Third-Generation Anticancer Photodynamic Therapy Systems Based on Star-like Anionic Polyacrylamide Polymer, Gold Nanoparticles, and Temoporfin Photosensitizer
Abstract
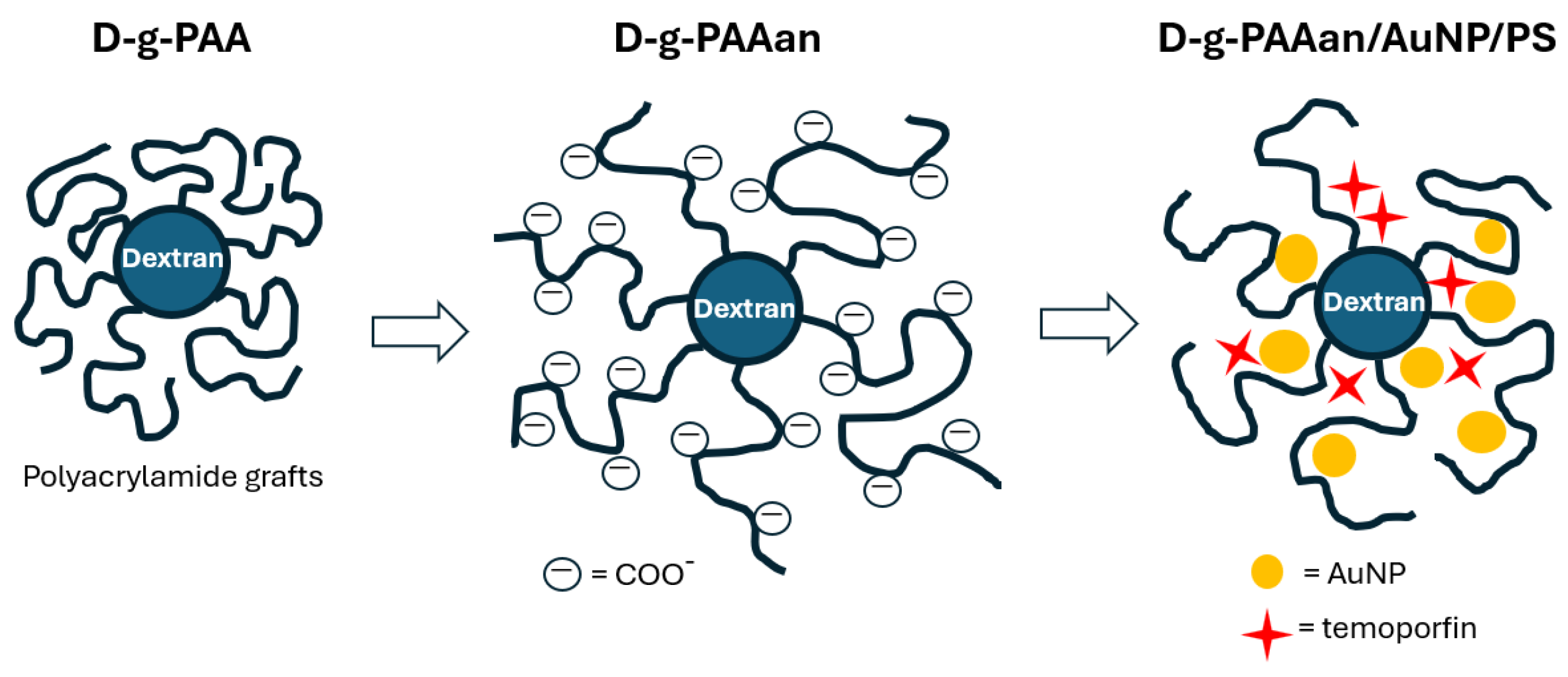
1. Introduction
2. Results and Discussion
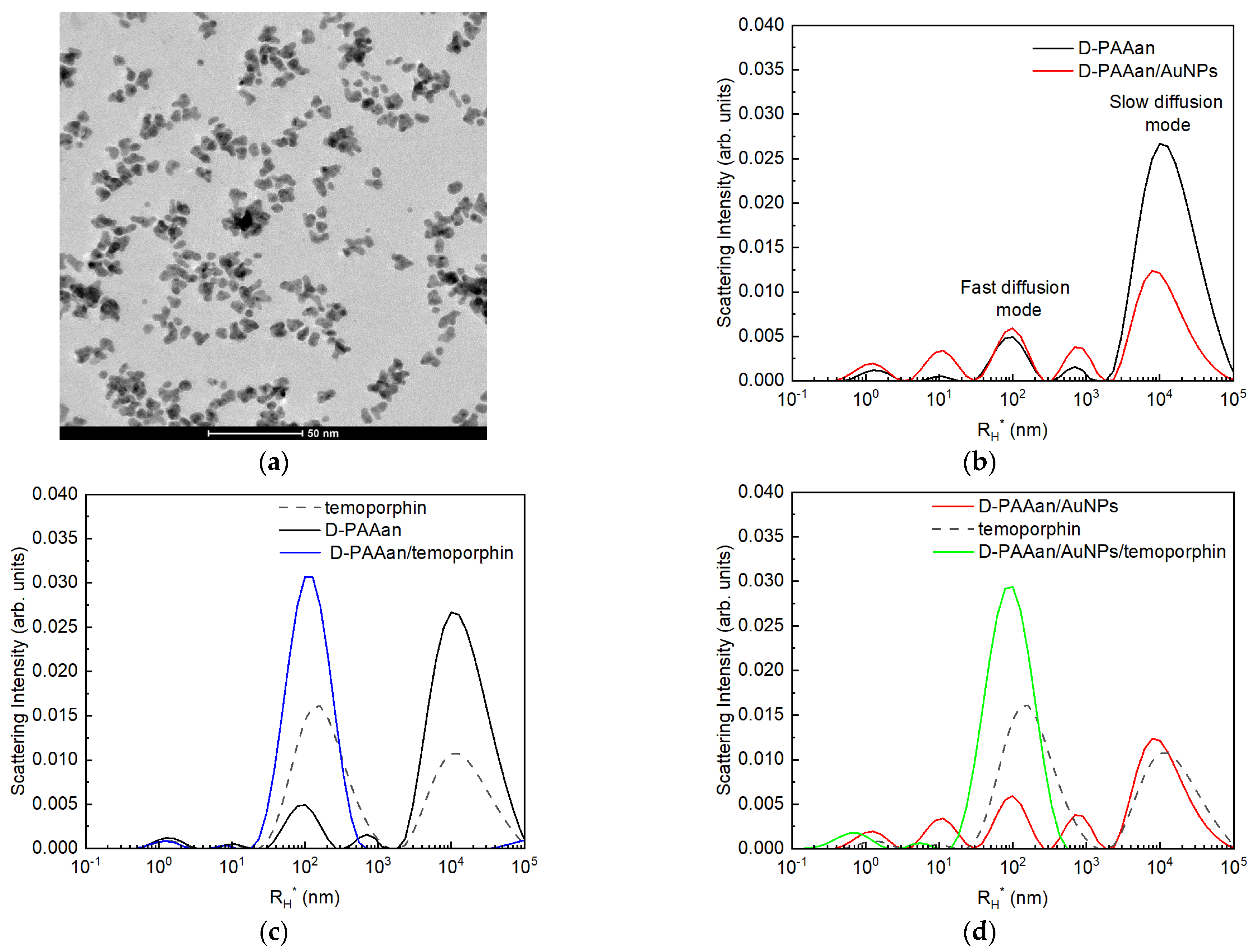
2.1. Size Characteristics of Nanohybrids in Aqueous Solution
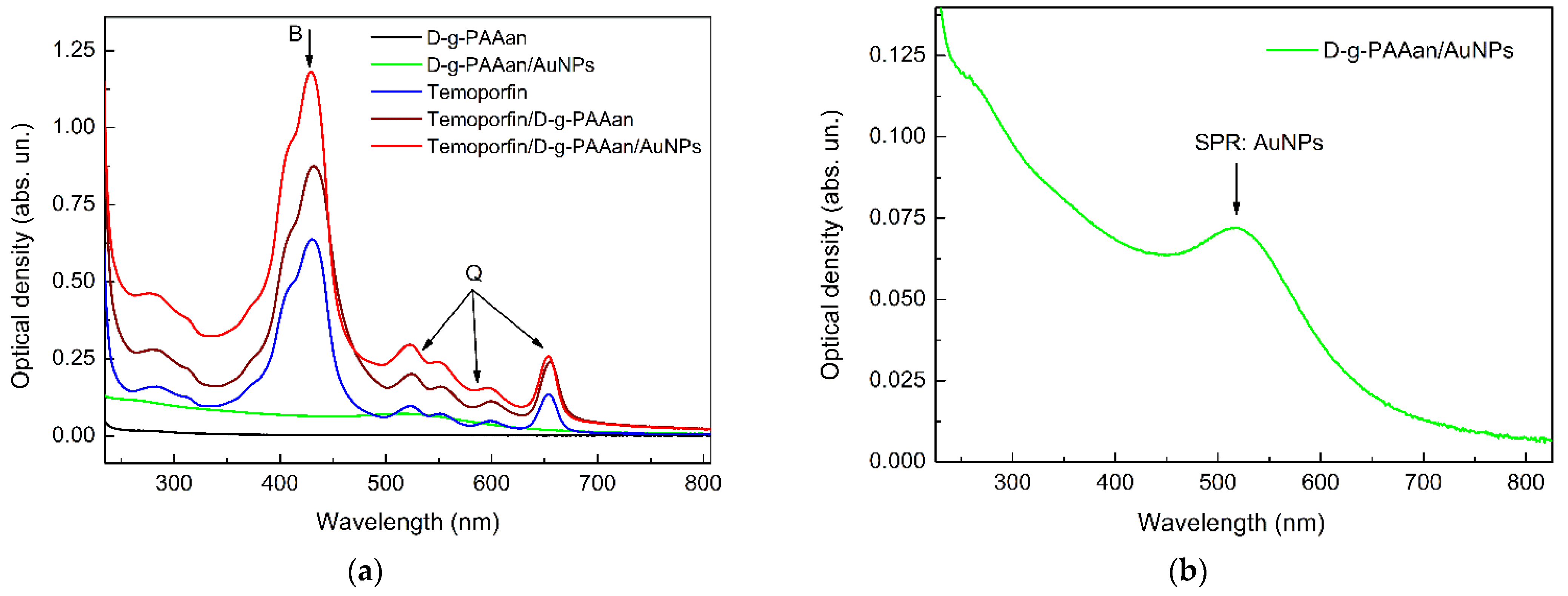
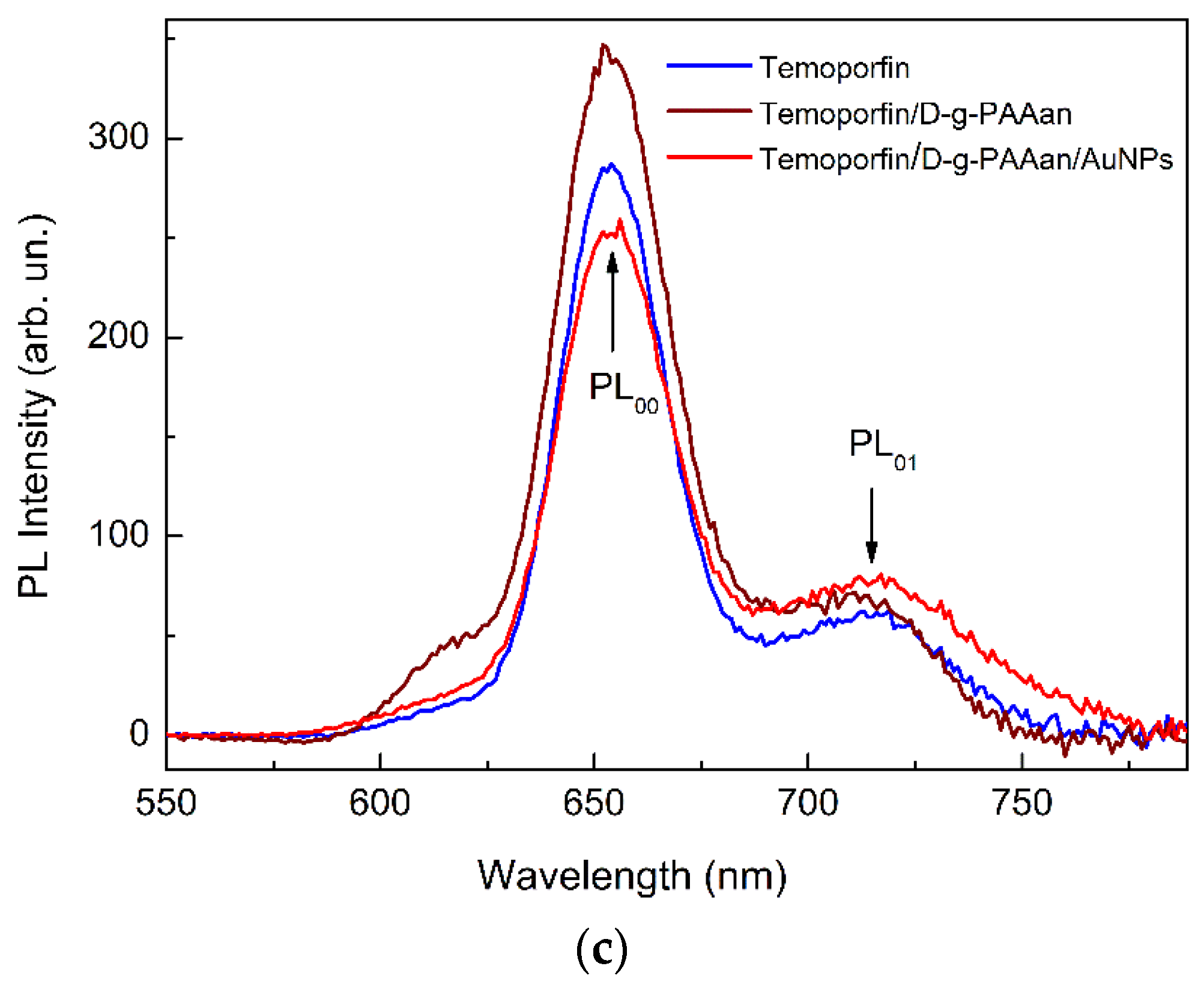
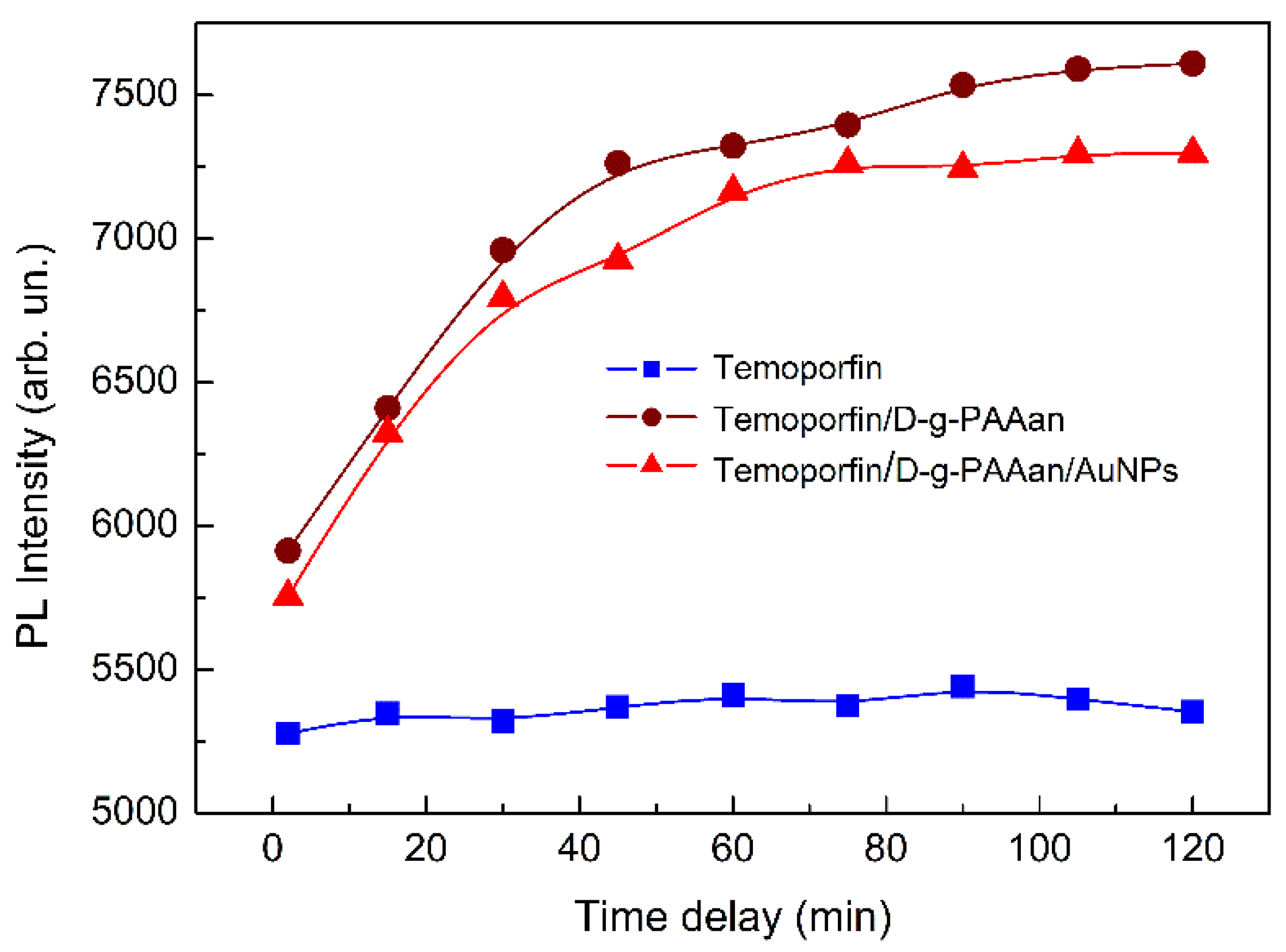
2.2. Optical Properties of Temoporfin/D-g-PAAan and Temoporfin/D-g-PAAan/AuNPs Nanohybrids
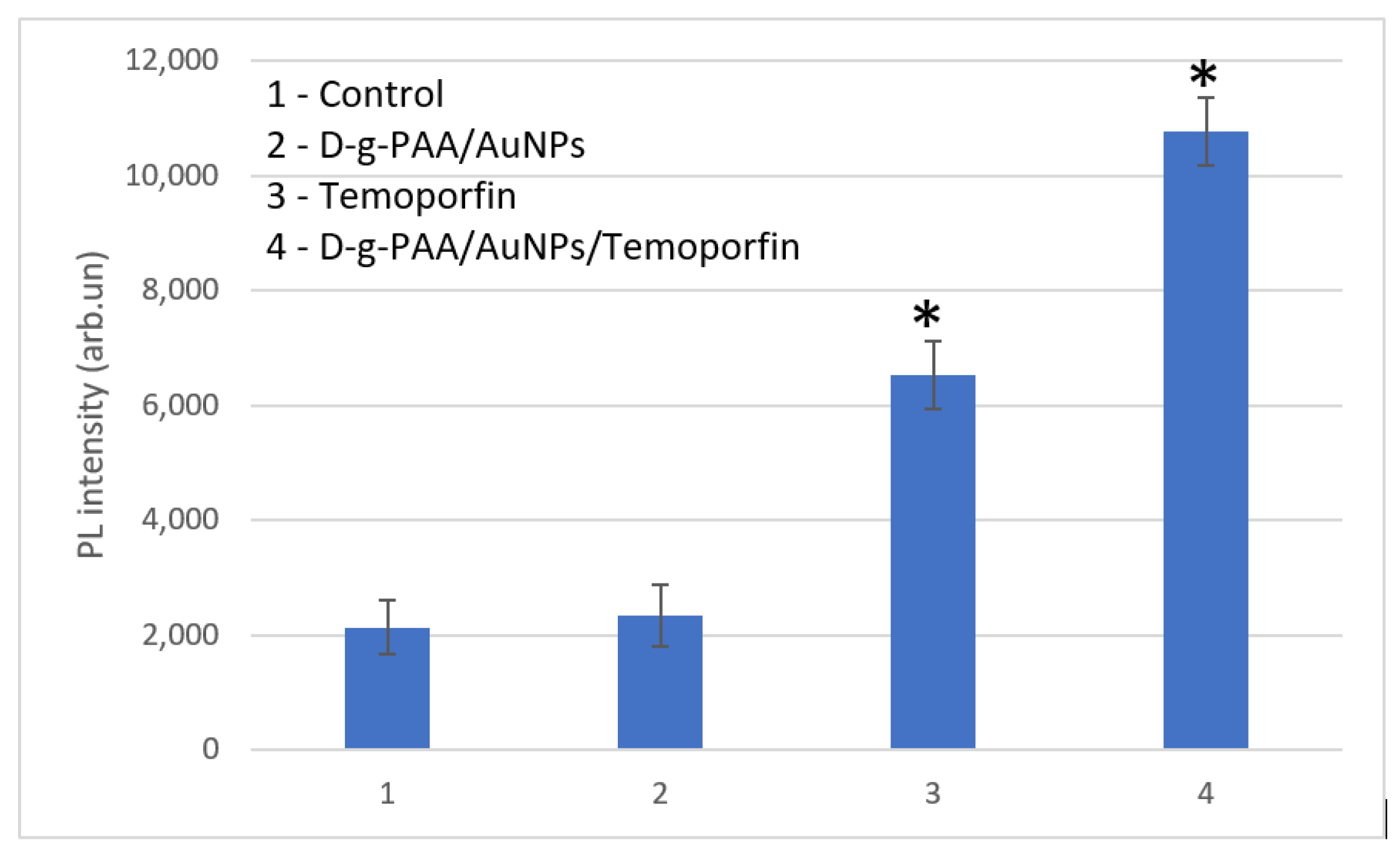
2.3. ROS Generation
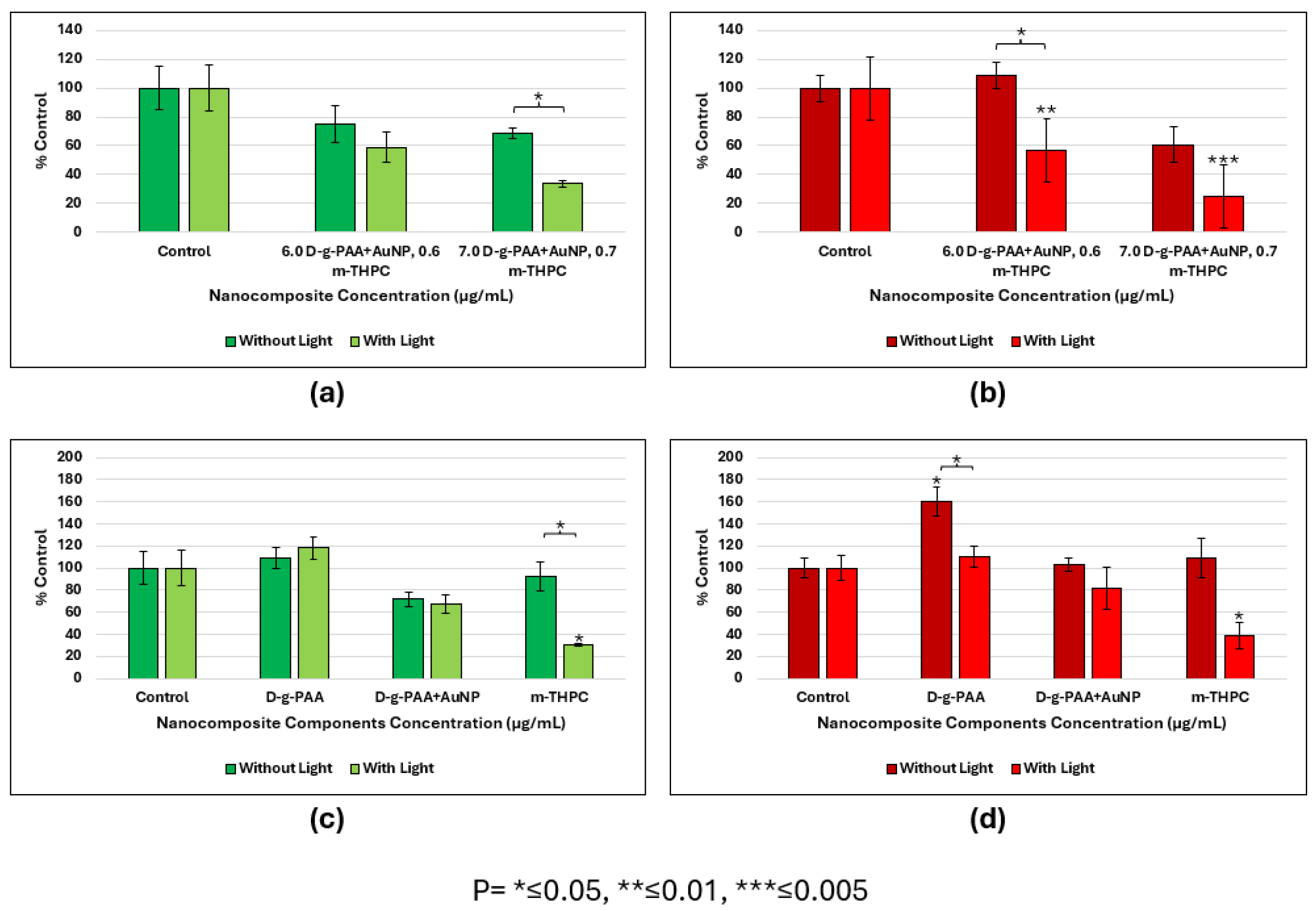
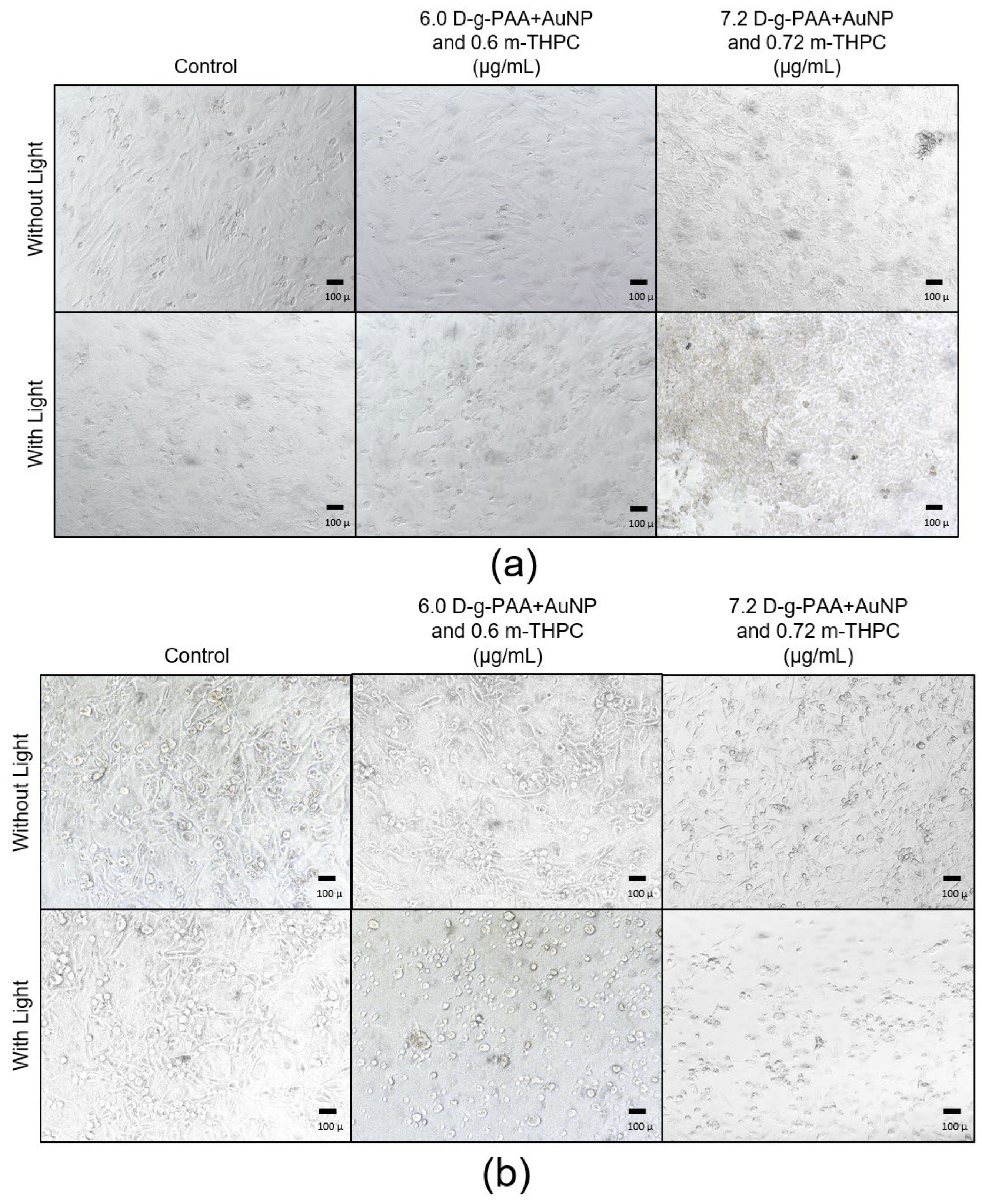
2.4. Photodynamic Therapy In Vitro
3. Materials and Methods
3.1. Materials
3.2. Polymer Nanocarrier
3.3. Nanosystem Preparation
3.4. Optical Characterization
3.5. Dynamic Light Scattering Characterization
3.6. ROS Detection In Situ
3.7. In Vitro Cell Culture
3.8. Photodynamic Therapy Protocol
3.9. Cell Counts and Morphology
3.10. Statistical Analysis and Figures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Itoo, A.M.; Paul, M.; Padaga, S.G.; Ghosh, B.; Biswas, S. Nanotherapeutic Intervention in Photodynamic Therapy for Cancer. ACS Omega 2022, 7, 45882–45909. [Google Scholar] [CrossRef]
- Hussain, Z.; Qi, Q.; Zhu, J.; Anderson, K.E.; Ma, X. Protoporphyrin IX-induced phototoxicity: Mechanisms and therapeutics. Pharmacol. Ther. 2023, 248, 108487. [Google Scholar] [CrossRef]
- Hamidi, A.; Shamlouei, H.R.; Maleki, A.; Goodajdar, B.M. Improving the Optical Properties of Porphyrin Ring with Different Substitutions: As Candidate Using in Photosensitizer. Int. J. Nanoelectron. Mater. 2020, 13, 411–420. [Google Scholar]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Hu, J.-J.; Lei, Q.; Zhang, X.-Z. Recent advances in photonanomedicines for enhanced cancer photodynamic therapy. Prog. Mater. Sci. 2020, 114, 100685. [Google Scholar] [CrossRef]
