Comparative Studies of Bioactivities and Chemical Components in Fresh and Black Garlics
Abstract
1. Introduction
2. Results and Discussion
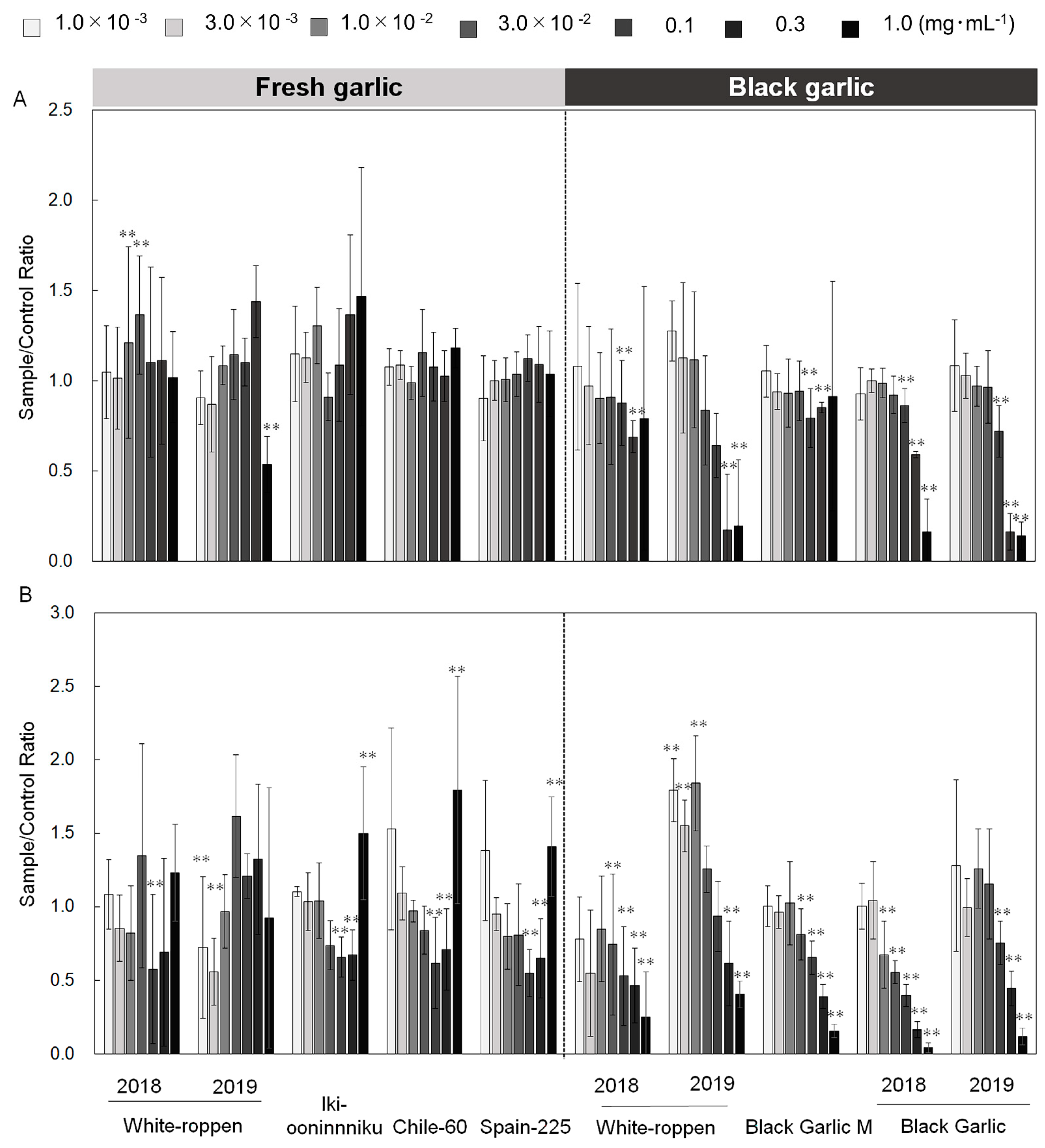
2.1. Simultaneous Monitoring of Superoxide and Intracellular Calcium Ions in Neutrophils by Chemiluminescence and Fluorescence
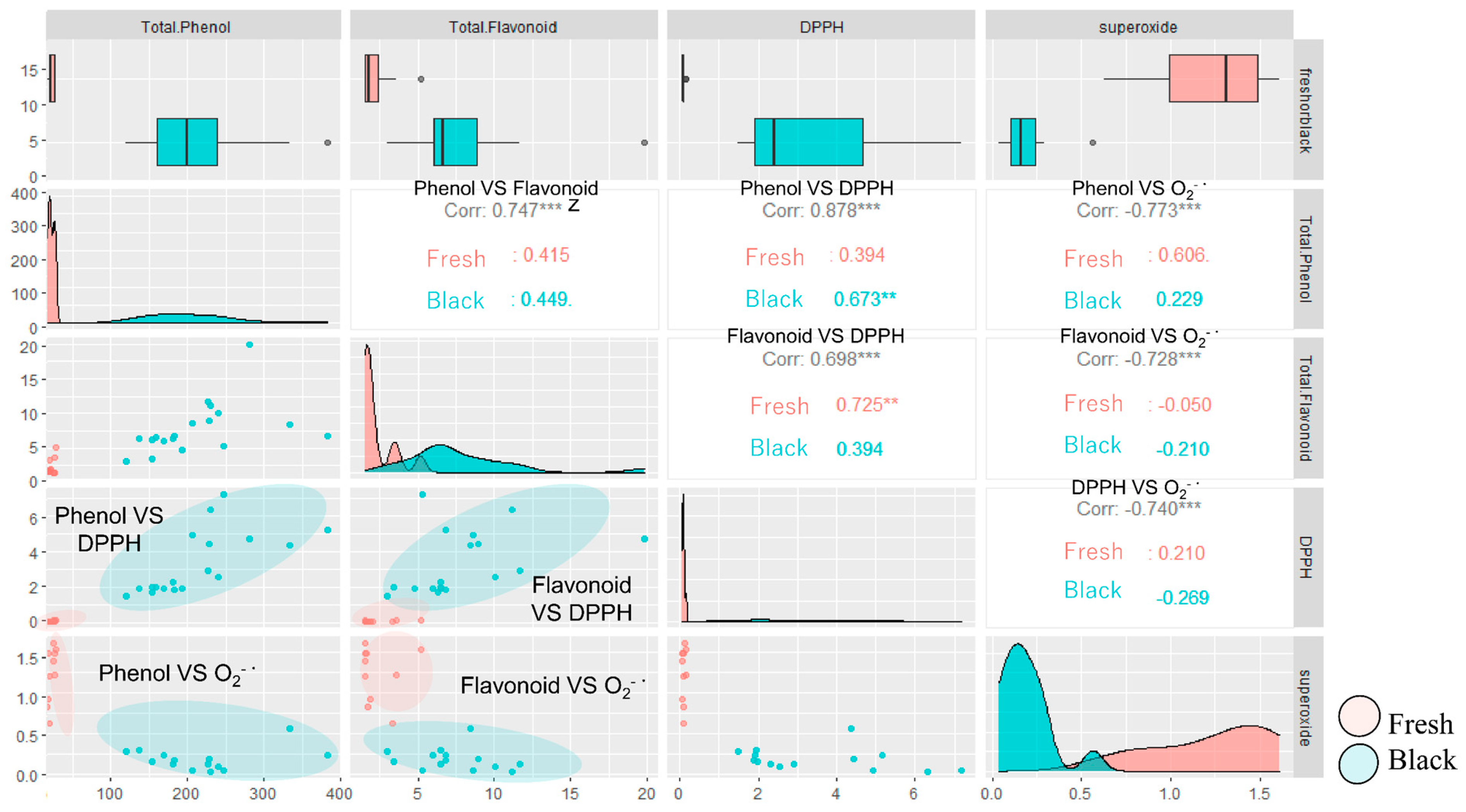
2.2. Evaluation of Antioxidant Activity
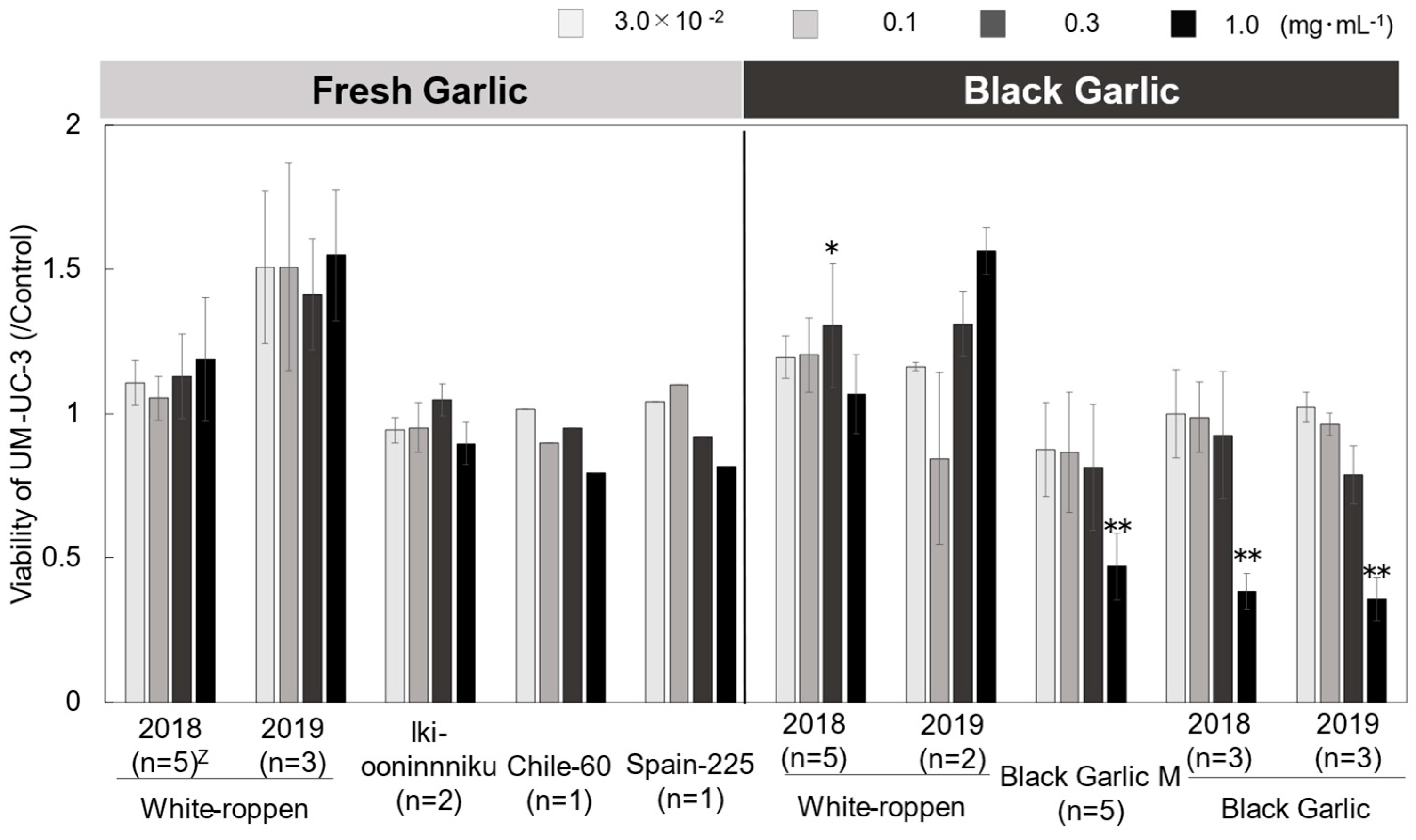
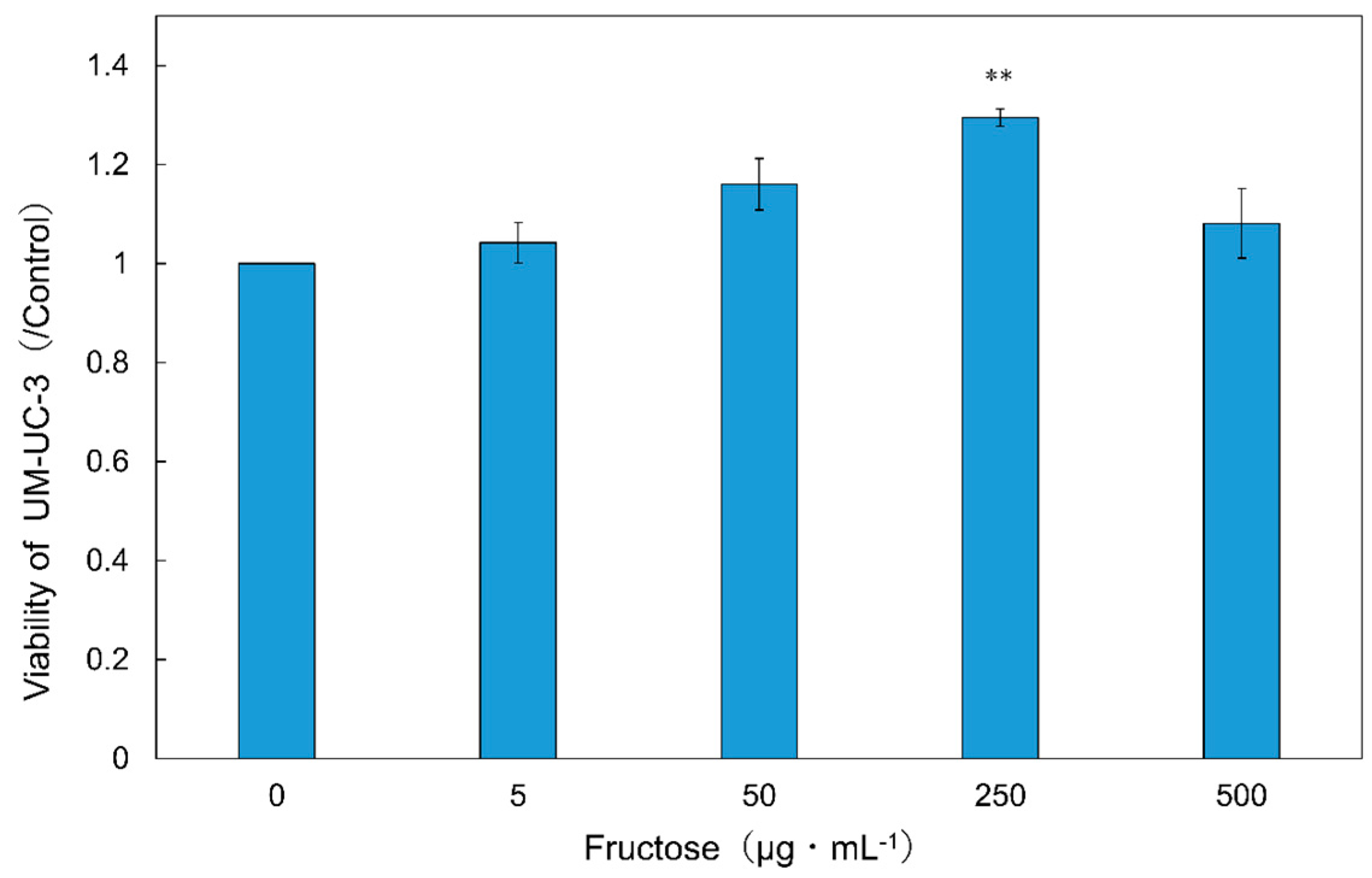
2.3. Evaluation of Anticancer Effect
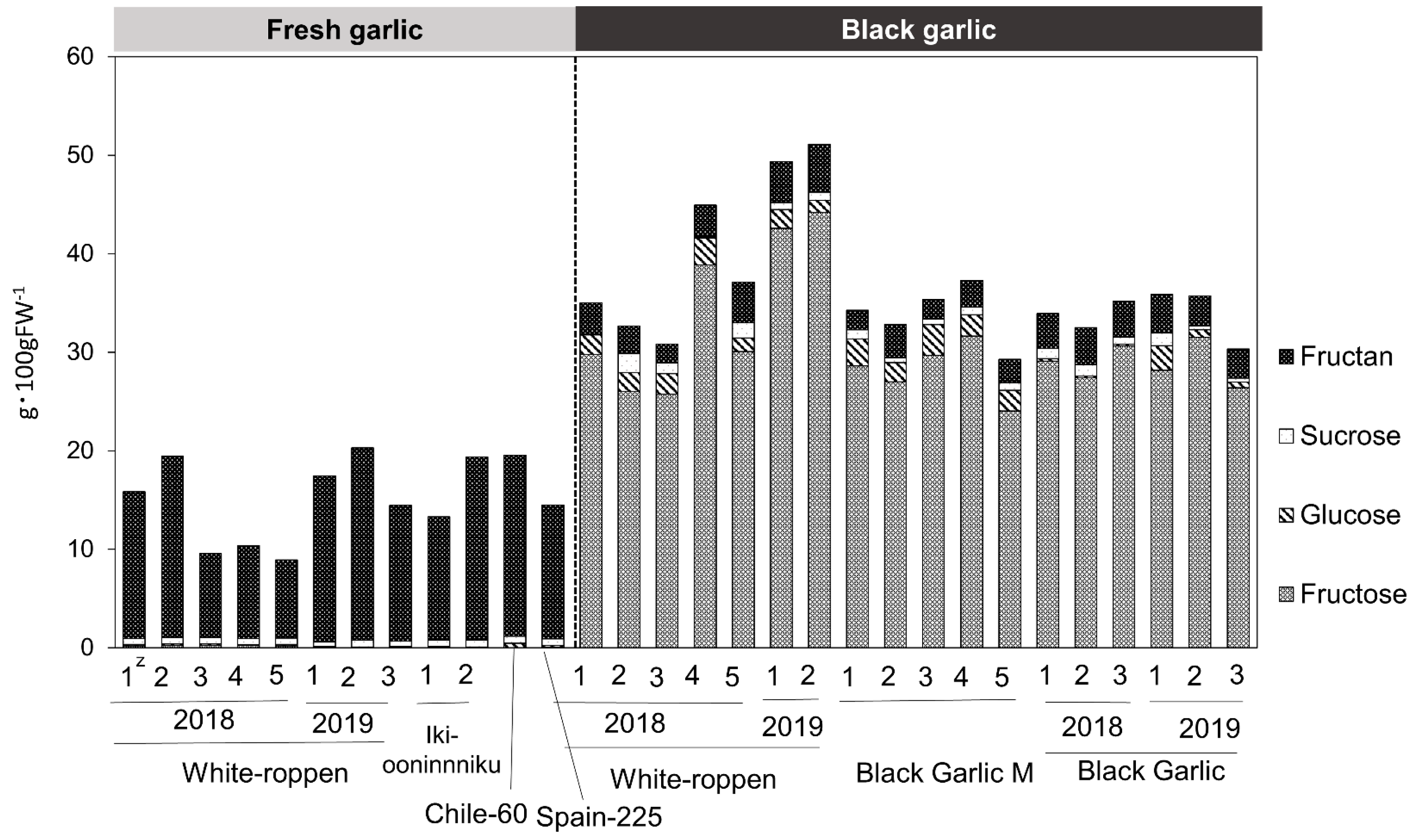
2.4. Amino Acid and Sugar Contents
3. Materials and Methods
3.1. Plant Materials
3.2. Sample Preparation
3.2.1. Hot Ethanol Extraction
3.2.2. Dimethyl Sulfoxide (DMSO) Extraction
3.3. Simultaneous Evaluation for Antioxidant, Anti-Inflammatory, and Innate Immune Activation
3.4. DPPH Radical Scavenging Activity
3.5. Measurement of Cancer Cell Viability with CCK-8 Kit
3.6. Determination of Soluble Sugars (Glucose, Fructose, and Sucrose)
3.7. Determination of Fructan Content
3.8. Determination of Total Phenolic Compounds
3.9. Determination of Total Flavonoid Compounds
3.10. Determination of Amino Acid Contents
