; Salakhutdinov, N.F. Camphor-based symmetric diimines as inhibitors of influenza virus reproduction. Bioorg. Med. Chem. 2014, 22, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wu, T.; Huang, L. Therapeutic and delivery strategies of phytoconstituents for renal fibrosis. Adv. Drug Deliv. Rev. 2021, 177, 113911. [Google Scholar] [CrossRef]
- Rodrigues, T.; Reker, D.; Schneider, P.; Schneider, G. Counting on natural products for drug design. Nat. Chem. 2016, 8, 531–541. [Google Scholar] [CrossRef]
- Giménez, B.G.; Santos, M.S.; Ferrarini, M.; Fernandes, J.P. Evaluation of blockbuster drugs under the rule-of-five. Pharmazie 2010, 65, 148–152. [Google Scholar]
- Lipinski, C.A. Rule of five in 2015 and beyond: Target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug Deliv. Rev. 2016, 101, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Su, R.; Nie, S.; Sun, M.; Zhang, J.; Wu, D.; Moustaid-Moussa, N. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J. Nutr. Biochem. 2014, 25, 363–376. [Google Scholar] [CrossRef]
- Armendáriz-Barragán, B.; Zafar, N.; Badri, W.; Galindo-Rodríguez, S.A.; Kabbaj, D.; Fessi, H.; Elaissari, A. Plant extracts: From encapsulation to application. Expert Opin. Drug Deliv. 2016, 13, 1165–1175. [Google Scholar] [CrossRef]
- Nyhan, B.; Reifler, J. Does correcting myths about the flu vaccine work? An experimental evaluation of the effects of corrective information. Vaccine 2015, 33, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Elbahesh, H.; Gerlach, T.; Saletti, G.; Rimmelzwaan, G.F. Response Modifiers: Tweaking the Immune Response against Influenza A Virus. Front. Immunol. 2019, 10, 809. [Google Scholar] [CrossRef] [PubMed]








| No | Natural Resource | Active Compound | Strain | Activities | Ref. | |
|---|---|---|---|---|---|---|
| 1 | Paratetilla sp. sponge-derived fungus, Penicillium sclerotiorum OUCMDZ-3839 | Sclerotiorin E | H1N1 A/PR/8/34 | IC50 = 79 μM | [51] | |
| 2 | (+) Sclerotiorin | H1N1 A/PR/8/34 | IC50 = 129 μM | |||
| 3 | TL-1-monoactate | H1N1 A/PR/8/34 | IC50 = 115 μM | |||
| 4 | Ochrephilone | H1N1 A/PR/8/34 | IC50 = 151 μM | |||
| 5 | 8-acetyldechloroisochromophilone III | H1N1 A/PR/8/34 | IC50 = 91 μM | |||
| 6 | Scleratioramine | H1N1 A/PR/8/34 | IC50 = 134 μM | |||
| 7 | Isochromophilone IX | H1N1 | IC50 = 157 μM | |||
| 8 | Marine-derived fungus Aspergillus terreus SCSGAF0162 | Asperterrestide A | A/WSN/33 (H1N1) | IC50 = 15 μM | [52] | |
| 9 | Marine-derived fungus Aspergillus terreus OUCMDZ-1925 | Rubrolides S | H1N1 A/PR/8/34 | IC50 = 87 µM | [53] | |
| 10 | Aspergillus sp. SCSIO XWS02F40 | Asteltoxin E | H1N1 | IC50 = 4 µM | [54] | |
| 11 | Streptomyces sp. OUCMDZ-3434 | Wailupemycin J | H1N1 | 47.8% inhibition at 50 μg/mL | [55] | |
