Optimization of the Extraction Process and Biological Activities of Triterpenoids of Schisandra sphenanthera from Different Medicinal Parts and Growth Stages
Abstract
1. Introduction
2. Results
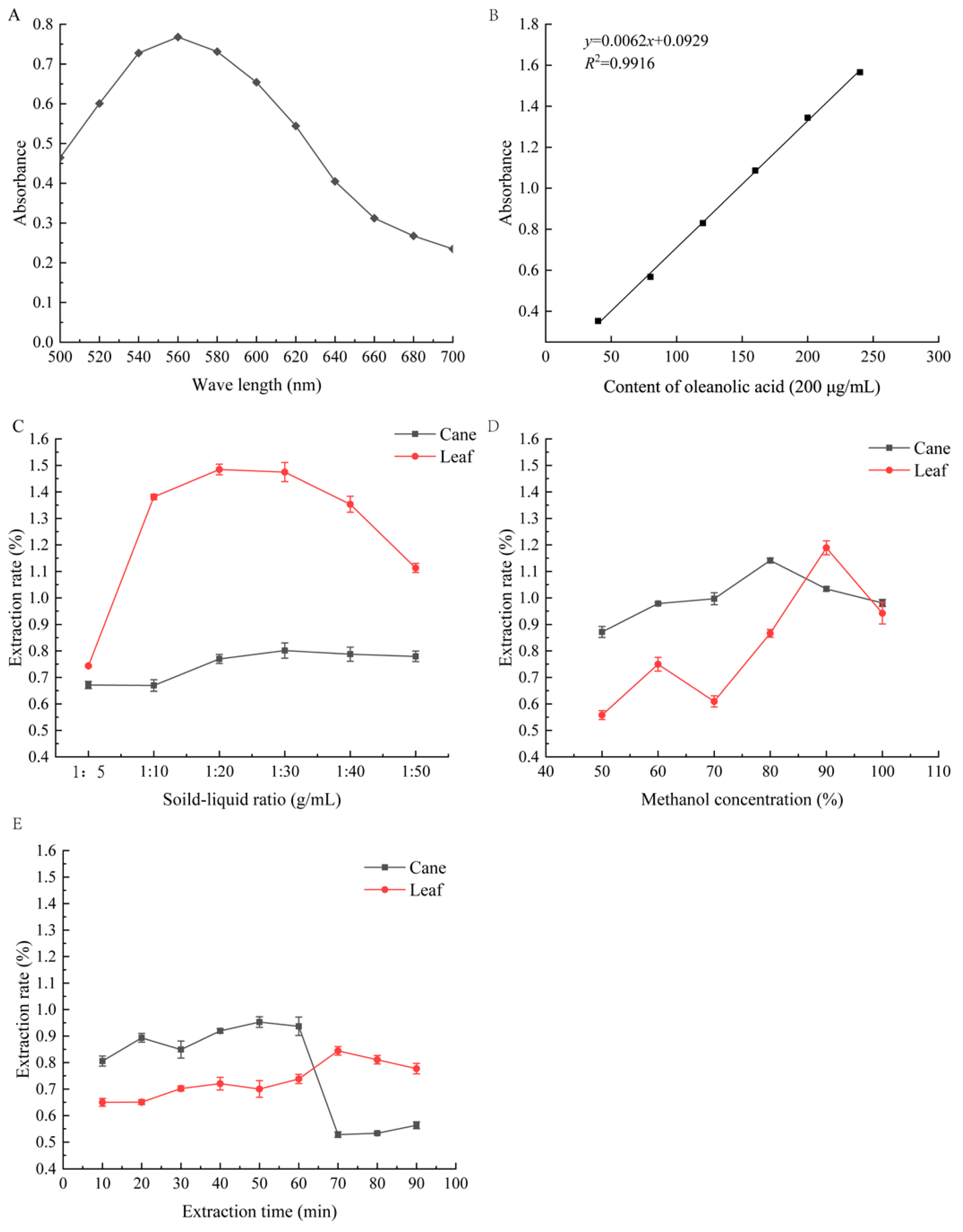
2.1. Optimization of Extraction Rate
2.1.1. Influence of Single Factors on the Extraction Rate
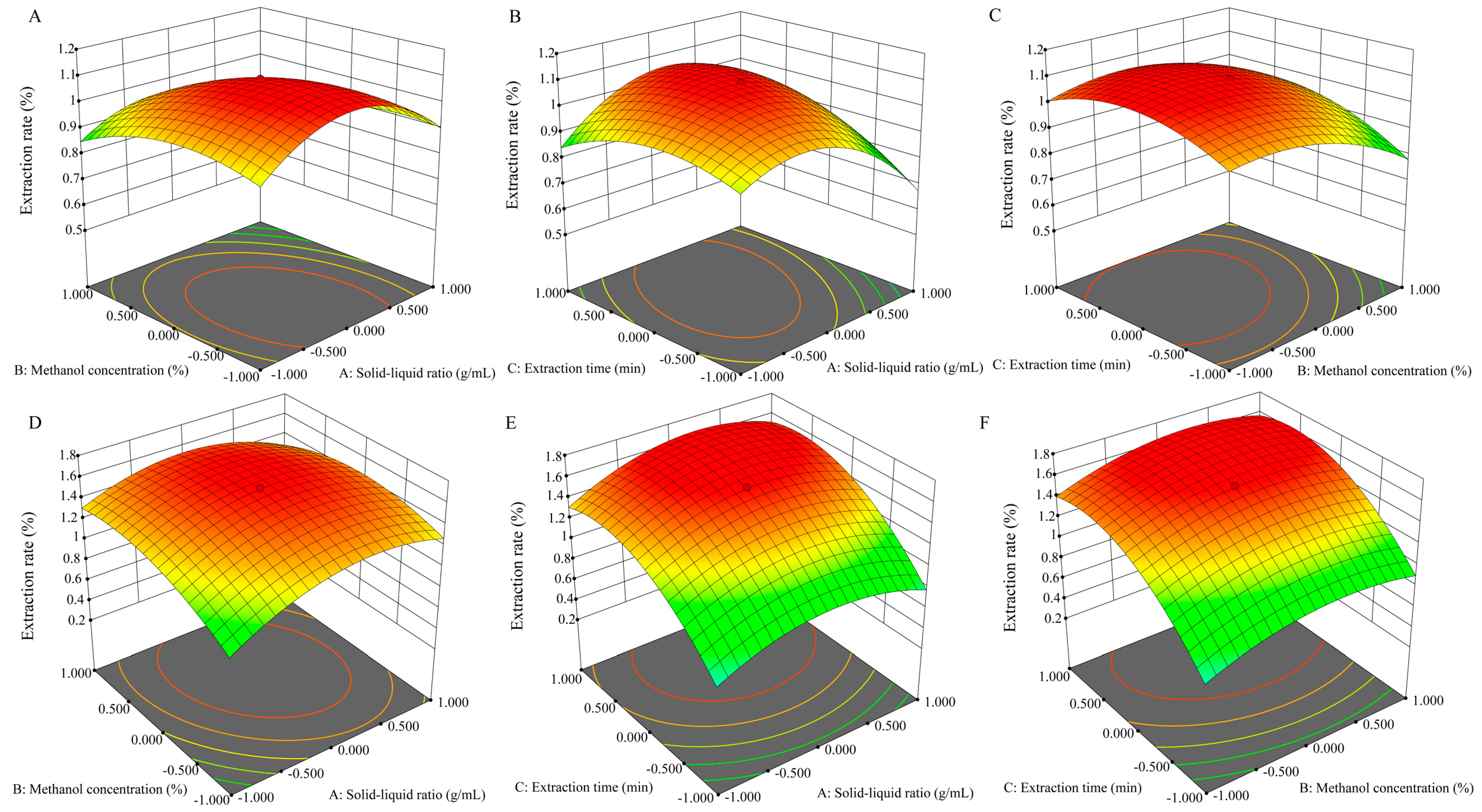
2.1.2. Optimization of Extraction for Total Triterpenoids by RSM
2.2. Total Triterpene Content and Purification
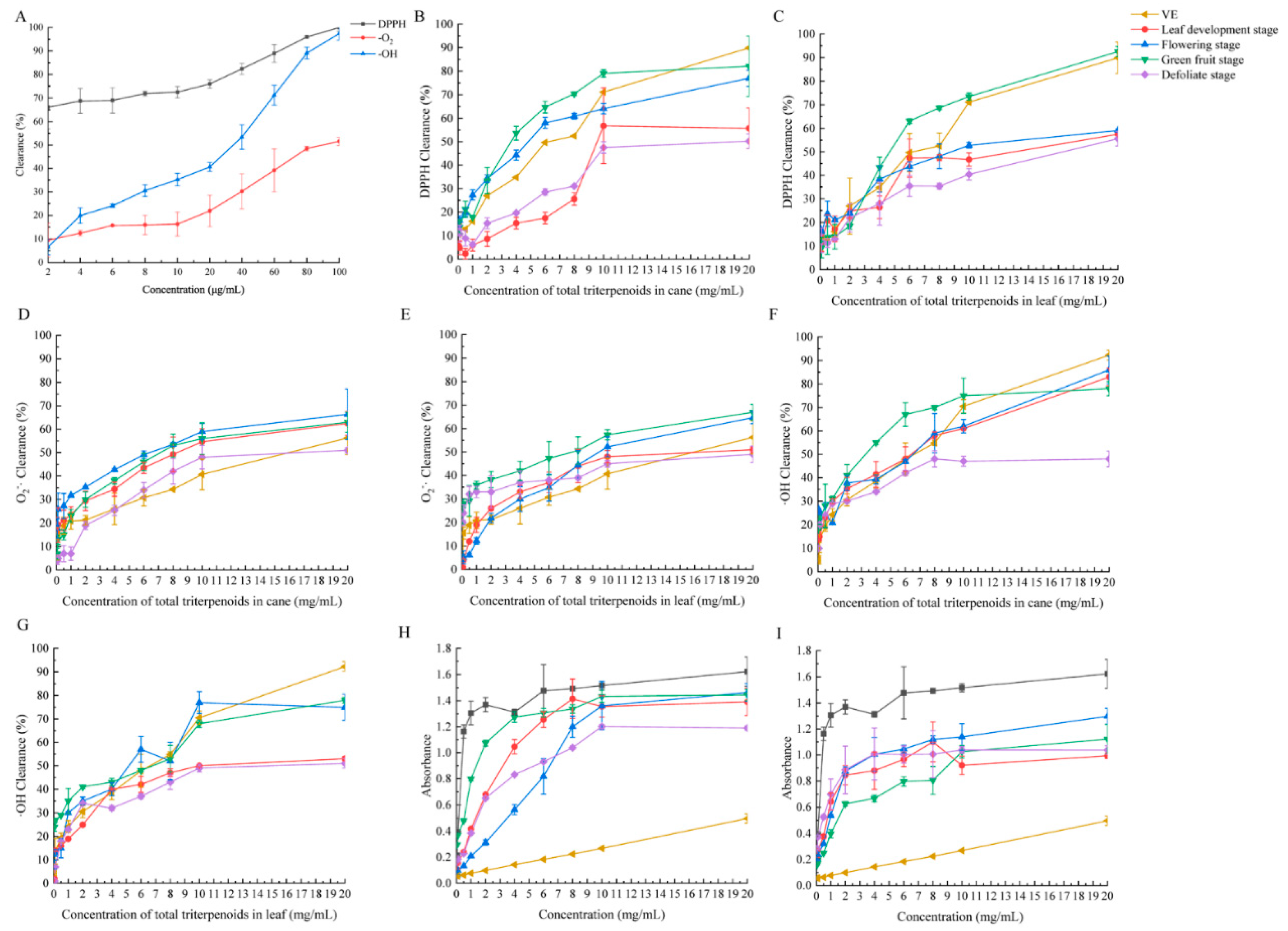
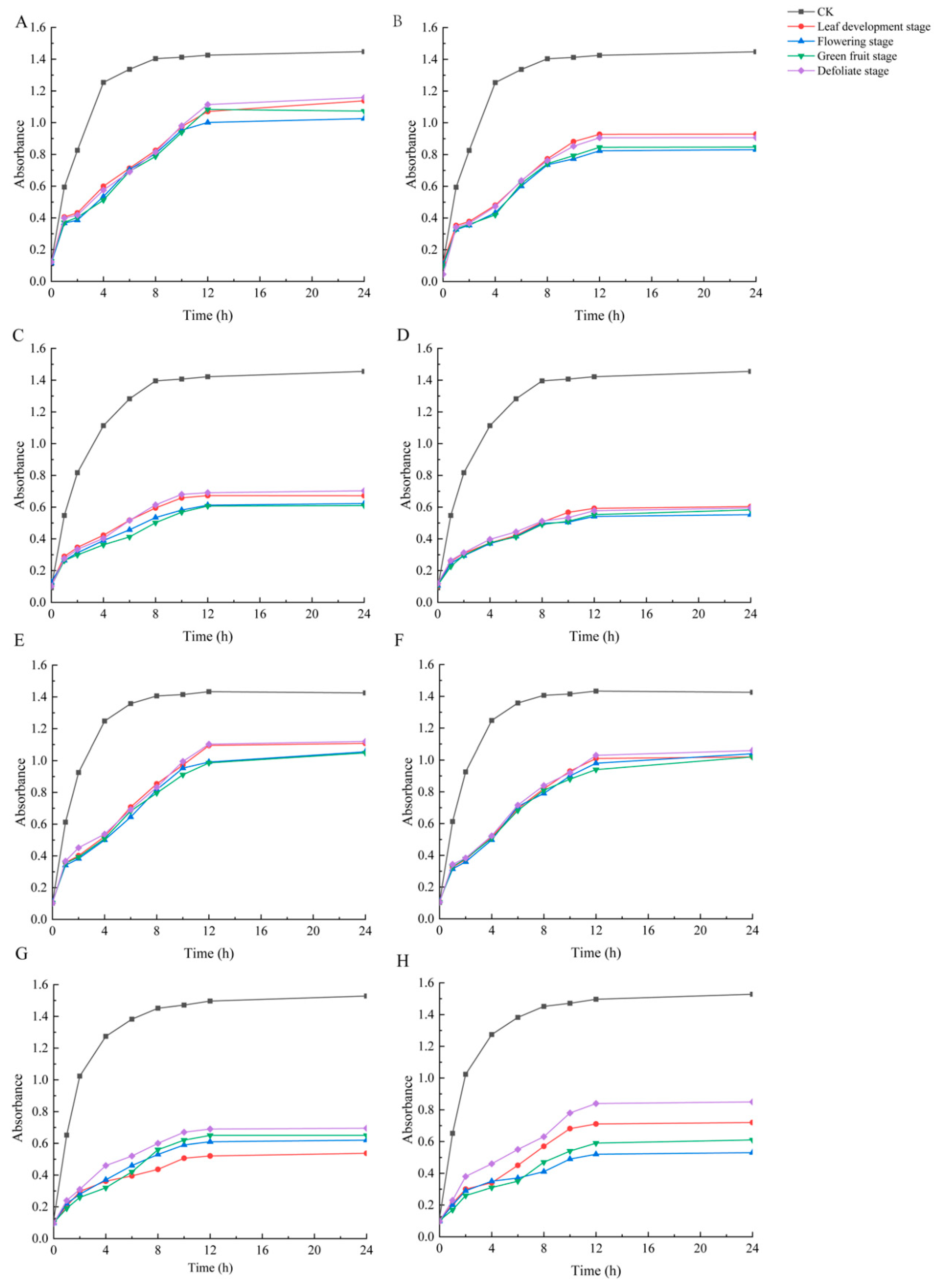
2.3. Antioxidant Activity Determination
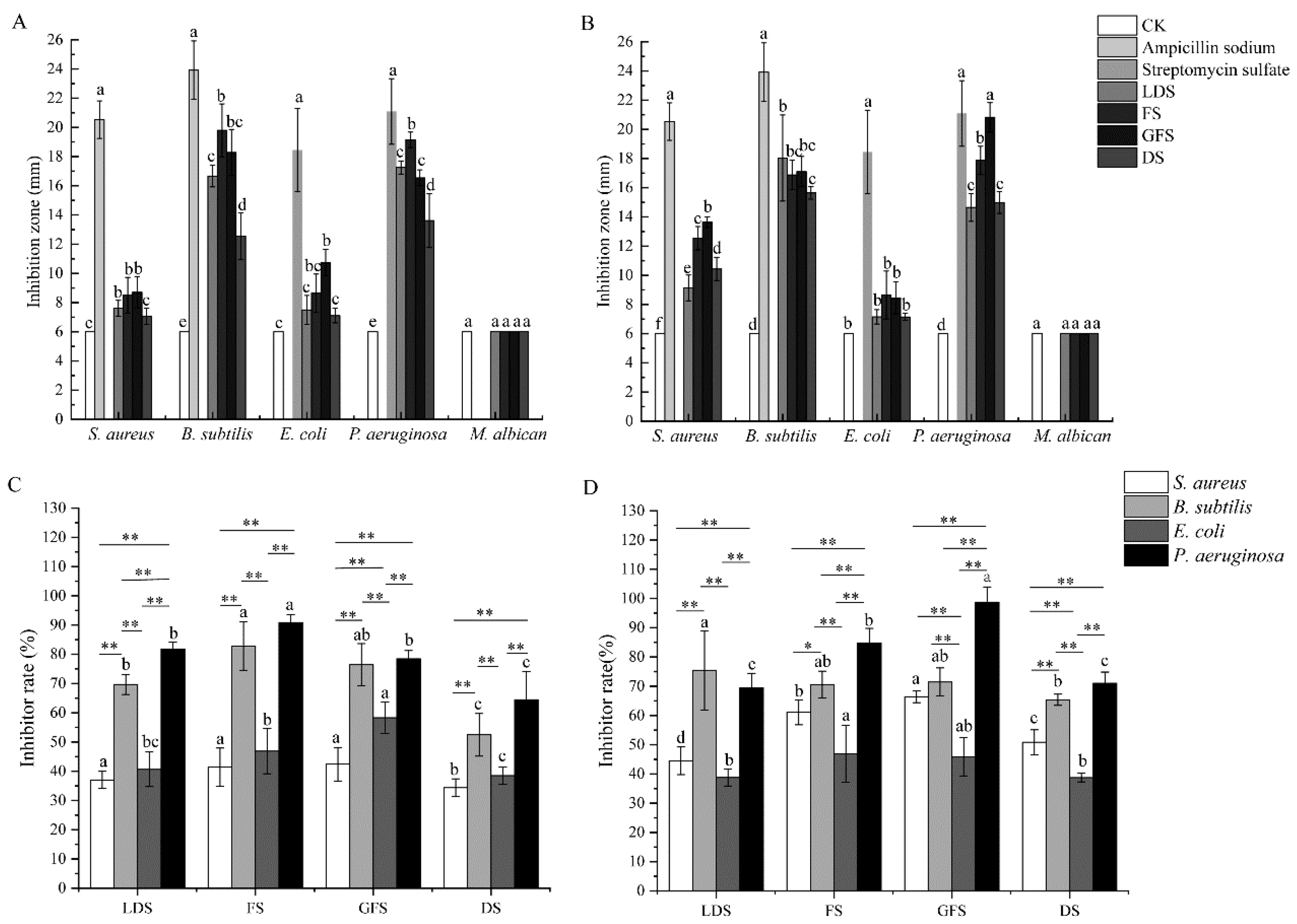
2.4. Determination of Antibacterial Activity
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Extraction of the Total Triterpenoids
4.2.1. Extraction Method for Triterpenoid Optimization
4.2.2. Determination of Total Triterpenoid Content
4.2.3. Purification of Total Triterpenoid Extract
4.3. Antioxidant Ability
4.3.1. The Scavenging Ability of Three Radicals
4.3.2. Determination of Reduction Power
4.4. Antibacterial Ability
4.4.1. Strain Activation and Bacterial Suspension Preparation
4.4.2. Determination of Inhibition Zone
4.4.3. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saunders, R.M.K. Monograph of Schisandra (Schisandraceae). Syst. Bot. Monogr. 2000, 58, 1–146. [Google Scholar] [CrossRef]
- The State Pharmacopoeia Commission of P. R. China. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; p. 263. ISBN 9787506773379. [Google Scholar]
- Guo, Y.L.; Wei, H.Y.; Lu, C.Y.; Gao, B.; Gu, W. Predictions of potential geographical distribution and quality of Schisandra sphenanthera under climate change. PeerJ 2016, 4, e2554. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Gu, W.; Dai, A.H.; Wei, H.Y. Assessing habitat suitability based on geographic information system (GIS) and fuzzy: A case study of Schisandra sphenanthera Rehd. et Wils. in Qinling Mountains, China. Ecol. Model. 2012, 242, 105–115. [Google Scholar] [CrossRef]
