Qualitative and Quantitative Analysis of Chemical Components in Yinhua Pinggan Granule with High-Performance Liquid Chromatography Coupled with Q-Exactive Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of Chromatographic Conditions
2.2. Qualitative Analysis of YPG by HPLC–Q-Exactive MS
2.3. Identification of Several Specific Compounds in YPG
2.3.1. Phenolic Acids
2.3.2. Flavonoids
2.3.3. Alkaloids
2.3.4. Terpenoids
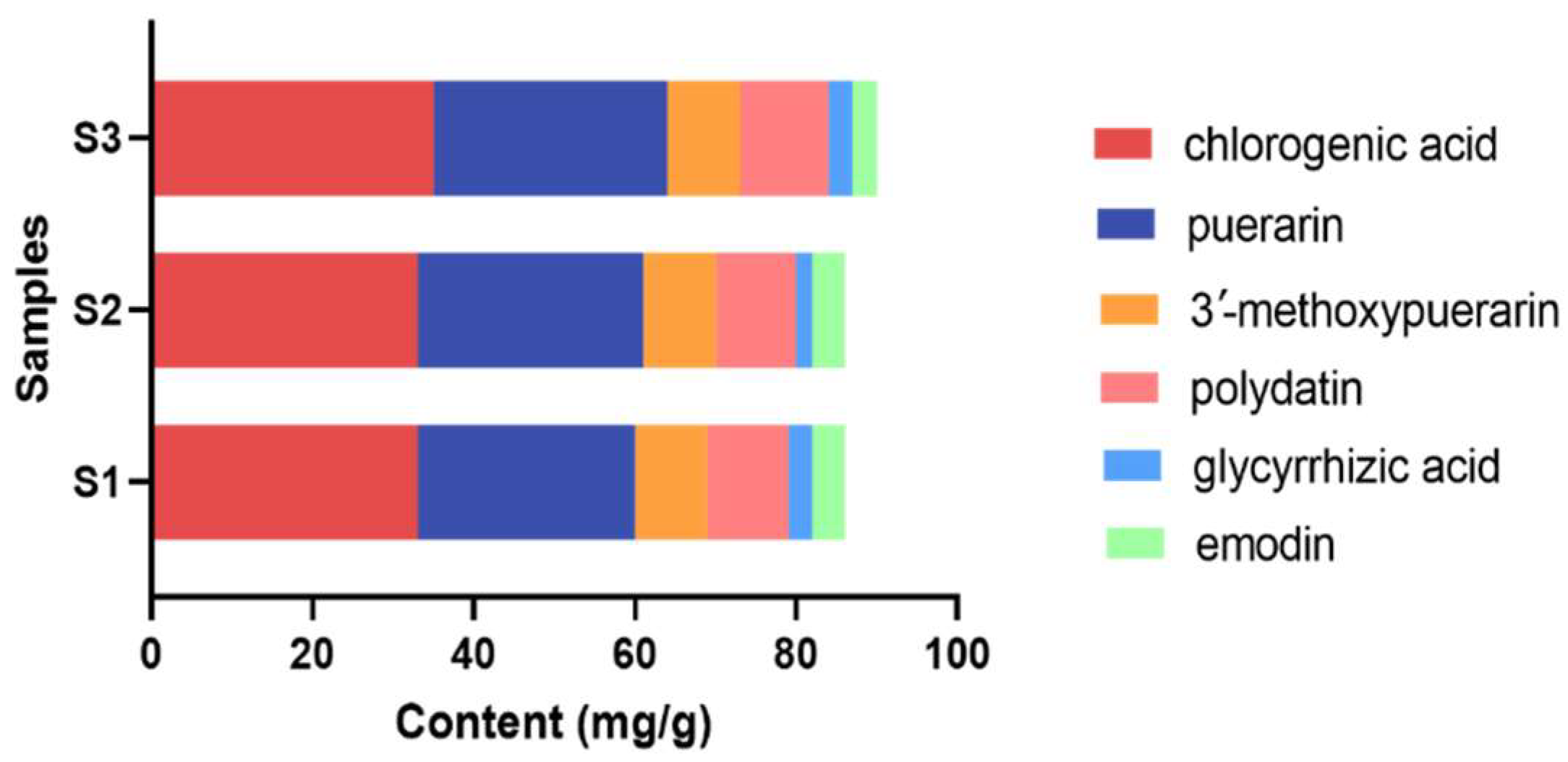
2.4. Quantitative Analysis, Total Phenolic Content, and DPPH Radical Scavenging Activity of YPG
3. Materials and Methods
3.1. Reagents and Materials
3.2. Sample Preparation
3.3. Preparation of Standard Solutions
3.4. Qualitative Analysis with HPLC–Q-Exactive MS
3.5. Quantitative Analysis by HPLC-DAD
3.6. Quantitative Analysis Method Validation
3.7. Determination of Total Phenolic Contents
3.7.1. Total Phenol Standard Curve Drawing
3.7.2. Total Phenol Content in the Sample
3.8. In Vitro Antioxidant Activity Evaluation—DPPH Radical Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, H.; Zhou, H.; Yang, J.; Lu, Y.; He, Y.; Wan, H. Preliminary study of Yinhuapinggan granule against H1N1 influenza virus infection in mice through inhibition of apoptosis. Pharm. Biol. 2020, 58, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Zhou, H.; Wan, H.; Yang, J.; Lu, Y.; He, Y.; Wan, H. Antiviral effects and mechanisms of Yinhuapinggan granule against H1N1 influenza virus infection in RAW264.7 cells. Inflammopharmacology 2018, 26, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Du, H.; Zhou, H.; Yang, J.; Zhu, J.; Tong, X.; Yang, Y.; Wan, J.; Fan, Y.; Lu, Y.; et al. Screening of Antiviral Components of Yinhuapinggan Granule and Protective Effects of Yinhuapinggan Granule on MDCK Cells with Influenza A/H1N1 Virus. Biomed Res. Int. 2022, 2022, 1040129. [Google Scholar] [CrossRef]
- Wang, J.; Hu, H.; Du, H.; Luo, M.; Cao, Y.; Xu, J.; Chen, T.; Guo, Y.; Li, Q.; Chen, W.; et al. Clinical Efficacy Protocol of Yinhuapinggan Granules: A Randomized, Double-Blind, Parallel, and Controlled Clinical Trial Program for the Intervention of Community-Acquired Drug-Resistant Bacterial Pneumonia as a Complementary Therapy. Front. Pharmacol. 2022, 13, 852604. [Google Scholar] [CrossRef]
- Xie, K.; Guan, S.; Jing, H.; Ji, W.; Kong, X.; Du, S.; Jia, M.; Wang, H. Efficacy and safety of traditional Chinese medicine adjuvant therapy for severe pneumonia: Evidence mapping of the randomized controlled trials, systematic reviews, and meta-analyses. Front. Pharmacol. 2023, 14, 1227436. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, X.; Wang, L.; Zhang, J.; Lv, R.; Tan, L.; Chen, Y.; Tao, R.; Li, X.; Chen, Y.; et al. Improvement influenza vaccine immune responses with traditional Chinese medicine and its active ingredients. Front. Microbiol. 2023, 14, 1111886. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Sun, J.; Zeng, J.; Ji, E.; Xu, J.; Tang, C.; Huo, H.; Zhang, Y.; Li, H.; Yang, H. Precise Investigation of the Efficacy of Multicomponent Drugs Against Pneumonia Infected with Influenza Virus. Front. Pharmacol. 2021, 12, 604009. [Google Scholar] [CrossRef]
- Lee, D.Y.W.; Li, Q.Y.; Liu, J.; Efferth, T. Traditional Chinese herbal medicine at the forefront battle against COVID-19: Clinical experience and scientific basis. Phytomedicine 2021, 80, 153337. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Chen, C.; Gu, Y.; Zhu, C.; Wang, S.; Chen, J.; Zhang, L.; Lv, L.; Zhang, G.; et al. Identifying potential anti-COVID-19 pharmacological components of traditional Chinese medicine Lianhuaqingwen capsule based on human exposure and ACE2 biochromatography screening. Acta Pharm. Sin. B 2021, 11, 222–236. [Google Scholar] [CrossRef]
- Li, R.; Hou, Y.; Huang, J.; Pan, W.; Ma, Q.; Shi, Y.; Li, C.; Zhao, J.; Jia, Z.; Jiang, H.; et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 2020, 156, 104761. [Google Scholar]
- Qian, X.; Nie, L.; Zhao, H.; Dai, Z.; Ma, S.; Liu, J.; Kuang, Y. Discovery and molecular elucidation of the anti-influenza material basis of Banlangen granules based on biological activities and ultra-high performance liquid chromatography coupled with quadrupole-orbitrap mass spectrometry. J. Ethnopharmacol. 2022, 298, 115683. [Google Scholar] [CrossRef]
- Fu, S.; Cheng, R.; Deng, Z.; Liu, T. Qualitative analysis of chemical components in Lianhua Qingwen capsule by HPLC-Q Exactive-Orbitrap-MS coupled with GC-MS. J. Pharm. Anal. 2021, 11, 709–716. [Google Scholar] [CrossRef]
- Hogenboom, A.C.; van Leerdam, J.A.; de Voogt, P. Accurate mass screening and identification of emerging contaminants in environmental samples by liquid chromatography-hybrid linear ion trap Orbitrap mass spectrometry. J. Chromatogr. A 2009, 1216, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Wan, H.; Bao, Y.; He, Y.; Li, C.; Wan, H. Rapid identification, quantitation, and antioxidant activity evaluation of the components in Guanxin Shutong capsule with liquid chromatography and mass spectrometry. J. Pharm. Biomed. Anal. 2023, 224, 115194. [Google Scholar] [CrossRef]
- Shah, M.A.; Kang, J.B.; Park, D.J.; Kim, M.O.; Koh, P.O. Chlorogenic acid alleviates cerebral ischemia-induced neuroinflammation via attenuating nuclear factor kappa B activation. Neurosci. Lett. 2022, 773, 136495. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia / Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Devel. Ther. 2020, 14, 51–60. [Google Scholar] [CrossRef]
- Song, M. Chemistry of the Chinese herbal medicine Puerariae Radix (Ge-Gen): A review. J. Chinese Pharm. Sci. 2014, 23, 347–360. [Google Scholar] [CrossRef]
- Chen, X.; Li, R.; Liang, T.; Zhang, K.; Gao, Y.; Xu, L. Puerarin improves metabolic function leading to hepatoprotective effects in chronic alcohol-induced liver injury in rats. Phytomedicine 2013, 20, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wang, S.; Liang, P.; Wang, Y.; Zhang, X.; Jia, Q.; Fu, J.; Han, S.; He, L. Screening and evaluation of anti-SARS-CoV-2 components from Ephedra sinica by ACE2/CMC-HPLC-IT-TOF-MS approach. Anal. Bioanal. Chem. 2021, 413, 2995–3004. [Google Scholar] [CrossRef]
- Zhao, Z.; Xiao, Y.; Xu, L.; Liu, Y.; Jiang, G.; Wang, W.; Li, B.; Zhu, T.; Tan, Q.; Tang, L.; et al. Glycyrrhizic Acid Nanoparticles as Antiviral and Anti-inflammatory Agents for COVID-19 Treatment. ACS Appl. Mater. Interfaces 2021, 13, 20995–21006. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.; Yuan, B.; Liu, Y.; Liu, C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 2015, 5, 310–315. [Google Scholar] [CrossRef]
- Yu, S.; Zhu, Y.; Xu, J.; Yao, G.; Zhang, P.; Wang, M.; Zhao, Y.; Lin, G.; Chen, H.; Chen, L.; et al. Glycyrrhizic acid exerts inhibitory activity against the spike protein of SARS-CoV-2. Phytomedicine 2021, 85, 153364. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.B. Simple and rapid method for the analysis of phenolic compounds in beverages and grains. J. Agric. Food Chem. 2011, 59, 1565–1571. [Google Scholar] [CrossRef]
- Silva, V.; Genta, G.; Möller, M.N.; Masner, M.; Thomson, L.; Romero, N.; Radi, R.; Fernandes, D.C.; Laurindo, F.R.; Heinzen, H.; et al. Antioxidant activity of uruguayan propolis. In vitro and cellular assays. J. Agric. Food Chem. 2011, 59, 6430–6437. [Google Scholar] [CrossRef]
- Li, J.; Fang, L.; Zhang, Y.; Wang, X.; Zhang, Q. Research progress on chemical constituents and pharmacological activities of Herba Ephedrae. Mod. Chin. Med. 2012, 14, 21–27. (In Chinese) [Google Scholar]
- Shang, X.; Pan, H.; Li, M.; Miao, X.; Ding, H. Lonicera japonica Thunb.: Ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine. J. Ethnopharmacol. 2011, 138, 1–21. [Google Scholar] [CrossRef]
- Liang, C.; Wang, S.; Chen, S.; Wang, Y.; Li, J.; Chang, Y. Research development on chemical composition and pharmacology of Polygoni Cuspidati Rhizoma et Radix. Chin. Tradit. Herb. Drugs 2022, 53, 1264–1276. (In Chinese) [Google Scholar]
- Zhu, W.; Li, J.; Meng, X.; Zhang, P.; Wu, W.; Liu, R. Research advances in chemical constituents and pharmacological activities of Pueraria genus. China J. Chin. Mater. Medica 2021, 46, 1311–1331. (In Chinese) [Google Scholar]
- Gong, X.; Liu, W.; Cao, L.; Yu, J.; Si, D.; Li, J.; Tu, P.; Li, J.; Song, Y. Rapid chemome profiling of chemical components of Lonicerae Japonicae Flos using DI-MS /MSALL. Chin. J. Chin. Mater. Med. 2021, 46, 2220–2228. (In Chinese) [Google Scholar]
- Miao, S.; Zhang, Q.; Bi, X.; Cui, J.; Wang, M. A review of the phytochemistry and pharmacological activities of Ephedra herb. Chin. J. Nat. Med. 2020, 18, 321–344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z. Test of Thrombolysis Effect and Study on Chemical Composition of Non-Ephedrine Alkaloids Part in Ephedra sinica Stapf. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2004. (In Chinese). [Google Scholar]
