Abstract
We present a Raman spectroscopy study of the vibrational properties of free-base meso-tetra(4-pyridyl) porphyrin polycrystals under various temperature and hydrostatic pressure conditions. The combination of experimental results and Density Functional Theory (DFT) calculations allows us to assign most of the observed Raman bands. The modifications in the Raman spectra when excited with and laser lights indicate that a resonance effect in the band is taking place. The pressure-dependent results show that the resonance conditions change with increasing pressure, probably due to the shift of the electronic transitions. The temperature-dependent results show that the relative intensities of the Raman modes change at low temperatures, while no frequency shifts are observed. The experimental and theoretical analysis presented here suggest that these molecules are well represented by the point symmetry group.
1. Introduction
Over the past few decades, porphyrin molecules have attracted a great deal of attention given their central role in numerous fundamental natural processes [1]. The interplay between the structural and spectroscopic properties of these molecules enables the optimization of their electronic characteristics aiming at specific applications [2,3].
The structure of porphyrins consists of a macrocycle formed by four pyrrolic rings interconnected with methyne bridges, and this arrangement is upheld by the insertion of either two hydrogen atoms (free-base porphyrins) or a metal ion (in metalloporphyrins) at the center of the macrocycle [1,4]; see Figure 1. The study of the optical properties associated with distinct porphyrins is driven by their cyclic conjugation, which leads to a pronounced absorption of near-ultraviolet and visible light as well as a red emission that is readily observable with the naked eye [1,4]. Additionally, these molecules present intriguing nonlinear optical traits [5,6]. Their absorption spectra are primarily composed of two characteristic bands known as the -bands (or Soret bands), localized in the blue region of the spectrum, and the -bands, found in the green-red portion of the spectrum [1,4,7,8]. These spectroscopic responses are related to porphyrin’s electronic and vibronic properties, tuned through the modification of its structure, such as the substitution of the central ion and the addition of outlying and axial groups [3,6,7,8,9,10]. Those are very desirable possibilities since they create opportunities to employ porphyrin derivatives in many applications such as (i) photovoltaic cells [11,12,13], (ii) sensors [8,14], (iii) cancer treatment [15,16,17], and (iv) fluorescence imaging [18,19], among others.
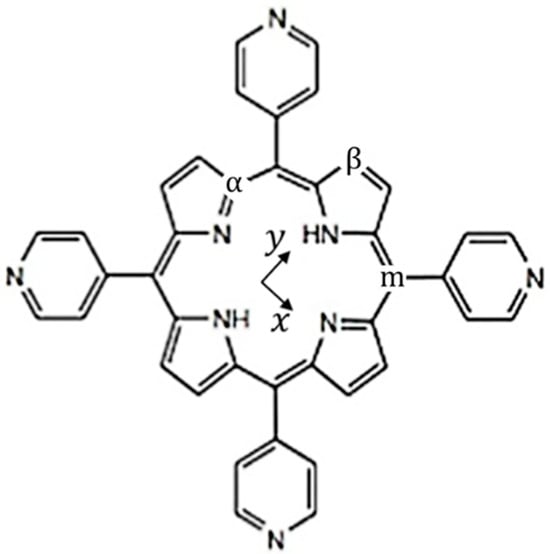
Figure 1.
Schematic representation of free-base tetrapyridyl porphyrin (H2TPyP). Within the macrocycle’s plane, two distinct directions are defined: (i) the x-direction containing only nitrogen atoms, and (ii) the y-direction containing nitrogen atoms bonded with hydrogen. The indices and give the carbon positions in the pyrrolic rings, and indicates the carbon position in the methynic bridge. The carbon atoms occupying these positions are labeled as follows: , linked to the central nitrogen atoms; , located at the outer edge of the macrocycle; and (meso-carbon), connecting the pyrrolic rings.
Dissolved in organic solvents, free-base tetrapyridyl porphyrin (H2TPyP), as shown in Figure 1, depicts a complex -band with multiple electronic transitions and their corresponding vibronic progressions [20].
While the optical properties of tetrapyridyl porphyrins have been extensively studied [6,10,20,21], their vibrational properties, especially in their crystalline form, remain poorly explored. Although the vibrational modes of other porphyrins have been investigated [22,23,24,25], their behaviors are substantially different from H2TPyP’s vibrational modes. In addition, the few studies of H2TPyP modes lack proper assignments and detailed descriptions of their symmetries [26,27], which are intimately connected with porphyrin’s vibronic transitions [20]. In this context, Raman spectroscopy emerges as a non-invasive, fast, and reproducible method to study the properties of these vibrational modes under different thermodynamic conditions, e.g., low temperatures and high pressures [28,29,30,31,32,33].
In the present work, we combine Raman spectroscopy measurements with first-principle calculations to provide assignments for the Raman modes in poly-crystals of free-base tetrapyridyl porphyrin or C-H2TPyP (see Figure S1 in Supplementary Materials). The evolution of the assigned modes in C-H2TPyP under high pressures, low temperatures, and different excitation energies is addressed. In addition, we elucidate the modifications in porphyrin’s resonance conditions under high pressures, along with possible symmetry changes occurring at both high pressures and low temperatures.
2. Results and Discussion
2.1. Raman Bands Assignments
The Raman spectra of C-H2TPyP show a rich distribution of bands, ranging from to ; see Figure 2.
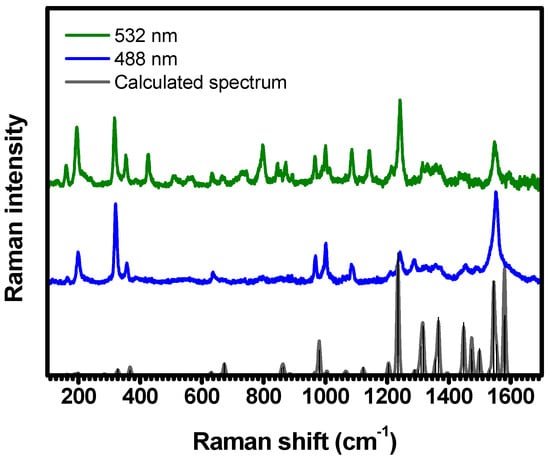
Figure 2.
Raman spectra of C-H2TPyP experimentally obtained with excitation centered at 488 nm (represented by a blue solid line) and 532 nm (green solid line), and the DFT–calculated Raman spectrum for the H2TPyP molecule (gray solid line). In the theoretical spectrum, Raman intensity (in A4/amu) refers to the Raman activity (scattering factor).
These spectra were acquired by exciting the sample at (resonant with the absorption band), and at (resonant with the intersection between the and bands, referred to herein as the band) [20]. These vibronic progressions arise from the coupling of the electronic absorption band with the vibrational modes centered around ( band) [20]. To provide a clearer depiction of the investigated modes, we present and discuss the results by zooming into the specific spectral regions, as depicted in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10 and Figure 11. The experimental spectra were deconvoluted using Lorentzian functions. This constitutes a conventional approach in Raman spectroscopy, stemming from the intrinsic properties of Raman scattering (RS). The semi-classical interpretation of RS relies on the forced damped oscillator model, which follows the Lorentzian function. In the quantum mechanical framework, each vibration exhibits a Lorentzian probability of light scattering [34]. The fitting procedure enables us to identify the spectral band positions with a deviation of . The corresponding center-peak wavenumbers () of the Raman bands, obtained with and , are listed in Table 1. Illustrations with an overview of the identified vibration patterns are provided in Table S2 in Supplementary Materials.
