Photocatalytic Degradation of Quinolones by Magnetic MOFs Materials and Mechanism Study
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisations
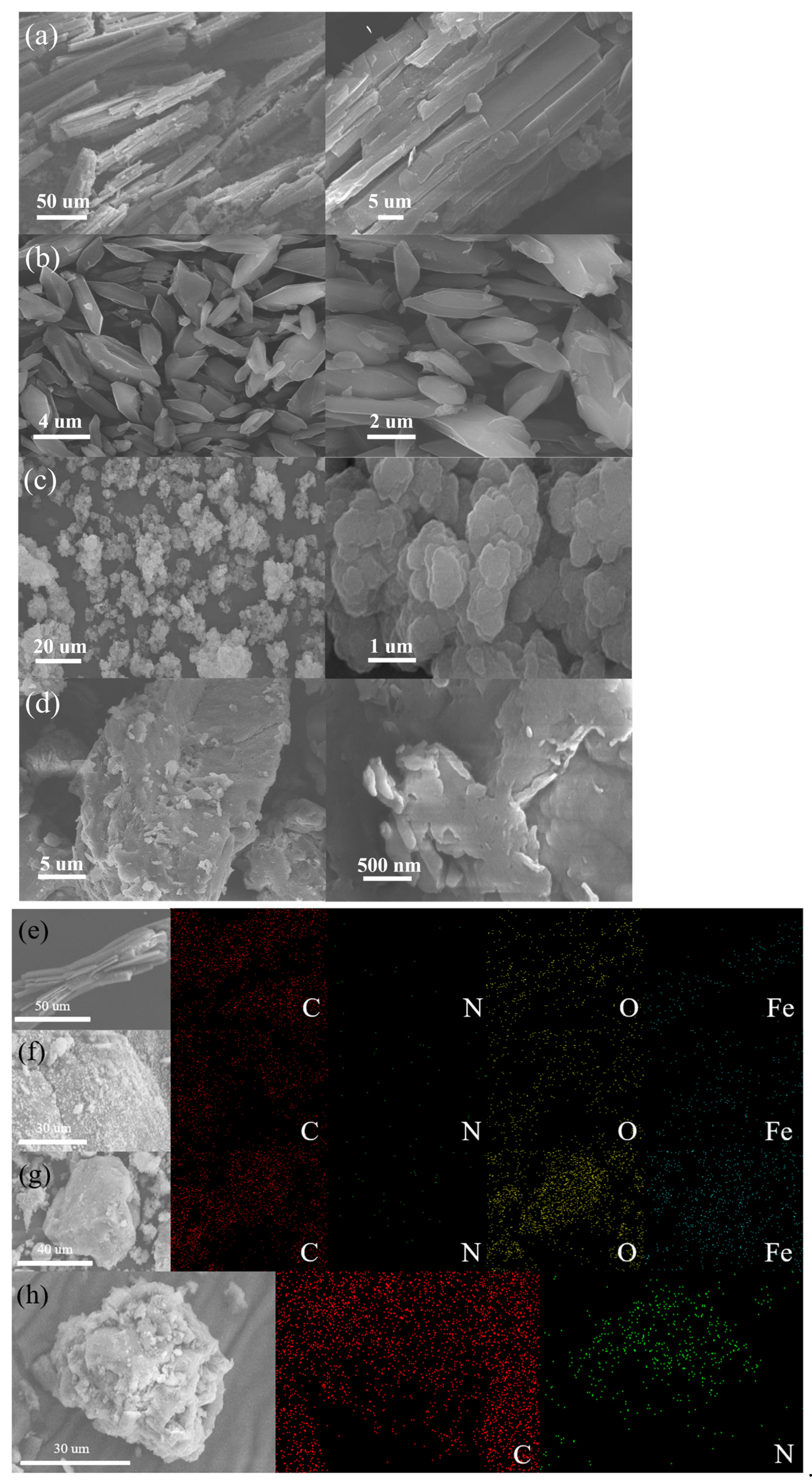
2.1.1. SEM Analysis
2.1.2. XRD Analysis
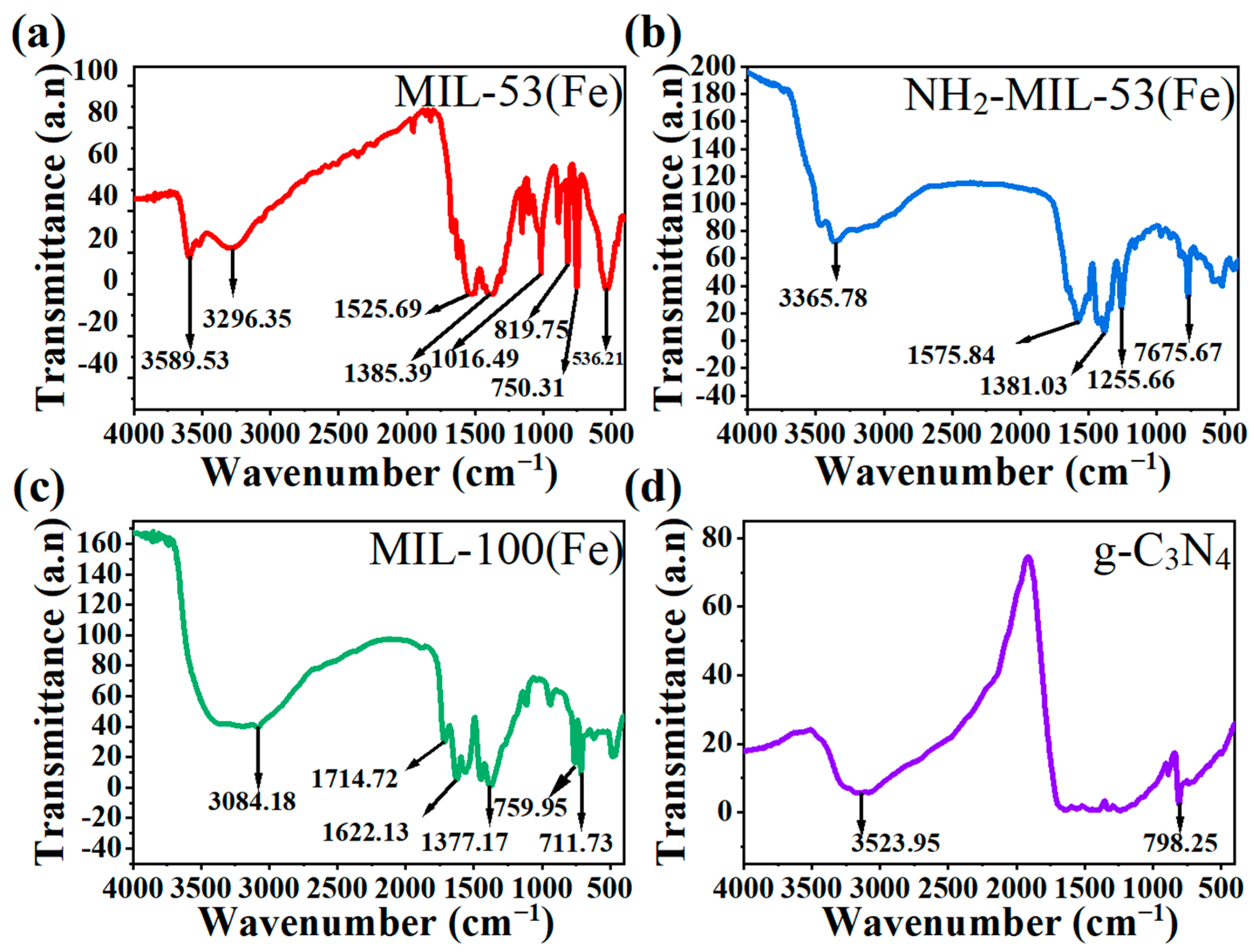
2.1.3. FT-IR Analysis
2.1.4. XPS Analysis
2.1.5. BET Analysis
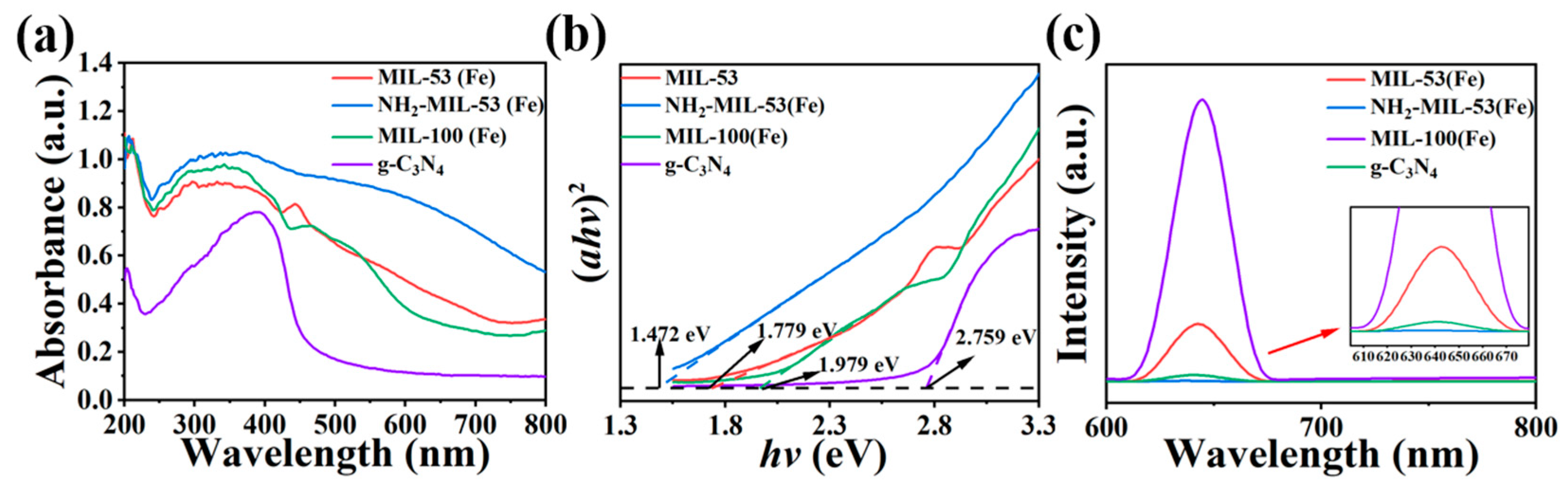
2.2. Optical Characteristic Analysis
2.3. Electrochemical Analysis
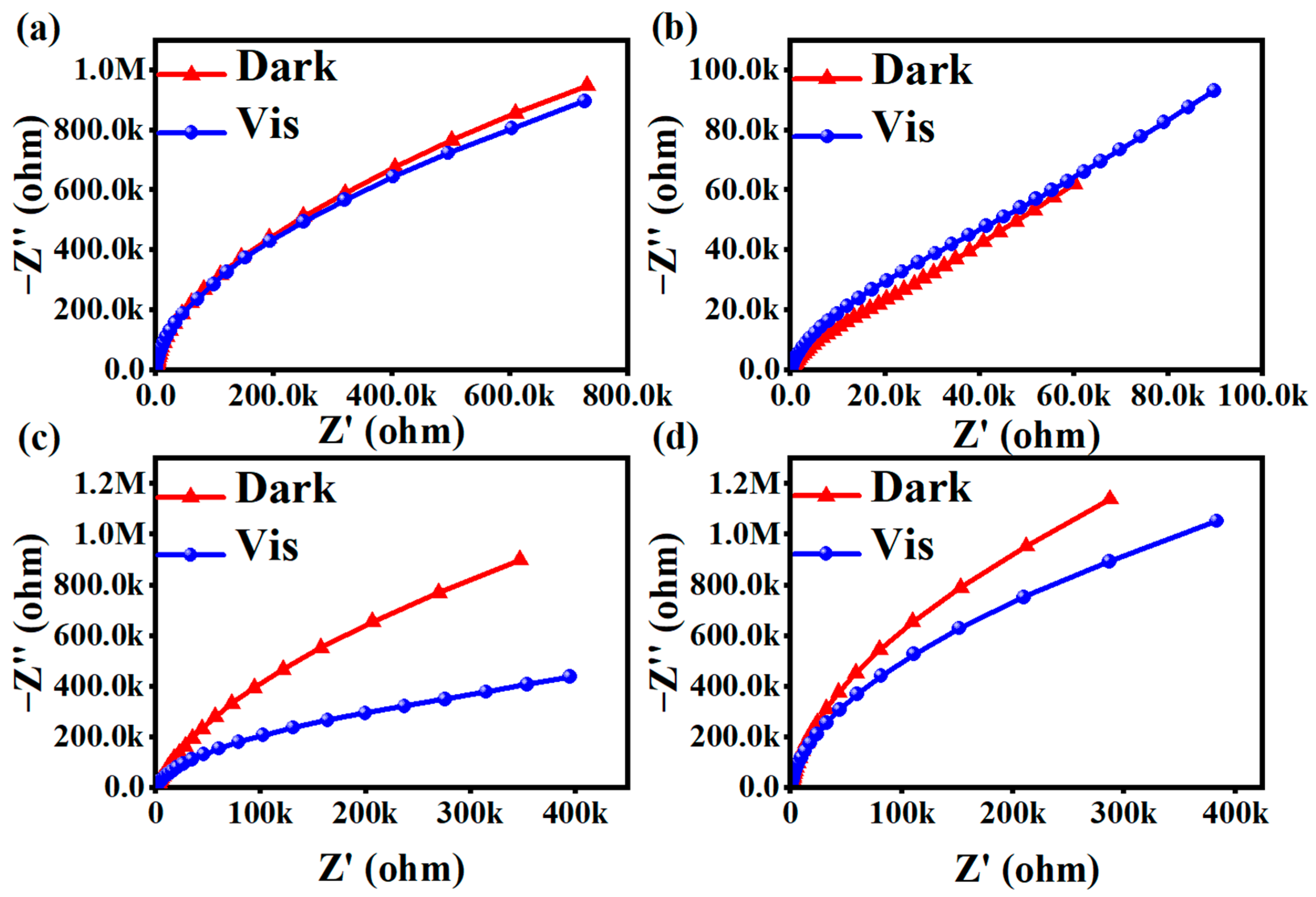
