Plant In Vitro Cultures of Coleus scutellarioides (L.) Benth. “Electric Lime” and Possibilities of Modification in the Biosynthesis of Volatile Compounds
Abstract
1. Introduction
2. Results
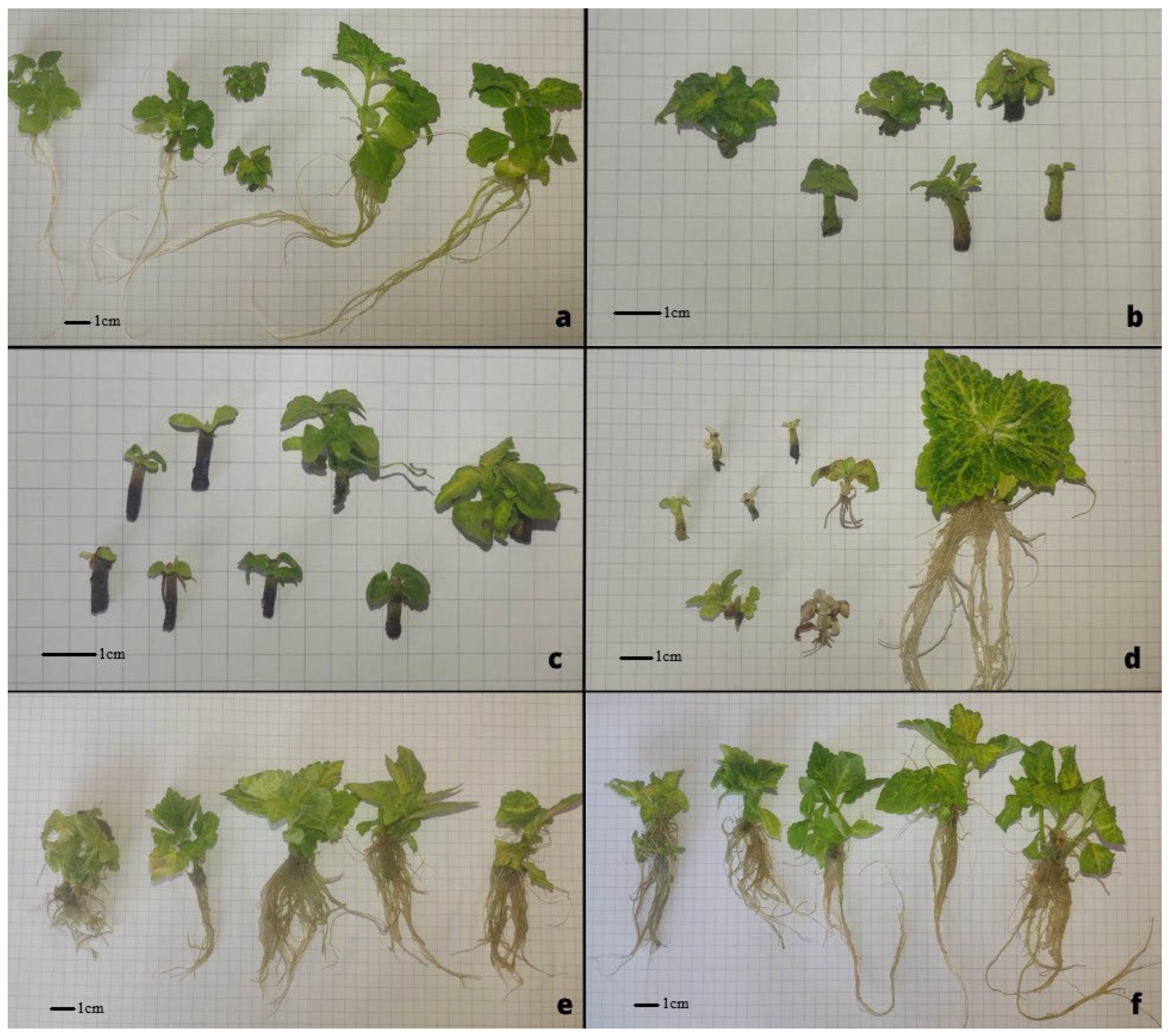
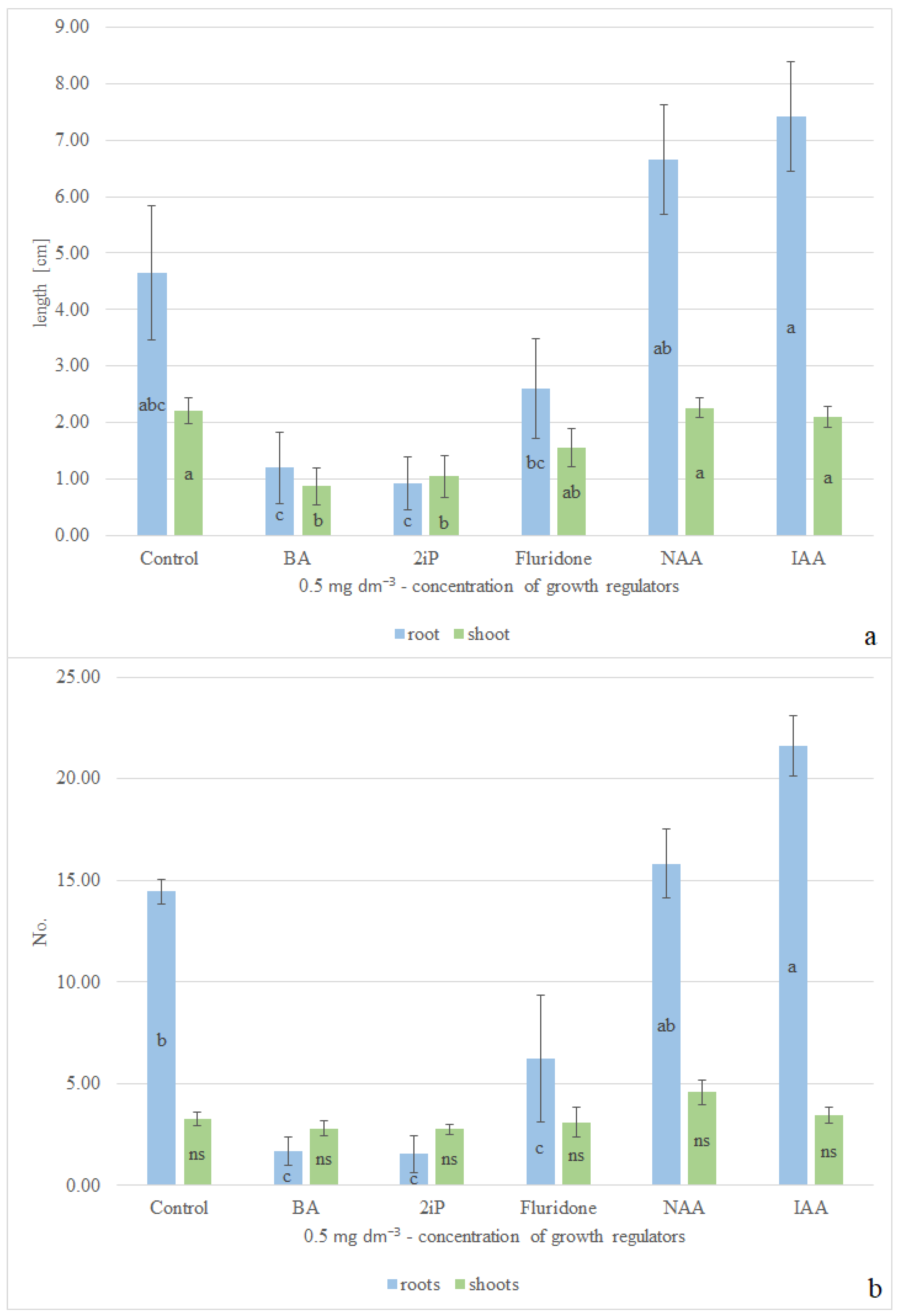
2.1. Plant In Vitro Cultures
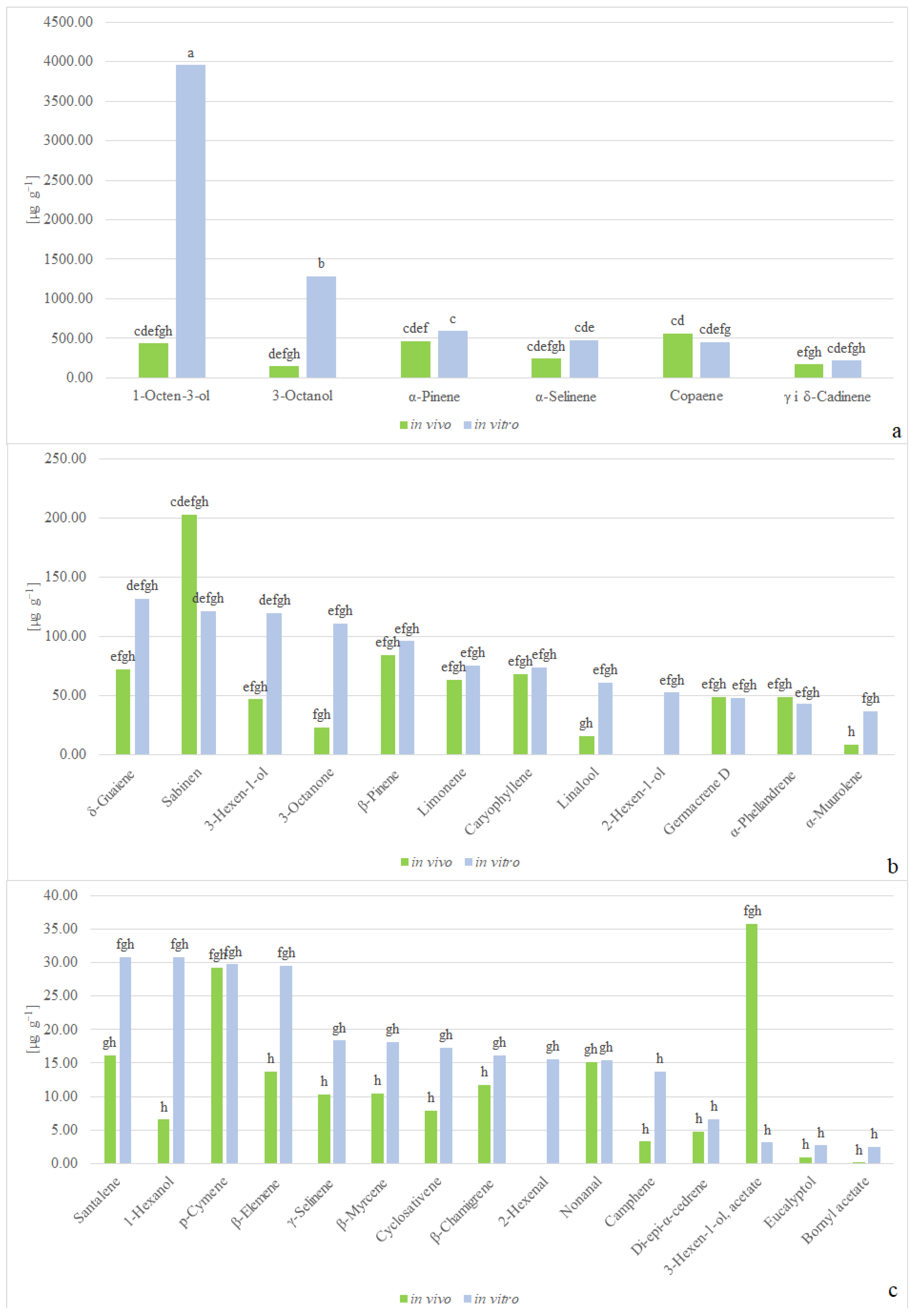
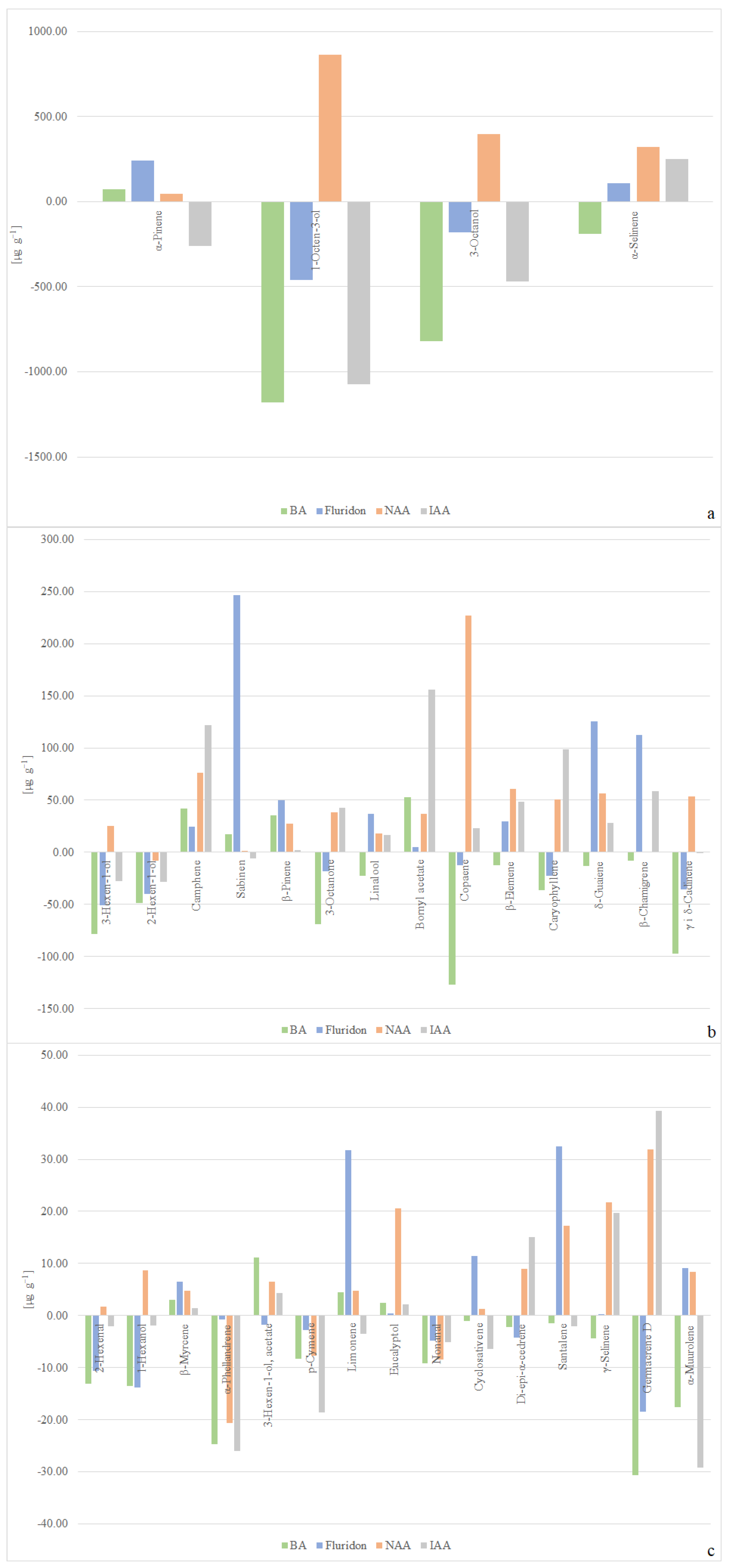
2.2. Volatile Compounds Composition
3. Discussion
3.1. Discussion of In Vitro Cultivation
3.2. Discussion of Volatile Compounds
4. Materials and Methods
4.1. Micropropagation
4.2. Chemical Compounds Analysis—Aroma Profiling
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paton, A.J.; Mwanyambo, M.; Govaerts, R.H.A.; Smitha, K.; Suddee, S.; Phillipson, P.B.; Wilson, T.C.; Forster, P.I.; Culham, A. Nomenclatural changes in Coleus and Plectranthus (Lamiaceae): A tale of more than two genera. PhytoKeys 2019, 129, 1–158. [Google Scholar] [CrossRef] [PubMed]
- Paton, A.J.; Mwanyambo, M.; Culham, A. Phylogenetic study of Plectranthus, Coleus and allies (Lamiaceae): Taxonomy, distribution and medicinal use. Bot. J. Linn. Soc. 2018, 188, 355–376. [Google Scholar] [CrossRef]
- Plectranthus Scutellarioides. Available online: http://www.missouribotanicalgarden.org (accessed on 15 March 2023).