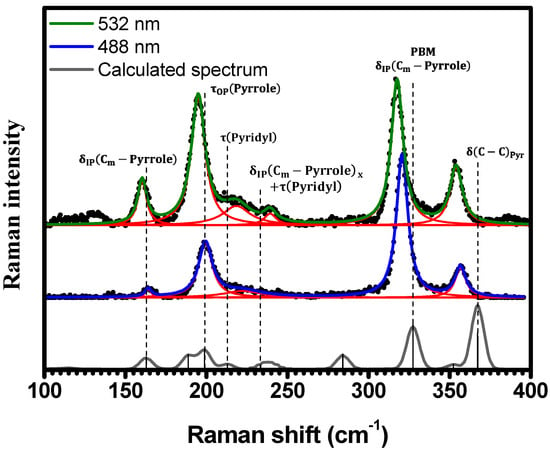
Figure 3.
Experimental Raman spectra (top and middle) measured for C-H2TPyP (black dots) and calculated (bottom) for the H2TPyP molecule (gray solid line) under ambient conditions in the spectral range of . The experimental spectra were obtained by exciting the sample at (middle spectrum) and (top spectrum). The blue () and green () solid curves represent the fittings obtained through the deconvolution process using Lorentzian functions (red solid lines). In the theoretical spectrum, Raman intensity (in A4/amu) refers to the Raman activity (scattering factor).
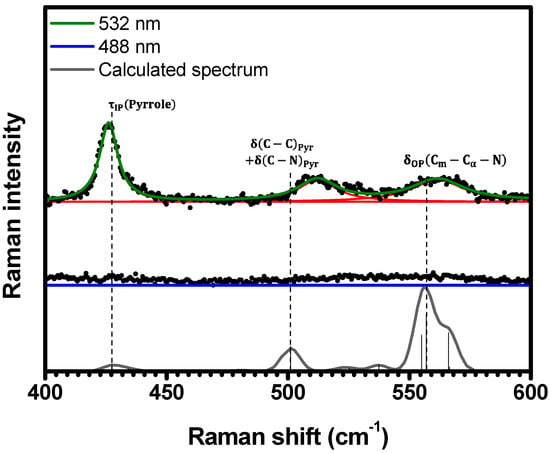
Figure 4.
Experimental Raman spectra (top and middle) measured for C-H2TPyP (black dots) and calculated (bottom) for the H2TPyP molecule (gray solid line) under ambient conditions in the spectral range of . The experimental spectra were obtained by exciting the sample at 488 nm (middle spectrum) and 532 nm (top spectrum). The blue (488 nm) and green (532 nm) solid curves represent the fittings obtained through the deconvolution process using Lorentzian functions (red solid lines). In the theoretical spectrum, Raman intensity (in A4/amu) refers to the Raman activity (scattering factor).
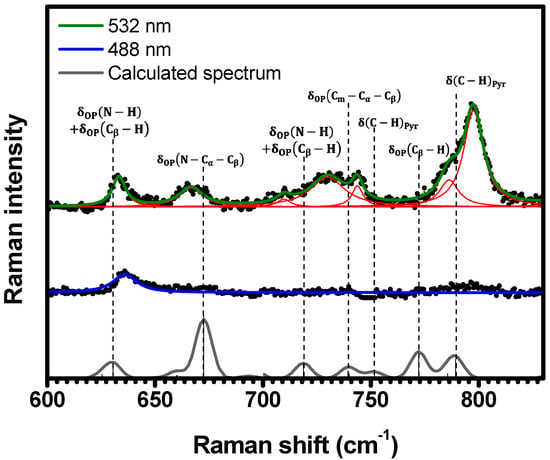
Figure 5.
Experimental Raman spectra (top and middle) measured for C-H2TPyP (black dots) and calculated (bottom) for the H2TPyP molecule (gray solid line) under ambient conditions in the spectral range of . The experimental spectra were obtained by exciting the sample at (middle spectrum) and (top spectrum). The blue () and green () solid curves represent the fittings obtained through the deconvolution process using Lorentzian functions (red solid lines). In the theoretical spectrum, Raman intensity (in A4/amu) refers to the Raman activity (scattering factor).
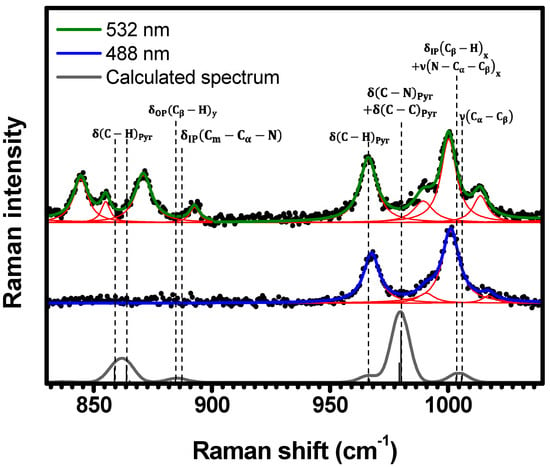
Figure 6.
Experimental Raman spectra (top and middle) measured for C-H2TPyP (black dots) and calculated (bottom) for the H2TPyP molecule (gray solid line) under ambient conditions in the spectral range of . The experimental spectra were obtained by exciting the sample at (middle spectrum) and (top spectrum). The blue () and green () solid curves represent the fittings obtained through the deconvolution process using Lorentzian functions (red solid lines). In the theoretical spectrum, Raman intensity (in A4/amu) refers to the Raman activity (scattering factor).
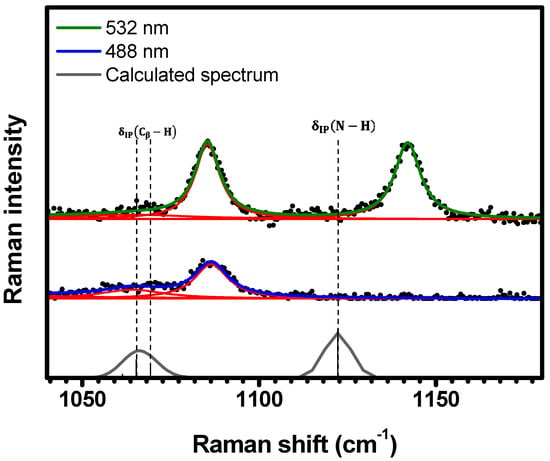
Figure 7.
Experimental Raman spectra (top and middle) measured for C-H2TPyP (black dots) and calculated (bottom) for the H2TPyP molecule (gray solid line) under ambient conditions in the spectral range of . The experimental spectra were obtained by exciting the sample at (middle spectrum) and (top spectrum). The blue () and green () solid curves represent the fittings obtained through the deconvolution process using Lorentzian functions (red solid lines). In the theoretical spectrum, Raman intensity (in A4/amu) refers to the Raman activity (scattering factor).
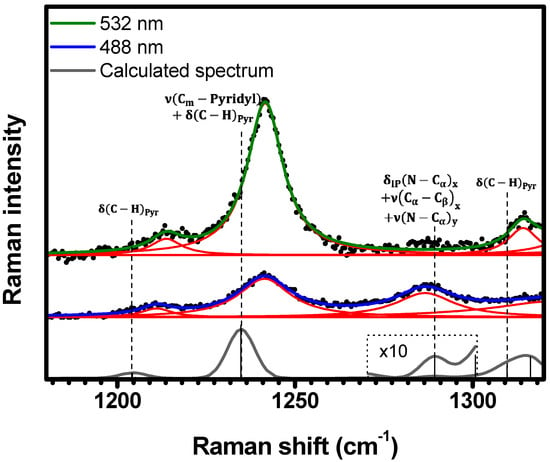
Figure 8.