- Zang, X.; Shang, M.; Xu, F.; Liang, J.; Wang, X.; Mikage, M.; Cai, S. A-type proanthocyanidins from the stems of Ephedra sinica (Ephedraceae) and their antimicrobial activities. Molecules 2013, 18, 5172–5189. [Google Scholar] [CrossRef]
- Li, R. Chinese Formula Mahuangtang Chemical Composition Analysis and Spectrum-Effect Relation Research. Master’s Thesis, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2015. (In Chinese). [Google Scholar]
- Lin, K. Studies on Fingerprint for Herba Ephedrae and Determination for Alkaloids Contents in Herba Ephedrae with HPLC. Master’s Thesis, Chongqing Medical University, Chongqing, China, 2006. (In Chinese). [Google Scholar]
- Song, W.; Qiao, X.; Chen, K.; Wang, Y.; Ji, S.; Feng, J.; Li, K.; Lin, Y.; Ye, M. Biosynthesis-Based Quantitative Analysis of 151 Secondary Metabolites of Licorice to Differentiate Medicinal Glycyrrhiza Species and Their Hybrids. Anal. Chem. 2017, 89, 3146–3153. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Jin, Y.; Wang, Y.; Zhao, H.; Li, J.; Pu, G.; Zhang, L.; Yang, H.; Zhang, Y.; Zhang, L. Research progress on chemical constituents, pharmacological activities and in vivo metabolism of phenolic acids in Lonicera japonica Thunb. Chin. Tradit. Pat. Med. 2022, 44, 864–871. (In Chinese) [Google Scholar]
- Zhang, Y.; Huang, X.; Chen, Y.; Li, J.; Yu, K. Chemical constituents and their biosynthesis mechanisms of Polygonum cuspidatum. Chin. J. Chin. Mater. Med. 2020, 45, 4364–4372. (In Chinese) [Google Scholar]
- Cai, Z.; Liao, H.; Wang, C.; Chen, J.; Tan, M.; Mei, Y.; Wei, L.; Chen, H.; Yang, R.; Liu, X. A comprehensive study of the aerial parts of Lonicera japonica Thunb. based on metabolite profiling coupled with PLS-DA. Phytochem. Anal. 2020, 31, 786–800. [Google Scholar] [CrossRef]
- Li, N.; Zhang, C.; Zhong, G.; Xiu, L.; Liu, H.; Chen, S.; Chen, F.; Li, M.; Liao, W.; Ren, Y. Research progress on chemical constituents and pharmacological effects of different varieties of Glycyrrhizae Radix et Rhizoma and predictive analysis of quality markers. Chin. Tradit. Herbal. Drugs 2021, 52, 7680–7692. (In Chinese) [Google Scholar]
- Ma, P. Pharmacognostic Studies of Polygonum cuspidatum Sieb. Et Zucc.(Polydonaceae). Ph.D. Thesis, Peking Union Medical College, Beijing, China, 2013. (In Chinese). [Google Scholar]
- Wu, J.; Wang, C.; Yu, H. Chemical Constituents and Pharmacological Effect of Lonicerae Japonicae Flos. Chin. J. Exp. Tradit. Med. Form 2019, 25, 225–234. (In Chinese) [Google Scholar]
- Xiang, C.; Qiao, X.; Ye, M.; Guo, D. Classification and distribution analysis of components in Glycyrrhiza using licorice compounds database. Acta Pharm. Sin. 2012, 47, 1023–1030. (In Chinese) [Google Scholar]
- Tan, G.; Zhu, Z.; Zhang, H.; Zhao, L.; Liu, Y.; Dong, X.; Lou, Z.; Zhang, G.; Chai, Y. Analysis of phenolic and triterpenoid compounds in licorice and rat plasma by high-performance liquid chromatography diode-array detection, time-of-flight mass spectrometry and quadrupole ion trap mass spectrometry. Rapid. Commun. Mass Spectrom. 2010, 24, 209–218. [Google Scholar] [CrossRef]
- Wang, X.; Qin, Y.; Sun, J.; Hua, l.; Luo, W. Research progress on chemical constituents, pharmacological actives, clinical applications and quality control of Polygoni cuspidati folium. Asia-Pac. Tradit. Med. 2019, 15, 196–200. (In Chinese) [Google Scholar]
- Zhang, Y.; Yang, J.; Song, J.; Gao, S.; Zhang, Q.; Wang, B.; Zhao, Y. Discussion on mechanism of Ephedra Herba in treatment of heart failure based on network pharmacology. Drug Eval. Res. 2021, 44, 2189–2202. (In Chinese) [Google Scholar]
- Liu, G.; Wu, Y.; Liu, Y.; Li, X.; Yang, W. Chemical Constituents in Glycyrrhiza uralensis: A Review. Mod. Chin. Med. 2021, 23, 2006–2016. (In Chinese) [Google Scholar]
- Cheng, M.; Zhang, J.; Yang, L.; Shen, S.; Li, P.; Yao, S.; Qu, H.; Li, J.; Yao, C.; Wei, W.; et al. Recent advances in chemical analysis of licorice (Gan-Cao). Fitoterapia 2021, 149, 104803. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ji, S.; Song, W.; Kuang, Y.; Lin, Y.; Tang, S.; Cui, Z.; Qiao, X.; Yu, S.; Ye, M. Glycybridins A-K, Bioactive Phenolic Compounds from Glycyrrhiza glabra. J. Nat. Prod. 2017, 80, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Montoro, P.; Maldini, M.; Russo, M.; Postorino, S.; Piacente, S.; Pizza, C. Metabolic profiling of roots of liquorice (Glycyrrhiza glabra) from different geographical areas by ESI/MS/MS and determination of major metabolites by LC-ESI/MS and LC-ESI/MS/MS. J. Pharm. Biomed. Anal. 2011, 54, 535–544. [Google Scholar] [CrossRef]
- Frattaruolo, L.; Carullo, G.; Brindisi, M.; Mazzotta, S.; Bellissimo, L.; Rago, V.; Curcio, R.; Dolce, V.; Aiello, F.; Cappello, A.R. Antioxidant and Anti-Inflammatory Activities of Flavanones from Glycyrrhiza glabra L. (licorice) Leaf Phytocomplexes: Identification of Licoflavanone as a Modulator of NF-kB/MAPK Pathway. Antioxidants 2019, 8, 186. [Google Scholar] [CrossRef]
- Cao, Y. Research on Chemical Components and α-glucosidase Inhibiting Activity of Huzhang. Master’s Thesis, Jilin Agricultural University, Jilin, China, 2015. (In Chinese). [Google Scholar]
- Qiu, Z.; Liu, Z.; Pang, J.; Wu, H.; Liu, X.; Yang, Z.; Li, X.; Chen, J. A network pharmacology study with molecular docking to investigate the possibility of licorice against posttraumatic stress disorder. Metab. Brain Dis. 2021, 36, 1763–1777. [Google Scholar] [CrossRef]
- Lu, Y.; Ding, H.; Shi, Z.; Lin, H.; Zhang, G. Study on the mechanism of action of Ephedra Herba Decoction against influenza A virus based on network pharmacology. TMR Modern Herb. Med. 2022, 5, 0502001. [Google Scholar] [CrossRef]
- Qiao, X.; Song, W.; Ji, S.; Wang, Q.; Guo, D.; Ye, M. Separation and characterization of phenolic compounds and triterpenoid saponins in licorice (Glycyrrhiza uralensis) using mobile phase-dependent reversed-phase×reversed-phase comprehensive two-dimensional liquid chromatography coupled with mass spectrometry. J. Chromatogr. A. 2015, 1402, 36–45. [Google Scholar]
- Qiao, X.; Liu, C.; Ji, S.; Lin, X.; Guo, D.; Ye, M. Simultaneous determination of five minor coumarins and flavonoids in Glycyrrhiza uralensis by solid-phase extraction and high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Planta Med. 2014, 80, 237–242. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Shao, Y.; Ma, G.; Song, D.; Xu, G.; Wang, Z. Wide Identification of the Compounds in Licorice and Exploration of the Mechanism for Prostatitis Treatment by Combining UHPLC-LTQ-Orbitrap MS with Network Pharmacology. ChemistrySelect 2019, 4, 3011–3017. [Google Scholar] [CrossRef]
- Wang, K.; Meng, Y.; Lu, X.; Pang, X.; Wang, Y.; Ji, S.; Deng, Z.; Ma, P. Study on the Mechanism of Mahuang (Ephedrae Herba)-Xingren (Armeniacae Semen Amarum) in the Treatment of Bronchial Asthma. J. Liaoning Univ. Tradit. Chin. Med. 2021, 23, 205–212. (In Chinese) [Google Scholar]
- Fan, J.; Kuang, Y.; Dong, Z.; Yi, Y.; Zhou, Y.; Li, B.; Qiao, X.; Ye, M. Prenylated Phenolic Compounds from the Aerial Parts of Glycyrrhiza uralensis as PTP1B and α-Glucosidase Inhibitors. J. Nat. Prod. 2020, 83, 814–824. [Google Scholar] [CrossRef]
- Chin, Y.W.; Jung, H.A.; Liu, Y.; Su, B.N.; Castoro, J.A.; Keller, W.J.; Pereira, M.A.; Kinghorn, A.D. Antioxidant constituents of the roots and stolons of licorice (Glycyrrhiza glabra). J. Agric. Food Chem. 2007, 55, 4691–4697. [Google Scholar] [CrossRef]
- Mitscher, L.A.; Park, Y.H.; Clark, D.; Beal, J.L. Antimicrobial agents from higher plants. Antimicrobial isoflavanoids and related substances from Glycyrrhiza glabra L. var. typica. J. Nat. Prod. 1980, 43, 259–269. [Google Scholar] [CrossRef]
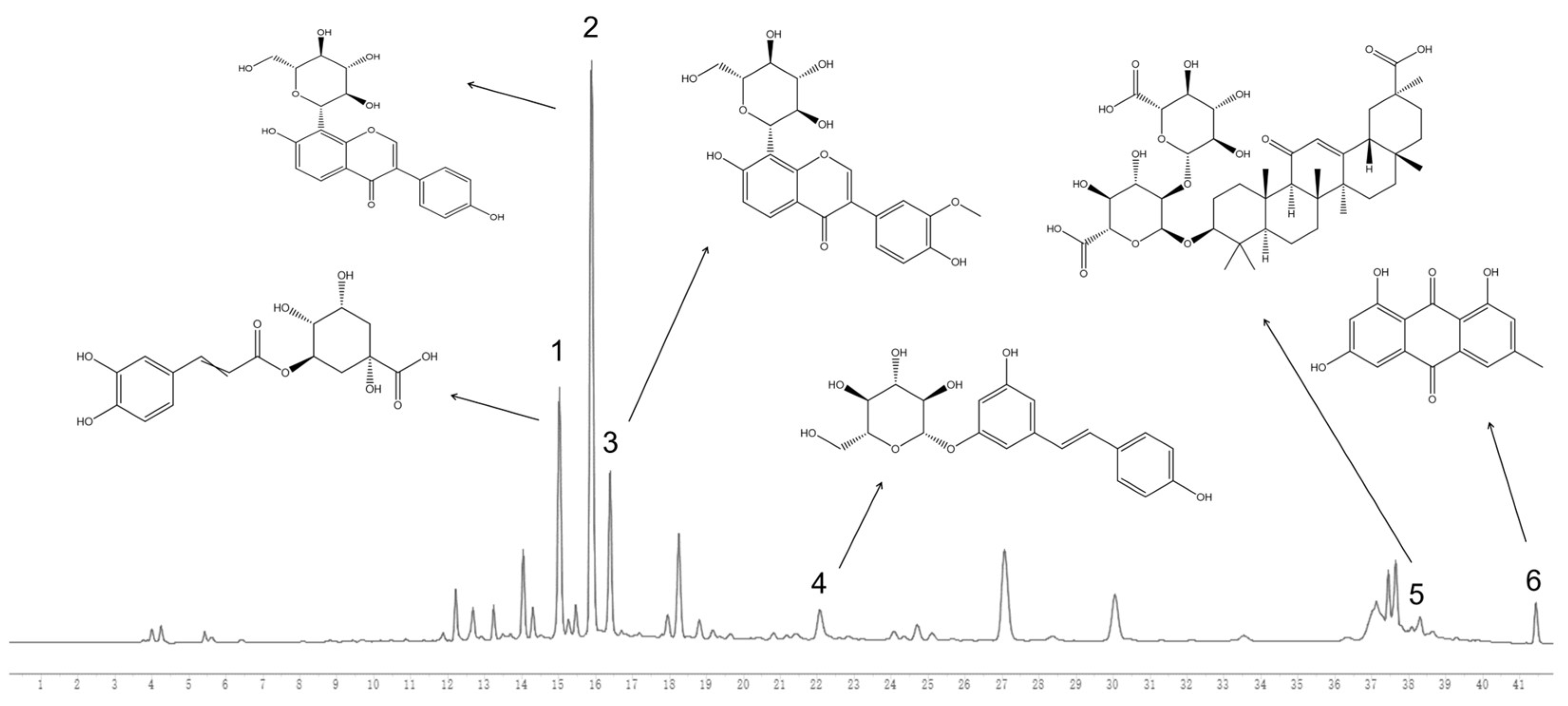
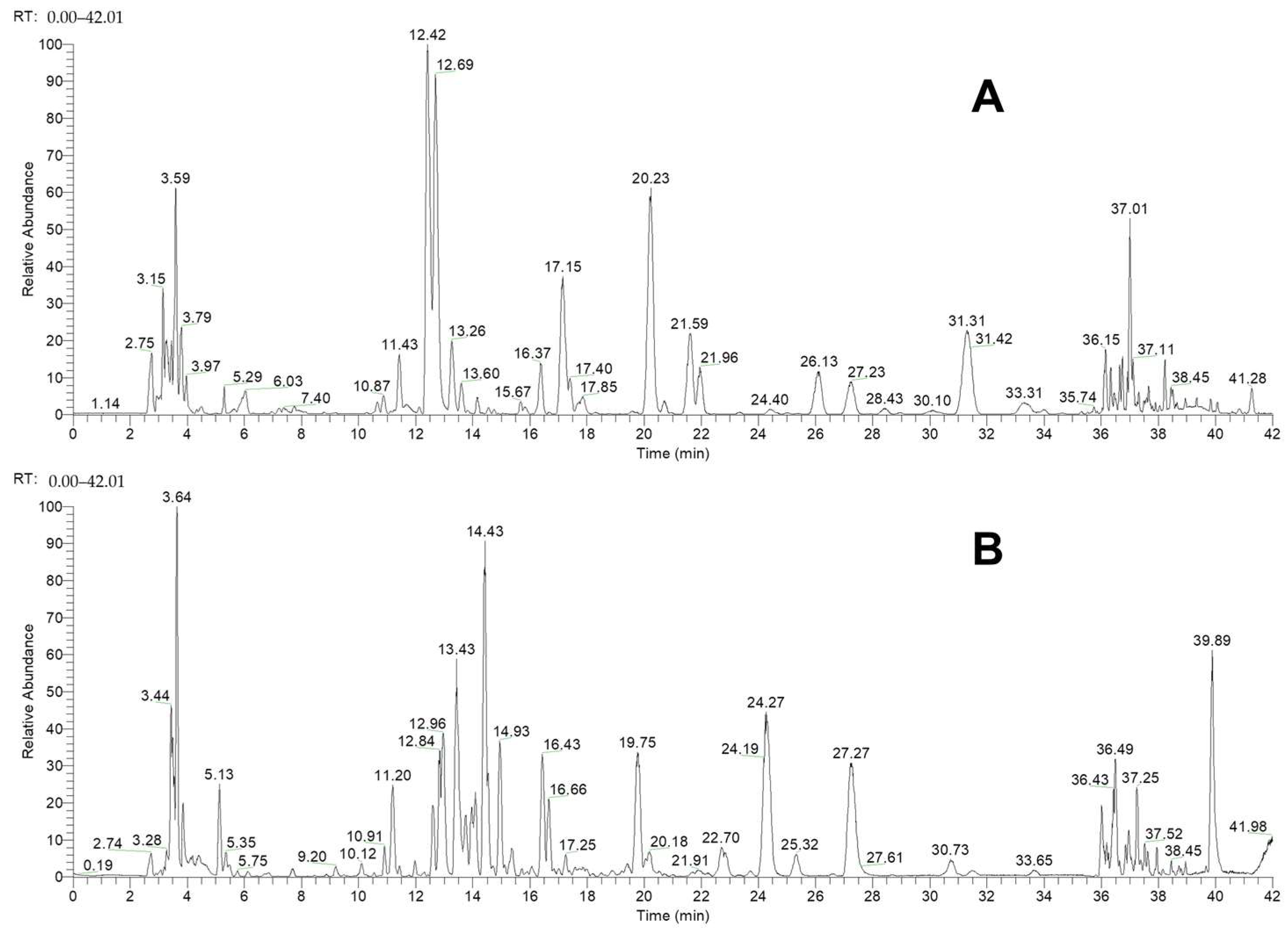
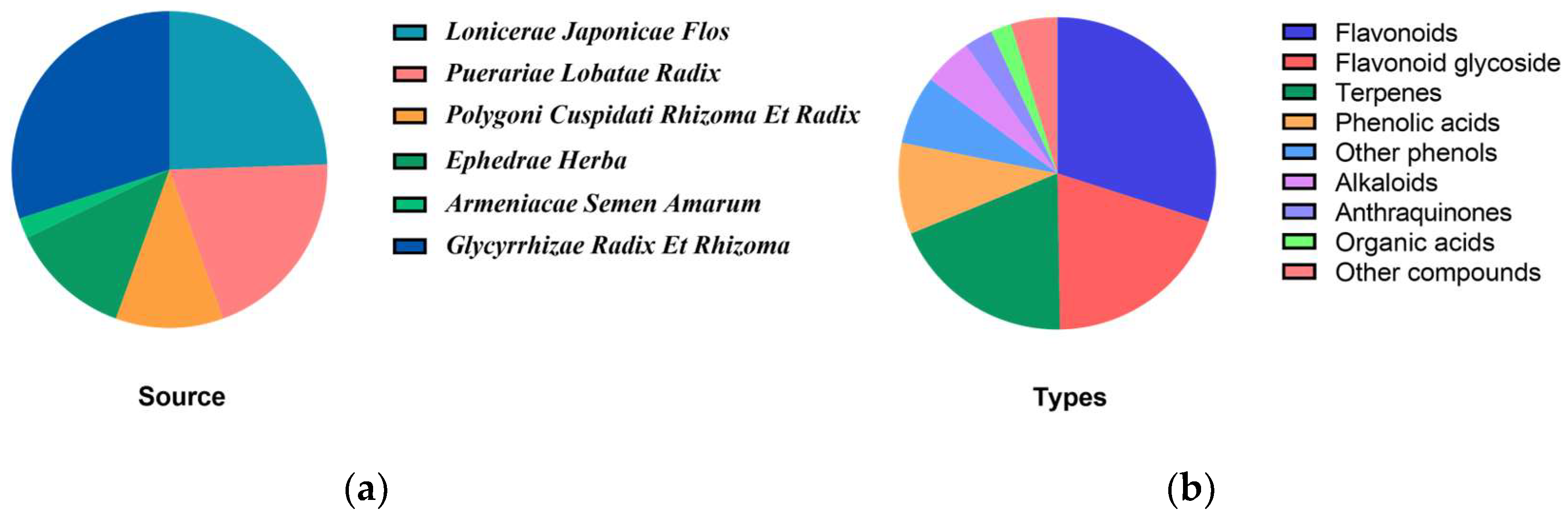
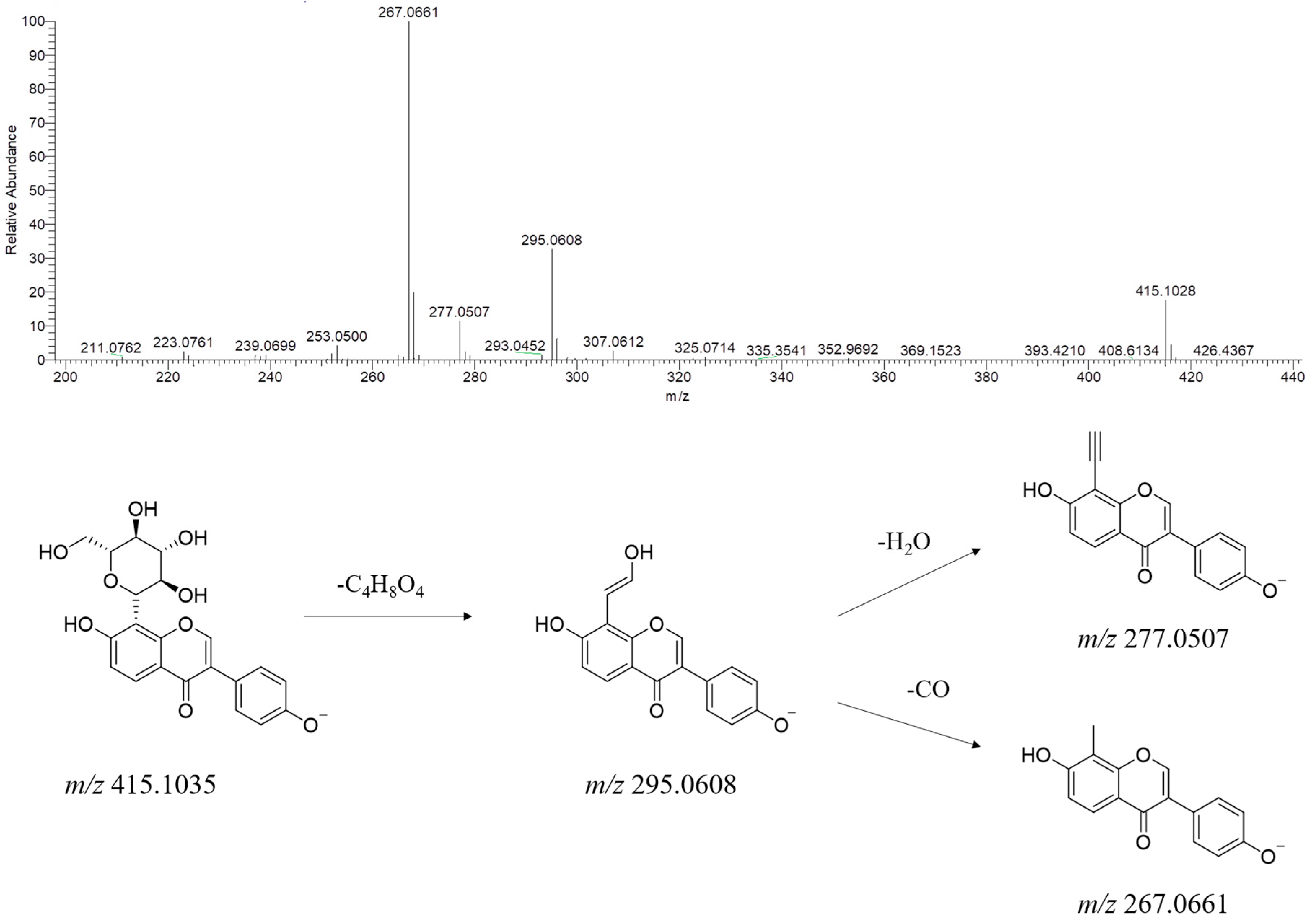
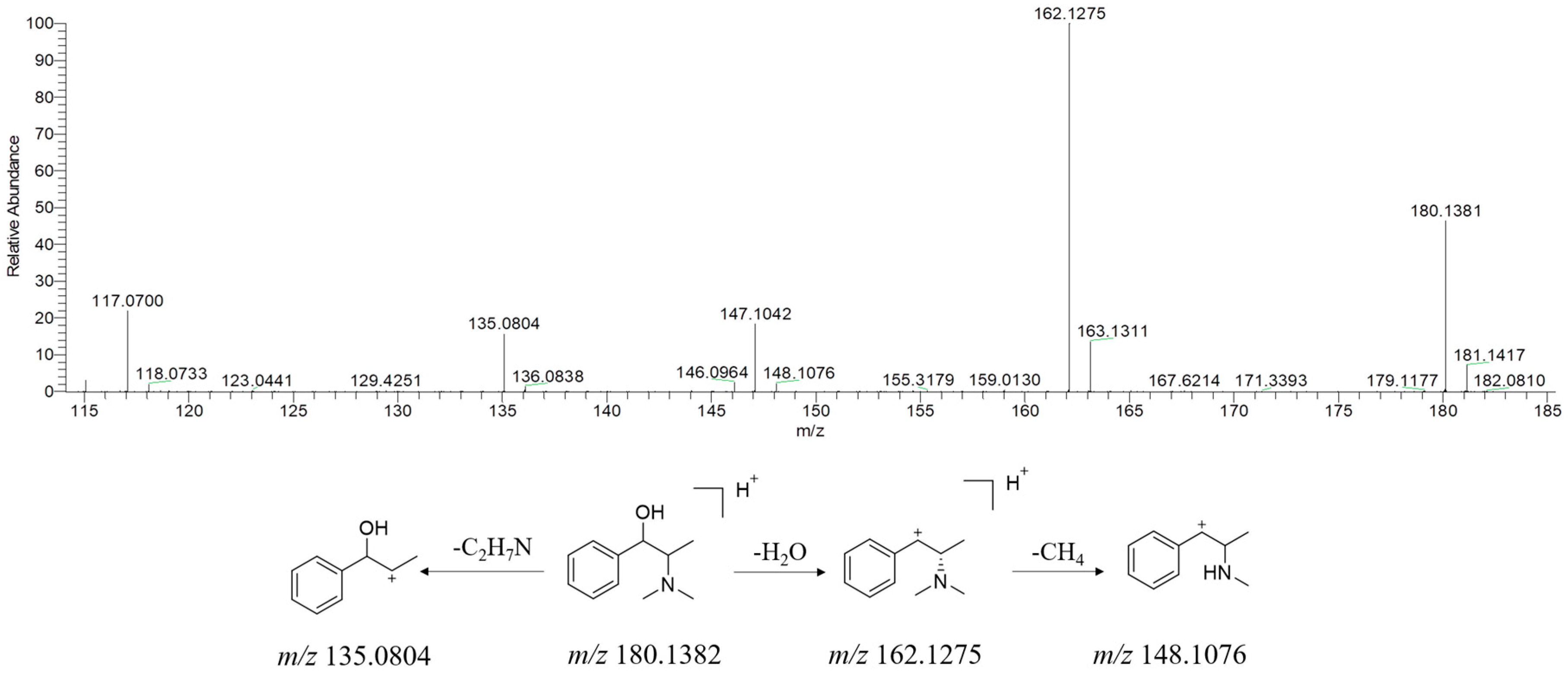
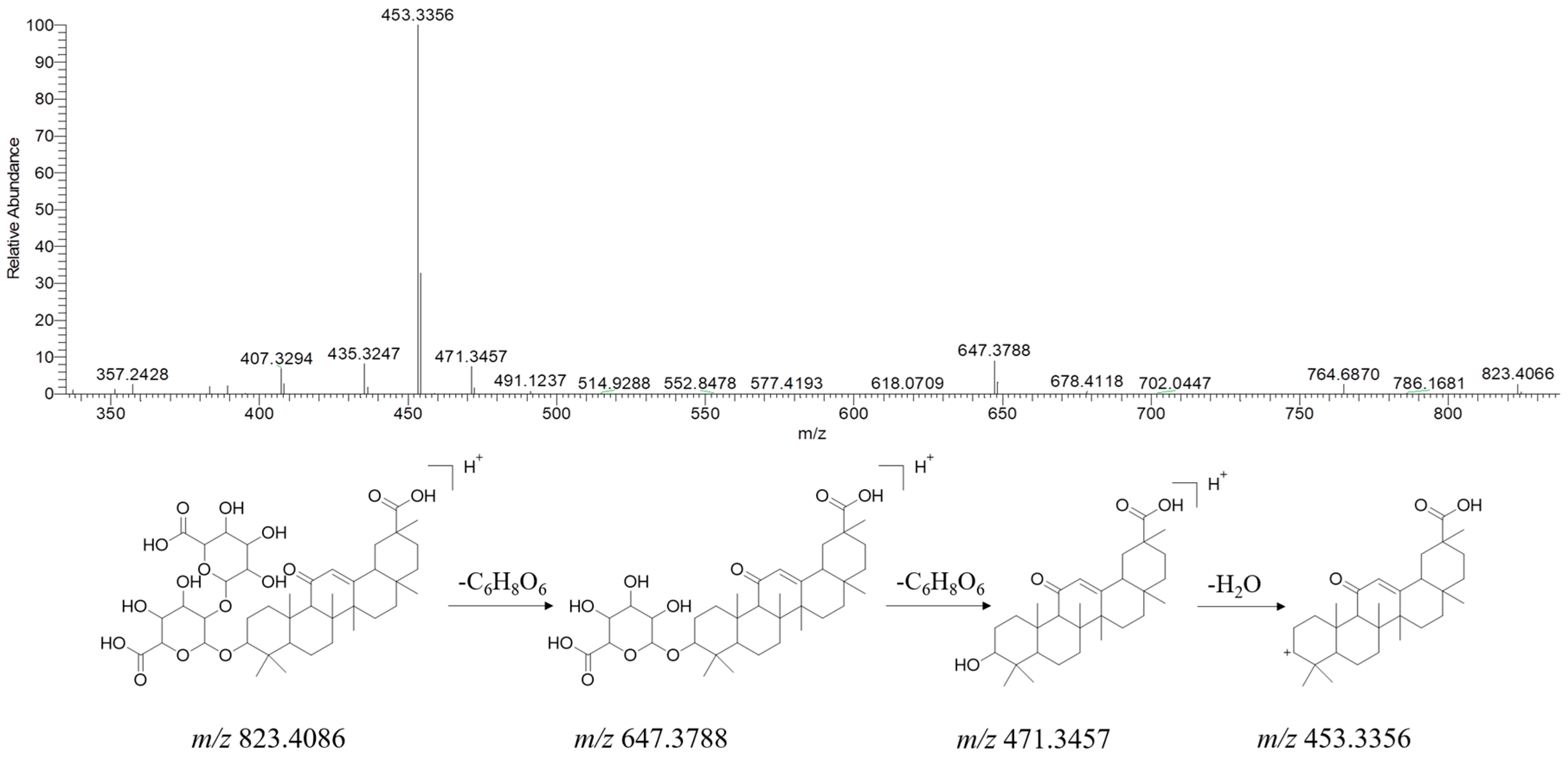
| No. | Name | RT (min) | Formula | Ion Type | Molecular Ion (m/z) | Main Product Ion (m/z) |
|---|---|---|---|---|---|---|
| 1 | ephedrannin A | 3.40 | C30H20O11 | [M + H]+ | 557.1104 | 395.1281, 215.0650, 177.0543, 145.0284 |
| 2 | glucose | 3.63 | C6H12O6 | [M + H]+ | 181.0705 | 163.0792, 144.0655, 109.0286, 81.0340 |
| [M − H]− | 179.0551 | 161.0445, 141.0181, 117.0181, 87.0073 | ||||
| 3 | secologanic acid | 3.67 | C16H22O10 | [M − H]− | 373.1134 | 347.9473, 189.0156, 161.0234, 135.0440 |
| 4 | D-mannitol | 3.82 | C6H14O6 | [M + H]+ | 183.0862 | 147.0650, 129.0543, 104.1073, 69.0342 |
| 5 | sucrose | 3.86 | C12H22O11 | [M + H]+ | 343.1227 | 306.1183, 145.0495, 127.0390, 85.0290 |
| [M − H]− | 341.1083 | 261.7968, 179.0559, 113.0230, 59.0125 | ||||
