Co-Assembled Supramolecular Organohydrogels of Amphiphilic Zwitterion and Polyoxometalate with Controlled Microstructures
Abstract
1. Introduction
2. Results and Discussion
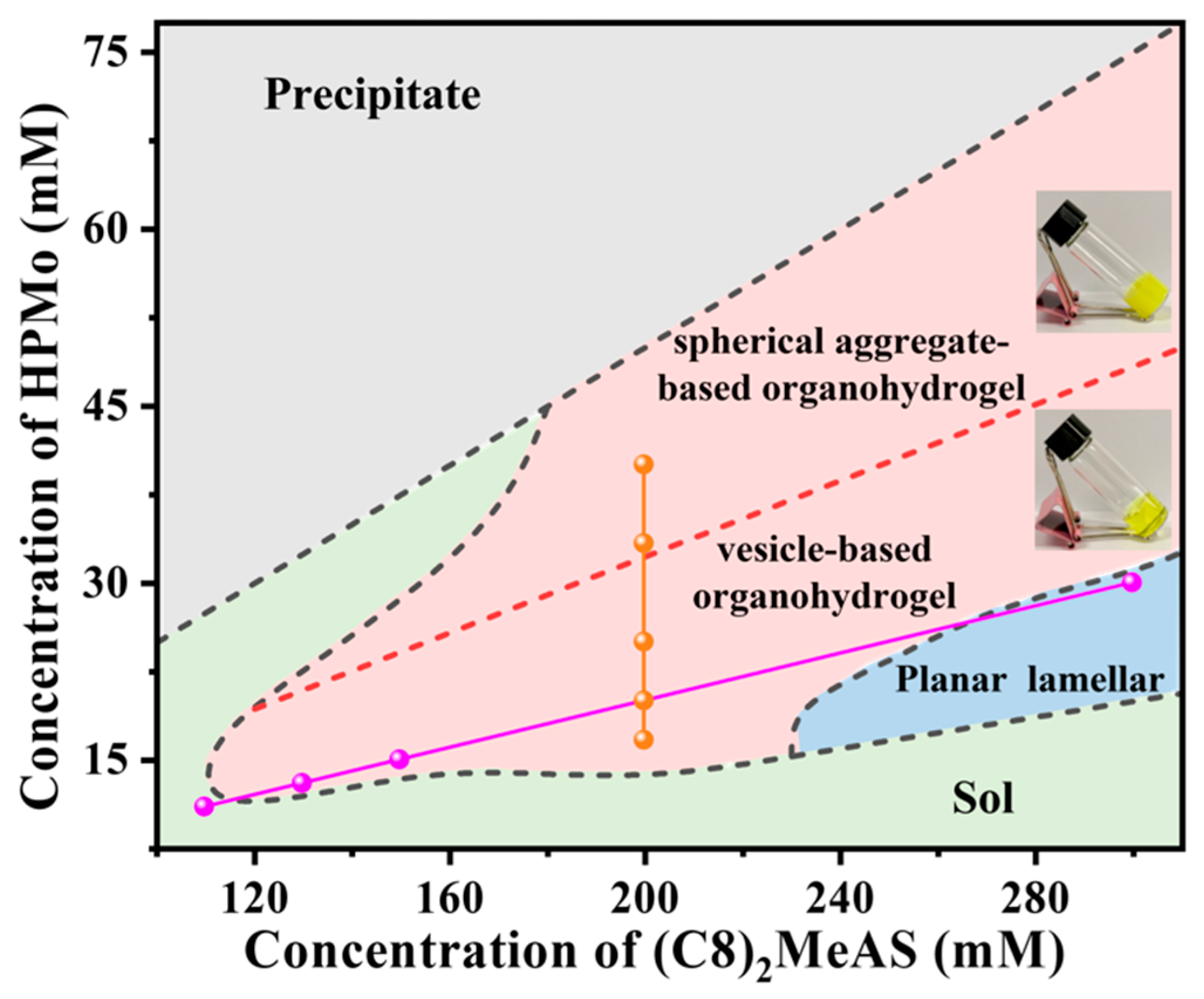
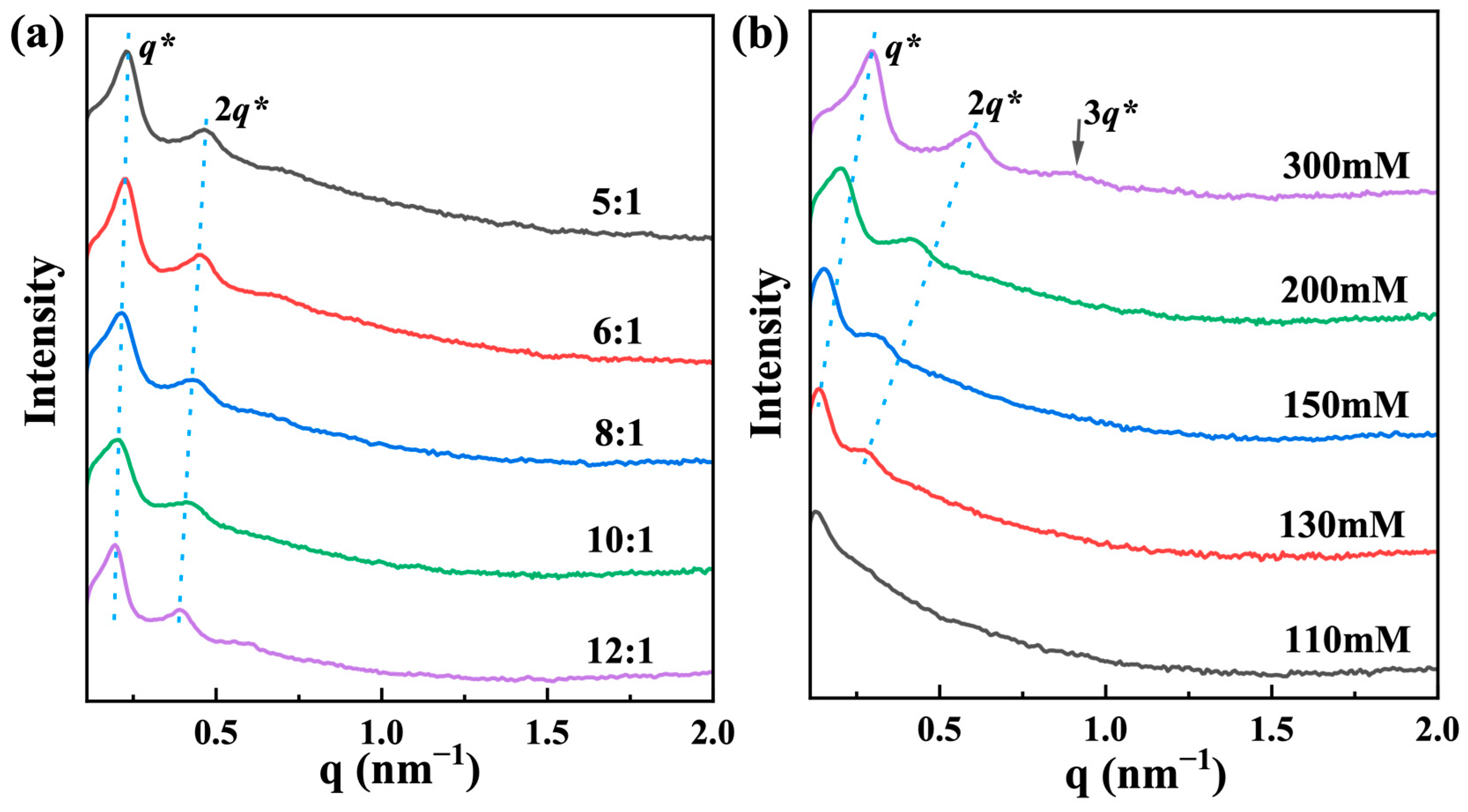
2.1. Phase Diagram and Morphologies of the Organohydrogels
2.2. Rheological Properties of the Organohydrogels
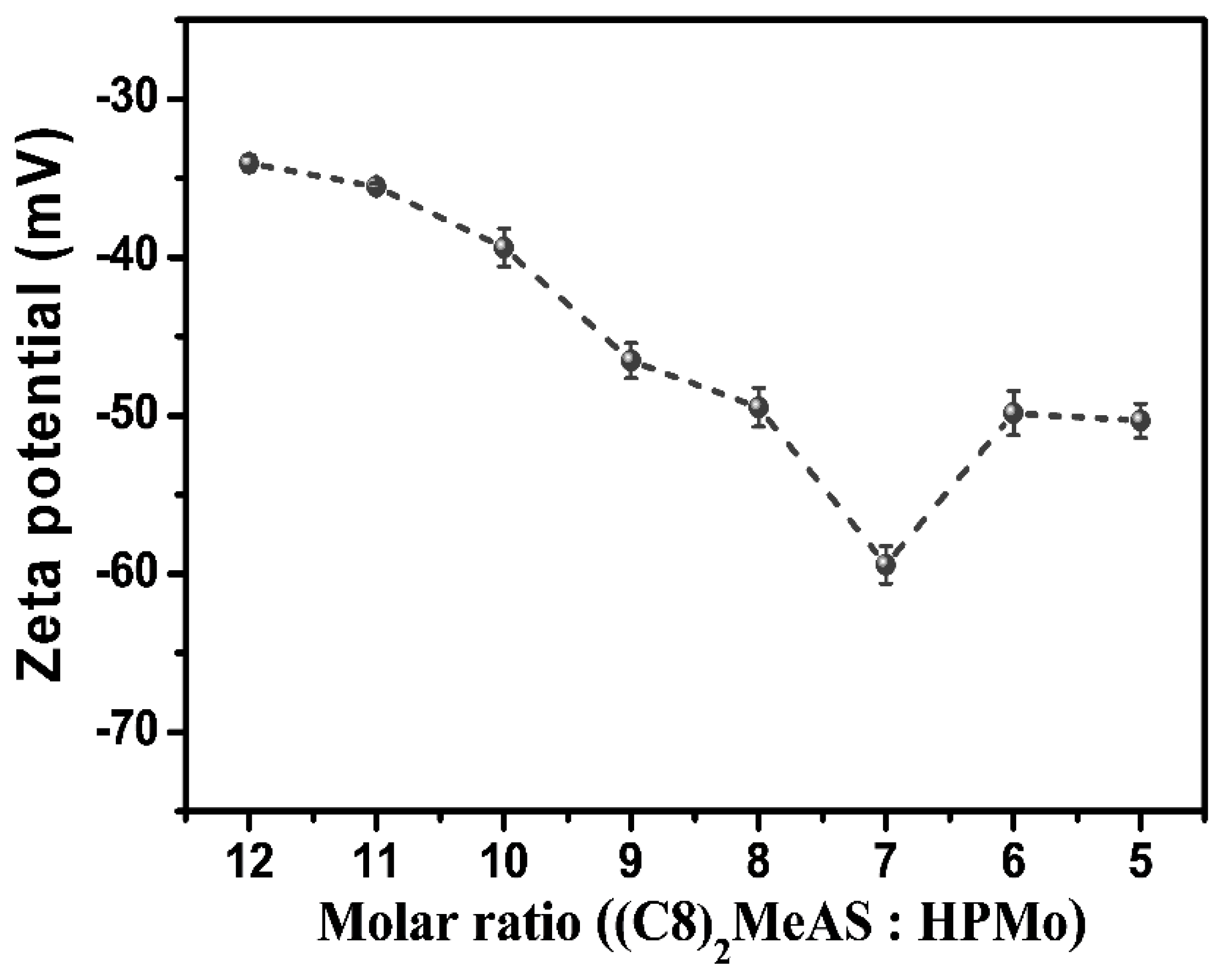
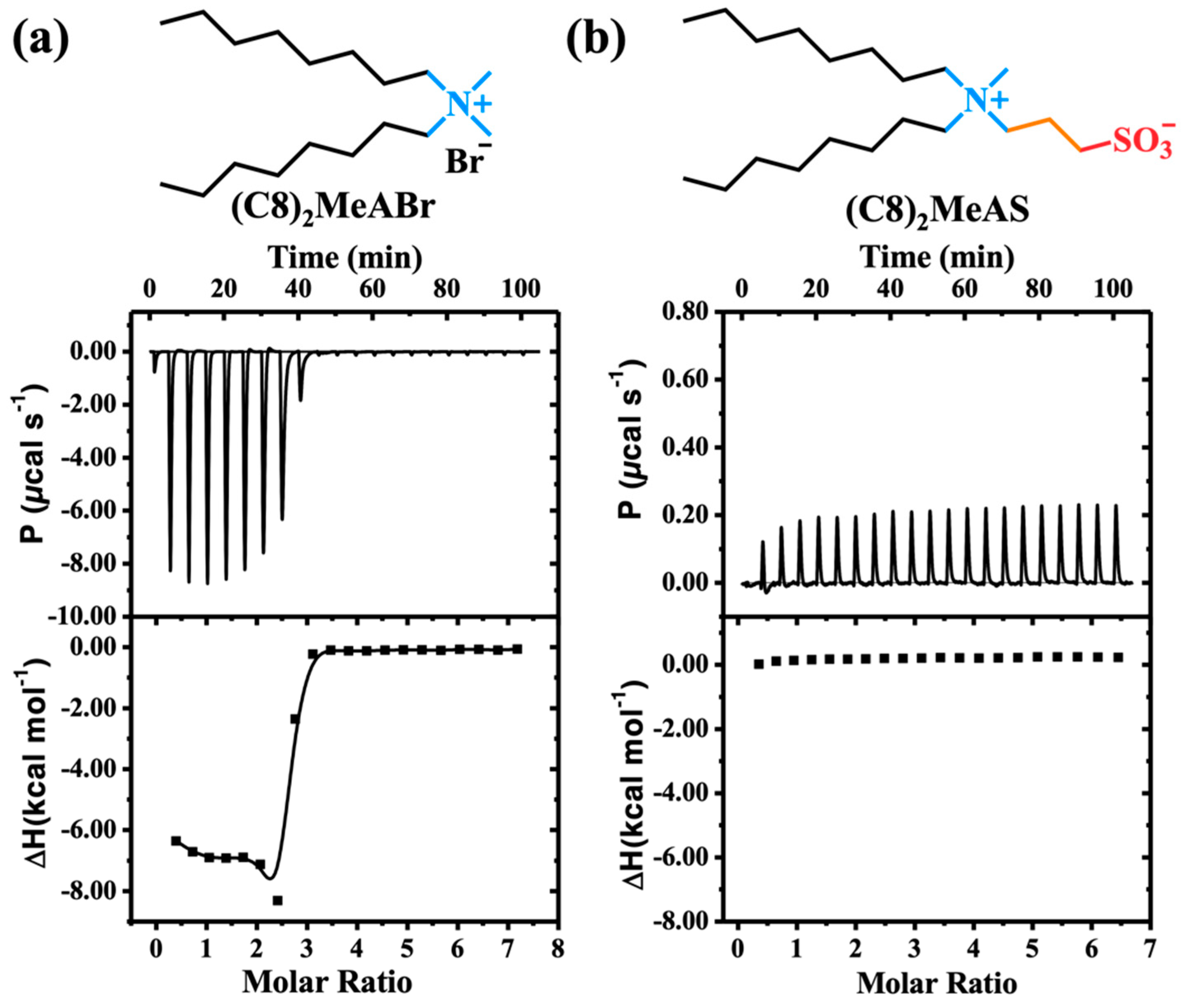
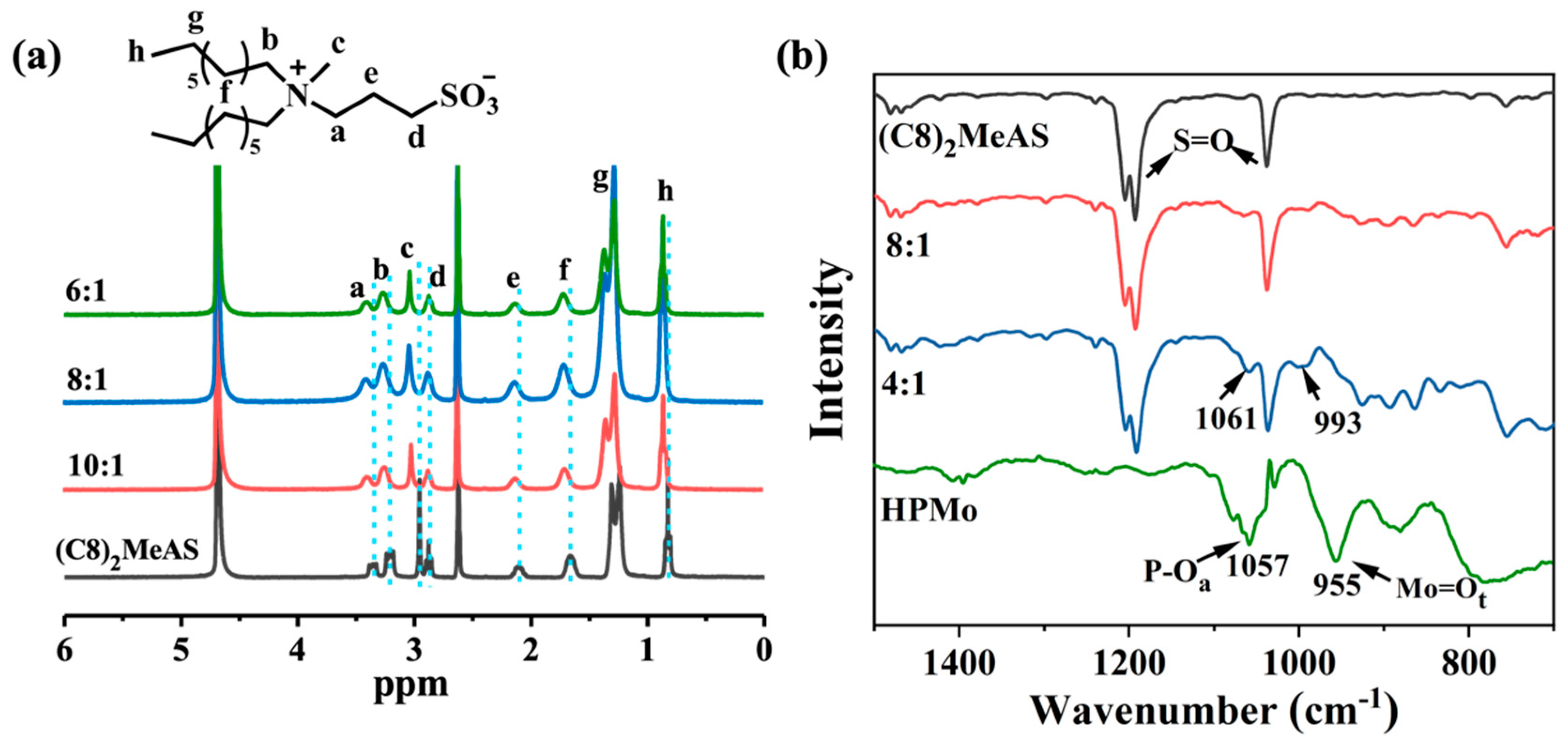
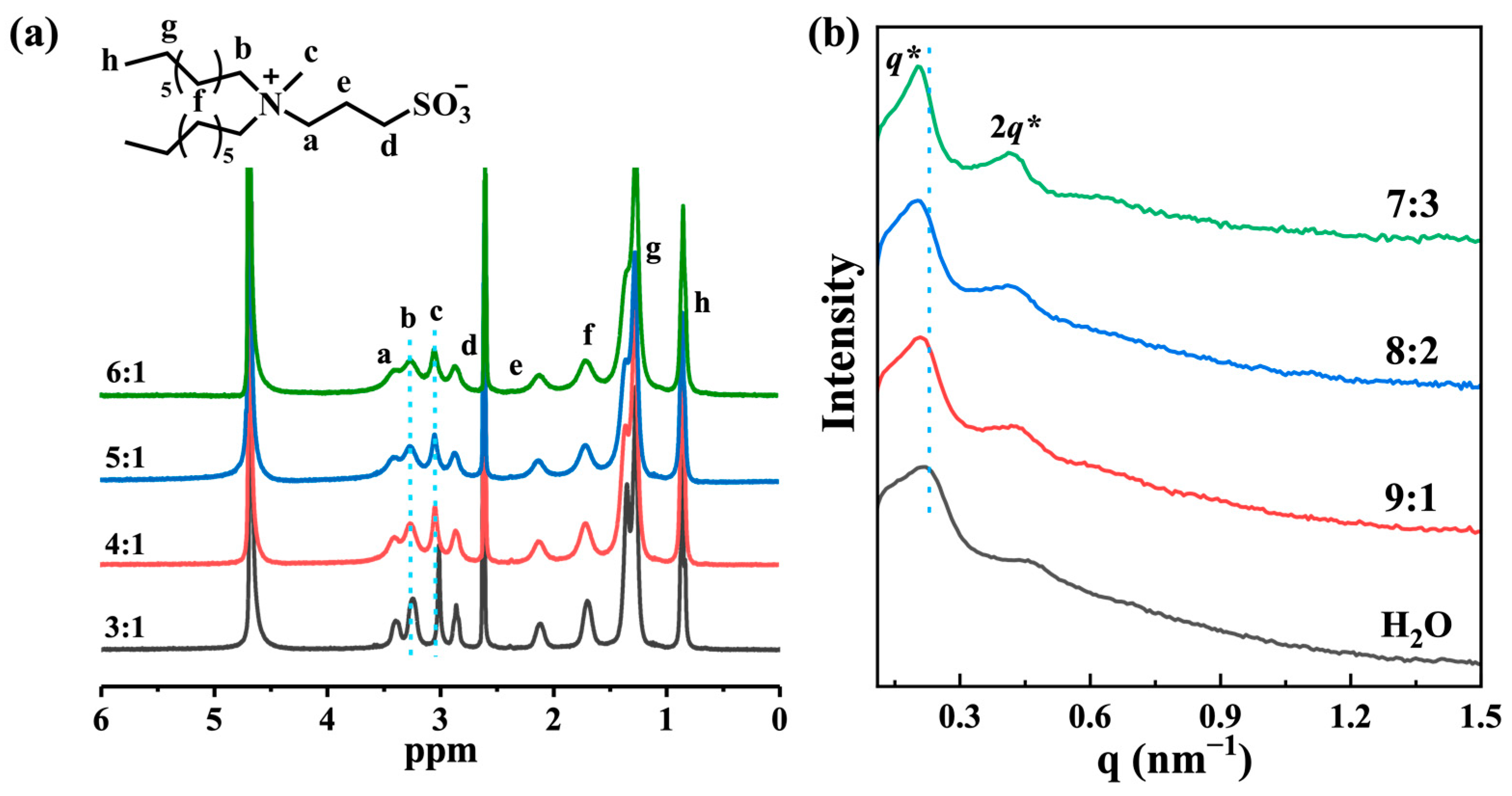
2.3. Co-Assembly Mechanism of the Formation of Organohydrogels
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Dioctylmethylammonium Propanesulfonate ((C8)2MeAS)
3.3. Sample Preparation
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, D.; Thangavel, G.; Lee, J.; Lv, J.; Li, Y.; Ciou, J.-H.; Xiong, J.; Park, T.; Lee, P.S. A Supramolecular Gel-Elastomer System for Soft Iontronic Adhesives. Nat. Commun. 2023, 14, 1990. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.M.; Ayres, N. Dynamic Covalent Bonds in Self-Healing, Shape Memory, and Controllable Stiffness Hydrogels. Polym. Chem. 2020, 11, 1410–1423. [Google Scholar] [CrossRef]
- Phillip, R.A.; Chivers, D.K.S. Shaping and Structuring Supramolecular Gels. Nat. Rev. 2019, 4, 463–478. [Google Scholar]
- Panja, S. Stimuli Responsive Dynamic Transformations in Supramolecular Gels. Chem. Soc. Rev. 2021, 50, 5165–5200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Yin, Y.; Liu, J.; Li, P.; Zhao, Y.; Bai, D.; Zhao, H.; Han, X.; Chen, Q. High-Strength and Injectable Supramolecular Hydrogel Self-Assembled by Monomeric Nucleoside for Tooth-Extraction Wound Healing. Adv. Mater. 2022, 34, 2108300. [Google Scholar] [CrossRef] [PubMed]
