Mercury Adsorption and Oxidation Performance of an Iron-Based Oxygen Carrier during Coal Chemical Looping Process
Abstract
1. Introduction
2. Results and Discussion
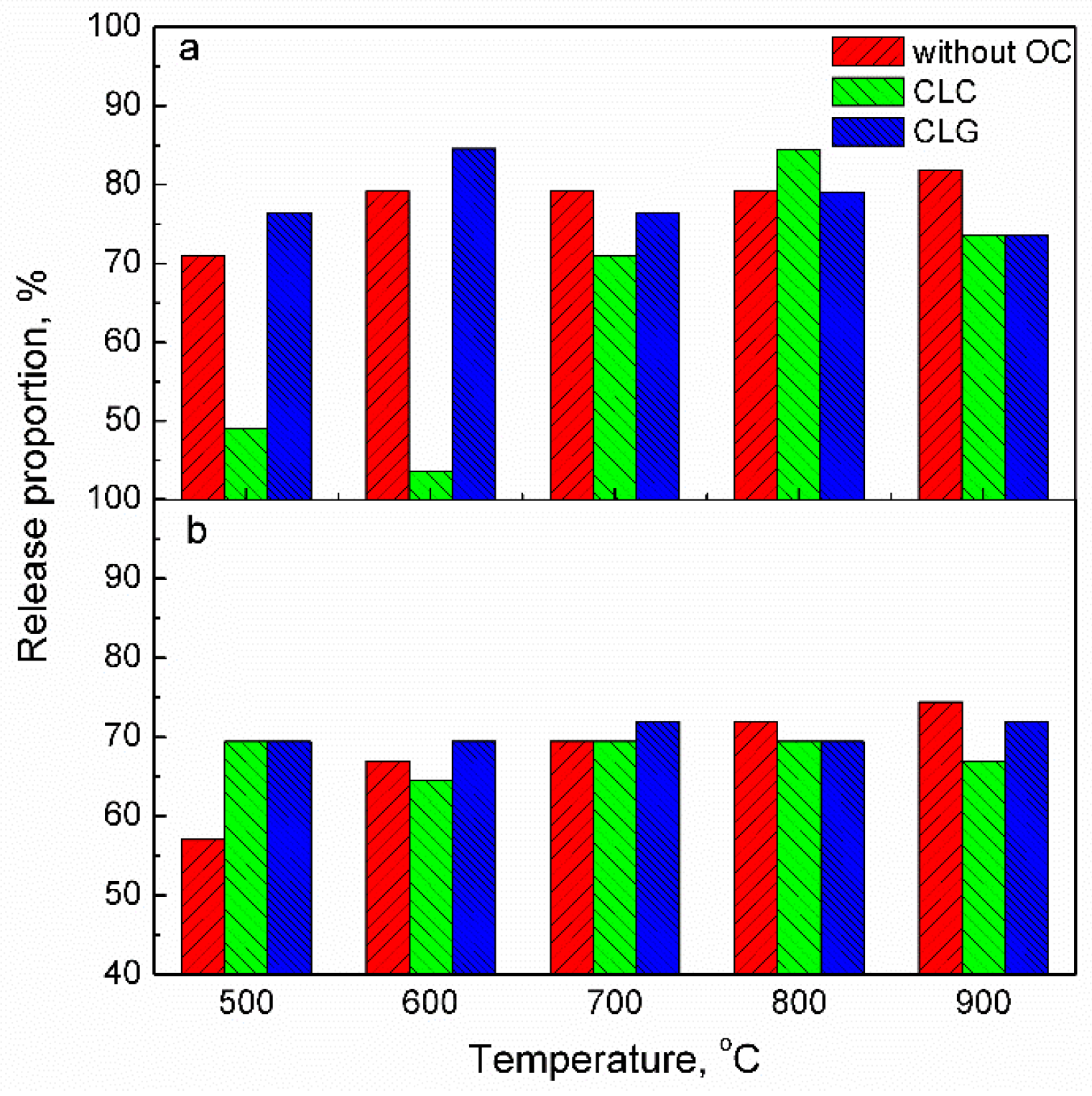
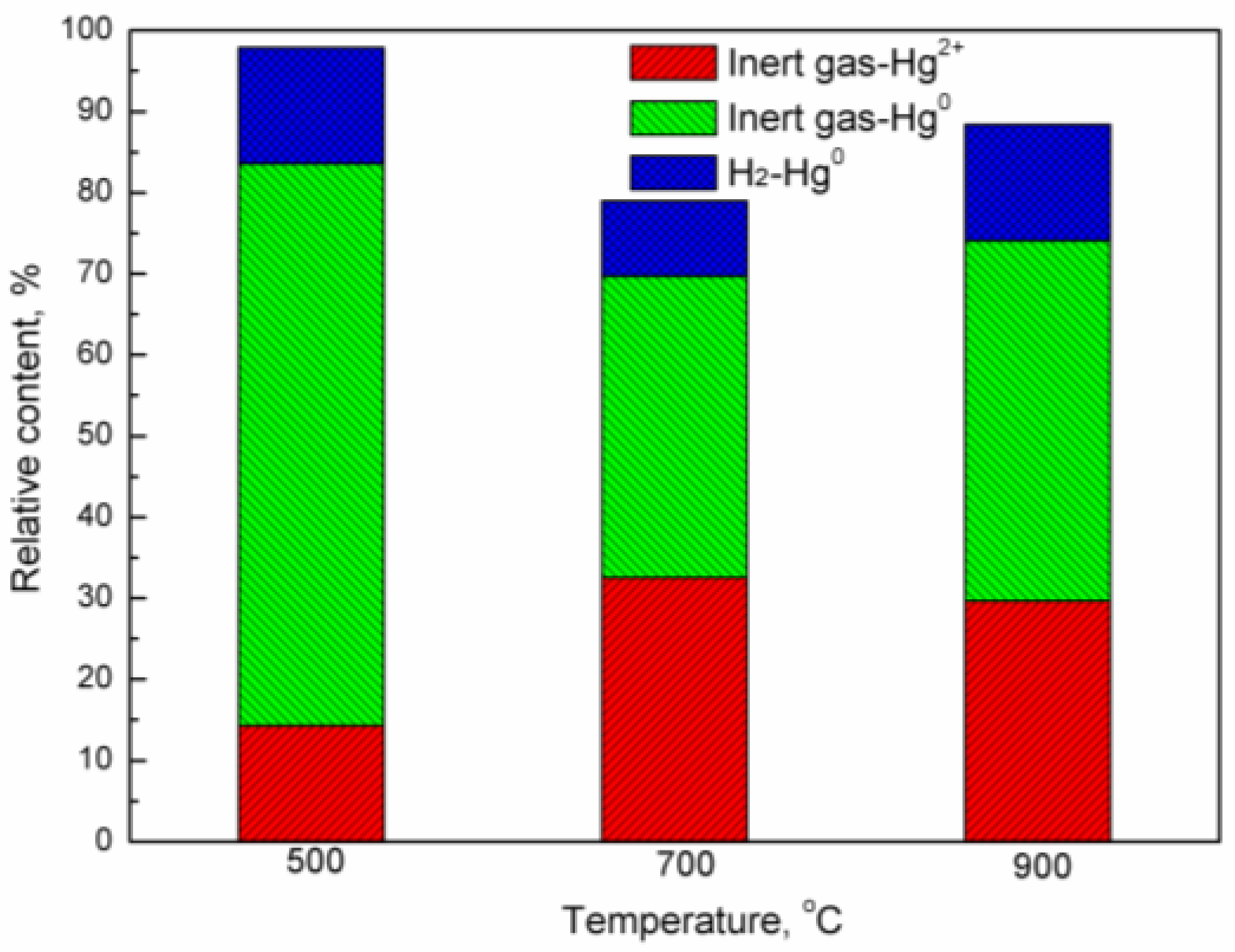
