Labdanum Resin from Cistus ladanifer L. as a Source of Compounds with Anti-Diabetic, Neuroprotective and Anti-Proliferative Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Yields and Chemical Characterization of Labdanum and Fraction Extracts
2.2. Evaluation of Anti-Diabetic and Neuroprotective Potential of Labdanum and Fraction Extracts
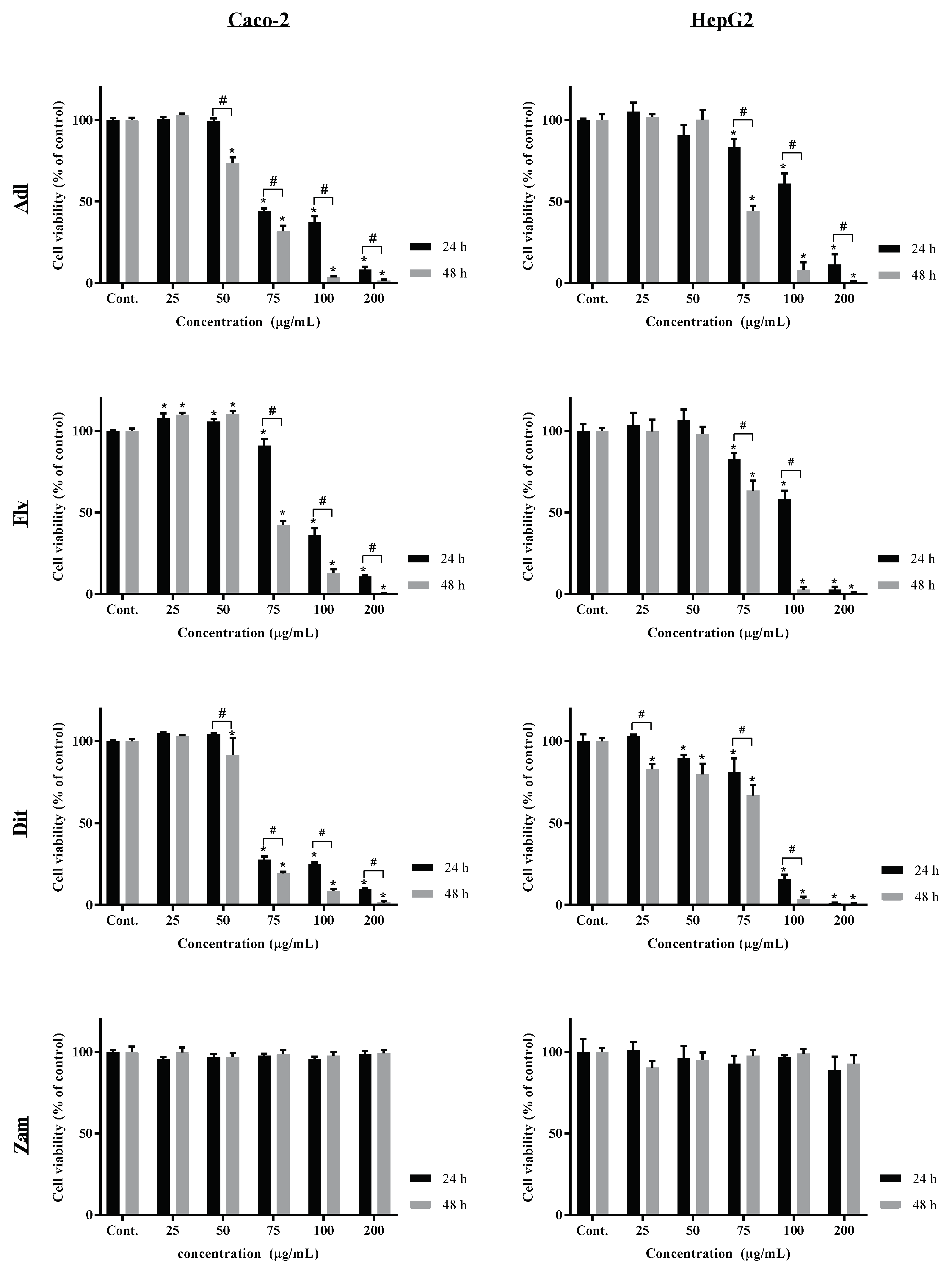
2.3. Anti-Proliferative/Cytotoxic Activity of Labdanum Absolutes and Fractions
3. Materials and Methods
3.1. Labdanum Resin Extraction and Purification
3.2. Gas Chromatography Coupled to an Electron Ionization Mass Spectrometer (GC-EI-MS)
3.3. α-Amylase, α-Glucosidase, and Acetylcholinesterase (AChE) Inhibition
3.4. Caco-2 and HepG2 Cell Cytotoxicity Assay
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godinho-Ferreira, P.; Azevedo, A.; Rego, F. Carta da tipologia florestal de Portugal Continental. Silva Lusit. 2005, 13, 1–34. [Google Scholar]
- Demoly, J.P.; Montserrat, P. Cistus. In Flora Ibérica; Castroviejo, S., Ed.; CSIC (Centro Superior Investigacion Cientifica): Madrid, Spain, 1993; pp. 319–337. [Google Scholar]
- Montero, G.; López-Leiva, C.; Ruiz-Peinado, R.; López-Senespleda, E.; Onrubia, R.; Pasalodos, M. Producción de Biomasa Y Fijación de Carbono Por Los Matorrales Españoles Y Por El Horizonte Orgánico Superficial de Los Suelos Forestales; Ministerio De Agricultura, Pesca Y Alimentación, Secretaría General Técnica Spain: Madrid, Spain, 2020; pp. 100–101.
- Mendes, P.; Meireles, C.; Vila-Viçosa, C.; Musarella, C.; Pinto-Gomes, C. Best management practices to face degraded territories occupied by Cistus ladanifer shrublands–Portugal case study. Plant Biosyst. -Int. J. Deal. All Asp. Plant Biol. 2015, 149, 494–502. [Google Scholar]
- Frazão, D.F.; Gonçalves, J.C.; Silva, A.M.; Delgado, F. Rockrose Land Management: Contribution of Periodic Harvesting to Increase Value and to Control Cistus ladanifer L. Shrublands. Forests 2023, 14, 638. [Google Scholar] [CrossRef]
- Raimundo, J.R.; Frazão, D.F.; Domingues, J.L.; Quintela-Sabarís, C.; Dentinho, T.P.; Anjos, O.; Alves, M.; Delgado, F. Neglected Mediterranean plant species are valuable resources: The example of Cistus ladanifer. Planta 2018, 248, 1351–1364. [Google Scholar] [CrossRef] [PubMed]