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Shigyo, M.; Khar, A.; Abdelrahman, M. The Allium Genomes: Compendium of Plant Genomes; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Ahmed, T.; Wang, C.-K. Black Garlic and Its Bioactive Compounds on Human Health Diseases: A Review. Molecules 2021, 26, 5028. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Kang, D. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules 2017, 22, 919. [Google Scholar] [CrossRef]
- Ichikawa, M.; Yoshida, J.; Ide, N.; Sasaoka, T.; Yamaguchi, H.; Ono, K. Tetrahydro-b-Carboline Derivatives in Aged Garlic Extract Show Antioxidant Properties. J. Nutr. 2006, 136, 726S–731S. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kang, M.J.; Hong, S.S.; Choi, Y.; Shin, J.H. Antiinflammatory Effects of Functionally Active Compounds Isolated from Aged Black Garlic. Phytotherapy Res. 2017, 31, 53–61. [Google Scholar] [CrossRef]
- Elkhawas, Y.A.; Seleem, M.; Shabayek, M.I.; Majrashi, T.A.; Al-Warhi, T.; Eldehna, W.M.; Mostafa, N.M. Phytochemical profiling and mechanistic evaluation of black garlic extract on multiple sclerosis rat model. J. Funct. Foods 2023, 111, 105900. [Google Scholar] [CrossRef]
- Battin, E.E.; Brumaghim, J.L. Antioxidant Activity of Sulfur and Selenium: A Review of Reactive Oxygen Species Scavenging, Glutathione Peroxidase, and Metal-Binding Antioxidant Mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Horie, T.; Awazu, S.; Itakura, Y.; Fuwa, T. Identified Diallyl Polysulfides from