2.3.1. AC Impedance Analysis
2.3.2. Photocurrent Analysis
2.4. Photocatalytic Activities
2.4.1. Photocatalytic Performance of Different Materials
2.4.2. Effect of pH on the Degradation of Floxacin
2.4.3. Effect of Different Inputs on the Degradation of Floxacin
2.4.4. Effect of Different Ions on the Degradation of Floxacin
2.4.5. Photocatalytic Cycle
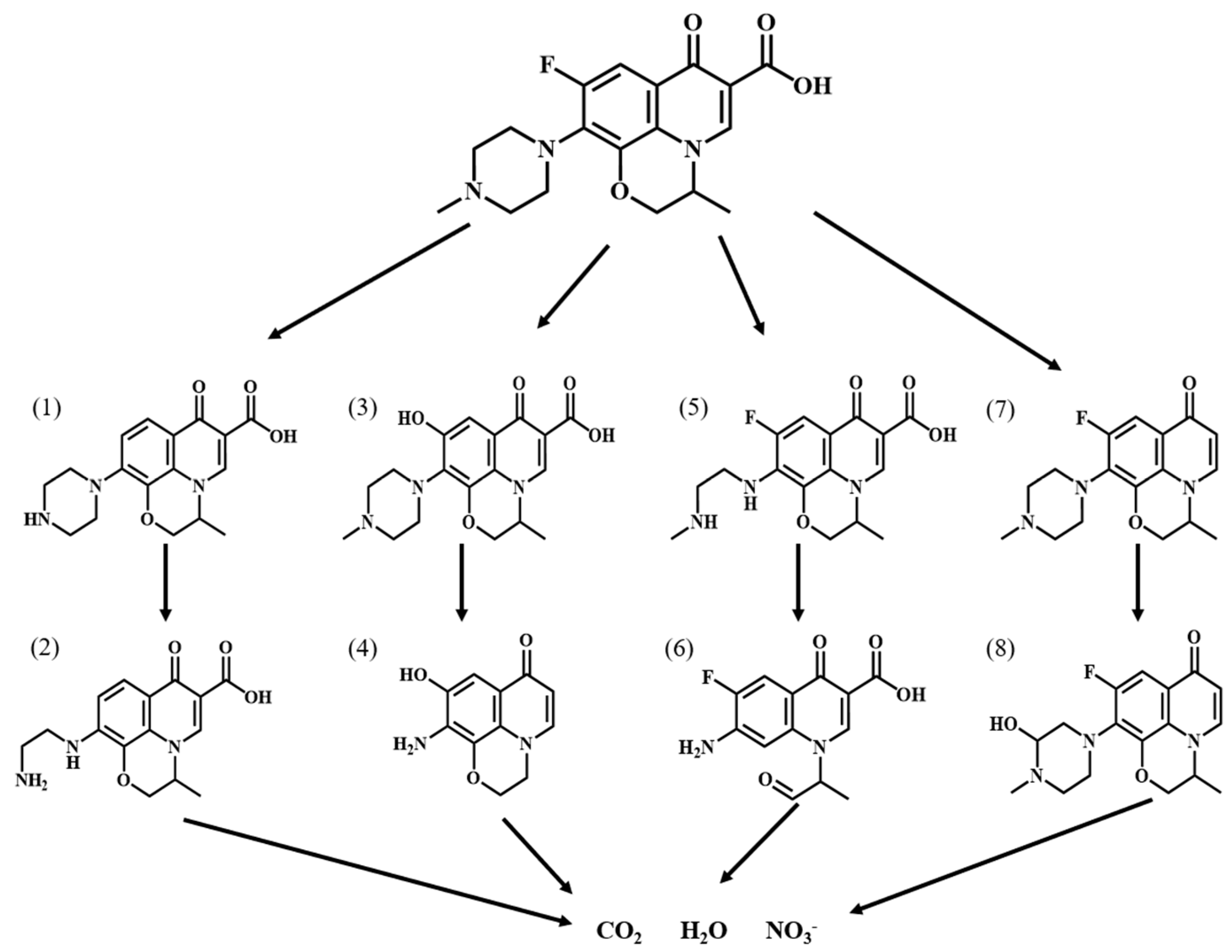
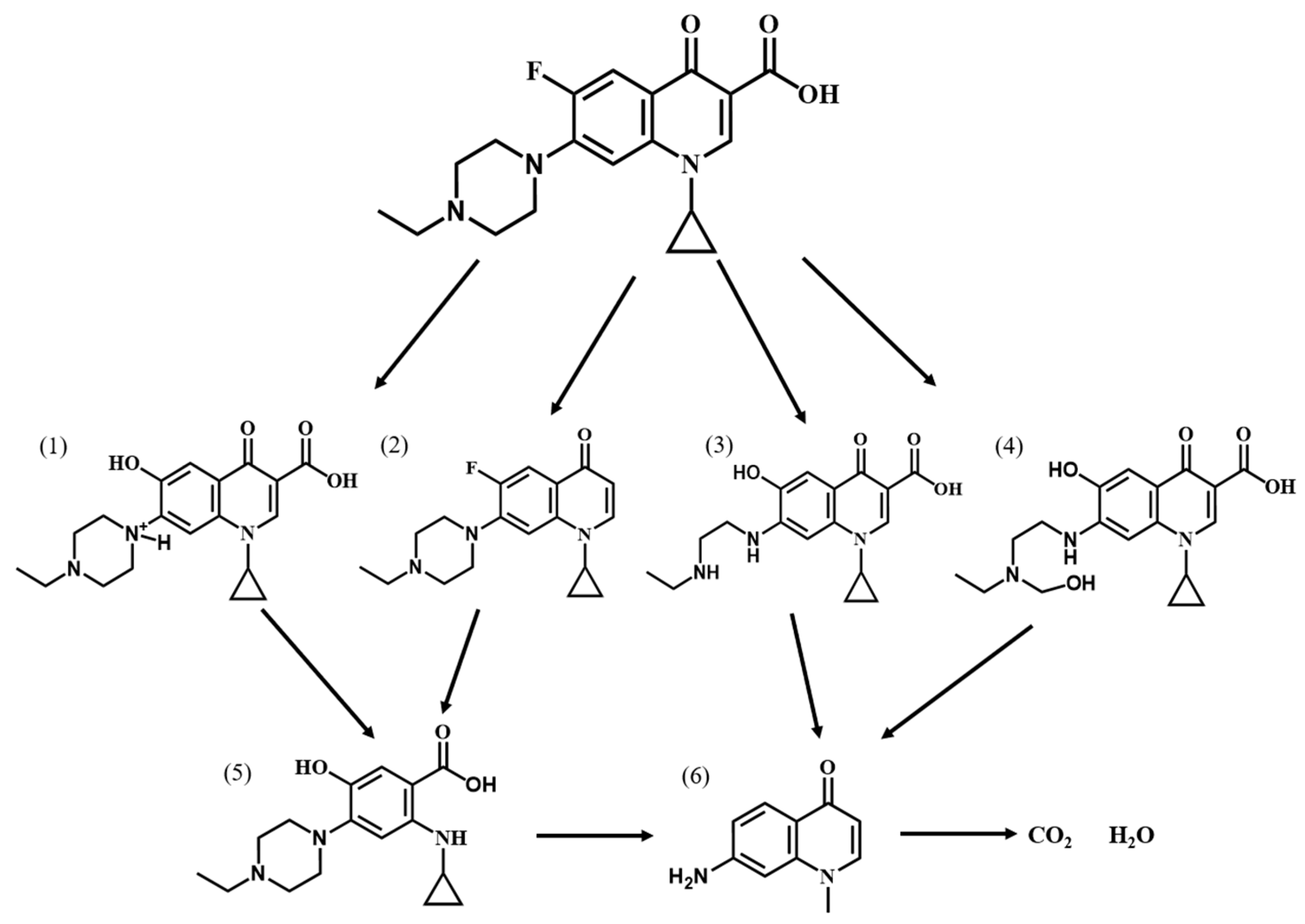
2.4.6. Degradation Pathway
2.4.7. Photocatalytic Reaction Mechanism
3. Experimental Sections
3.1. Experimental Materials
3.2. Preparation of Photocatalyst
3.2.1. Preparation of MIL-53(Fe)
3.2.2. Preparation of NH2-MIL-53(Fe)
3.2.3. Preparation of MIL-100(Fe)
3.2.4. Preparation of g-C3N4
3.3. Characterisation of Photocatalysts
3.4. Electrochemical Tests
3.5. Photocatalytic Tests
3.6. Photocatalytic Degradation Mechanism
3.7. Liquid Chromatography Analysis Conditions
4. Conclusions
- Fluorescence and UV diffuse reflectance analyses of the four materials showed that the photogenerated carrier separation efficiency of NH2-MIL-53(Fe) was the best, followed by MIL-100(Fe).
- According to electrochemical tests on the four materials, the MIL-100(Fe) materials had a large separation rate of photogenerated electron–hole pairs. The time–current curves indicated that MIL-100(Fe) produced the largest photocurrent and the best photocatalytic performance.
