Synthesis and Biological Properties of Fluorescent Strigolactone Mimics Derived from 1,8-Naphthalimide
Abstract
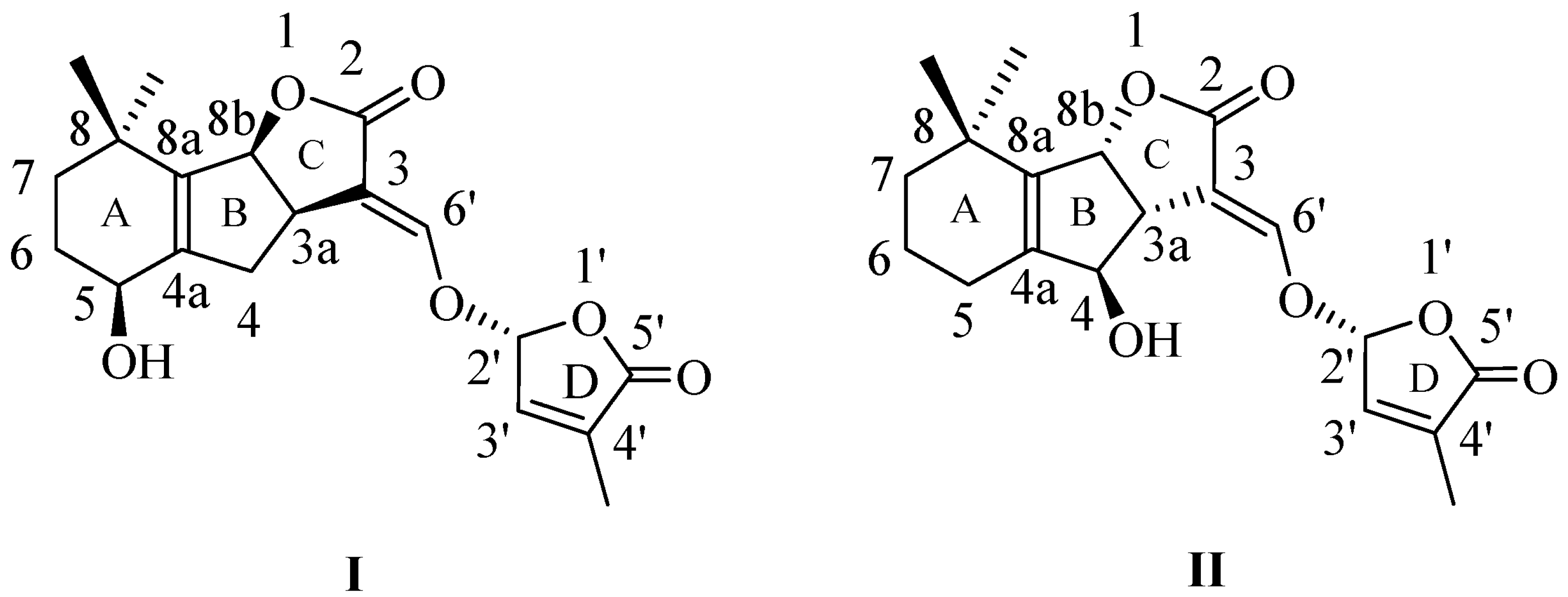
1. Introduction
2. Results and Discussion
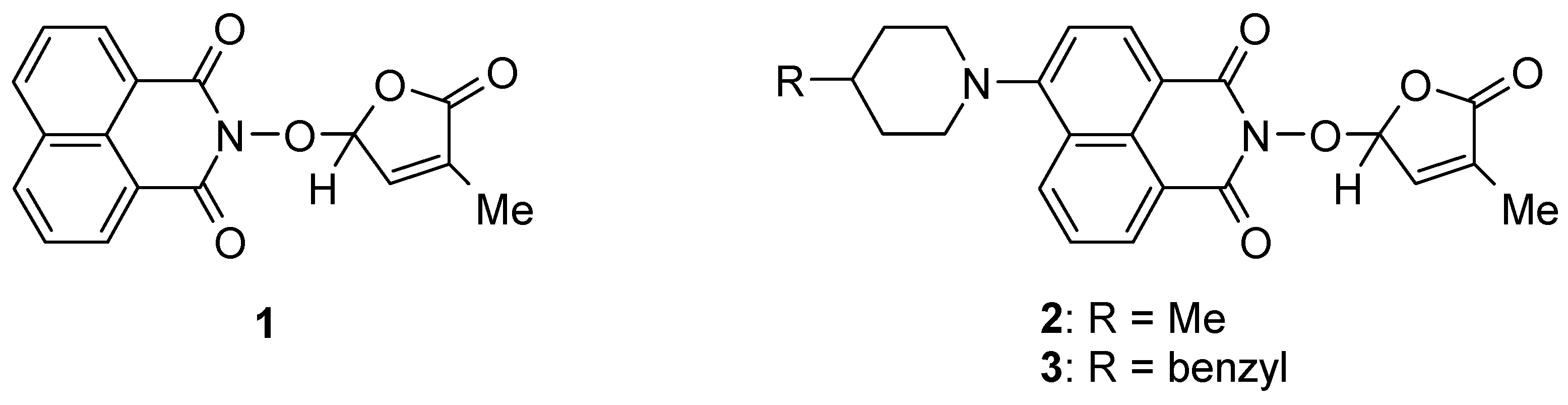
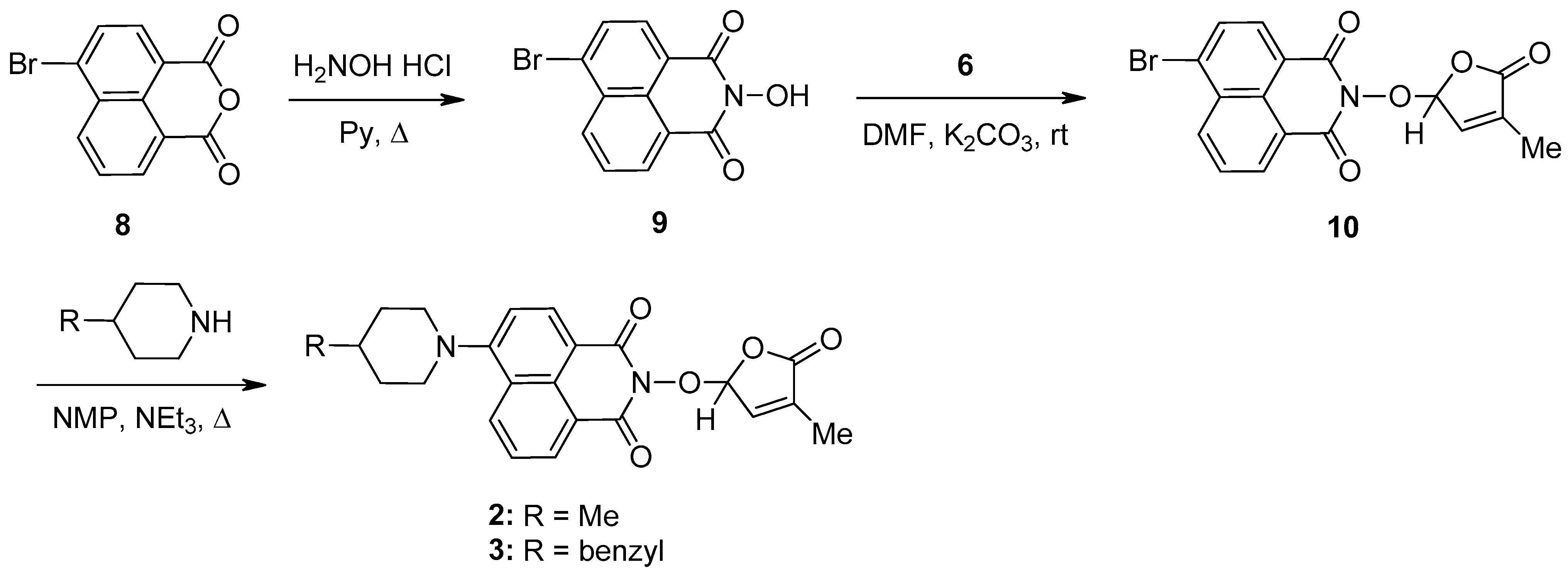
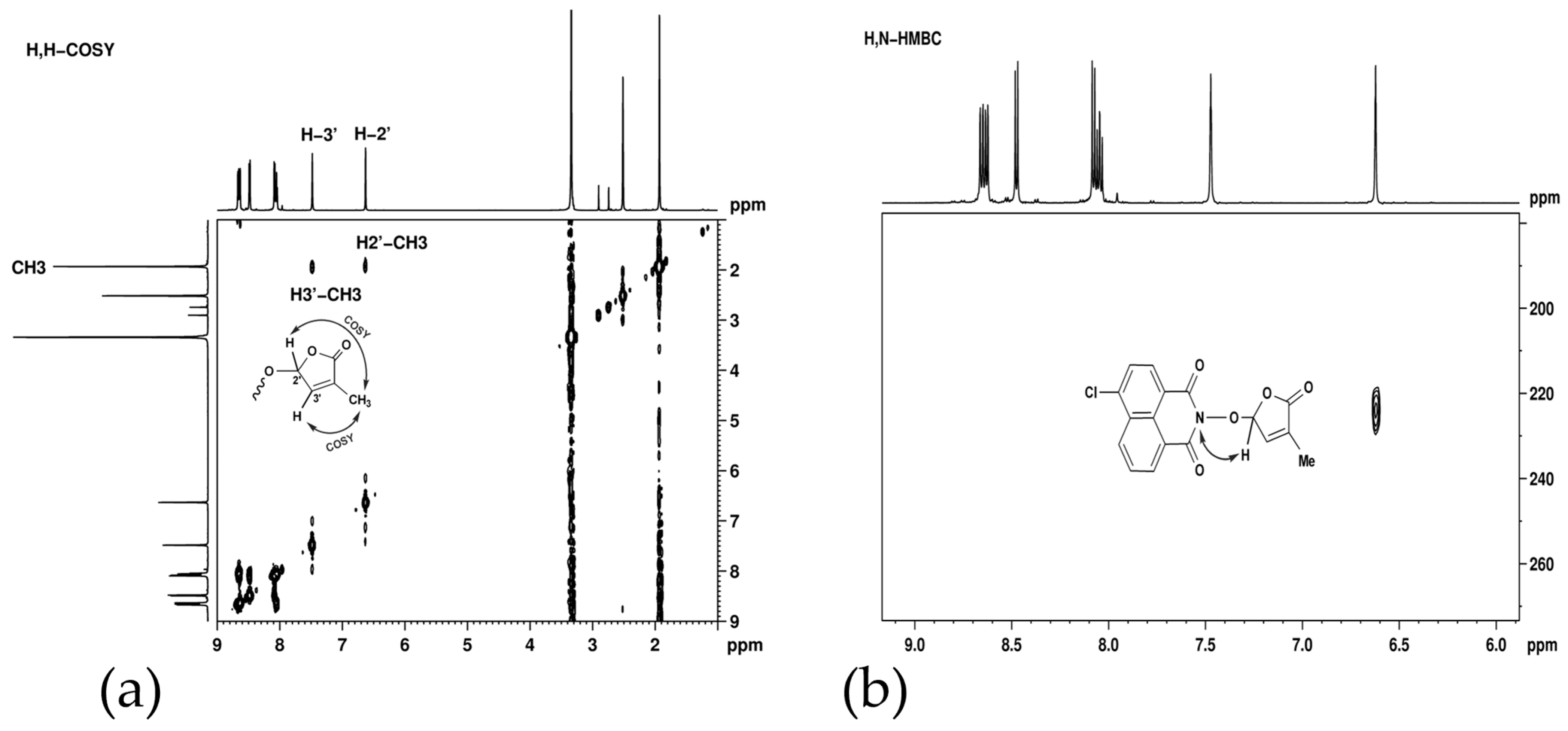
2.1. Synthesis of 1,8-Naphthalimide-Derived Strigolactone Mimics
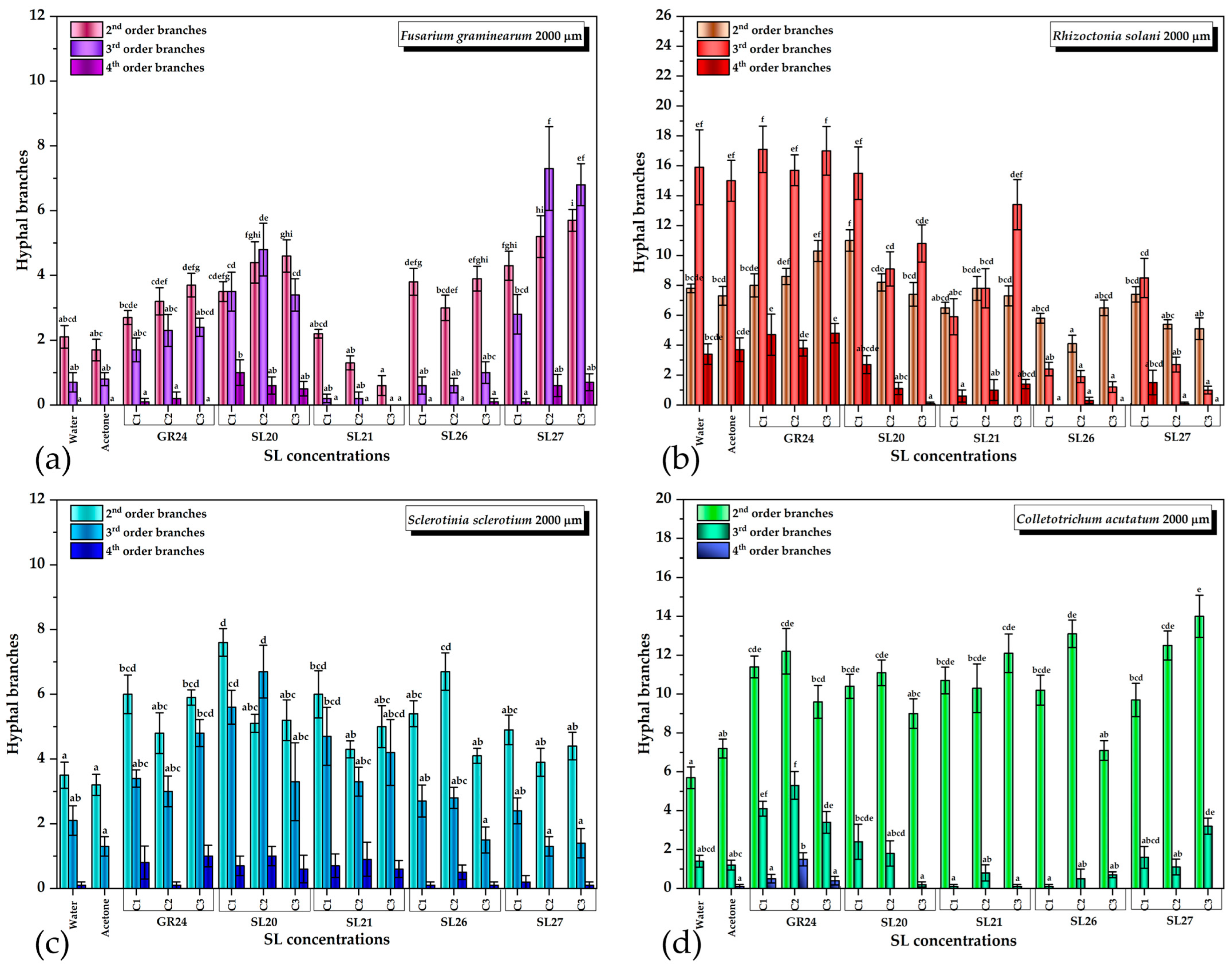
2.2. Biological Activity of Synthesized Compounds
3. Materials and Methods
3.1. Materials
3.2. Structural and Physical–Chemical Characterization of the Synthesized Compounds
