Abstract
To further extend the structure-activity relationships (SARs) of 5-aminopyrazoles (5APs) and identify novel compounds able to interfere with inflammation, oxidative stress, and tumorigenesis, 5APs 1–4 have been designed and prepared. Some chemical modifications have been inserted on cathecol function or in aminopyrazole central core; in detail: (i) smaller, bigger, and more lipophilic substituents were introduced in meta and para positions of catechol portion (5APs 1); (ii) a methyl group was inserted on C3 of the pyrazole scaffold (5APs 2); (iii) a more flexible alkyl chain was inserted on N1 position (5APs 3); (iv) the acylhydrazonic linker was moved from position 4 to position 3 of the pyrazole scaffold (5APs 4). All new derivatives 1–4 have been tested for radical scavenging (DPPH assay), anti-aggregating/antioxidant (in human platelets) and cell growth inhibitory activity (MTT assay) properties. In addition, in silico pharmacokinetics, drug-likeness properties, and toxicity have been calculated. 5APs 1 emerged to be promising anti-proliferative agents, able to suppress the growth of specific cancer cell lines. Furthermore, derivatives 3 remarkably inhibited ROS production in platelets and 5APs 4 showed interesting in vitro radical scavenging properties. Overall, the collected results further confirm the pharmaceutical potentials of this class of compounds and support future studies for the development of novel anti-proliferative and antioxidant agents.
1. Introduction
Pyrazole nucleus has been extensively investigated as pharmacophore [1,2,3,4,5,6,7,8,9,10,11] and several aminopyrazoles bearing a free amino group showed relevant biological activity in different therapeutic areas [12,13,14,15,16]. Specifically, 5-aminopyrazoles (5APs) have been deeply studied for their anti-inflammatory and anticancer activity [17,18], providing useful ligands for receptors or enzymes, as p38 MAPK [19,20,21,22], COX [23], carbonic anhydrase [24], and other different targets involved in cancer progression [25,26], as demonstrated by the recent approval of 5AP Pirtobrutinib, a reversible BTK inhibitor, clinically used for the treatment of mantle cell lymphoma (MCL) [27,28,29,30,31,32,33].
In the effort to synthetize new pyrazole-based compounds able to interfere with inflammation, oxidative stress, and tumorigenesis, we recently reported derivatives I (Figure 1) endowed with excellent anti-proliferative activity. Molecular docking and molecular dynamic simulations suggested the ability of the most active compound Ia (Figure 1) to interact with polymeric tubulin α/tubulin β/stathmin4 complex at the colchicine binding site [34]. By applying a ring opening strategy, we obtained 5APs II (Figure 1), able to strongly inhibit ROS and superoxide anion production, lipid peroxidation, and NADPH oxidase in thrombin-stimulated human platelets and ROS formation in EAhy926 cells [35].
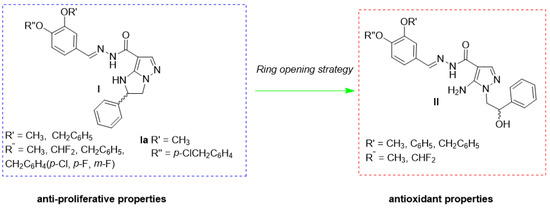
Figure 1.
Chemical structure of previous compounds I and derived 5APs II.
To further extend the structure-activity relationships (SARs) of this class of compounds and verify their biological profile, a new library (eighteen compounds) of 5APs 1–4 was designed and synthesized (Figure 2 and Table 1). In detail: (i) in 5APs 1, smaller, bigger, and more lipophilic substituents, similar to previous II, were introduced in meta and para positions of catechol portion; (ii) in 5APs 2, a methyl group on C3 of the pyrazole scaffold was inserted to increase steric hindrance; (iii) in 5APs 3, a more flexible alkyl chain was inserted on N1 position; (iv) finally, in 5APs 4, the acylhydrazonic substituent was shifted from position 4 to position 3 of the pyrazole scaffold.
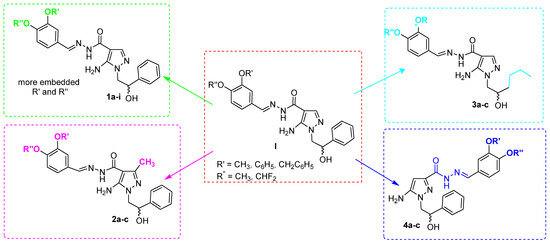
Figure 2.
Chemical structure of previous 5-APs I and new 5APs 1–4.

Table 1.
Different decoration of novel compounds 1–4.
With the aim to investigate the antioxidant and anti-cancer activity of this novel library, in analogy with previous derivatives I and II, all new derivatives 1–4 have been tested for: (i) in vitro radical-scavenging activity (DPPH test); (ii) anti-aggregating/antioxidant activity in human platelets; and (iii) cell growth inhibitory activity. In addition, in silico pharmacokinetics, drug-likeness properties, and toxicity have been calculated.
2. Results
2.1. Chemistry
5APs 1–4 were synthesized in good-to-moderate yields through the three-step sequential procedure (Scheme 1). Briefly, the condensation of the proper ethyl cyanoacrylate 5a–c with suitable hydrazinoethanol 6a,b [36,37,38] led to 5APs 7a–d, as previously reported [37,38,39]. These intermediates were then transformed in the corresponding hydrazides 8a–d by reaction with hydrazine monohydrate. Finally, the reaction between carbohydrazide intermediates 8 and the suitable benzaldehydes 9a–l (commercially available or prepared via literature methods [40,41]) allowed the isolation of the desired compounds 1–4 (Table 1).
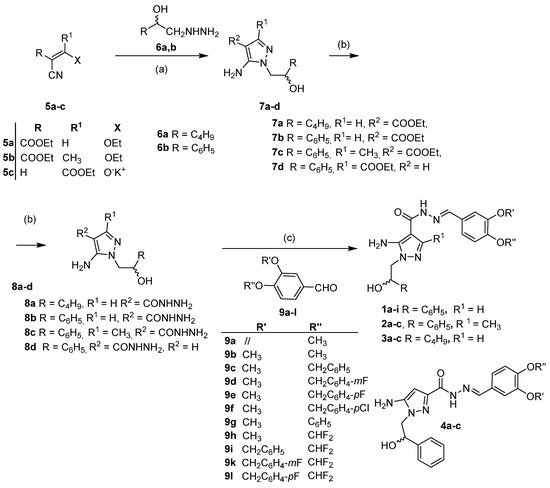
Scheme 1.
Synthesis of compounds 1–4. Reagents and conditions: (a) Toluene, 70–80 °C, 8 h (7b, 7d) or absolute ethanol (abs. EtOH), reflux (7a, 7c); (b) NH2NH2·H2O, 120–130 °C, 3–4 h (8a–c) or r.t., 6 h (8d); (c) abs. EtOH, 9a–l, reflux 1–18 h.
As previously reported, the final reaction proved to be stereoselective and only hydrazones E were isolated, as assessed by NMR spectral analyses [34].
2.2. In Vitro Antioxidant Activity (DPPH Assay)
The antioxidant activity of the new synthesized compounds was measured in vitro using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay [42,43]. The results were calculated as Trolox equivalent and expressed as percentage of antioxidant activity (AA%) (Table 2). The best AA% values have been obtained for 4b and 4c (27.65 and 15.47%, respectively); intermediate activity was shown by 1d, 1h, 1i, 2b, 2c, 3c, and 4a (AA% range = 4.22–6.12%), while all other tested compounds resulted ineffective.

Table 2.
Evaluation of antioxidant activity percent (AA%) using DPPH assay.
2.3. Inhibiting Effect on Human Platelet Aggregation and ROS Production
As previously reported for compounds I and II, platelets could be considered inflammatory cells and could represent a simple, economic, and suitable cellular model based on a causal relationship between inflammation and tumorigenesis [34]. Inflammation and thrombosis are two critical, closely interconnected processes in the response to injury and infection. This correlation has long been recognized, particularly in atherosclerotic cardiovascular disease [44] as well in cancer [45].
Oxidative stress has been associated with several pathological conditions (e.g., cancer, diabetes, metabolic disorders, atherosclerosis, and cardiovascular diseases) [46] and plays a significant role in promoting inflammation and platelet activation [47]. Thus, we tested the new 5AP library on human platelets to verify the inhibitory activity on aggregation and ROS production. As reported in Table 3, the newly synthetized compounds affected platelet aggregation and ROS production. Thus, the majority of the tested derivatives displayed IC50 ≤ 200 μM on both tested parameters; 3b and 3c showed the higher inhibitory properties against both platelet aggregation and ROS production (IC50 values between 113 and 139 μM). It should also be noted that three compounds have intermediate IC50 values (around 400 μM), while five are virtually inactive.