- Hyam, R.; Pankhurst, R.J. Plants and Their Names: A Concise Dictionary, 1st ed.; Oxford University Press: Oxford, UK, 1995; p. 399. [Google Scholar]
- Rață, G.; Sala, F.; Samfira, I. Agricultural English, 1st ed.; Cambridge Scholars Publishing: Cambridge, UK, 2012; p. 191. [Google Scholar]
- Bauer, N.; Vuković, R.; Likić, S.; Jelaska, S. Potential of Different Coleus blumei Tissues for Rosmarinic Acid Production. Food Technol. Biotechnol. 2015, 53, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ayu, A.C.; Ida, M.; Moelyono, M.; Fakhriati, S.G. Total Anthocyanin Content and Identification of Anthocyanidin From Plectranthus scutellarioides (L.) R. Br Leaves. Res. J. Chem. Environ. 2018, 22, 11–16. [Google Scholar]
- Cretton, S.; Saraux, N.; Monteillier, A.; Righi, D.; Marcourt, L.; Genta-Jouve, G.; Wolfender, J.L.; Cuendet, M.; Christen, P. Anti-inflammatory and antiproliferative diterpenoids from Plectranthus scutellarioides. Phytochemistry 2018, 154, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Rakainsa, S.K.; Nisa, K.; Morita, H. Three new abietane-type diterpenoids from the leaves of Indonesian Plectranthus scutellarioides. Fitoterapia 2018, 127, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Moektiwardoyo, M.; Levita, J.; Sidiq, S.P.; Ahmad, K.; Mustarichie, R.; Subarnas, A.; Supriyatna, A. The determination of quercetin in Plectranthus scutellarioides (L.) R.Br. leaves extract and its In SilicoStudy on Histamine H4 Receptor. Maj. Farm. Indones. 2011, 22, 191–196. [Google Scholar]
- Osman, A.R. Genetic Variability and total phenolic compounds among six Coleus blumei varieties using RAPD Analysis. J. Appl. Sci. Res. 2013, 9, 1395–1400. [Google Scholar]
- Mariano, H.A.L.; Sucaldito, D.R.; Atienza, T.V. Phytochemical Screening and Partial Characterization through GC-MS of Leaf Crude Extract of Plectranthus scutellarioides (Mayana), and Plectranthus amboinicus (Oregano) and Their Larvicidal Activity Against 2nd–3rd Instar Larvae of Aedes aegypti in Comparison to Azadirachta indica. In Proceedings of the DLSU Research Congress. De La Salle University, Manila, Philippines, 20–22 June 2017; pp. 1–8. [Google Scholar]
- Moron, M.L.; Acero, L.H. Mayana (Coleus blumei) Leaves Ointment in Wound Healing of Albino Rats (Rattus albus). Int. J. Food Eng. 2017, 3, 18–22. [Google Scholar] [CrossRef][Green Version]
- Mustarichie, R.; Moektiwardojo, M.; Dewi, W.A. Isolation, Identification, and Characteristic of Essential Oil of Iler (Plectranthus scutellarioides (L.) R.Br leaves. J. Pharm. Sci. Res. 2017, 9, 2218–2223. [Google Scholar]
- Aziz, P.; Muhammad, N.; Intisar, A.; Abid, M.A.; Din, M.I.; Yaseen, M.; Kousar, R.; Aamir, A.; Ejaz, Q.; Ejaz, R. Constituents and antibacterial activity of leaf essential oil of Plectranthus scutellarioides. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 1247–1252. [Google Scholar] [CrossRef]
- Karo, M.B.; Hatta, M.; Patellongi, I.; Natzir, R.; Tambaip, T. IgM antibody and colony fungal load impacts of orally administered ethanol extract of Plectranthus scutellarioides on mice with systemic candidiasis. J. Pharm. Pharmacogn. Res. 2018, 6, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Moektiwardoyo, M.; Rositah, S.; Kusuma, A.F. Immunostimulatory activity of Plectranthus scutellarioides (L.) R.Br. leaves ethanolic extract and its fraction on rat using carbon clearance method. Drug Invent. Today 2019, 11, 2990–2995. [Google Scholar]
- Prabowo, Y.; Saptarini, N.M.; Sumiwi, S.A.; Levita, J.; Wicaksono, I.A.; Moektiwardoyo, M. Plectranthus scutellarioides (L.) Reduces the Rectal Temperature of Diphteria-Pertussis-Tetanus Vaccine-Induced Mice. Pharmacol. Clin. Pharm. Res. 2019, 4, 27. [Google Scholar] [CrossRef]
- Ragas, C.Y.; Templora, V.F.; Rideout, J.A. Diastereomeric Diterpenes from Coleus blumei. Chem. Pharm. Bull. 2001, 49, 927–929. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salaeh, A.; Augusti, R.S.; Susilawati, Y.; Sumiwi, S.A.; Moektiwardojo, M. Antidiabetic Activity of Fractions and Subfraction of Iler [Plectranthus scutellarioides (L.) R. Br.] Leaves on Diabetic Mice Induced by Alloxan. Res. J. Chem. Environ. 2018, 22, 5–10. [Google Scholar] [CrossRef]
- Astuti, A.D.; Yasir, B.; Subehan; Alam, G. Comparison of two varieties of Plectranthus scutellarioides based on extraction method, phytochemical compound, and cytotoxicity. J. Phys. Conf. Ser. 2019, 1341, 072012. [Google Scholar] [CrossRef]
- Bourdy, G.; Walter, A. Maternity and medicinal plants in Vanuatu I. The cycle of reproduction. J. Ethnopharmacol. 1992, 37, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Cordero, C.; Ligsay, A.; Alejandro, G. Ethnobotanical documentation of medicinal plants used by the Ati tribe in Malay, Aklan, Philippines. J. Complement. Med. Res. 2020, 11, 170. [Google Scholar] [CrossRef]
- Satrija, F.; Retnani, E.B.; Ridwan, Y.; Tiuria, R. Potential Use of Herbal Anthelmintics as Alternative Antiparasitic Drugs for Small Holder Farms in Developing Countries. Livestock Community and Environment. In Proceedings of the 10th Conference of the Association of Institutions for Tropical Veterinary Medicine, Copenhagen, Denmark, 20–23 August 2001; Kyvsgaard, N.C., Monrad, J., Eds.; Royal Veterinary and Agricultural University, Department of Clinical Studies and Department of Animal Science and Animal Health: Copenhagen, Denmark, 2002. [Google Scholar]
