The Involvement of Ascorbic Acid in Cancer Treatment
Abstract
1. Introduction
2. Absorption, Transport, and Metabolism of VC
3. Oral VC for Cancer Prevention
4. High-Dose Intravenous Administration of VC in Cancer Therapy
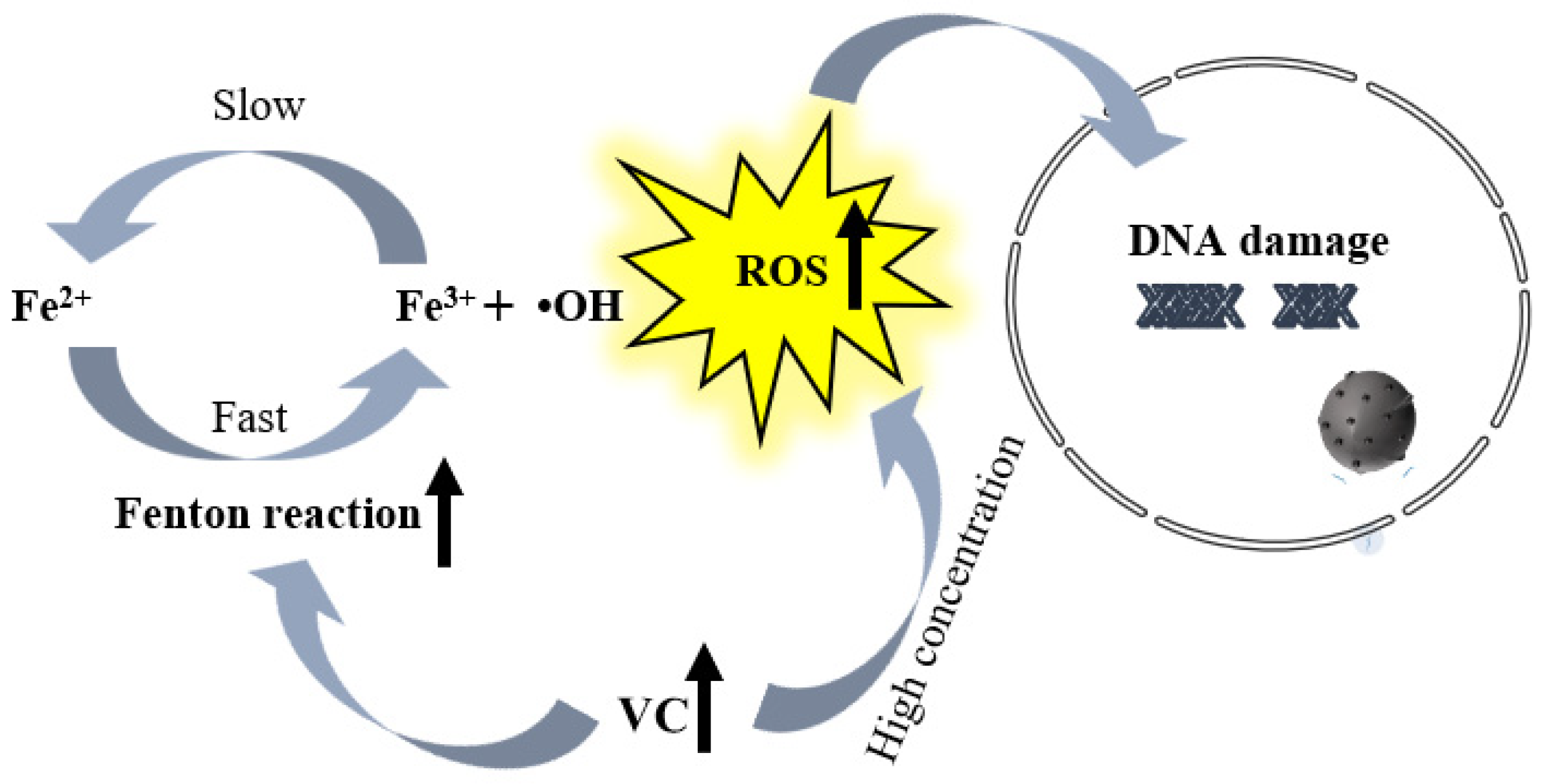
5. The Mechanism of VC on Tumors
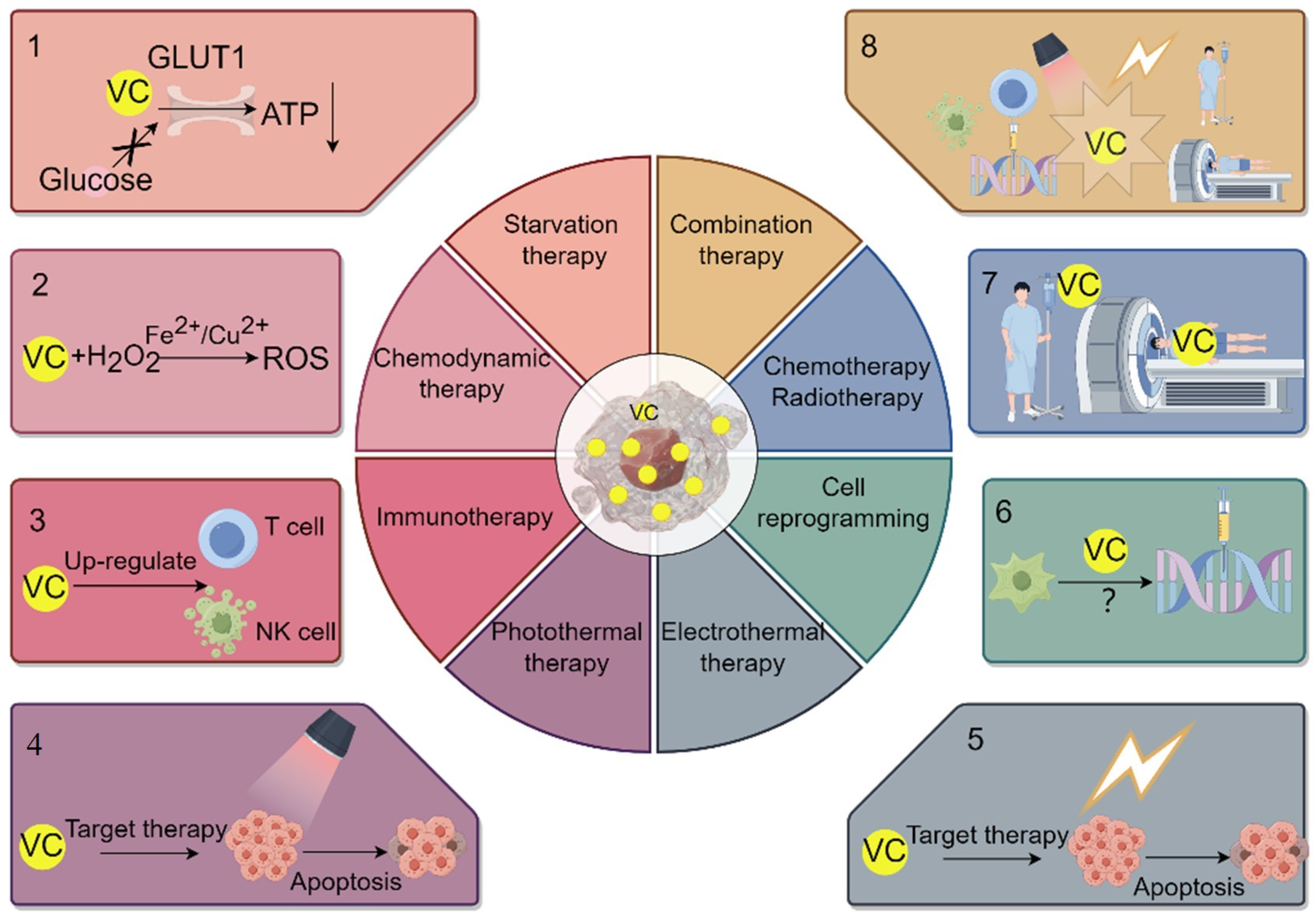
6. The Application of VC Delivery System in the Treatment of Cancer
7. VC Enhances the Efficacy of Starvation Therapy
8. The Application and Impact of VC in Chemodynamic Therapy for Cancer
9. Application and Influence of VC in Immunotherapy for Cancer
10. The Application and Impact of VC in Photothermal/Photodynamic Therapy and Electrothermal Therapy for Treating Cancer
11. The Application and Impact of VC in Cancer Cell Reprogramming Therapy
12. Application and Impact of VC on Radiotherapy/Chemotherapy and Combined Cancer Treatment
13. Clinical Prospects of VC in Cancer Treatment
14. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| VC | Vitamin C |
| CDT | chemodynamic therapy |
| PTT | photothermal therapy |
| PDT | photodynamic therapy |
| ROS | Reactive oxygen species |
References
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; De Pinto, M.C. Vitamin C in plants: From functions to biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Figueroa-Méndez, R.; Rivas-Arancibia, S. Vitamin C in health and disease: Its role in the metabolism of cells and redox state in the brain. Front. Physiol. 2015, 6, 397. [Google Scholar] [CrossRef]
- Zhitkovich, A. Nuclear and cytoplasmic functions of vitamin C. Chem. Res. Toxicol. 2020, 33, 2515–2526. [Google Scholar] [CrossRef]
- Kojo, S. Vitamin C: Basic metabolism and its function as an index of oxidative stress. Curr. Med. Chem. 2004, 11, 1041–1064. [Google Scholar] [CrossRef]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and cardiovascular disease: An update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef]
- Guo, X.; Tang, Y.; Sun, X.; Tang, K. Metabolic regulation of vitamins C and E in higher plants. Plant Physiol. J. 2011, 47, 731–744. [Google Scholar]
- Vargas, J.A.; Leonardo, D.A.; D’Muniz Pereira, H.; Lopes, A.R.; Rodriguez, H.N.; Cobos, M.; Marapara, J.L.; Castro, J.C.; Garratt, R.C. Structural characterization of l-galactose dehydrogenase: An essential enzyme for vitamin c biosynthesis. Plant Cell Physiol. 2022, 63, 1140–1155. [Google Scholar] [CrossRef] [PubMed]
- Vissers, M.C.; Das, A.B. Potential mechanisms of action for vitamin C in cancer: Reviewing the evidence. Front. Physiol. 2018, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Kaźmierczak-Barańska, J.; Boguszewska, K.; Adamus-Grabicka, A.; Karwowski, B.T. Two faces of vitamin C—Antioxidative and pro-oxidative agent. Nutrients 2020, 12, 1501. [Google Scholar] [CrossRef]
- Jenab, M.; Riboli, E.; Ferrari, P.; Sabate, J.; Slimani, N.; Norat, T.; Friesen, M.; Tjønneland, A.; Olsen, A.; Overvad, K. Plasma and dietary vitamin C levels and risk of gastric cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST). Carcinogenesis 2006, 27, 2250–2257. [Google Scholar] [CrossRef]
- Lin, X.-J.; Zou, X.-L.; Zhao, Z.-Y.; Wang, J.; Yang, Z.; Ni, X.; Wei, J. Effect of High Dose Vitamin C on Proliferation and Apoptosis of Acute Myeloid Leukemia Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2020, 28, 833–841. [Google Scholar]
- Khazaei, S.; Nilsson, L.; Adrian, G.; Tryggvadottir, H.; Konradsson, E.; Borgquist, S.; Isaksson, K.; Ceberg, C.; Jernström, H. Impact of combining vitamin C with radiation therapy in human breast cancer: Does it matter? Oncotarget 2022, 13, 439. [Google Scholar] [CrossRef]
- Blaszczak, W.; Barczak, W.; Masternak, J.; Kopczyński, P.; Zhitkovich, A.; Rubiś, B. Vitamin C as a Modulator of the Response to Cancer Therapy. Molecules 2019, 24, 453. [Google Scholar] [CrossRef]
- Munoz-Montesino, C.; Peña, E.; Roa, F.J.; Sotomayor, K.; Escobar, E.; Rivas, C.I. Transport of vitamin C in cancer. Antioxid. Redox Signal. 2021, 35, 61–74. [Google Scholar] [CrossRef]
- Uchiyama, S. Chemically amplified current response of vitamin C based on the cyclic reaction between L-ascorbic acid and dehydroascorbic acid, using dithiothreithol and ascorbate oxidase. Talanta 1992, 39, 1289–1292. [Google Scholar] [CrossRef]
- Lee, H.J.; Shin, C.; Chun, Y.S.; Kim, J.; Jung, H.; Choung, J.; Shim, S.M. Physicochemical properties and bioavailability of naturally formulated fat-soluble vitamins extracted from agricultural products for complementary use for natural vitamin supplements. Food Sci. Nutr. 2020, 8, 5660–5672. [Google Scholar] [CrossRef]
- Eggersdorfer, M. What is the optimal intake of vitamin C? Proc. Nutr. Soc. 2020, 79, E622. [Google Scholar] [CrossRef]