- Uchida, J.; Soberats, B.; Gupta, M.; Kato, T. Advanced Functional Liquid Crystals. Adv. Mater. 2022, 34, 2109063. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Jiang, F.; Li, B.; Wu, L. Multiple Modulations for Supramolecular Hydrogels of Bola-Form Surfactants Bearing Rigid and Flexible Groups. Soft Matter 2019, 15, 5034–5041. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, P.; Du, J.; Wang, Q.; Chen, X.; Zhao, L. Review on High-Temperature-Resistant Viscoelastic Surfactant Fracturing Fluids: State-of-the-Art and Perspectives. Energy Fuels 2023, 37, 9790–9821. [Google Scholar] [CrossRef]
- Tabet, A.; Forster, R.A.; Parkins, C.C.; Wu, G.; Scherman, O.A. Modulating Stiffness with Photo-Switchable Supramolecular Hydrogels. Polym. Chem. 2019, 10, 467–472. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Chen, Y.; Rehman, H.U.; Guo, Y.; Li, H.; Liu, H. Ionic Conductive Organohydrogels with Dynamic Pattern Behavior and Multi-Environmental Stability. Adv. Funct. Mater. 2021, 31, 2101464. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Moran, S.E.; An, H.Z.; Doyle, P.S. Mesoporous Organohydrogels from Thermogelling Photocrosslinkable Nanoemulsions. Nat. Mater. 2012, 11, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Dong, D.; Yao, M.; Yu, Q.; Sun, X.; Guo, Q.; Zhang, H.; Yao, F.; Li, J. Freezing-Tolerant Supramolecular Organohydrogel with High Toughness, Thermoplasticity, and Healable and Adhesive Properties. ACS Appl. Mater. Interfaces 2019, 11, 21184–21193. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.M.; Guillemot, G.; Galambos, T.; Amin, S.S.; Hampson, E.; Mall Haidaraly, K.; Newton, G.N.; Izzet, G. Supramolecular Assemblies of Organo-Functionalised Hybrid Polyoxometalates: From Functional Building Blocks to Hierarchical Nanomaterials. Chem. Soc. Rev. 2022, 51, 293–328. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-C.; Xia, X.-X.; Fan, R.-X.; Qian, Z.-G. Programmable Electrostatic Interactions Expand the Landscape of Dynamic Functional Hydrogels. Chem. Mater. 2020, 32, 1937–1945. [Google Scholar] [CrossRef]
- Pruksawan, S.; Lim, J.W.R.; Lee, Y.L.; Lin, Z.; Chee, H.L.; Chong, Y.T.; Chi, H.; Wang, F. Enhancing Hydrogel Toughness by Uniform Cross-Linking Using Modified Polyhedral Oligomeric Silsesquioxane. Commun. Mater. 2023, 4, 75. [Google Scholar] [CrossRef]
- Sun, X.; Yao, F.; Li, J. Nanocomposite Hydrogel-Based Strain and Pressure Sensors: A Review. J. Mater. Chem. A 2020, 8, 18605–18623. [Google Scholar] [CrossRef]
- Zhou, W.; Hu, Z.; Wei, J.; Dai, H.; Chen, Y.; Liu, S.; Duan, Z.; Xie, F.; Zhang, W.; Guo, R. Quantum Dots-Hydrogel Composites for Biomedical Applications. Chin. Chem. Lett. 2022, 33, 1245–1253. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Li, H.; Wu, L. Ionic Complexes of Metal Oxide Clusters for Versatile Self-Assemblies. Acc. Chem. Res. 2017, 50, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, T. Competition and Cooperation among Different Attractive Forces in Solutions of Inorganic–Organic Hybrids Containing Macroionic Clusters. Langmuir 2019, 35, 7603–7616. [Google Scholar] [CrossRef]