- Yang, K.; Qiu, J.; Huang, Z.; Yu, Z.; Wang, W.; Hu, H.; You, Y. A comprehensive review of ethnopharmacology, phytochemistry, pharmacology, and pharmacokinetics of Schisandra chinensis (Turcz.) Baill. and Schisandra sphenanthera Rehd. et Wils. J. Ethnopharmacol. 2022, 284, 114759. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Wei, N.Y.; Wang, Z.Z. LC Analysis of lignans from Schisandra sphenanthera Rehd. et Wils. Chromatographia 2008, 67, 979–983. [Google Scholar] [CrossRef]
- Wang, X.R.; Liu, Y.; Zhou, S.C.; Qin, X.L.; Gu, W. Comparison on the compositions of essential oils from the seed and pulp of Schisandra sphenanthera Rehd. et Wils. J. Essent. Oil Bear. Plants 2017, 20, 1066–1073. [Google Scholar] [CrossRef]
- Wang, X.R.; Liu, Y.; Niu, Y.Y.; Wang, N.X.; Gu, W. The chemical composition and functional properties of essential oils from four species of Schisandra growing wild in the Qinling Mountains, China. Molecules 2018, 23, 1645. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, J.; Li, W.; Wang, C.; Li, H.; Ju, W.; Chen, J.; Sun, J. Characteristics and antioxidant activity of lignans in Schisandra chinensis and Schisandra sphenanthera from different locations. Chem. Biodivers. 2018, 15, e1800030. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tang, R.; Liu, T.; Dai, W.; Liu, Q.; Gong, G.; Song, S.; Hu, M.; Huang, L.; Wang, Z. Physicochemical properties, antioxidant activity and immunological effects in vitro of polysaccharides from Schisandra sphenanthera and Schisandra chinensis. Int. J. Biol. Macromol. 2019, 131, 744–751. [Google Scholar] [CrossRef]
- Mai, N.T.; Doan, V.V.; Lan, H.T.T.; Anh, B.T.M.; Hoang, N.H.; Tai, B.H.; Nhiem, N.X.; Yen, P.H.; Park, S.J.; Seo, Y.; et al. Chemical constituents from Schisandra sphenanthera and their cytotoxic activity. Nat. Prod. Res. 2021, 35, 3360–3369. [Google Scholar] [CrossRef]
- Liang, C.Q.; Luo, R.H.; Yan, J.M.; Li, Y.; Li, X.N.; Shi, Y.M.; Shang, S.Z.; Gao, Z.H.; Yang, L.M.; Zheng, Y.T.; et al. Structure and bioactivity of triterpenoids from the stems of Schisandra sphenanthera. Arch. Pharm. Res. 2014, 37, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.M.; Wu, W.M.; Song, J.; Ruan, H.L. Triterpenoids and lignans from the fruit of Schisandra sphenanthera. Fitoterapia 2017, 116, 10–16. [Google Scholar] [CrossRef]