Experimental Raman spectra (top and middle) measured for C-H2TPyP (black dots) and calculated (bottom) for the H2TPyP molecule (gray solid line) under ambient conditions in the spectral range of . The experimental spectra were obtained by exciting the sample at (middle spectrum) and (top spectrum). The blue () and green () solid curves represent the fittings obtained through the deconvolution process using Lorentzian functions (red solid lines). In the theoretical spectrum, Raman intensity (in A4/amu) refers to the Raman activity (scattering factor).
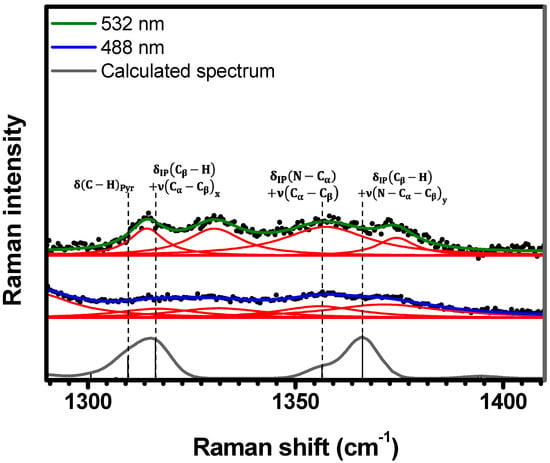
Figure 9.
Experimental Raman spectra (top and middle) measured for C-H2TPyP (black dots) and calculated (bottom) for the H2TPyP molecule (gray solid line) under ambient conditions in the spectral range of . The experimental spectra were obtained by exciting the sample at (middle spectrum) and (top spectrum). The blue () and green () solid curves represent the fittings obtained through the deconvolution process using Lorentzian functions (red solid lines). In the theoretical spectrum, Raman intensity (in A4/amu) refers to the Raman activity (scattering factor).
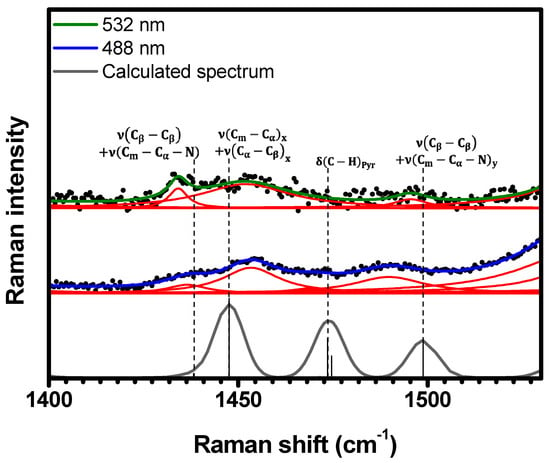
Figure 10.
Experimental Raman spectra (top and middle) measured for C-H2TPyP (black dots) and calculated (bottom) for the H2TPyP molecule (gray solid line) under ambient conditions in the spectral range of . The experimental spectra were obtained by exciting the sample at (middle spectrum) and (top spectrum). The blue () and green () solid curves represent the fittings obtained through the deconvolution process using Lorentzian functions (red solid lines). In the theoretical spectrum, Raman intensity (in A4/amu) refers to the Raman activity (scattering factor).
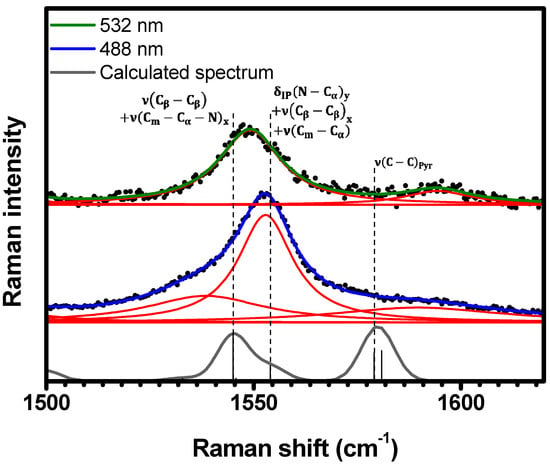
Figure 11.
Experimental Raman spectra (top and middle) measured for C-H2TPyP (black dots) and calculated (bottom) for the H2TPyP molecule (gray solid line) under ambient conditions in the spectral range of . The experimental spectra were obtained by exciting the sample at (middle spectrum) and (top spectrum). The blue () and green () solid curves represent the fittings obtained through the deconvolution process using Lorentzian functions (red solid lines). In the theoretical spectrum, Raman intensity (in A4/amu) refers to the Raman activity (scattering factor).

Table 1.
H2TPyP experimental and DFT–calculated Raman modes. In the table, stands for stretching; for bending; and for twist modes, respectively. The index “Pyr” identifies Raman modes related to the pyridyl ring. The indexes “IP” and “OP” stand for in-plane and out-of-plane modes, respectively. The indexes “” and “” indicate vibrations only in the respective direction.
In Figure 3, the spectral region of is displayed. Five Raman bands are observed for both excitations and are located at (), (), (), (), and () when excited at (). A Raman band at is observed in the spectrum obtained with excitation, presenting no corresponding band in the spectrum obtained with . Within this same spectral region, theoretical calculations predict nine Raman-active vibrational modes for the H2TPyP molecule. These modes are assigned to the following vibrations ( stands for out-of-plane, and stands for in-plane): at ; at ; at ; at ; at ; at ; at ; at ; and at . Despite the shifts when compared to experimental results, the calculations indicate that the vibrations at , , , , , and correspond to the six observed Raman bands, as shown in Figure 3 and summarized in Table 1 and Table S2 in SI (illustrations 1–6). No Raman bands were observed below under ambient conditions or either excitation wavelengths. We note that the in-phase mode at , from now on designated as “Pophyrin’s Breathing Mode (PBM)”, represents the breathing of porphyrin’s central ring.
As depicted in Figure 3, it is evident that the spectrum acquired with excitation at exhibits greater resolution compared to the spectrum acquired with excitation at . This observation aligns with the fact that the absorbance at is approximately twice that at [20], potentially resulting in a stronger resonance effect.
Within the spectral range of , no Raman bands were detected in the spectrum at , as shown in Figure 4. Nevertheless, at , three distinct Raman bands emerge at , , and , indicating the resonance of these modes with electronic transition. The observed Raman bands are assigned to the vibrations at , at , and at in the calculated spectrum, respectively; see Table 1 and Table S2 in SI (illustrations 7–9).
Figure 5 presents the Raman spectra within the range of . Several Raman bands are resonant when porphyrins are excited under (, , , , , , and ), whereas the spectrum obtained with excitation exhibits only one Raman band at (corresponding to at ). According to DFT calculations (see Table 1 and Table S2 in SI (illustrations 10–16)), these Raman bands are assigned to the following vibrations: at , at , at , at , at , at , and at .