- By comparing the photocatalytic degradation effects of four materials, it was found that MIL-100(Fe) had the best catalytic ability in degrading the ofloxacin and enrofloxacin. The degradation effect was best under acidic conditions. The addition of other ions did not affect the degradation rate to a great extent, the optimal amount of the photocatalyst added was 0.08 g. The degradation on floxacin by MIL-100(Fe) exhibited a good cycling performance.
- The ▪O2− played a major role in the photocatalytic reaction process, followed by holes (h+).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, L.; Zheng, H.; Gao, Q. Government environmental regulation strategy for new pollutants control in mariculture. Mar. Policy 2023, 150, 105545. [Google Scholar] [CrossRef]
- Kong, H.M.; Zhao, X.H.; Xu, W.; Dai, Y.H.; Zhang, J.Y. Occurrence and risk assessment of antibiotics in groundwater environment in China. Environ. Eng. 2022, 41, 219–226. [Google Scholar]
- Ma, J.; Cui, Y.; Li, A.; Zou, X.; Ma, C.; Chen, Z. Antibiotics and antibiotic resistance genes from wastewater treated in constructed wetlands. Ecol. Eng. 2022, 177, 106548. [Google Scholar] [CrossRef]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.H.O. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions-a review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Feng, M.; Wang, Z.; Dionysiou, D.D.; Sharma, K.V. Metal-mediated oxidation of fluoroquinolone antibiotics in water: A review on kinetics, transformation products, and toxicity assessment. J. Hazard. Mater. 2018, 344, 1136–1154. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Wang, B.; Chen, M.; Feng, Q.; Zhang, X.; Wang, S.; Zhao, R.; Jiang, T. Adsorption and photocatalytic degradation of quinolone antibiotics from wastewater using functionalized biochar. Environ. Pollut. 2023, 336, 122409. [Google Scholar] [CrossRef]
- Huang, F.; Zou, S.; Deng, D.; Lang, H.; Liu, F. Antibiotics in a typical karst river system in China: Spatiotemporal variation and environmental risks. Sci. Total Environ. 2019, 650 Pt 1, 1348–1355. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Jin, X.; Zheng, X.; Wang, Y.; Wei, D.; Zhang, Q.; Feng, Y.; Xie, Z.; Chen, P.; et al. Highly active metal-free carbon dots/g-C3N4 hollow porous nanospheres for solar-light-driven PPCPs remediation: Mechanism insights, kinetics and effects of natural water matrices. Water Res. 2020, 172, 115492. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, Q.; Pan, Y.; Xu, S.; Li, H.; Tang, J. The research status, potential hazards and toxicological mechanisms of fluoroquinolone antibiotics in the environment. Antibiotics 2023, 12, 1058. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, B.; Cao, J.; Wen, X.; Luo, M.; Xing, S.; Chen, D.; Feng, C.; Huang, G.; Jin, Y. High-performance Pd nanocatalysts based on the novel N-doped Ti3C2 support for ethanol electrooxidation in alkaline media. Electrochim. Acta 2021, 390, 138902. [Google Scholar] [CrossRef]
- Guo, Y.; Qi, P.S.; Liu, Y.Z. A review on advanced treatment of pharmaceutical wastewater. IOP Conf. Ser. Earth Environ. Sci. 2017, 63, 012025. [Google Scholar] [CrossRef]
- Khalid, N.R.; Majid, A.; Tahir, M.B.; Niaz, N.A.; Khalid, S. Carbonaceous-TiO2 nanomaterials for photocatalytic degradation of pollutants: A review. Ceram. Int. 2017, 43, 14552–14571. [Google Scholar] [CrossRef]
- Filipe, O.M.S.; Santos, E.B.H.; Otero, M.; Gonalves, E.A.C.; Neves, M.G.P.M.S. Photodegradation of metoprolol in the presence of aquatic fulvic acids. Kinetic studies, degradation pathways and role of singlet oxygen, OH radicals and fulvic acids triplet states. J. Hazard. Mater. 2019, 385, 121523. [Google Scholar] [CrossRef]
- Fanourakis, S.K.; Peña-Bahamonde, J.; Bandara, P.C.; Rodrigues, D.F. Nano-based adsorbent and photocatalyst use for pharmaceutical contaminant removal during indirect potable water reuse. Npj Clean. Water 2020, 3, 1. [Google Scholar] [CrossRef]