- Biolandes. Cistus Labdanum in Andalusia. Available online: https://www.biolandes.com/en-cistus-labdanum.php?voyage=o&lg=en (accessed on 24 February 2022).
- Lawrence, B.M. Progress in essential oils. Cistus and labdanum isolates and extracts. Perfum. Flavourist 1999, 24, 41–50. [Google Scholar]
- Sosa, T.; Alías, J.C.; Escudero, J.C.; Chaves, N. Interpopulational variation in the flavonoid composition of Cistus ladanifer L. exudate. Biochem. Syst. Ecol. 2005, 33, 353–364. [Google Scholar] [CrossRef]
- Alías, J.C.; Sosa, T.; Valares, C.; Escudero, J.C.; Chaves, N. Seasonal variation of Cistus ladanifer L. diterpenes. Plants 2012, 1, 6–15. [Google Scholar] [CrossRef] [PubMed]
- de Pascual, J.; Urones, J.; Basabe, M.; Marcos, I.; Montaña, A. Nuevo studio sobre componentes de Cistus ladaniferus L. Stvdia Chem. 1984, IX, 31–47. [Google Scholar]
- Greche, H.; Mrabet, N.; Ismaili-Alaoui, M.; Hajajji, N.; Bousta, D.; Dahchour, A.; Boukir, A.; Benjilali, B. Chemical composition, antibacterial and antifungal activities of Moroccan Cistus ladanifer L. leaves extracts. In Recherches sur les Plantes Aromatiques et Médicinales, Imprimerie Al Maarif Al Jadida, Rabat; Greche, H., Ennabili, A., Eds.; INPMA©: Rabat, Morocco, 2009; pp. 201–213. [Google Scholar]
- Burguer, L. Investigação e Comparação de Metodologias de Extração de lábdano OBTIDO a Partir de Cistus ladanifer L. Master’s Thesis, Instituto Politécnico de Bragança, Universidadad de Salamanca, Bragança, Portugal, 2016. [Google Scholar]
- Vogt, T.; Proksch, P.; Gülz, P.-G. Epicuticular flavonoid aglycones in the genus Cistus, Cistaceae. J. Plant Physiol. 1987, 131, 25–36. [Google Scholar] [CrossRef]
- Valares, C.M.; Sosa Díaz, T.; Alías Gallego, J.C.; Chaves Lobón, N. Quantitative variation of flavonoids and diterpenes in leaves and stems of Cistus ladanifer L. at different ages. Molecules 2016, 21, 275. [Google Scholar] [CrossRef]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A Review on Cistus sp.: Phytochemical and Antimicrobial Activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lirola, M.J.; González-Tejero, M.R.; Molero-Mesa, J. Ethnobotanical Resources in the Province of Almería, Spain: Campos de Nijar. Econ. Bot. 1996, 50, 40–56. [Google Scholar] [CrossRef]