- Zakaria, Z.; Aziz, R.; Lachimanan, Y.L.; Sreenivasan, S.; Rathinam, X. Antioxidant Activity of Coleus blumei, Orthosiphon Stamineus, Ocimum basilicum and Mentha arvensis from Lamiaceae Family. Int. J. Nat. Eng. Sci. 2008, 2, 93–95. [Google Scholar]
- Thiem, B.; Kikowska, G. Zapewnienie jakości roślin leczniczych rozmnażanych w kulturach in vitro. Herba Pol. 2008, 54, 168–178. [Google Scholar]
- Cho, K.H.; Laux, V.Y.; Wallace-Springer, N.; Clark, D.G.; Folta, K.M.; Colquhoun, T.A. Effects of Light Quality on Vegetative Cutting and In Vitro Propagation of Coleus (Plectranthus scutellarioides). HortScience 2019, 54, 926–935. [Google Scholar] [CrossRef]
- Ibrahim, K.M.; Musbah, H.M. Increasing poly phenols in Coleus blumei at the cellular and intact plant levels using PEG stress. Res. J. Pharm. Technol. 2018, 11, 321. [Google Scholar] [CrossRef]
- Medina, T.; Lourdes, B.; Cardenas, L.B. Comparative Culture Response of Three Coleus blumei Benth. Varieties As Basis For Explant Selection for Callus Induction. J. Nat. Stud. 2017, 16, 1–10. [Google Scholar]
- Sahu, R.; Dewanjee, S. Differential physiological and biochemical responses under variable culture conditions in micro-propagated Solenostemon scutellarioides: An important ornamental plant. Nat. Prod. Bioprospecting 2012, 2, 160–165. [Google Scholar] [CrossRef]
- Qian, J.; Liu, G.; Liu, X.; Han, X.; Liu, H. Influence of growth regulators and sucrose concentrations on growth and rosmarinic acid production in calli and suspension cultures of Coleus blumei. Nat. Prod. Res. 2009, 23, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytologist. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Prins, C.L.; Vieira, I.J.; Freitas, S.P. Growth regulators and essential oil production. Braz. J. Plant Physiol. 2010, 22, 91–102. [Google Scholar] [CrossRef]
- Jamwal, K.; Bhattacharya, S.; Puri, S. Plant growth regulator mediated consequences of secondary metabolites in medicinal plants. J. Appl. Res. Med. Aromat. Plants 2018, 9, 26–38. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Łyczko, J.; Godyla-Jabłoński, M.; Pachura, N.; Adamenko, K.; Klemens, M.; Szumny, A. Natural Appetite Control: Consumer Perception of Food-Based Appetite Regulating Aromas. Nutrients 2023, 15, 2996. [Google Scholar] [CrossRef]
- Bourdon, P.A.; Zottele, M.; Zafar, Z.; Baxter, I.; Midthassel, A.; Myrta, A.; Wechselberger, K.F.; Strasser, H.; Butt, T.M. Behavioral response of three subterranean pests (Agriotes lineatus, Diabrotica virgifera virgifera, Phyllopertha horticola) to the fungal volatile organic compounds 1-octen-3-ol and 3-octanone. Arthropod-Plant Interact. 2023, 17, 473–483. [Google Scholar] [CrossRef]
- Hummadi, E.H.; Dearden, A.; Generalovic, T.; Clunie, B.; Harrott, A.; Cetin, Y.; Demirbek, M.; Khoja, S.; Eastwood, D.; Dudley, E.; et al. Volatile organic compounds of Metarhizium brunneum influence the efficacy of entomopathogenic nematodes in insect control. Biol. Control 2021, 155, 104527. [Google Scholar] [CrossRef] [PubMed]
- Tonks, A.J.; Roberts, J.M.; Midthassel, A.; Pope, T. Exploiting volatile organic compounds in crop protection: A systematic review of 1-octen-3-ol and 3-octanone. Ann. Appl. Biol. 2023, 183, 121–134. [Google Scholar] [CrossRef]
- Khoshnazar, M.; Parvardeh, S.; Bigdeli, M.R. Alpha-pinene exerts neuroprotective effects via anti-inflammatory and anti-apoptotic mechanisms in a rat model of focal cerebral ischemia-reperfusion. J. Stroke Cerebrovasc. Dis. 2020, 29, 104977. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation by α-pinene on autonomic nervous activity. J. Wood Sci. 2016, 62, 568–572. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Yang, Y. α-Pinene inhibits human prostate cancer growth in a mouse xenograft model. Chemotherapy 2018, 63, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, S.L.D.; Dias, D.A.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic potential of α-and β-pinene: A miracle gift of nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Allenspach, M.; Steuer, C. α-Pinene: A never-ending story. Phytochemistry 2021, 190, 112857. [Google Scholar] [CrossRef]
- Pragadheesh, V.S.; Saroj, A.; Yadav, A.; Samad, A.; Chanotiya, C.S. Compositions, enantiomer characterization and antifungal activity of two Ocimum essential oils. Ind. Crops Prod. 2013, 50, 333–337. [Google Scholar] [CrossRef]
- Oladimeji, A.O.; Babatunde, O.; Musa, R.T.; M’civer, F.A.; Lawal, A.T.; Ogunwande, I.A. GC-MS analysis and cytotoxic activity of essential oils from the leaves of Abrus precatorius L. Gaertn. Asian Pac. J. Trop. Dis. 2016, 6, 372–375. [Google Scholar] [CrossRef]
- Rojas-Armas, J.P.; Arroyo-Acevedo, J.L.; Palomino-Pacheco, M.; Ortiz-Sánchez, J.M.; Calva, J.; Justil-Guerrero, H.J.; Castro-Luna, A.; Ramos-Cevallos, N.; Cieza-Macedo, E.C.; Herrera-Calderon, O. Phytochemical constituents and ameliorative effect of the essential oil from Annona muricata L. leaves in a murine model of breast cancer. Molecules 2022, 27, 1818. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.; Ray, A.; Sahoo, A.; Das, P.K.; Dash, K.T.; Kar, S.K.; Nayak, S.; Panda, P.C. Chemical composition and biological activities of leaf essential oil of Syzygium myrtifolium from eastern India. J. Essent. Oil Bear. Plants 2021, 24, 582–595. [Google Scholar] [CrossRef]