3.3. Synthesis of 6-Chloro-2-(4-methyl-5-oxo-2,5-dihydro-furan-2-yloxy)-benzo[de]isoquinoline-1,3-dione (7) SL-26 or 6-Bromo-2-(4-methyl-5-oxo-2,5-dihydro-furan-2-yloxy)-benzo[de]isoquinoline-1,3-dione (10) SL-27
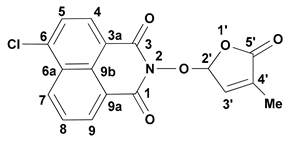
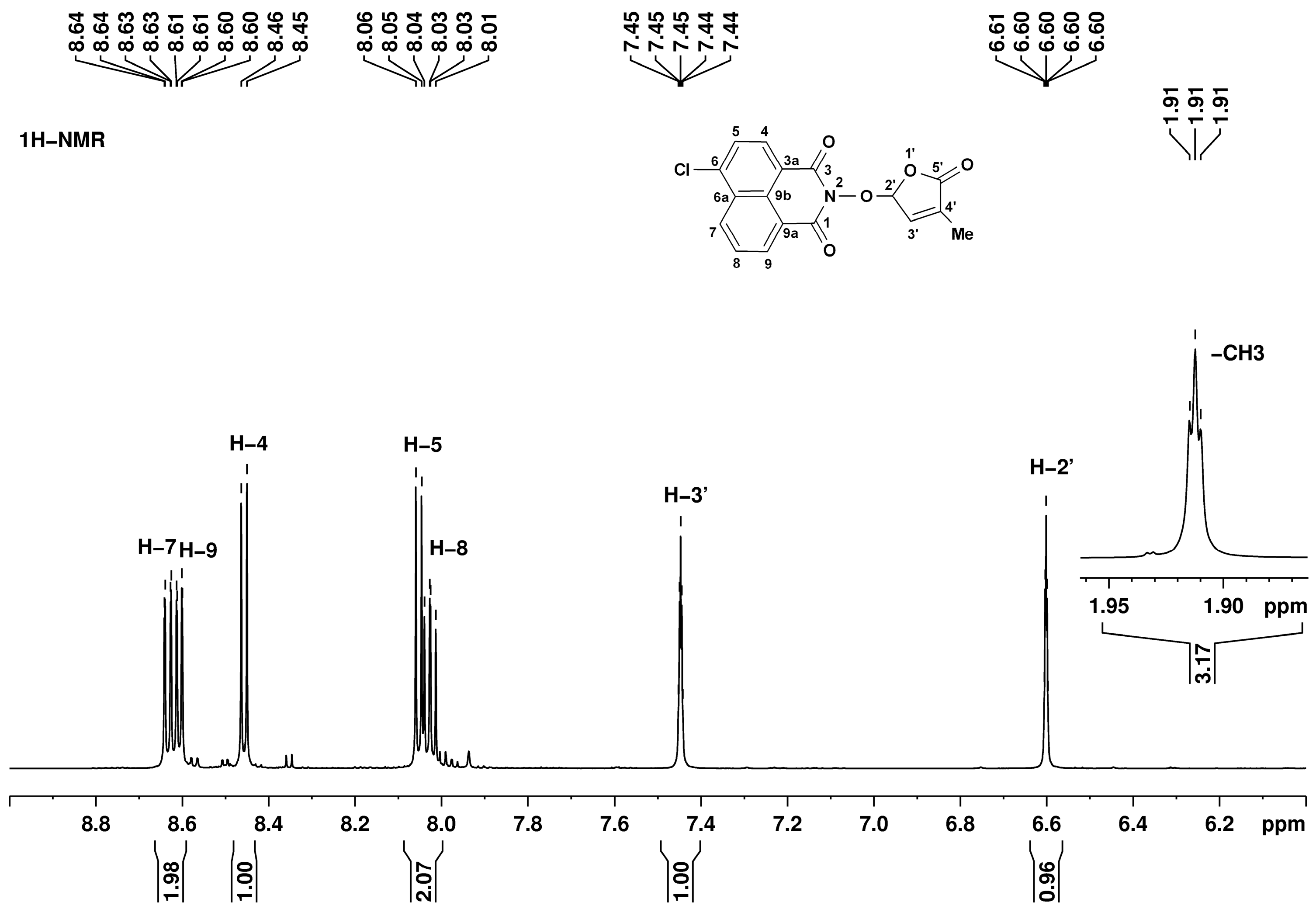
- 6-Chloro-2-(4-methyl-5-oxo-2,5-dihydro-furan-2-yloxy)-benzo[de]isoquinoline-1,3-dione (7) SL-26. Light beige crystals, m.p. 215–217 °C (CHCl3/MeOH). Yield 67% (2.3 g). Anal. Calcd. for C17H10ClNO5 (343.73): C, 59.40; H, 2.93; N, 4.07%. Found: C, 58.57; H, 3.02; N, 3.95%. FTIR (KBr, νmax): 2922, 1777, 1727, 1684, 1589, 1367, 1234, 1192, 1090, 1029 cm−1. 