an Aged Garlic Extract which Protects the Membranes from Lipid Peroxidation. Planta Medica 1992, 58, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Elosta, A.; Slevin, M.; Rahman, K.; Ahmed, N. Aged garlic has more potent antiglycation and antioxidant properties compared to fresh garlic extract in vitro. Sci. Rep. 2017, 7, 39613. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Shahiduzzaman, M.; Rahim, M.A.; Paul, M.; Sarkar, R.; Chaity, F.S.; Uddin, M.N.; Rana, G.M.M.; Yeasmin, M.S.; Kibria, A.; et al. Bioactive properties and organosulfur compounds profiling of newly developed garlic varieties of Bangladesh. Food Chem. X 2023, 17, 100577. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Kazumura, K.; Sato, Y.; Satozono, H.; Koike, T.; Tsuchiya, H.; Hiramatsu, M.; Katsumata, M.; Okazaki, S. Simultaneous monitoring of superoxides and intracellular calcium ions in neutrophils by chemiluminescence and fluorescence: Evaluation of action mechanisms of bioactive compounds in foods. J. Pharm. Biomed. Anal. 2013, 84, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yan, Y.; Yu, Q.; Deng, Y.; Wu, D.; Wang, Y.; Ge, Y.; Li, S.; Zhao, J. Comparison of Immunomodulatory Effects of Fresh Garlic and Black Garlic Polysaccharides on RAW 264.7 Macrophages. J. Food Sci. 2017, 82, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Sunanta, P.; Chung, H.H.; Kunasakdakul, K.; Ruksiriwanich, W.; Jantrawut, P.; Hongsibsong, S.; Sommano, S.R. Genomic relationship and physiochemical properties among raw materials used for Thai black garlic processing. Food Sci. Nutr. 2020, 8, 4534–4545. [Google Scholar] [CrossRef] [PubMed]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Kazumura, K.; Sato, Y.; Satozono, H.; Mochizuki, M.; Wu, X.; Tsuchiya, H.; Koike, T.; Okazaki, S.; Osawa, T. Health progress for human on Anthocyan assessed by new evaluation method using the innate immune response of neutrophils. In Proceedings of the Annual Meeting 2013 Sendai of Japan Society for Bioscience, Biotechnology, and Agrochemistry, Sendai, Japan, 24–28 March 2013; p. 1513. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; So, K.-F.; Wong, N.-K.; Xiao, J. Anti-cancer activities of Sallylmercaptocysteine from aged garlic. Chin. J. Nat. Med. 2019, 17, 43–49. [Google Scholar]
- Dirsch, V.M.; Gerbes, A.L.; Vollmar, A.M. Ajoene, a Compound of Garlic, Induces Apoptosis in Human Promyeloleukemic Cells, Accompanied by Generation of Reactive Oxygen Species and Activation of Nuclear Factor κB. Mol. Pharmacol. 1998, 53, 402–407. [Google Scholar] [CrossRef]
- Ting, K.K.Y. Fructose-induced metabolic reprogramming of cancer cells. Front. Immunol. 2024, 15, 1375461. [Google Scholar] [CrossRef] [PubMed]
