C(sp)-C(sp) Lever-Based Targets of Orientational Chirality: Design and Asymmetric Synthesis
Abstract
1. Introduction
2. Results and Discussion
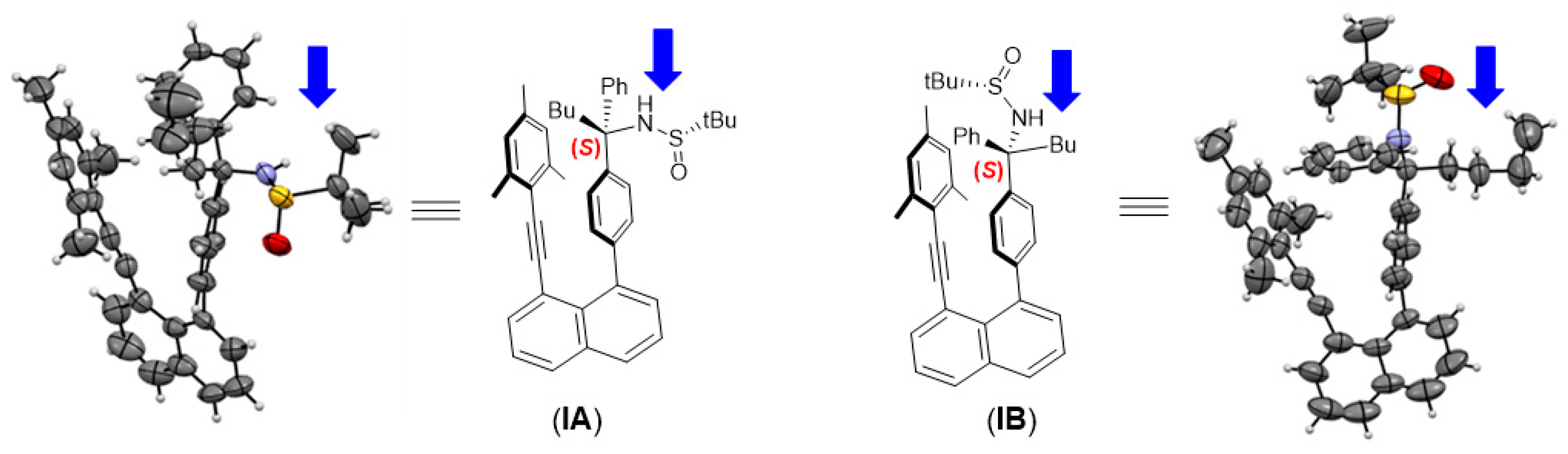
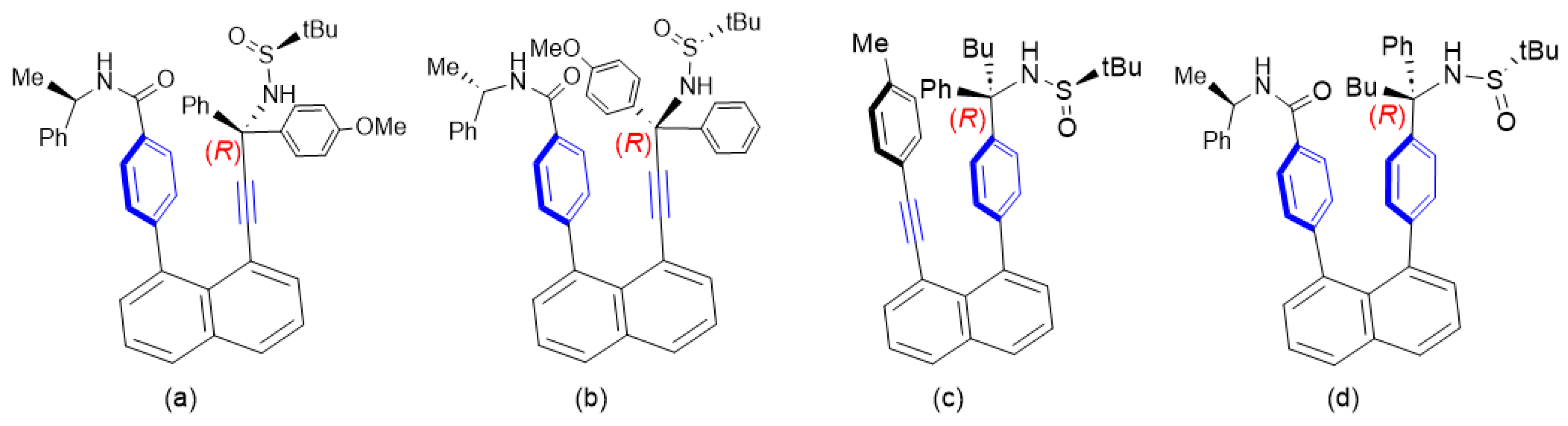
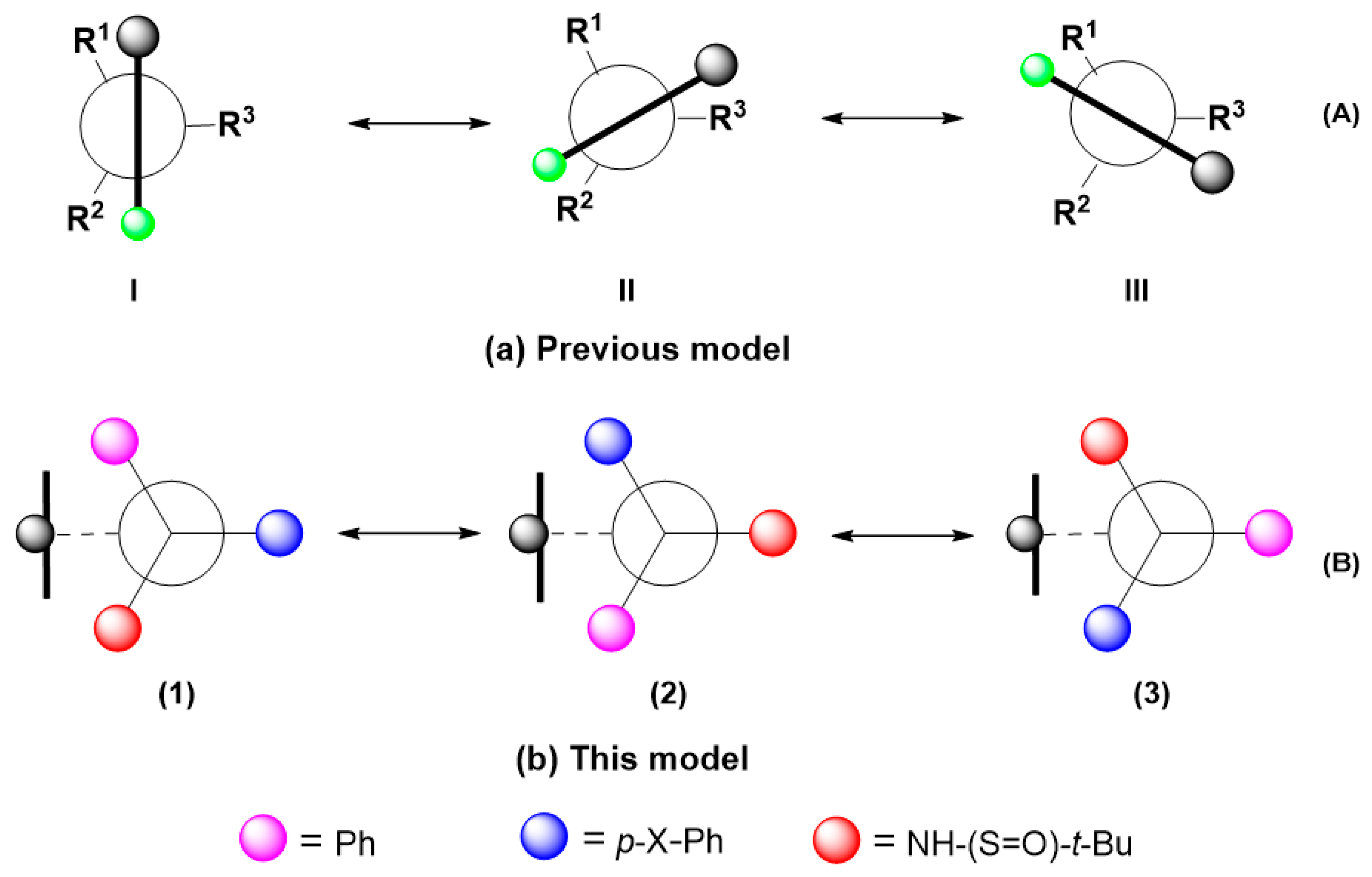
2.1. Structural Design and Models
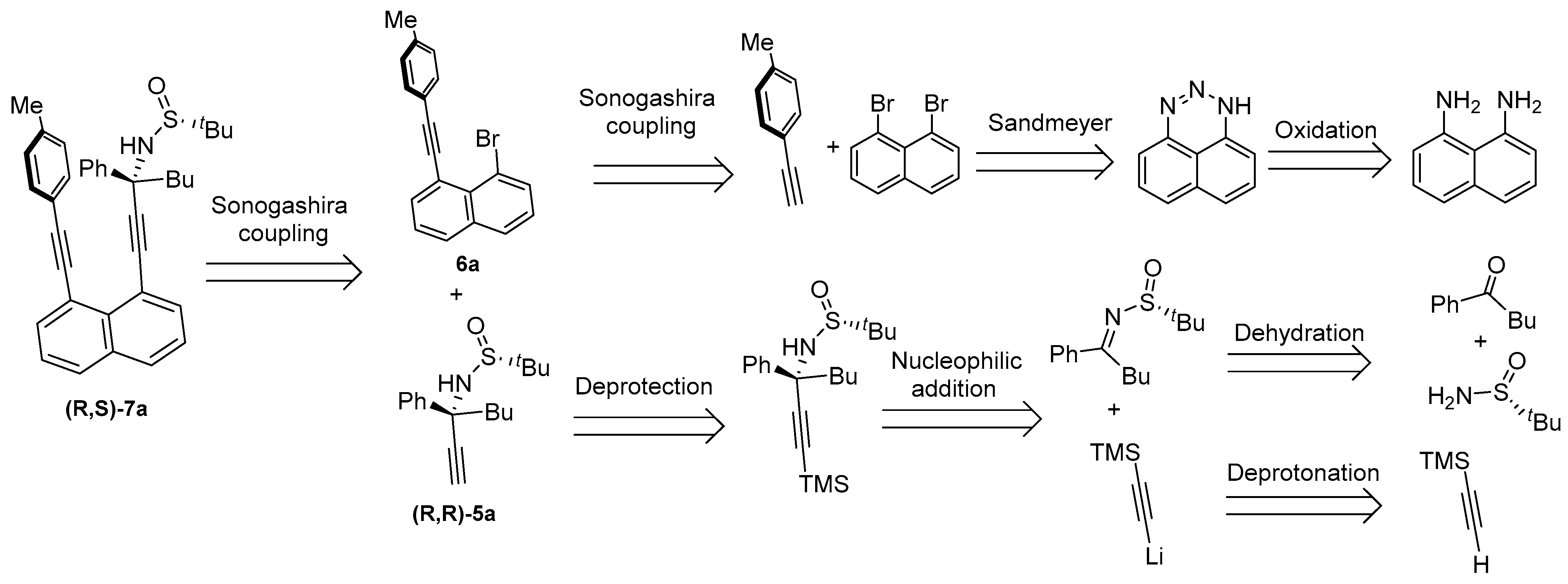
2.2. Retro-Synthetic Analysis (RSA)
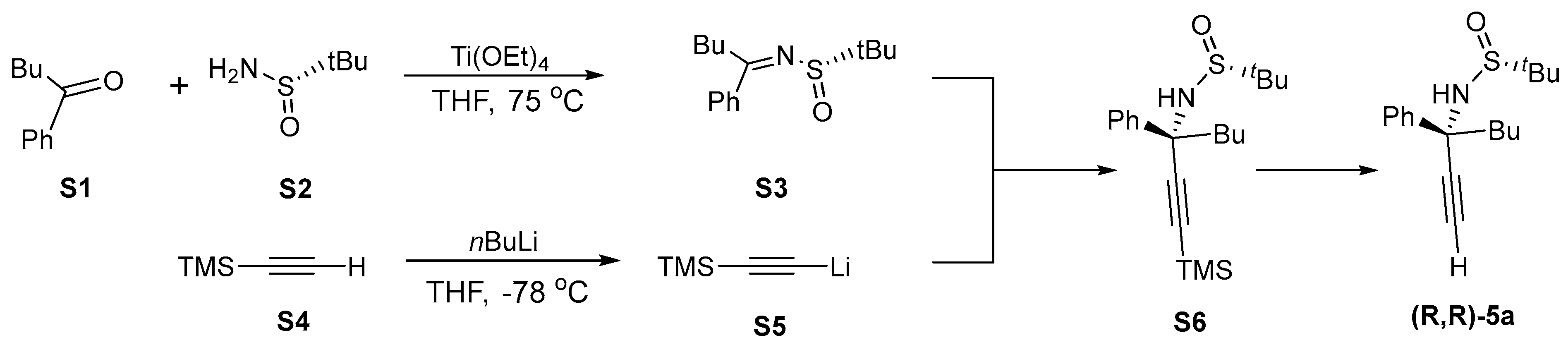
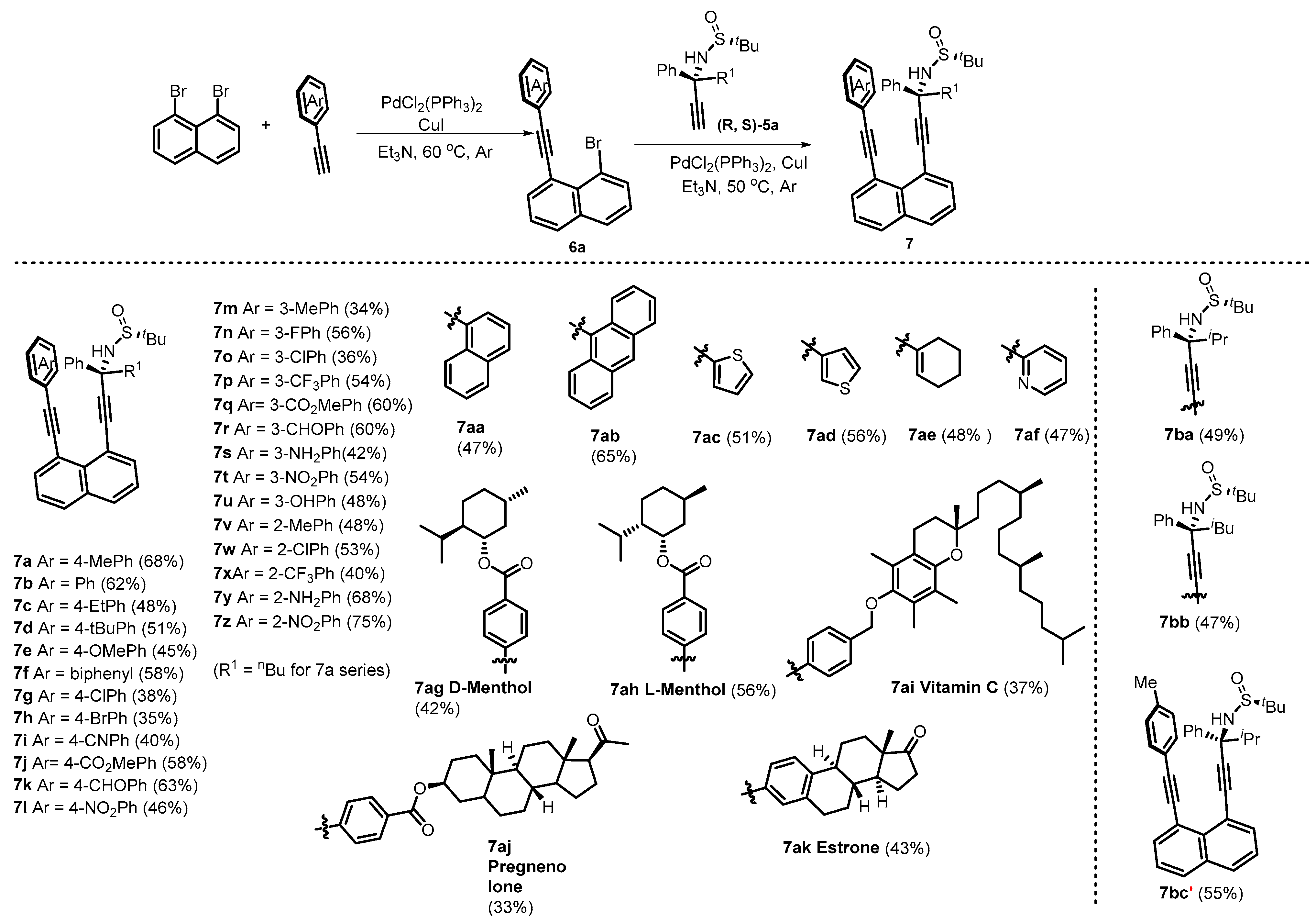
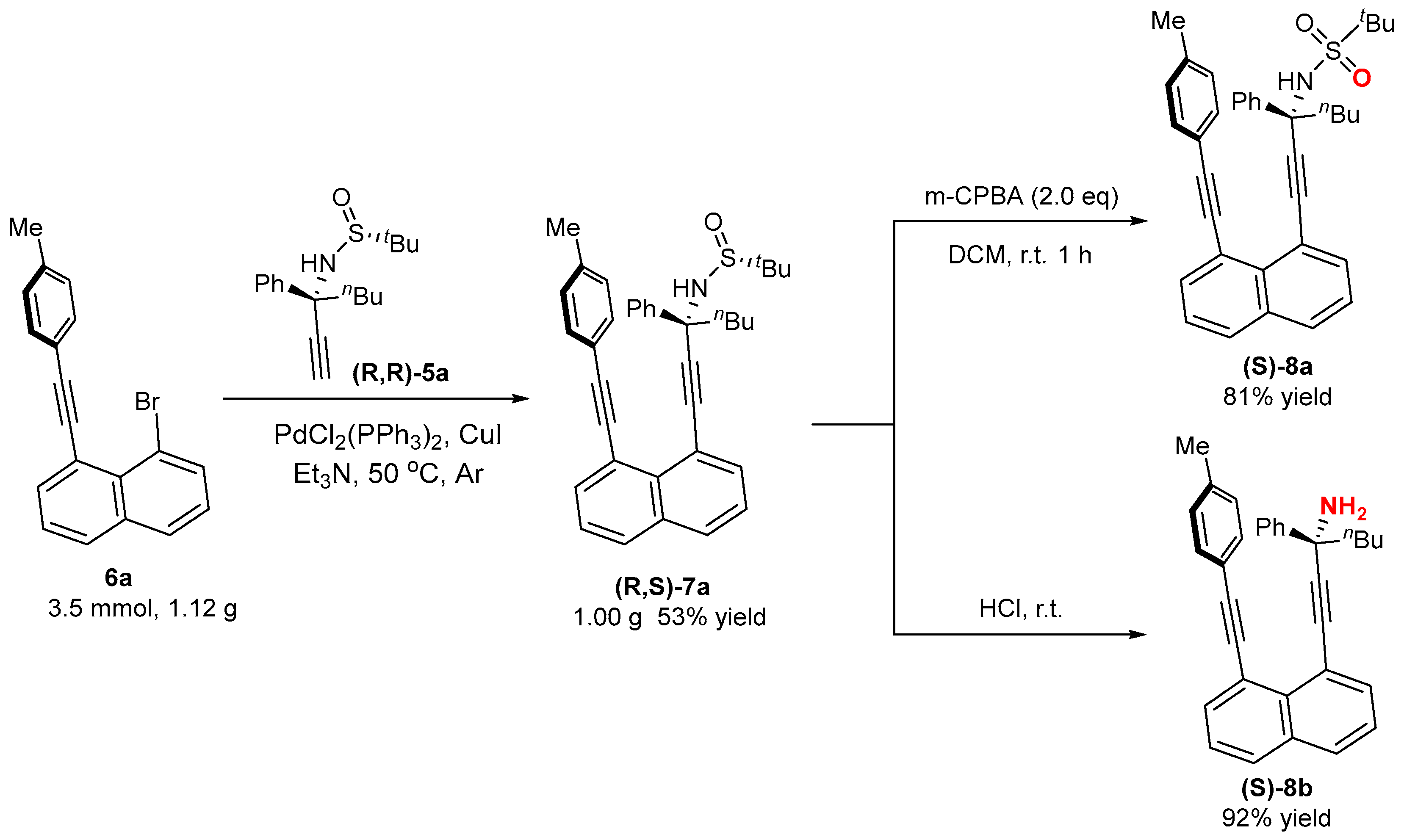
2.3. Asymmetric Synthesis
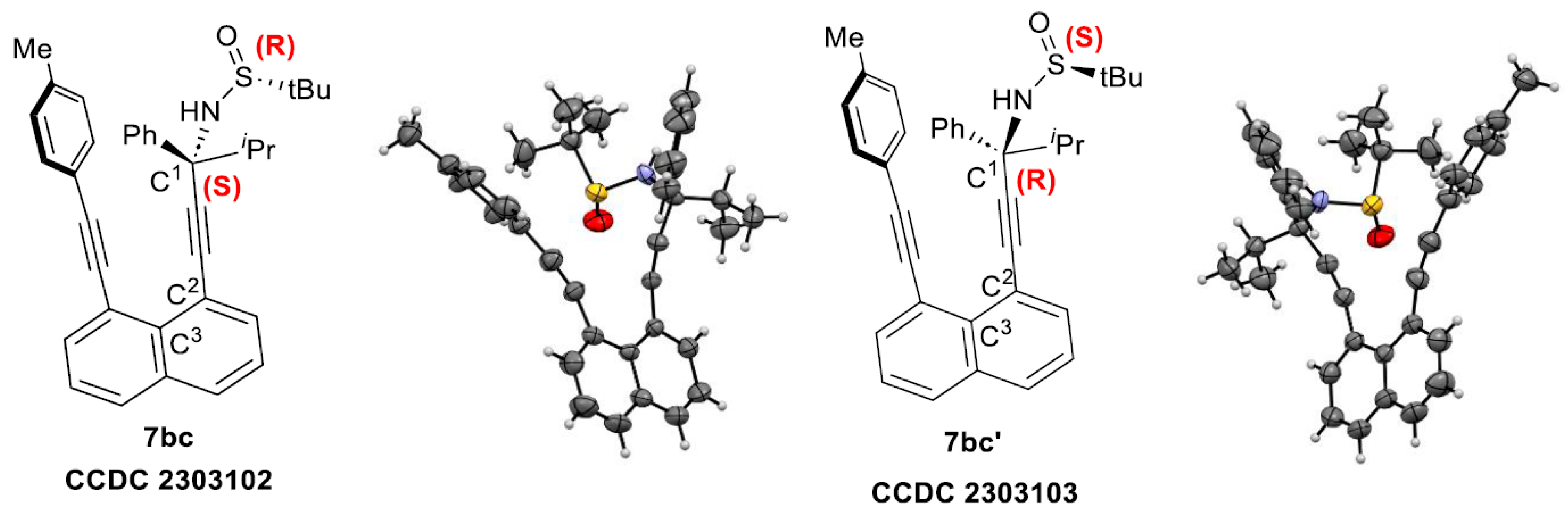
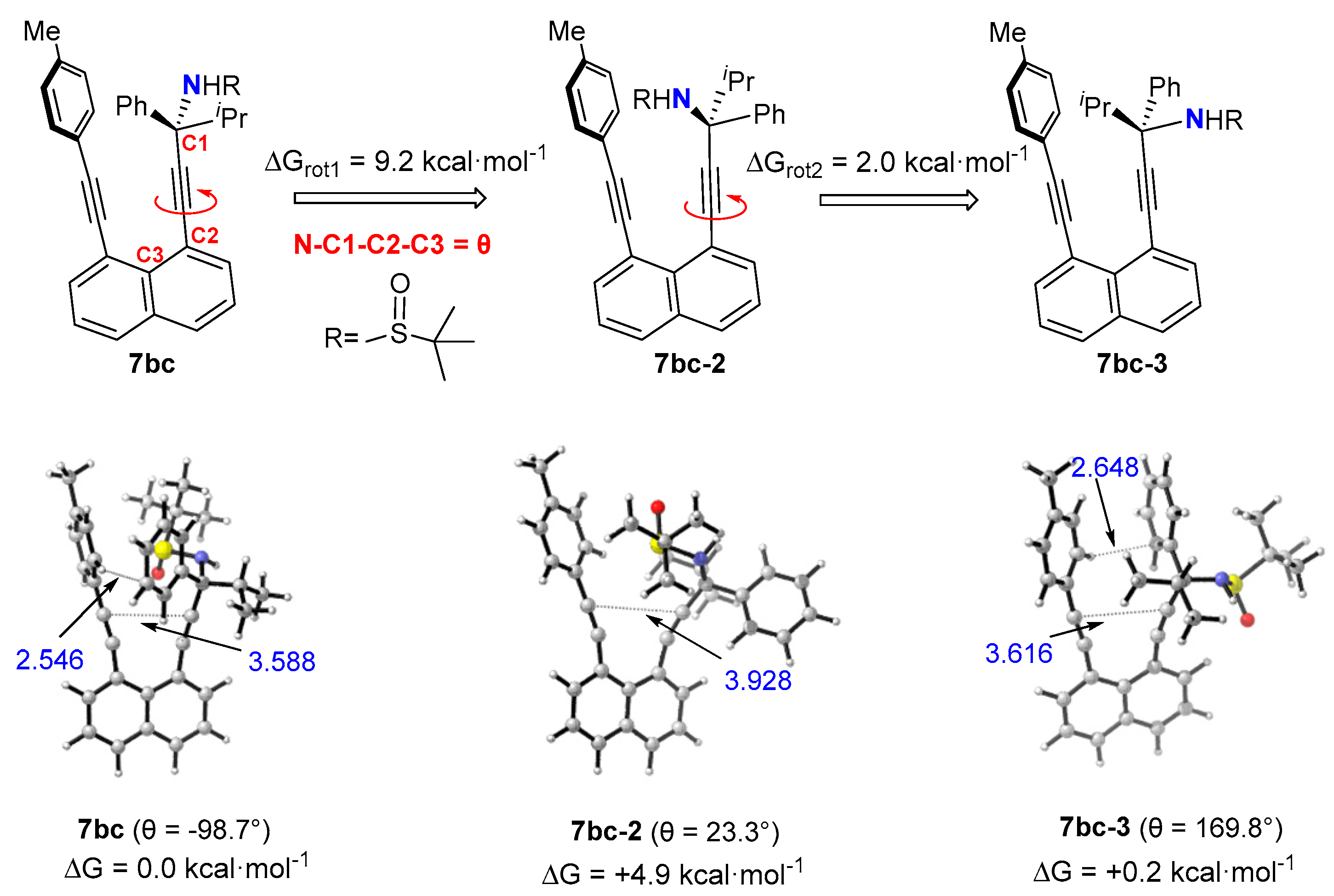
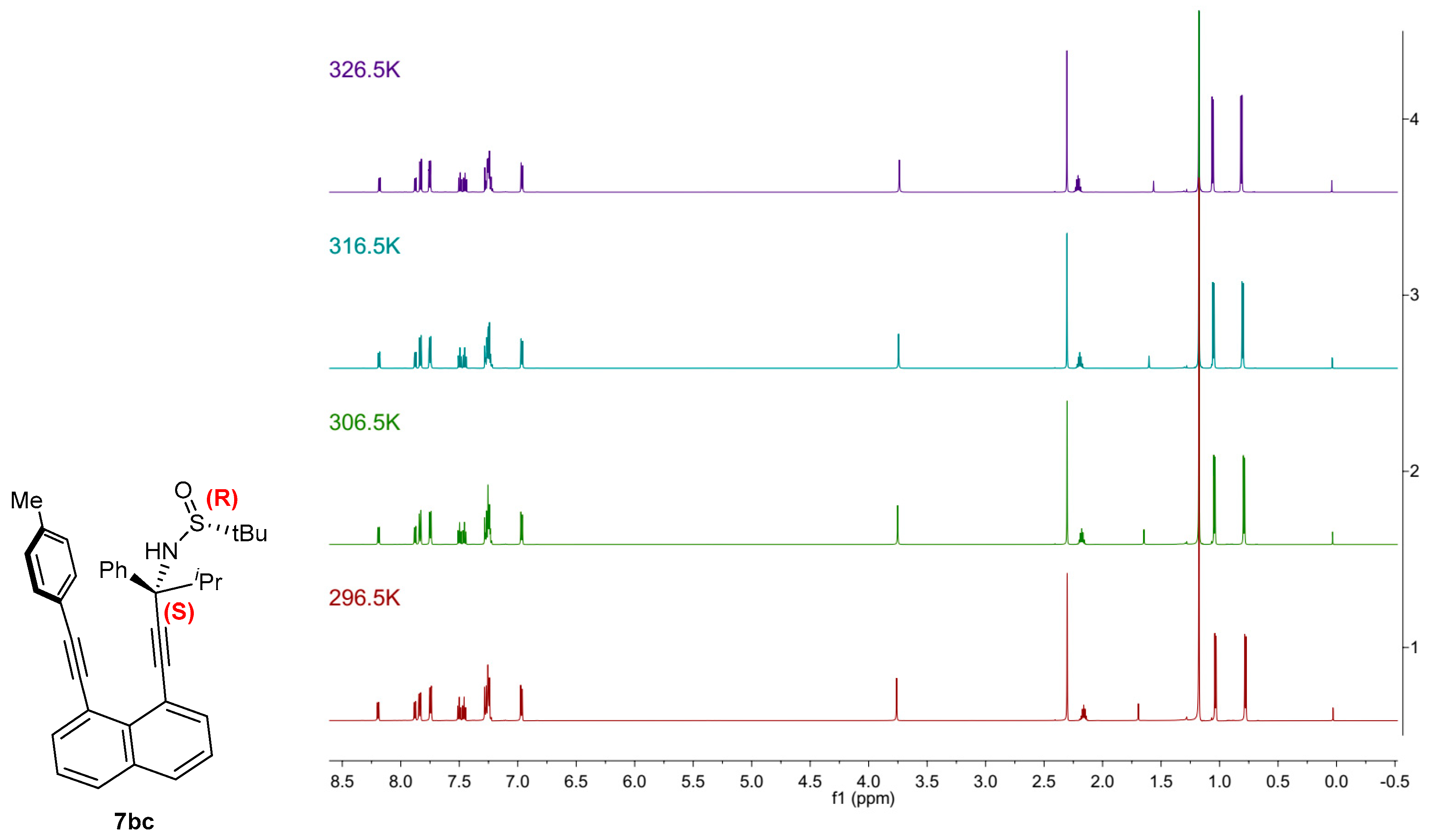
3. Computational and VT NMR Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taniguchi, K.; Maeda, R.; Ando, T.; Okumura, T.; Nakazawa, N.; Hatori, R.; Nakamura, M.; Hozumi, S.; Fujiwara, H.; Matsuno, K. Chirality in Planar Cell Shape Contributes to Left-Right Asymmetric Epithelial Morphogenesis. Science 2011, 333, 339–341. [Google Scholar] [CrossRef]
- Eliel, E.L.; Wilen, S.H. Stereochemistry of Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Feringa, B.L.; van Delden, R.A. Absolute Asymmetric Synthesis: The Origin, Control, and Amplification of Chirality. Angew. Chem. Int. Ed. Engl. 1999, 38, 3418–3438. [Google Scholar] [CrossRef]
- Zhang, J.; Kürti, L. Multi-Layer 3D Chirality: Its Enantioselective Synthesis and Aggregation-Induced Emission. Natl. Sci. Rev. 2021, 8, nwaa205. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Yang, Z.; Katakam, N.; Rouh, H.; Ahmed, S.; Unruh, D.; Surowiec, K.; Li, G. Multilayer 3D Chirality and Its Synthetic Assembly. Research 2019, 2019, 6717104. [Google Scholar] [CrossRef]
- Jin, S.; Wang, Y.; Tang, Y.; Wang, J.-Y.; Xu, T.; Pan, J.; Zhang, S.; Yuan, Q.; Rahman, A.U.; McDonald, J.D.; et al. Orientational Chirality, Its Asymmetric Control, and Computational Study. Research 2022, 2022, 0012. [Google Scholar] [CrossRef]
- Bryliakov, K.P. Chemical Mechanisms of Prebiotic Chirality Amplification. Research 2020, 2020, 5689246. [Google Scholar] [CrossRef]
- Moser, H.E.; Dervan, P.B. Sequence-Specific Cleavage of Double Helical DNA by Triple Helix Formation. Science 1987, 238, 645–650. [Google Scholar] [CrossRef]
