Abstract
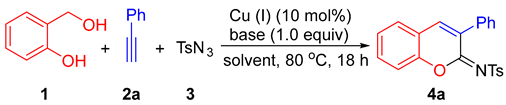
In this paper, an alternative and efficient copper(I)-catalyzed synthesis of 2-sulfonyliminocoumarins is developed through a three-component reaction of ortho-hydroxybenzyl alcohol, alkynes, and p-toluenesulfonyl azide. The proposed route for access to the 2-iminocoumarin ring involves a [4 + 2] hetero-Diels-Alder reaction between ortho-quinone methide and ketenimine intermediates generated in situ.
1. Introduction
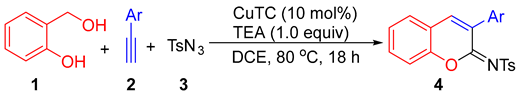
Coumarin-containing compounds are important oxygen-heterocycles that possess notable pharmacological and biological activities [1,2,3,4]. The synthesis, transformation, and biological activity of 2-iminocoumarins are of significant interest in synthetic chemistry and medicinal chemistry [5,6,7,8]. In addition, the N-sulfonylimino group is a key motif in organic transformations [9,10,11]; thus, the synthesis of 2-sulfonyliminocoumarins has been well-developed recently (Scheme 1). This includes copper(I)-catalyzed three-component cyclocoupling reactions of alkyne, sulfonyl azide, and ortho-functionalized phenols, such as salicylaldehydes (Equation (1)) [12,13], ortho-ethynylphenols (Equation (2)) [14], and ortho-hydroxybenzonitriles (Equation (3)) [15]; copper(I)-catalyzed cyclocoupling reactions of ynal, phenol, and sulfonyl azide (Equation (4)) [16]; as well as metal catalyst-free cyclocoupling reactions of salicylaldehydes, arylacetonitriles, and arylsulfonyl chlorides in 2-methyl-THF (tetrahydrofuran) solvent (Equation (5)) [17]. Very recently, a Rh(I)/Ag(I)-catalyzed reaction of aryl thiocarbamates, internal alkynes, and sulfonamides was also reported to efficiently afford 2-sulfonyliminocoumarins [18].
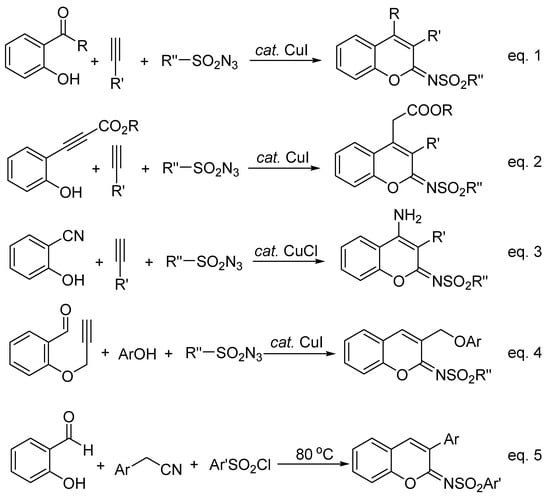
Scheme 1.
Recent reports on the synthesis of 2-sulfonyliminocoumarins using phenols.
In a continuation of our studies on the synthesis of oxygen-heterocyclic compounds [19,20,21,22] and the development of alkyne annulations [23], we are interested in establishing an alternative synthetic procedure for the formation of 2-sulfonyliminocoumarins. This involves a three-component cyclocoupling reaction of alkyne, sulfonyl azide, and ortho-hydroxybenzyl alcohol, which are cheaper and more stable substrates compared with those used in known procedures. As depicted in Scheme 2, the new designed procedure for accessing 2-sulfonyliminocoumarins involves the formation of two key intermediates: ortho-quinone methide [24,25,26] and ketenimine [12,27,28,29], followed by their regioselective [4 + 2] hetero-Diels-Alder reaction and oxidative dehydrogenative aromatization (ODA).
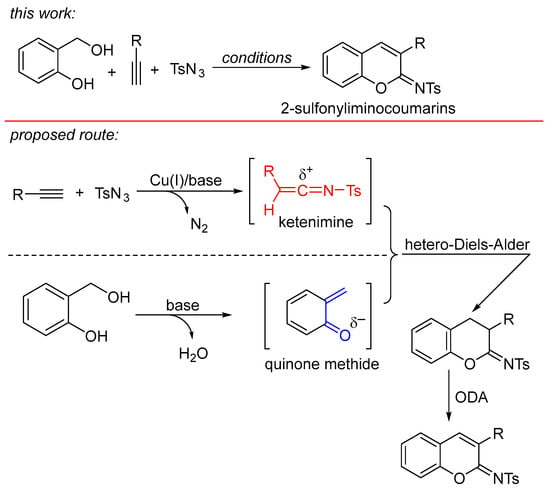
Scheme 2.