- Carr, A.C.; Block, G.; Lykkesfeldt, J. Estimation of Vitamin C Intake Requirements Based on Body Weight: Implications for Obesity. Nutrients 2022, 14, 1460. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.G.; Pullar, J.M.; Cook, J.; Spencer, E.S.; Vissers, M.C.; Carr, A.C.; Hampton, M.B. Peroxiredoxin 2 oxidation reveals hydrogen peroxide generation within erythrocytes during high-dose vitamin C administration. Redox Biol. 2021, 43, 101980. [Google Scholar] [CrossRef] [PubMed]
- Roa, F.J.; Peña, E.; Gatica, M.; Escobar-Acuña, K.; Saavedra, P.; Maldonado, M.; Cuevas, M.E.; Moraga-Cid, G.; Rivas, C.I.; Muñoz-Montesino, C. Therapeutic Use of Vitamin C in Cancer: Physiological Considerations. Front. Pharmacol. 2020, 11, 211. [Google Scholar] [CrossRef]
- Dell’Aglio, E.; Mhamdi, A. What Are the Roles for Dehydroascorbate Reductases and Glutathione in Sustaining Ascorbate Accumulation? Plant Physiol. 2020, 183, 11–12. [Google Scholar] [CrossRef]
- Wang, M.; He, J.; Li, S.; Cai, Q.; Zhang, K.; She, J. Structural basis of vitamin C recognition and transport by mammalian SVCT1 transporter. Nat. Commun. 2023, 14, 1361. [Google Scholar] [CrossRef] [PubMed]
- Salazar, K.; Espinoza, F.; Cerda-Gallardo, G.; Ferrada, L.; Magdalena, R.; Ramírez, E.; Ulloa, V.; Saldivia, N.; Troncoso, N.; Oviedo, M.J. SVCT2 overexpression and ascorbic acid uptake increase cortical neuron differentiation, which is dependent on vitamin c recycling between neurons and astrocytes. Antioxidants 2021, 10, 1413. [Google Scholar] [CrossRef] [PubMed]
- Tsiaoussis, G.I.; Christaki, E.; Apidianakis, Y. I Can C Clearly Now: How EPEC Inhibits Gut Vitamin C Transport by Dysregulating SVCT. Dig. Dis. Sci. 2020, 66, 2140–2142. [Google Scholar] [CrossRef] [PubMed]
- Rebane-Klemm, E.; Truu, L.; Reinsalu, L.; Puurand, M.; Shevchuk, I.; Chekulayev, V.; Timohhina, N.; Tepp, K.; Bogovskaja, J.; Afanasjev, V. Mitochondrial respiration in KRAS and BRAF mutated colorectal tumors and polyps. Cancers 2020, 12, 815. [Google Scholar] [CrossRef] [PubMed]
- Das, U.N. Vitamin C for type 2 diabetes mellitus and hypertension. Arch. Med. Res. 2019, 50, 11–14. [Google Scholar] [CrossRef]
- Hamid, M.; Mansoor, S.; Amber, S.; Zahid, S. A quantitative meta-analysis of vitamin C in the pathophysiology of Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 970263. [Google Scholar] [CrossRef]
- Hemilä, H.; Chalker, E. Bias against vitamin C in mainstream medicine: Examples from trials of vitamin C for infections. Life 2022, 12, 62. [Google Scholar] [CrossRef]
- Ganguly, S.; Chandra, A.; Chatterjee, I.B. Pathobiology of cigarette smoke-induced invasive cancer of the renal pelvis and its prevention by vitamin C. Toxicol. Rep. 2018, 5, 1002–1010. [Google Scholar] [CrossRef]
- Didier, A.J.; Stiene, J.; Fang, L.; Watkins, D.; Dworkin, L.D.; Creeden, J.F. Antioxidant and Anti-Tumor Effects of Dietary Vitamins A, C, and E. Antioxidants 2023, 12, 632. [Google Scholar] [CrossRef]
- Larsson, S.C.; Mason, A.M.; Vithayathil, M.; Carter, P.; Kar, S.; Zheng, J.-S.; Burgess, S. Circulating vitamin C and digestive system cancers: Mendelian randomization study. Clin. Nutr. 2022, 41, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Arif, M.A.; Inam-ur-Raheem, M.; Khalid, W.; Lima, C.M.G.; Jha, R.P.; Khalid, M.Z.; Santana, R.F.; Sharma, R.; Alhasaniah, A.H.; Emran, T.B. Effect of Antioxidant-Rich Moringa Leaves on Quality and Functional Properties of Strawberry Juice. Evid.-Based Complement. Altern. Med. 2022, 2022, 8563982. [Google Scholar] [CrossRef]
- Mussa, A.; Mohd Idris, R.A.; Ahmed, N.; Ahmad, S.; Murtadha, A.H.; Tengku Din, T.A.D.A.A.; Yean, C.Y.; Wan Abdul Rahman, W.F.; Mat Lazim, N.; Uskoković, V. High-dose vitamin C for cancer therapy. Pharmaceuticals 2022, 15, 711. [Google Scholar] [CrossRef]
- Wang, G.; Yin, T.; Wang, Y. In vitro and in vivo assessment of high-dose vitamin C against murine tumors. Exp. Ther. Med. 2016, 12, 3058–3062. [Google Scholar] [CrossRef]
- Hoffer, L.J.; Robitaille, L.; Zakarian, R.; Melnychuk, D.; Kavan, P.; Agulnik, J.; Cohen, V.; Small, D.; Miller Jr, W.H. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: A phase I-II clinical trial. PLoS ONE 2015, 10, e0120228. [Google Scholar] [CrossRef] [PubMed]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, physiological role, kinetics, deficiency, use, toxicity, and determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Mullarky, E.; Lu, C.; Bosch, K.N.; Kavalier, A.; Rivera, K.; Roper, J.; Chio, I.I.C.; Giannopoulou, E.G.; Rago, C. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science 2015, 350, 1391–1396. [Google Scholar] [CrossRef]