- Wagner, I.; Musso, H. New Naturally Occurring Amino Acids. Angew. Chem. Int. Ed. Engl. 1983, 22, 816–828. [Google Scholar] [CrossRef]
- Dunitz, J.D. Pauling’s Left-Handed α-Helix. Angew. Chem. Int. Ed. Engl. 2001, 40, 4167–4173. [Google Scholar] [CrossRef] [PubMed]
- Hruby, V.J.; Li, G.; Haskell-Luevano, C.; Shenderovich, M. Design of Peptides, Proteins, and Peptidomimetics in Chi Space. Biopolymers 1997, 43, 219–266. [Google Scholar] [CrossRef]
- Smith, D.A.; Jones, R.M. The Sulfonamide Group as a Structural Alert: A Distorted Story? Curr. Opin. Drug Discov. Devel. 2008, 11, 72–79. [Google Scholar]
- Hu, M.; Feng, H.-T.; Yuan, Y.-X.; Zheng, Y.-S.; Tang, B.Z. Chiral AIEgens—Chiral Recognition, CPL Materials and Other Chiral Applications. Coord. Chem. Rev 2020, 416, 213329. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, C.-F. Helicenes: Synthesis and Applications. Chem. Rev. 2012, 112, 1463–1535. [Google Scholar] [CrossRef]
- Oki, O.; Kulkarni, C.; Yamagishi, H.; Meskers, S.C.J.; Lin, Z.-H.; Huang, J.-S.; Meijer, E.W.; Yamamoto, Y. Robust Angular Anisotropy of Circularly Polarized Luminescence from a Single Twisted-Bipolar Polymeric Microsphere. J. Am. Chem. Soc. 2021, 143, 8772–8779. [Google Scholar] [CrossRef]
- Liu, T.-T.; Yan, Z.-P.; Hu, J.-J.; Yuan, L.; Luo, X.-F.; Tu, Z.-L.; Zheng, Y.-X. Chiral Thermally Activated Delayed Fluorescence Emitters-Based Efficient Circularly Polarized Organic Light-Emitting Diodes Featuring Low Efficiency Roll-Off. ACS Appl. Mater. Interfaces 2021, 13, 56413–56419. [Google Scholar] [CrossRef]
- Zhao, T.; Han, J.; Duan, P.; Liu, M. New Perspectives to Trigger and Modulate Circularly Polarized Luminescence of Complex and Aggregated Systems: Energy Transfer, Photon Upconversion, Charge Transfer, and Organic Radical. Acc. Chem. Res. 2020, 53, 1279–1292. [Google Scholar] [CrossRef]
- Li, J.; Hou, C.; Huang, C.; Xu, S.; Peng, X.; Qi, Q.; Lai, W.-Y.; Huang, W. Boosting Circularly Polarized Luminescence of Organic Conjugated Systems via Twisted Intramolecular Charge Transfer. Research 2020, 2020, 3839160. [Google Scholar] [CrossRef]