Designed hetero-Diels-Alder reaction of ortho-quinone methide with ketenimine for access to 2-sulfonyliminocoumarins.
2. Results and Discussion
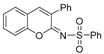
Based on the above-mentioned formation of ketenimine in the presence of copper (I) salts [12,27,28,29], we examined reactions using various copper (I) salts as catalysts and simple bases in different solvents under an air atmosphere. As summarized in Table 1, in THF solvent with triethylamine (TEA) as the base, CuCl, CuBr, and CuI showed a similar and effective catalytic activity, catalyzing cyclocondensation of 2-(hydroxymethyl)phenol (1), phenyl acetylene (2a), and p-toluenesulfonyl azide (3) at 80 °C, affording the desired product 4a in 65%, 70%, and 65% isolated yields, respectively (entries 1–3). The structure of 4a was confirmed unambiguously through X-ray diffraction studies [30 and see Supplementary Materials]. When 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) and K2CO3 were used as bases, the 4a yield decreased greatly (entries 4–5). The screening of solvents using TEA showed that 1,2-dichloroethane (DCE) was a better solvent for CuI-catalyzed formation of 4a (entry 6). DCE was the best solvent when compared with THF, dioxane, DMF (N,N-dimethylformamide), and toluene, with the use of CuTC (copper(I)-thiophene-2-carboxylate) as catalyst to afford 4a in 82% yield (entries 7–11). When 5.0 mol% of CuTC was used, the yield of 4a decreased to 51% (entry 12), and in the absence of a catalyst, no desired product formed at all (entry 13). In addition, under a nitrogen atmosphere, the yield of 4a decreased to 11%.

Table 1.
Optimization of the reaction conditions a.
Then, we examined the reactions of 2-(hydroxymethyl)phenol (1) and p-toluenesulfonyl azide (3) with various aromatic terminal alkynes under the optimized reaction condition (indicated in entry 11 of Table 1) to explore the functional group tolerance. As shown in Table 2, compared with 2a, 1-naphthyl acetylene (2b) and 2-naphthyl acetylene (2c) underwent the cyclocondensation to provide slightly lower yields of 4b and 4c. Both electron-rich and electron-poor aromatic terminal alkynes showed a good reactivity, resulting in yields of 62–81% for the corresponding products, and the reactions of the electron-rich aromatic terminal alkynes resulted in improved yields for the products. For example, the reactions of aromatic terminal alkynes bearing electron-donating groups, such as Me (2d–2f), Et (2g), n-Pr (2h), t-Bu (2i), and MeO (2j–2k), produced the desired products with yields of 65–81% (4d–4k). Because of the steric hindrance, ortho- and meta-substituents resulted in a slightly lower yield (4d vs. 4e, 4j vs. 4k). The reactions of electron-poor aromatic terminal alkynes with F (2l–2m), Cl (2n), and Br (2o) groups afforded the products (4l–4o) in 62–73% yields, and these products could easily further derivate with the possible activation of the carbon–halogen bond. In addition, heteroaromatic terminal alkynes (2p) could be tolerated in this reaction, resulting in a yield of 76% for the desired product (4p). Moreover, the reaction of 1, 2a, and benzenesulfonyl azide resulted in a yield of 55% for the corresponding product 4q, while the reaction of 1, 2a, and methanesulfonyl azide did not afford the expected product at all. Note that when aliphatic terminal alkynes were used, none of the expected products formed either.

Table 2.
Scope for the formation of 2-sulfonyliminocoumarins a.
3. Materials and Methods
3.1. General Methods
Column chromatography was performed with silica gel and analytical thin-layer chromatography (TLC) was performed on 0.2 mm silica-gel-coated glass sheets. All yields refer to isolated yields. Nuclear magnetic resonance (NMR) spectra were recorded on JEOL 400 (Tokyo, Japan) using CDCl3 or DMSO-d6 as solvents at 298 K. 1H NMR (400 MHz) chemical shifts (δ) referenced the internal standard TMS (δ = 0.00 ppm). 13C{1H} NMR (101 MHz) chemical shifts referenced internal solvent CDCl3 (δ = 77.16 ppm) or DMSO-d6 (δ = 39.52 ppm). High-resolution mass spectra (HRMS) with electron spray ionization (ESI) were obtained with a micrOTOF-Q spectrometer (Agilent, California, CA, USA). Single-crystal X-ray diffraction data were obtained using a SuperNova diffractometer (Tokyo, Japan) with Cu Kα radiation at a low temperature (173.15 K).