1H NMR (600 MHz, DMSO-d6), δ (ppm): 1.93 (t, J = 1.4 Hz, 3H, CH3), 6.62 (quintet, J = 1.4 Hz, 1H, H-2′), 7.45 (quintet, J = 1.4 Hz, 1H, H-3′), 8.05 (dd, J = 8.3, 7.4 Hz, 1H, H-8), 8.08 (d, J = 7.9 Hz, 1H, H-5), 8.45 (d, J = 7.9 Hz, 1H, H-4), 8.63 (dd, J = 7.3, 1.0 Hz, 1H, H-9), 8.66 (dd, J = 8.4, 1.0 Hz, 1H, H-7). 13C NMR (150.9 MHz, DMSO-d6), δ (ppm): 10.2 (CH3), 104.0 (CH-2′), 121.8 (C-3a), 123.1 (C-9a), 127.9 (CH-5 and C-9b), 128.6 (C-6a), 128.8 (CH-8), 130.7 (CH-7), 131.2 (CH-4), 132.0 (CH-9), 134.3 (C-4′), 138.1 (C-6), 141.7 (CH-3′), 159.7 (CO-3), 159.9 (CO-1), 170.9 (CO-5′). 15N NMR (60.8 MHz, DMSO-d6), δ (ppm): 223.9 (N-2). HRMS-ESI (m/z): [M + Na]+ for C17H10ClNNaO5, calcd. 366.0140, found 366.0150.