Table 3.
Inhibitory effect of new APs 1–4 and reference compounds ASA (acetylsalicylic acid) and NAC (N-acetylcysteine) on platelet aggregation and ROS production on human platelets expressed as IC50 (μM) values.
2.4. Cell Growth Inhibitory Activity
APs 1–4 were tested for anti-proliferative activity on a panel of 60 different cancer cell lines by National Cancer Institute (Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, Table 4) [48].

Table 4.
Cell growth percent values of most active 5APS 1–4 on different cancer cell lines at 10 μM concentration. For each compound, only cell lines with a growth percent values ≤ 50% are indicated.
Compounds 2 (3-methyl substituted pyrazoles) and 4 (3-benzylidenecarbohydrazide pyrazoles) did not show a relevant anti-cancer effect, thus indicating that 3-unsubstitued pyrazole scaffold is as key determinant for anti-proliferative activity. Conversely, compounds 1, close analogues of previous II, and derivatives 3, characterized by a more flexible chain on N1, evidenced a good percentage of cancer cell growth inhibition in different cancer cell lines (Table 4).
Interestingly, among compounds 1, only derivatives with hindered substituents on catechol portion (i.e., OPh, OCH2Ar) and more strictly related to previous I showed growth percent inhibition values lower than 40% against selected cancer cell lines (1c and 1f on breast cancer cell lines; 1d on leukemia cell lines and breast cancer cell lines, 1e on renal cell lines; Table 4). Remarkably, compound 1e proved to selectively block the growth of renal cancer cell line CAKI-1.
Within series 3, compounds 3a and 3c showed a relevant anti-proliferative activity against different leukemic cell lines.
The significant cell growth inhibitory activity of 1c, 1d, 1f, and 1g against breast cancer cell lines prompted us to further evaluate (at fixed concentration of 10 µM, MTT assay) their effect against other breast adenocarcinoma cancer cell lines (namely, MCF7, MDA-MB231, and SK-BR3) using Cisplatin as reference compound (Table 5). 5-APs 1c and 1d were inactive against all three cell lines, 1f selectively inhibited SK-BR3 cell lines (65% of cell growth), while 1g showed remarkable action against all breast cancer cell lines, particularly against SKBR3, evidencing a GI50 value lower than reference compound Cisplatin (14.4 μM versus 26 μM).

Table 5.
Cell growth percent values of 1c, 1d, 1f, and 1g and Cisplatin, used as reference compound, on three breast cancer cell lines at 10 μM concentration. Data are mean values for three separate experiments. Variation among triplicate samples is less than 10%. For 1g, GI50 values (μM) were also reported.
2.5. Pharmacokinetic Properties, Druglikness and Toxicity Prediction
To evaluate the pharmaceutical relevance of this new library of 5APs, the pharmacokinetics and drug-likeness properties of all compounds were calculated by SwissADME (Tables S1 and S2, Supporting Information) [49].
Collectively the considered compounds are characterized by eight to twelve rotatable bonds, five to eight H-bond acceptors, three H-bond donors, and TPSA values of 123.99 A2 except 1a and 2a (114.76 A2), thus supporting a good capacity of all derivatives to permeate lipophilic barriers. In detail, none of the novel 5APs are able to pass brain-blood barrier (BBB), whereas gastrointestinal (GI) absorption is predicted high for derivatives 1a–g, 2a,3a, and 4a. Except for 1i, all 5APs are predicted to be moderately soluble with a further gain in water solubility for derivatives 3a,b (ESOL method) [50]. A Lipinski violation (MW > 500 Da) was identified for compounds 1e–i, 2b, 2c, 3c, 4b, and 4c, but no compound showed any pan-assay interference compound (PAINS) alerts. Finally, the presence of a C=N functionality was identified as a limitation, according to the Brenk filter [51].
In addition, the toxicity profiles of all novel compounds were predicted using ProTox webserver (Table S3, Supporting Information) [52,53]. According to the simulation, all APs were predicted to belong to toxicity class 5 (predicted LD50 of 4540 mg/kg), with the exception of compounds 1g, 1i, and 2a (predicted LD50 = 1000 mg/kg, class 4) and derivative 4c (LD50 = 6000 mg/kg, class 6). No novel derivatives would show any hepatotoxicity, cardiotoxicity, and nephrotoxicity [54], but for most of them (except 1a, 2c, and 3a–c), immunotoxicity (B cell growth inhibition) has been predicted [55]. Finally, no toxicity targets pharmacophores (Novartis off-targets, Adenosine A2a receptor, Adrenergic beta 2 receptor, Androgen receptor, Amine oxidase A, Corticotropin-releasing hormone receptor 1, Dopamine D3 receptor, Estrogen receptor 1, Estrogen receptor 2, Glucocorticoid receptor, Histamine H1 receptor, Nuclear receptor subfamily 1 group I member 2, Opiod receptor kappa 1, Progesterone receptor, Phosphodiesterase 4D, Prostaglandin G/H synthase 1) have been detected.
3. Discussion and Conclusions
The biological results reported here pointed at 5AP scaffold as an interesting chemotype to obtain new potential antioxidant/anti-inflammatory/anti-proliferative agents. In detail, the following SARs have been defined (Figure 3):
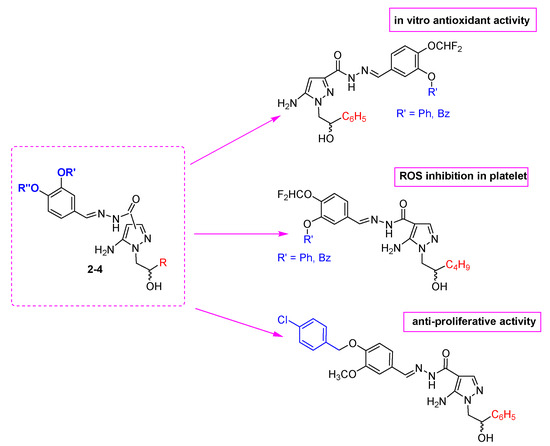
Figure 3.
Schematic representation of SARs of novel 5APs here reported.
- (i)
- The presence of the acylhydrazone moiety at position 3 of the pyrazole core increases the radical scavenging activity (compare 4 with 1–3);
- (ii)
- The best ROS inhibitors in human platelets are 5APs 3 (particularly 3b and 3c, with IC50 values of 113–115 μM), characterized in N1 by a more flexible alkyl chain (hydroxyhexyl). Furthermore, the substituents on the cathecol portions of these compounds (difluoromethoxy in para position and phenoxy or benzyloxy in meta position) are the same of II, previously identified as potent ROS inhibitors. In addition, 3c showed significative anti-cancer profile against different leukemic cell lines. These data confirmed the key role of a flexible hydroxyalkyl chain on N1 position, not only for ROS production inhibition, but also for anti-cancer activity;
- (iii)
- 5APs 1 showed promising anti-proliferative activity, probably related to the chemical similarity of these compounds with previous derivatives I. Of note is the fact that 1g (active against breast cancer cell lines) bears the same substituents on the catechol fragment of its precursor Ia.
- (iv)
- Finally, compounds 2, bearing an additional methyl group on C3 position of pyrazole nucleus, did not show a relevant biological activity, confirming that the increase of steric hindrance in this position is detrimental not only for antioxidant activity and ROS production inhibition in platelets but also for anti-proliferative activity.
Collectively, the results here reported extend the SARs on 5AP scaffold, confirming its role as important chemo-type to obtain compounds able to counteract cancer and inflammation.
4. Materials and Methods
4.1. Chemical Part
4.1.1. General Information
Chiminord (Milan, Italy) and Aldrich Chemical (Milan, Italy) purchased all chemicals. Solvents were reagent grade. Unless otherwise stated, all commercial reagents were used without further purification. Organic solutions were dried over anhydrous sodium sulphate. A thin layer chromatography (TLC) system was used for routine monitoring of the course of reactions and confirming the purity of analytical samples. Detection of spots was performed using UV light. Merck silica gel, 230–400 mesh, was used for chromatography. Flash chromatography was performed using Isolera one instrument (Biotage, Uppsala, Sweden) using Silicagel column. Melting points are not “corrected” and were measured with a Buchi M-560 instrument (Buchi instruments, Flawil, Switzerland). NMR spectra were recorded on JEOL JNM-ECZR (400 MHz, Tokyo, Japan) instruments (Figures S1–S42, Supporting Information) using CDCl3 or DMSO-d6 as solvent; chemical shifts are reported as δ (ppm) and signals were characterized as s (singlet), d (doublet), t (triplet), n t (near triplet), q (quartet), m (multiplet), br s (broad signal); J are reported in Hz.