- Ryu, K.; Ide, N.; Matsuura, H.; Itakura, Y. Nα-(1-Deoxy-D-fructos-1-yl)-L-Arginine, an Antioxidant Compound Identified in Aged Garlic Extract. J. Nutr. 2001, 131, 972S–976S. [Google Scholar] [CrossRef] [PubMed]
- Saito, H. Garlic Science; Asakura Publishing Co., Ltd.: Tokyo, Japan, 2008. [Google Scholar]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. The Comparison of the Contents of Sugar, Amadori, and Heyns Compounds in Fresh and Black Garlic. J. Food Sci. 2016, 81, C1662–C1668. [Google Scholar] [CrossRef] [PubMed]
- Shemesh-Mayer, E.; Ben-Michael, T.; Rotem, N.; Rabinowitch, H.D.; Doron-Faigenboim, A.; Kosmala, A.; Perlikowski, D.; Sherman, A.; Kamenetsky, R. Garlic (Allium sativum L.) fertility: Transcriptome and proteome analyses provide insight into flower and pollen development. Front. Plant Sci. 2015, 6, 271. [Google Scholar] [CrossRef] [PubMed]
- Hirata, S.; Abdelrahman, M.; Yamauchi, N.; Shigyo, M. Diversity evaluation based on morphological, physiological and isozyme variation in genetic resources of garlic (Allium sativum L.) collected worldwide. Genes Genet. Syst. 2016, 91, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Tsuda, K.; Muto, N.; Nakatani, S. Changes in Antioxidant Activity during Fruit Development in Citrus Fruit. Hort. Res. 2002, 1, 63–66. [Google Scholar] [CrossRef][Green Version]