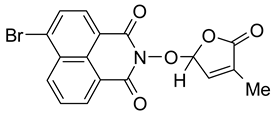
- 6-Bromo-2-(4-methyl-5-oxo-2,5-dihydro-furan-2-yloxy)-benzo[de]isoquinoline-1,3-dione (10). SL-27. Light beige crystals, m.p. 238–241 °C (MeNO2). Yield 65% (2.5 g). Anal. Calcd. for C17H10BrNO5 (388.18): C, 52.60; H, 2.60; N, 3.61%. Found: C, 51.89; H, 2.70; N, 3.51%. ATR-FTIR (solid, νmax): 1775, 1721, 1679, 1579, 1359, 1336, 1229, 1094, 1028 cm−1. 1H NMR (600 MHz, DMSO-d6), δ (ppm): 1.93 (bs, 3H, CH3), 6.62 (bs, 1H, H-2′), 7.48 (bs, 1H, H-3′), 8.03 (t, J = 7.8 Hz, 1H, H-8), 8.26 (d, J = 7.8 Hz, 1H, H-5), 8.37 (d, J = 7.8 Hz, 1H, H-4), 8.59 (d, J = 8.5, 1H, H-9), 8.62 (d, J = 7.2 Hz, 1H, H-7). 13C NMR (150.9 MHz, DMSO-d6), δ (ppm): 10.2 (CH3), 104.1 (CH-2′), 122.4 (C-3a), 123.1 (C-9a), 127.8 (C-9b), 129.0 (CH-8), 129.9 (C-6), 130.0 (C-6a), 131.4 (CH-4), 131.5 (CH-5), 132.0 (CH-7), 133.3 (CH-9), 134.3 (C-4′), 141.8 (CH-3′), 159.9 (CO-3), 160.0 (CO-1), 170.9 (CO-5′). 15N NMR (60.8 MHz, DMSO-d6), δ (ppm): 223.0 (N-2). HRMS-ESI (m/z): [M + Na]+ for C17H10BrNO5, calcd. 409.9640, found 409.9655.
3.4. New Synthetic Procedure for SL Mimics 2 and 3
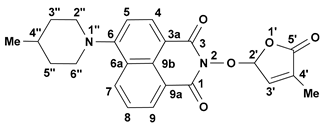
- 2-(4-Methyl-5-oxo-2,5-dihydrofuran-2-yloxy)-6-(4-methylpiperidin-1-yl)-benzo[de]isoquinoline-1,3-dione (2): SL-20. Orange crystals, m.p. 198–200 °C (CHCl3). Yield: 60% (1.45 g). Anal. Calcd. for C23H22N2O5 (406.44): C, 67.97; H, 5.46; N, 6.89%. Found: C, 68.10; H, 5.54; N, 5.93%. FTIR (KBr, νmax): 3432, 2916, 1784, 1715, 1676, 1584, 1457, 1363, 1229, 1193, 1088 cm−1. 1H NMR (600 MHz, DMSO-d6), δ (ppm): 1.04 (d, J = 6.4 Hz, 3H, CH3-4″), 1.52 (dd, J = 22.6, 11.2 Hz, 1H, CH2-3″A), 1.63–1.65 (m, 1H, CH-4″), 1.82 (d, J = 11.9 Hz, 1H, CH2-3″B), 1.92 (bs, 3H, CH3-4′), 2.93 (t, J = 11.9 Hz, 1H, CH2-2″A), 3.56 (d, J = 11.9 Hz, 1H, CH2-2″B), 6.60 (bs, 1H, H-2′), 7.34 (d, J = 8.2 Hz, 1H, H-5), 7.47 (bs, 1H, H-3′), 7.83 (t, J = 7.8 Hz, 1H, H-8), 8.40 (d, J = 8.1 Hz, 1H, H-4), 8.44 (d, J = 8.4 Hz, 1H, H-7), 8.50 (d, J = 7.1 Hz, 1H, H-9). 13C NMR (150.9 MHz, DMSO-d6), δ (ppm): 10.2 (CH3-4′), 21.7 (CH3-4″), 30.2 (CH-4″), 33.9 (CH2-3″), 53.2 and 53.3 (CH2-2″ and CH2-6″), 104.0 (CH-2′), 114.6 (C-3a), 115.1 (CH-5), 122.7 (C-9a), 125.5 (C-6a), 125.9 (CH-8), 128.7 (C-9b), 131.1 (CH-9), 131.4 (CH-7), 132.8 (CH-4), 134.2 (C-4′), 141.9 (CH-3′), 157.2 (C-6), 159.9 (CO-3), 160.4 (CO-1), 171.0 (CO-5′). 15N NMR (60.8 MHz, DMSO-d6), δ (ppm): 73.6 (N-1″), 223.6 (N-2). HRMS-ESI (m/z): [M + Na]+ for C23H22N2NaO5, calcd. 429.1421, found 429.1425 [67].
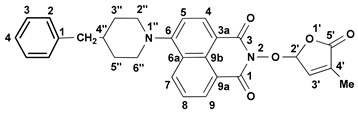
- 2-(4-Methyl-5-oxo-2,5-dihydro-furan-2-yloxy)-6-(4-benzyl-piperidin-1-yl)-benzo[de]isoquinoline-1,3-dione (3): SL-21. Orange crystals, m.p. 182–184 °C (CHCl3). Yield: 58% (1.67 g). Anal. Calcd. for C29H26N2O5 (482.54): C, 72.19; H, 5.43; N, 5.81%. Found: C, 72.06; H, 5.33; N, 5.93%. FTIR (KBr, νmax): 2911, 2794, 1784, 1718, 1684, 1584, 1452, 1364, 1235, 1177, 1026 cm−1. 1H NMR (600 MHz, DMSO-d6), δ (ppm): 1.58 (dd, J = 22.6, 11.2 Hz, 1H, CH2-3″A), 1.77 (d, J = 11.3 Hz, 2H, CH2-3″B and CH-4″), 1.91 (bs, 3H, CH3-4′), 2.65 (d, J = 6.5 Hz, 2H, CH2-4″), 2.87 (t, J = 12.1 Hz, 1H, CH2-2″A), 3.56 (d, J = 11.6 Hz, 1H, CH2-2″B), 6.60 (bs, 1H, H-2′), 7.21 (t, J = 7.2 Hz, 1H, H-4Ph), 7.24 (d, J = 7.3 Hz, 2H, H-2Ph), 7.31 (d, J = 7.2 Hz, 1H, H-5), 7.32 (t, J = 8.0 Hz, 2H, H-3Ph), 7.46 (bs, 1H, H-3′), 7.82 (t, J = 7.9 Hz, 1H, H-8), 8.39 (d, J = 8.2 Hz, 1H, H-4), 8.42 (d, J = 8.3 Hz, 1H, H-7), 8.49 (d, J = 7.2 Hz, 1H, H-9). 13C NMR (150.9 MHz, DMSO-d6), δ (ppm): 10.3 (CH3-4′), 31.8 (CH2-3″), 37.3 (CH-4″), 42.3 (CH2-4″), 53.2 and 53.3 (CH2-2″ and CH2-6″), 104.0 (CH-2′), 114.7 (C-3a), 115.1 (CH-5), 122.7 (C-9a), 125.5 (C-6a), 125.9 (CH-4Ph), 126.0 (CH-8), 128.2 (CH-3Ph), 128.7 (C-9b), 129.1 (CH-2Ph), 131.2 (CH-9), 131.4 (CH-7), 132.8 (CH-4), 134.2 (C-4′), 140.2 (C-1Ph), 141.9 (CH-3′), 157.1 (C-6), 160.0 (CO-3), 160.5 (CO-1), 171.1 (CO-5′). 15N NMR (60.8 MHz, DMSO-d6), δ (ppm): 74.2 (N-1″), 223.4 (N-2). HRMS-ESI (m/z): [M + Na]+ for C29H26N2NaO5, calcd. 505.1734, found 505.1738 [67].