The spectral region of (Figure 6) displays eight resonant Raman bands (, , , , , , , and ) under excitation. However, when excited under , only the higher energy bands at , , , and are resonant. The DFT calculations (see Table 1 and Table S2 in SI (illustrations 17–24)) suggest the following assignments to these bands: two at and , at , at , at , at , at , and at .
As shown in Figure 7, the spectral region of exhibits three resonant Raman bands under excitation: , , and . The spectrum acquired with displays two bands at and (the same bands observed at ). These bands are assigned to the vibrations at and , and the vibration at , respectively; see Table 1 and Table S2 in SI (illustrations 25–27).
Figure 8 shows the spectral range of . In this range, three Raman bands are resonant under both (, , and ), and (, , and ) excitations. The lower energy bands and at ( and at ) are assigned to the calculated vibrations at , and at , respectively. The theoretical mode at is assigned to the band at , while the mode at is assigned to the band at ; see Table 1 and Table S2 in SI (illustrations 28–31).
The spectral range of exhibits the same four resonant Raman bands under both and excitations (see Figure 9) centered at (the same band discussed above in Figure 8), , , and . The main difference between the two spectra lay on the intensity (i.e., resonant effects) of the bands: the bands at appear less structured when compared with the band at . According to the DFT calculations, these bands are assigned to the following vibrations: at , at , at , and at ; see Table 1 and Table S2 in SI (illustrations 31–34).
The spectral range , shown in Figure 10, exhibits three common Raman bands for each excitation. At (), these bands are centered at (), (), and (). The DFT calculations indicate that these bands correspond to the following vibrations: at , at , and at ; see Table 1 and Table S2 in SI (illustrations 35, 36 and 38). The vibration at is only resonant at (see Table 1 and Table S2 in SI (illustrations 35–38).)
As shown in Figure 11, the spectral range exhibits three resonant Raman bands centered at , , and in the spectrum. The lower energy Raman band () is absent in the spectrum, while the other two are also resonant, with their centers ( and ) slightly redshifted. These bands are assigned to the vibrations at , at , and at ; see Table 1 and Table S2 in SI (illustrations 39–41).
Table 1 provides assignments for a total of forty-one vibrational modes, comprising fifteen that resonate exclusively with electronic transition, three resonating only with vibronic progression, and twenty-three that resonate with both. It is noteworthy that reference [20] elucidated, via the deconvolution of the absorbance UV-Vis spectrum, that the vibronic progression arises from the coupling between electronic transition and a vibrational mode centered at . This mode closely aligns in energy with () which resonates with both () and (); see Figure 8 and Table 1.
2.2. Hydrostatic Pressure Experiments
To explore the structural stability of C-H2TPyP, studies were conducted under high-pressure conditions, from to . In Figure 12, the Raman spectra acquired from samples under ambient conditions and submitted to various loads are presented. To facilitate a more comprehensive discussion, the Raman spectra are divided into three distinct wavenumber regions: , , and . It is worth noting that even at a very low pressure (), some modes that were not visible under ambient conditions become apparent. The lack of theoretical prediction for some of these modes as vibrational modes of the H2TPyP molecule suggests that the bands in the low-wavenumber region () are associated with the lattice vibration of C-H2TpyP, i.e., librations and the torsion of porphyrin’s ring.
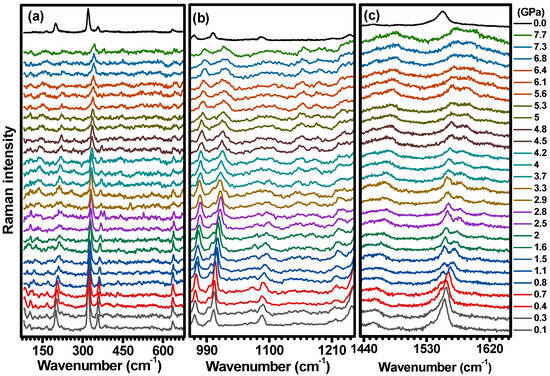
Figure 12.
Raman spectrum of C-H2TpyP excited at with pressures ranging from to for three distinct wavenumber regions: (a) , (b) , and (c) . The spectrum at 0.0 GPA was acquired at ambient conditions and it is being shown as a reference.
Figure 13 shows the evolution of the Raman band frequencies with increasing pressure, presenting distinct rates , as summarized in Table 2. Notably, at pressures of , , , and , changes in the wavenumber displacement are evident for some Raman bands, as indicated in Table 2.
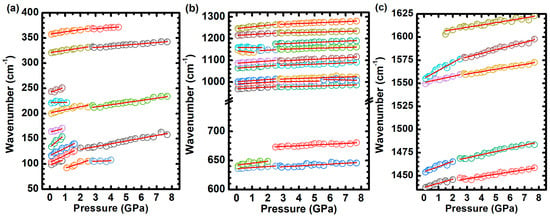
Figure 13.
Evolutions of the frequencies of the Raman bands with increasing pressure. The evolution of the Raman bands with pressure is analyzed for three distinct wavenumber regions: (a) , (b) , and (c) , to facilitate a more comprehensive discussion.

Table 2.
Experimental rates for the observed Raman bands. CLV stands for Crystal Lattice Vibration. Some Raman modes present two slopes with pressure; their intercept and at such pressures are indicated as follows: # 0.8 GPa, **, *, and $. The numbers in brackets are the errors in the intercept and rates obtained from fitting.
The crystal lattice frequency vibrations undergo high blueshift rates (). The vibrations initially at and disappear at , and the vibration initially at disappears at . The remaining lattice vibrations at and have their shift rates decreased at and at , respectively. Moreover, the vibration () undergoes a blueshift at a rate of , manifesting a gradual decrease in intensity, followed by an increase in its Full Width at Half Maximum (FWHM). Both () and () vibrations display a blueshift at comparable rates of and , with observed until , while remains up to . The () vibration undergoes a high blueshift rate (). In contrast, a minor displacement rate is identified for the () mode () throughout the entire process. This rate is notably lower when compared to the displacement rates of other vibrational modes within the range of to . Furthermore, the disappearance of some lattice modes beyond and the observed increase in the FWHM bands suggest the initiation of an amorphization process.
In the region ranging from and , most of the Raman bands undergo a gradual blueshift, except for the band centered around , which initially displays a redshift at a rate of (see Table 2 and Figure 13b). In the region within and , the vibrations (), (), (), and () undergo a blueshift, presenting rates from to . The vibrations () and () undergo an initial blueshift, with rates around . The former Raman band disappears at , and the latter has its shift rate greatly decreased at the same pressure.
Figure 14 shows representative C-H2TPyP Raman spectra for selected hydrostatic pressures. When compared with the spectrum at (the lowest pressure in the experiment), the spectrum at shows three new Raman modes centered at , , and ; see Figure 14a. In addition, the intensity of the Raman mode at (see illustration 24 in Table S2 in Supplementary Materials) increases relative to the intensity of the mode at (see illustration 23 in Table S2 in Supplementary Materials), making both modes (see Table 1), which are associated with a distinct stretching of the carbons and , distinguishable.
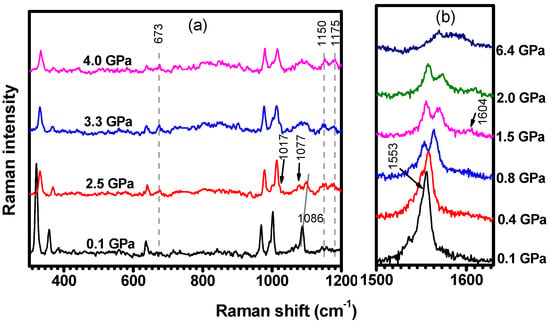
Figure 14.