- Lin, X.; Xu, D.; Zheng, J.; Song, M.; Che, G.; Wang, Y.; Yang, Y.; Liu, C.; Zhao, L.; Chang, L. Graphitic carbon nitride quantum dots loaded on leaf-like InVO4/BiVO4 nanoheterostructures with enhanced visible-light photocatalytic activity. J. Alloys Compd. 2016, 688, 891–898. [Google Scholar] [CrossRef]
- Yu, B.; Chang, H.; Wei, W.; Yu, H.; Chen, Z.; Cheng, X.; Chen, D.; Jin, Y.; Han, D.; Xu, W. Highly Effective Removal of Ciprofloxacin Antibiotic from Water by Magnetic Metal–Organic Framework. Water 2023, 15, 2531. [Google Scholar] [CrossRef]
- Yu, H.; Xu, W.; Chang, H.; Xu, G.; Li, L.; Zang, J.; Huang, R.; Zhu, L.; Yu, B. Electrocatalytic Ni-Co Metal Organic Framework for Efficient Urea Oxidation Reaction. Processes 2023, 11, 3035. [Google Scholar] [CrossRef]
- Yu, H.; Xu, G.; Wen, C.; Yu, B.; Jin, Y.; Yin, X.B. Multi-level Reactive Oxygen Species Amplifier to Enhance Photo-/Chemo-Dynamic/Ca2+ Overload Synergistic Therapy. ACS Appl. Mater. Interfaces 2024, 16, 18459–18473. [Google Scholar] [CrossRef] [PubMed]
- Oveisi, M.; Mahmoodi, N.M.; Asli, M.A. Facile and green synthesis of metal-organic framework/inorganic nanofiber using electro- spinning for recyclable visible-light photocatalysis. Clean. Prod. 2019, 222, 669–684. [Google Scholar] [CrossRef]
- Li, X.; Guo, W.; Liu, Z.; Wang, R.; Liu, H. Quinone-modified NH2-MIL-101(Fe) composite as a redox mediator for improved degradation of bisphenol A. J. Hazard. Mater. 2017, 324, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Geng, N.; Chen, W.; Xu, H.; Ding, M.; Lin, T.; Wu, Q.; Zhang, L. Insights into the novel application of Fe-MOFs in ultrasound assisted heterogeneous Fenton system: Efficiency, kinetics and mechanism. Ultrason. Sonochem. 2021, 72, 105411. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, Y.; Liu, X.; Si, Y.; Tang, Y.; Wang, X. MoS2-modified MIL-53(Fe) for synergistic adsorption-photocatalytic degradation of tetracycline. Environ. Sci. Pollut. Res. 2023, 30, 23086–23095. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Liu, L.X.; Liu, L.; You, Z.H.; Guo, H.X.; Chen, Z.X. Ultrasound Assisted Synthesis of Nanoscale NH2-MIL-53(Fe) for the Adsorption of Dye. Struct. Chem. 2021, 40, 42–46. [Google Scholar]
- Song, G.; Shi, G.; Chen, L.; Wang, X.; Sun, J.; Yu, L.; Xie, X. Different degradation mechanisms of low-concentration ozone for MIL-100 (Fe) and MIL-100 (Mn) over wide humidity fluctuation. Chemosphere 2022, 308, 136352. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wen, N.; Zhang, W.; Yu, L.; Shen, J.; Li, S.; Lv, Y. Preparation of g-C3N4/TCNQ Composite and Photocatalytic Degradation of Pefloxacin. Micromachines 2023, 14, 941. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Dong, W.; Li, X.; Wang, D.; Yang, Q.; Deng, P.; Huang, J. Modified MIL-100 (Fe) for enhanced photocatalytic degradation of tetracycline under visible-light irradiation. J. Colloid. Interface Sci. 2020, 574, 364–376. [Google Scholar] [CrossRef]
- Ma, X.; Wang, W.; Sun, C.; Li, H.; Sun, J.; Liu, X. Adsorption performance and kinetic study of hierarchical porous Fe-based MOFs for toluene removal. Sci. Total Environ. 2021, 793, 148622. [Google Scholar] [CrossRef]
- Lv, J.; Feng, S.; Ding, Y.; Chen, C.; Zhang, Y.; Lei, W.; Hao, Q.; Chen, S.M. A high-performance fluorescent probe for dopamine detection based on g-C3N4 nanofibers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 212, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kuila, U.; Prasad, M. Specific surface area and pore-size distribution in clays and shales. Geophys. Prospect. 2013, 61, 341–362. [Google Scholar] [CrossRef]
- Hu, J.; Tang, S.; Zhang, S. Investigation of pore structure and fractal characteristics of the lower silurian longmaxi shales in western Hunan and Hubei provinces in China. Nat. Gas Sci. Eng. 2016, 28, 522–535. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.F.; Cao, L.Y.; Li, J.Y.; Ouyang, H.B.; Yao, C.Y. Microwave hydrothermal synthesis of Sr2+ doped ZnO crystallites with enhanced photocatalytic properties. Ceram. Int. 2014, 40, 2647–2653. [Google Scholar] [CrossRef]