| 12 | R-wailupemycin K | H1N1 | 42.5% inhibition at 50 μg/mL | |||
| 13 | 5-deoxyenterocin | H1N1 | 60.6% inhibition at 50 μg/mL | |||
| 14 | M. senhousei | Pyropheophoride a | H1N1 A/PR/8/34 | IC50 = 0.17 µg/mL | [56] | |
| 15 | The South China Sea soft coral Sarcophyton sp. | (24R)-methylcholest-7-en-3β,5α,6β-triol | H1N1 A/PR/8/34 | IC50 = 19.6 µg/mL | [57] | |
| 16 | The South China Sea soft coral Sarcophyton sp. | (24S)-ergost-3β,5α,6β, 11α-tetraol | H1N1 A/PR/8/34 | IC50 = 36.7 µg/mL | [57] | |
| 17 | Cleistocalyx operculatus leaves | 2′,4′dihydroxy-6′-methoxy-3′,5′-dimethylchalcone | H1N1 A/PR/8/34 | IC50 = 8 μM | [58] | |
| 18 | Myricetin-3′,5′-dimethylether 3-O-β-D-galactopyranoside | H1N1 A/PR/8/34 | IC50 = 9 μM | |||
| 19 | Pentarhizidium orientale | Demethoxymatteucinol | H1N1 A/PR/8/34 | IC50 = 30 µM | [59] | |
| 20 | Matteucinol | H1N1 A/PR/8/34 | IC50 = 25 µM | |||
| 21 | Matteucin | H1N1 A/PR/8/34 | IC50 = 24 µM | |||
| 22 | Methoxymatteucin | H1N1 A/PR/8/34 | IC50 = 25 M | |||
| 23 | 3′-hydroxy-5′-methoxy-6,8dimethylhuazhongilexone | H1N1 A/PR/8/34 | IC50 = 24 µM | |||
| 24 | Bee pollen | Kaempferol-3-sophoroside | H1N1 | IC50 = 86 µM | [60] | |
| 25 | Kaempferol-3-neohesperidoside | H1N1 | IC50 = 56 µM | |||
| 26 | Kaempferol-3-sambubioside | H1N1 | IC50 = 45 µM | |||
| 27 | Kaempferol-3-glucoside | H1N1 | IC50 = 36 µM | |||
| 28 | Quercetin-3-sophoroside | H1N1 | IC50 = 88 µM | |||
| 29 | Bee pollen/Rhodiola rosea/Salvia plebeia R. Br. | Luteolin | H1N1 | IC50 = 11-18 µM | ||
| 30 | Bee pollen | Chelianthifoline | H1N1 | IC50 = 101 µM | ||
| 31 | Rhodiola rosea | Apigenin | rvH1N1 | IC50 = 33 µM | [61] | |
| 32 | Kaempferol | rvH1N1 | IC50 = 11 µM | |||
| 33 | Quercetin | rvH1N1 | IC50 = 2 µM | |||
| 34 | Herbacetin | rvH1N1 | IC50 = 9 µM | |||
| 35 | Gossypetin | rvH1N1 | IC50 = 3 µM | |||
| 36 | Cosmosiin | rvH1N1 | IC50 = 47 µM | |||
| 37 | Astragalin | rvH1N1 | IC50 = 38 µM | |||
| 38 | Rhodiolinin | rvH1N1 | IC50 = 10 µM | |||
| 39 | Rhodionin | rvH1N1 | IC50 = 32 µM | |||
| 40 | Rhodiosin | rvH1N1 | IC50 = 57 µM | |||
| 41 | Linocinamarin | rvH1N1 | IC50 = 44 µM | |||
| 42 | Rutin | rvH1N1 | IC50 = 34 µM | |||
| 43 | Nicotiflorin | rvH1N1 | IC50 = 32 µM | |||
| 44 | Elderberries | 5,7,3′,4′-tetra-O-methylquercetin | H1N1 A/PR/8/34 | IC50 = 0.4 µM | [62] | |
| 45 | (±)-Dihydromyricetin | H1N1 A/PR/8/34 | IC50 = 9 µM | |||
| 46 | Salvia plebeia R. Br. | Hispidulin | H1N1 A/PR/8/34 | IC50 = 20 µM | [63] | |
| 47 | Nepetin | H1N1 A/PR/8/34 | IC50 = 11 µM | |||
| 48 | Rosmarinic acid methyl ester | H1N1 A/PR/8/34 | IC50 = 17 µM | |||
| - | Honeysuckle | Acids-flavonoids | H1N1 | EC50 = 4 µg/mL | [64] | |
| 49 | Commelina communis L. | Homonojirimycin | A/PR/8/34 (H1N1) | EC50 = 10 μg/mL | [65] | |