- Percheron, F. Dosage colorimetrique du fructose et des fructo-franosides par l’ acide thiobarbiturique. Compte Rendu 1962, 255, 2521–2522. [Google Scholar]
- Folin, O.; Denis, W. A Colorimetric Method for The Determination of Phenols (and Phenol Derivatives) in Urine. J. Biol. Chem. 1915, 22, 305–308. [Google Scholar] [CrossRef]
- Vu, Q.H.; Hang, T.T.M.; Yaguchi, S.; Ono, Y.; Pham, T.M.P.; Yamauchi, N.; Shigyo, M. Assessment of biochemical and antioxidant diversities in a shallot germplasm collection from Vietnam and its surrounding countries. Genet. Resour. Crop Evol. 2013, 60, 1297–1312. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]


| Materials | Number of Bulbs | Collected Site | Accession Information | |
|---|---|---|---|---|
| Fresh garlic | ‘White-roppen’ 2018 | 5 | Japanese local market | — |
| ‘White-roppen’ 2019 | 3 | Japanese local market | — | |
| ‘Iki-ooninnniku’ | 2 | Saga University, Japan | Hirata et al. [28] | |
| ‘Chile-60’ | 1 | Chile | Etoh [29] | |
| ‘Spain-225’ | 1 | Spain | Hirata et al. [28] | |
| Black garlic | ‘White-roppen’ 2018 | 5 | Processed White-roppen 2018 | — |
| ‘White-roppen’ 2019 | 2 | Processed White-roppen 2019 | — | |
| Black Garlic M | 5 | Japanese local market | — | |
| Black Garlic 2018 | 3 | IPB Shop Official | — | |