Caution! p-Toluenesulfonyl azide (TsN3, 3) can undergo explosive decomposition at 120°. Although it is safe at 80 °C in a sealed vessel under standard reaction conditions, it is potentially explosive. Therefore, all of the reactions were carried out behind a safety shield in a hood.
3.2. Typical Experimental Procedure for the Synthesis of 4-Methyl-N-(3-phenyl-2H-chromen-2-ylidene)benzenesulfonamide (4a)
A mixture of 2-(hydroxymethyl)phenol (1, 124.1 mg, 1.0 mmol), phenyl acetylene (2a, 102.0 mg, 1.0 mmol), sulfonyl azide (3, 197.0 mg, 1.0 mmol), TEA (102.0 mg, 1.0 mmol), CuTC (19.0 mg, 0.1 mmol), and DCE (4.0 mL) under an air atmosphere in a 25 mL screw-capped thick-walled Pyrex tube was stirred at 80 °C for 18 h in an oil bath. After the reaction mixture was cooled to room temperature, it was poured into a solvent mixture of water (50.0 mL) and ethyl acetate (20.0 mL), and the two phases were then separated. The aqueous layer was extracted with ethyl acetate (3 × 20.0 mL). The combined organic solvent was dried over anhydrous Na2SO4. After removal of the organic solvent under reduced pressure, the residue was purified by column chromatography on silica gel with petroleum ether/ethyl acetate (gradient mixture ratio from 100:0 to 80:20) as the eluent to afford 4a as a pale yellow solid (255.2 mg, 0.82 mmol, 82%). The single crystals of 4a were obtained by slow evaporation of its solution in a mixture solvent of petroleum ether and CH2Cl2 at room temperature.
3.3. Characterization Data of Products
4-Methyl-N-(3-phenyl-2H-chromen-2-ylidene)benzenesulfonamide (4a) [12]: Pale yellow solid (255.2 mg, 82%). 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.3 Hz, 2H), 7.71 (s, 1H), 7.62–7.27 (m, 11H), 2.40 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.5, 152.4, 143.1, 139.7, 139.4, 134.5, 132.0, 130.3, 129.3, 129.2, 129.1, 128.4, 128.2, 127.3, 125.8, 119.9, 116.6, 21.6. HRMS (ESI) m/z: [M + H]+ Calcd for C22H18NO3S 376.1002, found 376.1006.
4-Methyl-N-(3-(naphthalen-1-yl)-2H-chromen-2-ylidene)benzenesulfonamide (4b): White solid (235.8 mg, 65%). 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.0 Hz, 2H), 7.75 (s, 1H), 7.71– 7.37 (m, 11H), 7.13 (d, J = 8.0 Hz, 2H), 2.34 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 158.0, 152.9, 142.9, 141.9, 139.2, 133.7, 132.4, 132.3, 131.5, 130.0, 129.8, 129.5, 129.1, 128.6, 128.3, 127.8, 127.1, 126.6, 126.5, 126.1, 125.4, 125.3, 119.6, 116.9, 21.6. HRMS (ESI) m/z: [M + H]+ Calcd for C26H20NO3S 426.1158, found 426.1162.
4-Methyl-N-(3-(naphthalen-2-yl)-2H-chromen-2-ylidene)benzenesulfonamide (4c): White solid (246.8 mg, 68%). m.p. 208.4–209.7 °C. 1H NMR (400 MHz, CDCl3) δ 8.04 (s, 1H), 7.93 (d, J = 8.2 Hz, 2H), 7.80–7.76 (m, 6H), 7.71 (d, J = 8.5 Hz, 1H), 7.60–7.46 (m, 3H), 7.35 (t, J = 7.5 Hz, 1H), 7.26–7.26 (d, J = 2.6 Hz, 2H), 2.40 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.6, 152.4, 143.5, 143.1, 140.09, 139.2, 133.4, 133.0, 132.0, 129.7, 129.3, 128.6, 128.4, 128.3, 127.77, 127.71, 127.2, 126.9, 126.6, 126.4, 125.9, 119.9, 116.6, 21.5. HRMS (ESI) m/z: [M + H] + Calcd for C26H20NO3S 426.1158, found 426.1162.