- Jin, G.R.; He, R.Y.; Liu, Q.; Dong, Y.Q.; Lin, M.; Li, W.F.; Xu, F. Theranostics of Triple-Negative Breast Cancer Based on Conjugated Polymer Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 10634–10646. [Google Scholar] [CrossRef]
- Zhang, H.J.; Liang, Y.C.; Zhao, H.; Qi, R.L.; Chen, Z.; Yuan, H.X.; Liang, H.Y.; Wang, L. Dual-mode antibacterial conjugated polymer nanoparticles for photothermal and photodynamic therapy. Macromol. Biosci. 2020, 20, 1900301. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Xie, Y.; Li, J.; Peng, Z.H.; Sheinin, Y.; Zhou, J.P.; Oupicky, D. Tumor-penetrating nanoparticles for enhanced anticancer activity of combined photodynamic and hypoxia-activated therapy. ACS Nano 2017, 11, 2227–2238. [Google Scholar] [CrossRef]
- Wu, X.J.; Gao, Y.Q.; Dong, C.M. Polymer/gold hybrid nanoparticles: From synthesis to cancer theranostic applications. RSC Adv. 2015, 5, 13787–13796. [Google Scholar] [CrossRef]
- Calavia, P.G.; Bruce, G.; Pérez-García, L.; Russell, D.A. Photosensitiser-gold nanoparticle conjugates for photodynamic therapy of cancer. Photochem. Photobiol. Sci. 2018, 17, 1534–1552. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Weersink, R.A.; Wilson, B.C.; Zheng, G. Photodynamic and photothermal therapies: Synergy opportunities for nanomedicine. ACS Nano 2023, 17, 7979–8003. [Google Scholar] [CrossRef] [PubMed]
- Nkune, N.W.; Abrahamse, H. Anti-Hypoxia Nanoplatforms for enhanced photosensitizer uptake and photodynamic therapy effects in cancer cells. Int. J. Mol. Sci. 2023, 24, 2656. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Yang, T.; Mao, C.B. Enhancement of photodynamic cancer therapy by physical and chemical factors. Angew. Chem. Int. Ed. 2019, 58, 14066–14080. [Google Scholar] [CrossRef] [PubMed]