| Black Garlic 2019 | 3 | IPB Shop Official | — | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuse, K.; Hirata, S.; Abdelrahman, M.; Nakajima, T.; Iuchi, Y.; Kambayashi, S.; Okuda, M.; Kazumura, K.; Manochai, B.; Shigyo, M. Comparative Studies of Bioactivities and Chemical Components in Fresh and Black Garlics. Molecules 2024, 29, 2258. https://doi.org/10.3390/molecules29102258
Matsuse K, Hirata S, Abdelrahman M, Nakajima T, Iuchi Y, Kambayashi S, Okuda M, Kazumura K, Manochai B, Shigyo M. Comparative Studies of Bioactivities and Chemical Components in Fresh and Black Garlics. Molecules. 2024; 29(10):2258. https://doi.org/10.3390/molecules29102258
Chicago/Turabian StyleMatsuse, Kanako, Sho Hirata, Mostafa Abdelrahman, Tetsuya Nakajima, Yoshihito Iuchi, Satoshi Kambayashi, Masaru Okuda, Kimiko Kazumura, Benya Manochai, and Masayoshi Shigyo. 2024. "Comparative Studies of Bioactivities and Chemical Components in Fresh and Black Garlics" Molecules 29, no. 10: 2258. https://doi.org/10.3390/molecules29102258
APA StyleMatsuse, K., Hirata, S., Abdelrahman, M., Nakajima, T., Iuchi, Y., Kambayashi, S., Okuda, M., Kazumura, K., Manochai, B., & Shigyo, M. (2024). Comparative Studies of Bioactivities and Chemical Components in Fresh and Black Garlics. Molecules, 29(10), 2258. https://doi.org/10.3390/molecules29102258