Raman spectra ranging from (a) to , and (b) from to . The spectra were excited at and acquired under different hydrostatic pressures.
The Raman band initially at (out-of-phase bending of the pair; see illustration 26 in Table S2 in Supplementary Materials) undergoes a frequency upshift and an intensity decrease, favoring the observation of the lower energy band at (in-phase bending of the pair; see illustration 25 in Table S2 in Supplementary Materials), whose main change is connected to its intensity increase. These two bands start fading and lose resolution when the pressure is further increased to . It is important to comment that the in-phase bending of the pair at appears at when measured at ambient conditions. With increasing pressure, the inactive vibration at ambient conditions centered at (see Figure 15) becomes active with the frequency slightly upshifted to . It is worth mentioning that the modes centered at and only undergo a slight enhancement of their intensities.
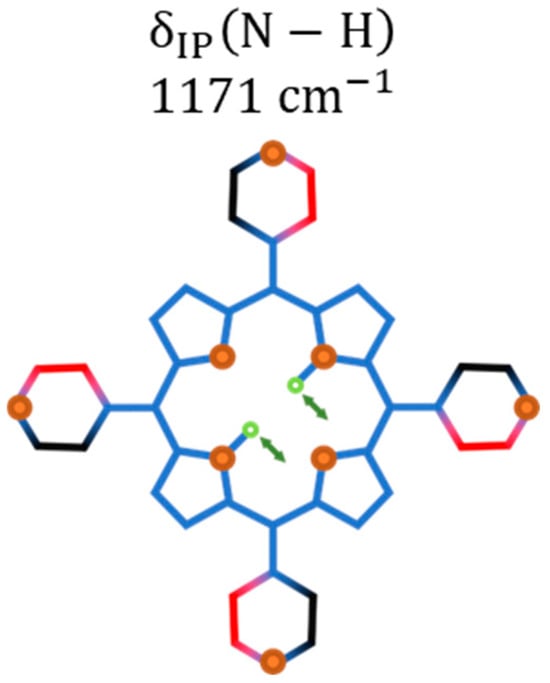
Figure 15.
Schematic illustrations of the Raman vibration activated at higher pressures: bending of the bonds; stands for in-plane.
From Figure 12 and Figure 14b, it is noteworthy that the modes centered at (; see illustration 39 in Table S2 in Supplementary Materials) and at (; see illustration 40 in Table S2 in Supplementary Materials) gradually upshift in frequency for pressures up to . Initially, displays an higher upshift rate of compared to the observed for . However, beyond , both rates decrease to and to , respectively. This implies that after , upshifts more than one and a half times when compared with . This observation explains the observed splitting in the Raman bands with increasing pressure. Their relative intensities present an interesting behavior: the intensity of the mode is first enhanced and then suppressed with increasing pressure, while the intensity of the mode is continuously suppressed. Finally, the Raman-active vibration theoretically centered at (see illustration 41 in Table S2 in Supplementary Materials) undergoes both a frequency upshift to and a substantial enhancement of its intensity with increasing pressure.
In addition to structural modifications observed, our findings indicate the influence of pressure load on resonance conditions of C-H2TPyP, probably due to modifications in the and bands’ energy gap. Indeed, as mentioned in Section 2.1, some of the Raman bands observed at () do not have a correspondent in the spectra at (), evidencing the resonance effect [31,33,34], which occurs when the different regions of the optical absorption spectrum (i.e., the and the bands) are excited [34]. It is also known that the resonance conditions of vibrational modes are often affected by external stimuli (e.g., temperature and pressure) that perturb the molecular geometry [35,36].
A new Raman mode at is observed at with excitation at , as shown in Figure 16a, and its intensity increases with compression, up to . Furthermore, as shown in Figure 16b, the Raman-active vibration centered at (not present in the spectrum at ) has emerged in the spectrum acquired at with the excitation at . These bands are assigned to the and vibrations, respectively, as seen in Table 1 and Table S2 in SI (illustrations 4 and 11). Although not present when the sample is excited at , these bands are resonant with the excitation at ambient conditions. The Raman features centered at , and (Figure 16c,d), which are assigned to the , , and vibrations (Table 1 and Table S2 in SI (illustrations 24, 27, and 41)), present the same behavior: they appear in the spectrum obtained at with excitation at , but they are not present when the pressure is set to . In addition, these bands are also resonant with the excitation at ambient conditions. These results suggest that the resonance conditions of the porphyrin molecules are changing with changing pressure. In other words, the increase in pressure seems to result in an increased energy separation between electronic levels. Therefore, bands which are resonant at (ambient conditions) become resonant at for higher pressures.
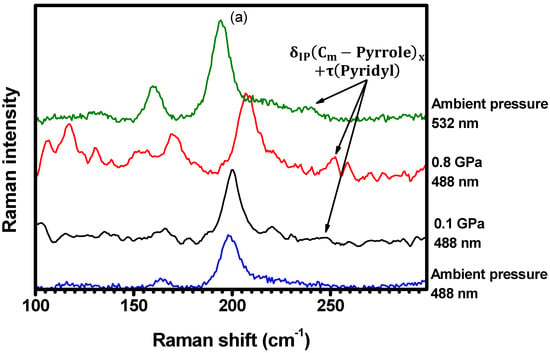
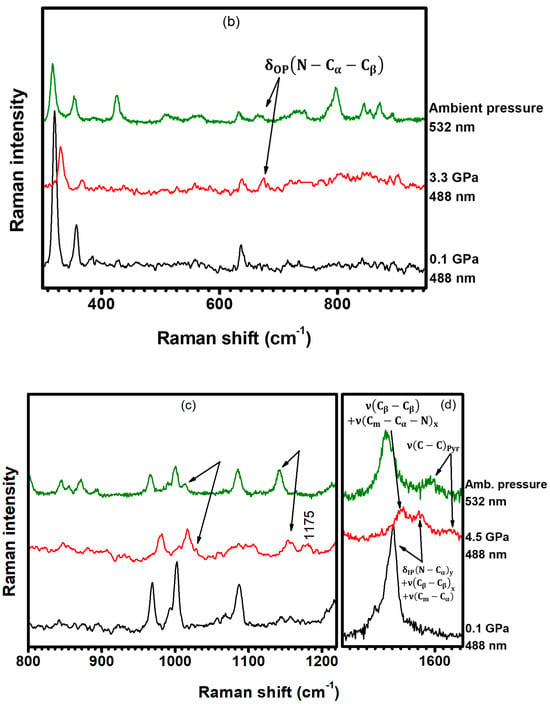
Figure 16.
Raman spectra ranging from (a) to , (b) to , (c) to , and (d) from to . The spectra were acquired under different hydrostatic pressures and excited at both and (ambient conditions).
It is also important to note that the results associated with the molecules’ decompression show that the frequency shifts are reversible for most bands, but the vibrations between and present signs of irreversibility (see Figure S3 in Supplementary Materials).
2.3. Low-Temperature Experiments
Temperature-dependent Raman spectroscopy has also been performed to complement the understand of porphyrin’s vibrational properties. The C-H2TPyP molecules were submitted to temperatures ranging from to . Differently from the behavior presented at variable pressures, no shifts in the Raman band centers or broadenings of the bands’ linewidths were detected in this range of temperatures, as shown in Figure 17.