- Gustafson, J.L.; Lim, D.; Miller, S.J. Dynamic Kinetic Resolution of Biaryl Atropisomers via Peptide-Catalyzed Asymmetric Bromination. Science 2010, 328, 1251–1255. [Google Scholar] [CrossRef]
- Phipps, R.J.; Hamilton, G.L.; Toste, F.D. The Progression of Chiral Anions from Concepts to Applications in Asymmetric Catalysis. Nat. Chem. 2012, 4, 603–614. [Google Scholar] [CrossRef]
- Dong, S.; Feng, X.; Liu, X. Chiral Guanidines and Their Derivatives in Asymmetric Synthesis. Chem. Soc. Rev. 2018, 47, 8525–8540. [Google Scholar] [CrossRef]
- Cui, X.; Xu, X.; Lu, H.; Zhu, S.; Wojtas, L.; Zhang, X.P. Enantioselective Cyclopropenation of Alkynes with Acceptor/Acceptor-Substituted Diazo Reagents via Co(II)-Based Metalloradical Catalysis. J. Am. Chem. Soc. 2011, 133, 3304–3307. [Google Scholar] [CrossRef]
- Ma, C.; Sheng, F.-T.; Wang, H.-Q.; Deng, S.; Zhang, Y.-C.; Jiao, Y.; Tan, W.; Shi, F. Atroposelective Access to Oxindole-Based Axially Chiral Styrenes via the Strategy of Catalytic Kinetic Resolution. J. Am. Chem. Soc. 2020, 142, 15686–15696. [Google Scholar] [CrossRef]
- Li, G.-Q.; Gao, H.; Keene, C.; Devonas, M.; Ess, D.H.; Kürti, L. Organocatalytic Aryl–Aryl Bond Formation: An Atroposelective [3,3]-Rearrangement Approach to BINAM Derivatives. J. Am. Chem. Soc. 2013, 135, 7414–7417. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, M.; Guan, Y.; Zhang, Y.; Hong, X.; Wei, C.; Zheng, P.; Wei, D.; Fu, Z.; Chi, Y.R.; et al. Desymmetrization of Cyclic 1,3-Diketones under N-Heterocyclic Carbene Organocatalysis: Access to Organofluorines with Multiple Stereogenic Centers. Research 2021, 2021, 9867915. [Google Scholar] [CrossRef]
- Huang, S.; Wen, H.; Tian, Y.; Wang, P.; Qin, W.; Yan, H. Organocatalytic Enantioselective Construction of Chiral Azepine Skeleton Bearing Multiple-Stereogenic Elements. Angew. Chem. Int. Ed. Engl. 2021, 60, 21486–21493. [Google Scholar] [CrossRef]