- You, C.; Qin, D.; Wang, Y.; Lan, W.; Li, Y.; Yu, B.; Peng, Y.; Xu, J.; Dong, J. Plant triterpenoids regulate endophyte community to promote medicinal plant Schisandra sphenanthera growth and metabolites accumulation. J. Fungi 2021, 7, 788. [Google Scholar] [CrossRef]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2014, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.F.; Wang, Y.K.; Liu, R.T.; Wu, J.P.; Xu, K.P. Review of natural plant-derived seco-triterpenoids and derived saponins from 2020 to 2023: New compounds, distributions, diverse activities and structure-activity relationships. Phytochem. Rev. 2024. [Google Scholar] [CrossRef]
- Peña, R.D.L.; Hodgson, H.; Liu, J.C.T.; Stephenson, M.J.; Martin, A.C.; Owen, C.; Harkess, A.; Leebens-Mack, J.; Jimenez, L.E.; Osbourn, A.; et al. Complex scaffold remodeling in plant triterpene biosynthesis. Science 2023, 379, 361–368. [Google Scholar] [CrossRef]
- Shi, Y.M.; Xiao, W.L.; Pu, J.X.; Sun, H.D. Triterpenoids from the Schisandraceae family: An update. Nat. Prod. Rep. 2015, 32, 367–410. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, Y.; Zhang, Z.P.; Wu, D.D.; Zhuang, L.X.; Algradi, A.M.; Kuang, H.X.; Yang, B.Y. Schisandraceae triterpenoids: A review of phytochemistry, bioactivities and synthesis. Fitoterapia 2022, 161, 105230. [Google Scholar] [CrossRef]
- Qiu, F.; Liu, H.; Duan, H.; Chen, P.; Lu, S.J.; Yang, G.Z.; Lei, X.X. Isolation, structural elucidation of three new triterpenoids from the stems and leaves of Schisandra chinensis (Turcz) Baill. Molecules 2018, 23, 1624. [Google Scholar] [CrossRef] [PubMed]
- Pandey, D.K.; Kaur, P. Optimization of extraction parameters of pentacyclic triterpenoids from Swertia chirata stem using response surface methodology. 3 Biotech 2018, 8, 152. [Google Scholar] [CrossRef]