2.1. Effect of Oxygen Carrier on Release Behavior of Mercury in Coal
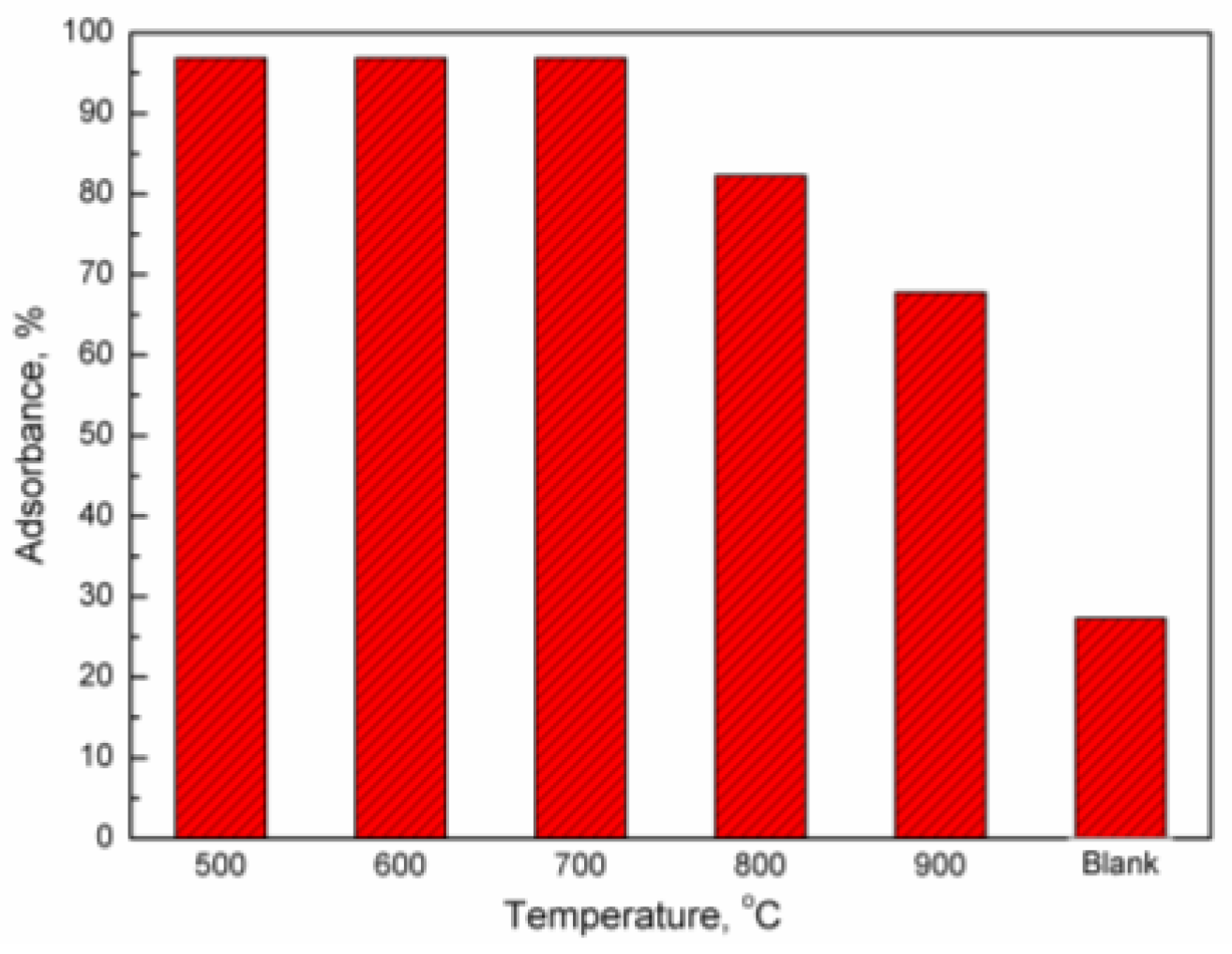
2.2. Adsorption of Mercury Vapor by Iron-Based Oxygen Carrier
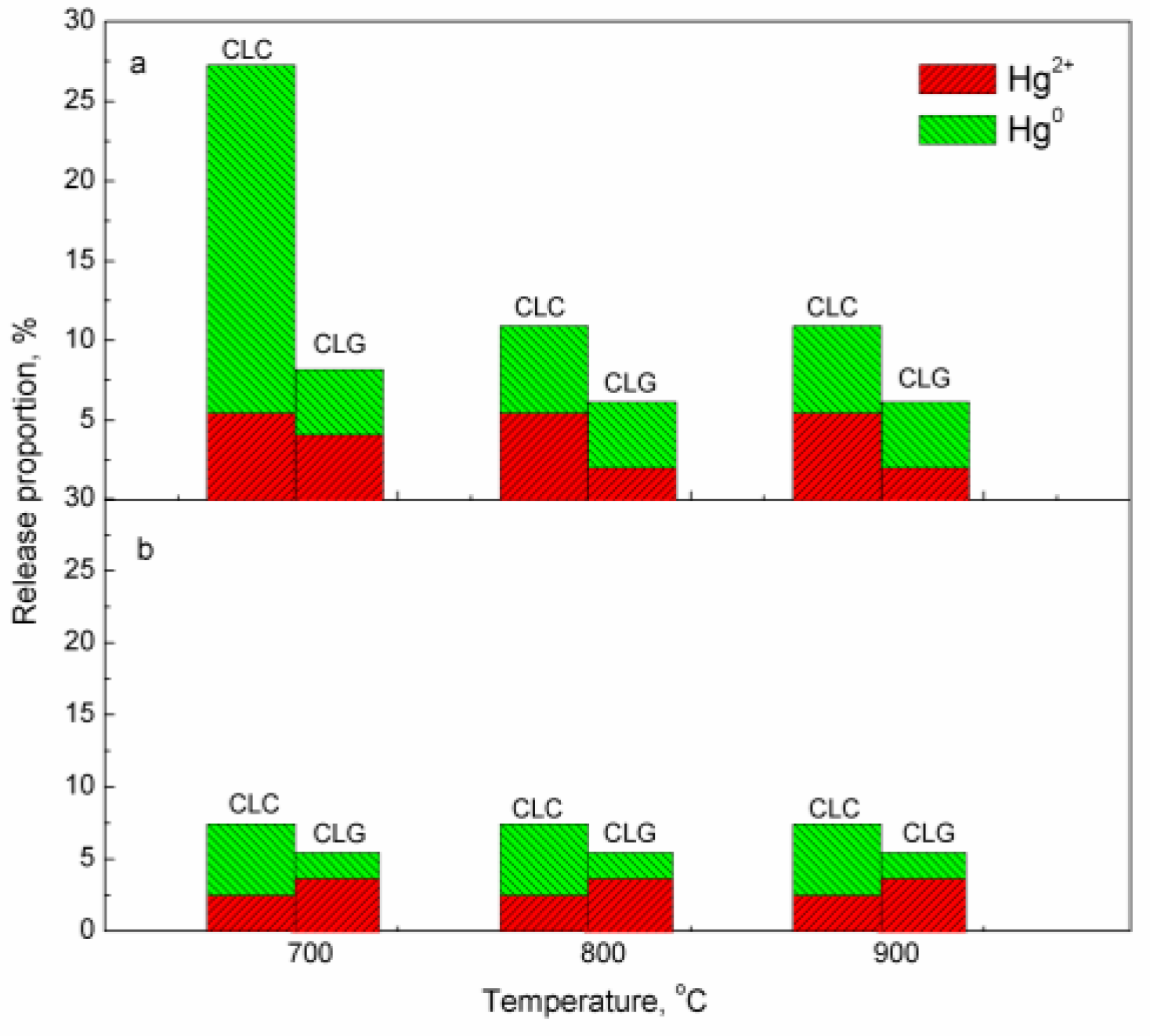
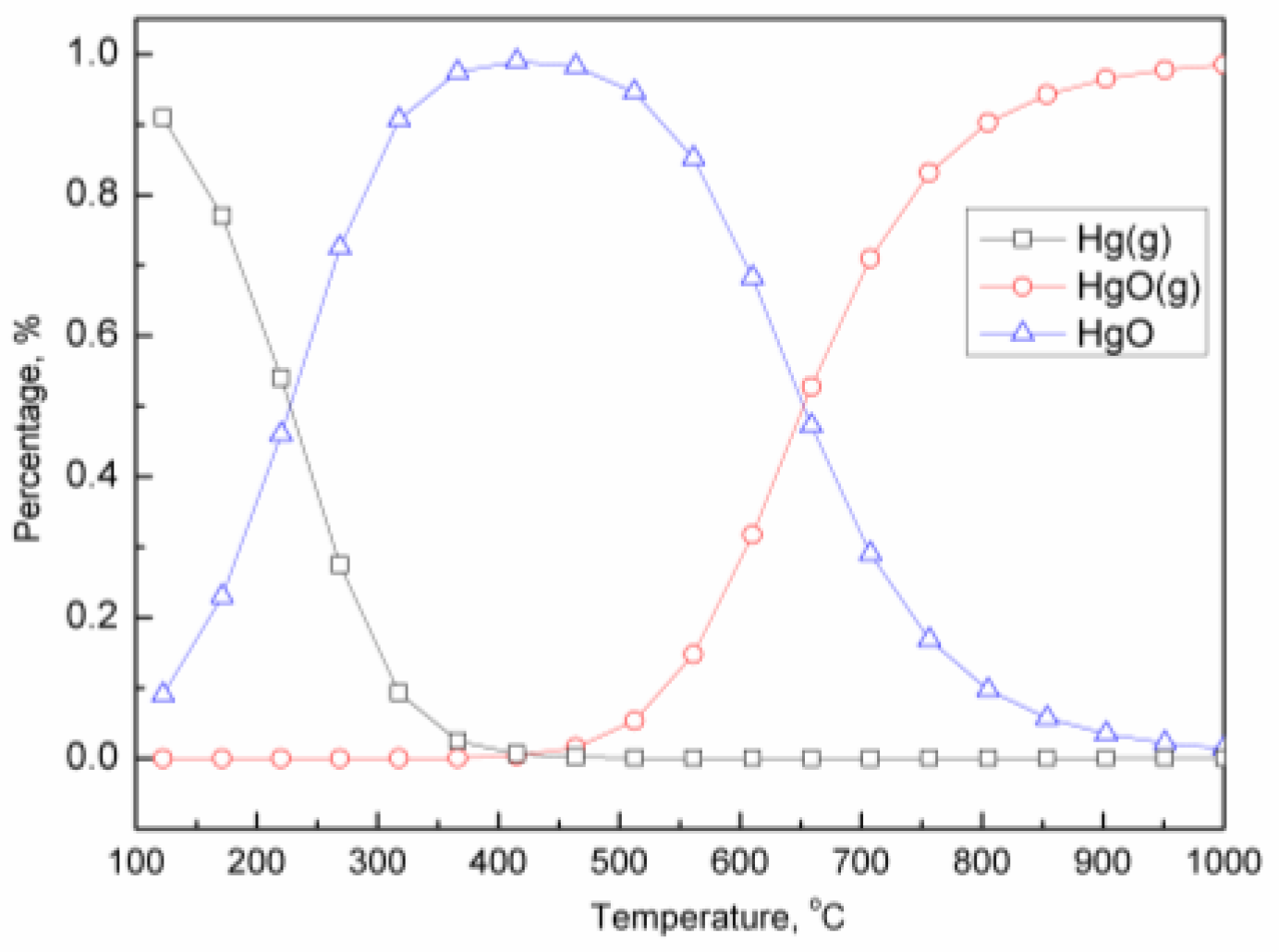