| 50 | Jishengella endophytica 161111 | Perlolyrine | H1N1 A/PR/8/34 | IC50 = 38 μg/mL | [66] | |
| 51 | 1-hydroxy-β-carboline | H1N1 A/PR/8/34 | IC50 = 25 μg/mL | |||
| 52 | Lumichrome | H1N1 A/PR/8/34 | IC50 = 40 μg/mL | |||
| 53 | 1H-indole-3-carboxaldehyde | H1N1 A/PR/8/34 | IC50 = 46 μg/mL | |||
| 54 | Marine sponges Pericharax heteroraphis | Leucettamine C | H1N1 A/PR/8/34 | 33% Inhibition at 50 μg/mL | [67] | |
| - | Peganum harmala L. | The crude extract | H1N1 A/PR/8/34 | IC50 = 10 µg/mL | [68] | |
| - | Total alkaloid | H1N1 A/PR/8/34 | IC50 = 6 µg/mL | |||
| 55 | Papaver somniferum | Papaverine | A/WSN/33 (H1N1) | IC50 = 17 µM | [69] | |
| 56 | I. indigotica leaves | Isatidifoliumosides | Isatidifoliumosides/Epiisatidifoliumosides C (53/54) in the 3:2 ratio | H1N1 A/PR/8/34 | IC50 = 65 µmol/L | [70] |
| 57 | Epiisatidifoliumosides C | |||||
| 58 | Sonneratia paracaseolaris | Paracaseolins A | H1N1 | IC50 = 28 µg/mL | [71] | |
| - | Ganoderma lingzhi | Hot water extract | (A/California/04/2009/(H1N1)) | IC50 = 15 µg/mL | [72] | |
| 59 | Trichoderma atroviride FKI-3849. | Wickerol A | A/PR/8/34 (H1N1) | IC50 = 0.1 mg/mL | [73] | |
| 60 | Wickerol B | A/PR/8/34 (H1N1) | IC50 = 5 mg/mL | |||
| 61 | Lemnalia sp. (No. XSSC201907) | Neolemnane sesquiterpene lineolemnenes F | H1N1 | IC50 = 6 µM | [74] | |
| 62 | Celastrus aculeatus Merr. | Aculeatusane A | A/GZ/GIRD07/09 (H1N1) | IC50 = 23 µM | [75] | |
| 63 | Calotropis gigantea (Asclepiadaceae) | (+)-pinoresinol 4-O-[6″-O-vanilloyl]- β-D-glucopyranoside | A/PR/8/34 (H1N1) | IC50 = 25 µM | [76] | |
| 64 | Tea polyphenols | (-)-epigallocatechin (EGC) | A/PR/8/34 (H1N1) | IC50 = 31 µg/mL | [77] | |
| 65 | (-)-epigallocatechingallate (EGCG) | A/PR/8/34 (H1N1) | IC50 = 56 µg/mL | |||
| 66 | Procyanidin B-2 | A/PR/8/34 (H1N1) | IC50 = 51 µg/mL | |||
| 67 | Procyanidin B-23,3′-di-O-gallate | A/PR/8/34 (H1N1) | IC50 = 35 µg/mL | |||
| 68 | Theaflavin | A/PR/8/34 (H1N1) | IC50 = 16 µg/mL | |||
| - | Gyrodinium impudium | The sulfated exopolysaccharide, p-KG03 | A/PR/8/34 (H1N1) | EC50 = 0.5 µg/mL | [78] | |
| - | wild-type (WT) H1N1 | EC50 = 0.2 µg/mL | ||||
| - | A. nodosum | Cold water extract | A/PR/8/34 (H1N1) | IC50 = 112 µg/mL | [79] | |
| - | Hot water extract | A/PR/8/34 (H1N1) | IC50 = 101 µg/mL | |||
| - | 2% Na2CO3 extract | A/PR/8/34 (H1N1) | IC50 = 152 µg/mL | |||
| - | 0.5 mol−1NaOH extract | A/PR/8/34 (H1N1) | IC50 = 191 µg/mL | |||
| - | F. vesiculosus | Cold water extract | A/PR/8/34 (H1N1) | IC50 = 75 µg/mL | ||
| - | Hot water extract | A/PR/8/34 (H1N1) | IC50 = 181 µg/mL | |||
| - | 2% Na2CO3 extract | A/PR/8/34 (H1N1) | IC50 = 177 µg/mL | |||
| - | 0.5 mol−1 NaOH extract | A/PR/8/34 (H1N1) | IC50 = 125 µg/mL | |||