Elemental analysis was determined with an elemental analyzer EA 1110 (Fison-Instruments, Milan, Italy) and the purity of all synthesized compounds was >95%; products are considered pure when the difference between calculated and found values is ± than 0.4.
4.1.2. Synthesis
Synthesis of compounds 7a–d and 8b are yet reported [34,37,38].
Synthesis of Carbohydrazide 8a, 8c, and 8d
Suitable compound 7 (5 mmol) and hydrazine monohydrate (0.25 g, 0.25 mL, 5 mmol) are heated to 120–130 °C for 4 h (8a and 8c) or at room temperature for 6 h (8d). After cooling to room temperature, H2O is added (5 mL) and the white solid obtained is filtered, washed several times with H2O, and recrystallized from a mixture of ethanol/methanol (1:2) (8a, 8c) or absolute ethanol (8d).
- 5-Amino-1-(2-hydroxyhexyl)-1H-pyrazole-4-carbohydrazide 8a. Yield: 78%. M.p.: 140–142 °C. 1H-NMR (400 MHz, CDCl3): δ 0.86 (t, J = 5.4 Hz, 3H, CH3), 1.15–1.58 (m, 6H, 3CH2), 4.56–4.66 (m, 2H, CH2N pyraz.), 4.92 (br s, 2H, NH2, exchangeable with D2O), 5.02 (d, J = 4.4, 1H, OH, exchangeable with D2O), 5.13–5.18 (m, 1H, CHOH), 6.20 (s, 2H, NH2, exchangeable with D2O), 7.65 (s, 1H, H-3 pyraz.), 8.97 (br s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 164.48, 149,03, 138.30, 97.48, 70.38, 53.18, 35.00, 27.54, 22.74, 14.05. Anal calcd. for C10H19N5O2. Calcd: %C 49.79, %H 7.94, %N 29.02; found: %C 49.72, %H 7.62, %N 29.30.
- 5-Amino-1-(2-hydroxy-2-phenylethyl)-3-methyl-1H-pyrazole-4-carbohydrazide 8c. Yield: 85%. M.p.: 224–226 °C. 1H-NMR (400 MHz, CDCl3): δ 2.24 (s, 3H, CH3), 3.83–3.94 (m, 2H, CH2N), 4.28 (br s, 2H, NH2, exchangeble with D2O), 4.84–4.88 (m, 1H, CHOH), 5.78 (d, J = 4.4, 1H, OH, exchangeble with D2O), 6.03 (s, 2H, NH2, exchangeble with D2O), 7.23–7.48 (m, 5H, 5Ar), 8.02 (s, 1H, CONH, exchangeble with D2O). 13C-NMR (101 MHz, CDCl3): δ 166.87, 151.19, 150.25, 142.40, 128.56, 128.19, 127.25, 93.34, 73.03, 56.46, 14.93. Anal calcd. for C13H17N5O2. Calcd: %C 56.71, %H 6.22, %N 25.44; found: %C 56.94, %H 6.02, %N 25.06.
- 5-Amino-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-3-carbohydrazide 8d. Yield: 85%. M.p.: 72–73 °C. 1H-NMR (400 MHz, CDCl3): δ 3.92–4.08 (m, 2H, CH2N), 4.90–4.94 (m, 1H, CHOH), 5.23 (br s., 2H, NH2, exchangeble with D2O), 5.30 (d, J = 4.4, 1H, OH, exchangeble with D2O), 5.66 (s, 1H, H-4 pyraz.), 5.91 (s, 2H, NH2, exchangeble with D2O), 7.11–7.54 (m, 5H, 5Ar), 8.01 (br s, 1H, CONH, exchangebles with D2O). 13C-NMR (101 MHz, CDCl3): δ 162.76, 56.25, 150.62, 145.20, 142.39, 128.56, 128.19, 127.25, 87.47, 72.89. Anal calcd. for C12H15N5O2. Calcd: %C 55.16, %H 5.79, %N 26.80; found: %C 55.00, %H 5.49, %N 26.46.
Synthesis of Final Compounds 1–4
To a solution of suitable carbohydrazide 8a–d (1 mmol) in absolute ethanol (10 mL), the suitable aldehyde 9a–l (1 mmol), solved in absolute ethanol (2 mL), is added dropwise, then the reaction mixture is heated at reflux for 1–18 h. After cooling to room temperature, the solvent is removed under reduced pressure to obtain yellow/white solids, which are filtered and recrystallized from abs. ethanol (compounds 1) or diethyl ether (compounds 2–4).
- (E)-5-Amino-1-(2-hydroxy-2-phenylethyl)-N′-(4-methoxybenzylidene)-1H-pyrazole-4-carbohydrazide 1a. Yield: 61%. M.p.: 233–234 °C. 1H-NMR (400 MHz, CDCl3): δ 3.76 (s, 3H, OCH3), 3.84–4.13 (m, 2H, CH2N), 4.85–4.88 (m, 1H, CHOH), 5.68 (d, J = 4.6 Hz, 1H, OH, exchangeable with D2O), 6.12 (s, 2H, NH2, exchangeable with D2O), 7.25–7.46 (m, 9H, 5Ar + H-2 Ar + H-3 Ar + H-5 Ar + H-6 Ar), 7.86 (s, 1H, H-3 pyraz.), 8.11 (s, 1H, CH=N), 10.98 (s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 164.49, 161.94, 149.28, 148.53, 142.40, 136.23, 128.80, 128.56, 128.19, 127.25, 126.79, 114.41, 113.80, 97.65, 70.60, 55.72, 55.35. Anal calcd. for C20H21N5O3. Calcd: %C 63.31, %H 5.58, %N 18.46; found: %C 63.38, %H 5.62, %N. 18.22.
- (E)-5-Amino-N′-(3,4-dimethoxybenzylidene)-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carbohydrazide 1b. Yield: 32%. M.p.: 89–90 °C. 1H-NMR (400 MHz, CDCl3): δ 3.74 (s, 3H, OCH3), 3.79 (s, 3H, OCH3), 3.89–4.10 (m, 2H, CH2N), 4.86–4.89 (m, 1H, CHOH), 5.67 (d, J = 4.7 Hz, 1H, OH, exchangeable with D2O), 6.26 (s, 2H, NH2, exchangeable with D2O), 6.87–7.42 (m, 8H, 5Ar + H-2 Ar + H-5 Ar + H-6 Ar), 7.85 (s, 1H, H-3 pyraz.), 8.11 (s, 1H, CH=N), 11.02 (s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 160.95, 151.87, 150.32, 149.28, 144.57, 142.40, 136.31, 128.56, 128.19, 127.99, 127.25, 124.16, 111.32, 109.92, 97.65, 70.76, 55.94, 55.93, 55.72. Anal calcd. for C21H23N5O4. Calcd: %C 61.60, %H 5.66, %N 17.10; found: %C 61.25, %H 5.38, %N 17.14.
- (E)-5-Amino-1-(2-hydroxy-2-phenylethyl)-N′-(4-methoxy-3-phenoxybenzylidene)-1H-pyrazole-4-carbohydrazide 1c. Yield: 32%. M.p.: 190–191 °C. 1H-NMR (400 MHz, CDCl3): δ 3.77 (s, 3H, OCH3), 3.88–4.07 (m, 2H, CH2N), 4.83–4.85 (m, 1H, CHOH), 5.66 (d, J = 4.7 Hz, 1H, OH, exchangeable with D2O), 6.30 (s, 2H, NH2, exchangeable with D2O), 6.73–7.49 (m, 13H, 10Ar + H-5 Ar + H-6 Ar + H-2 Ar), 7.77 (s, 1H, H-3 pyraz.), 8.15 (s, 1H, CH=N), 11.01 (s, 1H, CONH, exchangeable with D2O). 13C NMR (101 MHz, DMSO-d6): δ 157.89, 152.89, 143.17, 130.41, 128.65, 128.55, 127.92, 126.72, 123.15, 117.08, 72.00, 56.40, 54.46. Anal calcd. for C26H25N5O4. Calcd: %C 66.23, %H 5.34, %N 14.85; found: %C 65.86, %H 5.31, %N 14.38.