- Rouh, H.; Tang, Y.; Xu, T.; Yuan, Q.; Zhang, S.; Wang, J.-Y.; Jin, S.; Wang, Y.; Pan, J.; Wood, H.L.; et al. Aggregation-Induced Synthesis (AIS): Asymmetric Synthesis via Chiral Aggregates. Research 2022, 2022, 9865108. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Yuan, Q.; Zhang, S.; Wang, J.-Y.; Jin, S.; Xu, T.; Pan, J.; Surowiec, K.; Li, G. Aggregation-Induced Catalysis: Asymmetric Catalysis with Chiral Aggregates. Research 2023, 2023, 0163. [Google Scholar] [CrossRef]
- Lorion, M.M.; Maindan, K.; Kapdi, A.R.; Ackermann, L. Heteromultimetallic Catalysis for Sustainable Organic Syntheses. Chem. Soc. Rev. 2017, 46, 7399–7420. [Google Scholar] [CrossRef]
- Akiyama, T. Stronger Brønsted Acids. Chem. Rev. 2007, 107, 5744–5758. [Google Scholar] [CrossRef]
- Yu, J.; Shi, F.; Gong, L.-Z. Brønsted-Acid-Catalyzed Asymmetric Multicomponent Reactions for the Facile Synthesis of Highly Enantioenriched Structurally Diverse Nitrogenous Heterocycles. Acc. Chem. Res. 2011, 44, 1156–1171. [Google Scholar] [CrossRef]
- Liu, Y.; Li, W.; Zhang, J. Chiral Ligands Designed in China. Natl. Sci. Rev. 2017, 4, 326–358. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Tan, B. Construction of Axially Chiral Compounds via Asymmetric Organocatalysis. Acc. Chem. Res. 2018, 51, 534–547. [Google Scholar] [CrossRef]
- Zhao, P.; Li, Z.; He, J.; Liu, X.; Feng, X. Asymmetric Catalytic 1,3-Dipolar Cycloaddition of α-Diazoesters for Synthesis of 1-Pyrazoline-Based Spirochromanones and Beyond. Sci. China Chem. 2021, 64, 1355–1360. [Google Scholar] [CrossRef]
- Liu, D.; Li, B.; Chen, J.; Gridnev, I.D.; Yan, D.; Zhang, W. Ni-Catalyzed Asymmetric Hydrogenation of N-Aryl Imino Esters for the Efficient Synthesis of Chiral α-Aryl Glycines. Nat. Commun. 2020, 11, 5935. [Google Scholar] [CrossRef]
- Ge, Y.; Qin, C.; Bai, L.; Hao, J.; Liu, J.; Luan, X. A Dearomatization/Debromination Strategy for the [4+1] Spiroannulation of Bromophenols with α,β-Unsaturated Imines. Angew. Chem. Int. Ed Engl. 2020, 59, 18985–18989. [Google Scholar] [CrossRef]
- Chen, J.-J.; Fang, J.-H.; Du, X.-Y.; Zhang, J.-Y.; Bian, J.-Q.; Wang, F.-L.; Luan, C.; Liu, W.-L.; Liu, J.-R.; Dong, X.-Y.; et al. Enantioconvergent Cu-Catalysed N-Alkylation of Aliphatic Amines. Nature 2023, 618, 294–300. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Liang, H.; Cao, Z.; Zhao, X.; He, Y.; Wang, S.; Pang, J.; Zhou, Z.; Ke, Z.; et al. Enantioselective Synthesis of Axially Chiral Biaryl Monophosphine Oxides via Direct Asymmetric Suzuki Coupling and DFT Investigations of the Enantioselectivity. ACS Catal. 2014, 4, 1390–1397. [Google Scholar] [CrossRef]