- Bencheikh, N.; Elbouzidi, A.; Kharchoufa, L.; Ouassou, H.; Alami Merrouni, I.; Mechchate, H.; Es-safi, I.; Hano, C.; Addi, M.; Bouhrim, M.; et al. Inventory of Medicinal Plants Used Traditionally to Manage Kidney Diseases in North-Eastern Morocco: Ethnobotanical Fieldwork and Pharmacological Evidence. Plants 2021, 10, 1966. [Google Scholar] [CrossRef] [PubMed]
- Papaefthimiou, D.; Papanikolaou, A.; Falara, V.; Givanoudi, S.; Kostas, S.; Kanellis, A.K. Genus Cistus: A model for exploring labdane-type diterpenes’ biosynthesis and a natural source of high value products with biological, aromatic, and pharmacological properties. Front. Chem. 2014, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Vieira, R.; Souto, S.B.; Sánchez-López, E.; Machado, A.L.; Severino, P.; Jose, S.; Santini, A.; Fortuna, A.; García, M.L.; Silva, A.M.; et al. Sugar-Lowering Drugs for Type 2 Diabetes Mellitus and Metabolic Syndrome-Review of Classical and New Compounds: Part-I. Pharmaceuticals 2019, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Frazão, D.F.; Martins-Gomes, C.; Steck, J.L.; Keller, J.; Delgado, F.; Gonçalves, J.C.; Bunzel, M.; Pintado, C.M.B.S.; Díaz, T.S.; Silva, A.M. Labdanum Resin from Cistus ladanifer L.: A Natural and Sustainable Ingredient for Skin Care Cosmetics with Relevant Cosmeceutical Bioactivities. Plants 2022, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Sayah, K.; Marmouzi, I.; Naceiri Mrabti, H.; Cherrah, Y.; Faouzi, M.E.A. Antioxidant Activity and Inhibitory Potential of Cistus salviifolius (L.) and Cistus monspeliensis (L.) Aerial Parts Extracts against Key Enzymes Linked to Hyperglycemia. BioMed Res. Int. 2017, 2017, 2789482. [Google Scholar] [CrossRef] [PubMed]
- Crespo Martín, J.M.; Cardenal Galván, J.A.; Peral Pacheco, D.; Vallejo Villalobos, J.R. Jara pringosa (Cistus ladanifer), usos, utilidades y curiosidades en Extremadura. Rev. De Estud. Extrem. 2009, 65, 1637–1650. [Google Scholar]
- González, J.; Vallejo, J.R.; Amich, F. Cistus ladanifer L. En: Inventario Español de los Conocimientos Tradicionales Relativos a la Biodiversidad; Fase II (Tomo 2); Ministerio de Agricultura y Pesca, Alimentación y Medio Ambiente (MAPAMA): Madrid, Spain, 2018; pp. 47–55.