| - | Red algae Eucheuma denticulatum | 85% ethanol extract | H1N1 | IC50 = 277 µg/mL | [80] | |
| - | Water extract | H1N1 | IC50 = 366 µg/mL | |||
| - | 4% NaOH extract | H1N1 | IC50 = 436 µg/mL | |||
| 69 | Roots of Ilex asprella | Asprellcoside A | A/PR/8/34 (H1N1) | EC50 = 4 mM | [81] | |
| 70 | 3,4,5-trimethoxyphe β-D-5-O-caffeoyl-Apiofuranosyl-(16) -β-D-Glucopyranoside | A/PR/8/34 (H1N1) | EC50 = 2 mM | |||
| 71 | Angelica dahurica | Isoimperatorin | A/PR/8/34 (H1N1) | EC50 = 8 µM | [82] | |
| 72 | Oxypeucedanin | A/PR/8/34 (H1N1) | EC50 = 6 µM | |||
| 73 | Oxypeucedanin hydrate | A/PR/8/34 (H1N1) | EC50 = 11 µM | |||
| 74 | Imperatorin | A/PR/8/34 (H1N1) | EC50 = 11 µM | |||
| 75 | Gentiopicroside derivatives | 2′,3′,6′-Tri-O-benzoyl-4′- O-methylsulfonyl gentiopicroside | A/WSN/33 (H1N1) | IC50 = 39.5 µM | [83] | |
| 76 | Gentiopicroside derivatives | 4′-fluoro-4′-deoxy gentiopicroside | A/WSN/33 (H1N1) | IC50 = 45.2 µM | [83] | |
| 77 | Gentiopicroside derivatives | 2′,3′,6′-Tri-O-benzoyl-4′,5′-olefin gentiopicroside | A/WSN/33 (H1N1) | IC50 = 44.0 µM | [83] | |
| 78 | Triptolide derivatives | 4-(((5bS, 6aS, 7aR, 8R, 8aS, 9aS, 9bS, 10aS, 10bS)-8a-isopropyl-10bmethyl-3-oxo 1, 2,3, 5,5b, 6,6a, 8,8a, 9a, 9b, 10b dodecahydrotris(oxireno) [2′, 3′:4b, 5;2″, 3″:6, 7;2‴, 3‴:8a, 9] phenanthro[1, 2-c] furan-8-yl)oxy)-2, 2-dimethyl-4 oxobutanoic acid | A/WSN/33 (H1N1) | EC50 = 3.24 µM | [84] | |
| 79 | (-)-Borneol derivatives | N,N,N-Trimethyl-2-oxo-2-((1S,2R,4S)-1,7,7-trimethylbicyclo [2.2.1]heptan-2-yloxy)ethanaminium iodide | A/PR/8/34 (H1N1) | IC50= 2.4 μM | [85] | |
| 80 | Andrographolide derivatives | 14-alpha-lipoyl andrographolide | A/PR/8/34 (H1N1) | EC50 = 7.2 µM | [86] | |
| 81 | Resveratrol derivatives | R42 | A/PR/8/34 (H1N1) | IC50 = 3.56 µM | [87] | |
| 82 | Pterodontic acid derivatives | Pterodontic acid derivatives compound 15 | H1N1 | IC50 = 9.92 µM | [88] | |
| 83 | Gastrodin derivatives | Methyl 4-fluoro-3-((2S,3R,4S,5R,6R)-3,4,5-triacetoxy-6-(acetoxymethyl)-tetrahydro-2H-pyran-2-yloxy) benzoate | A/FM/1/47(H1N1) | IC50 = 34.4 μM | [89] | |
| 84 | Mannich bases of abietic acid derivatives | Mannich bases of abietic acid derivatives compound 12 | A/PR/8/34 (H1N1) | IC50 = 5.0 μM | [90] | |
| 85 | Terpenophenols and some of their N- and O-derivatives | 2-(1,7,7-Trimethylbicyclo[2.2.1]hept-exo-2-yl)cyclohexa-2,5-dien-1,4-dione | A/PR/8/34 (H1N1) | IC50 = 0.5 µM | [91] | |
| 86 | Ferulic acid derivatives | (E)-3-(4-Hydroxy-3-methoxyphenyl)-1-(4-methylpiperazin-1-yl)-prop-2-en-1-one | H1N1 | IC50 = 12.77 ± 0.47 μg/mL | [92] | |
| No | Name | MW | Alog P | Hdon | Hacc | OB (%) | Cao-2 | BBB | DL | RBN |
|---|---|---|---|---|---|---|---|---|---|---|
| 24 | Kaempferol-3-sophoroside | 610.57 | −2.07 | 10 | 16 | 5.30 | −2.42 | −3.27 | 0.71 | 7 |