- Liao, G.; Yao, Q.-J.; Zhang, Z.-Z.; Wu, Y.-J.; Huang, D.-Y.; Shi, B.-F. Scalable, Stereocontrolled Formal Syntheses of (+)-isoschizandrin and (+)-steganone: Development and Applications of Palladium(II)-catalyzed Atroposelective C−H Alkynylation. Angew. Chem. Int. Ed Engl. 2018, 57, 3661–3665. [Google Scholar] [CrossRef]
- Dai, L.; Liu, Y.; Xu, Q.; Wang, M.; Zhu, Q.; Yu, P.; Zhong, G.; Zeng, X. A Dynamic Kinetic Resolution Approach to Axially Chiral Diaryl Ethers by Catalytic Atroposelective Transfer Hydrogenation. Angew. Chem. Int. Ed Engl. 2023, 62, e202216534. [Google Scholar] [CrossRef]
- Zhou, Q.-L. (Ed.) Privileged Chiral Ligands and Catalysts, 1st ed.; Wiley-VCH Verlag: Weinheim, Germany, 2011. [Google Scholar]
- Zhang, R.; Ge, S.; Sun, J. SPHENOL, A New Chiral Framework for Asymmetric Synthesis. J. Am. Chem. Soc. 2021, 143, 12445–12449. [Google Scholar] [CrossRef]
- Fu, G.C. Enantioselective Nucleophilic Catalysis with “Planar-Chiral” Heterocycles. Acc. Chem. Res. 2000, 33, 412–420. [Google Scholar] [CrossRef]
- Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Asymmetric Catalysis with Chiral Ferrocene Ligands. Acc. Chem. Res. 2003, 36, 659–667. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, G.; Jin, S.; Liu, Y.; Ma, L.; Zhang, S.; Rouh, H.; Ali, A.I.M.; Wang, J.-Y.; Xu, T.; et al. From Center-to-Multilayer Chirality: Asymmetric Synthesis of Multilayer Targets with Electron-Rich Bridges. J. Org. Chem. 2022, 87, 5976–5986. [Google Scholar] [CrossRef]
- Jin, S.; Wang, J.-Y.; Tang, Y.; Rouh, H.; Zhang, S.; Xu, T.; Wang, Y.; Yuan, Q.; Chen, D.; Unruh, D.; et al. Central-to-Folding Chirality Control: Asymmetric Synthesis of Multilayer 3D Targets with Electron-Deficient Bridges. Front. Chem. 2022, 10, 860398. [Google Scholar] [CrossRef]
- Tang, Y.; Jin, S.; Zhang, S.; Wu, G.-Z.; Wang, J.-Y.; Xu, T.; Wang, Y.; Unruh, D.; Surowiec, K.; Ma, Y.; et al. Multilayer 3D Chiral Folding Polymers and Their Asymmetric Catalytic Assembly. Research 2022, 2022, 9847949. [Google Scholar] [CrossRef]