- (E)-5-Amino-N′-(3-(benzyloxy)-4-methoxybenzylidene)-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carbohydrazide 1d. Yield: 62%. M.p.: 161–163 °C. 1H-NMR (400 MHz, CDCl3): δ 3.77 (s, 3H, OCH3), 3.91–4.09 (m, 2H, CH2N), 4.89–4.90 (m, 1H, CHOH), 5.10 (s, 2H, CH2O), 5.68 (d, J = 4.6 Hz, 1H, OH, exchangeable with D2O), 6.39 (s, 2H, NH2, exchangeable with D2O), 6.92–7.60 (m, 13H, 10Ar + H-5 Ar + H-6 Ar + H-2 Ar), 7.84 (s, 1H, H-3 pyraz.), 8.10 (s, 1H, CH=N), 10.99 (s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 160.95, 152.83, 149.28, 149.26, 144.57, 142.40, 137.41, 135.44, 128.56, 128.50, 128.19, 128.16, 128.12, 127.71, 127.25, 124.42, 112.85, 112.10, 97.65, 71.88, 71.05, 56.07, 55.72. Anal calcd. for C27H27N5O4. Calcd: %C 66.79, %H 5.61, %N 14.42; found: %C 66.66, %H 5.77, %N 14.48.
- (E)-5-Amino-N′-(4-((4-fluorobenzyl)oxy)-3-methoxybenzylidene)-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carbohydrazide 1e. Yield: 75%. M.p.: 136–137 °C. 1H-NMR (400 MHz, CDCl3): δ 3.78 (s, 3H, OCH3), 3.91–4.12 (m, 2H, CH2N), 4.91–4.93 (m, 1H, CHOH), 5.04 (s, 2H, CH2O), 5.69 (d, J = 4.6 Hz, 1H, OH, exchangeable with D2O), 6.28 (s, 2H, NH2, exchangeable with D2O), 7.00–7.56 (m, 12H, 9Ar + H-5 Ar + H-6 Ar + H-2 Ar), 7.86 (s, 1H, H-3 pyraz.), 8.10 (s, 1H, CH=N), 11.04 (s, 1H, CONH, exchangeable with D2O). 13C NMR (101 MHz, DMSO-d6): δ 163.59, 161.17, 149.89, 149.63, 143.19, 133.63, 133.60, 130.74, 130.65, 128.67, 128.41, 127.94, 126.73, 115.92, 115.71, 113.81, 72.04, 69.68, 55.94, 54.51, 40.50. Anal calcd. for C27H26N5O4F. Calcd: %C 64.40, % H 5.20, %N 13.91; found: %C 64.55, %H 5.37, %N 13.51.
- (E)-5-Amino-N′-(4-((3-fluorobenzyl)oxy)-3-methoxybenzylidene)-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carbohydrazide 1f. Yield: 64%. M.p.: 123–125 °C. 1H-NMR (400 MHz, CDCl3): δ 3.81 (s, 3H, OCH3), 3.90–4.12 (m, 2H, CH2N), 4.89–4.91 (m, 1H, CHOH), 5.12 (s, 2H, CH2O), 5.68 (d, J = 4.6 Hz, 1H, OH, exchangeable with D2O), 6.31 (s, 2H, NH2, exchangeable with D2O), 6.96–7.48 (m, 12H, 9Ar + H-5 Ar + H-6 Ar + H-2 Ar), 7.86 (s, 1H, H-3 pyraz.), 8.10 (s, 1H, CH=N), 11.03 (s, 1H, CONH, exchangeable with D2O). 13C NMR (101 MHz, DMSO-d6): δ 163.93, 161.51, 149.90, 149.47, 143.19, 140.40, 140.32, 131.09, 131.01, 128.67, 128.55, 127.94, 126.73, 124.23, 124.20, 115.33, 115.12, 115.00, 114.78, 113.87, 72.03, 69.55, 55.99, 54.50, 40.70, 40.49. Anal calcd. for C27H26N5O4F. Calcd: %C 64.40, %H 5.20, %N 13.91; found: %C 64.82, %H 5.16, %N 13.78.
- (E)-5-Amino-N′-(4-((4-chlorobenzyl)oxy)-3-methoxybenzylidene)-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carbohydrazide 1g. Yield: 48%. M.p.: 120–123 °C. 1H-NMR (400 MHz, CDCl3): δ 3.79 (s, 3H, OCH3), 3.85–4.13 (m, 2H, CH2N), 4.88–4.91 (m, 1H, CHOH), 5.15 (s, 2H, CH2O), 5.67 (d, J = 4.4 Hz, 1H, OH, exchangeable with D2O), 6.36 (s, 2H, NH2, exchangeable with D2O), 6.99–7.49 (m, 12H, 9Ar + H-5 Ar + H-6 Ar + H-2 Ar), 7.77 (s, 1H, H-3 pyraz.), 8.12 (s, 1H, CH=N), 11.02 (s, 1H, CONH, exchangeable with D2O). 13C NMR (101 MHz, DMSO-d6): δ 149.89, 149.51, 143.19, 136.47, 133.05, 130.19, 129.01, 128.67, 128.49, 127.94, 126.73, 72.03, 69.53, 55.97, 54.50, 40.50. Anal calcd. for C27H26N5O4Cl. Calcd: %C 62.37, %H 5.04, %N 13.47; found: %C 62.39, %H 5.28, %N 13.86.
- (E)-5-Amino-N′-(4-(difluoromethoxy)-3-((3-fluorobenzyl)oxy)benzylidene)-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carbohydrazide 1h. Yield: 82%. M.p.: 148–153 °C. 1H-NMR (400 MHz, CDCl3): δ 3.90–4.12 (m, 2H, CH2N), 4.85–4.87 (m, 1H, CHOH), 5.23 (s, 2H, CH2O), 5.68 (d, J =4.4, 1H, OH, exchangeable with D2O), 6.37 (s, 2H, NH2, exchangeable with D2O), 7.15 (t, J = 70 Hz, 1H, OCHF2), 7.21–7.50 (m, 12H, 9Ar + H-5 Ar + H-6 Ar + H-2 Ar), 7.96 (s, 1H, H-3 pyraz.), 8.21 (s, 1H, CH=N), 11.18 (s, 1H, CONH, exchangeable with D2O). 13C NMR (101 MHz, DMSO-d6): δ 163.98, 161.56, 150.30, 143.17, 141.20, 139.98, 139.91, 133.76, 131.17, 131.08, 128.67, 127.94, 126.74, 123.97, 122.21, 119.88, 117.31, 115.44, 115.23, 114.74, 114.52, 72.03, 69.63, 54.49. Anal calcd. for C27H24N5O4F3. Calcd: %C 60.11, %H 4.48, %N 12.98; found: %C 60.23, %H 4.60, %N 13.12.
- (E)-5-Amino-N′-(3-((4-chlorobenzyl)oxy)-4-(difluoromethoxy)benzylidene)-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-4-carbohydrazide 1i. Yield: 81%. M.p.: 106–108 °C. 1H-NMR (400 MHz, CDCl3): δ 3.83–4.14 (m, 2H, CH2N), 4.87–4.90 (m, 1H, CHOH), 5.23 (s, 2H, CH2O), 5.68 (d, J = 4.4, 1H, OH, exchangeable with D2O), 6.32 (s, 2H, NH2, exchangeable with D2O), 7.13 (t, J = 70 Hz, 1H, OCHF2), 7.21–7.53 (m, 12H, 9Ar + H-5 Ar + H-6 Ar + H-2 Ar), 7.94 (s, 1H, H-3 pyraz.), 8.18 (s, 1H, CH=N), 11.18 (s, 1H, CONH, exchangeable with D2O). 13C NMR (101 MHz, DMSO-d6): δ 150.28, 143.17, 141.23, 136.07, 133.68, 133.17, 130.98, 129.98, 129.11, 128.90, 128.67, 127.95, 126.75, 122.07, 119.83, 117.26, 114.69, 112.19, 72.04, 69.66, 54.49, 40.70, 38.25. Anal calcd. for C27H24N5O4ClF2. Calcd: %C 58.33, %H 4.35, %N 12.60; found: %C 58.10, %H 4.33, %N 12.35.
- (E)-5-Amino-N′-(4-(difluoromethoxy)-3-methoxybenzylidene)-1-(2-hydroxy-2-phenylethyl)-3-methyl-1H-pyrazole-4-carbohydrazide 2a. Yield: 46%. M.p.: 193–195 °C. 1H-NMR (400 MHz, CDCl3): δ 2.31 (s, 3H, CH3), 3.65–4.12 (m, 5H, CH2N + OCH3), 4.83–4.86 (m, 1H, CHOH), 5.70 (d, J = 4.6 Hz, 1H, OH, exchangeable with D2O), 6.05 (s, 2H, NH2, exchangeable with D2O), 7.28 (t, J = 70, 1H, OCHF2), 7.37–7.67 (m, 8H, 5 Ar + H-5 Ar + H-6 Ar + H-2 Ar), 8.25 (s, 1H, CH=N), 11.42 (s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 166.24, 151.94, 150.72, 149.40, 144.53, 144.45, 142.40, 128.30, 128.19, 127.25, 121.90, 118.71, 118.69, 117.87, 115.77, 113.66, 110.53, 93.67, 73.03, 56.45, 56.23, 14.93. Anal calcd. for C22H23N5O4F2. Calcd: %C 57.51, %H 5.05, %N 15.24; found: %C 57.31, %H 5.26, %N 15.64.