4-Methyl-N-(3-(p-tolyl)-2H-chromen-2-ylidene)benzenesulfonamide (4d) [17]: White solid (260.0 mg, 80%). 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.2 Hz, 2H), 7.65 (s, 1H), 7.53–7.47 (m, 4H), 7.41 (d, J = 8.2 Hz, 1H), 7.31–7.26 (m, 3H), 7.16 (d, J = 8.0 Hz, 2H), 2.38 (s, 3H), 2.34 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.5, 152.1, 143.0, 139.3, 139.2, 139.0, 131.7, 131.4, 129.9, 129.2, 128.9, 128.2, 127.3, 125.7, 119.8, 116.2, 21.5, 21.3. HRMS (ESI) m/z: [M + H]+ Calcd for C23H20NO3S 390.1158, found 390.1162.
4-Methyl-N-(3-(o-tolyl)-2H-chromen-2-ylidene)benzenesulfonamide (4e): White solid (232.2 mg, 71%). 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 8.2 Hz, 2H), 7.61–7.55 (m, 2H), 7.50–7.47 (m, 2H), 7.33–7.24 (m, 2H), 7.20–7.10 (m, 5H), 2.38 (s, 3H), 2.25 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.3, 152.7, 143.0, 140.8, 139.4, 136.9, 134.5, 132.0, 131.4, 130.3, 129.8, 129.2, 128.9, 128.2, 127.3, 125.9, 125.8, 119.6, 116.7, 21.6, 21.5. HRMS (ESI) m/z: [M + H]+ Calcd for C23H20NO3S 390.1158, found 390.1162.
4-Methyl-N-(3-(m-tolyl)-2H-chromen-2-ylidene)benzenesulfonamide (4f): White solid (255.0 mg, 78%). m.p. 177.0–179.0 °C 1H NMR (400 MHz, CDCl3) δ 7.94 (d, J = 8.2 Hz, 2H), 7.69 (s, 1H), 7.57–7.45 (m, 2H), 7.45–7.26 (m, 7H), 7.19 (d, J = 8.0 Hz, 1H), 2.39 (s, 3H), 2.36 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.5, 152.3, 143.0, 139.5, 137.9, 134.4, 131.9, 130.3, 129.8, 129.28, 129.26, 128.2, 127.2, 126.3, 125.8, 119.9, 116.6, 21.6, 21.5. HRMS (ESI) m/z: [M + H]+ Calcd for C23H20NO3S 390.1158, found 390.1162.
N-(3-(4-Ethylphenyl)-2H-chromen-2-ylidene)-4-methylbenzenesulfonamide (4g): White solid (255.7 mg, 75%). m.p. 189.0–190.0 °C. 1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 8.2 Hz, 2H), 7.67 (s, 1H), 7.52–7.49 (m, 4H), 7.42 (d, J = 8.2 Hz, 1H), 7.30–7.27 (m, 3H), 7.22 –7.20 (m, 2H), 2.66 (q, J = 7.6 Hz, 2H), 2.39 (s, 3H), 1.25 (t, J = 7.6, 3H). 13C NMR (101 MHz, CDCl3) δ 157.6, 152.2, 145.4, 143.1, 139.3, 139.2, 131.8, 131.7, 130.0, 129.2, 129.1, 128.2, 127.8, 127.4, 125.7, 119.9, 116.3, 28.7, 21.6, 15.4. HRMS (ESI) m/z: [M + H]+ Calcd for C24H22NO3S 404.1315, found 404.1319.
4-Methyl-N-(3-(4-propylphenyl)-2H-chromen-2-ylidene)benzenesulfonamide (4h): White solid (277.2 mg, 78%). m.p. 199.0–201.0 °C. 1H NMR (400 MHz, CDCl3) δ 7.99–7.94 (m, 2H), 7.68–7.67 (m, 1H), 7.57–7.41 (m, 5H), 7.36–7.26 (m, 3H), 7.23–7.18 (m, 2H), 2.61 (t, J = 7.6, 2H), 2.40 (s, 3H), 1.72–1.60 (m, 2H), 0.97 (t, J = 7.2, 3H). 13C NMR (101 MHz, CDCl3) δ 157.6, 152.2, 143.9, 143.1, 139.4, 139.2, 131.8, 130.1, 129.2, 129.0, 128.4, 128.1, 127.4, 125.7, 120.0, 116.4, 37.9, 24.4, 21.6, 13.9. HRMS (ESI) m/z: [M + H]+ Calcd for C25H24NO3S 418.1471, found 418.1475.