- Tong, S.; Li, J.-T.; Liang, D.-D.; Zhang, Y.-E.; Feng, Q.-Y.; Zhang, X.; Zhu, J.; Wang, M.-X. Catalytic Enantioselective Synthesis and Switchable Chiroptical Property of Inherently Chiral Macrocycles. J. Am. Chem. Soc. 2020, 142, 14432–14436. [Google Scholar] [CrossRef]
- Wang, J.-Y.; Tang, Y.; Wu, G.-Z.; Zhang, S.; Rouh, H.; Jin, S.; Xu, T.; Wang, Y.; Unruh, D.; Surowiec, K.; et al. Asymmetric Catalytic Assembly of Triple-Columned and Multilayered Chiral Folding Polymers Showing Aggregation-Induced Emission (AIE). Chem-Eur. J. 2022, 28, e202200183. [Google Scholar] [CrossRef]
- Wu, X.; Witzig, R.M.; Beaud, R.; Fischer, C.; Häussinger, D.; Sparr, C. Catalyst Control over Sixfold Stereogenicity. Nat. Catal 2021, 4, 457–462. [Google Scholar] [CrossRef]
- Bertuzzi, G.; Corti, V.; Izzo, J.A.; Ričko, S.; Jessen, N.I.; Jørgensen, K.A. Organocatalytic Enantioselective Construction of Conformationally Stable C(Sp2)-C(Sp3) Atropisomers. J. Am. Chem. Soc. 2022, 144, 1056–1065. [Google Scholar] [CrossRef]
- Casarini, D.; Lunazzi, L.; Mazzanti, A.; Foresti, E. Conformational Studies by Dynamic NMR. 64.1Stereomutations of Atropisomers and of Conformational Enantiomers in Ethers of Hindered Naphthylcarbinols. J. Org. Chem. 1998, 63, 4746–4754. [Google Scholar] [CrossRef]
- Casarini, D.; Coluccini, C.; Lunazzi, L.; Mazzanti, A. Structure, Conformation, and Stereodynamics of the Atropisomers of Highly Hindered Benzyl Ethers. J. Org. Chem. 2006, 71, 4490–4496. [Google Scholar] [CrossRef]
- Jin, S.; Xu, T.; Tang, Y.; Wang, J.-Y.; Wang, Y.; Pan, J.; Zhang, S.; Yuan, Q.; Rahman, A.U.; Aquino, A.J.A.; et al. A New Chiral Phenomenon of Orientational Chirality, Its Synthetic Control and Computational Study. Front. Chem. 2022, 10, 1110240. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, T.; Jin, S.; Wang, J.-Y.; Yuan, Q.; Liu, H.; Tang, Y.; Zhang, S.; Yan, W.; Jiao, Y.; et al. Design and Asymmetric Control of Orientational Chirality by Using the Combination of C(sp2)-C(sp) Levers and Achiral N-Protecting Group. Chem. Eur. J. 2024, e202400005. [Google Scholar] [CrossRef]