| 6 | allantoin | 3.99 | C4H6N4O3 | [M + H]+ | 159.0512 | 142.0862, 114.0915, 99.0193, 70.0658 |
| * 7 | quinic acid | 4.13 | C7H12O6 | [M + H]+ | 193.0706 | 157.0492, 147.0652, 129.0546, 111.0443 |
| 8 | salicylic acid | 4.14 | C7H6O3 | [M + H]+ | 139.0389 | 122.0714, 111.0443, 97.0287, 85.0289 |
| 9 | 4-aminophenol | 5.72 | C6H7NO | [M + H]+ | 110.0604 | 87.0046, 81.0340, 78.9949 |
| * 10 | citric acid | 5.59 | C6H8O7 | [M + H]+ | 193.0343 | 161.0595, 151.0388, 133.0647, 105.0702 |
| [M − H]− | 191.0188 | 173.0085, 129.0180, 111.0075, 87.0071 | ||||
| 11 | guanosine | 6.34 | C10H13N5O5 | [M + H]+ | 284.0986 | 258.4957, 152.0566, 135.0302, 110.0351 |
| [M − H]− | 282.0843 | 169.3754, 150.0411, 88.1632, 61.9871 | ||||
| 12 | adenosine | 6.46 | C10H13N5O4 | [M + H]+ | 268.1037 | 213.3238, 169.7115, 136.0617, 85.0288 |
| * 13 | gallic acid | 7.23 | C7H6O5 | [M + H]+ | 171.0291 | 154.0974, 130.0863, 115.0392, 70.0658 |
| 14 | coumalic acid | 7.40 | C6H4O4 | [M + H]+ | 141.0184 | 113.9639, 90.9481, 72.9378, 56.9430 |
| 15 | tachioside | 8.05 | C13H18O8 | [M − H]− | 301.0926 | 283.1918, 257.0457, 221.1909, 151.0029 |
| 16 | isotachioside | 8.27 | C13H18O8 | [M − H]− | 301.0926 | 284.0321, 243.0658, 178.9978, 151.0026 |
| 17 | ephedroxane | 8.27 | C11H13NO2 | [M + H]+ | 192.1019 | 164.9844, 146.9612, 106.0654, 87.0445 |
| 18 | quinaldic acid | 8.27 | C10H7NO2 | [M + H]+ | 174.0551 | 146.9612, 128.9507, 105.9351, 55.9352 |
| * 19 | hordenine | 8.56 | C10H15NO | [M + H]+ | 166.1225 | 151.0101, 121.0649, 103.0546, 93.0703 |
| 20 | leonuriside A | 8.99 | C14H20O9 | [M − H]− | 331.1035 | 285.0384, 253.0501, 169.0130, 125.0231 |
| 21 | 4-vinylguaiacol | 9.19 | C9H10O2 | [M + H]+ | 151.0755 | 133.0761, 123.9456, 119.0493, 91.0547 |
| 22 | 7α-morroniside | 9.50 | C17H26O11 | [M − H]− | 405.1401 | 371.0939, 243.0672, 191.0199, 111.0070 |
| 23 | tetramethylpyrazine | 9.83 | C8H12N2 | [M + H]+ | 137.1075 | 111.0080, 93.0704, 68.9978 |
| 24 | shuangkangsu | 10.49 | C20H30O14 | [M − H]− | 493.1560 | 447.2225, 431.0965, 269.0450, 169.0130 |
| 25 | epi-gallocatechin | 10.69 | C15H14O7 | [M + H]+ | 307.0807 | 289.1790, 243.1704, 208.9966, 139.0389 |
| [M − H]− | 305.0665 | 247.5994, 219.0664, 165.0182, 125.0232 | ||||
| 26 | robinin | 10.73 | C33H40O19 | [M + H]+ | 741.2226 | 678.4389, 579.1688, 381.0964, 297.0752 |
| 27 | 5-(hydroxymethyl)furfural | 10.85 | C6H6O3 | [M + H]+ | 127.0393 | 111.9689, 110.0238, 84.9603, 55.9352 |
| 28 | N-ethylbenzylamine | 10.90 | C8H11N | [M + H]+ | 122.0967 | 107.0732, 105.0336, 95.0495, 88.0237 |
| 29 | vicenin-2 | 11.00 | C27H30O15 | [M + H]+ | 595.1641 | 433.1127, 415.1019, 313.0700, 283.0596 |
| [M − H]− | 593.1510 | 539.2719, 521.2607, 463.2737, 226.9863 | ||||
| 30 | 4′-hydroxyacetophenone | 11.09 | C8H8O2 | [M + H]+ | 137.0599 | 122.0364, 116.9720, 95.0497, 55.9352 |
| 31 | 6-hydroxykynurenic acid | 11.35 | C10H7NO4 | [M + H]+ | 206.0445 | 178.0497, 148.1121, 117.0699, 90.0797 |
| [M − H]− | 204.0293 | 168.1795, 160.0393, 132.0446, 110.1680 | ||||
| 32 | chlorogenic acid butyl ester | 11.36 | C20H26O9 | [M − H]− | 409.1495 | 365.0681, 337.0357, 241.0023, 169.0135 |
| 33 | mirificin-4′-O-glucoside | 11.73 | C32H38O18 | [M + H]+ | 711.2118 | 579.1680, 417.1183, 399.1065, 297.0753 |
| [M − H]− | 709.1986 | 487.1239, 457.1142, 294.0533, 266.0583 | ||||
| 34 | mandelonitrile | 11.82 | C8H7NO | [M + H]+ | 134.0600 | 106.0654, 91.0544, 79.0548 |
| 35 | kakkalide | 11.83 | C28H32O15 | [M + H]+ | 609.1803 | 447.1279, 411.1072, 327.0857, 297.0755 |
| [M − H]− | 607.1663 | 588.1254, 487.1243, 309.0403, 281.0458 | ||||
| 36 | mahuannin A | 11.87 | C30H24O10 | [M − H]− | 543.1324 | 528.5940, 497.2623, 381.1213, 265.0987 |
| 37 | 2,6-dihydroxybenzoic acid | 11.92 | C17H24O9 | [M + H]+ | 373.1480 | 308.2842, 237.3755, 151.0375, 107.0485 |
| 38 | 8-epi-loganic acid | 11.96 | C16H24O10 | [M + H]+ | 377.1438 | 357.1662, 339.1559, 265.0587, 237.0277 |
| [M − H]− | 375.1292 | 315.8566, 265.2063, 201.0163, 113.0231 | ||||
| 39 | swertiamarin | 12.02 | C16H22O10 | [M − H]− | 373.1134 | 357.0130, 295.0618, 201.0158, 135.0433 |
| 40 | 8-epi-loganin | 12.30 | C17H26O10 | [M − H]− | 389.1448 | 371.8339, 345.1187, 227.0693, 185.0593 |
| 41 | 5-methoxysalicylic acid | 12.31 | C8H8O4 | [M + H]+ | 169.0498 | 151.0391, 128.9508, 111.0444, 93.0339 |
| 42 | norephedrine | 12.65 | C9H13NO | [M + H]+ | 152.1068 | 134.0964, 117.0700, 115.0545, 91.0547 |
| 43 | 7-epi-vogeloside | 12.81 | C17H24O10 | [M − H]− | 387.1295 | 341.1095, 272.9591, 227.0690, 179.0566 |
| 44 | loganic acid | 12.88 | C16H24O10 | [M − H]− | 375.1292 | 287.1191, 201.0159, 189.0158, 135.0440 |
| * 45 | protocatechuic acid | 12.90 | C7H6O4 | [M + H]+ | 155.0339 | 137.0233, 117.0701, 107.0495, 72.9379 |
| 46 | polygalin B | 13.12 | C28H32O15 | [M + H]+ | 609.1803 | 555.7846, 447.1268, 285.0752, 270.0516 |
| [M − H]− | 607.1663 | 460.8990, 325.0714, 310.0492, 282.0534 | ||||
| 47 | lonijaposide B | 13.15 | C25H32NO12 | [M − H]− | 537.1833 | 511.3804, 375.0705, 335.0791, 201.0155 |
| 48 | 3′-hydroxypuerarin | 13.17 | C21H20O10 | [M − H]− | 431.0975 | 415.0348, 311.0557, 283.0609, 255.0659 |
| 49 | norpseudoephedrine | 13.19 | C9H13NO | [M + H]+ | 152.1068 | 134.0964, 117.0700, 106.0655 |
| 50 | loganin | 13.30 | C17H26O10 | [M − H]− | 389.1448 | 371.8339, 326.0798, 227.0693, 185.0593 |
| 51 | secologanoside | 13.43 | C16H22O11 | [M + H]+ | 391.1222 | 239.0796, 241.0385, 163.0388, 151.0389 |
| [M − H]− | 389.1083 | 280.5215, 194.8876, 121.0647, 95.0489 | ||||
| 52 | leucodelphidin | 13.74 | C15H14O8 | [M − H]− | 321.0607 | 305.2140, 265.0519, 253.0507, 186.9385 |
| 53 | secologanin | 13.83 | C17H24O10 | [M − H]− | 387.1295 | / |
| 54 | glucoisoliquiritin | 14.04 | C27H32O14 | [M − H]− | 579.1715 | 529.4413, 491.9212, 463.1199, 255.0662 |
| 55 | glucoliquiritin apioside | 14.07 | C32H40O18 | [M − H]− | 711.2134 | 678.4865, 549.119, 457.1141, 255.0664 |
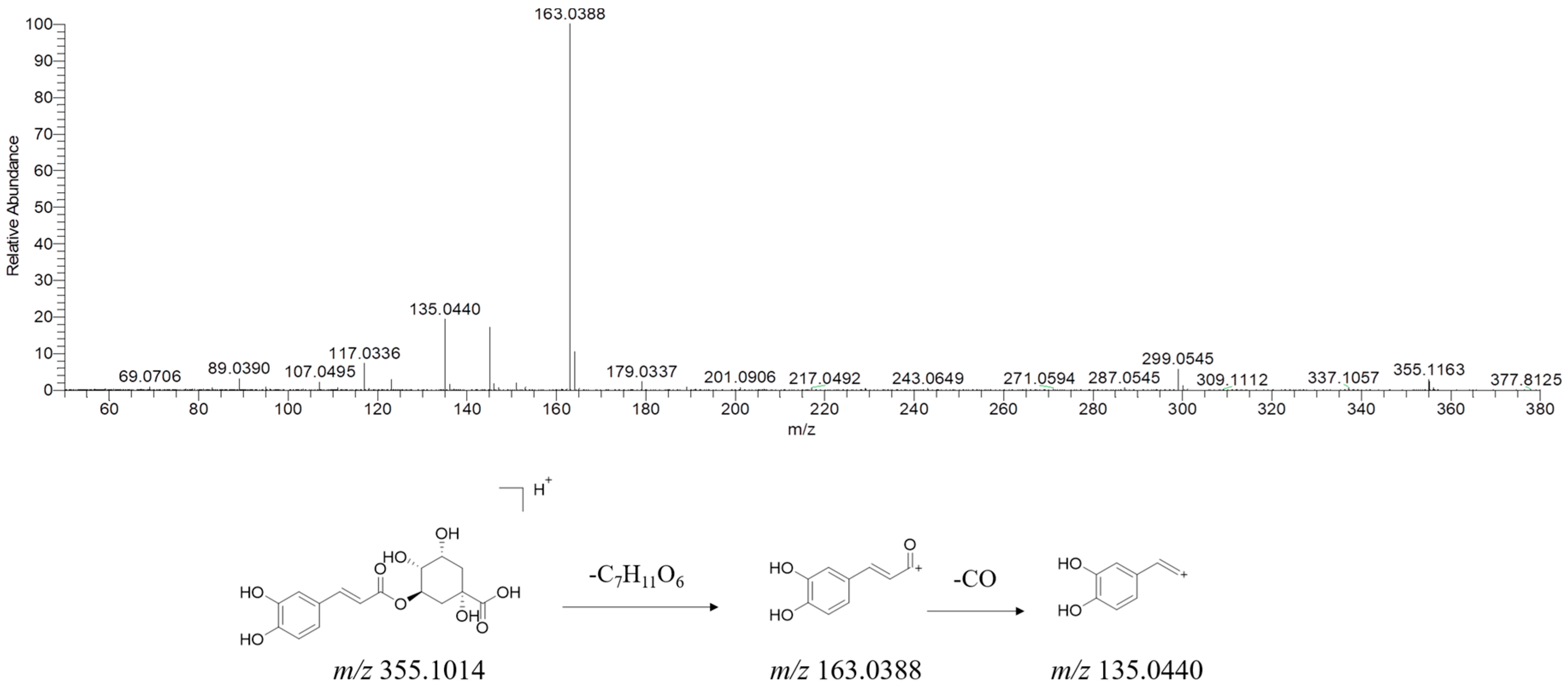
| * 56 | chlorogenic acid | 14.15 | C16H18O9 | [M + H]+ | 355.1014 | 163.0388, 135.0440 |
| [M − H]− | 353.0871 | 191.0553, 161.0234, 135.0440, 127.0385 | ||||
| * 57 | neochlorogenic acid | 14.22 | C16H18O9 | [M + H]+ | 355.1014 | 338.1603, 289.0706, 235.0594, 163.0390 |
| * 58 | catechin | 14.25 | C15H14O6 | [M + H]+ | 291.0857 | 255.7885, 107.0648, 139.0389, 123.0442 |
| [M − H]− | 289.0714 | 245.0817, 203.0705, 151.0387.123.0439 | ||||
| 59 | ephedrine | 14.36 | C10H15NO | [M + H]+ | 166.1225 | 148.1119, 133.0886, 117.0700, 91.0548 |
| * 60 | cryptochlorogenic acid | 14.37 | C16H18O9 | [M + H]+ | 355.1014 | 337.0915, 235.0589, 205.0494, 163.0388 |
| 61 | pseudoephedrine | 14.43 | C10H15NO | [M + H]+ | 166.1225 | 148.1119, 133.0887, 117.0701, 91.0546 |
| 62 | ephedrannin D4 | 14.44 | C30H24O14 | [M − H]− | 607.1089 | 563.1151, 487.1202, 413.0903, 267.0680 |
| 63 | puerarin 6″-O-xyloside | 14.61 | C26H28O13 | [M + H]+ | 549.1590 | 417.1175, 381.0964, 297.0753, 267.0648 |
| [M − H]− | 547.1447 | 437.0846, 295.0609, 277.0504, 267.0661 | ||||
| 64 | chrysoeriol 7-O-neohesperidoside | 14.65 | C28H32O15 | [M + H]+ | 609.1803 | 447.1284, 429.1182, 327.0859, 285.0755 |
| [M − H]− | 607.1663 | 547.1422, 487.1246, 295.0607, 267.0660 | ||||
| * 65 | p-hydroxybenzoic acid | 14.70 | C7H6O3 | [M + H]+ | 139.0389 | 121.0286, 111.0443, 93.0339 |
| * 66 | amygdalin | 14.72 | C20H27NO11 | [M + H]+ | 458.1647 | 355.1036, 213.0755, 163.0389, 107.0495 |
| [M − H]− | 456.1505 | 382.6093, 323.0968, 256.1356, 161.0449 | ||||
| 67 | methyl caffeate | 14.83 | C10H10O4 | [M + H]+ | 195.0652 | 177.0544, 163.0388, 145.0283, 117.0336 |
| 68 | 3,4-dimethyl-5-phenyloxazolidine | 14.94 | C11H15NO | [M + H]+ | 178.1227 | 162.1274, 147.1040, 117.0700, 105.0702 |
| 69 | apigenin 5-rhamnoside | 15.01 | C21H20O9 | [M + H]+ | 417.1167 | 381.0964, 321.0743, 297.0753, 267.0647 |
| [M − H]− | 415.1027 | 295.0608, 267.0661, 253.0509, 223.0762 | ||||
| * 70 | puerarin | 15.02 | C21H20O9 | [M + H]+ | 417.1167 | 399.1072, 381.0956, 363.0844, 255.0646 |
| [M − H]− | 415.1035 | 295.0608, 277.0507, 267.0661 | ||||
| 71 | mirificin | 15.04 | C26H28O13 | [M + H]+ | 549.1590 | 417.1168, 399.1069, 297.0754, 267.0648 |
| [M − H]− | 547.1447 | 418.4808, 295.0609, 267.0660, 114.2369 | ||||
| 72 | tectorigenin 7-O-xylosylglucoside | 15.07 | C27H30O15 | [M + H]+ | 595.1641 | 379.0811, 325.0695, 216.0653, 121.0283 |
| [M − H]− | 593.1510 | 495.0386, 473.1082, 310.0499, 282.0529 | ||||
| 73 | methylephedrine | 15.14 | C11H17NO | [M + H]+ | 180.1382 | 162.1275, 148.1076, 135.0804 |
| 74 | methylpseudoephedrine | 15.18 | C11H17NO | [M + H]+ | 180.1382 | 162.1275, 147.1042, 135.0803, 117.0700 |
| 75 | 7-O-ethylsweroside | 15.26 | C18H26O10 | [M − H]− | 401.1451 | 325.7480, 269.1024, 253.0505, 178.0263 |