- Chandra, M.; Prakash, O.; Kumar, R.; Bachheti, R.K.; Bhushan, B.; Kumar, M.; Pant, A.K. β-Selinene-rich essential oils from the parts of Callicarpa macrophylla and their antioxidant and pharmacological activities. Medicines 2017, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-CoA reductase activity. PLoS ONE 2011, 6, 20516. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Kim, J.; Moon, B.S.; Park, S.M.; Jung, D.E.; Kang, S.Y.; Lee, S.J.; Oh, S.J.; Kwon, S.H.; Nam, M.H.; et al. Camphene attenuates skeletal muscle atrophy by regulating oxidative stress and lipid metabolism in rats. Nutrients 2020, 12, 3731. [Google Scholar] [CrossRef] [PubMed]
- Hachlafi, N.E.; Aanniz, T.; Menyiy, N.E.; Baaboua, A.E.; Omari, N.E.; Balahbib, A.; Shariat, M.A.; Zengin, G.; Fikri-Benbrahim, K.; Bouyahya, A. In vitro and in vivo biological investigations of camphene and its mechanism insights: A review. Food Rev. Int. 2023, 39, 1799–1826. [Google Scholar] [CrossRef]
- Hall, D.R.; Beevor, P.S.; Cork, A.; Nesbitt, B.F.; Vale, G.A. 1-Octen-3-ol: A potent olfactory stimulant and attractant for tsetse isolated from cattle odours. Int. J. Trop. Insect Sci. 1984, 5, 335–339. [Google Scholar] [CrossRef]
- Kline, D.L.; Allan, S.A.; Bernier, U.R.; Welch, C.H. Evaluation of the enantiomers of 1-octen-3-ol and 1-octyn-3-ol as attractants for mosquitoes associated with a freshwater swamp in Florida, USA. Med. Vet. Entomol. 2007, 21, 323–331. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Peng, Y.; Yang, W.; Shi, J. Antifungal activity of 1-octen-3-ol against Monilinia fructicola and its ability in enhancing disease resistance of peach fruit. Food Control 2022, 135, 108804. [Google Scholar] [CrossRef]
- Kishimoto, K.; Matsui, K.; Ozawa, R.; Takabayashi, J. Volatile 1-octen-3-ol induces a defensive response in Arabidopsis thaliana. J. Gen. Plant Pathol. 2007, 73, 35–37. [Google Scholar] [CrossRef]
- Yavasoglu, S.İ.; Wood, M.J.; Alkhaibari, A.M.; Touray, M.; Butt, T. Potential of 3-octanone as a lure and kill agent for control of the Brown garden snail. J. Invertebr. Pathol. 2023, 198, 107920. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; El-Daly, N.; El-Khateeb, A.Y.; Hassan, S.; Elsherbiny, E.A. Chemical Composition, Antioxidant, Antitumor and Antifungal Activities of Methanolic Extracts of Coleus blumei, Plectranthus amboinicus and Salvia splendens (Lamiaceae). J. Agric. Chem. Biotechnol. 2021, 12, 177–187. [Google Scholar] [CrossRef]
- Affonso, V.R.; Bizzo, H.R.; Lima, S.S.D.; Esquibel, M.A.; Sato, A. Solid phase microextraction (SPME) analysis of volatile compounds produced by in vitro shoots of Lantana camara L. under the influence of auxins and cytokinins. J. Braz. Chem. Soc. 2007, 18, 1504–1508. [Google Scholar] [CrossRef]
- Ibrahim, M.; Agarwal, M.; Yang, J.O.; Abdulhussein, M.; Du, X.; Hardy, G.; Ren, Y. Plant Growth Regulators Improve the Production of Volatile Organic Compounds in Two Rose Varieties. Plants 2019, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Łyczko, J.; Piotrowski, K.; Kolasa, K.; Galek, R.; Szumny, A. Mentha piperita L. Micropropagation and the Potential Influence of Plant Growth Regulators on Volatile Organic Compound Composition. Molecules 2020, 25, 2652. [Google Scholar] [CrossRef]
- Zielińska, S.; Piątczak, E.; Kalemba, D.; Matkowski, A. Influence of plant growth regulators on volatiles produced by in vitro grown shoots of Agastache rugosa (Fischer & C.A.Meyer) O. Kuntze. Plant Cell Tissue Organ Cult. 2011, 107, 161–167. [Google Scholar]
- Khanam, D.; Mohammad, F. Effect of structurally different plant growth regulators (PGRs) on the concentration, yield, and constituents of peppermint essential oil. J. Herbs Spices Med. Plants 2017, 23, 26–35. [Google Scholar] [CrossRef]
- Castilho, C.V.V.; Leitão, S.G.; Silva, V.D.; Miranda, C.D.O.; Santos, M.C.D.S.; Bizzo, H.R.; Silva, N.C.B.D. In vitro propagation of a carvacrol-producing type of Lippia origanoides Kunth: A promising oregano-like herb. Ind. Crops Prod. 2019, 130, 491–498. [Google Scholar] [CrossRef]
- Rajkumari, S.; Sanatombi, K. Secondary metabolites content and essential oil composition of in vitro cultures of Zingiber montanum (Koenig) Link ex A. Dietr. Biotechnol. Lett. 2020, 42, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, M.; Swain, D.K.; Bhadoria, P.B.S. Effect of IAA and BAP application in varying concentration on seed yield and oil quality of Guizotia abyssinica (L.f.) Cass. Ann. Agric. Sci. 2022, 67, 15–23. [Google Scholar] [CrossRef]
- Ji, X.H.; Wang, Y.T.; Zhang, R.; Wu, S.J.; An, M.M.; Li, M.; Wang, C.Z.; Chen, X.L.; Zhing, Y.M.; Chen, X.S. Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tissue Organ Cult. 2015, 120, 325–337. [Google Scholar] [CrossRef]
- Popova, L. Effect of fluridone on plant development and stress-induced ABA accumulation in Vicia faba L. plants. Bulg. J. Plant Physiol. 1995, 21, 42–50. [Google Scholar]
- Grzyb, M.; Kalandyk, A.; Mikuła, A. Effect of TIBA, fluridone and salicylic acid on somatic embryogenesis and endogenous hormone and sugar contents in the tree fern Cyathea delgadii Sternb. Acta Physiol. Plant. 2018, 40, 1–11. [Google Scholar] [CrossRef]