- Moccia, F.; Montagna, D. Transient Receptor Potential Ankyrin 1 (TRPA1) Channel as a Sensor of Oxidative Stress in Cancer Cells. Cells 2023, 12, 1261. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.A.; Taylor, J.A.; Chen, Q.; Woolbright, B.L.; Chen, P.; Wulff-Burchfield, E.M.; Holzbeierlein, J.; Jensen, R.A.; Drisko, J.A. IV vitamin C with chemotherapy for cisplatin ineligible bladder cancer patients (CI-MIBC) first-stage analysis NCT04046094. J. Clin. Oncol. 2022, 40, e16540. [Google Scholar] [CrossRef]
- Nibbe, P.; Schleusener, J.; Siebert, S.; Borgart, R.; Brandt, D.; Westphalen, R.; Schüler, N.; Berger, B.; Peters, E.M.; Meinke, M.C. Oxidative stress coping capacity (OSC) value: Development and validation of an in vitro measurement method for blood plasma using electron paramagnetic resonance spectroscopy (EPR) and vitamin C. Free Radic. Biol. Med. 2023, 194, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.; Feng, X.; Wu, X.; Tan, X.; Liu, Z.; Li, L.; Huang, Y.; Teng, F.; Li, Y. Encapsulating vitamins C and E using food-grade soy protein isolate and pectin particles as carrier: Insights on the vitamin additive antioxidant effects. Food Chem. 2023, 418, 135955. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, M.; Karimi, T.; Faraoni, I.; Graziani, G. High-dose vitamin C: Preclinical evidence for tailoring treatment in cancer patients. Cancers 2021, 13, 1428. [Google Scholar] [CrossRef]
- Yue, X.; Rao, A. TET family dioxygenases and the TET activator vitamin C in immune responses and cancer. Blood J. Am. Soc. Hematol. 2020, 136, 1394–1401. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xu, S.; Fu, B.; Tang, W.; Zaky, M.Y.; Tian, R.; Yao, R.; Zhang, S.; Zhao, Q.; Nian, W. Vitamin C-induced competitive binding of HIF-1α and p53 to ubiquitin E3 ligase CBL contributes to anti-breast cancer progression through p53 deacetylation. Food Chem. Toxicol. 2022, 168, 113321. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Afolabi, H.A.; Syed, N.H.; Talib, M.; Murtadha, A.H.; Hajissa, K.; Mokhtar, N.F.; Mohamud, R.; Hassan, R. The NF-κB Transcriptional Network Is a High-Dose Vitamin C-Targetable Vulnerability in Breast Cancer. Biomedicines 2023, 11, 1060. [Google Scholar] [CrossRef]
- Das, S.; Reddy, R.C.; Chadchan, K.S.; Patil, A.J.; Biradar, M.S.; Das, K.K. Nickel and oxidative stress: Cell signaling mechanisms and protective role of vitamin C. Endocr. Metab. Immune Disord.-Drug Targets (Former.Curr. Drug Targets-Immune Endocr. Metab. Disord.) 2020, 20, 1024–1031. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, J.; Zhang, Y.; Wang, L.; Yu, M.; Wang, F. Vitamin C decreases VEGF expression levels via hypoxia-inducible factor-1α dependent and independent pathways in lens epithelial cells. Mol. Med. Rep. 2020, 22, 436–444. [Google Scholar] [CrossRef]
- Li, W.-N.; Zhang, S.-J.; Feng, J.-Q.; Jin, W.-L. Repurposing vitamin C for cancer treatment: Focus on targeting the tumor microenvironment. Cancers 2022, 14, 2608. [Google Scholar] [CrossRef] [PubMed]
- Khuntia, A.; Daharwal, N.; Mitra, J. Complex Coacervated Vitamin C Microparticles Embedded Biodegradable Packaging: A Potential Bioactive Film for Storing Perishable Foods. J. Biosyst. Eng. 2022, 47, 286–301. [Google Scholar] [CrossRef]
- Phan, A.D.T.; Adiamo, O.; Akter, S.; Netzel, M.E.; Cozzolino, D.; Sultanbawa, Y. Effects of drying methods and maltodextrin on vitamin C and quality of Terminalia ferdinandiana fruit powder, an emerging Australian functional food ingredient. J. Sci. Food Agric. 2021, 101, 5132–5141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, L.; Zhu, D.; Wu, Y.; Qin, Y.; Ou, W.; Song, L.; Zhang, Q. Influence of composition on the encapsulation properties of P/O/W multiple emulsions for Vitamin C. J. Dispers. Sci. Technol. 2018, 40, 1637–1644. [Google Scholar] [CrossRef]
- Yan, B.; Davachi, S.M.; Ravanfar, R.; Dadmohammadi, Y.; Deisenroth, T.W.; Van Pho, T.; Odorisio, P.A.; Darji, R.H.; Abbaspourrad, A. Improvement of vitamin C stability in vitamin gummies by encapsulation in casein gel. Food Hydrocoll. 2021, 113, 106414. [Google Scholar] [CrossRef]
- Khuntia, A.; Kumar, R.; Premjit, Y.; Mitra, J. Release behavior of vitamin C nanoliposomes from starch–vitamin C active packaging films. J. Food Process Eng. 2022, 45, e14075. [Google Scholar] [CrossRef]
- Ugolotti, A.; Dolce, M.; Di Valentin, C. Vitamin C Affinity to TiO2 Nanotubes: A Computational Study by Hybrid Density Functional Theory Calculations. Nanomaterials 2024, 14, 261. [Google Scholar] [CrossRef]