| 76 | isoviolanthin | 15.47 | C27H30O14 | [M + H]+ | 579.1692 | 417.1190, 399.1072, 297.0753, 267.0649 |
| [M − H]− | 577.1554 | 531.2839, 518.0385, 283.0610, 268.0376 | ||||
| * 77 | 3′-methoxypuerarin | 15.53 | C22H22O10 | [M + H]+ | 447.1277 | 285.0751, 270.0516, 225.0542, 137.0232 |
| [M − H]− | 445.1134 | 430.0887, 367.1027, 327.1080, 215.0089 | ||||
| * 78 | glycitin | 15.54 | C22H22O10 | [M + H]+ | 447.1277 | 429.1190, 411.1062, 327.0855, 297.0754 |
| [M − H]− | 445.1135 | 379.8243, 325.0714, 282.0530, 254.0597 | ||||
| 79 | benzyl alcohol | 15.67 | C7H8O | [M + H]+ | 109.0653 | 94.0148, 91.0546, 87.0045, 81.0704 |
| 80 | methyl,4-hydroxycinnamate | 15.68 | C10H10O3 | [M + H]+ | 179.0705 | 162.1276, 147.1041, 117.0700, 109.0651 |
| 81 | γ-octalactone | 15.94 | C8H14O2 | [M + H]+ | 143.1068 | 128.9508, 116.9721, 113.9639, 84.9603 |
| 82 | (6S-9R)-roseoside | 15.98 | C19H30O8 | [M + H]+ | 387.2004 | 369.1341, 297.0762, 267.0641, 151.0388 |
| 83 | syringin | 15.98 | C17H24O9 | [M + H]+ | 373.1480 | 308.0842, 292.1605, 237.3755, 151.0375 |
| 84 | 5,7-dihydroxyisobenzofuran | 15.99 | C8H6O4 | [M + H]+ | 167.0339 | 148.1120, 133.0885, 117.0700, 111.0442 |
| 85 | kingiside | 16.01 | C17H24O11 | [M − H]− | 403.1240 | 371.1025, 310.7592, 243.0664, 174.8555 |
| * 86 | puerarin-7-O-glucoside | 16.04 | C27H30O14 | [M + H]+ | 579.1692 | 561.1633, 399.1074, 297.0753, 267.0647 |
| 87 | sweroside | 16.09 | C16H22O9 | [M + H]+ | 359.1333 | 297.8041, 265.6036, 197.0808, 127.0391 |
| * 88 | caffeic acid | 16.29 | C9H8O4 | [M + H]+ | 181.0494 | 163.0388, 145.0284, 135.0441, 117.0337 |
| [M − H]− | 179.0341 | 164.0098, 135.0440, 112.1822, 107.0492 | ||||
| 89 | ephedrannin D1 | 16.53 | C30H24O13 | [M + H]+ | 593.1279 | 576.3641, 447.1271, 327.0849, 297.0745 |
| 90 | 5-p-coumaroylquinic acid | 16.70 | C16H18O8 | [M + H]+ | 339.1071 | 266.4322, 245.8672, 147.0439, 119.0494 |
| [M − H]− | 337.0930 | 191.0553, 163.0390, 119.0470, 93.0332 | ||||
| 91 | loniceracetalide A | 16.71 | C21H32O11 | [M − H]− | 459.1869 | / |
| 92 | piceatannol 3′-O-glucoside | 16.77 | C20H22O9 | [M − H]− | 405.1190 | 359.0753, 243.0659, 201.0549, 159.0440 |
| 93 | lonijaposide D | 16.82 | C26H32NO13 | [M − H]− | 565.1774 | 550.4224, 519.2438, 445.1137, 325.0718 |
| 94 | ephedralone | 16.90 | C11H9NO4 | [M + H]+ | 220.0602 | 192.0652, 164.0699, 151.4024, 119.0490 |
| [M − H]− | 218.0452 | 174.0550, 159.0315, 144.0077, 131.0365 | ||||
| 95 | neochlorogenic acid methyl ester | 17.85 | C17H20O9 | [M + H]+ | 369.1173 | 191.9904, 177.0544, 145.0283, 117.0336 |
| [M − H]− | 367.1029 | 255.0194, 191.0552, 173.0447, 134.0361 | ||||
| 96 | isoschaftoside | 17.86 | C26H28O14 | [M + H]+ | 565.1542 | 479.5669, 415.1016, 313.0699, 283.0596 |
| [M − H]− | 563.1340 | 341.0680, 311.0559, 283.0609, 149.0235 | ||||
| 97 | 2,6-dihydroxyphenylacetic acid | 18.07 | C8H8O4 | [M + H]+ | 169.0498 | 151.0389, 146.9612, 128.9508, 123.0441 |
| 98 | schaftoside | 18.35 | C26H28O14 | [M + H]+ | 565.1542 | 520.6823, 433.1122, 313.0700, 283.0597 |
| [M − H]− | 563.1340 | 529.1846, 341.0670, 311.0558, 283.0610 | ||||
| * 99 | vanillic acid | 18.50 | C8H8O4 | [M + H]+ | 169.0498 | 151.0388, 146.9612, 128.9508, 123.0442 |
| 100 | flavoyadorinin B | 18.51 | C23H24O11 | [M + H]+ | 477.1379 | 327.1659, 279.0375, 204.5438, 145.0499 |
| 101 | ephedrannin D2 | 18.54 | C30H24O13 | [M − H]− | 591.1150 | 547.1453, 462.7491, 285.0408, 253.0507 |
| * 102 | cinnamic acid | 18.59 | C9H8O2 | [M + H]+ | 149.0597 | 133.0885, 121.0648, 103.0548, 95.0497 |
| 103 | hydroxyphenylacetic acid | 18.65 | C8H8O3 | [M + H]+ | 153.0546 | 135.1167, 112.0395, 90.9481, 72.9378 |
| 104 | 3-O-caffeoylshikimic acid | 18.81 | C16H16O8 | [M + H]+ | 337.0910 | 181.0494, 163.0388, 145.0283, 95.0495 |
| [M − H]− | 335.0771 | 269.1091, 179.0345, 161.0233, 133.0282 | ||||
| 105 | leucopelargonidin | 18.82 | C15H14O6 | [M + H]+ | 291.0857 | 273.0753, 207.0647, 147.0440, 139.0389 |
| [M − H]− | 289.0714 | 245.0818, 203.0705, 151.0392, 123.0441 | ||||
| 106 | licuraside | 19.43 | C26H30O13 | [M − H]− | 549.1609 | 502.1001, 429.1062, 255.0660, 119.0490 |
| 107 | genistein 7-O-glucoside | 20.25 | C21H20O10 | [M + H]+ | 433.1120 | 271.0595, 215.0700, 137.0231 |
| * 108 | liquiritin apioside | 20.29 | C26H30O13 | [M − H]− | 549.1609 | 482.5059, 297.0778, 255.0660, 135.0076 |
| 109 | isoliquiritin apioside | 20.34 | C26H30O13 | [M − H]− | 549.1609 | 488.6683, 429.1148, 255.0659, 135.0076 |
| * 110 | liquiritin | 20.38 | C21H22O9 | [M − H]− | 417.1186 | 402.1664, 373.0210, 255.0662, 119.0490 |
| 111 | secologanin dimethyl acetal | 20.39 | C19H30O11 | [M − H]− | 433.1706 | / |
| 112 | 3-methoxyphenol | 20.50 | C7H8O2 | [M + H]+ | 125.0599 | 102.9706, 97.0287, 84.9602 |
| 113 | piceid gallate A | 20.63 | C27H26O13 | [M − H]− | 557.1293 | / |
| * 114 | polydatin | 20.92 | C20H22O8 | [M − H]− | 389.1236 | 227.0707, 185.0598, 159.0808, 143.0491 |
| 115 | lonicerin | 21.02 | C27H30O15 | [M + H]+ | 595.1641 | 433.1108, 313.0717, 271.0596, 215.0697 |
| [M − H]− | 593.1510 | 430.4479, 329.5679, 285.0396, 227.0705 | ||||
| 116 | isoliquiritin | 21.24 | C21H22O9 | [M − H]− | 417.1186 | 255.0660, 153.0182, 135.0075, 119.0489 |
| * 117 | quercetin 3-glucoside | 21.69 | C21H20O12 | [M − H]− | 463.0880 | 300.0272, 271.0247, 255.0296, 151.0027 |
| 118 | kaempferol 7-O-glucopyranoside | 21.69 | C21H20O11 | [M + H]+ | 449.1072 | 330.0535, 287.0545, 203.4280, 153.0181 |
| [M − H]− | 447.0931 | 410.9457, 325.0732, 285.0397, 256.0383 | ||||
| 119 | neoisoliquiritin | 22.95 | C21H22O9 | [M − H]− | 417.1186 | 374.0878, 255.0660, 153.0183, 135.0076 |
| * 120 | coumarin | 23.54 | C9H6O2 | [M + H]+ | 147.0442 | 131.9743, 119.0493, 113.9640 |
| * 121 | daidzein | 23.56 | C15H10O4 | [M − H]− | 253.0502 | 224.0468, 209.0598, 197.0602, 135.0076 |
| 122 | rhoifolin | 23.76 | C27H30O14 | [M + H]+ | 579.1692 | 515.2410, 429.1205, 327.0858, 297.0754 |
| * 123 | isochlorogenic acid A | 23.89 | C25H24O12 | [M − H]− | 515.1185 | 353.0883, 335.0772, 173.0445, 135.0440 |
| 124 | reynoutrin | 24.65 | C20H18O11 | [M − H]− | 433.0771 | / |
| 125 | avicularin | 25.43 | C20H18O11 | [M − H]− | 433.0771 | / |
| * 126 | resveratroloside | 25.46 | C20H22O8 | [M + H]+ | 391.1375 | 229.0856, 211.0759, 135.0440, 107.0495 |
| 127 | liquiritigenin 7,4′-diglucoside | 25.61 | C27H32O14 | [M + H]+ | 581.1848 | 538.0963, 431.0979, 311.0434, 287.0430 |
| 128 | centauroside | 25.64 | C34H46O19 | [M − H]− | 757.2545 | 679.1150, 525.1623, 458.1185, 254.0573 |
| 129 | 3,4-dicaffeoylquinic acid | 25.75 | C25H24O12 | [M − H]− | 515.1185 | 437.3583, 353.0874, 191.0552, 135.0440 |
| 130 | herniarin | 26.18 | C10H8O3 | [M + H]+ | 177.0546 | 149.0597, 145.0283, 117.0336, 89.0390 |
| 131 | catechin-5-O-β-D-glucopyranoside | 26.54 | C21H24O11 | [M − H]− | 451.1243 | 313.0739, 289.0719, 191.0340, 167.0340 |
| 132 | vanillin | 26.58 | C8H8O3 | [M + H]+ | 153.0546 | 131.9743, 125.0597, 111.0443, 93.0338 |
| 133 | 4,7-dihydroxyflavone 7-D-glucoside | 26.84 | C21H20O9 | [M + H]+ | 417.1167 | 338.5892, 255.0647, 227.0695, 199.0747 |
| 134 | methyl chlorogenate | 27.00 | C17H20O9 | [M + H]+ | 369.1173 | 313.0666, 285.0745, 207.0644, 161.0596 |
| 135 | ketologanin | 27.07 | C17H24O10 | [M + H]+ | 389.1433 | 371.1681, 324.1584, 225.0426, 151.0388 |
| 136 | naringin | 27.96 | C27H32O14 | [M + H]+ | 581.1848 | 449.1047, 431.0979, 329.0610, 311.0434 |
| 137 | (E)-aldosecologanin | 28.10 | C34H46O19 | [M − H]− | 757.2545 | 679.1150, 595.2075, 525.1623, 458.1185 |
| 138 | dihydrocaffeic acid | 28.25 | C9H10O4 | [M + H]+ | 183.0649 | 165.0545, 151.0389, 123.0441, 113.9639 |
| 139 | p-coumaric acid | 28.25 | C9H8O3 | [M + H]+ | 165.0544 | 137.0597, 133.0283, 109.0650, 79.0547 |
| 140 | secoxyloganin | 28.25 | C17H24O11 | [M + H]+ | 405.1379 | 373.2119, 309.2449, 165.0545, 151.0389 |
| 141 | benzoic acid | 28.26 | C7H6O2 | [M + H]+ | 123.0441 | 105.0450, 95.0495, 67.0549 |
| 142 | 1,5-dicaffeoylquinic acid | 28.63 | C25H24O12 | [M − H]− | 515.1185 | 454.9042, 353.0873, 191.0552, 173.0446 |
| 143 | vogeloside | 28.65 | C17H24O10 | [M + H]+ | 389.1433 | 233.2362, 195.0655, 151.0389, 107.0495 |
| 144 | 3-O-caffeoylquinic acid methyl ester | 28.94 | C17H20O9 | [M + H]+ | 369.1173 | 207.0649, 177.0546, 148.0514, 107.0857 |
| 145 | quercitrin | 30.05 | C21H20O11 | [M + H]+ | 449.1072 | 330.0535, 287.0545, 269.0448, 153.0181 |
| [M − H]− | 447.0931 | 403.1030, 241.0501, 197.0599, 174.9555 | ||||
| 146 | 4-feruloylquinic acid | 30.26 | C17H20O9 | [M + H]+ | 369.1173 | 239.4636, 207.0649, 177.0539, 148.0516 |
| * 147 | naringenin | 31.32 | C15H12O5 | [M − H]− | 271.0609 | 230.0589, 177.0189, 151.0026, 119.0490 |
| 148 | kuzubutenolide A | 31.41 | C23H24O10 | [M + H]+ | 461.1433 | 299.0909, 253.0853, 193.0497, 107.0494 |