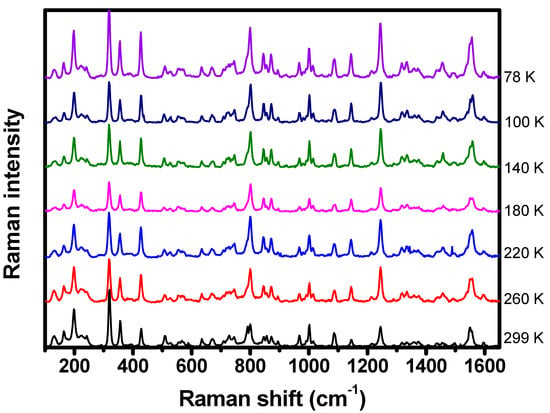
Figure 17.
Raman spectrum of C-H2TPyP excited at with temperatures ranging from to .
The analysis of the relative intensities of the Raman bands with respect to the PBM ( intensity at 299 shows that the intensities of most of the Raman modes remain essentially unchanged. However, some vibrations have their intensities greatly altered at lower temperatures, such as the vibration at , whose relative intensity has an initial value of at room temperature (299 ) that is increased to at (see Figure 18a), and the mode at , whose relative intensity goes from to when the temperature is lowered from 299 to 180 ; see Figure 18b.
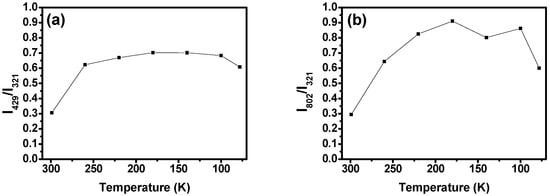
Figure 18.
(a) (at ) and (b) (at ) Raman modes’ relative intensities as a function of temperature. The relative intensities plotted here are the ratio of the modes’ absolute intensities with relation to the absolute intensity of the PBM mode () at 299 . The errors associated with the measurements of the relative intensities are lower than 0.1% for all acquired spectra.
Overall, the effects observed in the relative intensities of the Raman bands are indicative of perturbations in the molecular symmetry at lower temperatures, which are likely altering the Raman activities of the modes. The graphs containing the temperature-dependent relative intensities for each assigned mode are available in Supplementary Materials; see Table S3.
Finally, the literature reports that isolated H2TPyP belongs to the point group [37]. For this symmetry, it is expected that antisymmetric vibrational modes with respect to the molecule inversion center, called odd modes, will not be Raman-active [38,39]. Despite that, some vibrations observed in this work, at ambient conditions, higher pressures, and lower temperatures, are odd modes (for instance, () and ()). The observation of such modes in the Raman spectra strongly indicates that a reduction in the planarity of the molecule, and consequently a change in its symmetry, is taking place. We hypothesize that this planarity reduction could be associated with a saddle-shaped conformation (already observed in porphyrins [40]), due to local fields in the porphyrin crystal. Our theoretical predictions could only describe our experimental results after considering such symmetry change, predicting that H2TPyP assumes the point group. In addition, the new vibration observed at at higher pressures is also an odd mode and possesses an antisymmetric vibration in the plane. Its appearance indicates a further planarity modification with pressure, without further symmetry changes. Lastly, the changes in the relative intensities observed both at high pressures and low temperatures also indicate changes in the molecular symmetry.
3. Materials and Methods
C-H2TPyP was synthesized following the procedures described in reference [41], and the spectrometric analysis of the resulting crystals are in good agreement with the literature [42].
The vibrational properties of C-H2TPyP were investigated via Raman spectroscopy using a T64000 spectrometer from Horiba Jobin Yvon (Lille Country, France). The scattered light was collected using a magnification objective lens in a backscattering configuration. The spectral resolution was . The measurements were conducted under both ambient conditions and high pressures. C-H2TPyP was excited using two different laser lines: for both ambient and high-pressure conditions, and for ambient conditions only. No fluorescence background was observed upon sample excitation at 488 nm. However, the issue of fluorescence background arises when the sample is excited with 532 nm. To address this problem, a baseline correction of the spectrum is performed. The baseline determination proceeds as follows: First, a numerical derivative of the experimental data is calculated. Since the fluorescence bands are generally much broader profiles compared to Raman bands, the first derivative is used to distinguish them. In the derivative spectrum, each Raman band appears as two symmetric bands around zero and the fluorescence signal grows as a background curve with the increase in the wavenumber. This fluorescence curve is then adjusted using a multiparametric function, integrated, and subsequently employed as the baseline for the original dataset.
The high-pressure Raman spectra were measured using a diamond anvil cell (μ-scope DAC HT(S)) from Almax easyLab (Diksmuider, Belgium). A mineral oil, specifically Nujol, was used as the pressure-transmitting medium [43]. The sample was loaded into a hole drilled in a stainless-steel gasket (thickness of ), using an electric discharge machine from Almax easyLab. Pressure measurements were calibrated by monitoring the shifts in the ruby fluorescence lines [44,45]. The increase in fluorescence background originating from C-H2TPyP upon its insertion into the pressure cell makes it impractical to acquire the Raman signal using excitation in high-pressure experiments.
The low-temperature Raman spectra measurements were performed with the Janis ST-500 cryostat from Lake shore Cryotornics (Westerville, OH, USA) and the samples, after being properly accommodated in the cryostat, were excited with a () CW laser using objective lens with numerical aperture 0.60. The scattered light was acquired in a backscattering configuration, using an Andor SR303i spectrometer operating with a grating, coupled to an iDUS CCD camera.
Our theoretical approach was based on the Density Functional Theory (DFT) formalism as implemented in the ORCA code [46], considering isolated molecules. We employed a polarized triple-zeta basis set (def2-TZVP) and the Generalized Gradient Approximation (GGA) within the Perdew–Burke–Ernzerhof (PBE) parametrization for the exchange–correlation functional. The calculated main bond lengths and angles are provided in supporting information (see Table S1 in Supplementary Materials) and were computed with both GGA (PBE) and META-GGA (M06-L). In Figure S2 in Supplementary Materials, the structure of H2TPyP computed with GGA (PBE) is used as reference to the analysis of Table S1 in Supplementary Materials. We found an excellent agreement between the two functionals, with the largest absolute difference in bond lengths being only . Following the complete geometry optimization, the Raman spectra were determined numerically, with the best approximation to the experimental data achieved using the PBE functional, which is consistent with previous studies [47].