- Yakavets, I.; Millard, M.; Zorin, V.; Lassalle, H.P.; Bezdetnaya, L. Current state of the nanoscale delivery systems for temoporfin-based photodynamic therapy: Advanced delivery strategies. J. Control. Release 2019, 304, 268–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, W.Z.; Zhang, T.X.; Jiang, X.Q.; Hu, Y. Hybrid nanoparticle composites applied to photodynamic therapy: Strategies and applications. J. Mater. Chem. B 2020, 8, 4726–4737. [Google Scholar] [CrossRef] [PubMed]
- Kruger, C.A.; Abrahamse, H. Utilisation of targeted nanoparticle photosensitiser drug delivery systems for the enhancement of photodynamic therapy. Molecules 2018, 23, 2628. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Chota, A.; Sarbadhikary, P.; Abrahamse, H. Fundamentals and applications of metal nanoparticle- enhanced singlet oxygen generation for improved cancer photodynamic therapy. Front. Chem. 2022, 10, 964674. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Koczorowski, T.; Glowacka-Sobotta, A.; Michalak, M.; Mlynarczyk, D.T.; Güzel, E.; Goslinski, T.; Sobotta, L. Connections between Metallic Nanoparticles and Chlorin e6—An Overview of Physicochemical and Biological Properties and Prospective Medical Applications. Appl. Sci. 2023, 13, 3933. [Google Scholar] [CrossRef]
- Setaro, F.; Wennink, J.W.H.; Mäkinen, P.I.; Holappa, L.; Trohopoulos, P.N.; Ylä-Herttuala, S.; van Nostrum, C.F.; de la Escosura, A.; Torres, T. Amphiphilic phthalocyanines in polymeric micelles: A supramolecular approach toward efficient third-generation photosensitizers. J. Mater. Chem. B 2020, 8, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Gualdesi, M.S.; Vara, J.; Aiassa, V.; Alvarez Igarzabal, C.I.; Ortiz, C.S. New poly(acrylamide) nanoparticles in the development of third generation photosensitizers. Dye. Pigment. 