- Goggin, D.E.; Powles, S.B. Fluridone: A combination germination stimulant and herbicide for problem fields? Pest Manag. Sci. 2014, 70, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Mialoundama, A.S.; Heintz, D.; Debayle, D.; Rahier, A.; Camara, B.; Bouvier, F. Abscisic acid negatively regulates elicitor-induced synthesis of capsidiol in wild tobacco. Plant Physiol. 2009, 150, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Farias-Soares, F.L.; Steiner, N.; Schmidt, É.C.; Pereira, M.L.; Rogge-Renner, G.D.; Bouzon, Z.L.; Floh, E.S.I.; Guerra, M.P. The transition of proembryogenic masses to somatic embryos in Araucaria angustifolia (Bertol.) Kuntze is related to the endogenous contents of IAA, ABA and polyamines. Acta Physiol. Plant. 2014, 36, 1853–1865. [Google Scholar] [CrossRef]
- Karppinen, K.; Tegelberg, P.; Häggman, H.; Jaakola, L. Abscisic acid regulates anthocyanin biosynthesis and gene expression associated with cell wall modification in ripening bilberry (Vaccinium myrtillus L.) fruits. Front. Plant Sci. 2018, 9, 406326. [Google Scholar] [CrossRef]
- Saffari, V.R.; Khalighi, A.H.M.A.D.; Lesani, H.O.S.S.E.I.N.; Babalar, M.E.S.B.A.H.; Obermaier, J.F. Effects of different plant growth regulators and time of pruning on yield components of Rosa damascena Mill. Int. J. Agric. Biol. 2004, 6, 1040–1042. [Google Scholar]
- da Silva, B.O.; Amaral, A.C.F.; Ferreira, J.L.P.; Santiago, L.J.M.; Louro, R.P. Micropropagation and in vitro production of secondary metabolites of Croton floribundus Spreng. Vitr. Cell. Dev. Biol. Plant 2013, 49, 366–372. [Google Scholar] [CrossRef]
- Morais, D.R.; Rotta, E.M.; Sargi, S.C.; Bonafe, E.G.; Suzuki, R.M.; Souza, N.E.; Matsushita, M.; Visentainer, J.V. Proximate composition, mineral contents and fatty acid composition of the different parts and dried peels of tropical fruits cultivated in Brazil. J. Braz. Chem. Soc. 2017, 28, 308–318. [Google Scholar] [CrossRef]
- Evans, W.C. Trease and Evans Pharmacognosy, 16th ed.; Sauders Elsevier: Edinburgh, UK, 2009; p. 94. [Google Scholar]
- Khan, M.M.A.; Quasar, N.; Afreen, R. Nanotized form of indole acetic acid improve biochemical activities, the ultrastructure of glandular trichomes and essential oil production in Ocimum tenuiflorum L. Ind. Crops Prod. 2023, 193, 116117. [Google Scholar] [CrossRef]
- Kecis, H.; Bagues, M.; Abdelouhab, Y.; Mekircha, F.; Gali, L.; Kadi, K.; Addad, D.; Nagaz, K.; Brahmi, F.; Kouba, Y. Different indole-3-acetic acid and 6 benzyl amino purine concentrations affect biomass, phenolic profile, and bioactivity in Mentha rotundifolia L. J. Food Meas. Charact. 2023, 17, 4551–4564. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Q.; Zhang, Y.; Wu, C.; Zheng, Y.; Tong, F.; Zhang, L.; Lu, R.; Pan, X.; Tan, H.; et al. Effects of exogenous indole-3-acetic acid on the density of trichomes, expression of artemisinin biosynthetic genes, and artemisinin biosynthesis in Artemisia annua. Biotechnol. Appl. Biochem. 2023, 70, 1870–1880. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, J.; Song, M.; Li, R.; Yang, Y.; Ye, X.; Chen, X. Analysis of the mechanism of exogenous indole-3-acetic acid on the enrichment of d-glucose in Chlorococcum humicola cultured by sludge extracts. Sci. Total Environ. 2023, 902, 166124. [Google Scholar] [CrossRef]
- Hakim, W.H.A.; Erfianti, T.; Dhiaurahman, A.N.; Maghfiroh, K.Q.; Amelia, R.; Nurafifah, I.; Kurnianto, D.; Siswanti, D.U.; Suyono, E.A.; Marno, S.; et al. The Effect of IAA Phytohormone (Indole-3-Acetic Acid) on the Growth, Lipid, Protein, Carbohydrate, and Pigment Content in Euglena sp. Malays. J. Fundam. Appl. Sci. 2023, 19, 513–524. [Google Scholar] [CrossRef]
- Schmiderer, C.; Grausgruber-Gröger, S.; Grassi, P.; Steinborn, R.; Novak, J. Influence of gibberellin and daminozide on the expression of terpene synthases and on monoterpenes in common sage (Salvia officinalis). J. Plant Physiol. 2010, 167, 779–786. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Crafts-Brandner, S.J.; ParÉ, P.W.; Henneberry, T.J. Exogenous methyl jasmonate induces volatile emissions in cotton plants. J. Chem. Ecol. 2001, 27, 679–695. [Google Scholar] [CrossRef]
- Naeem, M.M.M.A.; Khan, M.M.A.; Moinuddin; Idrees, M.; Aftab, T. Triacontanol-mediated regulation of growth and other physiological attributes, active constituents and yield of Mentha arvensis L. Plant Growth Regul. 2011, 65, 195–206. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F.A. Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oils by Ion Trap Mass Spectroscopy; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Abdi, H.; Williams, L.J. Tukey’s Honestly Significant Difference (HSD) Test. Encycl. Res. Des. 2010, 3, 1–5. [Google Scholar]
| Quantity of a Compound per Internal Standard [µg g−1] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time [min] | Compound | Literature Retention Index | Calculated Retention Index | Cultivation Conditions | |||||
| In Vivo | Control In Vitro | BA [0.5 mg dm−3] | Fluridone [0.5 mg dm−3] | NAA [0.5 mg dm−3] | IAA [0.5 mg dm−3] | ||||
| 6.4 | 2-Hexenal | 822 | 838 | - | 15.57 ± 2.39 nm | 2.51 ± 0.18 n | 5.01 ± 1.65 n | 17.31 ± 3.24 nm | 13.48 ± 4.66 nm |
| 6.4 | 3-Hexen-1-ol | 838 | 839 | 46.78 ± 9.80 nml | 119.66 ± 12.17 nmlk | 41.40 ± 4.98 nml | 68.85 ± 13.08 nml | 144.78 ± 15.83 nmlkj | 92.11 ± 17.75 nmlk |