N-(3-(4-(t-Butyl)phenyl)-2H-chromen-2-ylidene)-4-methylbenzenesulfonamide (4i): White solid (298.9 mg, 81%). m.p. 193.2–194.9 °C. 1H NMR (400 MHz, CDCl3) δ 7.98 (d, J = 8.2 Hz, 2H), 7.69 (s, 1H), 7.59–7.49 (m, 4H), 7.42–7.34 (m, 3H), 7.34–7.26 (m, 3H), 2.40 (s, 3H), 1.34 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 157.6, 152.3, 143.1, 139.38, 139.31, 131.8, 131.5, 130.1, 129.3, 128.8, 128.2, 127.5, 125.8, 125.4, 120.0, 116.4, 34.8, 31.3, 21.6. HRMS (ESI) m/z: [M + H]+ Calcd for C26H26NO3S 432.1628, found 432.1632.
N-(3-(4-Methoxyphenyl)-2H-chromen-2-ylidene)-4-methylbenzenesulfonamide (4j) [12]: White solid (259.2 mg, 76%). 1H NMR (400 MHz, CDCl3) δ 1H NMR (400 MHz, CDCl3) δ 7.96 (d, J = 8.3 Hz, 2H), 7.66 (s, 1H), 7.58–7.43 (m, 4H), 7.35–7.27 (m, 4H), 6.92 (d, J = 8.3 Hz, 2H), 3.84 (s, 3H), 2.40 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 160.3, 157.6, 152.1, 143.0, 139.3, 138.5, 131.6, 130.5, 129.7, 129.2, 128.0, 127.3, 126.7, 125.7, 120.0, 116.4, 113.7, 55.4, 21.6. HRMS (ESI) m/z: [M + H]+ Calcd for C23H20NO4S 406.1108, found 406.1112.
N-(3-(3-Methoxyphenyl)-2H-chromen-2-ylidene)-4-methylbenzenesulfonamide (4k): White solid (222.8 mg, 65%). 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.2 Hz, 2H), 7.71 (s, 1H), 7.58–7.50 (m, 2H), 7.44 (d, J = 8.2 Hz, 1H), 7.35–7.28 (m, 4H), 7.16–7.13 (m, 2H), 6.91– 6.88 (m, 1H), 3.73 (s, 3H), 2.39 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 159.3, 157.3, 152.2, 143.1, 139.8, 139.4, 135.6, 132.0, 129.7, 129.3, 129.2, 128.3, 127.2, 125.8, 121.4, 119.7, 116.4, 115.0, 114.5, 55.3, 21.5. HRMS (ESI) m/z: [M + H]+ Calcd for C23H20NO4S 406.1108, found 406.1112.
N-(3-(4-Fluorophenyl)-2H-chromen-2-ylidene)-4-methylbenzenesulfonamide (4l): White solid (240.2 mg, 73%). m.p. 194.2–196.0 °C. 1H NMR (400 MHz, CDCl3) δ 7.92 (d, J = 8.3 Hz, 2H), 7.78 (d, J = 8.2 Hz, 1H), 7.67 (s, 1H), 7.60–7.49 (m, 3H), 7.44–7.42 (m, 1H), 7.33 (t, J = 7.5 Hz, 1H), 7.27–7.26 (m, 2H), 7.05 (t, J = 8.6 Hz, 2H), 2.38 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 163.1 (d, JC-F = 249.1 Hz), 157.4, 152.3, 143.2, 139.6, 139.2, 132.1, 131.1, 130.4, 129.7, 129.3, 128.2, 127.3, 126.5, 125.9, 119.7, 116.5, 115.5, 115.2, 21.6. HRMS (ESI) m/z: [M + H]+ Calcd for C22H17FNO3S 394.0908, found 394.0904.