- Tehrani, E.; Amiri, S. Synthesis and characterization PVA electro-spun nanofibers containing encapsulated vitamin C in chitosan microspheres. J. Text. Inst. 2022, 113, 212–223. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, Y.; Chen, Y.; Yang, Z.; Zhang, L.; Xiao, W.; Yang, J.; Guo, L.; Wu, Y. Dual-targeting for brain-specific liposomes drug delivery system: Synthesis and preliminary evaluation. Bioorg. Med. Chem. 2018, 26, 4677–4686. [Google Scholar] [CrossRef]
- Ghanbari-Movahed, M.; Shiri Varnamkhasti, B.; Shourian, M. Inhibiting Notch activity in breast cancer stem cells by functionalized gold nanoparticles with gamma-secretase inhibitor DAPT and vitamin C. Chem. Pap. 2022, 76, 1157–1170. [Google Scholar] [CrossRef]
- Icten, O.; Hosmane, N.S.; Kose, D.A.; Zumreoglu-Karan, B. Magnetic nanocomposites of boron and vitamin C. New J. Chem. 2017, 41, 3646–3652. [Google Scholar] [CrossRef]
- Korany, M.; Mahmoud, B.; Ayoub, S.M.; Sakr, T.M.; Ahmed, S.A. Synthesis and radiolabeling of vitamin C-stabilized selenium nanoparticles as a promising approach in diagnosis of solid tumors. J. Radioanal. Nucl. Chem. 2020, 325, 237–244. [Google Scholar] [CrossRef]
- Sayman, H.B.; Toplutas, K.N.; Tunick, J.; Aras, O. 99mTc-Vitamin C SPECT/CT imaging in SARS-CoV-2 associated pneumonia. Nucl. Med. Rev. 2022, 25, 127–128. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, S.; Ueda, K.; Jayanchandran, G.; Xu, K.; Minna, J.D.; Roth, J.A.; Ji, L. Synergistic and selective inhibition of NSCLC cell growth via a caspase-independent cell death pathway by tumor suppressor 101F6 nanoparticles plus vitamin C in vitro and in vivo. J. Clin. Oncol. 2006, 24, 13086. [Google Scholar] [CrossRef]
- Talib, W.H.; Jum’AH, D.A.A.; Attallah, Z.S.; Jallad, M.S.; Al Kury, L.T.; Hadi, R.W.; Mahmod, A.I. Role of vitamins A, C, D, E in cancer prevention and therapy: Therapeutic potentials and mechanisms of action. Front. Nutr. 2023, 10, 1281879. [Google Scholar] [CrossRef] [PubMed]
- Di Tano, M.; Raucci, F.; Vernieri, C.; Caffa, I.; Buono, R.; Fanti, M.; Brandhorst, S.; Curigliano, G.; Nencioni, A.; de Braud, F. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat. Commun. 2020, 11, 2332. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Yu, D.; Hou, G.; Wu, J.; Li, F. CircRBM33 downregulation inhibits hypoxia-induced glycolysis and promotes apoptosis of breast cancer cells via a microRNA-542-3p/HIF-1α axis. Cell Death Discov. 2022, 8, 126. [Google Scholar] [CrossRef]
- Pujalte-Martin, M.; Belaïd, A.; Bost, S.; Kahi, M.; Peraldi, P.; Rouleau, M.; Mazure, N.M.; Bost, F. Targeting cancer and immune cell metabolism with the complex I inhibitors metformin and IACS-010759. Mol. Oncol. 2024. early view. [Google Scholar] [CrossRef]
- Xuan, W.; Xia, Y.; Li, T.; Wang, L.; Liu, Y.; Tan, W. Molecular self-assembly of bioorthogonal aptamer-prodrug conjugate micelles for hydrogen peroxide and pH-independent cancer chemodynamic therapy. J. Am. Chem. Soc. 2019, 142, 937–944. [Google Scholar] [CrossRef]
- Pal, S.; Jana, N.R. Enhanced therapeutic applications of vitamin c via nanotechnology-based pro-oxidant properties: A review. ACS Appl. Nano Mater. 2022, 5, 4583–4596. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y.; Cheng, B. A tumor microenvironment-responsive microneedle patch for chemodynamic therapy of oral squamous cell carcinoma. Nanoscale Adv. 2023, 5, 6162–6169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Z.; Wang, S.; Ma, Q.; Li, L.; Wu, X.; Guo, Q.; Tao, L.; Shen, X. Boosting ROS-Mediated Lysosomal Membrane Permeabilization for Cancer Ferroptosis Therapy. Adv. Healthc. Mater. 2023, 12, 2202150. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.E.; Ahmed, S.A.; Amer, S.A.; Al-Gabri, N.A.; Ahmed, A.I.; Abdel-Warith, A.-W.A.; Younis, E.-S.M.; Metwally, A.E. Influence of vitamin C feed supplementation on the growth, antioxidant activity, immune status, tissue histomorphology, and disease resistance in Nile tilapia, Oreochromis niloticus. Aquac. Rep. 2020, 18, 100545. [Google Scholar] [CrossRef]
- Magrì, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chilà, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707. [Google Scholar] [CrossRef]
- Wang, A.; Yang, M.; Liang, R.; Zhu, F.; Zhu, F.; Liu, X.; Han, Y.; Lin, R.; Wang, X.; Li, D. Mouse double minute 2 homolog-mediated ubiquitination facilitates forkhead box P3 stability and positively modulates human regulatory T cell function. Front. Immunol. 2020, 11, 1087. [Google Scholar] [CrossRef] [PubMed]
- Léonce, K.; Xu, Y.; Peters, C.; Junyi, H.; Yangzhe, W.; Zhinan, Y.; Dieter, K. Vitamin C promotes the proliferation and effector functions of human γδ T cells. Cell. Mol. Immunol. 2020, 17, 462–473. [Google Scholar]