3.5. Fungal Strain Cultivation and Experimental Design
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone Signaling and Evolution. Annu. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Chory, J. The Many Models of Strigolactone Signaling. Trends Plant Sci. 2020, 25, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Smith, S.M.; Huang, J. Origins of strigolactone and karrikin signaling in plants. Trends Plant Sci. 2022, 27, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Proust, H.; Hoffmann, B.; Xie, X.; Yoneyama, K.; Schaefer, D.G.; Yoneyama, K.; Nogué, F.; Rameau, C. Strigolactones regulate protonema branching and act as a quorum sensing-like signal in the moss Physcomitrella patens. Development 2011, 138, 1531–1539. [Google Scholar] [CrossRef]
- Wheeldon, C.D.; Hamon-Josse, M.; Lund, H.; Yoneyama, K.; Bennett, T. Environmental strigolactone drives early growth responses to neighboring plants and soil volume in pea. Curr. Biol. 2022, 32, 3593–3600.e3. [Google Scholar] [CrossRef]
- Akiyama, K.; Matsuzaki, K.; Hayashi, H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 2005, 435, 824–827. [Google Scholar] [CrossRef]
- Akiyama, K.; Ogasawara, S.; Ito, S.; Hayashi, H. Structural requirements of strigolactones for hyphal branching in AM fungi. Plant Cell Physiol. 2010, 51, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.J.; Roux, C.; Lopez-Raez, J.A.; Becard, G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends Plant Sci. 2007, 12, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.J.; Fernández-Aparicio, M.; Castellanos-Morales, V.; García-Garrido, J.M.; Ocampo, J.A.; Delgado, M.J.; Vierheilig, H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa). Soil Biol. Biochem. 2010, 42, 383–385. [Google Scholar] [CrossRef]
- Foo, E.; Davies, N.W. Strigolactones promote nodulation in pea. Planta 2011, 234, 1073–1081. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Rehman, N.U.; Yu, S.; Zhou, Y.; Haq, B.u.; Wang, J.; Li, P.; Zeng, Z.; Zhao, J. GmMAX2–D14 and–KAI interaction-mediated SL and KAR signaling play essential roles in soybean root nodulation. Plant J. 2020, 101, 334–351. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Rincon-Florez, V.A.; Brewer, P.B.; Beveridge, C.A.; Dennis, P.G.; Schenk, P.M. The ability of plants to produce strigolactones affects rhizosphere community composition of fungi but not bacteria. Rhizosphere 2019, 9, 18–26. [Google Scholar] [CrossRef]
- Liu, F.; Rice, J.H.; Lopes, V.; Grewal, P.; Lebeis, S.L.; Hewezi, T.; Staton, M.E. Overexpression of strigolactone-associated genes exerts fine-tuning selection on soybean rhizosphere bacterial and fungal microbiome. Phytobiomes J. 2020, 4, 239–251. [Google Scholar] [CrossRef]
- Kim, B.; Westerhuis, J.A.; Smilde, A.K.; Flokova, K.; Suleiman, A.K.A.; Kuramae, E.E.; Bouwmeester, H.J.; Zancarini, A. Effect of strigolactones on recruitment of the rice root-associated microbiome. FEMS Microbiol. Ecol. 2022, 98, fiac010. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.E.; Whichard, L.P.; Turner, B.; Wall, M.E.; Egley, G.H. Germination of Witchweed (Striga lutea Lour.): Isolation and Properties of a Potent Stimulant. Science 1966, 154, 1189–1190. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Takeuchi, Y. Strigolactones: Structures and biological activities. Pest Manag. Sci. 2009, 65, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yoneyama, K.; Yoneyama, K. The strigolactone story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, K.; Hayashi, H. Strigolactones: Chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann. Bot. 2006, 97, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Dor, E.; Joel, D.M.; Kapulnik, Y.; Koltai, H.; Hershenhorn, J. The synthetic strigolactone GR24 influences the growth pattern of phytopathogenic fungi. Planta 2011, 234, 419–427. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Umehara, M.; Hanada, A.; Yoshida, S.; Akiyama, K.; Arite, T.; Takeda-Kamiya, N.; Magome, H.; Kamiya, Y.; Shirasu, K.; Yoneyama, K.; et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature 2008, 455, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Resnick, N.; Mayzlish-Gati, E.; Kaplan, Y.; Wininger, S.; Hershenhorn, J.; Koltai, H. Strigolactones interact with ethylene and auxin in regulating root-hair elongation in Arabidopsis. J. Exp. Bot. 2011, 62, 2915–2924. [Google Scholar] [CrossRef] [PubMed]
- Koltai, H. Strigolactones are regulators of root development. New Phytol. 2011, 190, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Kapulnik, Y.; Delaux, P.M.; Resnick, N.; Mayzlish-Gati, E.; Wininger, S.; Bhattacharya, C.; Sejalon-Delmas, N.; Combier, J.P.; Becard, G.; Belausov, E.; et al. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta 2011, 233, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Boyer, F.D.; de Saint Germain, A.; Pillot, J.P.; Pouvreau, J.B.; Chen, V.X.; Ramos, S.; Stevenin, A.; Simier, P.; Delavault, P.; Beau, J.M.; et al. Structure-activity relationship studies of strigolactone-related molecules for branching inhibition in garden pea: Molecule design for shoot branching. Plant Physiol. 2012, 159, 1524–1544. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Pospisil, T. Structure and activity of strigolactones: New plant hormones with a rich future. Mol. Plant 2013, 6, 38–62. [Google Scholar] [CrossRef] [PubMed]
- Boyer, F.-D.; de Saint Germain, A.; Pouvreau, J.-B.; Clavé, G.; Pillot, J.-P.; Roux, A.; Rasmussen, A.; Depuydt, S.; Lauressergues, D.; dit Frey, N.F. New strigolactone analogs as plant hormones with low activities in the rhizosphere. Mol. Plant 2014, 7, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Pospisil, T.; Cavar Zeljkovic, S. Strigolactones: New plant hormones in action. Planta 2016, 243, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, H.J.; Fonne-Pfister, R.; Screpanti, C.; De Mesmaeker, A. Strigolactones: Plant Hormones with Promising Features. Angew. Chem. Int. Ed. Engl. 2019, 58, 12778–12786. [Google Scholar] [CrossRef]