- Corey, E.J.; Cheng, X.-M. The Logic of Chemical Synthesis; Wiley-Interscience: New York, NY, USA, 2009. [Google Scholar]
- Sonogashira, K. Development of Pd–Cu Catalyzed Cross-Coupling of Terminal Acetylenes with Sp2-Carbon Halides. J. Organomet. Chem. 2002, 653, 46–49. [Google Scholar] [CrossRef]
- Davis, A.F.; Chen, B.-C. Asymmetric Synthesis of Amino Acids Using Sulfinimines (Thiooxime S-Oxides). Chem. Soc. Rev. 1998, 27, 13. [Google Scholar] [CrossRef]
- Robak, M.T.; Herbage, M.A.; Ellman, J.A. Synthesis and Applications of Tert-Butanesulfinamide. Chem. Rev. 2010, 110, 3600–3740. [Google Scholar] [CrossRef]
- Noland, W.E.; Narina, V.S.; Britton, D. Synthesis and Crystallography of 8-Halonaphthalene-1-Carbonitriles and Naphthalene-1,8-Dicarbonitrile. J. Chem. Res. 2011, 35, 694–697. [Google Scholar] [CrossRef]
- Mothana, S.; Grassot, J.-M.; Hall, D.G. Multistep Phase-Switch Synthesis by Using Liquid-Liquid Partitioning of Boronic Acids: Productive Tags with an Expanded Repertoire of Compatible Reactions. Angew. Chem. Int. Ed. Engl. 2010, 49, 2883–2887. [Google Scholar] [CrossRef]
- Gaussian 16, rev. C.01. Available online: https://gaussian.com/gaussian16/ (accessed on 6 May 2024).
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154123. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Phys. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Andrae, D.; Haußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjustedab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta. 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Puthoff, H. Source of vacuum electromagnetic zero-point energy. Phys. Rev. A 1989, 40, 4857–4862. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Truhlar, Performance of SM6, SM8, and SMD on the SAMPL1 Test Set for the Prediction of Small-Molecule Solvation Free Energies. J. Phys. Chem. B 2009, 113, 4538–4543. [Google Scholar] [CrossRef]
- Legault, C.Y. CYLView, 1.0b, Université de Sherbrook. 2009. Available online: www.cylview.org (accessed on 6 May 2024).