- Morante-Palacios, O.; Godoy-Tena, G.; Calafell-Segura, J.; Ciudad, L.; Martínez-Cáceres, E.M.; Sardina, J.L.; Ballestar, E. Vitamin C enhances NF-κB-driven epigenomic reprogramming and boosts the immunogenic properties of dendritic cells. Nucleic Acids Res. 2022, 50, 10981–10994. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Almonte-Loya, A.; Lay, F.-Y.; Hsu, M.; Johnson, E.; González-Avalos, E.; Yin, J.; Bruno, R.S.; Ma, Q.; Ghoneim, H.E. Epigenetic remodeling by vitamin C potentiates plasma cell differentiation. eLife 2022, 11, e73754. [Google Scholar] [CrossRef]
- Luchtel, R.A.; Bhagat, T.; Pradhan, K.; Jacobs Jr, W.R.; Levine, M.; Verma, A.; Shenoy, N. High-dose ascorbic acid synergizes with anti-PD1 in a lymphoma mouse model. Proc. Natl. Acad. Sci. USA 2020, 117, 1666–1677. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.-H.; Hua, X.; Hong, H.-Q.; Shi, W.; Liu, K.-X.; Liu, Z.-X.; Huang, P. Genetically predicted vitamin C levels significantly affect patient survival and immunotypes in multiple cancer types. Front. Immunol. 2023, 14, 1177580. [Google Scholar] [CrossRef]
- Nomoto, T.; Komoto, K.; Nagano, T.; Ishii, T.; Guo, H.; Honda, Y.; Ogura, S.i.; Ishizuka, M.; Nishiyama, N. Polymeric iron chelators for enhancing 5-aminolevulinic acid-induced photodynamic therapy. Cancer Sci. 2023, 114, 1086–1094. [Google Scholar] [CrossRef]
- Ortega-Liebana, M.C.; Encabo-Berzosa, M.M.; Ruedas-Rama, M.J.; Hueso, J.L. Nitrogen-Induced Transformation of Vitamin C into Multifunctional Up-converting Carbon Nanodots in the Visible–NIR Range. Chem. Eur. J. 2017, 23, 3067–3073. [Google Scholar] [CrossRef]
- Yang, D.-P.; Liu, X.; Teng, C.P.; Owh, C.; Win, K.Y.; Lin, M.; Loh, X.J.; Wu, Y.-L.; Li, Z.; Ye, E. Unexpected formation of gold nanoflowers by a green synthesis method as agents for a safe and effective photothermal therapy. Nanoscale 2017, 9, 15753–15759. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.V.; Hoang, G.; Nguyen, T.P.; Kim, H.H.; Mondal, S.; Manivasagan, P.; Moorthy, M.S.; Lee, K.D.; Junghwan, O. Chitosan as a stabilizer and size-control agent for synthesis of porous flower-shaped palladium nanoparticles and their applications on photo-based therapies. Carbohydr. Polym. 2019, 205, 340–352. [Google Scholar] [CrossRef]
- Forika, G.; Balogh, A.; Vancsik, T.; Zalatnai, A.; Petovari, G.; Benyo, Z.; Krenacs, T. Modulated electro-hyperthermia resolves radioresistance of Panc1 pancreas adenocarcinoma and promotes DNA damage and apoptosis in vitro. Int. J. Mol. Sci. 2020, 21, 5100. [Google Scholar] [CrossRef]
- Ou, J.; Zhu, X.; Chen, P.; Du, Y.; Lu, Y.; Peng, X.; Bao, S.; Wang, J.; Zhang, X.; Zhang, T. A randomized phase II trial of best supportive care with or without hyperthermia and vitamin C for heavily pretreated, advanced, refractory non-small-cell lung cancer. J. Adv. Res. 2020, 24, 175–182. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Y.; Deng, H. Powering reprogramming with vitamin C. Cell Stem Cell 2010, 6, 1–2. [Google Scholar] [CrossRef][Green Version]
- Ramezankhani, B.; Taha, M.F.; Javeri, A. Vitamin C counteracts miR-302/367-induced reprogramming of human breast cancer cells and restores their invasive and proliferative capacity. J. Cell. Physiol. 2019, 234, 2672–2682. [Google Scholar] [CrossRef]
- Tobias, I.C.; Kao, M.-M.C.; Parmentier, T.; Hunter, H.; LaMarre, J.; Betts, D.H. Targeted expression profiling reveals distinct stages of early canine fibroblast reprogramming are regulated by 2-oxoglutarate hydroxylases. Stem Cell Res. Ther. 2020, 11, 528. [Google Scholar] [CrossRef]
- Abiri, B.; Vafa, M. Vitamin C and cancer: The role of vitamin C in disease progression and quality of life in cancer patients. Nutr. Cancer 2021, 73, 1282–1292. [Google Scholar] [CrossRef]
- Lee, S.J.; Jeong, J.-H.; Lee, I.H.; Lee, J.; Jung, J.H.; Park, H.Y.; Lee, D.H.; Chae, Y.S. Effect of high-dose vitamin C combined with anti-cancer treatment on breast cancer cells. Anticancer Res. 2019, 39, 751–758. [Google Scholar] [CrossRef]
- Ghavami, G.; Sardari, S. Synergistic effect of vitamin C with Cisplatin for inhibiting proliferation of gastric Cancer cells. Iran. Biomed. J. 2020, 24, 119. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Wang, T.; Li, Q.; Francis, P.S.; Barrow, C.J.; Duan, W.; Yang, W. Switching off the interactions between graphene oxide and doxorubicin using vitamin C: Combining simplicity and efficiency in drug delivery. J. Mater. Chem. B 2018, 6, 1251–1259. [Google Scholar] [CrossRef]
- El Sadda, R.R.; Elshahawy, Z.R.; Saad, E.A. Biochemical and pathophysiological improvements in rats with thioacetamide induced-hepatocellular carcinoma using aspirin plus vitamin C. BMC Cancer 2023, 23, 175. [Google Scholar] [CrossRef]