2021, 184, 108856. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, X.; Chen, L.; Gong, X.; Yang, H.; Duan, X.; Zhu, Y. Multifunctional Gold Nanoparticles in Cancer Diagnosis and Treatment. Int. J. Nanomed. 2022, 17, 2041–2067. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.Y. Plasmon-enhanced light–matter interactions and applications. npj Comput. Mater. 2019, 5, 45. [Google Scholar] [CrossRef]
- Baumberg, J.J.; Aizpurua, J.; Mikkelsen, M.H.; Smith, D.R. Extreme nanophotonics from ultrathin metallic gaps. Nat. Mater. 2019, 18, 668–678. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Zhang, Y.; Aslan, K.; Previte, M.J.; Geddes, C.D. Metal-enhanced singlet oxygen generation: A consequence of plasmon enhanced triplet yields. J. Fluoresc. 2007, 17, 345–349. [Google Scholar] [CrossRef]
- Macia, N.; Kabanov, V.; Heyne, B. Rationalizing the plasmonic contributions to the enhancement of singlet oxygen production. J. Phys. Chem. C 2020, 124, 3768–3777. [Google Scholar] [CrossRef]
- Planas, O.; Macia, N.; Agut, M.; Nonell, S.; Heyne, B. Distance-dependent plasmon-enhanced singlet oxygen production and emission for bacterial inactivation. J. Am. Chem. Soc. 2016, 138, 2762–2768. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, D.; Zhang, C.; Liu, H.; Hao, M.; Kan, S.; Liu, D.; Liu, W. The Applications of Gold Nanoparticles in the Diagnosis and Treatment of Gastrointestinal Cancer. Front. Oncol. 2022, 11, 819329. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Kardani, A.; Yaghoobi, H.A. comprehensive review of cancer therapies mediated by conjugated gold nanoparticles with nucleic acid. Int. J. Biol. Macromol. 2023, 253, 127184. [Google Scholar] [CrossRef] [PubMed]
- Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Virych, P.A.; Chumachenko, V.A.; Kuziv, Y.I.; Marinin, A.I.; Cheng, L.; Nie, G. Thermoresponsive zinc tetraphenylporphyrin photosensitizer/dextran graft poly(N-isopropylacrylamide) copolymer/Au nanoparticles hybrid nanosystem: Potential for photodynamic therapy applications. Nanomaterials 2022, 12, 2655. [Google Scholar] [CrossRef] [PubMed]
- Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Virych, P.A.; Chumachenko, V.A.; Kuziv, Y.I.; Naumenko, A.P.; Marinin, A.I. Plasmonic enhancement of the antibacterial photodynamic efficiency of a zinc tetraphenylporphyrin photosensitizer/dextran graft polyacrylamide anionic copolymer/Au nanoparticles hybrid nanosystem. RSC Adv. 2022, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Yeshchenko, O.A.; Kutsevol, N.V.; Tomchuk, A.V.; Khort, P.S.; Virych, P.A.; Chumachenko, V.A.; Kuziv, Y.I.; Naumenko, A.P.; Marinin, A.I. Zinc tetraphenylporphyrin / dextran-graft-polyacrylamide/gold nanoparticles hybrid nanosystem for photodynamic therapy: Plasmonic enhancement effect. Nanomed. Res. J. 2022, 7, 173. [Google Scholar]
- Chumachenko, V.A.; Shton, I.O.; Shishko, E.D.; Kutsevol, N.V.; Marinin, A.I.; Gamaleia, N.F. Branched Copolymers Dextran-Graft-Polyacrylamide as Nanocarriers for Delivery of Gold Nanoparticles and Photosensitizers to Tumor Cells. In Nanophysics, Nanophotonics, Surface Studies, and Applications; Fesenko, O., Yatsenko, L., Eds.; Springer: Cham, Switzerland, 2016; Volume 183, pp. 379–390. [Google Scholar]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical development of photodynamicagents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Kutsevol, N.; Bezugla, T.; Bezuglyi, M.; Rawiso, M. Branched Dextran-graft-Polyacrylamide Copolymers as Perspective Materials for Nanotechnology. Macromol. Symp. 2012, 317–318, 82–90. [Google Scholar] [CrossRef]