- Moss, D.E. Improving anti-neurodegenerative benefits of acetylcholinesterase inhibitors in Alzheimer’s disease: Are irreversible inhibitors the future? Int. J. Mol. Sci. 2020, 21, 3438. [Google Scholar] [CrossRef]
- Harvey, D.J.; Vouros, P. Mass spectrometric fragmentation of trimethylsilyl and related alkylsilyl derivatives. Mass Spectrom. Rev. 2020, 39, 105–211. [Google Scholar] [CrossRef]
- Jurčević Šangut, I.; Šarkanj, B.; Karalija, E.; Šamec, D. A Comparative Analysis of Radical Scavenging, Antifungal and Enzyme Inhibition Activity of 3′-8″-Biflavones and Their Monomeric Subunits. Antioxidants 2023, 12, 1854. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Taghouti, M.; Schäfer, J.; Bunzel, M.; Silva, A.M.; Nunes, F.M. Chemical characterization and bioactive properties of decoctions and hydroethanolic extracts of Thymus carnosus Boiss. J. Funct. Foods 2018, 43, 154–164. [Google Scholar] [CrossRef]
- Papoutsis, K.; Zhang, J.; Bowyer, M.C.; Brunton, N.; Gibney, E.R.; Lyng, J. Fruit, vegetables, and mushrooms for the preparation of extracts with α-amylase and α-glucosidase inhibition properties: A review. Food Chem. 2020, 338, 128119. [Google Scholar] [CrossRef] [PubMed]
- Standl, E.; Theodorakis, M.J.; Erbach, M.; Schnell, O.; Tuomilehto, J. On the potential of acarbose to reduce cardiovascular disease. Cardiovasc. Diabetol. 2014, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Poovitha, S.; Parani, M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.). BMC Complement. Altern. Med. 2016, 16 (Suppl. S1), 185. [Google Scholar] [CrossRef]
- Silva, A.M.; Félix, L.M.; Teixeira, I.; Martins-Gomes, C.; Schäfer, J.; Souto, E.B.; Santos, D.J.; Bunzel, M.; Nunes, F.M. Orange thyme: Phytochemical profiling, in vitro bioactivities of extracts and potential health benefits. Food Chem. X 2021, 12, 100171. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, D.P. Antioxidant and Antidiabetic Properties of Medicinal Plant Infusions. Master’s Thesis, Universidade do Algarve, Faro, Portugal, 2016. [Google Scholar]
- Santos, T.C.d.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.d.A. Naturally occurring acetylcholinesterase inhibitors and their potential use for Alzheimer’s disease therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Ben Jemia, M.; Senatore, F.; Bruno, M.; Menichini, F.; Tundis, R. Chemistry and functional properties in prevention of neurodegenerative disorders of five Cistus species essential oils. Food Chem. Toxicol. 2013, 59, 586–594. [Google Scholar] [CrossRef]
- Lai Shi Min, S.; Liew, S.Y.; Chear, N.J.Y.; Goh, B.H.; Tan, W.-N.; Khaw, K.Y. Plant Terpenoids as the Promising Source of Cholinesterase Inhibitors for Anti-AD Therapy. Biology 2022, 11, 307. [Google Scholar] [CrossRef]
- Khan, H.; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Howes, M.-J.R.; Houghton, P.J. Ethnobotanical Treatment Strategies Against Alzheimers Disease. Curr. Alzheimer Res. 2012, 9, 67–85. [Google Scholar] [CrossRef]
- Pohanka, M. Acetylcholinesterase inhibitors: A patent review (2008–present). Expert Opin. Ther. Pat. 2012, 22, 871–886. [Google Scholar] [CrossRef] [PubMed]