| 149 | pueroside A | 31.42 | C29H34O14 | [M + H]+ | 607.2010 | 461.1439, 376.1363, 299.0908, 107.0494 |
| 150 | epicatechingallate | 31.75 | C22H18O10 | [M + H]+ | 443.0962 | 390.0869, 291.0855, 273.0755, 123.0441 |
| 151 | garbanzol | 31.76 | C15H12O5 | [M + H]+ | 273.0752 | 242.4491, 189.0543, 153.0180, 123.0441 |
| 152 | chrysoeriol 7-O-glucopyranoside | 31.80 | C22H22O11 | [M + H]+ | 463.1220 | 445.1107, 427.1008, 343.0803, 313.0704 |
| 153 | sophoraside A | 31.92 | C24H26O10 | [M + H]+ | 475.1587 | 313.1061, 267.1010, 253.0853, 107.0494 |
| [M − H]− | 473.1447 | 377.9086, 311.0924, 267.1024, 252.0786 | ||||
| 154 | vitexin | 32.25 | C21H20O10 | [M + H]+ | 433.1120 | 415.1018, 397.0909, 313.0698, 283.0597 |
| [M − H]− | 431.0975 | 269.0453, 240.0423, 225.0551, 193.4129 | ||||
| 155 | 5-O-coumaroylcaffeoylquinic acid | 32.38 | C25H24O11 | [M + H]+ | 501.1379 | 483.1254, 320.0835, 255.0652, 163.0388 |
| [M − H]− | 499.1244 | 431.0978, 291.0275, 269.0454, 240.0423 | ||||
| * 156 | resveratrol | 32.64 | C14H12O3 | [M + H]+ | 229.0856 | 211.0747, 183.0808, 135.0441, 107.0494 |
| * 157 | ferulic acid | 32.90 | C10H10O4 | [M + H]+ | 195.0652 | 177.0544, 163.0389, 138.0661, 107.0494 |
| [M − H]− | 193.0498 | 165.0005, 134.0361, 126.9024, 102.9472 | ||||
| * 158 | isoferulic acid | 32.91 | C10H10O4 | [M + H]+ | 195.0652 | 177.0544, 163.0388, 149.0596, 109.0287 |
| [M − H]− | 193.0498 | 161.0233, 149.0236, 134.0363, 121.0281 | ||||
| 159 | kudzusaponin A1 | 34.62 | C52H84O23 | [M − H]− | 1075.5314 | 1029.5265, 763.3842, 603.3890, 485.3619 |
| 160 | hyperoside | 35.15 | C21H20O12 | [M + H]+ | 465.1023 | 447.1085, 303.0492, 286.0449, 257.0425 |
| 161 | polygalin A | 35.16 | C23H24O11 | [M + H]+ | 477.1379 | 355.1165, 315.0853, 271.0960, 229.0856 |
| [M − H]− | 475.1241 | 267.0660, 252.0424, 201.9968, 132.0607 | ||||
| 162 | 7-hydroxy-4-methoxy-5-methylcoumarin | 35.24 | C11H10O4 | [M + H]+ | 207.0650 | 189.0544, 161.0599, 150.0261, 123.0807 |
| 163 | glycitin-6″-O-xylosyl | 36.12 | C27H30O14 | [M + H]+ | 579.1692 | 433.1124, 337.0699, 313.0699, 283.0596 |
| 164 | cuspidatumin A | 36.14 | C14H12O4 | [M + H]+ | 245.0805 | 229.0854, 161.0122, 121.0286, 98.9757 |
| [M − H]− | 243.0661 | 225.1119, 207.1026, 174.9554, 146.9600 | ||||
| 165 | 3,5-dicaffeoylquinic acid methyl ester | 36.23 | C26H26O12 | [M + H]+ | 531.1486 | 513.1385, 369.1514, 283.0595, 163.0388 |
| * 166 | rutin | 36.27 | C27H30O16 | [M + H]+ | 611.1602 | 465.1010, 303.0493, 257.0441, 229.0495 |
| * 167 | taxifolin | 36.27 | C15H12O7 | [M + H]+ | 305.0653 | 287.1236, 269.1125, 227.1023, 191.0814 |
| 168 | pueroside B | 36.31 | C30H36O15 | [M + H]+ | 637.2119 | 475.1591, 313.1064, 267.1011, 107.0494 |
| 169 | pueroside C | 36.31 | C24H26O10 | [M + H]+ | 475.1587 | 457.3117, 313.1034, 249.1549, 107.0486 |
| 170 | macranthoidin B | 36.35 | C65H106O32 | [M + H]+ | 1399.6711 | 1075.5695, 943.5251, 795.2725, 633.2202 |
| [M − H]− | 1397.6552 | 1073.5525, 911.5010, 749.4481, 603.3898 | ||||
| 171 | kudzusaponin SA2 | 36.39 | C47H76O19 | [M + H]+ | 945.5027 | 848.4162, 763.4678, 679.2439, 421.3453 |
| 172 | macranthoidin A | 36.40 | C59H96O27 | [M + H]+ | 1237.6183 | 1076.5618, 943.5206, 751.4630, 603.2128 |
| [M − H]− | 1235.6036 | 1189.5997, 1073.5534, 911.5006, 749.4482 | ||||
| 173 | kudzusaponin SA4 | 36.40 | C47H74O20 | [M + H]+ | 959.4806 | 892.2470, 764.6243, 615.3878, 421.3457 |
| 174 | saponin 1 | 36.43 | C58H94O26 | [M + H]+ | 1207.6077 | 1075.5693, 913.5162, 751.4610, 603.2120 |
| [M − H]− | 1205.5934 | 881.4901, 749.4479, 603.3898, 471.3479 | ||||
| 175 | 24-hydroxy-licorice-saponin A3 | 36.45 | C48H72O22 | [M + H]+ | 1001.4559 | 825.4282, 763.0059, 631.3789, 469.3288 |
| 176 | 3,4-O-dicaffeoylquinic acid methyl ester | 36.46 | C26H26O12 | [M + H]+ | 531.1486 | 319.0808, 271.0598, 177.0545, 163.0388 |
| [M − H]− | 529.1344 | 443.6241, 367.1035, 191.0554, 135.1440 | ||||
| 177 | isoquercetin | 36.47 | C21H20O12 | [M + H]+ | 465.1023 | 303.0494, 257.0439, 229.0495, 153.0182 |
| 178 | soyasaponin A3 | 36.48 | C48H78O19 | [M + H]+ | 959.5183 | 813.4605, 439.3565, 141.0181, 85.0289 |
| [M − H]− | 957.5065 | 911.5010, 749.4482, 587.3950, 471.3475 | ||||
| 179 | dipsacoside B | 36.49 | C53H86O22 | [M − H]− | 1073.5519 | 912.0020, 749.4480, 585.3804, 471.3478 |
| 180 | kudzusaponin B1 | 36.52 | C48H76O21 | [M + H]+ | 989.4921 | 843.4330, 681.3870, 469.3314, 141.0181 |
| [M − H]− | 987.4794 | 926.4868, 763.7924, 661.3583, 503.3387 | ||||
| 181 | saponin 4 | 36.55 | C58H94O27 | [M − 2H]2− | 610.2908 | / |
| 182 | licoricesaponin A3 | 36.57 | C48H72O21 | [M + H]+ | 985.4612 | 809.4323, 615.3887, 453.3356, 189.1634 |
| [M − H]− | 983.4483 | 943.1790, 821.3969, 645.3637, 351.0566 | ||||
| 183 | neoliquiritin | 36.58 | C21H22O9 | [M + H]+ | 419.1326 | 315.0854, 257.0803, 217.0483, 124.0392 |
| 184 | 6″-O-malonyldaidzin | 36.58 | C24H22O12 | [M + H]+ | 503.1170 | 480.9303, 392.3837, 255.0647, 199.0751 |
| 185 | (2E)-1-(2,3-dihydroxy-4-methoxyphenyl)-3-(4-hydroxyphenyl)-2-propen--one | 36.58 | C16H14O5 | [M + H]+ | 287.0908 | 245.0804, 207.0649, 193.0492, 121.0285 |
| [M − H]− | 285.0765 | 270.0532, 177.0185, 150.0311, 108.0206 | ||||
| 186 | loniceroside D | 36.59 | C53H86O23 | [M + H]+ | 1091.5607 | 1033.7538, 945.5055, 783.4556, 421.3455 |
| [M − H]− | 1089.5470 | 1071.5394, 943.4768, 882.4898, 763.4315 | ||||
| 187 | akebiasaponin D | 36.60 | C47H76O18 | [M + H]+ | 929.5079 | 767.4589, 635.4064, 437.3408, 189.1637 |
| 188 | kudzusaponin A2 | 36.61 | C42H68O16 | [M + H]+ | 829.4560 | 764.7684, 649.3955, 455.3521, 269.0806 |
| [M − H]− | 827.4425 | 763.3452, 677.4987, 516.0891, 333.8636 | ||||
| 189 | isorhamnetin 3-O-glucopyranoside | 36.62 | C22H22O12 | [M + H]+ | 479.1170 | 397.5868, 317.0649, 274.0458, 120.0809 |
| * 190 | astragalin | 36.62 | C21H20O11 | [M + H]+ | 449.1072 | 409.0180, 346.9581, 287.0544, 252.9790 |
| [M − H]− | 447.0931 | 316.5085, 284.0322, 255.0294, 227.0343 | ||||
| 191 | 7,4′-dihydroxyflavone | 36.63 | C15H10O4 | [M + H]+ | 255.0645 | 227.0696, 199.0752, 137.0234, 91.0546 |
| [M − H]− | 253.0502 | 224.0470, 208.0522, 135.0076, 91.0174 | ||||
| 192 | isorhamentin 3-O-rutinoside | 36.66 | C28H32O16 | [M + H]+ | 625.1743 | 479.1161, 317.0652, 302.0414, 85.0289 |
| [M − H]− | 623.1613 | 527.7530, 415.1031, 252.0425, 223.0404 | ||||
| * 193 | 4′-methoxypuerarin | 36.67 | C22H22O9 | [M + H]+ | 431.1326 | 395.1120, 365.1009, 311.0910, 271.0595 |
| 194 | 4,5-O-dicaffeoylquinic acid methyl ester | 36.67 | C26H26O12 | [M − H]− | 529.1344 | 483.1268, 463.2749, 367.1032, 253.0501 |
| 195 | quercetin 3-O-arabinoside | 36.67 | C20H18O11 | [M + H]+ | 435.0917 | 303.0501, 271.0596, 153.0180, 121.0280 |
| 196 | loniceroside A | 36.69 | C52H84O21 | [M − H]− | 1043.5422 | 1025.5223, 763.3167, 709.8038, 532.3125 |
| * 197 | rhein | 36.71 | C15H8O6 | [M + H]+ | 285.0392 | 269.0440, 257.0428, 151.0385, 121.0283 |
| [M − H]− | 283.0246 | 268.0373, 217.0500, 175.0391, 133.0284 | ||||
| 198 | 3,4,5-tricaffeoylquinic acid | 36.72 | C34H30O15 | [M + H]+ | 679.1633 | 499.1226, 322.2479, 163.0387, 135.0440 |
| [M − H]− | 677.1511 | 515.1179, 353.0875, 173.0446, 135.0440 | ||||
| 199 | choerospondin | 36.74 | C21H22O10 | [M + H]+ | 435.1296 | 303.0501, 271.0596, 231.0647, 153.0180 |
| 200 | 4,5-dicaffeoylquinic acid | 36.75 | C25H24O12 | [M + H]+ | 517.1326 | 499.1223, 453.8935, 269.0803, 163.0387 |
| 201 | pollenitin B | 36.76 | C22H22O12 | [M + H]+ | 479.1170 | 412.8673, 317.0651, 302.0415, 274.0472 |
| 202 | medicarpin3-O-glucoside | 36.77 | C22H24O9 | [M + H]+ | 433.1482 | 312.0939, 271.0596, 214.2812, 153.0182 |
| 203 | lonfuranacid A | 36.77 | C12H20O5 | [M + H]+ | 245.1377 | 229.0853, 189.1119, 125.0962, 97.1015 |
| 204 | questin | 36.78 | C16H12O5 | [M + H]+ | 285.0751 | 270.0518, 253.0490, 242.0574, 153.0179 |
| 205 | Tricin 7-O-glucoside | 36.78 | C23H24O12 | [M + H]+ | 493.1326 | 331.0807, 315.0493, 287.0537, 270.0518 |
| 206 | tectoridin | 36.79 | C22H22O11 | [M + H]+ | 463.1220 | 301.0700, 286.0467, 258.0517, 153.0181 |
| * 207 | liquiritigenin | 36.80 | C15H12O4 | [M + H]+ | 257.0805 | 239.0705, 211.0756, 147.0439, 137.0232 |
| [M − H]− | 255.0660 | 209.0605, 153.0183, 135.0077, 119.0490 | ||||
| 208 | subproside V | 36.80 | C54H88O24 | [M − H]− | 1119.5575 | 1073.5519, 911.5007, 749.4478, 603.3897 |
| 209 | loniceroside E | 36.81 | C53H86O21 | [M − H]− | 1057.5570 | 1039.5623, 849.4960, 763.3219, 413.0908 |
| 210 | kudzusaponin A5 | 36.82 | C48H78O20 | [M + H]+ | 975.5131 | 829.4545, 764.4423, 667.4020, 455.3510 |
| 211 | torachrysone | 36.82 | C14H14O4 | [M + H]+ | 247.0960 | 229.0856, 214.0621, 201.0907, 198.0673 |
| [M − H]− | 245.0812 | 230.0579, 215.0343, 202.0625, 159.0440 | ||||