- Rameau, C.; Goormachtig, S.; Cardinale, F.; Bennett, T.; Cubas, P. Strigolactones as plant hormones. In Strigolactones—Biology and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 47–87. [Google Scholar]
- Kleman, J.; Matusova, R. Strigolactones: Current research progress in the response of plants to abiotic stress. Biologia 2022, 78, 307–318. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Tran, L.P. Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef] [PubMed]
- Saeed, W.; Naseem, S.; Ali, Z. Strigolactones biosynthesis and their role in abiotic stress resilience in plants: A critical review. Front. Plant Sci. 2017, 8, 279971. [Google Scholar] [CrossRef] [PubMed]
- Al-Babili, S.; Bouwmeester, H.J. Strigolactones, a novel carotenoid-derived plant hormone. Annu. Rev. Plant Biol. 2015, 66, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, K.; Xie, X.; Yoneyama, K.; Kisugi, T.; Nomura, T.; Nakatani, Y.; Akiyama, K.; McErlean, C.S. Which are the major players, canonical or non-canonical strigolactones? J. Exp. Bot. 2018, 69, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Guercio, A.M.; Palayam, M.; Shabek, N. Strigolactones: Diversity, perception, and hydrolysis. Phytochem. Rev. 2023, 22, 339–359. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Mwakaboko, A.S.; Reizelman, A.; Anilkumar, G.; Sethumadhavan, D. Structure and function of natural and synthetic signalling molecules in parasitic weed germination. Pest Manag. Sci. 2009, 65, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.W.; Gowada, G.; Hassanali, A.; Knox, J.; Monaco, S.; Razavi, Z.; Rosebery, G. The preparation of synthetic analogues of strigol. J. Chem. Soc. Perkin Trans. 1 1981, 1734–1743. [Google Scholar] [CrossRef]
- Mangnus, E.M.; Zwanenburg, B. Synthesis, structural characterization, and biological evaluation of all four enantiomers of strigol Analog GR7. J. Agric. Food Chem. 1992, 40, 697–700. [Google Scholar] [CrossRef]
- Mangnus, E.M.; Van Vliet, L.A.; Vandenput, D.A.; Zwanenburg, B. Structural modifications of strigol analogs. Influence of the B and C rings on the bioactivity of the germination stimulant GR24. J. Agric. Food Chem. 1992, 40, 1222–1229. [Google Scholar] [CrossRef]
- Mangnus, E.M.; Dommerholt, F.J.; De Jong, R.L.; Zwanenburg, B. Improved synthesis of strigol analog GR24 and evaluation of the biological activity of its diastereomers. J. Agric. Food Chem. 1992, 40, 1230–1235. [Google Scholar] [CrossRef]
- Nefkens, G.H.; Thuring, J.W.J.; Beenakkers, M.F.; Zwanenburg, B. Synthesis of a phthaloylglycine-derived strigol analogue and its germination stimulatory activity toward seeds of the parasitic weeds Striga hermonthica and Orobanche crenata. J. Agric. Food Chem. 1997, 45, 2273–2277. [Google Scholar] [CrossRef]
- Mwakaboko, A.S.; Zwanenburg, B. Single step synthesis of strigolactone analogues from cyclic keto enols, germination stimulants for seeds of parasitic weeds. Bioorg. Med. Chem. 2011, 19, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Mwakaboko, A.S.; Zwanenburg, B. Strigolactone analogs derived from ketones using a working model for germination stimulants as a blueprint. Plant Cell Physiol. 2011, 52, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Lachia, M.; Jung, P.M.; De Mesmaeker, A. A novel approach toward the synthesis of strigolactones through intramolecular [2+2] cycloaddition of ketenes and ketene-iminiums to olefins. Application to the asymmetric synthesis of GR-24. Tetrahedron Lett. 2012, 53, 4514–4517. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Mwakaboko, A.S. Strigolactone analogues and mimics derived from phthalimide, saccharine, p-tolylmalondialdehyde, benzoic and salicylic acid as scaffolds. Biorg. Med. Chem. 2011, 19, 7394–7400. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Nayak, S.K.; Charnikhova, T.V.; Bouwmeester, H.J. New strigolactone mimics: Structure–activity relationship and mode of action as germinating stimulants for parasitic weeds. Bioorg. Med. Chem. Lett. 2013, 23, 5182–5186. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, M.; Soudek, P.; Vanek, T. Triazolide Strigolactone Mimics Influence Root Development in Arabidopsis. J. Nat. Prod. 2017, 80, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Heugebaert, T.; Matthys, C.; Van Deun, R.; Boyer, F.D.; Goormachtig, S.; Stevens, C.; Geelen, D. A fluorescent alternative to the synthetic strigolactone GR24. Mol. Plant 2013, 6, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, C.; Bonfante, P.; Deagostino, A.; Kapulnik, Y.; Larini, P.; Occhiato, E.G.; Prandi, C.; Venturello, P. A new class of conjugated strigolactone analogues with fluorescent properties: synthesis and biological activity. Org. Biomol. Chem. 2009, 7, 3413–3420. [Google Scholar] [CrossRef] [PubMed]
- Fornier, S.D.; de Saint Germain, A.; Retailleau, P.; Pillot, J.-P.; Taulera, Q.; Andna, L.; Miesch, L.; Rochange, S.; Pouvreau, J.-B.; Boyer, F.-D. Noncanonical Strigolactone Analogues Highlight Selectivity for Stimulating Germination in Two Phelipanche ramosa Populations. J. Nat. Prod. 2022, 85, 1976–1992. [Google Scholar] [CrossRef]
- Fukui, K.; Ito, S.; Asami, T. Selective Mimics of Strigolactone Actions and Their Potential Use for Controlling Damage Caused by Root Parasitic Weeds. Mol. Plant 2013, 6, 88–99. [Google Scholar] [CrossRef]
- Cala, A.; Ghooray, K.; Fernández-Aparicio, M.; Molinillo, J.M.; Galindo, J.C.; Rubiales, D.; Macías, F.A. Phthalimide-derived strigolactone mimics as germinating agents for seeds of parasitic weeds. Pest Manag. Sci. 2016, 72, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Oancea, F.; Georgescu, E.; Matusova, R.; Georgescu, F.; Nicolescu, A.; Raut, I.; Jecu, M.-L.; Vladulescu, M.-C.; Vladulescu, L.; Deleanu, C. New strigolactone mimics as exogenous signals for rhizosphere organisms. Molecules 2017, 22, 961. [Google Scholar] [CrossRef] [PubMed]