- Kutsevol, N.; Bezuglyi, M.; Rawiso, M.; Bezugla, T. Star-like Destran-graft-(polyacrylamide-co-polyacrylic acid) Copolymers. Macromol. Symp. 2014, 335, 12–16. [Google Scholar] [CrossRef]
- Yeshchenko, O.A.; Khort, P.S.; Kutsevol, N.V.; Prokopets, V.M.; Kapush, O.; Dzhagan, V. Temperature driven plasmon-exciton coupling in thermoresponsive dextran-graft-PNIPAM/Au nanoparticle/CdTe quantum dots hybrid nanosystem. Plasmonics 2021, 16, 1137–1150. [Google Scholar] [CrossRef]
- De Vetta, M.; Baig, O.; Steen, D.; Nogueira, J.J.; González, L. Assessing configurational sampling in the quantum mechanics/molecular mechanics calculation of temoporfin absorption spectrum and triplet density of states. Molecules 2018, 23, 2932. [Google Scholar] [CrossRef]
- Aslanoglu, B.; Yakavets, I.; Zorin, V.; Lassalle, H.-P.; Ingrosso, F.; Monari, A.; Catak, S. Optical properties of photodynamic therapy drugs in different environments: The paradigmatic case of temoporfin. Phys. Chem. Chem. Phys. 2020, 22, 16956–16964. [Google Scholar] [CrossRef]
- Mattioli, E.J.; Ulfo, L.; Marconi, A.; Pellicioni, V.; Costantini, P.E.; Marforio, T.D.; Di Giosia, M.; Danielli, A.; Fimognari, C.; Turrini, E.; et al. Carrying temoporfin with human serum albumin: A new perspective for photodynamic application in head and neck cancer. Biomolecules 2023, 13, 68. [Google Scholar] [CrossRef]
- Gierlich, P.; Mucha, S.; Robbins, E.; Gomes-Da-Silva, L.; Matczyszyn, K.; Senge, M.O. One-photon and two-photon photophysical properties of tetrafunctionalized Temoporfin (mTHPC) derivatives as potential agents for two-photon induced photodynamic therapy. ChemPhotoChem 2021, 6, e20210024. [Google Scholar]