2.3. Oxidation of Mercury Vapor by Iron-Based Oxygen Carrier
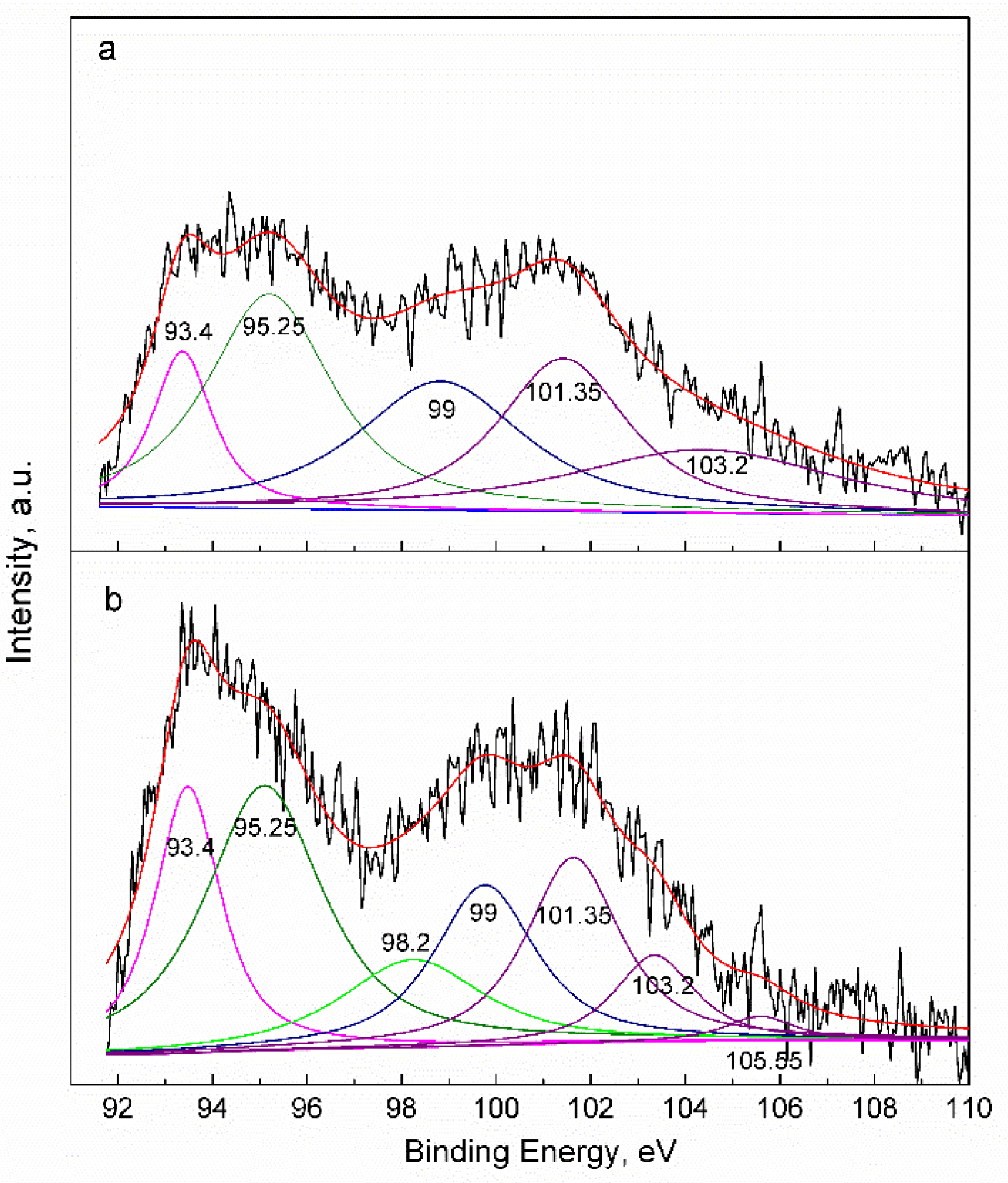
2.4. Effect Mechanism of Iron-Based Oxygen Carrier on Mercury
3. Experimental and Methods
3.1. Preparation and Characterization of Materials