| 212 | macranthoside B | 36.83 | C53H86O22 | [M + H]+ | 1075.5658 | 943.5235, 781.4703, 619.4197, 437.3409 |
| 213 | glycyroside | 36.83 | C27H30O13 | [M + H]+ | 563.1743 | 431.1331, 413.1223, 311.0907, 281.0803 |
| [M − H]− | 561.1608 | 523.2799, 339.0867, 309.0767, 266.0582 | ||||
| 214 | ohyscion | 36.84 | C16H12O5 | [M + H]+ | 285.0751 | 270.0518, 242.0567, 189.4096, 113.0597 |
| [M − H]− | 283.0609 | 268.0375, 240.0419, 211.0391, 184.0518 | ||||
| 215 | afzelin | 36.88 | C21H20O10 | [M + H]+ | 433.1120 | 418.8996, 271.0596, 243.0644, 215.0699 |
| [M − H]− | 431.0975 | 269.0454, 240.0424, 225.0552, 152.9942 | ||||
| 216 | kudzusaponin SA3 | 36.89 | C53H86O23 | [M + H]+ | 1091.5607 | 929.5117, 767.4597, 635.4111, 437.3414 |
| [M − H]− | 1089.5470 | 1043.5427, 881.4904, 749.4480, 603.3900 | ||||
| 217 | 22β-acetoxyglycyrrhizin | 36.91 | C44H64O18 | [M + H]+ | 881.4143 | 705.3826, 511.3415, 451.3196, 107.0859 |
| 218 | kudzusaponin C1 | 36.93 | C54H88O23 | [M + H]+ | 1105.5767 | 959.5132, 797.498, 603.4246, 423.3591 |
| 219 | questinol | 36.94 | C16H12O6 | [M + H]+ | 301.0701 | 286.0460, 269.0439, 167.0338, 134.0362 |
| 220 | 3′-methoxydaidzin | 36.96 | C22H22O10 | [M + H]+ | 447.1277 | 384.1155, 327.0859, 285.0752, 229.0857 |
| 221 | loniceroside B | 36.96 | C58H94O25 | [M + H]+ | 1191.6127 | 817.3367, 763.4253, 619.4137, 437.3397 |
| 222 | citreorosein | 36.97 | C15H10O6 | [M + H]+ | 287.0542 | 271.0596, 269.0443, 259.0960, 217.0491 |
| [M − H]− | 285.0402 | 268.0367, 257.0461, 196.0532, 133.0284 | ||||
| 223 | herbacetin | 37.01 | C15H10O7 | [M + H]+ | 303.0492 | 286.0430, 257.0442, 229.0497, 153.0181 |
| [M − H]− | 301.0351 | 284.0315, 273.0407, 178.9976, 151.0026 | ||||
| 224 | betulonic acid | 37.02 | C30H46O3 | [M + H]+ | 455.3507 | 409.3467, 388.4104, 203.1793, 189.1635 |
| 225 | kudzusaponin A3 | 37.05 | C48H78O20 | [M + H]+ | 975.5131 | 829.4545, 667.4020, 455.3510, 141.0181 |
| * 226 | ononin | 37.08 | C22H22O9 | [M + H]+ | 431.1326 | 269.0805, 254.0569, 213.0910, 107.0494 |
| 227 | kaempferol 3-O-rutinoside | 37.11 | C27H30O15 | [M + H]+ | 595.1641 | 525.0455, 433.1108, 287.0544, 271.0596 |
| 228 | isobavachalcone | 37.11 | C20H20O4 | [M + H]+ | 325.1429 | 309.0781, 285.0754, 189.0906, 95.0163 |
| 229 | liqcoumarin | 37.18 | C12H10O4 | [M + H]+ | 219.0648 | 201.0910, 174.0674, 133.1012, 105.0702 |
| 230 | isokaempferide | 37.19 | C16H12O6 | [M + H]+ | 301.0701 | 283.0596, 255.0636, 227.0698, 123.1169 |
| 231 | uralsaponin F | 37.21 | C44H64O19 | [M + H]+ | 897.4094 | 763.6343, 679.2714, 527.3329, 334.7203 |
| 232 | daidzein 4′,7-diglucoside | 37.25 | C27H30O14 | [M + H]+ | 579.1692 | 503.0054, 447.1283, 285.0753, 229.0858 |
| 233 | liquoric acid | 37.26 | C30H44O5 | [M + H]+ | 485.3245 | 323.1276, 255.0648, 199.0751, 163.0385 |
| 234 | isoorientin | 37.27 | C21H20O11 | [M + H]+ | 449.1072 | 330.0535, 287.0545, 153.0181, 135.0439 |
| 235 | isorhodoptilometrin | 37.31 | C17H14O6 | [M + H]+ | 315.0856 | 300.0623, 272.0670, 153.0185, 95.0858 |
| [M − H]− | 313.0715 | 298.0479, 270.0529, 227.0343, 183.0454 | ||||
| 236 | chrysophanol | 37.34 | C15H10O4 | [M + H]+ | 255.0645 | 237.0549, 227.0692, 199.0751, 187.0725 |
| 237 | licoricesaponin G2 | 37.34 | C42H62O17 | [M + H]+ | 839.4040 | 663.3734, 487.3410, 469.3306, 141.0181 |
| [M − H]− | 837.3906 | 763.7259, 724.4032, 351.0571, 193.0345 | ||||
| 238 | kudzusaponin SA1 | 37.35 | C42H68O15 | [M + H]+ | 813.4611 | 764.7317, 439.3548, 141.0181, 95.0860 |
| [M − H]− | 811.4482 | 765.4406, 603.3923, 432.7037, 283.0584 | ||||
| 239 | 1,4-dicaffeoylquinic acid | 37.38 | C25H24O12 | [M + H]+ | 517.1326 | 460.9336, 414.0990, 269.0804, 213.0906 |
| 240 | ethyl caffeate | 37.38 | C11H12O4 | [M + H]+ | 209.0806 | 163.0388, 145.1011, 135.0441, 117.0337 |
| [M − H]− | 207.0654 | 179.0341, 161.0233, 135.0441, 121.0284 | ||||
| 241 | apigenin 7-glucoside | 37.41 | C21H20O10 | [M + H]+ | 433.1120 | 379.0797, 337.0701, 313.0699, 271.0596 |
| [M − H]− | 431.0975 | 311.0562, 269.0453, 225.0554, 152.9949 | ||||
| * 242 | daidzin | 37.42 | C21H20O9 | [M + H]+ | 417.1167 | 387.2100, 297.0747, 255.0648, 199.0753 |
| 243 | 2,5-dimethyl-7-hydroxychromenone | 37.46 | C11H10O3 | [M + H]+ | 191.0699 | 151.0387, 131.0856, 107.0861, 95.0860 |
| 244 | prunetin | 37.46 | C16H12O5 | [M + H]+ | 285.0751 | 253.0489, 242.0572, 211.0750, 151.0390 |
| [M − H]− | 283.0609 | 268.0380, 240.0423, 197.0600, 168.0650 | ||||
| 245 | glabrolide | 37.47 | C30H44O4 | [M + H]+ | 469.3298 | 233.1540, 175.1479, 135.1167, 107.0858 |
| 246 | pinocembrin | 37.53 | C15H12O4 | [M + H]+ | 257.0805 | 239.0701, 229.0850, 211.0752, 147.0440 |
| 247 | polygonin B | 37.54 | C26H26O13 | [M + H]+ | 547.1430 | 299.0909, 284.0674, 239.0702, 163.0385 |
| * 248 | glycyrrhizic acid | 37.56 | C42H62O16 | [M + H]+ | 823.4086 | 647.3788, 471.3457, 453.3356 |
| [M − H]− | 821.3959 | 763.8063, 469.3315, 351.0569, 193.0347 | ||||
| 249 | echinatin | 37.56 | C16H14O4 | [M + H]+ | 271.0960 | 254.2115, 147.0438, 137.0596, 123.0441 |
| [M − H]− | 269.0816 | 251.0708, 225.0552, 151.0030, 119.0490 | ||||
| 250 | hesperetin | 37.62 | C16H14O6 | [M + H]+ | 303.0853 | 258.0517, 153.0183, 106.0866, 88.0762 |
| [M − H]− | 301.0714 | 273.0774, 255.0296, 230.0580, 183.0447 | ||||
| 251 | (S)-naringenin | 37.62 | C15H12O5 | [M + H]+ | 273.0752 | 189.0543, 153.0180, 123.0441 |
| 252 | puerol B | 37.64 | C18H16O5 | [M + H]+ | 313.1063 | 267.1011, 253.0854, 107.0495 |
| [M − H]− | 311.0922 | 296.0689, 267.1026, 252.0789, 161.0233 | ||||
| 253 | coumestrol | 37.72 | C15H8O5 | [M + H]+ | 269.0437 | 254.0572, 241.0492, 213.0543, 185.0595 |
| [M − H]− | 267.0297 | 251.0709, 225.0550, 181.0649, 151.0026 | ||||
| 254 | polygonin A | 37.73 | C25H24O13 | [M + H]+ | 533.1273 | 488.1885, 360.1438, 285.0753, 270.0517 |
| [M − H]− | 531.1141 | 341.9318, 253.0502, 229.0135, 191.0555 | ||||
| 255 | tricin | 37.73 | C17H14O7 | [M + H]+ | 331.0804 | 315.0492, 302.0406, 270.0519, 73.0291 |
| [M − H]− | 329.0664 | 271.0247, 211.1332, 171.1017 | ||||
| 256 | neobavaisoflavone | 37.76 | C20H18O4 | [M + H]+ | 323.1272 | 308.0663, 267.0647, 255.0648, 239.0698 |
| 257 | biochanin | 37.76 | C16H12O5 | [M + H]+ | 285.0751 | 270.0516, 253.0491, 225.0540, 137.0233 |
| [M − H]− | 283.0609 | 268.0377, 240.0423, 224.0474, 135.0075 | ||||
| 258 | gancaonin V | 37.76 | C19H20O4 | [M + H]+ | 313.1425 | 281.1162, 244.0359, 153.0181 |
| [M − H]− | 311.1285 | 296.0687, 267.1025, 252.0789, 161.0232 | ||||
| 259 | 6,7-dimethoxycoumarin | 37.76 | C11H10O4 | [M + H]+ | 207.0650 | 189.1636, 175.0388, 148.0517, 91.0547 |
| 260 | puerol A | 37.78 | C17H14O5 | [M + H]+ | 299.0908 | 284.0674, 256.0726, 239.0698, 95.0163 |
| [M − H]− | 297.0764 | 281.0457, 256.0376, 239.0346, 151.0025 | ||||
| 261 | biapigenin | 37.78 | C30H18O10 | [M + H]+ | 539.0958 | 522.9716, 387.0856, 286.0465, 184.0731 |
| [M − H]− | 537.0822 | 521.0623, 417.0622, 375.0506, 331.0608 | ||||
| 262 | 2-methoxy-6-acetyl-7-methyljuglone | 37.79 | C14H12O5 | [M + H]+ | 261.0754 | 243.0648, 215.0699, 200.0466, 187.0754 |
| [M − H]− | 259.0608 | 243.1414, 231.0657, 216.0422, 188.0471 | ||||
| 263 | kudzusaponin SB1 | 37.79 | C53H86O22 | [M + H]+ | 1075.5658 | 943.5235, 751.4603, 437.3405, 189.1636 |
| [M − H]− | 1073.5519 | 911.5006, 749.4489, 603.3903, 471.3480 | ||||
| 264 | diosmetin | 37.81 | C16H12O6 | [M + H]+ | 301.0701 | 286.0468, 258.0523, 241.0491, 88.0762 |
| [M − H]− | 299.0557 | 284.0325, 256.0372, 227.0344, 151.0030 | ||||
| 265 | (-)-epiafzelechin | 37.82 | C15H14O5 | [M + H]+ | 275.0906 | 257.0795, 217.0492, 189.0544, 107.0495 |
| [M − H]− | 273.0766 | 258.0532, 230.0579, 215.0343, 135.0076 | ||||
| 266 | licoricesaponin E2 | 37.85 | C42H60O16 | [M + H]+ | 821.3938 | 764.7926, 451.3198, 173.1327, 121.1012 |
| 267 | methyl glycyrrhizate | 37.86 | C43H64O16 | [M + H]+ | 837.4255 | 764.7705, 663.3716, 469.3308, 141.0181 |
| 268 | licoisoflavanone | 37.88 | C20H18O6 | [M + H]+ | 355.1169 | 337.1062, 299.0546, 179.0337, 123.0441 |
| [M − H]− | 353.1027 | 335.0921, 312.0276, 217.0863, 189.0913 | ||||
| 269 | 3-methoxyherbacetin | 37.89 | C16H12O7 | [M + H]+ | 317.0647 | 302.0411, 237.0383, 153.0181, 127.0391 |
| [M − H]− | 315.0506 | 300.0272, 272.0323, 188.0482, 112.9845 | ||||
| 270 | erybacin B | 37.89 | C19H18O5 | [M + H]+ | 327.1222 | 271.0597, 117.0367, 95.0163, 77.0059 |
| [M − H]− | 325.1076 | 309.2072, 297.0051, 197.1174, 171.1016 | ||||
| 271 | soyasaponin I | 37.91 | C48H78O18 | [M + H]+ | 943.5241 | 797.4680, 764.6400, 423.3611, 85.0289 |
| [M − H]− | 941.5101 | 912.5775, 763.8070, 615.3933, 438.3518 | ||||
| 272 | licoricesaponin B2 | 37.93 | C42H64O15 | [M + H]+ | 809.4296 | 633.3988, 439.3564, 285.2223, 107.0859 |
| [M − H]− | 807.4168 | 763.8304, 520.9705, 351.0565, 193.0345 | ||||