| 27 | Kaempferol-3-glucoside | 448.41 | −0.32 | 7 | 11 | 2.77 | −1.36 | −1.99 | 0.74 | 4 |
| 28 | Quercetin-3-sophoroside | 626.57 | −2.33 | 11 | 17 | 3.37 | −3.39 | 0.67 | 0.30 | 7 |
| 29 | Luteolin | 286.25 | 2.07 | 4 | 6 | 36.16 | 0.19 | −0.84 | 0.25 | 1 |
| 32 | Kaempferol | 286.25 | 1.77 | 4 | 6 | 41.88 | 0.26 | −0.55 | 0.24 | 1 |
| 33 | Quercetin | 302.25 | 1.50 | 5 | 7 | 46.43 | 0.05 | −0.77 | 0.28 | 1 |
| 34 | Herbacetin | 302.25 | 1.50 | 5 | 7 | 36.07 | 0.12 | −0.65 | 0.27 | 1 |
| 35 | Gossypetin | 318.25 | 1.24 | 6 | 8 | 35.00 | −0.09 | −1.02 | 0.31 | 1 |
| 36 | Cosmosiin | 432.41 | 0.43 | 6 | 10 | 9.68 | −1.08 | −2.26 | 0.74 | 4 |
| 37 | Astragalin | 448.41 | −0.32 | 7 | 11 | 14.03 | −1.24 | −1.97 | 0.74 | 4 |
| 42 | Rutin | 610.57 | −1.45 | 10 | 16 | 3.20 | −1.93 | −2.75 | 0.68 | 6 |
| 43 | Nicotiflorin | 594.57 | −1.18 | 9 | 15 | 3.64 | −1.77 | −2.55 | 0.73 | 6 |
| 46 | Hispidulin | 300.28 | 2.32 | 3 | 6 | 30.97 | 0.48 | −0.49 | 0.27 | 2 |
| 47 | Nepetin | 316.28 | 2.05 | 4 | 7 | 26.75 | 0.37 | −0.78 | 0.31 | 2 |
| 50 | Perlolyrine | 264.30 | 3.20 | 2 | 3 | 65.95 | 0.88 | 0.15 | 0.27 | 2 |
| 53 | 1H-indole-3-carboxaldehyde | 145.17 | 1.88 | 1 | 1 | 19.82 | 1.25 | 1.17 | 0.04 | 1 |
| 55 | Papaverine | 339.42 | 3.50 | 0 | 5 | 64.04 | 1.22 | 0.57 | 0.38 | 6 |
| 65 | (-)-epigallocatechin | 306.29 | 1.65 | 6 | 7 | 24.18 | −0.22 | −0.82 | 0.27 | 1 |
| 66 | Procyanidin B-2 | 578.56 | 3.36 | 10 | 12 | 3.01 | −1.14 | −2.02 | 0.66 | 3 |
| 67 | ProcyanidinB-23,3′-di-O-gallate | 902.88 | −0.13 | 16 | 22 | 3.01 | −3.60 | −4.93 | 0.17 | 9 |
| 71 | Isoimperatorin | 270.30 | 3.65 | 0 | 4 | 45.46 | 0.97 | 0.66 | 0.23 | 3 |
| 72 | Oxypeucedanin | 286.30 | 2.00 | 0 | 5 | 24.90 | 0.85 | 0.11 | 0.30 | 3 |
| 74 | Imperatorin | 270.30 | 3.65 | 0 | 4 | 34.55 | 1.13 | 0.92 | 0.22 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Han, Q.; Li, X.; Liu, X.; Jiao, W. Natural Product-Derived Phytochemicals for Influenza A Virus (H1N1) Prevention and Treatment. Molecules 2024, 29, 2371. https://doi.org/10.3390/molecules29102371
Li R, Han Q, Li X, Liu X, Jiao W. Natural Product-Derived Phytochemicals for Influenza A Virus (H1N1) Prevention and Treatment. Molecules. 2024; 29(10):2371. https://doi.org/10.3390/molecules29102371
Chicago/Turabian StyleLi, Ruichen, Qianru Han, Xiaokun Li, Xinguang Liu, and Weijie Jiao. 2024. "Natural Product-Derived Phytochemicals for Influenza A Virus (H1N1) Prevention and Treatment" Molecules 29, no. 10: 2371. https://doi.org/10.3390/molecules29102371
APA StyleLi, R., Han, Q., Li, X., Liu, X., & Jiao, W. (2024). Natural Product-Derived Phytochemicals for Influenza A Virus (H1N1) Prevention and Treatment. Molecules, 29(10), 2371. https://doi.org/10.3390/molecules29102371