- Ibrahim, S.S.A.; El-Aal, S.A.A.; Reda, A.M.; Achy, S.E.; Shahine, Y. Anti-neoplastic action of Cimetidine/Vitamin C on histamine and the PI3K/AKT/mTOR pathway in Ehrlich breast cancer. Sci. Rep. 2022, 12, 11514. [Google Scholar] [CrossRef]
- Mozdarani, H.; Ghoraeian, P. Modulation of gamma-ray-induced apoptosis in human peripheral blood leukocytes by famotidine and vitamin C. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2008, 649, 71–78. [Google Scholar] [CrossRef]
- Prisyanto, R.; Santoso, D.R.; Juswono, U.P.; Cahyati, Y. The Combination Effect of Vitamin C and E on the Number of Hemoglobin, Leukocyte and Platelet Post-Irradiation of Gamma Ray. Nat. B J. Health Environ. Sci. 2014, 2, 289–295. [Google Scholar] [CrossRef]
- Konopacka, M.; Widel, M.; Rzeszowska-Wolny, J. Modifying effect of vitamins C, E and beta-carotene against gamma-ray-induced DNA damage in mouse cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 1998, 417, 85–94. [Google Scholar] [CrossRef]
- Taper, H.S.; Keyeux, A.; Roberfroid, M. Potentiation of radiotherapy by nontoxic pretreatment with combined vitamins C and K3 in mice bearing solid transplantable tumor. Anticancer Res. 1996, 16, 499–503. [Google Scholar]
- Zhang, F.; Cheng, K.; Huang, Z.Y.; Hou, X.L.; Zhang, X.S.; Zhong, Z.T.; Hu, Y.G.; Lei, X.L.; Li, Y.; Zhang, P.J. Tumor Microenvironment-Responsive Nanocarrier Based on VOx Nanozyme Amplify Oxidative Stress for Tumor Therapy. Adv. Funct. Mater. 2023, 33, 2212740. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Wang, H.; Wang, T.; Pan, H.; Ji, W.; Chang, J. A synergistic cancer immunotherapy nano-system for preventing tumor growth. Chem. Eng. J. 2020, 380, 122472. [Google Scholar] [CrossRef]
- Wang, X.; Lee, J.; Xie, C. Autophagy Regulation on Cancer Stem Cell Maintenance, Metastasis, and Therapy Resistance. Cancers 2022, 14, 381. [Google Scholar] [CrossRef]
- Satheesh, N.J.; Samuel, S.M.; Büsselberg, D. Combination therapy with vitamin C could eradicate cancer stem cells. Biomolecules 2020, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wu, R.; Long, Y.; Peng, L.; Yang, T.; Zhang, B.; Shi, X.; Liu, J.; Zhang, X. Role of Fe, Transferrin and Transferrin Receptor in Anti-Tumor Effect of Vitamin C. Cancers 2022, 14, 4507. [Google Scholar] [CrossRef] [PubMed]
- Campbell, E.J.; Vissers, M.C.; Wohlrab, C.; Hicks, K.O.; Strother, R.M.; Bozonet, S.M.; Robinson, B.A.; Dachs, G.U. Pharmacokinetic and anti-cancer properties of high dose ascorbate in solid tumours of ascorbate-dependent mice. Free Radic. Biol. Med. 2016, 99, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.H.; Mok, K.L. High dose vitamin C induced methemoglobinemia and hemolytic anemia in glucose-6-phosphate dehydrogenase deficiency. Am. J. Emerg. Med. 2020, 38, 2488.e3–2488.e5. [Google Scholar] [CrossRef]
- Wang, F.; He, M.-M.; Wang, Z.-X.; Li, S.; Jin, Y.; Ren, C.; Shi, S.-M.; Bi, B.-T.; Chen, S.-Z.; Lv, Z.-D. Phase I study of high-dose ascorbic acid with mFOLFOX6 or FOLFIRI in patients with metastatic colorectal cancer or gastric cancer. BMC Cancer 2019, 19, 460. [Google Scholar] [CrossRef]

| Category | Methods | Format | Function |
|---|---|---|---|
| Enveloping | Composite and cohesion | Different concentrations of gelatin and pectin are used as wall materials to form complex coacervates with different concentrations of VC. | The concentration of the wall material will affect the loading and release of VC. |
| Spray drying | Maltodextrin serves as the carrier material for loading VC. | Enhancing the stability and encapsulation efficiency of fruit juice powder. | |
| Emulsification | P/O/W and W/O/W | The P/O/W system demonstrates superior encapsulation stability. | |
| Microcapsule granulation | VC capsules were prepared in casein gel | This capsule shows significant effectiveness in enhancing the stability of vitamins and delaying degradation. | |
| Nanoliposomes | Preparation of nanoliposomes containing VC. | It demonstrates a high encapsulation efficiency (94.18%) and storage stability. | |
| Control of Carrier Structure and Properties | Modification of Titanium Dioxide Nanotube Carriers | Altering the geometric factors of titanium dioxide. | Enhancing the adsorption capacity of VC. |
| Surface Modification or Functionalization | Crosslinking Functionalization | Cross-linked preparation of VC-encapsulated chitosan microspheres. | To achieve control over the release of VC. |
| Functionalized Targeting Capability | Synthesis of brain-targeted glucose- VC derivatives | To achieve control over the release of VC. | |
| Functionalization of Au-AA-DAPT NPs. | VC enhances the targeting ability of Au-AA-DAPT NPs towards CSC and suppresses Notch activity in breast cancer stem cells. | ||