- Törmö, P.; Barnes, W.L. Strong coupling between surface plasmon polaritons and emitters: A Review. Rep. Prog. Phys. 2015, 78, 013901. [Google Scholar] [CrossRef] [PubMed]
- Rodarte, A.L.; Tao, A.R. Plasmon-exciton coupling between metallic nanoparticles and dye monomers. J. Phys. Chem. C 2017, 121, 3496–3502. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.L. Novotny, Enhancement and quenching of single-molecule fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef]
- Roller, E.M.; Argyropoulos, C.; Högele, A.; Liedl, T.; Pilo-Pais, M. Plasmon-exciton coupling using DNA templates. Nano Lett. 2016, 16, 5962–5966. [Google Scholar] [CrossRef] [PubMed]
- Dolinnyi, A.I. Nanometric rulers based on plasmon coupling in pairs of gold nanoparticles. J. Phys. Chem. C 2015, 119, 4990–5001. [Google Scholar] [CrossRef]
- Su, Q.; Jiang, C.; Gou, D.; Long, Y. Surface plasmon-assisted fluorescence enhancing and quenching: From theory to application. ACS Appl. Bio Mater. 2021, 4, 4684–4705. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef]
- Sedlák, M. What Can Be Seen by Static and Dynamic Light Scattering in Polyelectrolyte Solutions and Mixtures? Langmuir 1999, 15, 4045–4051. [Google Scholar] [CrossRef]
- Daliang, Y.; Yingying, Z.; Zheng, Z.; Yiming, R.; Ziwei, L.; Lili, S.; Sen, H. Improved detection of reactive oxygen species by DCFH-DA: New insight into self-amplification of fluorescence signal by light irradiation. Sens. Actuators B Chem. 2021, 339, 129878. [Google Scholar]
| Sample | Zeta-Potential, mV |
|---|---|
| D-g-PAAan | −70.1 |
| D-g-PAAan/AuNPs | −45.8 |
| Temoporfin | −2.1 |
| D-g-PAAan/Temoporfin | −19.04 |
| D-g-PAAan/AuNPs/Temoporfin | −14.55 |
| Sample | Normalized Absorption (Total Optical Density), rel. un | Normalized Total PL Intensity, rel. un. | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 1 | 2 | 3 | |