- (E)-5-Amino-N′-(4-(difluoromethoxy)-3-phenoxybenzylidene)-1-(2-hydroxy-2-phenylethyl)-3-methyl-1H-pyrazole-4-carbohydrazide 2b. Yield: 25%. M.p.: 203–206 °C. 1H-NMR (400 MHz, CDCl3): δ 2.51 (s, 3H, CH3), 3.71–4.10 (m, 2H, CH2N), 4.84–4.85 (m, 1H, CHOH), 5.71 (d, J = 4.6 Hz, 1H, OH, exchangeable with D2O), 5.95 (s, 2H, NH2, exchangeable with D2O), 6.94–7.53 (m, 14H, 10Ar + H-5 Ar + H-6 Ar + H-2 Ar + OCHF2), 8.21 (s, 1H, CH=N), 10.32 (s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 166.24, 156.83, 152.85, 150.72, 147.66, 145.72, 144.77, 142.40, 130.02, 128.19, 127.25, 124.60, 122.31, 122.17, 120.07, 118.73, 117.96, 115.73, 115.71, 115.69, 114.87, 92.84, 74.52, 54.27, 16.24. Anal calcd. for C27H25N5O4F2. Calcd: %C 62.18, %H 4.83, %N 13.43; found: %C 62.37, %H 4.25, %N 13.34.
- (E)-5-Amino-N′-(3-(benzyloxy)-4-(difluoromethoxy)benzylidene)-1-(2-hydroxy-2-phenylethyl)-3-methyl-1H-pyrazole-4-carbohydrazide 2c. Yield: 75%. M.p.: 188–190 °C. 1H-NMR (400 MHz, CDCl3): δ 2.29 (s, 3H, CH3), 3.73–4.15 (m, 2H, CH2N), 4.79–4.82 (m, 1H, CHOH), 5.21 (s, 2H, CH2O), 5.71 (d, J =4.4, 1H, OH, exchangeable with D2O), 6.03 (s, 2H, NH2, exchangeable with D2O), 7.17 (t, J = 70 Hz, 1H, OCHF2), 7.30–7.63 (m, 13H, 10Ar + H-5 Ar + H-6 Ar + H-2 Ar), 8.22 (s, 1H, CH=N), 10.42 (s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 166.24, 154.48, 150.58, 150.56, 145.94, 144.67, 142.40, 137.49, 128.56, 128.50, 128.22, 128.19, 128.16, 127.71, 127.25, 122.14, 119.79, 117.68, 115.58, 112.92, 112.89, 112.87, 111.68, 95.24, 72.49, 71.24, 54.32, 16.33. Anal calcd. for C28H27N5O4F2. Calcd: %C 62.80, %H 5.08, %N 13.08; found: %C 62.72, %H 5.18, %N 13.51.
- (E)-5-Amino-N′-(4-(difluoromethoxy)-3-methoxybenzylidene)-1-(2-hydroxyhexyl)-1H-pyrazole-4-carbohydrazide 3a. Yield: 52%. M.p.: 164–165 °C. 1H-NMR (400 MHz, CDCl3): δ 0.85 (s, 3H, CH3), 1.07–1.48 (m, 6H, 3CH2), 3.64–4.03 (m, 5H, OCH3 + CH2N), 4.93–4.97 (s, 1H, CHOH), 5.67 (d, J = 4.5 Hz, 1H, OH, exchangeable with D2O), 6.34 (s, 2H, NH2, exchangeable with D2O), 7.12 (t, J = 67 Hz, 1H, OCHF2), 7.40–7.54 (m, 3H, H-5 Ar + H-6 Ar + H-2 Ar), 7.99 (s, 1H, H-3 pyraz.), 8.18 (s, 1H, CH=N), 11.22 (s, 1H, CONH exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 158.95, 151.94, 149.02, 144.53, 135.49, 128.30, 121.90, 118.69, 115.77, 113.66, 110.53, 97.46, 70.38, 56.23, 55.36, 32.62, 27.54, 22.74, 16.41. Anal calcd. for C19H25N5O4F2. Calcd: %C 53.64, %H 5.92, %N 16.46; found: %C 53.75, %H 5.81, %N 16.36.
- (E)-5-Amino-N′-(4-(difluoromethoxy)-3-phenoxybenzylidene)-1-(2-hydroxyhexyl)-1H-pyrazole-4-carbohydrazide 3b. Yield: 73%. M.p.: 140–141 °C. 1H-NMR (400 MHz, CDCl3): δ 0.86 (t, 3H, CH3), 1.14–1.52 (m, 6H, 3CH2), 3.65–3.97 (m, 2H, CH2N), 4.92–4.94 (m, 1H, CHOH), 6.01 (d, J = 4.4 Hz, 1H, OH, exchangeable with D2O), 6.29 (s, 2H, NH2, exchangeable with D2O), 6.83 (t, J = 67 Hz, 1H, OCHF2), 7.08–7.63 (m, 8H, 5 Ar + H-5 Ar + H-6 Ar + H-2 Ar), 7.79 (s, 1H, H-3 pyraz.), 8.15 (s, 1H, CH=N), 11.18 (s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 165.06, 156.83, 149.02, 147.65, 145.13, 145.08, 144.73, 134.57, 130.02, 128.30, 124.60, 122.31, 118.73, 117.87, 115.76, 114.87, 113.66, 97.46, 70.38, 53.17, 32.77, 27.54, 24.62, 18.06. Anal calcd. for C24H27N5O4F2. Calcd: %C 59.13, %H 5.58, %N 14.37; found: %C 59.59, %H 5.46, %N 14.69.
- (E)-5-Amino-N′-(3-(benzyloxy)-4-(difluoromethoxy)benzylidene)-1-(2-hydroxyhexyl)-1H-pyrazole-4-carbohydrazide 3c. Yield: 52%. M.p.: 129–131 °C. 1H-NMR (400 MHz, CDCl3): δ 0.84 (t, J = 5.6 Hz, 3H, CH3), 1.18–1.65 (m, 6H, 3CH2), 3.65–3.99 (m, 2H, CH2N), 4.85–4.86 (m, 1H, CHOH), 5.23 (s, 2H, CH2O), 6.03 (d, J = 4.4 Hz, 1H, OH, exchangeable with D2O), 6.35 (s, 2H, NH2, exchangeable with D2O), 7.01 (t, J = 67 Hz, 1H, OCHF2), 7.15–7.63 (m, 8H, 5 Ar + H-5 Ar + H-6 Ar + H-2 Ar), 7.97 (s, 1H, H-3 pyraz.), 8.10 (s, 1H, CH=N), 11.22 (s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 162.95, 150.55, 149.02, 144.54, 137.49, 135.36, 128.22, 128.16, 127.71, 122.14, 118.44, 118.42, 116.84, 114.74, 112.63, 111.68, 95.84, 71.24, 70.38, 53.17, 31.77, 27.54, 24.31, 18.41. Anal calcd. for C25H29N5O4F2. Calcd: %C 59.87, %H 5.83, %N 13.96; found: %C 59.59, %H 5.77, %N 14.10.
- (E)-5-Amino-N′-(4-(difluoromethoxy)-3-methoxybenzylidene)-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-3-carbohydrazide 4a. Yield: 54%. M.p.: 196–197 °C. 1H-NMR (400 MHz, CDCl3): δ 3.86–3.94 (m, 2H, CH2N), 3.97 (s, 3H, OCH3), 4.52 (d, J = 4.4 Hz, 1H, OH, exchangeable with D2O), 5.10–5.13 (m, 1H, CHOH), 5.69 (s, 2H, NH2, exchangeable with D2O), 5.74 (s, 1H, H-4 pyraz.), 7.04 (t, J = 70 Hz, 1H, OCHF2), 7.10–7.51 (m, 8H, 5Ar + H-5 Ar + H-6 Ar + H-2 Ar), 8.55 (s, 1H, CH=N), 11.78 (s, 1H, CONH, exchangeable with D2O). 13C NMR (101 MHz, DMSO-d6): δ 166.71, 165.11, 150.37, 150.03, 137.50, 136.45, 97.39, 95.92, 69.85, 53.18, 34.39, 27.68, 22.69, 14.51. Anal calcd. for C21H21N5O4F2. Calcd: %C 56.60, %H 4.70, %N 15.72; found: %C 56.69, %H 4.42, %N 15.69.