- de Saint Germain, A.; Retailleau, P.; Norsikian, S.; Servajean, V.; Pelissier, F.; Steinmetz, V.; Pillot, J.-P.; Rochange, S.; Pouvreau, J.-B.; Boyer, F.-D. Contalactone, a contaminant formed during chemical synthesis of the strigolactone reference GR24 is also a strigolactone mimic. Phytochemistry 2019, 168, 112112. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, M.C.; Dakas, P.-Y.; De Mesmaeker, A. Synthetic Access to Noncanonical Strigolactones: Syntheses of Carlactonic Acid and Methyl Carlactonoate. J. Org. Chem. 2018, 83, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, B.; Blanco-Ania, D. Strigolactones: New plant hormones in the spotlight. J. Exp. Bot. 2018, 69, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Xi, Z. Strigolactone agonists/antagonists for agricultural applications: New opportunities. Adv. Agrochem. 2022, 1, 61–72. [Google Scholar] [CrossRef]
- Yao, R.; Ming, Z.; Yan, L.; Li, S.; Wang, F.; Ma, S.; Yu, C.; Yang, M.; Chen, L.; Chen, L. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature 2016, 536, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Conn, C.E.; Bythell-Douglas, R.; Neumann, D.; Yoshida, S.; Whittington, B.; Westwood, J.H.; Shirasu, K.; Bond, C.S.; Dyer, K.A.; Nelson, D.C. PLANT EVOLUTION. Convergent evolution of strigolactone perception enabled host detection in parasitic plants. Science 2015, 349, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Wang, F.; Ming, Z.; Du, X.; Chen, L.; Wang, Y.; Zhang, W.; Deng, H.; Xie, D. ShHTL7 is a non-canonical receptor for strigolactones in root parasitic weeds. Cell Res. 2017, 27, 838–841. [Google Scholar] [CrossRef]
- Prandi, C.; Occhiato, E.G.; Tabasso, S.; Bonfante, P.; Novero, M.; Scarpi, D.; Bova, M.E.; Miletto, I. New potent fluorescent analogues of strigolactones: Synthesis and biological activity in parasitic weed germination and fungal branching. Eur. J. Org. Chem. 2011, 2011, 3781–3793. [Google Scholar] [CrossRef]
- Prandi, C.; Rosso, H.; Lace, B.; Occhiato, E.G.; Oppedisano, A.; Tabasso, S.; Alberto, G.; Blangetti, M. Strigolactone analogs as molecular probes in chasing the (SLs) receptor/s: Design and synthesis of fluorescent labeled molecules. Mol. Plant 2013, 6, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Goossens, H.; Heugebaert, T.S.; Dereli, B.; Van Overtveldt, M.; Karahan, O.; Dogan, I.; Waroquier, M.; Van Speybroeck, V.; Aviyente, V.; Catak, S. Elucidating the structural isomerism of fluorescent strigolactone analogue CISA-1. Eur. J. Org. Chem. 2015, 2015, 1211–1217. [Google Scholar] [CrossRef]
- Lace, B.; Prandi, C. Shaping small bioactive molecules to untangle their biological function: A focus on fluorescent plant hormones. Mol. Plant 2016, 9, 1099–1118. [Google Scholar] [CrossRef] [PubMed]
- Parisotto, S.; Lace, B.; Artuso, E.; Lombardi, C.; Deagostino, A.; Scudu, R.; Garino, C.; Medana, C.; Prandi, C. Heck functionalization of an asymmetric aza-BODIPY core: Synthesis of far-red infrared probes for bioimaging applications. Org. Biomol. Chem. 2017, 15, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Oancea, A.; Georgescu, E.; Georgescu, F.; Nicolescu, A.; Oprita, E.I.; Tudora, C.; Vladulescu, L.; Vladulescu, M.-C.; Oancea, F.; Deleanu, C. Isoxazole derivatives as new nitric oxide elicitors in plants. Beilstein J. Org. Chem. 2017, 13, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Georgescu, E.; Oancea, A.; Georgescu, F.; Nicolescu, A.; Oprita, E.I.; Vladulescu, L.; Vladulescu, M.-C.; Oancea, F.; Shova, S.; Deleanu, C. Schiff bases containing a furoxan moiety as potential nitric oxide donors in plant tissues. PLoS ONE 2018, 13, e0198121. [Google Scholar] [CrossRef] [PubMed]
- Bala, I.; Trica, B.; Georgescu, F.; Georgescu, E.; Constantinescu-Aruxandei, D.; Shaposhnikov, S.; Oancea, F. The effect of a strigolactone mimic on growth and colony morphology in phytopathogenic fungi. AgroLife Sci. J. 2021, 10, 36–47. [Google Scholar] [CrossRef]
- Tariq, A.; Ullah, I.; Sardans, J.; Graciano, C.; Mussarat, S.; Ullah, A.; Zeng, F.; Wang, W.; Al-Bakre, D.A.; Ahmed, Z.; et al. Strigolactones can be a potential tool to fight environmental stresses in arid lands. Environ. Res. 2023, 229, 115966. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Xu, K.; Li, X.; Liu, J.; Altaf, M.A.; Fu, H.; Lu, X.; Cheng, S.; Wang, Z. Exogenous strigolactone enhanced the drought tolerance of pepper (Capsicum chinense) by mitigating oxidative damage and altering the antioxidant mechanism. Plant Cell Rep. 2024, 43, 106. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 426696. [Google Scholar] [CrossRef]
- Banerjee, S.; Veale, E.B.; Phelan, C.M.; Murphy, S.A.; Tocci, G.M.; Gillespie, L.J.; Frimannsson, D.O.; Kelly, J.M.; Gunnlaugsson, T. Recent advances in the development of 1,8-naphthalimide based DNA targeting binders, anticancer and fluorescent cellular imaging agents. Chem. Soc. Rev. 2013, 42, 1601–1618. [Google Scholar] [CrossRef] [PubMed]
- Bekere, L.; Gachet, D.; Lokshin, V.; Marine, W.; Khodorkovsky, V. Synthesis and spectroscopic properties of 4-amino-1,8-naphthalimide derivatives involving the carboxylic group: A new molecular probe for ZnO nanoparticles with unusual fluorescence features. Beilstein J. Org. Chem. 2013, 9, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Marinova, N.V.; Georgiev, N.I.; Bojinov, V.B. Facile synthesis, sensor activity and logic behaviour of 4-aryloxy substituted 1, 8-naphthalimide. J. Photochem. Photobiol. A 2013, 254, 54–61. [Google Scholar] [CrossRef]
- Saini, A.; Thomas, K.J.; Huang, Y.-J.; Ho, K.-C. Synthesis and characterization of naphthalimide-based dyes for dye sensitized solar cells. J. Mater. Sci. Mater. Electron. 2018, 29, 16565–16580. [Google Scholar] [CrossRef]
- Ulla, H.; Garudachari, B.; Satyanarayan, M.; Umesh, G.; Isloor, A. Blue organic light emitting materials: Synthesis and characterization of novel 1, 8-naphthalimide derivatives. Opt. Mater. 2014, 36, 704–711. [Google Scholar] [CrossRef]