4. Conclusions
Raman-active vibrations in H2TPyP have been poorly explored and, in this work, through the combination of experiments and DFT calculations, we provide a thorough discussion of such vibrations. Every measured Raman-active vibration within to that is resonant with either or is now assigned, with their symmetries and resonance properties properly addressed. In addition, the results show that the resonance conditions of active vibrations are tunable under hydrostatic pressure. In other words, bands which, under ambient conditions, are only active under excitation become readily active at with increasing pressure. Finally, H2TPyP has been reported to possess the point symmetry , but the experimental results presented here, combined with DFT calculations, suggest that these molecules are better described under the symmetry. The pressure- and temperature-dependent results indicate that molecular planarity is being further perturbed at lower temperatures and higher pressures.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29102362/s1, Figure S1: X-ray diffractogram of C-H2TPyP. The peaks reveal a crystalline structure of the investigated sample. Figure S2: Structure of H2TPyP molecule calculated with GGA (PBE) and used as reference to Table S2 in SI. Figure S3: Raman shift of the C-H2TPyP bands under compression (▲) and decompression (∇). Table S1: Main bond lengths determined by DFT calculations employing two choices of exchange-correlation functionals, GGA (PBE) and META-GGA (M06-L). The bonds are indicated in Figure S2, which presents the structure of optimized porphyrin. The two functionals are in excellent agreement with each other: the largest absolute difference is only 0.012 Å, while the largest percentage difference is 0.9%. Table S2: Schematic illustrations of the Raman modes observed in this work. The black and red lines indicate out-of-plane opposite bonds. The arrows are in-plane vibrations, and the symbols ⊙ and ⊗ are out-of-plane opposite vibrations. Green and purple arrows represent out-of-phase modes. The indexes “IP” and “OP” stand for in-plane and out-of-plane modes, respectively. The indexes “x” and “y” indicate vibrations only in the respective direction. Table S3: Raman relative intensity (Y-axis) of C-H2TPyP bands, relative to the PBM mode at 321 cm−1, as function of temperature (X-axis), ranging from 299 K to 76 K. The inserted values indicate the ratio between the intensity at the analyzed Raman band frequency (in cm−1) and the reference (PBM).
Author Contributions
Conceptualization, N.M.B.N., W.P. and P.T.A.; methodology, N.M.B.N., A.A.B., W.P., J.R.T.d.R., W.H.N.S., G.A.S.R., K.S., F.F.L., P.T.A., A.R.P. and M.M.; software, M.M., G.A.S.R. and W.H.N.S.; validation, N.M.B.N., J.R.T.d.R., P.T.A., W.P., A.A.B. and M.M.; formal analysis, J.R.T.d.R., N.M.B.N., W.P., F.F.L., A.R.P. and P.T.A.; investigation, J.R.T.d.R., F.F.L., W.H.N.S., G.A.S.R. and K.S.; resources, N.M.B.N., P.T.A., A.A.B., M.M., A.R.P. and W.P.; data curation, J.R.T.d.R.; writing—original draft preparation, N.M.B.N., J.R.T.d.R. and P.T.A.; writing—review and editing, N.M.B.N., J.R.T.d.R., F.F.L., M.M. and P.T.A.; visualization, J.R.T.d.R., N.M.B.N., P.T.A. and M.M.; supervision, N.M.B.N., M.M. and W.P.; project administration, N.M.B.N., P.T.A., A.A.B. and W.P.; funding acquisition, N.M.B.N., P.T.A., A.A.B. and W.P. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are indebted to Brazilian agencies CNPq under Grant No. [306147/2020-3], FAPESPA, and CAPES under Grant No. [Finance Code 001; AUXPE 88881.159129/2017-01]. NMBN acknowledges the support from the Brazilian National Council for Scientific and Technological Development (CNPq) (process number: 465572/2014-6), and the São Paulo Research Foundation. PTA is grateful to the National Science Foundation (NSF) under Grant No. [1848418] for the financial support. NMBN is especially grateful to the Fulbright Foundation for the Visiting Professor Scholarship Award.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Milgrom, L.R. The Colours of Life: An Introduction to the Chemistry of Porphyrins and Related Compounds, 1st ed.; Oxford University Press Inc.: Oxford, UK, 1997. [Google Scholar]
- Mills, A.; Lepre, A. Controlling the Response Characteristics of Luminescent Porphyrin Plastic Film Sensors for Oxygen. Anal. Chem. 1997, 69, 4653–4659. [Google Scholar] [CrossRef]
- Imahori, H.; Matsubara, Y.; Iijima, H.; Umeyama, T.; Matano, Y.; Ito, S.; Niemi, M.; Tkachenko, N.V.; Lemmetyinen, H. Effects of meso-Diarylamino Group of Porphyrins as Sensitizers in Dye-Sensitized Solar Cells on Optical, Electrochemical, and Photovoltaic Properties. J. Phys. Chem. C 2010, 114, 10656–10665. [Google Scholar] [CrossRef]
- Kalyanasundaram, K. Photochemistry of Polypyridine and Porphyrin Complexes; Academic Press: San Diego, CA, USA, 1992. [Google Scholar]
- Vijisha, M.V.; Ramesh, J.; Arunkumar, C.; Chandrasekharan, K. Nonlinear optical absorption and refraction properties of fluorinated trans-dicationic pyridinium porphyrin and its metal complexes. Opt. Mater. 2019, 98, 109474. [Google Scholar] [CrossRef]
- Neto, N.M.B.; De Boni, L.; Mendonça, C.R.; Misoguti, L.; Queiroz, S.L.; Dinelli, L.R.; Batista, A.A.; Zilio, S.C. Nonlinear Absorption Dynamics in Tetrapyridyl Metalloporphyrins. J. Phys. Chem. B 2005, 109, 17340–17345. [Google Scholar] [CrossRef] [PubMed]
- Gouterman, M. Spectra of porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Gouterman, M.; Wagnière, G.H.; Snyder, L.C. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar] [CrossRef]
- Senge, M.O.; Medforth, C.J.; Forsyth, T.P.; Lee, D.A.; Olmstead, M.M.; Jentzen, W.; Pandey, R.K.; Shelnutt, J.A.; Smith, K.M. Comparative Analysis of the Conformations of Symmetrically and Asymmetrically Deca- and Undecasubstituted Porphyrins Bearing Meso-Alkyl or -Aryl Groups. Inorg. Chem. 1997, 36, 1149–1163. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.M.S.; Sampaio, R.N.; Ito, A.; Batista, A.; Machado, A.; Araujo, P.; Neto, N.M.B. Evolution of electronic and vibronic transitions in metal(II) meso-tetra(4-pyridyl)porphyrins. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 215, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Birel, Ö.; Nadeem, S.; Duman, H. Porphyrin-Based Dye-Sensitized Solar Cells (DSSCs): A Review. J. Fluoresc. 2017, 27, 1075–1085. [Google Scholar] [CrossRef]
- Higashino, T.; Imahori, H. Porphyrins as excellent dyes for dye-sensitized solar cells: Recent developments and insights. Dalton Trans. 2015, 44, 448–463. [Google Scholar] [CrossRef] [PubMed]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-Substituted Porphyrins for Dye-Sensitized Solar Cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef] [PubMed]