- Rodrigues, V.H.; Portugal, I.; Silva, C.M. Experimental optimization of the supercritical fluid extraction of triterpenoids from Acacia dealbata Link. leaves. Sep. Purif. Technol. 2023, 306, 122637. [Google Scholar] [CrossRef]
- Sabaragamuwa, R.; Perera, C.O.; Fedrizzi, B. Ultrasound assisted extraction and quantification of targeted bioactive compounds of Centella asiatica (Gotu Kola) by UHPLC-MS/MS MRM tandem mass spectroscopy. Food Chem. 2022, 371, 131187. [Google Scholar] [CrossRef]
- Han, Z.Y.; Wu, J.T.; Lin, Y.X.; Bi, Y.; Naseem, A.; Hao, Z.C.; Pan, J.; Guan, W.; Kuang, H.X.; Chen, Q.S.; et al. Seven new triterpenoids from the roots of Adenophora tetraphylla (Thub.) Fisch. Fitoterapia 2024, 175, 105902. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.R.; Bai, L.; Lin, L.M.; Liao, D.F.; Gong, Y.; Liu, X.Q.; Wang, Z.M.; Li, C. Studies on the chemical constituents and quality evaluation of Rosa cymosa Tratt. Root. J.Sep. Sci. 2020, 43, 4379–4389. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.; Feng, X.; Cai, Y.S.; Ibrahim, S.A.; Liu, Y.; Huang, W. Regulation of tumor apoptosis of Poriae cutis-derived lanostane triterpenes by AKT/PI3K and MAPK signaling pathways in vitro. Nutrients 2023, 15, 4360. [Google Scholar] [CrossRef]
- Tan, M.; Zhao, Q.; Wang, X.; Zhao, B. Study on extraction, isolation, and biological activity of saponins from quinoa bran. J. Food Process. Preserv. 2022, 46, e17155. [Google Scholar] [CrossRef]
- Yang, W.; Liang, Y.; Liu, Y.; Yao, Y.; Yu, Z.; Chen, B.; Cai, Y.; Wei, M.; Zheng, G. Enhancement of hepatoprotective activity of limonin from citrus seeds against acetaminophen-induced liver injury by HSCCC purification and liposomal encapsulation. Fitoterapia 2024, 175, 105899. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhou, Q.Q.; Fan, S.; Liu, C.L.; Li, H.J. Response surface methodology optimization of extraction and enrichment conditions of total triterpenoid saponins from Celosiae semen and evaluation of its lipid-lowering activity. Biomed. Chromatogr. 2024, 38, e5835. [Google Scholar] [CrossRef]