N-(3-(2-Fluorophenyl)-2H-chromen-2-ylidene)-4-methylbenzenesulfonamide (4m) [17]: White solid (225.0 mg, 68%). 1H NMR (400 MHz, CDCl3) δ 7.90–7.75 (m, 3H), 7.70 (s, 1H), 7.59–7.47 (m, 2H), 7.44–7.40 (m, 1H), 7.39–7.30 (m, 2H), 7.25–7.06 (m, 4H), 2.37 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 159.9 (d, JC-F = 249.2 Hz), 157.0, 152.5, 143.3 (d, JC-F = 16.9 Hz), 139.2 (d, JC-F = 31.2 Hz), 132.4, 131.4, 130.9 (d, JC-F = 8.4 Hz), 129.6, 129.2, 128.4, 127.3, 126.4, 125.9, 124.1 (d, JC-F = 3.2 Hz), 122.2 (d, JC-F = 14.2 Hz), 119.3, 116.5, 115.8, 21.7. HRMS (ESI) m/z: [M + H]+ Calcd for C22H17FNO3S 394.0908, found 394.0912.
N-(3-(3-Chlorophenyl)-2H-chromen-2-ylidene)-4-methylbenzenesulfonamide (4n): White solid (235.6 mg, 68%). 1H NMR (400 MHz, CDCl3) δ 7.94 (d, J = 8.2 Hz, 2H), 7.72 (s, 1H), 7.62–7.48 (m, 5H), 7.39–7.28 (m, 5H), 2.41 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.1, 152.5, 143.2, 140.2, 139.3, 136.2, 134.2, 132.4, 129.6, 129.4, 129.3, 129.2, 128.9, 128.4, 127.4, 127.2. 126.0, 119.6, 116.7, 21.7. HRMS (ESI) m/z: [M + H]+ Calcd for C22H17ClNO3S 410.0612, found 410.0616.
N-(3-(3-Bromophenyl)-2H-chromen-2-ylidene)-4-methylbenzenesulfonamide (4o): White solid (242.2 mg, 62%). 1H NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.2 Hz, 2H), 7.75 (s, 1H), 7.73 (s, 1H), 7.62–7.27 (m, 5H), 7.38 (t, J = 7.5 Hz, 1H), 7.34–7.27 (m, 3H), 2.42 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 157.0, 152.5, 143.2, 140.1, 139.3, 136.4, 132.4, 132.1, 132.0, 129.9, 129.4, 128.7, 128.4, 127.9, 127.2, 126.0, 122.3, 119.6, 116.7, 21.7. HRMS (ESI) m/z: [M + H]+ Calcd for C22H17BrNO3S 456.0088, found 456.0092.
4-Methyl-N-(3-(thiophen-2-yl)-2H-chromen-2-ylidene)benzenesulfonamide (4p): Pale yellow solid (242.4 mg, 76%). m.p. 181.0–183.0 °C. 1H NMR (400 MHz, CDCl3) δ 8.02 (d, J = 7.6 Hz, 2 H), 7.96–7.63 (s, 1H), 7.69–7.68 (m, 1H), 7.55–7.40 (m, 3H), 7.38–7.28 (m, 4H), 7.06–7.03 (m, 1H), 2.41 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 155.5, 151.5, 143.3, 139.2, 135.5, 134.9, 131.8, 129.4, 128.9, 128.1, 127.7, 127.4, 127.1, 125.9, 123.1, 119.5, 116.4, 21.7. HRMS (ESI) m/z: [M + H]+ Calcd for C20H16NO3S2 382.0566, found 382.0570.
N-(3-Phenyl-2H-chromen-2-ylidene)benzenesulfonamide (4q): White solid (201.3 mg, 55%). m.p. 174.0–176.0 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.37 (s, 1H), 8.23 (d, J = 6.9 Hz, 1H), 8.07–7.93 (m, 2H), 7.88–7.70 (m, 6H), 7.69–7.51 (m, 5H). 13C NMR (100 MHz, DMSO-d6) δ 157.3, 151.4, 144.1, 141.6, 141.4, 134.3, 132.8, 132.5, 131.7, 129.1, 129.0, 128.9, 128.1, 127.1, 125.5, 119.6, 115.5. HRMS (ESI) m/z: [M + H]+ Calcd for C21H16NO3S 362.0870, found 362.0866.
4. Conclusions
In conclusion, the syntheses of 2-sulfonyliminocoumarins with decent yields and high atom utilization are developed via a CuTC-catalyzed three-component cyclocondendation of ortho-hydroxybenzyl alcohol, p-toluenesulfonyl azide, and various aromatic terminal alkynes in the presence of a base. The proposed way access the 2-iminocoumarin ring involves a [4 + 2] hetero-Diels-Alder reaction between ortho-quinone methide and ketenimine intermediate, which have an interesting application in the formation of functionalized oxygen-containing heterocyclic compounds.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29143426/s1: the copies of NMR charts of products, X-ray structural details of 4a. References [31,32,33] are cited in the Supplementary Materials.