- Loesche, A.; Wiemann, J.; Al Halabi, Z.; Karasch, J.; Sippl, W.; Csuk, R. Unexpected AChE inhibitory activity of (2E)α,β-unsaturated fatty acids. Bioorganic Med. Chem. Lett. 2018, 28, 3315–3319. [Google Scholar] [CrossRef] [PubMed]
- Lea, T. Caco-2 cell line. In The Impact of Food Bioactives on Health; Springer: Berlin/Heidelberg, Germany, 2015; pp. 103–111. [Google Scholar]
- Tham, N.T.; Hwang, S.-R.; Bang, J.-H.; Yi, H.; Park, Y.-I.; Kang, S.-J.; Kang, H.-G.; Kim, Y.-S.; Ku, H.-O. High-content analysis of in vitro hepatocyte injury induced by various hepatotoxicants. J. Vet. Sci. 2019, 20, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Saura, D.; Guillén, E.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Martins-Gomes, C.; Souto, E.B.; Cosme, F.; Nunes, F.M.; Silva, A.M. Thymus carnosus extracts induce anti-proliferative activity in Caco-2 cells through mechanisms that involve cell cycle arrest and apoptosis. J. Funct. Foods 2019, 54, 128–135. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Kukula-Koch, W.; Hoian, U.; Czop, M.; Strzępek-Gomółka, M.; Antosiewicz, B. Characterization of Cistus× incanus L. and Cistus ladanifer L. extracts as potential multifunctional antioxidant ingredients for skin protecting cosmetics. Antioxidants 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Andrade, D.; Gil, C.; Breitenfeld, L.; Domingues, F.; Duarte, A.P. Bioactive extracts from Cistus ladanifer and Arbutus unedo L. Ind. Crops Prod. 2009, 30, 165–167. [Google Scholar] [CrossRef]
- Skorić, M.; Todorović, S.; Gligorijević, N.; Janković, R.; Živković, S.; Ristić, M.; Radulović, S. Cytotoxic activity of ethanol extracts of in vitro grown Cistus creticus subsp. creticus L. on human cancer cell lines. Ind. Crops Prod. 2012, 38, 153–159. [Google Scholar] [CrossRef]
- Szeremeta, D.; Knaś, M.; Długosz, E.; Krzykała, K.; Mrozek-Wilczkiewicz, A.; Musioł, R.; Kowalska, T.; Ott, P.G.; Sajewicz, M.; Móricz, Á.M. Investigation of antibacterial and cytotoxic potential of phenolics derived from Cistus incanus L. by means of thin-layer chromatography-direct bioautography and cytotoxicity assay. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 349–357. [Google Scholar] [CrossRef]
- Azemard, C.; Menager, M.; Vieillescazes, C. Analysis of diterpenic compounds by GC-MS/MS: Contribution to the identification of main conifer resins. Anal. Bioanal. Chem. 2016, 408, 6599–6612. [Google Scholar] [CrossRef]
- Martins-Gomes, C.; Steck, J.; Keller, J.; Bunzel, M.; Nunes, F.M.; Silva, A.M. Molecular Characterization of Thymus capitellatus Extracts and Their Antioxidant, Neuroprotective and Anti-Proliferative Activities. Int. J. Mol. Sci. 2022, 23, 15187. [Google Scholar] [CrossRef] [PubMed]
- Torres-Naranjo, M.; Suarez, A.; Gilardoni, G.; Cartuche, L.; Flores, P.; Morocho, V. Chemical Constituents of Muehlenbeckia tamnifolia (Kunth) Meisn (Polygonaceae) and Its In Vitro alpha-Amilase and alpha-Glucosidase Inhibitory Activities. Molecules 2016, 21, 1461. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins-Gomes, C.; Ferreira, S.S.; Souto, E.B.; Andreani, T. Molecular Physicochemical Properties of Selected Pesticides as Predictive Factors for Oxidative Stress and Apoptosis-Dependent Cell Death in Caco-2 and HepG2 Cells. Int. J. Mol. Sci. 2022, 23, 8107. [Google Scholar] [CrossRef] [PubMed]