| 273 | ephedrannin B | 37.94 | C30H20O10 | [M + H]+ | 541.1106 | 415.0806, 389.1013, 171.0287, 153.0181 |
| [M − H]− | 539.0981 | 521.2609, 507.2097, 396.8802, 266.9637 | ||||
| 274 | medicarpin | 37.97 | C16H14O4 | [M + H]+ | 271.0960 | 253.0497, 229.0855, 197.0594, 121.0285 |
| * 275 | kaempferol | 37.99 | C15H10O6 | [M + H]+ | 287.0542 | 271.0556, 254.0524, 226.0577, 153.0181 |
| [M − H]− | 285.0402 | 268.0364, 257.0451, 241.0497, 211.0396 | ||||
| 276 | glyasperin D | 38.04 | C22H26O5 | [M + H]+ | 371.1844 | 315.1218, 303.1219, 167.0701, 123.0441 |
| 277 | isoliquiritigenin | 38.09 | C15H12O4 | [M + H]+ | 257.0805 | 239.0698, 211.0755, 147.0440, 137.0232 |
| [M − H]− | 255.0660 | 153.0182, 135.0077, 119.0489, 91.0175 | ||||
| 278 | 3′-hydroxydaidzein | 38.10 | C15H10O5 | [M + H]+ | 271.0594 | 253.0492, 243.0647, 215.0702, 153.0180 |
| 279 | kaikasaponin III | 38.15 | C48H78O17 | [M + H]+ | 927.5280 | 767.4596, 635.4124, 437.3406, 203.1794 |
| 280 | 3,4,3′,4′-tetrahydroxychalcone | 38.16 | C15H12O5 | [M + H]+ | 273.0752 | 245.0811, 171.0285, 153.0181, 123.0442 |
| 281 | araboglycyrrhizin | 38.21 | C41H62O14 | [M + H]+ | 779.4183 | / |
| 282 | macranthoside A | 38.22 | C47H76O17 | [M + H]+ | 913.5130 | 781.4694, 617.4044, 423.3610, 141.0180 |
| [M − H]− | 911.5004 | 749.4489, 603.3895, 471.3479, 423.3271 | ||||
| 283 | hydnocarpin | 38.23 | C25H20O9 | [M + H]+ | 465.1173 | 447.1065, 286.0468, 257.0440, 147.0438 |
| [M − H]− | 463.1033 | 447.2420, 285.0402, 255.0293, 208.9755 | ||||
| 284 | puerariafuran | 38.24 | C16H12O5 | [M + H]+ | 285.0751 | 270.0512, 253.0493, 242.0569, 211.0754 |
| 285 | vestitol | 38.29 | C16H16O4 | [M + H]+ | 273.1118 | 255.1017, 227.1794, 137.0233, 121.0285 |
| 286 | homobutein | 38.31 | C16H14O5 | [M + H]+ | 287.0908 | 269.0440, 241.0491, 185.0592, 151.0389 |
| 287 | glycycoumarin | 38.34 | C21H20O6 | [M + H]+ | 369.1324 | 351.1228, 297.0746, 193.0494, 165.0545 |
| 288 | licoricesaponin J2 | 38.34 | C42H64O16 | [M + H]+ | 825.4243 | 764.8267, 455.3507, 189.1634, 141.0181 |
| [M − H]− | 823.4119 | 763.2627, 473.1696, 351.0565, 193.0342 | ||||
| 289 | licoricesaponin C2 | 38.35 | C42H62O15 | [M + H]+ | 807.4139 | 764.8302, 678.4443, 631.3784, 437.3406 |
| [M − H]− | 805.4016 | 763.3167, 453.3408, 351.0559, 193.0349 | ||||
| 290 | 3′-hydroxy-4′-O-methylglabridin | 38.39 | C21H22O5 | [M − H]− | 353.1393 | / |
| 291 | blumenol A | 38.39 | C13H20O3 | [M + H]+ | 225.1483 | 210.1245, 167.9932, 114.0913, 95.0860 |
| 292 | dihydrodaidzein | 38.40 | C15H12O4 | [M + H]+ | 257.0805 | 239.0690, 229.0851, 211.0744, 147.0439 |
| * 293 | formononetin | 38.44 | C16H12O4 | [M + H]+ | 269.0803 | 254.0567, 213.0907, 118.0414, 95.0859 |
| [M − H]− | 267.0660 | 252.0423, 225.0553, 195.0443, 132.0204 | ||||
| 294 | lupiwighteone | 38.44 | C20H18O5 | [M + H]+ | 339.1219 | 322.2484, 283.0594, 271.0597, 209.1646 |
| * 295 | quercetin | 38.48 | C15H10O7 | [M + H]+ | 303.0492 | 285.0393, 257.0439, 229.0493, 153.0181 |
| [M − H]− | 301.0351 | 283.0246, 255.0298, 227.0342, 138.0312 | ||||
| 296 | glycyuralin E | 38.52 | C21H22O6 | [M + H]+ | 371.1480 | 353.1372, 339.1213, 285.0749, 167.0695 |
| [M − H]− | 369.1341 | 311.0558, 229.0865, 206.0213, 139.0390 | ||||
| 297 | estradiol | 38.53 | C18H24O2 | [M + H]+ | 273.1845 | 255.1007, 248.4772, 153.0180, 119.0856 |
| 298 | licoflavone A | 38.57 | C20H18O4 | [M + H]+ | 323.1272 | 280.0719, 267.0648, 254.0570, 239.0700 |
| 299 | irisolidone | 38.67 | C17H14O6 | [M + H]+ | 315.0856 | 297.0751, 226.0619, 199.0751, 153.0182 |
| [M − H]− | 313.0715 | 295.0610, 270.0479, 224.0468, 167.2795 | ||||
| 300 | 1-methoxyphaseollidin | 38.68 | C21H22O5 | [M + H]+ | 355.1532 | 299.0548, 221.1169, 165.0546, 123.0441 |
| [M − H]− | 353.1393 | 338.1162, 292.0359, 253.0505, 150.0311 | ||||
| 301 | cupressuflavone | 38.69 | C30H18O10 | [M + H]+ | 539.0958 | 497.0887, 403.0439, 377.0645, 335.0543 |
| [M − H]− | 537.0822 | 521.2611, 505.2242, 375.0520, 266.9636 | ||||
| 302 | licoarylcoumarin | 38.69 | C21H20O6 | [M + H]+ | 369.1324 | 313.0699, 271.0596, 243.0647, 147.0439 |
| 303 | isoformononetin | 38.71 | C16H12O4 | [M + H]+ | 269.0803 | 251.0697, 241.0828, 237.0537, 107.0855 |
| [M − H]− | 267.0660 | 252.0424, 241.0503, 197.0604, 96.9588 | ||||
| 304 | kakkasaponin I | 38.72 | C47H76O16 | [M − H]− | 895.5067 | 877.5569, 763.6689, 678.9240, 509.4025 |
| 305 | β-amyrone | 38.76 | C30H48O | [M + H]+ | 425.3767 | / |
| 306 | tuberosin | 38.82 | C20H18O5 | [M − H]− | 337.1080 | 309.0397, 281.0454, 254.0585, 203.1068 |
| 307 | glicophenone | 38.83 | C20H22O6 | [M + H]+ | 359.1482 | 301.0710, 283.0596, 175.0389, 153.0545 |
| [M − H]− | 357.1341 | 247.0974, 232.0737, 189.0186, 109.0282 | ||||
| 308 | 7,4′-dihydroxy-3′-methoxyisoflavan | 38.83 | C16H16O4 | [M + H]+ | 273.1118 | 245.1898, 163.0750, 137.0596, 123.0442 |
| [M − H]− | 271.0973 | 241.0499, 225.0550, 197.0596, 181.0652 | ||||
| 309 | 2′,3′-dihydro-7,7′-dihydroxy-5′-methoxy-2′,2′-dimethyl[3,6′-bi-4H-1-benzopyran]-4-one | 38.89 | C21H20O6 | [M + H]+ | 369.1324 | 313.0699, 285.0752, 270.0518, 243.0648 |
| [M − H]− | 367.1182 | 337.0717, 309.0403, 256.0376, 203.0708 | ||||
| 310 | 3,4-didehydroglabridin | 38.98 | C20H18O4 | [M + H]+ | 323.1272 | 267.0648, 255.0647, 239.0698, 95.0163 |
| [M − H]− | 321.1129 | 277.0503, 265.0505, 252.0424, 149.0598 | ||||
| 311 | glyasperin C | 38.98 | C21H24O5 | [M + H]+ | 357.1689 | 301.1063, 221.1165, 165.0546, 123.0441 |
| [M − H]− | 355.1547 | 298.0483, 229.0865, 174.0313, 125.0232 | ||||
| 312 | neouralenol | 39.01 | C20H18O7 | [M + H]+ | 371.1119 | 315.0856, 268.2631, 183.0287, 165.0181 |
| [M − H]− | 369.0976 | 351.0870, 310.0444, 283.0975, 193.0135 | ||||
| 313 | phaseol | 39.05 | C20H16O5 | [M + H]+ | 337.1065 | 319.0956, 283.0596, 255.0646, 163.0388 |
| 314 | eurycarpin A | 39.08 | C20H18O5 | [M + H]+ | 339.1219 | 322.2490, 293.0592, 163.0388, 114.0915 |
| [M − H]− | 337.1080 | 293.1182, 268.0376, 224.0470, 135.0077 | ||||
| 315 | glyurallin A | 39.10 | C21H20O5 | [M − H]− | 351.1236 | 335.0564, 323.0929, 308.0317, 191.0711 |
| 316 | sophoraisoflavone A | 39.10 | C20H16O6 | [M + H]+ | 353.1014 | 335.0906, 325.1064, 283.02599, 191.0343 |
| 317 | licocoumarone | 39.11 | C20H20O5 | [M + H]+ | 341.1379 | 323.1265, 267.0648, 209.1646, 114.0915 |
| [M − H]− | 339.1233 | 296.0677, 268.0377, 219.0656, 119.0490 | ||||
| 318 | dehydrovomifoliol | 39.18 | C13H18O3 | [M + H]+ | 223.1326 | 135.1167, 107.0858, 81.0704 |
| [M − H]− | 221.1177 | 205.1224, 164.0829, 148.0516, 118.5610 | ||||
| 319 | fallacinol | 39.20 | C16H12O6 | [M + H]+ | 301.0701 | 283.0598, 269.0440, 227.0701, 199.0752 |
| [M − H]− | 299.0557 | 284.0317, 255.0649, 240.0422, 212.0468 | ||||
| 320 | genkwanin | 39.23 | C16H12O5 | [M + H]+ | 285.0751 | 270.0519, 253.0494, 225.0542, 137.0233 |
| [M − H]− | 283.0609 | 268.0378, 240.0423, 186.6367, 118.3947 | ||||
| 321 | kanzonol U | 39.25 | C19H16O4 | [M + H]+ | 309.1115 | / |
| 322 | 2,3-dehydrokievitone | 39.26 | C20H18O6 | [M + H]+ | 355.1169 | 337.1066, 229.0854, 179.0338, 123.0442 |
| [M − H]− | 353.1027 | 284.0319, 243.1021, 216.0419, 201.0915 | ||||
| 323 | 2,3,4-trimethyl-5-phenyloxazolidine | 39.28 | C12H17NO | [M + H]+ | 192.1382 | 133.1011, 119.0493, 91.0547 |
| 324 | pratensein | 39.29 | C16H12O6 | [M + H]+ | 301.0701 | 283.0598, 269.0440, 227.0701, 199.0752 |
| 325 | lupenone | 39.32 | C30H48O | [M + H]+ | 425.3767 | / |
| 326 | corylifol B | 39.35 | C20H20O5 | [M + H]+ | 341.1379 | 267.0648, 209.1646, 114.0916 |
| [M − H]− | 339.1233 | 269.0453, 233.0818, 187.1117, 167.0340 | ||||
| 327 | 4-O-methylglabridin | 39.36 | C21H22O4 | [M + H]+ | 339.1586 | 322.2483, 209.1644, 114.0916, 95.0163 |
| 328 | luteone | 39.43 | C20H18O6 | [M + H]+ | 355.1169 | 338.3415, 299.0540, 267.0284, 239.0334 |
| [M − H]− | 353.1027 | 257.0063, 227.0702, 165.0179, 125.0232 | ||||
| 329 | glyinflanin H | 39.43 | C19H16O4 | [M + H]+ | 309.1115 | 291.1940, 223.0596, 113.0600, 95.0163 |
| 330 | butyl octyl phthalate | 39.44 | C20H30O4 | [M − H]− | 333.2062 | 293.0450, 281.0451, 252.0419, 201.0916 |
| 331 | glycyrrhetic acid 3-O-glucuronide | 39.45 | C36H54O10 | [M + H]+ | 647.3769 | 453.3359, 357.2422, 285.2203, 121.1012 |
| [M − H]− | 645.3641 | 580.9614, 521.2628, 469.3308, 322.6431 | ||||
| 332 | glyasperin A | 39.46 | C25H26O6 | [M − H]− | 421.1654 | 403.9289, 353.1024, 312.0273, 280.0371 |
| 333 | 1-methoxyphaseollin | 39.47 | C21H20O5 | [M − H]− | 351.1236 | 294.4448, 243.1023, 227.0710, 125.0232 |
| 334 | licochalcone D | 39.53 | C21H22O5 | [M + H]+ | 355.1532 | 338.3410, 311.0542, 193.0494, 135.0440 |
| 335 | wighteone | 39.54 | C20H18O5 | [M + H]+ | 339.1219 | 321.2453, 311.0548, 209.1647, 114.0916 |
| [M − H]− | 337.1080 | 321.0765, 309.1127, 253.0500, 209.0596 | ||||