- (E)-5-Amino-N′-(4-(difluoromethoxy)-3-phenoxybenzylidene)-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-3-carbohydrazide 4b. Yield: 59%. M.p.: 195–196 °C. 1H-NMR (400 MHz, CDCl3): δ 3.92–4.08 (m, 2H, CH2N), 4.96–4.98 (m, 1H, CHOH), 5.33 (d, J = 4.4 Hz, 1H, OH, exchangeable with D2O), 5.43 (s, 2H, NH2, exchangeable with D2O), 5.75 (s, 1H, H-4 pyraz.), 6.92–7.50 (m, 14H, 10Ar + H-5 Ar + H-6 Ar + H-2 Ar + OCHF2), 8.40 (s, 1H, CH=N), 11.41 (s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 162.64, 156.83, 151.88, 150.61, 145.08, 144.66, 142.39, 140.61, 130.02, 128.56, 128.32, 128.19, 127.25, 124.60, 122.31, 121.36, 119.25, 118.73, 117.15, 115.73, 115.70, 115.68, 114.87, 87.65, 71.77, 57.42. Anal calcd. for C26H23N5O4F2. Calcd: %C 61.53, %H 4.57, %N 13.80; found: %C 61.63, %H 4.95, %N 13.65.
- (E)-5-Amino-N′-(3-(benzyloxy)-4-(difluoromethoxy)benzylidene)-1-(2-hydroxy-2-phenylethyl)-1H-pyrazole-3-carbohydrazide 4c. Yield: 15%. M.p.: 136–137 °C. 1H-NMR (400 MHz, CDCl3): δ 4.00–4.14 (m, 2H, CH2N), 4.98–5.01 (m, 1H, CHOH), 5.17 (s, 2H, CH2O), 5.36 (d, J = 4.4 Hz, 1H, OH, exchangeable with D2O), 5.75 (s, 2H, NH2, exchangeable with D2O), 5.81 (s, 1H, H-4 pyraz.), 7.02 (t, J = 70 Hz, 1H, OCHF2), 7.22–7.53 (m, 13H, 10Ar + H-5 Ar + H-6 Ar + H-2 Ar), 8.43 (s, 1H, CH=N), 11.44 (s, 1H, CONH, exchangeable with D2O). 13C-NMR (101 MHz, CDCl3): δ 162.95, 153.71, 153.70, 150.61, 144.49, 144.43, 144.36, 142.39, 140.74, 137.49, 128.50, 128.19, 128.18, 128.16, 127.71, 127.25, 122.14, 120.67, 118.57, 116.46, 112.90, 112.88, 111.68, 86.98, 73.64, 71.24, 57.37. Anal calcd. for C27H25N5O4F2. Calcd: %C 62.18, %H 4.83, %N 13.43; found: %C 62.15, %H 4.40, %N 13.01.
4.2. In Vitro Antioxidant Activity (DPPH Assay)
The antioxidant activity was measured using the DPPH antioxidant assay. The assay is based on the bleaching rate of the stable radical DPPH [56]. Briefly, ca 3 mg of single compound was dissolved with methanol, then 0.1 mL of this solution was mixed with 3.9 mL of DPPH methanol solution (65 µM). Absorbance was measured at 517 nm after reacting for 30 min in the dark. Linear calibration curve was obtained using Trolox standards (range between 20 to 200 mg/L, R2 = 0.9988). The result was calculated as Trolox equivalents in mg/L and the percentage of antioxidant activity (AA%) was calculated from the ratio of decreasing absorbance of sample solution (A0 − As) to absorbance of blank DPPH solution (A0), as expressed in Equation (1) [57].
AA% = [(A0 − As)/A0] × 100
4.3. Human Platelet Assays
4.3.1. Material
2′,7′-Dichlorofluorescein diacetate (DCFH-DA) and thrombin were purchased from Sigma-Aldrich (St. Louis, MI, USA)/Merck Millipore (Burlington, VT, USA).
4.3.2. Blood Collection and Preparative Procedures
Washed platelets were prepared from freshly drawn venous blood obtained from healthy volunteers at the Centro Trasfusionale, Ospedale San Martino in Genoa. Donors declare that they have not taken any drugs known to interfere with platelet function for at least two weeks prior to blood collection and gave their informed consent. Blood was collected into anticoagulant solution containing 130 mM aqueous trisodium citrate (9:1). Whole blood is centrifuged at 100× g for 20 min to obtain platelet-rich plasma (PRP) that is further centrifuged at 1100× g for 15 min. The obtained pellet is washed once with an acidic solution containing 75 mM trisodium citrate, 42 mM citric acid, and 136 mM glucose (pH 5.2) and then resuspended in a pH 7.4 Hepes buffer solution (145 mM NaCl, 5 mM KCl, 1 mM MgSO4, 10 mM glucose, and 10 mM HEPES).
IC50 values reported represent the molar concentration of a compound required to inhibit 50% of the maximal effect induced by thrombin. The percentage of inhibition is calculated by comparing the inhibition of the maximal effect measured in the presence of the test compound with that measured in a control sample containing saline, under the same experimental conditions.
4.3.3. ROS Assay
ROS production was measured by the ROS-sensitive probe DCFH-DA, which, upon oxidation, forms the fluorescent compound DCF. The DCF formed by ROS is trapped inside the cells, allowing for the quantification of ROS levels [58]. Washed platelets (1.0 × 108/mL) were preincubated with either saline or test compounds for 15 min at 37 °C. Platelets were then stimulated by 0.1 U/mL thrombin for 15 min at 37 °C. After stimulation, the incubation was stopped by cooling in an ice bath; the samples were immediately analyzed using a flow cytometer (Merck Millipore Bioscience Guava easyCyte flow cytometer). IC50 values were calculated as described above.
4.3.4. Platelet Aggregation
Born’s method [59] is a well-established technique for studying platelet aggregation in response to various stimuli and it provides valuable information about the ability of compounds to modulate platelet function.
Washed platelets (3.0 × 108/mL) were preincubated for 3 min at 37 °C with either saline or test compounds and then stimulated by 0.1 U/mL thrombin. In a BioData Aggregometer (Bio-Data Corporation, Horsham, PA, USA), platelet aggregation is quantified by measuring the light transmission over 6 min at 37 °C. IC50 values were calculated as above reported.
4.4. Cell Growth Inibitory activity
1c, 1d, 1f, and 1g were tested at a fixed concentration of 10 μM against three different breast cancer cell lines (namely, MCF7, MDA-MB231, SK-BR3) using Cisplatin (Cis-Pt) as reference compound. Cisplatin was kindly provided by the pharmacy (UFA-Unità Farmaci Antiblastici) of the IRCCS Ospedale Policlinico San Martino.
MTT Assay
To perform MTT assay, SK-BR3 (breast adenocarcinoma, Biologic Bank and Cell Factory, IRCCS Policlinico San Martino, Genoa, Italy), MCF-7 (breast adenocarcinoma, Biologic Bank and Cell Factory, IRCCS Policlinico San Martino, Genoa, Italy), and MDA-MB231 (breast adenocarcinoma, Biologic Bank and Cell Factory, IRCCS Policlinico San Martino, Genoa, Italy) cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM) added to 10% Fetal bovine serum (FBS), 2 mM Glutamine, and 1% penstrep. Reagents were acquired from EuroClone (Milan, Italy) and incubated in a humidified environment at 37 °C with 5% CO2. All chemical compounds (1c, 1d, 1f, and 1g and Cisplatin, used as reference compound) were dissolved in DMSO to give a 10 mM stock solution. Then, after an intermediate dilution in growth medium, they were added to the cultured cells at a final working concentration of 10 μM and incubated for 48 h. At the end of the incubation, 30 μL of MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) at a concentration of 2 mg/mL in PBS, were added in each well and incubated 4 h. Finally, the supernatants were removed and 100 μL/well of DMSO were added to each well to dissolve the Formazan precipitates. After 20 min, the results were read at λ = 570 nm. Results are expressed as a percentage of the control samples, where cells have been treated with the same amount of DMSO but without any chemical compound. The assay was repeated three times, and a single compound was tested six times. Means and standard deviations were calculated.