Author Contributions
Investigation, writing—original draft preparation, D.M.K. and J.L.; supervision, writing—review and editing, R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 21673124 and 21972072.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.; Spillere, A.R.; das Neves, G.M.; Kagami, L.P.; von Poser, G.L.; Canto, R.F.S.; Eifler-Lima, V. Natural and synthetic coumarins as antileishmanial agents: A review. Eur. J. Med. Chem. 2020, 203, 112514. [Google Scholar] [CrossRef] [PubMed]
- Salehian, F.; Nadri, H.; Jalili-Baleh, L.; Youseftabar-Miri, L.; Bukhari, S.N.A.; Foroumadi, A.; Küçükkilinç, T.T.; Sharifzadeh, M.; Khoobi, M. A review: Biologically active 3,4-heterocycle-fused coumarins. Eur. J. Med. Chem. 2021, 212, 113034. [Google Scholar] [CrossRef] [PubMed]
- Deryabin, D.; Inchagova, K.; Rusakova, E.; Duskaev, G. Coumarin’s anti-quorum sensing activity can be enhanced when combined with other plant-derived small molecules. Molecules 2021, 26, 208. [Google Scholar] [CrossRef] [PubMed]
- Volmajer, J.; Toplak, R.; Leban, I.; Le Marechal, A.M. Synthesis of new iminocoumarins and their transformations into N-chloro and hydrazono compounds. Tetrahedron 2005, 61, 7012–7021. [Google Scholar] [CrossRef]
- Guo, D.; Chen, T.; Ye, D.; Xu, J.; Jiang, H.; Chen, K.; Wang, H.; Liu, H. Cell-permeable iminocoumarine-based fluorescent dyes for mitochondria. Org. Lett. 2011, 13, 2884–2887. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.-R.; Man, N.-N.; Yuan, W.-K.; Li, M. Direct construction of 2-aryliminochromenes from arynes, N, S-keteneacetals, and DMF. J. Org. Chem. 2016, 81, 5942–5948. [Google Scholar] [CrossRef] [PubMed]
- Perin, N.; Cindrić, M.; Vervaeke, P.; Liekens, S.; Mašek, T.; Starčević, K.; Hranjec, M. Benzazole substituted iminocoumarins as potential antioxidants with antiproliferative activity. Med. Chem. 2021, 17, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Laws, S.W.; Moore, L.C.; Di Maso, M.J.; Nguyen, Q.N.N.; Tantillo, D.J.; Shaw, J.T. Diastereoselective base-catalyzed formal [4 + 2] cycloadditions of N-sulfonyl imines and cyclic anhydrides. Org. Lett. 2017, 19, 2466–2469. [Google Scholar] [CrossRef]
- Hopkins, M.D.; Scott, K.A.; DeMier, B.C.; Morgan, H.R.; Macgruder, J.A.; Lamar, A.A. Formation of N-sulfonyl imines from iminoiodinanes by iodine-promoted, N-centered radical sulfonamidation of aldehydes. Org. Biomol. Chem. 2017, 15, 9209–9216. [Google Scholar] [CrossRef]
- Shirataki, H.; Ono, T.; Ohashi, M.; Ogoshi, S. Ni(0)-catalyzed three-component coupling reaction of tetrafluoroethylene and N-sulfonyl-substituted imines with silanes via aza-nickelacycles. Org. Lett. 2019, 21, 851–856. [Google Scholar] [CrossRef]
- Cui, S.-L.; Lin, X.-F.; Wang, Y.-G. Novel and efficient synthesis of iminocoumarins via copper-catalyzed multicomponent reaction. Org. Lett. 2006, 8, 4517–4520. [Google Scholar] [CrossRef]
- Mandal, P.K. Copper-catalyzed one-pot synthesis of glycosylated iminocoumarins and 3-triazolyl-2-iminocoumarins. RSC Adv. 2014, 4, 5803–5814. [Google Scholar] [CrossRef]
- Shen, Y.; Cui, S.; Wang, J.; Chen, X.; Lu, P.; Wang, Y. Copper-catalyzed three-component synthesis of 2-iminodihydrocoumarins and 2-iminocoumarins. Adv. Synth. Catal. 2010, 352, 1139–1144. [Google Scholar] [CrossRef]
- Yi, F.; Zhang, S.; Huang, Y.; Zhang, L.; Yi, W. An efficient one-pot protocol for the synthesis of polysubstituted 4-amino-iminocoumarins and 4-aminoquinolines by a copper-catalyzed three-component reaction. Eur. J. Org. Chem. 2017, 102–110. [Google Scholar] [CrossRef]