| Synthesis of magnetic nanocomposite materials containing single or double (ascorbic acid ester) complexes of boron and VC. | Used for targeted delivery and therapeutic applications. | ||
| Contrast medium | 99m Tc-VC | 99m Tc-VC emerges as a potential radiopharmaceutical with high radiolabeling efficiency for solid tumor imaging using SPECT/CT. | |
| Other | Gene regulation | The binding of exogenous VC with nanoparticle-mediated wt-101F6 gene transfer. | Facilitating intracellular uptake of VC. |
| Category | Method | Function | Object |
|---|---|---|---|
| Starvation therapy | Inhibition of glycolysis | Excessive amounts of VC and mutations (KRAS or BRAF) activate the downstream MAPK pathway, restricting glucose transport and ATP production, leading to energy crisis and cell death. | Gastric cancers and carcinoma of colon |
| VC inhibits the activation of HIF, thereby suppressing glycolysis, angiogenesis, etc., leading to energy crisis and cell death. | Breast cancer | ||
| High doses of VC can impair glycolysis. When combined with the anti-diabetic drug metformin, it can alleviate tumor burden by inhibiting mitochondrial complex I. | Acute myelocytic leukemia and other solid tumors | ||
| Chemodynamic therapy | Enhancement of Fenton Reaction | Loading VC and iron ions onto nano-microneedle patches, utilizing intracellular high ferric oxide (Fe3+) and VC to undergo Fenton reaction, leading to death and apoptosis of oral squamous cell carcinoma cells. | Oral squamous cell carcinoma |
| VC@N3AMcLAVs effectively enhances the generation of ROS, efficiently converting generated H2O2 into highly toxic •OH, initiating irreversible cell death of tumor cells through the iron death pathway. | Mouse colon cancer | ||
| immunotherapy | Reducing the risk of infection | Supplementing VC can elevate immunoglobulin levels, enhance lysozyme activity, thereby reducing the risk of infection. | Oreochromis niloticus L. |
| Modulating immune cells | VC can regulate various immune cell functions, thereby strengthening the anticancer capabilities of the immune system. | breast, colorectal, melanoma and pancreatic tumor model | |
| Transcriptional regulation factor expression | VC increases the stability of human forkhead box protein Foxp3 expression, regulates TET activity and Treg cell function, thereby enhancing the anticancer capabilities of immune cells. | Human Treg cells | |
| Influencing relevant metabolism | VC can enhance the metabolic vitality of γδ T cells, increase the proportion of cells in the G2/M phase, and strengthen glycolysis and oxidative respiration. | γδ T cells | |
| Influencing relevant gene expression | Pre-treatment with VC leads to significant demethylation of NF-κB/p65 binding sites, enhancing the binding of STAT3 at the Prdm1 promoter and downstream enhancers, thereby promoting plasma cell differentiation and enhancing the immune cell’s efficacy against tumors. | Dendritic cells | |
| Collaborative immune checkpoint therapy | High-dose VC can synergize with immune checkpoint therapies, such as anti-PD1 and anti-CTLA4, and act on immune checkpoint inhibitors to enhance treatment response to a variety of cancers. | Lymphoma mouse model/mammary gland/large intestine/melanoma and pancreatic mice | |
| Gene prediction of VC intake | Individually adjusting VC intake through personalized gene prediction enhances immune activity and improves the survival rate of cancer patients. | Breast cancer, head and neck squamous cell carcinoma, renal clear cell carcinoma and rectal adenocarcinoma | |
| photothermal/photodynamic therapy | Combined photosensitizer | VC and iron chelators (photosensitizers) enhance the generation of reactive oxygen species and the photothermal therapy to kill tumor cells. Iron chelators also enhance the efficacy of photodynamic therapy. | BALB/c mice model of mouse colon cancer and human gastric cancer cells |
| Preparation of VC Nanocomposites | By rational carbonization of VC, water-soluble, biocompatible, and photo-luminescent carbon nanodots were obtained, making them suitable for photodynamic therapy treatment. | Human astroglioma cells | |
| Starfruit juice rich in VC and polyphenol antioxidants was utilized to prepare gold nanoflowers, exhibiting robust absorption in the near-infrared region, suitable for photothermal therapy. | Mouse model of human breast cancer | ||
| Chitosan and VC were used for the green synthesis of porous flower-shaped palladium nanoparticles, exhibiting high efficiency in photothermal therapy and photoacoustic imaging. | Mouse model of Breast cancer | ||
| electrothermal therapy | Modulated Electrohyperthermia (mEHT) | Combining intravenous administration of VC with mEHT improved the quality of life for non-small cell lung cancer patients, extending both progression-free survival and overall survival. | Non-small cell lung cancer patients |