The GI50 values were calculated based on single concentration-response curves. Each experiment was repeated three times.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29102298/s1, Table S1. Predicted pharmacokinetics and drug-like properties of compounds 1a-i. Table S2. Predicted pharmacokinetics and drug-like properties of compounds 2a–c, 3a–c, 4a–c. Table S3. Predicted toxicity of compounds 1a–i, 2a–c, 3a–c, 4a–c. Figures S1–S42: 1H NMR (400 MHz) and 13C NMR (101 MHz) of compounds 8a,8c,8d and 1–4.
Author Contributions
Writing and conceptualization, C.B.; synthesis of compounds, F.R., B.T.; MTT and cell biology, M.P., E.I., C.R.; platelet aggregation and antioxidant inhibitory activity, M.G.S.; DPPH test E.R., D.C., C.V.; statistical analysis and in silico pharmacokinetics, drug-likeness, and toxicity properties, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
E.I.: C.R., M.P. acknowledge the support of the Italian Minister of Health (Ricerca Corrente).
Institutional Review Board Statement
Since blood for the experiments is collected during the voluntary blood donation in the transfusion center of the Hospital, under the Italian legislation it’s not necessary to obtain the Ethics Commission Authorization. Donors are healthy subjects who have not undergone any treatment.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in article and Supplementary Materials.
Acknowledgments
We thank M. Anzaldi and R. Raggio for recording elemental analysis, 1H and 13 C spectra. We thank Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute (NCI, http://dtp.cancer.gov Accessed on 1 May 2024) for cell growth inhibitory activityevaluation.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ebenezer, O.; Shapi, M.; Tuszynski, J.A. A Review of the Recent Development in the Synthesis and Biological Evaluations of Pyrazole Derivatives. Biomedicines 2022, 10, 1124–1180. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134–219. [Google Scholar] [CrossRef]
- Karati, D.; Mahadik, K.R.; Kumar, D. Pyrazole Scaffolds: Centrality in Anti-Inflammatory and Antiviral Drug Design. Med. Chem. 2022, 18, 1060–1072. [Google Scholar] [CrossRef]
- Karati, D.; Mahadik, K.R.; Trivedi, P.; Kumar, D. A Molecular Insight into Pyrazole Congeners as Antimicrobial, Anticancer, and Antimalarial Agents. Med. Chem. 2022, 18, 1044–1059. [Google Scholar] [CrossRef]
- Ansari, A.; Ali, A.; Asif, M. Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar] [CrossRef]
- Li, M.; Zhao, B.X. Progress of the synthesis of condensed pyrazole derivatives (from 2010 to mid-2013). Eur. J. Med. Chem 2014, 85, 311–340. [Google Scholar] [CrossRef]
- Dadiboyena, S.; Nefzi, A. Synthesis of functionalized tetrasubstituted pyrazolyl heterocycles--a review. Eur. J. Med. Chem. 2011, 46, 5258–5275. [Google Scholar] [CrossRef]
- Abu-Hashem, A.A.; Aly, A.S. Synthesis of New Pyrazole, Triazole, Thiazolidine, -Pyrimido [4,5-b] quinoline derivatives with Potential Antitumor Activity. Arch. Pharm. Res. 2012, 35, 437–445. [Google Scholar] [CrossRef]
- Abu-Hashem, A. Synthesis of new pyrazoles, oxadiazoles, triazoles, pyrrolotriazines and pyrrolotriazepines as potential cytotoxic agents. J. Heter. Chem. 2021, 58, 805–821. [Google Scholar] [CrossRef]
- Silva, V.L.; Silva, A.M.S. Special Issue “Recent Advances in the Synthesis, Functionalization and Applications of Pyrazole-Type Compounds”. Molecules 2021, 26, 4989. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.L.M.; Silva, A.M.S. Special Issue Recent Advances in the Synthesis, Functionalization and Applications of Pyrazole-Type Compounds II. Molecules 2023, 28, 5873. [Google Scholar] [CrossRef]
- Lusardi, M.; Spallarossa, A.; Brullo, C. Amino-Pyrazoles in Medicinal Chemistry: A Review. IJMS 2023, 24, 7834. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; Cherrah, Y.; Ramli, Y.; Karrouchi, K.; Ansar, M.; Faouzi, M.E.A. Overview of recent developments of pyrazole derivatives as an anticancer agent in different cell line. Bioorg. Chem. 2020, 97, 103470. [Google Scholar] [CrossRef]
- Küçükgüzel, Ş.G.; Şenkardeş, S. Recent advances in bioactive pyrazoles. Eur. J. Med. Chem. 2015, 97, 786–815. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Hymete, A.; El-Din A Bekhit, A.; Damtew, A.; Aboul-Enein, H.Y. Pyrazoles as promising scaffold for the synthesis of anti-inflammatory and/or antimicrobial agent: A review. Mini Rev. Med. Chem. 2010, 10, 1014–1033. [Google Scholar] [CrossRef]
- Bennani, F.E.; Doudach, L.; El Rhayam, Y.; Karrouchi, K.; Cherrah, Y.; Tarib, A.; Ansar, M.; Faouzi, M.E.A. Identification of the new progress on Pyrazole Derivatives Molecules as Antimicrobial and Antifungal Agents. West Afr. J. Med. 2022, 39, 1217–1244. [Google Scholar]
- Anwara, H.F.; Mohamed, H.E. Recent developments in aminopyrazole chemistry. ARKIVOC 2009, 198–250. Available online: https://www.arkat-usa.org/get-file/29257/ (accessed on 8 May 2024). [CrossRef]
- Abu Elmaati, T.M.; El-Taweel, F.M. New Trends in the Chemistry of 5-Aminopyrazoles. J. Heterocycl. Chem. 2004, 41, 109–134. [Google Scholar] [CrossRef]
- Goldstein, D.M.; Alfredson, T.; Bertrand, J.; Browner, M.F.; Clifford, K.; Dalrymple, S.A.; Dunn, J.; Freire-Moar, J.; Harris, S.; Labadie, S.S.; et al. Discovery of S-[5-Amino-1-(4-fluorophenyl)-1H-pyrazol-4-yl]-[3-(2,3-dihydroxypropoxy)phenyl]-methanone (RO3201195), an Orally Bioavailable and Highly Selective Inhibitor of p38 Map Kinase. J. Med. Chem. 2006, 49, 1562–1575. [Google Scholar] [CrossRef]
- Bagley, M.C.; Davis, T.; Dix, M.C.; Murziani, P.G.S.; Rokicki, M.J.; Kipling, D. Microwave-assisted synthesis of 5-aminopyrazol-4-yl ketones and the p38MAPK inhibitor RO3201195 for study in Werner syndrome cells. Bioorg. Med. Chem. Lett. 2008, 18, 3745–3748. [Google Scholar] [CrossRef]
- Röhm, S.; Berger, B.T.; Schröder, M.; Chaikuad, A.; Winkel, R.; Hekking, K.F.W.; Benningshof, J.J.C.; Müller, G.; Tesch, R.; Kudolo, M.; et al. Fast Iterative Synthetic Approach toward Identification of Novel Highly Selective p38 MAP Kinase Inhibitors. J. Med. Chem. 2019, 62, 10757–10782. [Google Scholar] [CrossRef] [PubMed]
- Röhm, S.; Schröder, M.; Dwyer, J.E.; Widdowson, C.S.; Chaikuad, A.; Berger, B.T.; Joerger, A.C.; Krämer, A.; Harbig, J.; Dauch, D.; et al. Selective targeting of the αC and DGF-out pocket in p38 MAPK. Eur. J. Med. Chem. 2020, 208, 112721–112755. [Google Scholar] [CrossRef] [PubMed]
- Fadaly, W.A.A.; Elshaier, Y.A.M.M.; Hassanein, E.H.M.; Abdellatif, K.R.A. New 1,2,4-triazole/pyrazole hybrids linked to oxime moiety as nitric oxide donor celecoxib analogs: Synthesis, cyclooxygenase inhibition antiinflammatory, ulcerogenicity, anti-proliferative activities, apoptosis, molecular modeling and nitric oxide release studies. Bioorg. Chem. 2020, 98, 103752–103771. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.S.; Abou-Seri, S.H.; Tanc, M.; Elaasser, M.M.; Abdel-Aziz, H.A.; Supuran, C.T. Isatin-pyrazole benzenesulfonamide hybrids potently inhibit tumor-associated carbonic anhydrase isoforms IX and XII. Eur. J. Med. Chem. 2015, 103, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Pippione, A.C.; Sainas, S.; Federico, A.; Lupino, E.; Piccinini, M.; Kubbutat, M.; Contreras, J.M.; Morice, C.; Barge, A.; Ducime, A.; et al. N-Acetyl-3-aminopyrazoles block the noncanonical NF-κB cascade by selectively inhibiting NIK. Med. Chem. Commun. 2018, 9, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.Y.; Saleh, N.M.; Kadh, M.S.; Abou-Amra, E.S. New fused pyrazolopyrimidine derivatives; heterocyclic styling, synthesis, molecular docking and anticancer evaluation. J. Heterocycl. Chem. 2020, 57, 2704–2721. [Google Scholar] [CrossRef]