- Murugavel, G.; Punniyamurthy, T. Novel copper-catalyzed multicomponent cascade synthesis of iminocoumarin aryl methyl ethers. Org. Lett. 2013, 15, 3828–3831. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.S.; Kumar, A.V. Three-component one-pot synthesis of N-arylsulfonyl-2-iminocoumarins. Tetrahedron 2018, 74, 1900–1907. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, S.; Jiang, W.; Zhu, F.; Chen, Y.; Zhao, Y. Multicomponent synthesis of iminocoumarins via rhodium-catalyzed C-H bond activation. J. Org. Chem. 2020, 85, 11006–11013. [Google Scholar] [CrossRef]
- Wu, B.; Hua, R. Palladium-catalyzed [3+2+1] cyclocarbonylative coupling of 1,3-cyclohexanediones, alkynes, and carbon monoxide: An atom-economic route to chromene-2,5-dione derivatives. Tetrahedron Lett. 2010, 51, 6433–6435. [Google Scholar] [CrossRef]
- Zheng, Q.; Hua, R.; Jiang, J.; Zhang, L. A general approach to arylated furans, pyrroles, and thiophenes. Tetrahedron 2014, 70, 8252–8256. [Google Scholar] [CrossRef]
- Nizami, T.A.; Hua, R. Synthesis of 3H-naphtho [2.1-b]pyran-2-carboxamides from cyclocoupling of β-naphthol, propargyl alcohols and isocyanide in the presence of Lewis acids. Tetrahedron 2018, 74, 3776–3780. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Hua, R. Base-promoted chemodivergent formation of 1,4-benzoxazepin-5(4H)-ones and 1,3-benzoxazin-4(4H)-ones switched by solvents. Molecules 2019, 24, 3773. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Hua, R. C–H activation and alkyne annulation via automatic or intrinsic directing groups: Towards high step economy. Chem. Rec. 2018, 18, 556–569. [Google Scholar] [CrossRef] [PubMed]
- Merijan, A.; Gardner, P.D. Quinone methides. Base-catalyzed condensation reactions of hydroxybenzyl alcohols and ethers. J. Org. Chem. 1965, 30, 3965–3967. [Google Scholar] [CrossRef]
- Pathak, T.P.; Sigman, M.S. Applications of ortho-quinone methide intermediates in catalysis and asymmetric synthesis. J. Org. Chem. 2011, 76, 9210–9215. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, A.A.; Scheidt, K.A. Emerging roles of in situ generated quinone methides in metal-free catalysis. J. Org. Chem. 2016, 81, 10145–10153. [Google Scholar] [CrossRef]
- Yoo, E.J.; Bae, I.; Cho, S.H.; Han, H.; Chang, S. A facile access to N-sulfonylimidates and their synthetic utility for the transformation to amidines and amides. Org. Lett. 2006, 8, 1347–1350. [Google Scholar] [CrossRef]
- Raushel, J.; Fokin, V.V. Efficient synthesis of 1-sulfonyl-1,2,3-triazoles. Org. Lett. 2010, 12, 4952–4955. [Google Scholar] [CrossRef]
- Yavari, I.; Ghazanfarpour-Darjani, M.; Nematpour, M. Copper-catalyzed tandem synthesis of 2-(sulfonylimino)alkanamides from N-sulfonylketenimines and alkyl isocyanides. Tetrahedron Lett. 2015, 56, 2416–2417. [Google Scholar] [CrossRef]
- The Structural Data for 4a Are Available Free of Charge from the Cambridge Crystallographic Data Centre with Reference Number CCDC2312012. Available online: https://www.ccdc.cam.ac.uk (accessed on 4 December 2023).
- Bruker. SHELXTL. Structure Determination Programs, Version 5.10; Bruker AXS Inc.: Madison, WI, USA, 1997. [Google Scholar]
- International Tables for X-ray Crystallography: Volume C, Tables 4.2.6.8 and 6.1.1.4; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989.
- Oxford. CrysAlisPro, Agilent Technologies, Version 1.171.36.32; Oxford Diffraction Ltd.: Abingdon, UK, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).