- Medel, S.; Bosch, P.; Grabchev, I.; de la Torre, M.C.; Ramírez, P. Click chemistry to fluorescent hyperbranched polymeric sensors. 2. Synthesis, spectroscopic and cation-sensing properties of new green fluorescent 1, 8-naphthalimides. Eur. Polym. J. 2016, 74, 241–255. [Google Scholar] [CrossRef]
- Nicolescu, A.; Airinei, A.; Georgescu, E.; Georgescu, F.; Tigoianu, R.; Oancea, F.; Deleanu, C. Synthesis, photophysical properties and solvatochromic analysis of some naphthalene-1, 8-dicarboxylic acid derivatives. J. Mol. Liq. 2020, 303, 112626. [Google Scholar] [CrossRef]
- Bala, I.; Airinei, A.; Georgescu, E.; Oancea, F.; Georgescu, F.; Nicolescu, A.; Tigoianu, R.; Deleanu, C. Photophysical and biological properties of a strigolactone mimic derived from 1, 8-naphthalic anhydride. Rev. Roum. Chim. 2022, 67, 51–62. [Google Scholar]
- Tigoianu, R.; Airinei, A.; Georgescu, E.; Nicolescu, A.; Georgescu, F.; Isac, D.L.; Deleanu, C.; Oancea, F. Synthesis and solvent dependent fluorescence of some piperidine-substituted naphthalimide derivatives and consequences for water sensing. Int. J. Mol. Sci. 2022, 23, 2760. [Google Scholar] [CrossRef]
- MacAlpine, G.A.; Raphael, R.A.; Shaw, A.; Taylor, A.W.; Wild, H.-J. Synthesis of the germination stimulant (±)-strigol. J. Chem. Soc. Perkin Trans. 1 1976, 410–416. [Google Scholar] [CrossRef]
- Sugamoto, K.; Matsushita, Y.i.; Kameda, Y.h.; Suzuki, M.; Matsui, T. Microwave-assisted synthesis of N-hydroxyphthalimide derivatives. Synth. Commun. 2005, 35, 67–70. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Yoshimura, M.; Sato, Y.; Kuwata, K.; Toh, S.; Holbrook-Smith, D.; Zhang, H.; McCourt, P.; Itami, K.; Kinoshita, T.; et al. PARASITIC PLANTS. Probing strigolactone receptors in Striga hermonthica with fluorescence. Science 2015, 349, 864–868. [Google Scholar] [CrossRef] [PubMed]
- de Saint Germain, A.; Clavé, G.; Boyer, F.-D. Synthesis of profluorescent strigolactone probes for biochemical studies. In Strigolactones: Methods in Molecular Biology; Prandi, C., Cardinale, F., Eds.; Humana: New York, NY, USA, 2021; Volume 2309, pp. 219–231. [Google Scholar]
- Wang, D.-W.; Yu, S.-Y.; Pang, Z.-L.; Ma, D.-J.; Liang, L.; Wang, X.; Wei, T.; Yang, H.-Z.; Ma, Y.-Q.; Xi, Z. Discovery of a broad-spectrum fluorogenic agonist for strigolactone receptors through a computational approach. J. Agric. Food Chem. 2021, 69, 10486–10495. [Google Scholar] [CrossRef] [PubMed]
- de Saint Germain, A.; Clave, G.; Schouveiler, P.; Pillot, J.P.; Singh, A.V.; Chevalier, A.; Daignan Fornier, S.; Guillory, A.; Bonhomme, S.; Rameau, C.; et al. Expansion of the Strigolactone Profluorescent Probes Repertory: The Right Probe for the Right Application. Front. Plant Sci. 2022, 13, 887347. [Google Scholar] [CrossRef] [PubMed]
- Belmondo, S.; Marschall, R.; Tudzynski, P.; López Ráez, J.A.; Artuso, E.; Prandi, C.; Lanfranco, L. Identification of genes involved in fungal responses to strigolactones using mutants from fungal pathogens. Curr. Genet. 2017, 63, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Yasui, R.; Kameoka, H.; Tamiru, M.; Cao, M.; Terauchi, R.; Sakurada, A.; Hirano, R.; Kisugi, T.; Hanada, A.; et al. Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat. Commun. 2019, 10, 191. [Google Scholar] [CrossRef]
- Fiorilli, V.; Forgia, M.; de Saint Germain, A.; D’Arrigo, G.; Cornu, D.; Le Bris, P.; Al-Babili, S.; Cardinale, F.; Prandi, C.; Spyrakis, F.; et al. A structural homologue of the plant receptor D14 mediates responses to strigolactones in the fungal phytopathogen Cryphonectria parasitica. New Phytol. 2022, 234, 1003–1017. [Google Scholar] [CrossRef]




| Sample | Solvent | λmax (nm) | λem (nm) | Δν (cm−1) | Φ (%) |
|---|---|---|---|---|---|
| 7 | PhMe | 330 sh, 343.5, 359 | 408.3 | 4621 | 5.49 |
| DCM | 255, 325 sh, 343.5, 358.5 | 382 sh, 394.4 | 3757 | 6.82 | |
| DMF | 325 sh, 342.5, 355 sh | 386 sh, 400 | 4197 | 0.01 | |
| 10 | PhMe | 325 sh, 345, 360 | 412 | 4713 | 0.65 |
| DCM | 325 sh, 345, 360 | 378, 396, 417 | 3733 | 1.57 | |
| DMF | 325 sh, 343, 356 | 387 sh, 402 | 4279 | 0.89 |
| Sample | Solvent | τ1 (ns) | τ2 (ns) | τ3 (ns) | a1 (%) | a2 (%) | a3 (%) |
|---|---|---|---|---|---|---|---|
| 7 | PhMe | 0.94 | 3.95 | - | 82.14 | 17.86 | - |
| DMF | 0.03 | - | - | 100 | - | - | |
| 10 | DCM | 0.03 | 1.39 | 7.26 | 24.95 | 14.51 | 60.54 |
| DMF | 0.33 | 4.99 | - | 53.86 | 46.14 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bala, I.-A.; Nicolescu, A.; Georgescu, F.; Dumitrascu, F.; Airinei, A.; Tigoianu, R.; Georgescu, E.; Constantinescu-Aruxandei, D.; Oancea, F.; Deleanu, C. Synthesis and Biological Properties of Fluorescent Strigolactone Mimics Derived from 1,8-Naphthalimide. Molecules 2024, 29, 2283. https://doi.org/10.3390/molecules29102283
Bala I-A, Nicolescu A, Georgescu F, Dumitrascu F, Airinei A, Tigoianu R, Georgescu E, Constantinescu-Aruxandei D, Oancea F, Deleanu C. Synthesis and Biological Properties of Fluorescent Strigolactone Mimics Derived from 1,8-Naphthalimide. Molecules. 2024; 29(10):2283. https://doi.org/10.3390/molecules29102283
Chicago/Turabian StyleBala, Ioana-Alexandra, Alina Nicolescu, Florentina Georgescu, Florea Dumitrascu, Anton Airinei, Radu Tigoianu, Emilian Georgescu, Diana Constantinescu-Aruxandei, Florin Oancea, and Calin Deleanu. 2024. "Synthesis and Biological Properties of Fluorescent Strigolactone Mimics Derived from 1,8-Naphthalimide" Molecules 29, no. 10: 2283. https://doi.org/10.3390/molecules29102283
APA StyleBala, I.-A., Nicolescu, A., Georgescu, F., Dumitrascu, F., Airinei, A., Tigoianu, R., Georgescu, E., Constantinescu-Aruxandei, D., Oancea, F., & Deleanu, C. (2024). Synthesis and Biological Properties of Fluorescent Strigolactone Mimics Derived from 1,8-Naphthalimide. Molecules, 29(10), 2283. https://doi.org/10.3390/molecules29102283