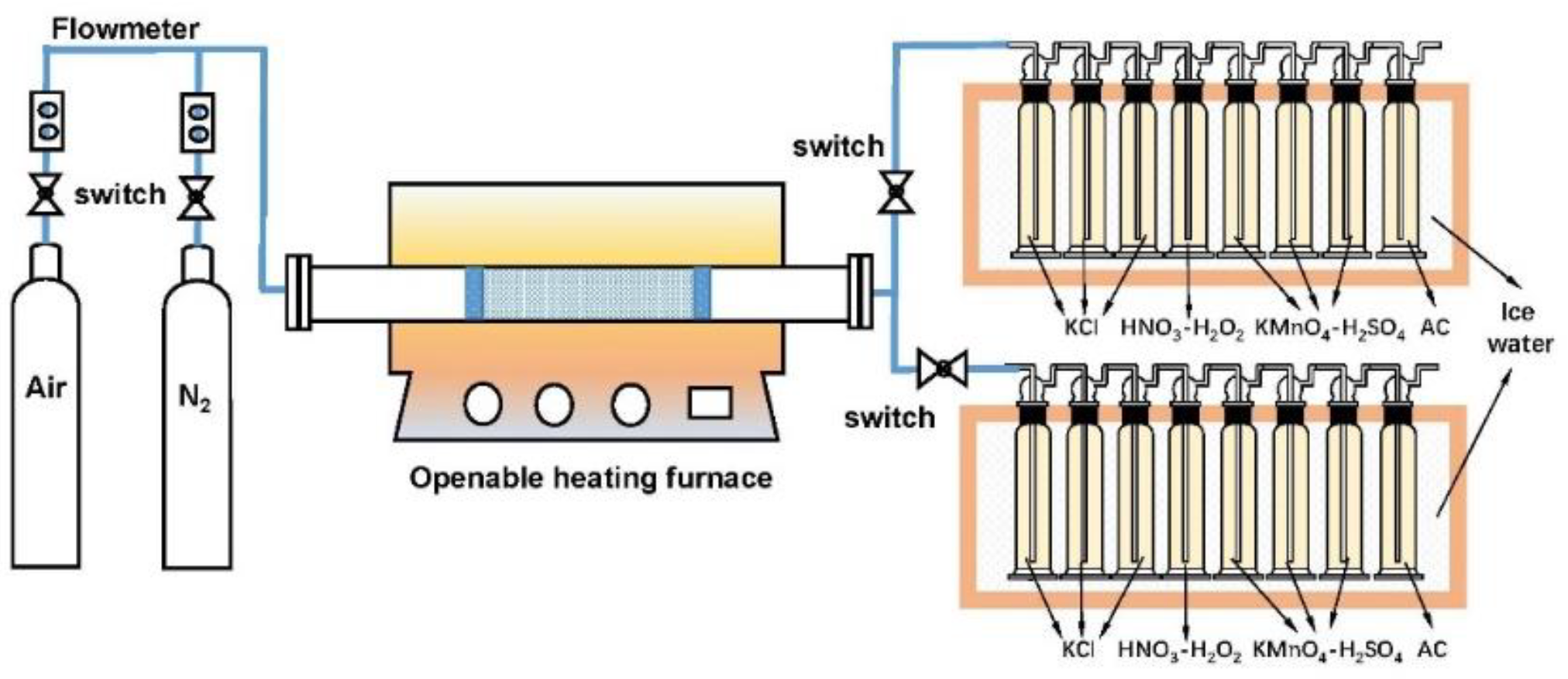
3.2. Experimental Setup and Procedure
3.3. Data Evaluation
4. Conclusions
- (1)
- The effect of an iron-based oxygen carrier on mercury release in chemical looping conversion is attributed to three aspects: the enhanced release rate of mercury from coal, the adsorption of mercury on the surface of the oxygen carrier, and the oxidation of gaseous mercury from Hg0 to Hg2+.
- (2)
- With the increasing temperature, the adsorbance of mercury by the iron-based oxygen carrier decreases, while its oxidation of mercury enhances. Even at 900 °C, the adsorbance of mercury by the oxygen carrier remained at 0.1687 g/g, with a relative content of Hg2+ at 22.55%.