| 0.072 μg/mL (Temoporfin), 0.72 μg/mL (D-g-PAAan/Au) | 1 | 1.38 | 1.58 | 1 | 1.24 | 1.51 |
| 0.72 μg/mL (Temoporfin), 7.2 μg/mL (D-g-PAAan/Au) | 4.32 | 10.11 | 11.66 | 2.60 | 3.53 | 3.28 |
| 7.2 μg/mL (Temoporfin), 72 μg/mL (D-g-PAAan/Au) | 45.68 | 80.45 | 95.24 | 6.18 | 7.22 | 5.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeshchenko, O.; Khort, P.; Fedotov, O.; Chumachenko, V.; Virych, P.; Warren, H.S.; Booth, B.W.; Bliznyuk, V.; Kutsevol, N. Third-Generation Anticancer Photodynamic Therapy Systems Based on Star-like Anionic Polyacrylamide Polymer, Gold Nanoparticles, and Temoporfin Photosensitizer. Molecules 2024, 29, 2224. https://doi.org/10.3390/molecules29102224
Yeshchenko O, Khort P, Fedotov O, Chumachenko V, Virych P, Warren HS, Booth BW, Bliznyuk V, Kutsevol N. Third-Generation Anticancer Photodynamic Therapy Systems Based on Star-like Anionic Polyacrylamide Polymer, Gold Nanoparticles, and Temoporfin Photosensitizer. Molecules. 2024; 29(10):2224. https://doi.org/10.3390/molecules29102224
Chicago/Turabian StyleYeshchenko, Oleg, Pavlo Khort, Oles Fedotov, Vasyl Chumachenko, Pavlo Virych, Hunter S. Warren, Brian W. Booth, Valery Bliznyuk, and Nataliya Kutsevol. 2024. "Third-Generation Anticancer Photodynamic Therapy Systems Based on Star-like Anionic Polyacrylamide Polymer, Gold Nanoparticles, and Temoporfin Photosensitizer" Molecules 29, no. 10: 2224. https://doi.org/10.3390/molecules29102224
APA StyleYeshchenko, O., Khort, P., Fedotov, O., Chumachenko, V., Virych, P., Warren, H. S., Booth, B. W., Bliznyuk, V., & Kutsevol, N. (2024). Third-Generation Anticancer Photodynamic Therapy Systems Based on Star-like Anionic Polyacrylamide Polymer, Gold Nanoparticles, and Temoporfin Photosensitizer. Molecules, 29(10), 2224. https://doi.org/10.3390/molecules29102224