- Nisar, A.; Zhuang, J.; Wang, X. Cluster-Based Self-Assembly: Reversible Formation of Polyoxometalate Nanocones and Nanotubes. Chem. Mater. 2009, 21, 3745–3751. [Google Scholar] [CrossRef]
- Li, H.; Sun, H.; Qi, W.; Xu, M.; Wu, L. Onionlike Hybrid Assemblies Based on Surfactant-Encapsulated Polyoxometalates. Angew. Chem. Int. Ed. 2007, 46, 1300–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Sun, H.; Wang, Y.; Eastoe, J.; Dong, S.; Hao, J. Self-Assembled Magnetic Viruslike Particles for Encapsulation and Delivery of Deoxyribonucleic Acid. Langmuir 2018, 34, 7171–7179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cui, J.; Hao, J.; Van Horn, J.D. Co-Assemblies of Polyoxometalate {Mo72Fe30}/Double-Tailed Magnetic-Surfactant for Magnetic-Driven Anchorage and Enrichment of Protein. J. Colloid Interface Sci. 2019, 536, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wu, A.; Yu, Y.; Gao, X.; Zheng, L. Polyoxometalate-Based Photochromic Supramolecular Hydrogels with Highly Ordered Spherical and Cylindrical Micellar Nanostructures. Chem. Eur. J. 2019, 25, 6203–6211. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Sun, P.; Sun, N.; Yu, Y.; Zheng, L. Coassembly of a Polyoxometalate and a Zwitterionic Amphiphile into a Luminescent Hydrogel with Excellent Stimuli Responsiveness. Chem. Eur. J. 2018, 24, 16857–16864. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Rajesh, K.; Stanton, J.F.; Baiz, C.R. Quantifying Hydrogen-Bond Populations in Dimethyl Sulfoxide/Water Mixtures. Angew. Chem. Int. Ed. 2017, 56, 11375–11379. [Google Scholar] [CrossRef]