- (3)
- The mercury adsorption on the surface of the iron-based oxygen carrier involves both chemisorption and physical absorption. Physical adsorption includes both Hg0 and Hg2+, while chemisorption refers to complex-compound formation between mercury and the iron-based oxygen carrier.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evan, J.G.; Henry, W.P. Constance Senior. Mercury Control for Coal-Derived Gas Streams; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2015; pp. 51–66. [Google Scholar]
- Wu, C.L.; Cao, Y.; Dong, Z.B.; Cheng, C.M.; Li, H.X.; Pan, W.P. Evaluation of mercury speciation and removal through air pollution control devices of a 190 MW boiler. J. Environ. Sci. 2010, 22, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, J.; Rupp, E.; Ying, S.C.; Lim, D.-H.; Negreira, A.S.; Kirchofer, A.; Feng, F.; Lee, K. Mercury adsorption and oxidation in coal combustion and gasification processes. Int. J. Coal Geol. 2012, 90–91, 4–20. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.Y.; Zhao, Y.C.; Zheng, C.G. Volatility and speciation of mercury during pyrolysis and gasification of five Chinese coals. Energy Fuel 2011, 25, 3988–3996. [Google Scholar] [CrossRef]
- Zhu, X.; Donat QI, F.; Müller, C.R.; Li, F.X. Chemical looping beyond combustion–a perspective. Energy Environ. Sci. 2020, 13, 772–804. [Google Scholar] [CrossRef]
- Adánez, J.; Abad, A.; Mendiara, T.; Gayán, P.; de Diego, L.F.; García-Labiano, F. Chemical looping combustion of solid fuels. Prog. Energ. Combust. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, H.; Sikarwar, V.S.; Zhao, M.; Park, A.-H.A.; Fennell, P.S.; Shen, L.H.; Fan, L.-S. Biomass-based chemical looping technologies: The good, the bad and the future. Energy Environ. Sci. 2017, 10, 1885–1910. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Guo, Q.J. Investigation into syngas generation from solid fuel using CaSO4-based chemical looping gasification process. Chin. J. Chem. Eng. 2013, 21, 127–134. [Google Scholar]
- Liu, Y.Z.; Jia, W.H.; Guo, Q.J.; Ryu, H.J. Effect of the gasifying medium on the coal chemical looping gasification with CaSO4 as oxygen carrier. Chin. J. Chem. Eng. 2014, 22, 1208–1214. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Zhou, L.; Li, B.; Wang, T.; Liu, H. A review on mercury removal in chemical looping combustion of coal. Sep. Purif. Technol. 2024, 337, 126352. [Google Scholar] [CrossRef]
- Liu, D.; Wang, C.; Fan, Y.; Liu, Q.; Wang, X.; Xu, K.; Jin, J.; Ma, J.; Ma, J. Mercury transformation and removal in chemical looping combustion of coal: A review. Fuel 2023, 347, 128440. [Google Scholar] [CrossRef]
- Mendiara, T.; Izquierdo, M.T.; Abad, A.; Gayán, P.; García-Labiano, F.; de Diego, L.F.; Adánez, J. Mercury release and speciation in chemical looping combustion of coal. Energy Fuels 2014, 28, 2786–2794. [Google Scholar] [CrossRef]
- Mendiara, T.; Izquierdo, M.T.; Abad, A.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Adánez, J. Release of pollutant components in CLC of lignite. Int. J. Greenh. Gas Control 2014, 22, 15–24. [Google Scholar] [CrossRef]
- Pérez, V.R.; Adánez, R.I.; Gayán, P.; Izquierdo, M.T.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Adánez, J. Sulphur, nitrogen and mercury emissions from coal combustion with CO2 capture in chemical looping with oxygen uncoupling (CLOU). Int. J. Greenh. Gas Control 2016, 46, 28–38. [Google Scholar] [CrossRef]
- Ma, J.C.; Mei, D.F.; Tian, X.; Zhang, S.B.; Yang, J.P.; Wang, C.Q.; Chen, G.P.; Zhao, Y.C.; Zheng, C.G.; Zhao, H.B. Fate of mercury in volatiles and char during in situ gasification chemical-looping combustion of coal. Environ. Sci. Technol. 2019, 53, 7887–7892. [Google Scholar] [CrossRef]
- An, M.; Ma, J.J.; Guo, Q.J. Transformation and migration of mercury during chemical-looping gasification of coal. Ind. Eng. Chem. Res. 2019, 58, 20481–20490. [Google Scholar] [CrossRef]
- An, M.; Guo, Q.J.; Wei, X.Y. Reaction mechanism of H2S with Hg0 on CuFe2O4 oxygen carrier with oxygen vacancy structure during coal chemical looping gasification. Fuel 2023, 333, 126477. [Google Scholar] [CrossRef]