- Wang, X.Y.; Su, J.Q.; Chu, X.L.; Zhang, X.Y.; Kan, Q.B.; Liu, R.X.; Fu, X. Adsorption and desorption characteristics of total flavonoids from Acanthopanax senticosus on macroporous adsorption resins. Molecules 2021, 26, 4162. [Google Scholar] [CrossRef]
- Liu, H.T.; Qi, Y.D.; Xu, L.J.; Peng, Y.; Zhang, B.G.; Xiao, P.G. Ethno-pharmacological investigation of Schisandraceae plants in China. China J. Chin. Mater. Med. 2012, 10, 1353–1359. [Google Scholar] [CrossRef]
- Xia, Y.G.; Yang, B.Y.; Kuang, H.X. Schisandraceae triterpenoids: A review. Phytochem. Rev. 2015, 14, 155–187. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Sun, Y.; Li, Y.; Jiang, Y.; Deng, C.; Song, X.; Zhang, D. A systematic review on triterpenoids from genus Schisandra: Botany, traditional use, pharmacology and modern application. ARAB J. Chem. 2023, 16, 105178. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Wang, W.G.; Li, H.M.; Zhang, R.B.; Li, H.Z.; Li, R.T. Schisanlactone H and sphenanthin A, new metabolites from Schisandra sphenanthera. J. Asian Nat. Prod. Res. 2009, 11, 861–866. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Wiśniewski, R. Determination of triterpenoids, carotenoids, chlorophylls, and antioxidant capacity in Allium ursinum L. at different times of harvesting and anatomical parts. Eur. Food Res. Technol. 2018, 244, 1269–1280. [Google Scholar] [CrossRef]
- Hao, J.; Si, Q.; Wang, Z.; Jia, Y.; Fu, Z.; Zhao, M.; Wilkes, A.; Ge, G. Optimization of extraction process and dynamic changes in triterpenoids of Lactuca indica from different medicinal parts and growth periods. Molecules 2023, 28, 3345. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, Z.; Chen, J.; Dong, Y.; Qu, K.; Guo, T.; Wang, F.; Liu, A.; Chen, S.; Li, X. Reactive oxygen species signaling in melatonin-mediated plant stress response. Plant Physiol. Bioch. 2024, 207, 108398. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Szakiel, A.; Głowacka, A.; Rozpara, E.; Marszałek, K.; Skąpska, S. Triterpenoids of three apple cultivars-biosynthesis, antioxidative and anti-inflammatory properties, and fate during processing. Molecules 2023, 28, 2584. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Dall’Acqua, S.; Sinan, K.I.; Uba, A.I.; Sut, S.; Peron, G.; Etienne, O.K.; Kumar, M.; Cespedes-Acuña, C.L.; Alarcon-Enos, J.; et al. Gathering scientific evidence for a new bioactive natural ingredient: The combination between chemical profiles and biological activities of Flueggea virosa extracts. Food Biosci. 2022, 49, 101967. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Le, N.T.; Le, N.T.N.; Duong, T.D.; Le, T.T.; Nguyen, H.T.T.; Phung, H.T.; Nguyen, H.T. Extraction, purification, and evaluation of bioactivities of total triterpenoids from Persimmon (Diospyros kaki L.f.) Leaves. Process Biochem. 2024, 139, 70–80. [Google Scholar] [CrossRef]
- Ayachi, A.; Ben Younes, A.; Ben Ammar, A.; Bouzayani, B.; Samet, S.; Siala, M.; Trigui, M.; Treilhou, M.; Téné, N.; Mezghani-Jarraya, R. Effect of the harvest season of Anthyllis henoniana stems on antioxidant and antimicrobial activities: Phytochemical profiling of their ethyl acetate extracts. Molecules 2023, 28, 3947. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Chen, K.; Zhang, L.; Li, Y. Triterpenoids from the roots of Sanguisorba officinalis and their Nrf2 stimulation activity. Phytochemistry 2023, 214, 113803. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Pan, Z.; Liu, Z.; Cheng, W.; Yu, T. Influence of cultivation substrate on antioxidant activities and triterpenoid profiles of the fruiting body of Ganoderma lucidum. Front. Nutr. 2024, 11, 1329579. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Li, Y.S.; Meng, X.J.; Xue, X.; Zhu, L.J. Study on antioxidant activity of triterpenes from caculis of Schisandra chinensis (Turcz.) baill. Sci. Technol. Food Ind. 2012, 33, 121–123, 128. [Google Scholar] [CrossRef]
- Qiu, M.Y.; Zhao, W.; Liu, Y.T.; Gong, P.H.; Shao, S.; Qi, B.; Yan, M.M.; Zhao, D.Q. Comparative study on antioxidation and α-glucosidase activity of Schisandra chinensis. Nat. Prod. Res. Dev. 2018, 30, 840–846. [Google Scholar] [CrossRef]