- Das, S.; Mondal, S.; Ghosh, S. Physicochemical Studies on the Micellization of Cationic, Anionic, and Nonionic Surfactants in Water–Polar Organic Solvent Mixtures. J. Chem. Eng. Data 2013, 58, 2586–2595. [Google Scholar] [CrossRef]
- Dong, R.; Zhong, Z.; Hao, J. Self-Assembly of Onion-like Vesicles Induced by Charge and Rheological Properties in Anionic–Nonionic Surfactant Solutions. Soft Matter 2012, 8, 7812. [Google Scholar] [CrossRef]
- Liu, X.; Gitsov, I. Nonionic Amphiphilic Linear Dendritic Block Copolymers. Solvent-Induced Self-Assembly and Morphology Tuning. Macromolecules 2019, 52, 5563–5573. [Google Scholar] [CrossRef]
- Nieh, M.-P.; Dolinar, P.; Kučerka, N.; Kline, S.R.; Debeer-Schmitt, L.M.; Littrell, K.C.; Katsaras, J. Formation of Kinetically Trapped Nanoscopic Unilamellar Vesicles from Metastable Nanodiscs. Langmuir 2011, 27, 14308–14316. [Google Scholar] [CrossRef]
- Cao, X.; Gao, A.; Hou, J.; Yi, T. Fluorescent Supramolecular Self-Assembly Gels and Their Application as Sensors: A Review. Coord. Chem. Rev. 2021, 434, 213792. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.-W.; Wu, Y.; Wang, Y.; Wu, L. Self-Assembly of an Europium-Containing Polyoxometalate and the Arginine/Lysine-Rich Peptides from Human Papillomavirus Capsid Protein L1 in Forming Luminescence-Enhanced Hybrid Nanoparticles. J. Phys. Chem. C 2015, 119, 8321–8328. [Google Scholar] [CrossRef]