- Pavinatto, F.J.; Gameiro, A.; Hidalgo, A.; Dinelli, L.; Romualdo, L.; Batista, A.; Neto, N.M.B.; Ferreira, M.; Oliveira, O. Langmuir and Langmuir–Blodgett (LB) films of tetrapyridyl metalloporphyrins. Appl. Surf. Sci. 2008, 254, 5946–5952. [Google Scholar] [CrossRef]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Sternberg, E.D.; Dolphin, D.; Brückner, C. Porphyrin-based photosensitizers for use in photodynamic therapy. Tetrahedron 1998, 54, 4151–4202. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Pollock, M.E.; Eugene, J.; Hammer-Wilson, M.; Berns, M.W. Photosensitization of experimental atheromas by porphyrins. J. Am. Coll. Cardiol. 1987, 9, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Schneckenburger, H.; Rück, A.; Bartos, B.; Steiner, R. Intracellular distribution of photosensitizing porphyrins measured by video-enhanced fluorescence microscopy. J. Photochem. Photobiol. B 1988, 2, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Lopes, J.M.S.; Sharma, K.; Sampaio, R.N.; Batista, A.; Ito, A.; Machado, A.; Araújo, P.; Neto, N.M.B. Novel insights on the vibronic transitions in free base meso-tetrapyridyl porphyrin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 209, 274–279. [Google Scholar] [CrossRef]
- Sampaio, R.N.; Gomes, W.R.; Araujo, D.M.S.; Machado, A.E.H.; Silva, R.A.; Marletta, A.; Borissevitch, I.E.; Ito, A.S.; Dinelli, L.R.; Batista, A.A.; et al. Investigation of ground- and excited-state photophysical properties of 5,10,15,20-tetra(4-pyridyl)-21H,23H-porphyrin with ruthenium outlying complees. J. Phys. Chem. A 2012, 116, 18–26. [Google Scholar] [CrossRef]
- Li, X.Y.; Czernuszewicz, R.S.; Kincaid, J.R.; Su, Y.O.; Spiro, T.G. Consistent porphyrin force field. 1. Normal-mode analysis for nickel porphine and nickel tetraphenylporphine from resonance Raman and infrared spectra and isotope shifts. J. Phys. Chem. 1990, 94, 31–47. [Google Scholar] [CrossRef]
- Li, X.Y.; Czernuszewicz, R.S.; Kincaid, J.R.; Stein, P.; Spiro, T.G. Consistent porphyrin force field. 2. Nickel octaethylporphyrin skeletal and substituent mode assignments from nitrogen-15, meso-d4, and methylene-d16 Raman and infrared isotope shifts. J. Phys. Chem. 1990, 94, 47–61. [Google Scholar] [CrossRef]
- Li, X.Y.; Czernuszewicz, R.S.; Kincaid, J.R.; Spiro, T.G. Consistent porphyrin force field. 3. Out-of-plane modes in the resonance Raman spectra of planar and ruffled nickel octaethylporphyrin. J. Am. Chem. Soc. 1989, 111, 7012–7023. [Google Scholar] [CrossRef]
- Aydin, M. DFT and Raman spectroscopy of porphyrin derivatives: Tetraphenylporphine (TPP). Vib. Spectrosc. 2013, 68, 141–152. [Google Scholar] [CrossRef]
- Šloufová-Srnová, I.; Vlčková, B. Two-dimensional Assembling of Au Nanoparticles Mediated by Tetrapyridylporphine Molecules. Nano Lett. 2002, 2, 121–125. [Google Scholar] [CrossRef]
- Siskova, K.; Vlckova, B.; Turpin, P.Y.; Thorel, A.; Grosjean, A. Porphyrins as SERRS spectral probes of chemically functionalized Ag nanoparticles. Vib. Spectrosc. 2008, 48, 44–52. [Google Scholar] [CrossRef]
- Maczka, M.; Paraguassu, W.; Freire, P.T.C.; Filho, A.G.S.; Filho, J.M.; Hanuza, J. High-pressure Raman scattering study of ferroelectric K3Nb3O6(BO3)2. Phys. Rev. B 2010, 82, 014106. [Google Scholar] [CrossRef]
- Jayaraman, A.; Wang, S.Y.; Sharma, S.K.; Ming, L.C. Pressure-induced phase transformations in HfO2 to 50 GPa studied by Raman spectroscopy. Phys. Rev. B 1993, 48, 9205–9211. [Google Scholar] [CrossRef] [PubMed]
- Pawbake, A.; Bellin, C.; Pawbake, A.; Paulatto, L.; Béneut, K.; Biscaras, J.; Narayana, C.; Narayana, D.J.; Shukla, A. Pressure-Induced Phase Transitions in Germanium Telluride: Raman Signatures of Anharmonicity and Oxidation. Phys. Rev. Lett. 2019, 122, 145701. [Google Scholar] [CrossRef]
- Chen, D.-M.; He, T.; Cong, D.-F.; Zhang, Y.-H.; Liu, F.-C. Resonance Raman Spectra and Excited-State Structure of Aggregated Tetrakis(4-sulfonatophenyl)porphyrin Diacid. J. Phys. Chem. A 2001, 105, 3981–3988. [Google Scholar] [CrossRef]
- Jorio, A.; Bellin, C.; Paulatto, L.; Béneut, K.; Biscaras, J.; Narayana, C.; Late, D.J.; Shukla, A. G-band resonant Raman study of 62 isolated single-wall carbon nanotubes. Phys. Rev. B 2002, 65, 155412. [Google Scholar] [CrossRef]
- Wood, B.R.; McNaughton, D. Raman excitation wavelength investigation of single red blood cells in vivo. J. Raman Spectrosc. 2002, 33, 517–523. [Google Scholar] [CrossRef]
- Ferraro, J.R.; Nakamoto, K.; Brown, C.W. Introductory Raman Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Li, J.M.; Yao, Y.K.; Sun, L.H.; Shan, X.Y.; Wang, C.; Lu, X.H. Double Resonance Raman Scattering in Single-Layer MoSe2 under Moderate Pressure. Chin. Phys. Lett. 2019, 36, 048201. [Google Scholar] [CrossRef]
- Saha, P.; Ghosh, B.; Mazumder, A.; Mukherjee, G.D. High pressure anomalies in exfoliated MoSe2: Resonance Raman and X-ray diffraction studies. Mater. Res. Express 2020, 7, 025902. [Google Scholar] [CrossRef]
- Khisa, J.; Derese, S.; Mack, J.; Amuhaya, E.; Nyokong, T. Synthesis, photophysical properties and photodynamic antimicrobial activity of meso 5,10,15,20-tetra(pyren-1-yl)porphyrin and its indium(III) complex. J. Porphyr. Phthalocyanines 2021, 25, 794–799. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Jorio, A.A. Group Theory: Application to the Physics of Condensed Matter; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Rousseau, D.L.; Bauman, R.P.; Porto, S.P.S. Normal mode determination in crystals. J. Raman Spectrosc. 1981, 10, 253–290. [Google Scholar] [CrossRef]
- Kingsbury, C.J.; Senge, M.O. The shape of porphyrins. In Coordination Chemistry Reviews; Elsevier B.V.: Amsterdam, The Netherlands, 2021; Volume 431. [Google Scholar] [CrossRef]
- Neto, N.M.B.; De Boni, L.; Rodrigues, J.J.; Misoguti, L.; Mendonça, C.R.; Dinelli, L.R.; Batista, A.A.; Zilio, S.C. Dynamic saturable optical nonlinearities in free base tetrapyridylporphyrin. J. Porphyr. Phthalocyanines 2003, 07, 452–456. [Google Scholar] [CrossRef]
- Fleischer, E.B. α,β,γ,δ-Tetra-(4-pyridyl)-porphine and Some of its Metal Complexes. Inorg. Chem. 1962, 1, 493–495. [Google Scholar] [CrossRef]
- Klotz, S.; Chervin, J.-C.; Munsch, P.; Le Marchand, G. Hydrostatic limits of 11 pressure transmitting media. J. Phys. D Appl. Phys. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Silvera, I.F.; Chijioke, A.D.; Nellis, W.J.; Soldatov, A.; Tempere, J. Calibration of the ruby pressure scale to 150 GPa. Phys. Status Solidi B 2007, 244, 460–467. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. Solid Earth 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Finkelshtein, E.I.; Shamsiev, R.S. Spectral and structural properties of carotenoids—DFT and thermochemical calculations. J. Mol. Struct. 2019, 1197, 583–593. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).