- Sayen, S.; Ortenbach-Lopez, M.; Guillon, E. Sorptive removal of enrofloxacin antibiotic from aqueous solution using a ligno-cellulosic substrate from wheat bran. J. Environ. Chem. Eng. 2018, 6, 5820–5829. [Google Scholar] [CrossRef]
- Najdanović, S.M.; Petrović, M.M.; Kostić, M.M.; Mitrović, J.Z.; Bojić, D.V.; Antonijević, M.D.; Bojić, A.L. Electrochemical synthesis and characterization of basic bismuth nitrate [Bi6O5 OH)3](NO3)5·2H2O: A potential highly efficient sorbent for textile reactive dye removal. Res. Chem. Intermed. 2020, 46, 661–680. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Asli, M.A.; Vossoughi, M. Metal-organic framework (MIL-100 (Fe)): Synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling. MRS Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, F.; He, Y.; Liu, X.; Song, C.; Xu, Y.; Zhang, Y. Heterogeneous fenton-like degradation of ofloxacin over sludge derived carbon as catalysts: Mechanism and performance. Sci. Total Environ. 2019, 654, 942–947. [Google Scholar] [CrossRef]
- Guo, H.; Cui, J.; Chai, X.; Shi, Y.; Gao, S.; Gao, J. Preparation of multilayer strontium-doped TiO2/CDs with enhanced photocatalytic efficiency for enrofloxacin removal. Environ. Sci. Pollut. Res. Int. 2023, 30, 68403–68416. [Google Scholar] [CrossRef]
- Prakash, K.; Saravanakumar, K.; Babu, S.G.; Muthuraj, V.; Karuthapandian, S.; Kalidass, S. Fabrication of Sm2(WO4)3/g-C3N4 heterojunction for boosting photodegradation of methyl parathion and ofloxacin: Characteristics, mechanism insight and pathways. J. Mol. Struct. 2024, 1305, 137729. [Google Scholar] [CrossRef]
- Nugroho, D.; Wannakan, K.; Nanan, S.; Benchawattananon, R. The Synthesis of carbon dots//zincoxide (CDs/ZnO-H400) by using hydrothermal methods for degradation of ofloxacin antibiotics and reactive red azo dye (RR141). Sci. Rep. 2024, 14, 2455. [Google Scholar] [CrossRef] [PubMed]
- Luu, T.V.H.; Tho, N.T.M.; Thuy, T.T.T.; Thong, L.N.; Dung, N.T.; Dang, P.H. Synthetization pill-like C-doped ZnO nano-photocatalyst for removing ofloxacin and methylene blue under visible light. J. Solgel Sci. Technol. 2024, 110, 204–220. [Google Scholar] [CrossRef]
- Zhang, Y.; He, K.; Chen, L.; Liu, W.; Yuan, J.; Gao, Y.; Qi, Y.; Liu, B. Photocatalytic degradation of enrofloxacin with CoAl-LDH mediated persulfate system: Efficiency evaluations and reaction pathways. Emerg. Contam. 2024, 10, 100328. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, X.; Yu, B.; Yao, J.; Han, D.; Jin, Y.; Wu, C.; Dai, G.; Xiong, X. Integrating CaIn2S4 nanosheets with Co3O4 nanoparticles possessing semiconducting and electrocatalytic properties for efficient photocatalytic H2 production. J. Funct. Biomater. 2024, 50, 3052–3063. [Google Scholar] [CrossRef]
- Ai, Y.; Hu, J.; Xiong, X.; Carabineiro, S.A.C.; Li, Y.; Sirotkin, N.; Agafonov, A.; Lv, K. Synergistic interfacial engineering of a S-scheme ZnO/In2S3 photocatalyst with S–O covalent bonds: A dual-functional advancement for tetracycline hydrochloride degradation and H2 evolution. Appl. Catal. B Environ. Energy 2024, 353, 124098. [Google Scholar] [CrossRef]
- Li, J.; Yao, J.; Yu, Q.; Zhang, X.; Carabineiro, S.A.C.; Xiong, X.; Wu, C.; Lv, K. Understanding the unique Ohmic-junction for enhancing the photocatalytic activity of CoS2/MgIn2S4 towards hydrogen production. Appl. Catal. B Environ. Energy 2024, 351, 123950. [Google Scholar] [CrossRef]
- Xu, W.; Xu, J.; Zhang, Q.; Yun, Z.; Zuo, Q.; Wang, L. Study on visible light photocatalytic performance of MIL-100 (Fe) modified by carbon nanodots. Environ. Sci. Pollut. Res. Int. 2022, 29, 55069–55080. [Google Scholar] [CrossRef]