- Cheng, C.-Y.; Wang, T.-Y.; Tung, S.-H. Biological Hydrogels Formed by Swollen Multilamellar Liposomes. Langmuir 2015, 31, 13312–13320. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Kim, H.; Ba, X.; Baumgartner, R.; Lee, J.S.; Tang, H.; Leal, C.; Cheng, J. Polypeptide Vesicles with Densely Packed Multilayer Membranes. Soft Matter 2015, 11, 4091–4098. [Google Scholar] [CrossRef] [PubMed]
- Himmelein, S.; Lewe, V.; Stuart, M.C.A.; Ravoo, B.J. A Carbohydrate-Based Hydrogel Containing Vesicles as Responsive Non-Covalent Cross-Linkers. Chem. Sci. 2014, 5, 1054–1058. [Google Scholar] [CrossRef]
- Briceño-Ahumada, Z.; Soltero, A.; Maldonado, A.; Perez, J.; Langevin, D.; Impéror-Clerc, M. On the Use of Shear Rheology to Formulate Stable Foams. Example of a Lyotropic Lamellar Phase. Colloids Surf. Physicochem. Eng. Asp. 2016, 507, 110–117. [Google Scholar] [CrossRef]
- Sato, D.; Obara, K.; Kawabata, Y.; Iwahashi, M.; Kato, T. Re-Entrant Lamellar/Onion Transition with Varying Temperature under Shear Flow. Langmuir 2013, 29, 121–132. [Google Scholar] [PubMed]
- Fujii, S.; Richtering, W. Shear Quench-Induced Disintegration of a Nonionic Surfactant C10E3 Onion Phase. Soft Matter 2013, 9, 5391. [Google Scholar] [CrossRef]
- Wu, A.; Gao, X.; Liang, L.; Sun, N.; Zheng, L. Interaction among Worm-like Micelles in Polyoxometalate-Based Supramolecular Hydrogel. Langmuir 2019, 35, 6137–6144. [Google Scholar] [CrossRef]
- Huang, X.; Han, Y.; Wang, Y.; Wang, Y. Aggregation Behavior of Nitrophenoxy-Tailed Quaternary Ammonium Surfactants. J. Phys. Chem. B 2007, 111, 12439–12446. [Google Scholar] [CrossRef]