- Wu, P.; Yu, L.; Gao, C.-H.; Cheng, Q.; Deng, S.; Jiao, Y.; Tan, W.; Shi, F. Design and Synthesis of Axially Chiral Aryl-Pyrroloindoles via the Strategy of Organocatalytic Asymmetric (2 + 3) Cyclization. Fundam. Res. 2023, 3, 237–248. [Google Scholar] [CrossRef]
- Mancinelli, M.; Bencivenni, G.; Pecorari, D.; Mazzanti, A. Stereochemistry and Recent Applications of Axially Chiral Organic Molecules. Eur. J. Org. Chem. 2020, 2020, 4070–4086. [Google Scholar] [CrossRef]



 | |||||
|---|---|---|---|---|---|
| Entry a | Cat | Base | Solvent | Temp. | Product (%) b |
| 1 | NiCl2(PPh3)2 | - | Et3N | 60 | n. r. |
| 2 | PdCl2(PPh3)2 | - | Et3N | 60 | 45 |
| 3 | PdCl2 | - | Et3N | 60 | n. r. |
| 4 | Pd(PPh3)4 | - | Et3N | 60 | 28 |
| 5 | Pd(OAc)2 | - | - | 60 | n. r. |
| 6 | PdCl2(dppf)2 | - | - | 60 | 42 |
| 7 | PdCl2(PPh3)2 | Et3N | THF | 60 | Trace |
| 8 | PdCl2(PPh3)2 | Cs2CO3 | THF | 60 | 37 |
| 9 | PdCl2(PPh3)2 | Et3N | DMF | 60 | 42 |
| 10 c | PdCl2(PPh3)2 | - | Et3N | 50 | 68 |
| 11 | PdCl2(PPh3)2 | - | Et3N | 70 | 52 |
| 12 | - | - | Et3N | 60 | n. r. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Wang, J.-Y.; Wang, Y.; Jin, S.; Tang, Y.; Zhang, S.; Yuan, Q.; Liu, H.; Yan, W.; Jiao, Y.; et al. C(sp)-C(sp) Lever-Based Targets of Orientational Chirality: Design and Asymmetric Synthesis. Molecules 2024, 29, 2274. https://doi.org/10.3390/molecules29102274
Xu T, Wang J-Y, Wang Y, Jin S, Tang Y, Zhang S, Yuan Q, Liu H, Yan W, Jiao Y, et al. C(sp)-C(sp) Lever-Based Targets of Orientational Chirality: Design and Asymmetric Synthesis. Molecules. 2024; 29(10):2274. https://doi.org/10.3390/molecules29102274
Chicago/Turabian StyleXu, Ting, Jia-Yin Wang, Yu Wang, Shengzhou Jin, Yao Tang, Sai Zhang, Qingkai Yuan, Hao Liu, Wenxin Yan, Yinchun Jiao, and et al. 2024. "C(sp)-C(sp) Lever-Based Targets of Orientational Chirality: Design and Asymmetric Synthesis" Molecules 29, no. 10: 2274. https://doi.org/10.3390/molecules29102274
APA StyleXu, T., Wang, J.-Y., Wang, Y., Jin, S., Tang, Y., Zhang, S., Yuan, Q., Liu, H., Yan, W., Jiao, Y., Yang, X.-L., & Li, G. (2024). C(sp)-C(sp) Lever-Based Targets of Orientational Chirality: Design and Asymmetric Synthesis. Molecules, 29(10), 2274. https://doi.org/10.3390/molecules29102274