| 6.7 | 2-Hexen-1-ol | 862 | 857 | - | 52.49 ± 17.68 nml | 4.12 ± 0.37 n | 12.39 ± 1.87 nm | 44.37 ± 7.67 nml | 23.84 ± 8.23 nml |
| 6.8 | 1-Hexanol | 868 | 858 | 6.55 ± 1.09 n | 30.84 ± 4.59 nml | 17.36 ± 0.87 nm | 17.02 ± 3.75 nm | 39.50 ± 5.76 nml | 28.91 ± 4.29 nml |
| 8.9 | α-Pinene | 935 | 940 | 458.43 ± 31.24 nmlkjihg | 590.82 ± 156.90 nmlkjihg | 665.35 ± 99.54 mlkjihgf | 833.88 ± 214.03 hgf | 637.49 ± 156.23 nmlkjihgf | 329.15 ± 51.61 nmlkjih |
| 9.4 | Camphene | 953 | 955 | 3.28 ± 1.55 n | 13.70 ± 1.35 nm | 55.74 ± 45.13 nml | 38.26 ± 6.49 nml | 90.07 ± 33.07 nmlk | 135.59 ± 25.74 nmlk |
| 10.4 | Sabinen | 976 | 979 | 202.69 ± 37.45 nmlkjih | 121.62 ± 36.26 nmlk | 139.29 ± 40.54 nmlk | 367.97 ± 108.69 nmlkjih | 122.85 ± 35.01 nmlk | 115.71 ± 24.83 nmlk |
| 10.5 | β-pinene | 978 | 981 | 84.47 ± 6.91 nmlk | 95.96 ± 24.65 nmlk | 131.44 ± 33.74 nmlk | 145.78 ± 34.80 nmlkj | 123.57 ± 27.51 nmlk | 98.22 ± 15.12 nmlk |
| 10.6 | 1-Octen-3-ol | 982 | 984 | 433.11 ± 41.43 nmlkjih | 3953.76 ± 486.22 b | 2772.06 ± 100.49 d | 3494.34 ± 497.67 cb | 4815.90 ± 882.53 a | 2882.59 ± 469.36 dc |
| 10.9 | 3-Octanone | 989 | 990 | 23.31 ± 6.69 nml | 111.03 ± 8.66 nmlk | 41.88 ± 2.41 nml | 92.63 ± 15.94 nmlk | 149.26 ± 15.23 nmlkj | 153.91 ± 34.77 nmlkji |
| 11.0 | β-Myrcene | 992 | 994 | 10.46 ± 1.12 nm | 18.13 ± 3.63 nm | 21.14 ± 4.71 nml | 24.61 ± 5.26 nml | 22.92 ± 2.79 nml | 19.52 ± 2.40 nm |
| 11.2 | 3-Octanol | 995 | 997 | 143.98 ± 16.14 nmlkj | 1279.46 ± 211.98 fe | 461.19 ± 11.92 nmlkjihg | 1100.13 ± 112.90 gfe | 1674.66 ± 355.52 e | 808.82 ± 85.25 ihgf |
| 11.5 | α-Phellandrene | 1002 | 1006 | 48.66 ± 10.94 nml | 42.75 ± 11.06 nml | 18.03 ± 6.67 nm | 41.98 ± 15.16 nml | 22.17 ± 5.61 nml | 16.79 ± 3.28 nm |
| 11.6 | 3-Hexen-1-ol acetate | 1005 | 1010 | 35.75 ± 8.64 nml | 3.14 ± 0.83 n | 14.27 ± 12.09 nm | 1.45 ± 0.34 n | 9.62 ± 0.79 nm | 7.43 ± 1.72 nm |
| 12.2 | p-Cymene | 1026 | 1029 | 29.24 ± 2.58 nml | 29.74 ± 6.76 nml | 21.47 ± 3.16 nml | 26.91 ± 5.20 nml | 22.05 ± 4.92 nml | 11.18 ± 1.53 nm |
| 12.4 | Limonene | 1032 | 1033 | 63.04 ± 6.72 nml | 75.27 ± 16.79 nmlk | 79.79 ± 19.49 nmlk | 106.95 ± 23.13 nmlk | 79.98 ± 16.67 nmlk | 71.81 ± 13.03 nmlk |
| 12.5 | Eucalyptol | 1033 | 1036 | 0.96 ± 0.29 n | 2.71 ± 0.62 n | 5.13 ± 0.80 n | 3.08 ± 0.61 n | 23.33 ± 19.23 nml | 4.87 ± 0.67 n |
| 15.1 | Linalool | 1103 | 1101 | 16.11 ± 1.50 nm | 61.27 ± 8.18 nml | 38.83 ± 4.98 nml | 97.84 ± 17.56 nmlk | 79.44 ± 15.77 nmlk | 78.16 ± 13.12 nmlk |
| 15.3 | Nonanal | 1111 | 1106 | 15.18 ± 4.83 nm | 15.39 ± 5.05 nm | 6.31 ± 0.49 n | 10.58 ± 1.81 nm | 6.97 ± 1.37 n | 10.33 ± 3.92 nm |
| 21.9 | Bornyl acetate | 1284 | 1288 | 0.13 ± 0.06 n | 2.53 ± 1.46 n | 55.14 ± 53.56 nml | 7.61 ± 1.27 nm | 39.17 ± 27.01 nml | 158.20 ± 34.67 nmlkji |
| 24.4 | Cyclosativene | 1368 | 1376 | 7.94 ± 3.67 nm | 17.28 ± 3.91 nm | 16.26 ± 1.95 nm | 28.74 ± 5.31 nml | 18.55 ± 0.93 nm | 10.82 ± 2.03 nm |
| 24.7 | Copaene | 1382 | 1383 | 556.89 ± 64.50 nmlkjihg | 451.63 ± 65.62 nmlkjihg | 324.44 ± 18.68 nmlkjih | 439.41 ± 99.17 nmlkjih | 678.83 ± 112.12 lkjihgf | 474.52 ± 94.36 nmlkjihg |
| 24.8 | Di-epi-α-cedrene | 1385 | 1389 | 4.79 ± 1.12 n | 6.65 ± 2.21 n | 4.47 ± 0.91 n | 2.36 ± 0.56 n | 15.60 ± 6.20 nm | 21.72 ± 4.98 nml |
| 25.0 | β-Elemene | 1394 | 1396 | 13.67 ± 5.64 nm | 29.52 ± 8.71 nml | 17.19 ± 4.94 nm | 59.44 ± 12.79 nml | 90.47 ± 11.29 nmlk | 77.86 ± 16.80 nmlk |
| 25.5 | Santalene | 1417 | 1418 | 16.13 ± 6.12 nm | 30.84 ± 7.95 nml | 29.43 ± 3.23 nml | 63.34 ± 16.39 nml | 48.10 ± 10.71 nml | 28.79 ± 5.26 nml |
| 25.7 | Caryophyllene | 1424 | 1428 | 68.19 ± 13.08 nml | 74.04 ± 15.96 nmlk | 37.80 ± 2.96 nml | 51.54 ± 6.10 nml | 124.70 ± 25.14 nmlk | 172.60 ± 32.90 nmlkji |
| 26.2 | δ-Guaiene | 1452 | 1455 | 72.10 ± 32.80 nmlk | 131.98 ± 29.36 nmlk | 119.01 ± 12.91 nmlk | 257.29 ± 67.63 nmlkjih | 188.24 ± 53.83 nmlkjih | 159.90 ± 30.39 nmlkji |
| 26.8 | γ-Selinene | 1479 | 1484 | 10.33 ± 1.40 nm | 18.36 ± 0.92 nm | 14.02 ± 0.03 nm | 18.69 ± 4.29 nm | 40.06 ± 4.64 nml | 38.07 ± 9.48 nml |
| 26.9 | β-Chamigrene | 1776 | 1488 | 11.74 ± 2.59 nm | 16.19 ± 4.02 nm | 8.35 ± 1.14 nm | 128.86 ± 44.60 nmlk | 16.27 ± 5.29 nm | 74.88 ± 19.32 nmlk |
| 26.9 | Germacrene D | 1481 | 1491 | 48.55 ± 8.57 nml | 47.91 ± 3.91 nml | 17.27 ± 0.46 nm | 29.51 ± 15.62 nml | 79.81 ± 10.28 nmlk | 87.15 ± 13.81 nmlk |
| 27.2 | α-Selinene | 1494 | 1504 | 239.47 ± 49.92 nmlkjih | 478.64 ± 65.16 nmlkjihg | 291.59 ± 4.57 nmlkjih | 588.20 ± 142.43 nmlkjihg | 798.35 ± 82.56 jihgf | 728.95 ± 139.46 kjihgf |
| 27.2 | α-Muurolene | 1499 | 1508 | 8.41 ± 2.62 nm | 36.63 ± 16.23 nml | 19.02 ± 7.28 nm | 45.69 ± 17.47 nml | 45.02 ± 14.83 nml | 7.45 ± 1.46 nm |
| 27.5 | γ i δ-Cadinene | 1513 | 1525 | 168.25 ± 70.16 nmlk | 215.98 ± 81.53 nmlk | 118.72 ± 45.39 nml | 180.36 ± 70.28 nmlk | 269.68 ± 103.93 nmlkjih | 215.64 ± 87.24 nmlk |
| 1524 | 1534 | ||||||||
| Content of Volatile Compounds [%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time [min] | Compound | Literature Retention Index | Calculated Retention Index | Cultivation Conditions | |||||
| In Vivo | Control In Vitro | BA [0.5 mg dm−3] | Fluridon [0.5 mg dm−3] | NAA [0.5 mg dm−3] | IAA [0.5 mg dm−3] | ||||
| 6.4 | 2-Hexenal | 822 | 838 | - | 0.19 ± 0.03 Δz | 0.04 ± 0.00 Δ | 0.06 ± 0.01 Δ | 0.16 ± 0.02 Δb | 0.18 ± 0.05 Δzd |