| cellular reprogramming | Reprogramming of mouse and human fibroblasts | In both mouse and human fibroblasts, the introduction of Oct4/Klf4/Sox2 or Oct4/Klf4/Sox2/cMyc transduction, combined with VC supplementation, significantly enhanced the reprogramming efficiency of both mouse and human fibroblasts. | Mouse and human fibroblasts |
| Reprogramming of embryonic stem cell epigenetic regulation | VC counteracted the reprogramming of human breast cancer cells induced by miR-302/367, restoring their invasive and proliferative capabilities. | Human breast cancer cells | |
| The influence of TET hydroxylase on somatic cell reprogramming | The association between TET hydroxylases and VC influences the reprogramming of somatic cells. The deficiency of TET hydroxylases may enhance the reprogramming efficiency. | somatic cell | |
| chemotherapy | Alleviating chemotherapy side effects | VC can enhance patients’ quality of life and alleviate side effects caused by chemotherapy through its antioxidative effects. | Pancreatic cancer/Cervical neoplasia/Renal cell carcinoma/Esophageal cancer/Prostate cancer patients |
| Combining VC with Chemotherapy Drugs | The combined use of high-dose VC with certain anticancer drugs can more comprehensively reduce the viability of cancer cells compared to using VC or chemotherapy alone. | Beast cancer cells and gastric Cancer cells | |
| Influence of VC and Chemotherapy Drugs on Tumor Microenvironment | The combination therapy of VC and cimetidine can inhibit the production of mast cell mediators (histamine, VEGF, and TNF-α), reduce the levels of the VEGF as a marker of angiogenesis, and restore oxidative stress and inflammatory status to achieve tumor growth inhibition. | Mouse model of breast cancer | |
| radiotherapy | Low-dose VC | Low-dose (10 μg/mL) VC exhibits protective effects against various doses of radiation, combating radiation-induced cell apoptosis. | Human peripheral blood leukocytes |
| VC and VE | The intake of VC and VE was found to reduce the levels of hemoglobin, leukocytes, and platelet decline caused by exposure to gamma rays. | Blood cells and hemoglobin | |
| High concentration of VC | High concentrations of VC can enhance the therapeutic effects of radiation in mice (400 mg/kg/day). | Mouse of erythrocytes and leukocytes | |
| VC and K3 | Pre-treating mice with VC and K3 was found to enhance the effectiveness of radiation therapy in mice with transplantable solid tumors. | Mouse with solid transplantable tumors | |
| combination therapies | Chemodynamic therapy/Photodynamic effect | VOx nanoparticles generate highly toxic hydroxyl radicals ∙OH through Fenton-like reactions and the formation of 1O2. The photodynamic effect of Ce6 can also produce more 1O2. | Mouse breast cancer cell mouse model |
| Photodynamic effect/Immunotherapy | MSN-ICG-YM155 as a prodrug exposes tumor antigens for cancer immunotherapy. MNP@nSiO2-anti-CD47 as a follow-up drug, in synergy with the prodrug, demonstrated potent anti-tumor immune effects on distant tumors. | Mouse skin melanoma cell model | |
| Others | Targeted effect of epigenetic modification by VC | VC affects the ability of epigenetic modification, and when administered with chemotherapy drugs, intravenous VC at pharmacological doses can selectively kill tumor cells and target CSCs. | Cancer Stem Cells |
| Individualized screening for VC-sensitive tumor types | 68Ga-citrate PET imaging technology evaluates the expression levels of tumor TF/TFR, thus selecting tumor types more sensitive to VC for personalized therapy. | Mouse model of human prostate cancer |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, D.; Liao, Y.; Na, J.; Wu, L.; Yin, Y.; Mi, Z.; Fang, S.; Liu, X.; Huang, Y. The Involvement of Ascorbic Acid in Cancer Treatment. Molecules 2024, 29, 2295. https://doi.org/10.3390/molecules29102295
Guo D, Liao Y, Na J, Wu L, Yin Y, Mi Z, Fang S, Liu X, Huang Y. The Involvement of Ascorbic Acid in Cancer Treatment. Molecules. 2024; 29(10):2295. https://doi.org/10.3390/molecules29102295
Chicago/Turabian StyleGuo, Di, Yuan Liao, Jintong Na, Liangliang Wu, Yao Yin, Zhengcheng Mi, Shixu Fang, Xiyu Liu, and Yong Huang. 2024. "The Involvement of Ascorbic Acid in Cancer Treatment" Molecules 29, no. 10: 2295. https://doi.org/10.3390/molecules29102295
APA StyleGuo, D., Liao, Y., Na, J., Wu, L., Yin, Y., Mi, Z., Fang, S., Liu, X., & Huang, Y. (2024). The Involvement of Ascorbic Acid in Cancer Treatment. Molecules, 29(10), 2295. https://doi.org/10.3390/molecules29102295