- Xu, W.; Shi, D.; Chen, K.; Popovich, D.G. TLC-Bioautography-Guided isolation and assessment of antibacterial compounds from Manuka (Leptospermum scoparium) leaf and branch extracts. Molecules 2024, 29, 717. [Google Scholar] [CrossRef]
- Wei, L.; Jing, B.; Li, X.; Hou, Y.; Xie, X.; Wang, Z.; Liu, Y.; Zhou, Y.; Chang, X.; Wang, W. Evaluation of nutritional ingredients, biologically active materials, and pharmacological activities of Stropharia rugosoannulata grown under the bamboo forest and in the greenhouse. J. Food Qual. 2021, 2021, 5478227. [Google Scholar] [CrossRef]
- Joshi, R.K. Bioactive Usual and unusual triterpenoids derived from natural sources used in traditional medicine. Chem. Biodivers. 2023, 20, e202200853. [Google Scholar] [CrossRef]
- Negi, A.; Pasam, T.; Farqadain, S.M.; Mahalaxm, Y.; Dandekar, M.P. In-vitro and preclinical testing of bacillus subtilis UBBS-14 probiotic in rats shows no toxicity. Toxicol. Res. 2024, 13, tfae021. [Google Scholar] [CrossRef] [PubMed]
- Onwumere-Idolor, O.S.; Kperegbeyi, J.I.; Imonikebe, U.G.; Okoli, C.E.; Ajibo, F.E.; Njoga, E.O. Epidemiology of multidrug-resistant zoonotic E. coli from beef processing and retail points in Delta State, Nigeria: Public health implications. Prev. Vet. Med. 2024, 224, 106132. [Google Scholar] [CrossRef]
- Chung, P.Y. Novel targets of pentacyclic triterpenoids in Staphylococcus aureus: A systematic review. Phytomedicine 2020, 73, 152933. [Google Scholar] [CrossRef]
- Shaukat, A.; Yang, C.; Yang, Y.; Guo, Y.F.; Jiang, K.; Guo, S.; Liu, J.; Zhang, T.; Zhao, G.; Ma, X.; et al. Ginsenosides Rb 1: A novel therapeutic agent in Staphylococcus aureus-induced acute lung injury with special reference to oxidative stress and apoptosis. Microb. Pathog. 2020, 143, 104109. [Google Scholar] [CrossRef] [PubMed]
- Subsomwong, P.; Teng, W.; Ishiai, T.; Narita, K.; Sukchawalit, R.; Nakane, A.; Asano, K. Extracellular vesicles from Staphylococcus aureus promote the pathogenicity of Pseudomonas aeruginosa. Microbiol. Res. 2024, 281, 127612. [Google Scholar] [CrossRef] [PubMed]