- Ghorishi, S.B.; Lee, C.W.; Jozewicz, W.S.; Kilgroe, J.D. Effects of fly ash transition metal content and flue gas HCl/SO2 ratio on mercury speciation in waste combustion. Environ. Eng. Sci. 2005, 22, 221–231. [Google Scholar] [CrossRef]
- Galbreath, K.C.; Zygrliche, C.J.; Tibbetts, J.E.; Schulz, R.L.; Dunham, G.E. Effects of NOx, α-Fe2O3, γ-Fe2O3, and HCl on mercury transformations in a 7-kW coal combustion system. Fuel Process. Technol. 2005, 86, 429–448. [Google Scholar] [CrossRef]
- Ni, M.G.; Liu, D.Y.; Jin, J.; Feng, L.; Liu, Z. Ranking Oxygen Carriers for Elemental Mercury Oxidation in Coal-Fired Chemical-Looping Combustion: A Thermodynamic Approach. Energy Fuels 2020, 34, 2355–2365. [Google Scholar] [CrossRef]
- Li, J.H.; Lin, C.F.; Qin, W.; Xiao, X.B.; Li, W. Synergetic effect of mercury adsorption on the catalytic decomposition of CO over perfect and reduced Fe2O3[001] Surface. Acta Phys.-Chim. Sin. 2016, 32, 2717–2723. [Google Scholar] [CrossRef]
- Zhang, J.J.; Qin, W.; Dong, C.Q.; Yang, Y.P. Density functional theory study of elemental mercury adsorption on Fe2O3[104] and it’s effect on carbon deposit during chemical looping combustion. Energy Fuels 2016, 30, 3413–3418. [Google Scholar] [CrossRef]
- Guo, P.; Guo, X.; Zheng, C.G. Roles of γ-Fe2O3 in fly ash for mercury removal: Results of density functional theory study. Appl. Surf. Sci. 2010, 256, 6991–6996. [Google Scholar] [CrossRef]
- Jung, J.E.; Geatches, D.; Lee, K.; Aboud, S.; Brown, G.E., Jr.; Wilcox, J. First-Principles investigation of mercury adsorption on the α-Fe2O3(11̅02) surface. J. Phys. Chem. C 2015, 119, 26512–26518. [Google Scholar] [CrossRef]
- Yin, L.B.; Zhuo, Y.Q.; Xu, Q.S. Mercury emission from coal-fired power plants in China. Proc. CSEE 2013, 33, 1–9. [Google Scholar]
- Guo, Q.J.; Cheng, Y.; Liu, Y.Z.; Jia, W.H.; Ryu, H.-J. Coal chemical looping gasification for syngas generation using an iron-based oxygen carrier. Ind. Eng. Chem. Res. 2014, 53, 78–86. [Google Scholar] [CrossRef]
- Zhang, J.S.; Guo, Q.J.; Liu, Y.Z.; Cheng, Y. Preparation and characterization of Fe2O3/Al2O3 using the solution combustion approach for chemical looping combustion. Ind. Eng. Chem. Res. 2012, 51, 12773–12781. [Google Scholar] [CrossRef]
- ASTMD678416; Standard Test Method for Mercury from Coal-Fired Stationary Sources (Ontario Hydro Method). EPA: Washington, DC, USA, 1999.
- Liu, Y.Z.; Guo, Q.J.; Cheng, Y.; Ryu, H.-J. Reaction mechanism of coal chemical looping process for syngas production with CaSO4 oxygen carrier in CO2 atmosphere. Ind. Eng. Chem. Res. 2012, 51, 10364–10373. [Google Scholar] [CrossRef]



| Proximate Analysis, Wad/% | Ultimate Analysis, Wad/% | Hg, μg·g−1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | A | V | FC | C | H | O | N | S | ||
| JYC | 1.88 | 8.36 | 34.08 | 55.68 | 74.74 | 4.58 | 8.66 | 1.28 | 0.50 | 0.229 |
| ZTC | 22.38 | 23.5 | 30.98 | 23.14 | 35.6 | 2.21 | 14.49 | 0.93 | 0.89 | 0.252 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Zhao, S.; Gao, M.; Liu, Y. Mercury Adsorption and Oxidation Performance of an Iron-Based Oxygen Carrier during Coal Chemical Looping Process. Molecules 2024, 29, 2195. https://doi.org/10.3390/molecules29102195
Hu G, Zhao S, Gao M, Liu Y. Mercury Adsorption and Oxidation Performance of an Iron-Based Oxygen Carrier during Coal Chemical Looping Process. Molecules. 2024; 29(10):2195. https://doi.org/10.3390/molecules29102195
Chicago/Turabian StyleHu, Guochao, Shuju Zhao, Minggang Gao, and Yongzhuo Liu. 2024. "Mercury Adsorption and Oxidation Performance of an Iron-Based Oxygen Carrier during Coal Chemical Looping Process" Molecules 29, no. 10: 2195. https://doi.org/10.3390/molecules29102195
APA StyleHu, G., Zhao, S., Gao, M., & Liu, Y. (2024). Mercury Adsorption and Oxidation Performance of an Iron-Based Oxygen Carrier during Coal Chemical Looping Process. Molecules, 29(10), 2195. https://doi.org/10.3390/molecules29102195