- Andreani, T.; Kiill, C.P.; de Souza, A.L.R.; Fangueiro, J.F.; Fernandes, L.; Doktorovová, S.; Santos, D.L.; Garcia, M.L.; Gremião, M.P.D.; Souto, E.B. Surface engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloids Surf. B Biointerfaces 2014, 123, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.M.; Martins-Gomes, C.; Souto, E.B.; Schäfer, J.; Santos, J.A.; Bunzel, M.; Nunes, F.M. Thymus zygis subsp. zygis an Endemic Portuguese Plant: Phytochemical Profiling, Antioxidant, Anti-Proliferative and Anti-Inflammatory Activities. Antioxidants 2020, 9, 482. [Google Scholar] [CrossRef]
- Geran, R.I.; Greenberg, N.H.; Donald, M.M.M. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. 1972, 3, 1–103. [Google Scholar]
| Extraction Process | Resin Yield (% dw/fw) | Absolute Yield (% dw/dw) |
|---|---|---|
| Adl | 5.79 ± 0.63 a | 72.61 ± 1.52 a |
| Zam | 0.23 ± 0.08 b | 71.03 ± 4.18 a |
| Experimental | NIST Library/Standard Identification | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak | RT (min) | RI (i.u.) | Extract | TMS (73) | NIST Match | Compound | NIST RI | |||
| Adl Fractions | ||||||||||
| Adl | Zam | Flv | Dit | |||||||
| 1 | 7.46 ± 0.02 | 1133 ± 1 | + | yes | 886 ± 48 | 3-acetoxy-3-hydroxypropionic acid, methyl ester | ||||
| 2 | 9.64 ± 0.03 | 1286 ± 2 | + | ++ | yes | 921 ± 48 | 3-phenylpropanoic acid (hydrocinnamic acid), TMS | 1279/1246 | ||
| 3 | 9.72 ± 0.02 | 1291 ± 1 | ++ | yes | 814 ± 78 | 3-methyl-hexanedioic acid, dimethyl ester | 1285/1253 | |||
| 4 | 10.37 ± 0.00 | 1341 ± 0 | + | - | ||||||
| 5 | 12.42 ± 0.10 | 1499 ± 8 | ++ | - | ||||||
| 6 | 13.36 ± 0.02 | 1571 ± 2 | ++ | 872 ± 15 | 4-hydroxy-α-methyl-benzenepropanol (rhododendrol) | 1585 | ||||
| 7 | 14.20 ± 0.07 | 1630 ± 4 | ++ | - | ||||||
| 8 | 14.98 ± 0.13 | 1681 ± 8 | +++ | yes | - | |||||
| 9 | 16.02 ± 0.28 | 1742 ± 16 | M | yes | - | |||||
| 10 | 18.58 ± 0.02 | 1875 ± 1 | + | yes | - | |||||
| 11 | 19.80 ± 0.03 | 1932 ± 1 | ++ | yes | 885 ± 18 | Hexadecanoic acid, methyl ester | 1926/1909 | |||
| 12 | 24.58 ± 0.04 | 2135 ± 2 | ++ | + | + | yes | 884 ± 40 | Octadecanoic acid, methyl ester | 2128/2110 | |
| 13 | 26.48 ± 0.02 | 2212 ± 1 | ++ | yes | - | |||||
| 14 | 26.59 ± 0.04 | 2216 ± 1 | + | - | ||||||
| 15 | 26.81 ± 0.04 | 2225 ± 2 | + | - | ||||||
| 16 | 27.00 ± 0.04 | 2233 ± 2 | M | ++ | + | + | yes | 862 ± 32 | Labd-8(20)-en-15-oic acid, methyl ester | |
| 17 | 27.21 ± 0.05 | 2242 ± 2 | +++ | ++ | + | yes | - | |||
| 18 | 27.51 ± 0.04 | 2254 ± 2 | + | - | - | |||||
| 19 | 27.91 ± 0.05 | 2269 ± 2 | +++ | ++ | + | + | yes | - | ||
| 20 | 29.65 ± 0.04 | 2339 ± 1 | ++ | + | + | yes | 837 ± 56 | Eicosanoic acid, methyl ester | 2329/2310 | |
| 22 | 31.08 ± 0.01 | 2395 ± 0 | + | + | M | yes | Labdanolic acid, TMS (standard) | |||
| 23 | 32.87 ± 0.04 | 2467 ± 1 | ++ | ++ | + | ++ | yes | 6-oxo-labd-7-en-15-oic acid/6-oxocativic acid, TMS (standard) | ||
| 24 | 33.46 ± 0.01 | 2492 ± 0 | + | yes | - | |||||
| 25 | 33.83 ± 0.01 | 2506 ± 0 | + | - | ||||||
| 26 | 34.11 ± 0.00 | 2515 ± 0 | + | yes | - | |||||
| 27 | 34.49 ± 0.05 | 2528 ± 2 | + | + | yes | - | ||||