- Borisov, V.B.; Siletsky, S.A.; Nastasi, M.R.; Forte, E. ROS defense systems and terminal oxidases in bacteria. Antioxidants 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, C.L.; Schmidt-Malan, S.M.; Karau, M.J.; Greenwood-Quaintance, K.; Hassett, D.J.; Mandrekar, J.N.; Patel, R. Exposure of bacterial biofilms to electrical current leads to cell death mediated in part by reactive oxygen species. PLoS ONE 2016, 11, e0168595. [Google Scholar] [CrossRef]
- Kirmani, F.; Saddiqe, Z.; Saleem, S.; Ali, F.; Haq, F. Phytochemical investigation and antibacterial activity of Curcuma longa against multi-drug resistant bacteria. S. Afr. J. Bot. 2024, 164, 137–145. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Q.; Li, J.; Shang, Q.; Jiang, J.; Pu, H.; Fang, X.; Qin, X.; Zhou, J.; Wang, N.; Wang, X.; et al. Optimization of the Extraction Process and Biological Activities of Triterpenoids of Schisandra sphenanthera from Different Medicinal Parts and Growth Stages. Molecules 2024, 29, 2199. https://doi.org/10.3390/molecules29102199
Zhao Q, Li J, Shang Q, Jiang J, Pu H, Fang X, Qin X, Zhou J, Wang N, Wang X, et al. Optimization of the Extraction Process and Biological Activities of Triterpenoids of Schisandra sphenanthera from Different Medicinal Parts and Growth Stages. Molecules. 2024; 29(10):2199. https://doi.org/10.3390/molecules29102199
Chicago/Turabian StyleZhao, Qiaozhu, Jianhua Li, Qianqian Shang, Jiafang Jiang, Han Pu, Xilin Fang, Xiaolu Qin, Jia Zhou, Nongxue Wang, Xiaorui Wang, and et al. 2024. "Optimization of the Extraction Process and Biological Activities of Triterpenoids of Schisandra sphenanthera from Different Medicinal Parts and Growth Stages" Molecules 29, no. 10: 2199. https://doi.org/10.3390/molecules29102199
APA StyleZhao, Q., Li, J., Shang, Q., Jiang, J., Pu, H., Fang, X., Qin, X., Zhou, J., Wang, N., Wang, X., & Gu, W. (2024). Optimization of the Extraction Process and Biological Activities of Triterpenoids of Schisandra sphenanthera from Different Medicinal Parts and Growth Stages. Molecules, 29(10), 2199. https://doi.org/10.3390/molecules29102199