| 336 | 2′-O-demethylbidwillol B | 39.54 | C19H18O4 | [M + H]+ | 311.1269 | 293.1166, 278.0932, 263.0694, 95.0163 |
| 337 | glycyrol | 39.63 | C21H18O6 | [M + H]+ | 367.1168 | 337.0697, 227.0702, 167.0337, 91.0547 |
| [M − H]− | 365.1026 | 335.0560, 307.0247, 295.0245, 254.0220 | ||||
| 338 | 3-hydroxyglabrol | 39.65 | C25H28O5 | [M − H]− | 407.1863 | 387.2756, 371.2437, 150.9878, 93.0001 |
| 339 | kumatakenin | 39.72 | C17H14O6 | [M + H]+ | 315.0856 | 255.0647, 227.0699, 153.0180, 60.0452 |
| [M − H]− | 313.0715 | 295.0606, 283.0609, 267.0663, 239.0712 | ||||
| 340 | eriodictyol | 39.73 | C15H12O6 | [M + H]+ | 289.0697 | 271.0959, 229.0855, 163.0388, 153.0181 |
| [M − H]− | 287.0559 | 272.0326, 258.0119, 216.0419, 155.1432 | ||||
| 341 | licoflavone B | 39.74 | C25H26O4 | [M + H]+ | 391.1897 | 358.2020, 323.1262, 267.0647, 195.0430 |
| 342 | gancaonin U | 39.76 | C24H28O4 | [M − H]− | 379.1908 | / |
| 343 | dehydroglyceollin I | 39.78 | C20H16O4 | [M + H]+ | 321.1115 | 306.0873, 187.0752, 159.0803, 147.0439 |
| [M − H]− | 319.0970 | 303.0658, 289.0504, 243.0657, 161.0233 | ||||
| 344 | tectorigenin | 39.80 | C16H12O6 | [M + H]+ | 301.0701 | 283.0598, 255.0646, 227.0698, 199.0748 |
| [M − H]− | 299.0557 | 284.0317, 267.0301, 240.0422, 212.0468 | ||||
| 345 | derrone | 39.87 | C20H16O5 | [M + H]+ | 337.1065 | 309.1118, 267.0650, 225.0545, 91.0549 |
| [M − H]− | 335.0921 | 319.0606, 305.0436, 278.3866, 158.8393 | ||||
| 346 | abyssinone II | 39.91 | C20H20O4 | [M + H]+ | 325.1429 | 269.0804, 241.0850, 135.0440, 95.0163 |
| [M − H]− | 323.1283 | 308.1031, 201.0914, 187.0761, 135.0441 | ||||
| 347 | corylin | 39.96 | C20H16O4 | [M + H]+ | 321.1115 | 306.0870, 279.0649, 265.0488, 137.0232 |
| 348 | ephedradine A | 39.98 | C28H36N4O4 | [M + H]+ | 493.2800 | 465.2870, 394.2122, 219.1489, 120.0809 |
| 349 | kudzusapogenol A | 40.02 | C30H50O5 | [M − H]− | 489.3575 | / |
| 350 | kanzonol Y | 40.04 | C25H30O5 | [M − H]− | 409.2015 | 391.2520, 373.2436, 235.0971, 177.0912 |
| 351 | kanzonol W | 40.11 | C20H16O5 | [M + H]+ | 337.1065 | 321.1119, 281.0443, 253.0488, 163.0388 |
| [M − H]− | 335.0921 | 320.0677, 291.1024, 199.0758, 135.0078 | ||||
| 352 | isoglycyrol | 40.13 | C21H18O6 | [M + H]+ | 367.1168 | 349.1074, 325.0702, 291.0630, 167.0338 |
| [M − H]− | 365.1026 | 349.0708, 309.0393, 216.0423, 192.0055 | ||||
| 353 | licoisoflavone B | 40.17 | C20H16O6 | [M + H]+ | 353.1014 | 311.0558, 299.0544, 153.0180, 95.0163 |
| [M − H]− | 351.0869 | 337.0660, 283.0974, 241.0864, 199.0756 | ||||
| 354 | 6,8-diprenylgenistein | 40.26 | C25H26O5 | [M + H]+ | 407.1844 | 339.1198, 283.0596, 237.0534, 91.0547 |
| [M − H]− | 405.1704 | 387.2755, 371.2439, 281.0460, 150.9878 | ||||
| 355 | parvisoflavone A | 40.29 | C20H16O6 | [M + H]+ | 353.1014 | 335.0906, 325.1068, 191.0328, 153.0180 |
| 356 | licoricidin | 40.30 | C26H32O5 | [M + H]+ | 425.2315 | 369.1328, 313.0703, 175.0388, 139.0389 |
| 357 | kanzonol C | 40.31 | C25H28O4 | [M + H]+ | 393.2051 | 376.1539, 329.0271, 268.0656, 215.0684 |
| * 358 | emodin | 40.43 | C15H10O5 | [M + H]+ | 271.0594 | 243.0650, 229.0493, 197.0596, 173.0591 |
| [M − H]− | 269.0450 | 241.0503, 225.0551, 197.0597, 181.0647 | ||||
| 359 | apigenin | 40.43 | C15H10O5 | [M + H]+ | 271.0594 | 229.0495, 201.0543, 173.0597, 91.0548 |
| [M − H]− | 269.0450 | 241.0501, 225.0551, 210.0314, 181.0644 | ||||
| 360 | genistein | 40.44 | C15H10O5 | [M + H]+ | 271.0594 | 243.0642, 229.0495, 201.0543, 371.0596 |
| [M − H]− | 269.0450 | 241.0504, 225.0550, 197.0597, 181.0647 | ||||
| 361 | lupalbigenin | 40.45 | C25H26O5 | [M + H]+ | 407.1844 | 373.1034, 283.0597, 213.0541, 149.0232 |
| 362 | angustone A | 40.51 | C25H26O6 | [M − H]− | 421.1654 | 404.9251, 352.0951, 269.0453, 201.0913 |
| 363 | dehydroglyasperin D | 40.59 | C22H24O5 | [M + H]+ | 369.1688 | 313.0700, 295.0597, 197.0441, 179.0337 |
| [M − H]− | 367.1545 | 351.9669, 322.9651, 269.0455, 240.0420 | ||||
| 364 | corymbosin | 40.63 | C19H18O7 | [M + H]+ | 359.1118 | 329.0648, 313.0695, 269.0804, 95.0163 |
| 365 | euchrenone a5 | 40.64 | C25H26O4 | [M + H]+ | 391.1897 | 358.2020, 267.0647, 239.0701, 149.0236 |
| [M − H]− | 389.1751 | 319.0978, 298.0473, 266.0580, 195.1691 | ||||
| 366 | paratocarpin L | 40.75 | C25H28O5 | [M − H]− | 407.1863 | / |
| 367 | diisobutyl phthalate | 40.79 | C16H22O4 | [M + H]+ | 279.1583 | 167.0340, 149.0232, 121.0284, 57.0706 |
| [M − H]− | 277.1438 | 245.3889, 193.7951, 134.0361, 121.0283 | ||||
| 368 | glyurallin B | 40.79 | C25H26O6 | [M + H]+ | 423.1793 | / |
| 369 | angustone B | 40.82 | C25H24O6 | [M + H]+ | 421.1637 | 365.1014, 309.0388, 281.0439, 140.0342 |
| [M − H]− | 419.1497 | 402.9280, 375.0866, 363.0872, 308.0323 | ||||
| 370 | licoagrocarpin | 40.93 | C21H22O4 | [M + H]+ | 339.1586 | / |
| 371 | palmitic acid | 41.04 | C16H32O2 | [M − H]− | 255.2322 | 170.3186, 162.0524, 116.9273, 74.0233 |
| 372 | 2′-hydroxyisolupalbigenin | 41.11 | C25H26O6 | [M − H]− | 421.1654 | 404.9261, 363.0872, 227.0711, 193.0862 |
| 373 | butesuperin A | 41.15 | C26H22O8 | [M + H]+ | 463.1375 | 445.1275, 283.0597, 255.0647, 161.0594 |
| * 374 | luteolin | 41.16 | C15H10O6 | [M − H]− | 285.0402 | 268.9432, 257.0451, 242.0536, 196.0504 |
| 375 | sophoracoumestan A | 41.19 | C20H14O5 | [M + H]+ | 335.0909 | 320.0672, 307.0952, 292.0722, 137.0237 |
| * 376 | glycyrrhetic acid | 41.41 | C30H46O4 | [M + H]+ | 471.3456 | 317.2107, 269.0803, 189.1636, 121.1013 |
| 377 | 8-prenylphaseollinisoflavan | 41.74 | C25H28O4 | [M + H]+ | 393.2051 | 339.0701, 269.0807, 167.0337, 149.0232 |
| [M − H]− | 391.1912 | 289.1443, 271.1335, 187.0393, 119.0490 | ||||
| 378 | stearic acid | 41.75 | C18H36O2 | [M − H]− | 283.2635 | / |
| 379 | puerarol | 41.75 | C25H24O5 | [M + H]+ | 405.1683 | 319.0950, 281.0439, 209.0591, 171.0138 |
| [M − H]− | 403.1546 | 387.2754, 371.2439, 333.0764, 150.9877 | ||||
| 380 | hederagenin | 41.79 | C30H48O4 | [M + H]+ | 473.3615 | 310.8371, 189.1640, 133.1015, 59.0162 |
| [M − H]− | 471.3470 | 429.2146, 403.1549, 319.0597, 280.0376 |
| Main Components | Calibration Curve | Linear Range (mg/mL) | R2 | LOD (μg/mL) | LOQ (μg/mL) | Repeatability RSD (%) | Stability RSD (%) | Intermediate Precision (%, n = 12) |
|---|---|---|---|---|---|---|---|---|
| chlorogenic acid | y = 12,757x – 376.29 | 0.05–2.00 | 0.9991 | 2.13 | 6.47 | 1.18% | 1.37% | 2.82% |
| puerarin | y = 37,995x + 303.18 | 0.05–1.60 | 0.9990 | 1.85 | 5.61 | 1.23% | 2.60% | 2.00% |
| 3′-methoxypuerarin | y = 25,755x + 64.034 | 0.04–1.50 | 0.9998 | 1.26 | 3.83 | 2.94% | 1.09% | 2.81% |
| polydatin | y = 9872.5x – 89.081 | 0.03–1.00 | 0.9992 | 1.25 | 3.80 | 2.27% | 3.10% | 3.45% |
| glycyrrhizic acid | y = 7190.5x + 101.33 | 0.02–1.00 | 0.9994 | 0.77 | 2.33 | 2.03% | 1.30% | 3.89% |
| emodin | y = 33,371x + 138.83 | 0.01–0.50 | 0.9992 | 0.38 | 1.14 | 1.26% | 3.59% | 3.53% |
| Main Components | Sample S1 (mg/g) | Sample S2 (mg/g) | Sample S3 (mg/g) | Content (mg/g) | Scavenging Percentage of DPPH Radical (%) |
|---|---|---|---|---|---|
| chlorogenic acid | 33.16 | 33.72 | 35.56 | 34.15 ± 1.25 | 59.2 |
| puerarin | 27.30 | 28.15 | 29.46 | 28.30 ± 1.09 | 49.6 |
| 3′-methoxypuerarin | 9.73 | 9.66 | 9.50 | 9.63 ± 0.12 | 58.9 |
| polydatin | 10.29 | 10.76 | 11.43 | 10.83 ± 0.57 | 58.0 |
| glycyrrhizic acid | 3.18 | 2.85 | 3.94 | 3.33 ± 0.56 | 54.6 |
| emodin | 4.15 | 4.48 | 3.80 | 4.14 ± 0.34 | 30.3 |
| total phenol | 144.11 | 147.89 | 147.99 | 144.66 ± 2.21 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalkun, I.; Wan, H.; Ye, L.; Yu, L.; He, Y.; Li, C.; Wan, H. Qualitative and Quantitative Analysis of Chemical Components in Yinhua Pinggan Granule with High-Performance Liquid Chromatography Coupled with Q-Exactive Mass Spectrometry. Molecules 2024, 29, 2300. https://doi.org/10.3390/molecules29102300
Yalkun I, Wan H, Ye L, Yu L, He Y, Li C, Wan H. Qualitative and Quantitative Analysis of Chemical Components in Yinhua Pinggan Granule with High-Performance Liquid Chromatography Coupled with Q-Exactive Mass Spectrometry. Molecules. 2024; 29(10):2300. https://doi.org/10.3390/molecules29102300
Chicago/Turabian StyleYalkun, Imranjan, Haofang Wan, Lulu Ye, Li Yu, Yu He, Chang Li, and Haitong Wan. 2024. "Qualitative and Quantitative Analysis of Chemical Components in Yinhua Pinggan Granule with High-Performance Liquid Chromatography Coupled with Q-Exactive Mass Spectrometry" Molecules 29, no. 10: 2300. https://doi.org/10.3390/molecules29102300
APA StyleYalkun, I., Wan, H., Ye, L., Yu, L., He, Y., Li, C., & Wan, H. (2024). Qualitative and Quantitative Analysis of Chemical Components in Yinhua Pinggan Granule with High-Performance Liquid Chromatography Coupled with Q-Exactive Mass Spectrometry. Molecules, 29(10), 2300. https://doi.org/10.3390/molecules29102300