- De, S.K. Pirtobrutinib: First Non-covalent Tyrosine Kinase Inhibitor for Treating Relapsed or Refractory Mantle Cell Lymphoma in Adults. Curr. Med. Chem. 2023; ahead of print. [Google Scholar] [CrossRef]
- Schultze, M.D.; Reeves, D.J. Pirtobrutinib: A New and Distinctive Treatment Option for B-Cell Malignancies. Ann. Pharmacother. 2024, 10600280231223737. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2024 update. Pharmacol. Res. 2024, 200, 107059. [Google Scholar] [CrossRef]
- Mato, A.R.; Shah, N.N.; Jurczak, W.; Cheah, C.Y.; Pagel, J.M.; Woyach, J.A.; Fakhri, B.; Eyre, T.A.; Lamanna, N.; Patel, M.R. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 2021, 397, 892–901. [Google Scholar] [CrossRef]
- Brandhuber, B.J.; Ku, N.C.Y.; Nanda, N.; Smith, S.A.; Tsai, D. Dosing of a Bruton’s Tyrosine Kinase inhibitor. PCT Int. Appl. WO 2021113497, 10 June 2021. [Google Scholar]
- Telaraja, D.; Kasamon, Y.L.; Collazo, J.S.; Leong, R.; Wang, K.; Li, P.; Dahmane, E.; Yang, Y.; Earp, J.; Grimstein, M.; et al. FDA Approval Summary: Pirtobrutinib for Relapsed or Refractory Mantle Cell Lymphoma. Clin. Cancer Res. 2024, 30, 17–22. [Google Scholar] [CrossRef]
- Gomez, E.B.; Ebata, K.; Randeria, H.S.; Rosendahl, M.S.; Cedervall, E.P.; Morales, T.H.; Hanson, L.M.; Brown, N.E.; Gong, X.; Stephens, J.R.; et al. Pirtobrutinib preclinical characterization: A highly selective, non-covalent (reversible) BTK inhibitor. Blood 2023, 142, 62–72. [Google Scholar] [CrossRef]
- Spallarossa, A.; Rapetti, F.; Signorello, M.G.; Rosano, C.; Iervasi, E.; Ponassi, M.; Brullo, C. Insights on pharmacological activity of imidazo-pyrazole scaffold. ChemMedChem 2023, 18, e202300252. [Google Scholar] [CrossRef]
- Brullo, C.; Russo, E.; Garibaldi, S.; Altieri, P.; Ameri, P.; Ravera, S.; Signorello, M.G. Inside the Mechanism of Action of Three Pyrazole Derivatives in Human Platelets and Endothelial Cells. Antioxidants 2023, 12, 216. [Google Scholar] [CrossRef]
- Benoit, G. Hydroxyalkyl hydrazines. Bull. Soc. Chim. Fr. 1939, 6, 708–715. [Google Scholar]
- Schenone, S.; Bruno, O.; Ranise, A.; Bondavalli, F.; Brullo, C.; Fossa, P.; Mosti, L.; Menozzi, G.; Carraro, F.; Naldini, A.; et al. New pyrazolo [3,4-d]pyrimidines endowed with A431 antiproliferative activity and inhibitory properties of Src phosphorylation. Bioorg. Med. Chem. Lett. 2004, 14, 2511–2517. [Google Scholar] [CrossRef]
- Lusardi, M.; Wehrle-Haller, B.; Sidibe, A.; Ponassi, M.; Iervasi, E.; Rosano, C.; Brullo, C.; Spallarossa, A. Novel 5-aminopyrazoles endowed with anti-angiogenetic properties: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2023, 260, 115727. [Google Scholar] [CrossRef]
- Meta, E.; Brullo, C.; Sidibè, A.; Imhof, B.A.; Bruno, O. Design, synthesis, and biological evaluation of new pyrazolyl-ureas and imidazopyrazolecarboxamides able to interfere with MAPK and PI3K upstream signalling involved in the angiogenesis. Eur. J. Med. Chem. 2017, 133, 24–35. [Google Scholar] [CrossRef]
- Zuo, S.J.; Li, S.; Yu, R.H.; Zheng, G.X.; Cao, Y.X.; Zhang, S.Q. Discovery of novel 3-benzylquinazolin-4(3H)-ones as potent vasodilative agents. Bioorg. Med. Chem. Lett. 2014, 24, 5597–5601. [Google Scholar] [CrossRef]
- Lampe, T.; Alonso-alija, C.; Stelte-ludwig, B.; Sandner, P.; Bauser, M.; Beck, H.; Lustig, K.; Rosentreter, U.; Stahl, E.; Takagi, H. Substituted 4-benzyloxy-phenylmethylamide Derivatives as Cold Menthol Receptor-1 (cmr-i) Antagonists for the Treatment of Urological Disorder. PCT Int. Appl. Bayer Heatlthcare, WO 2006040136, 20 April 2006. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press Ltd.: Cambridge, MA, USA; Elsevier Science Ltd.: London, UK, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Russo, E.; Spallarossa, A.; Comite, A.; Pagliero, M.; Guida, P.; Belotti, V.; Caviglia, D.; Schito, A.M. Valorization and Potential Antimicrobial Use of Olive Mill Wastewater (OMW) from Italian Olive Oil Production. Antioxidants 2022, 11, 903. [Google Scholar] [CrossRef]
- Croce, K.; Libby, P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr. Opin. Hematol. 2007, 14, 55–61. [Google Scholar] [CrossRef]
- Cuesta, Á.M.; Palao, N.; Bragado, P.; Gutierrez-Uzquiza, A.; Herrera, B.; Sánchez, A.; Porras, A. New and Old Key Players in Liver Cancer. Int. J. Mol. Sci. 2023, 24, 17152. [Google Scholar] [CrossRef]
- Taniyama, Y.; Griendling, K.K. Reactive oxygen species in the vasculature: Molecular and cellular mechanisms. Hypertension 2023, 42, 1075–1081. [Google Scholar] [CrossRef]
- Alexander, Y.; Osto, E.; Schmidt-Trucksäss, A.; Shechter, M.; Trifunovic, D.; Duncker, D.J.; Aboyans, V.; Bäck, M.; Badimon, L.; Cosentino, F.; et al. Endothelial function in cardiovascular medicine: A consensus paper of the European Society of Cardiology Working Groups on Atherosclerosis and Vascular Biology, Aorta and Peripheral Vascular Diseases, Coronary Pathophysiology and Microcirculation, and Thrombosis. Cardiovasc. Res. 2021, 117, 29–42. [Google Scholar] [CrossRef]
- Available online: http://dtp.cancer.gov (accessed on 30 March 2024).
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717–42729. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Model. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef]
- Available online: https://comptox.charite.de/protox3/index.php?site=models (accessed on 30 April 2024).
- Drwal, M.N.; Banerjee, P.; Dunkel, M.; Wettig, M.R.; Preissner, R. ProTox: A web server for the in silico prediction of rodent oral toxicity. Nucleic Acids Res. 2014, 42, W53–W58. [Google Scholar] [CrossRef]
- Banerjee, P.; Dehnbostel, F.O.; Preissner, R. Prediction Is a Balancing Act: Importance of Sampling Methods to Balance Sensitivity and Specificity of Predictive Models Based on Imbalanced Chemical Data Sets. Front. Chem. 2018, 6, 362. [Google Scholar] [CrossRef]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Mielnik, M.B.; Olsen, E.; Vogt, G.; Adeline, D.; Skrede, G. Grape seed extract as antioxidant in cooked, cold stored turkey meat. LWT-Food Sci. Technol. 2006, 39, 191–198. [Google Scholar] [CrossRef]
- Aree, T.; Jongrungruangchok, S. Structure–antioxidant activity relationship of β-cyclodextrin inclusion complexes with olive tyrosol, hydroxytyrosol and oleuropein: Deep insights from X-ray analysis, DFT calculation and DPPH assay. Carbohydr. Polym. 2018, 199, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Maresca, M.; Colao, C.; Leoncini, G. Generation of hydrogen peroxide in resting and activated platelets. Cell Biochem. Funct. 1992, 10, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Born, G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).