| 28 | 34.62 ± 0.05 | 2532 ± 2 | + | yes | 884 ± 1 | Docosanoic acid, methyl ester | ||||
| 29 | 38.39 ± 0.03 | 2696 ± 2 | + | yes | - | - | ||||
| 30 | 38.97 ± 0.03 | 2723 ± 2 | ++ | yes | 826 ± 4 | Octadecanoic acid, 2,3-hydropropyl ester | ||||
| 31 | 39.99 ± 0.00 | 2767 ± 0 | + | - | ||||||
| 32 | 43.72 ± 0.00 | 2934 ± 0 | ++ | Apigenin-4′,7-dimethyl ether | ||||||
| 33 | 44.40 ± 0.06 | 2963 ± 0 | +++ | Kaempferol-4′,3,7-trimethyl ether (methyljaranol) | ||||||
| 34 | 45.40 ± 0.01 | * | + | Apigenin-4′-methyl ether (acacetin) | ||||||
| 35 | 45.66 ± 0.01 | * | ++ | Apigenin-7-methyl ether (genkawin) | ||||||
| 36 | 46.10 ± 0.03 | * | ++ | + | M | Kaempferol-3,7-dimethyl ether (Jaranol) | ||||
| 37 | 47.83 ± 0.01 | * | ++ | Apigenin | ||||||
| Sample | α-Amylase Inhibition (%) | |
|---|---|---|
| 1 mg/mL | 0.5 mg/mL | |
| Adl * | 19.77 ± 0.95 b | 12.50 ± 1.58 b |
| Zam | n.a. | n.a. |
| Dit * | 39.99 ± 2.77 a | 29.82 ± 0.92 a |
| Flv * | 3.43 ± 0.10 c | 4.60 ± 0.10 c |
| Sample | α-Glucosidase Inhibition (%) | |
|---|---|---|
| 1 mg/mL | 0.5 mg/mL | |
| Adl | 14.02 ± 0.87 a | 13.15 ± 2.17 abc |
| Zam * | 24.13 ± 4.62 a | 13.66 ± 0.51 ab |
| Dit | 6.43 ± 1.37 b | 4.34 ± 0.58 cd |
| Flv | 15.39 ± 0.94 a | 11.78 ± 5.28 abc |
| Sample | Acetylcholinesterase Inhibition (%) | |
|---|---|---|
| 1 mg/mL | 0.5 mg/mL | |
| Adl * | 75.08 ± 0.86 a | 69.76 ± 0.58 a |
| Zam | 21.22 ± 6.87 bcd | 7.57 ± 1.29 cd |
| Dit * | 35.25 ± 0.44 bd | 24.08 ± 0.45 bc |
| Flv | 22.94 ± 2.64 cd | 22.91 ± 5.12 bcd |
| Sample | HepG2 | Caco-2 | ||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |||
| Adl | 77.42 ± 3.90 bC | # | 48.27 ± 1.25 aB | 52.84 ± 4.27 aB | # | 36.73 ± 2.00 aA |
| Zam | >200.00 | >200.00 | >200.00 | >200.00 | ||
| Dit | 60.29 ± 2.27 aB | 53.77 ± 4.08 aB | 44.58 ± 4.51 aA | 38.67 ± 2.01 aA | ||
| Flv | 76.73 ± 2.97 bC | # | 52.84 ± 1.23 aA | 69.50 ± 2.11 bB | # | 48.30 ± 2.67 bA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frazão, D.F.; Martins-Gomes, C.; Díaz, T.S.; Delgado, F.; Gonçalves, J.C.; Silva, A.M. Labdanum Resin from Cistus ladanifer L. as a Source of Compounds with Anti-Diabetic, Neuroprotective and Anti-Proliferative Activity. Molecules 2024, 29, 2222. https://doi.org/10.3390/molecules29102222
Frazão DF, Martins-Gomes C, Díaz TS, Delgado F, Gonçalves JC, Silva AM. Labdanum Resin from Cistus ladanifer L. as a Source of Compounds with Anti-Diabetic, Neuroprotective and Anti-Proliferative Activity. Molecules. 2024; 29(10):2222. https://doi.org/10.3390/molecules29102222
Chicago/Turabian StyleFrazão, David F., Carlos Martins-Gomes, Teresa Sosa Díaz, Fernanda Delgado, José C. Gonçalves, and Amélia M. Silva. 2024. "Labdanum Resin from Cistus ladanifer L. as a Source of Compounds with Anti-Diabetic, Neuroprotective and Anti-Proliferative Activity" Molecules 29, no. 10: 2222. https://doi.org/10.3390/molecules29102222
APA StyleFrazão, D. F., Martins-Gomes, C., Díaz, T. S., Delgado, F., Gonçalves, J. C., & Silva, A. M. (2024). Labdanum Resin from Cistus ladanifer L. as a Source of Compounds with Anti-Diabetic, Neuroprotective and Anti-Proliferative Activity. Molecules, 29(10), 2222. https://doi.org/10.3390/molecules29102222