- Deng, C.; Peng, L.; Ling, X.; Wang, T.; Xu, R.; Zhu, Y.; Wang, C.; Qian, X.; Wang, L.; Wu, Y.; et al. Construction of S-scheme Zn0. 2Cd0. 8S/biochar aerogel architectures for boosting photocatalytic hydrogen production under sunlight irradiation. J. Clean. Prod. 2023, 414, 137616. [Google Scholar] [CrossRef]
- Fu, Q.; Wu, Y. Adsorption behavior and mechanism of action of magnetic MIL-100 (Fe) on MB. Environ. Monit. Assess. 2023, 195, 745. [Google Scholar] [CrossRef]
- Abdpour, S.; Kowsari, E.; Moghaddam, M.R.A. Synthesis of MIL-100 (Fe)@ MIL-53 (Fe) as a novel hybrid photocatalyst and evaluation photocatalytic and photoelectrochemical performance under visible light irradiation. J. Solid. State Chem. 2018, 262, 172–180. [Google Scholar] [CrossRef]
- Najdanović, S.M.; Petrović, M.M.; Kostić, M.M.; Velinov, N.D.; Radović Vučić, M.D.; Matović, B.Ž.; Bojić, A.L. New way of synthesis of basic bismuth nitrate by electrodeposition from ethanol solution: Characterization and application for removal of RB19 from water. Arab. J. Sci. Eng. 2019, 44, 9939–9950. [Google Scholar] [CrossRef]


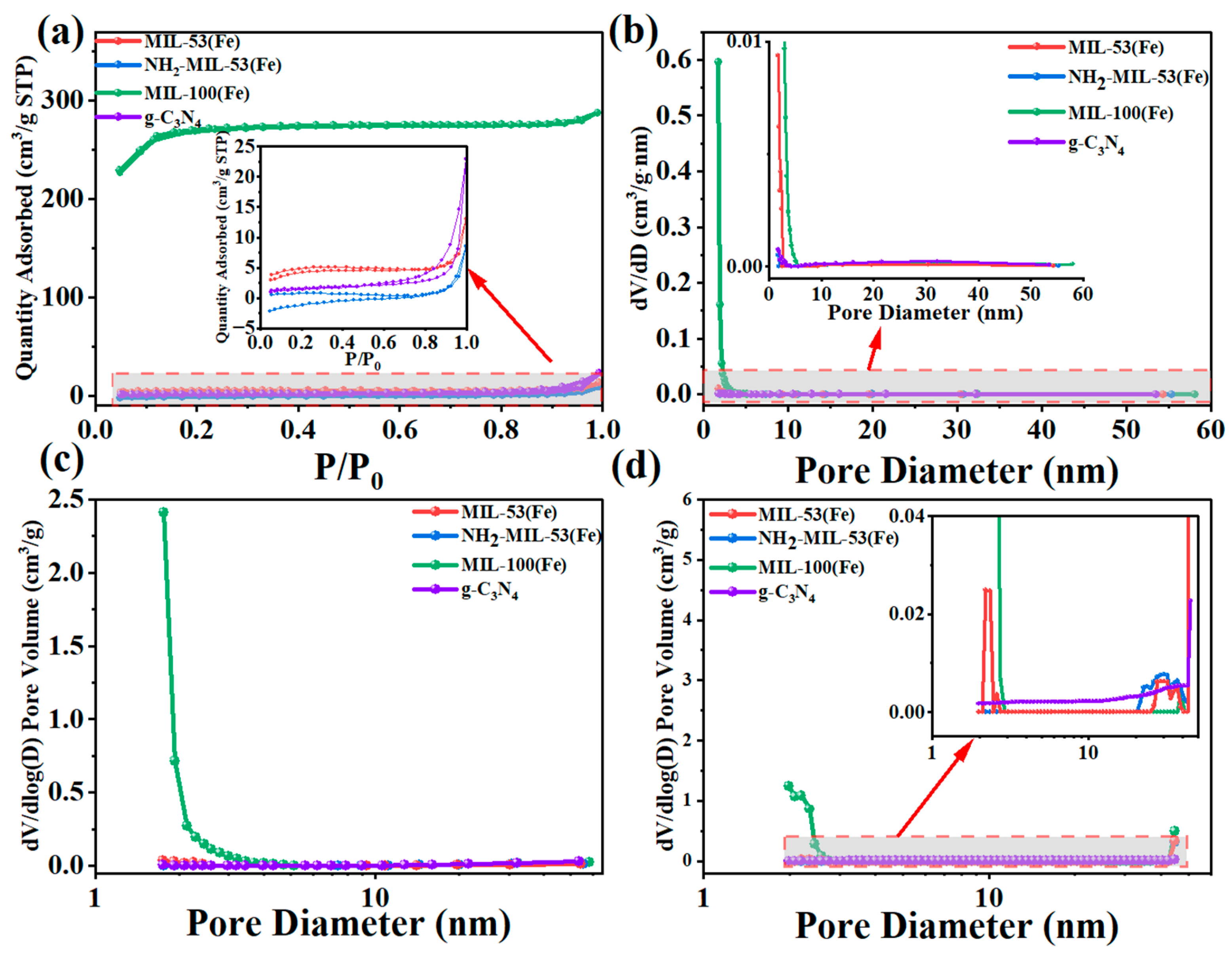





| Material | SBET m2/g | Vpore cm3/g | Pore Size/nm |
|---|---|---|---|
| MIL-53(Fe) | 17.04 | 0.02 | 4.73 |
| NH2-MIL-53(Fe) | 2.81 | 0.01 | 18.68 |
| MIL-100(Fe) | 900.15 | 0.45 | 1.98 |
| g-C3N4 | 52.72 | 0.19 | 14.05 |
| Photocatalytic reaction kinetic eigenvalues for degradation of OFL | ||||
| Materials | K | First-order kinetic equations | R2 | MRD (%) |
| MIL-53(Fe) | 0.32832 | ln(c0/c) = 0.32832t + 0.64929 | 0.77501 | 7.44271 |
| NH2-MIL-53(Fe) | 0.14225 | ln(c0/c) = 0.14225t + 0.15974 | 0.94451 | 9.14266 |
| MIL-100(Fe) | 0.76772 | ln(c0/c) = 0.76772t + 0.62241 | 0.96997 | 6.64729 |
| g-C3N4 | 0.11488 | ln(c0/c) = 0.11488t + 0.18819 | 0.89390 | 8.63929 |
| Photocatalytic reaction kinetic eigenvalues for degradation of ENR | ||||
| Materials | K | First-order kinetic equations | R2 | MRD (%) |
| MIL-53(Fe) | 0.20978 | ln(c0/c) = 0.20978t + 0.42368 | 0.77755 | 8.04445 |
| NH2-MIL-53(Fe) | 0.12618 | ln(c0/c) = 0.12618t + 0.13871 | 0.85743 | 4.61381 |
| MIL-100(Fe) | 1.17273 | ln(c0/c) = 1.17273t + 0.98767 | 0.89821 | 8.10197 |
| g-C3N4 | 0.99892 | ln(c0/c) = 0.99892t + 0.53164 | 0.80522 | 8.99683 |
| Catalysts (Weight) | Pollutant Concentration | Time (min) | Degradation Rate (%) | Refs. |
|---|---|---|---|---|
| Sm2(WO4)3@g-C3N4 (50 mg) | MP (20 mg dm−3) | 80 | 97.00 | [41] |
| Ofloxacin (20 mg dm−3) | 40 | 98.00 | ||
| CDs/ZnO-H400 (50 mg) | RR141 (10 mg dm−3) | 240 | 98.00 | [42] |
| Ofloxacin (10 mg dm−3) | 99.00 | |||
| C-dopedZnO (0.05 g) | MB (10 mg dm−3) | 180 | 92.00 | [43] |
| Ofloxacin (15 mg dm−3) | 93.00 | |||
| CoAl-LDH (150 mg) | Enrofloxacin (10 mg dm−3) | 60 | 97.72 | [44] |
| MIL-100 (0.08 g) | Ofloxacin (10 mg dm−3) | 180 | 95.10 | This work |
| Enrofloxacin (10 mg dm−3) | 99.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.; Xu, G.; Huang, X.; Xu, W.; Luo, F.; Zang, J.; Lin, X.; Huang, R.; Yu, H.; Yu, B. Photocatalytic Degradation of Quinolones by Magnetic MOFs Materials and Mechanism Study. Molecules 2024, 29, 2294. https://doi.org/10.3390/molecules29102294
Chang H, Xu G, Huang X, Xu W, Luo F, Zang J, Lin X, Huang R, Yu H, Yu B. Photocatalytic Degradation of Quinolones by Magnetic MOFs Materials and Mechanism Study. Molecules. 2024; 29(10):2294. https://doi.org/10.3390/molecules29102294
Chicago/Turabian StyleChang, Hongchao, Guangyao Xu, Xiantong Huang, Wei Xu, Fujuan Luo, Jiarong Zang, Xiaowei Lin, Rong Huang, Hua Yu, and Binbin Yu. 2024. "Photocatalytic Degradation of Quinolones by Magnetic MOFs Materials and Mechanism Study" Molecules 29, no. 10: 2294. https://doi.org/10.3390/molecules29102294
APA StyleChang, H., Xu, G., Huang, X., Xu, W., Luo, F., Zang, J., Lin, X., Huang, R., Yu, H., & Yu, B. (2024). Photocatalytic Degradation of Quinolones by Magnetic MOFs Materials and Mechanism Study. Molecules, 29(10), 2294. https://doi.org/10.3390/molecules29102294