- Gunaratne, K.D.D.; Johnson, G.E.; Andersen, A.; Du, D.; Zhang, W.; Prabhakaran, V.; Lin, Y.; Laskin, J. Controlling the Charge State and Redox Properties of Supported Polyoxometalates via Soft Landing of Mass-Selected Ions. J. Phys. Chem. C 2014, 118, 27611–27622. [Google Scholar] [CrossRef]
- Fong, C.; Le, T.; Drummond, C.J. Lyotropic Liquid Crystal Engineering–Ordered Nanostructured Small Molecule Amphiphileself-Assembly Materials by Design. Chem. Soc. Rev. 2012, 41, 1297–1322. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Wu, L. Organic−Inorganic Hybrid Supramolecular Gels of Surfactant-Encapsulated Polyoxometalates. Langmuir 2009, 25, 13194–13200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, X.; Li, W.; Li, B.; Wu, L. Solvent Dielectricity-Modulated Helical Assembly and Morphologic Transformation of Achiral Surfactant-Inorganic Cluster Ionic Complexes. Langmuir 2017, 33, 12750–12758. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Lu, F.; Liu, Y.; Sun, N.; Zheng, L. The Facile Construction of an Anion Exchange Membrane with 3D Interconnected Ionic Nano-Channels. Chem. Commun. 2017, 53, 767–770. [Google Scholar] [CrossRef] [PubMed]
- Li, B.-Y.; Li, Y.-C.; Lu, Z.-Y. The Important Role of Cosolvent in the Amphiphilic Diblock Copolymer Self-Assembly Process. Polymer 2019, 171, 1–7. [Google Scholar] [CrossRef]
- Hao, L.-S.; Wu, J.; Peng, Y.-R.; Wang, Y.; Xiao, K.; Hu, Y.; Nan, Y.-Q. Short-Chain n -Alcohol-Induced Changes in Phase Behaviors of Aqueous Mixed Cationic/Anionic Surfactant System. Langmuir 2018, 34, 7319–7333. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.M.; Passos, H.; Okafuji, A.; Tavares, A.P.M.; Ohno, H.; Freire, M.G.; Coutinho, J.A.P. An Integrated Process for Enzymatic Catalysis Allowing Product Recovery and Enzyme Reuse by Applying Thermoreversible Aqueous Biphasic Systems. Green Chem. 2018, 20, 1218–1223. [Google Scholar] [CrossRef]
- Sun, N.; Shi, L.; Lu, F.; Xie, S.; Sun, P.; Zheng, L. Spontaneous Vesicle Phase Formation by Linear Pseudo-Oligomeric Surfactant in Aqueous Solutions. Langmuir 2015, 31, 2281–2287. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.; Duan, Y.; Wang, C.; Sun, P.; Sun, N. Co-Assembled Supramolecular Organohydrogels of Amphiphilic Zwitterion and Polyoxometalate with Controlled Microstructures. Molecules 2024, 29, 2286. https://doi.org/10.3390/molecules29102286
Wei P, Duan Y, Wang C, Sun P, Sun N. Co-Assembled Supramolecular Organohydrogels of Amphiphilic Zwitterion and Polyoxometalate with Controlled Microstructures. Molecules. 2024; 29(10):2286. https://doi.org/10.3390/molecules29102286
Chicago/Turabian StyleWei, Peilin, Yu Duan, Chen Wang, Panpan Sun, and Na Sun. 2024. "Co-Assembled Supramolecular Organohydrogels of Amphiphilic Zwitterion and Polyoxometalate with Controlled Microstructures" Molecules 29, no. 10: 2286. https://doi.org/10.3390/molecules29102286
APA StyleWei, P., Duan, Y., Wang, C., Sun, P., & Sun, N. (2024). Co-Assembled Supramolecular Organohydrogels of Amphiphilic Zwitterion and Polyoxometalate with Controlled Microstructures. Molecules, 29(10), 2286. https://doi.org/10.3390/molecules29102286