| 6.4 | 3-Hexen-1-ol | 838 | 839 | 1.54 ± 0.23 Δzyxwvtsr | 1.44 ± 0.12 Δzyxwvtsr | 0.73 ± 0.11 Δzyx | 0.80 ± 0.05 Δzyxw | 1.36 ± 0.07 Δzyxwvts | 1.25 ± 0.17 Δzyxwv |
| 6.7 | 2-Hexen-1-ol | 862 | 857 | - | 0.60 ± 0.14 Δzy | 0.07 ± 0.01 Δ | 0.15 ± 0.02 Δ | 0.42 ± 0.07 Δz | 0.31 ± 0.10 Δz |
| 6.8 | 1-Hexanol | 868 | 858 | 0.21 ± 0.02 Δz | 0.37 ± 0.02 Δz | 0.30 ± 0.01 Δz | 0.19 ± 0.01 Δz | 0.37 ± 0.01 Δz | 0.39 ± 0.02 Δz |
| 8.9 | α-Pinene | 935 | 940 | 15.63 ± 2.16 fe | 6.87 ± 1.49 nmlkj | 11.50 ± 0.96 ihgf | 9.38 ± 1.14 lkjih | 5.76 ± 0.70 urqponmlk | 4.53 ± 0.47 zyxwvutsrqponm |
| 9.4 | Camphene | 953 | 955 | 0.11 ± 0.04 Δ | 0.17 ± 0.03 Δz | 0.88 ± 0.68 Δzyxw | 0.46 ± 0.07 Δzy | 0.86 ± 0.37 Δzyxw | 1.82 ± 0.06 Δzyxwvutsr |
| 10.4 | Sabinen | 976 | 979 | 6.83 ± 1.19 nmlkj | 1.43 ± 0.42 Δzyxwvtsr | 2.36 ± 0.51 Δzyxwvutsrqpo | 4.10 ± 0.83 Δzyxwvutsrqponm | 1.10 ± 0.18 Δzyxwv | 1.60 ± 0.29 Δzyxwvtsr |
| 10.5 | β-Pinene | 978 | 981 | 2.88 ± 0.40 Δzyxwvutsrqpon | 1.12 ± 0.24 Δzyxwv | 2.24 ± 0.41 Δzyxwvutsrqp | 1.65 ± 0.14 Δzyxwvtsr | 1.14 ± 0.22 Δzyxwv | 1.34 ± 0.03 Δzyxwvt |
| 10.6 | 1-Octen-3-ol | 982 | 984 | 14.44 ± 0.54 gfe | 47.35 ± 2.22 ba | 48.67 ± 2.37 a | 41.55 ± 3.36 dc | 44.23 ± 0.79 c | 39.01 ± 0.92 d |
| 10.9 | 3-Octanone | 989 | 990 | 0.75 ± 0.16 Δzyx | 1.37 ± 0.21 Δzyxwvts | 0.74 ± 0.06 Δzyx | 1.08 ± 0.02 Δzyxwv | 1.45 ± 0.29 Δzyxwvtsr | 2.05 ± 0.29 Δzyxwvutsrqp |
| 11.0 | β-Myrcene | 992 | 994 | 0.35 ± 0.01 Δz | 0.22 ± 0.04 Δz | 0.36 ± 0.06 Δz | 0.28 ± 0.01 Δz | 0.22 ± 0.03 Δz | 0.27 ± 0.01 Δz |
| 11.2 | 3-Octanol | 995 | 997 | 4.79 ± 0.23 yxwvutsrqponm | 15.19 ± 1.05 gfe | 8.12 ± 0.56 mlkji | 13.32 ± 1.65 hgf | 15.24 ± 0.66 gfe | 11.17 ± 0.64 jihg |
| 11.5 | α-Phellandrene | 1002 | 1006 | 1.57 ± 0.22 Δzyxwvtsr | 0.49 ± 0.07 Δzy | 0.31 ± 0.10 Δz | 0.47 ± 0.14 Δzy | 0.20 ± 0.02 Δz | 0.23 ± 0.04 Δz |
| 11.6 | 3-Hexen-1-ol. acetate | 1005 | 1010 | 1.17 ± 0.21 Δzyxwv | 0.04 ± 0.00 Δ | 0.27 ± 0.23 Δz | 0.02 ± 0.01 Δ | 0.09 ± 0.01 Δ | 0.10 ± 0.02 Δ |
| 12.2 | p-Cymene | 1026 | 1029 | 0.98 ± 0.05 Δzyxw | 0.35 ± 0.07 Δz | 0.38 ± 0.07 Δz | 0.31 ± 0.02 Δz | 0.20 ± 0.02 Δz | 0.15 ± 0.01 Δ |
| 12.4 | Limonene | 1032 | 1033 | 2.10 ± 0.10 Δzyxwvutsrqp | 0.88 ± 0.12 Δzyxw | 1.36 ± 0.24 Δzyxwvts | 1.23 ± 0.07 Δzyxwv | 0.74 ± 0.13 Δzyx | 0.97 ± 0.03 Δzyxw |
| 12.5 | Eucalyptol | 1033 | 1036 | 0.03 ± 0.01 Δ | 0.03 ± 0.01 Δ | 0.09 ± 0.01 Δ | 0.04 ± 0.00 Δ | 0.25 ± 0.21 Δz | 0.07 ± 0.01 Δ |
| 15.1 | -Linalool | 1103 | 1101 | 0.56 ± 0.13 Δzy | 0.73 ± 0.07 Δzyx | 0.67 ± 0.04 Δzy | 1.14 ± 0.02 Δzyxwv | 0.73 ± 0.03 Δzyx | 1.06 ± 0.02 Δzyxwv |
| 15.3 | Nonanal | 1111 | 1106 | 0.49 ± 0.12 Δzy | 0.18 ± 0.06 Δz | 0.11 ± 0.01 Δ | 0.13 ± 0.04 Δ | 0.06 ± 0.01 Δ | 0.17 ± 0.09 Δ |
| 21.9 | Bornyl acetate | 1284 | 1288 | 0.00 ± 0.00 Δ | 0.04 ± 0.02 Δ | 0.85 ± 0.82 Δzyxw | 0.09 ± 0.00 Δ | 0.43 ± 0.29 Δzy | 2.19 ± 0.44 Δzyxwvutsrqp |
| 24.4 | Cyclosativene | 1368 | 1376 | 0.25 ± 0.10 Δz | 0.20 ± 0.03 Δz | 0.29 ± 0.04 Δz | 0.33 ± 0.01 Δz | 0.18 ± 0.02 Δz | 0.15 ± 0.01 Δ |
| 24.7 | Copaene | 1382 | 1383 | 18.44 ± 0.27 e | 5.36 ± 0.18 vutsrqponml | 5.68 ± 0.22 utsrqponmlk | 5.04 ± 0.59 xwvutsrqponml | 6.27 ± 0.17 qponmlk | 6.40 ± 0.45 ponmlk |
| 24.8 | Di-epi-α-cedrene | 1385 | 1389 | 0.15 ± 0.02 Δ | 0.08 ± 0.02 Δ | 0.08 ± 0.01 Δ | 0.03 ± 0.00 Δ | 0.13 ± 0.03 Δ | 0.30 ± 0.05 Δz |
| 25.0 | β-Elemene | 1394 | 1396 | 0.48 ± 0.20 Δzy | 0.34 ± 0.08 Δz | 0.31 ± 0.10 Δz | 0.68 ± 0.06 Δzyx | 0.86 ± 0.14 Δzyxw | 1.03 ± 0.07 Δzyxwv |
| 25.5 | Santalene | 1417 | 1418 | 0.50 ± 0.17 Δzy | 0.36 ± 0.06 Δz | 0.51 ± 0.04 Δzy | 0.71 ± 0.10 Δzyx | 0.44 ± 0.05 Δzy | 0.39 ± 0.02 Δz |
| 25.7 | Caryophyllene | 1424 | 1428 | 2.22 ± 0.22 Δzyxwvutsrqp | 0.86 ± 0.07 Δzyxw | 0.66 ± 0.02 Δzy | 0.61 ± 0.04 Δzy | 1.14 ± 0.03 Δzyxwv | 2.33 ± 0.15 Δzyxwvutsrqpo |
| 26.2 | δ-Guaiene | 1452 | 1455 | 2.18 ± 0.95 Δzyxwvutsrqp | 1.54 ± 0.19 Δzyxwvtsr | 2.07 ± 0.13 Δzyxwvutsrqp | 2.89 ± 0.39 Δzyxwvutsrqpon | 1.67 ± 0.18 Δzyxwvtsr | 2.15 ± 0.08 Δzyxwvutsrqp |
| 26.8 | γ-Selinene | 1479 | 1484 | 0.34 ± 0.01 Δz | 0.22 ± 0.02 Δz | 0.25 ± 0.02 Δz | 0.21 ± 0.02 Δz | 0.38 ± 0.06 Δz | 0.50 ± 0.06 Δzy |
| 26.9 | β-Chamigrene | 1776 | 1488 | 0.38 ± 0.05 Δz | 0.19 ± 0.02 Δz | 0.14 ± 0.01 Δ | 1.41 ± 0.39 Δzyxwvtsr | 0.14 ± 0.02 Δ | 0.98 ± 0.11 Δzyxw |
| 26.9 | Germacrene D | 1481 | 1491 | 1.58 ± 0.13 Δzyxwvtsr | 0.58 ± 0.04 Δzy | 0.30 ± 0.02 Δz | 0.33 ± 0.16 Δz | 0.75 ± 0.09 Δzyx | 1.19 ± 0.06 Δzyxwv |
| 27.2 | α-Selinene | 1494 | 1504 | 7.76 ± 0.93 mlkji | 5.70 ± 0.03 usrqponmlk | 5.14 ± 0.37 wvutsrqponml | 6.67 ± 0.80 onmlk | 7.57 ± 0.88 mlkji | 9.78 ± 0.34 kjih |
| 27.2 | α-Muurolene | 1499 | 1508 | 0.27 ± 0.06 Δz | 0.40 ± 0.16 Δz | 0.34 ± 0.15 Δz | 0.49 ± 0.15 Δzy | 0.41 ± 0.11 Δz | 0.10 ± 0.01 Δ |
| 27.5 | γ i δ-Cadinene | 1513 | 1525 | 5.51 ± 2.19 uqponm | 2.55 ± 0.91 Δzyxwvutsrq | 2.08 ± 0.79 Δzyxwvtsr | 2.07 ± 0.74 Δzyxwvtsr | 2.51 ± 0.93 Δzyxwvutsrq | 2.93 ± 1.11 Δzyxwvutsrqpo |
| 1524 | 1534 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobina, M.; Łyczko, J.; Szumny, A.; Galek, R. Plant In Vitro Cultures of Coleus scutellarioides (L.) Benth. “Electric Lime” and Possibilities of Modification in the Biosynthesis of Volatile Compounds. Molecules 2024, 29, 2193. https://doi.org/10.3390/molecules29102193
Jakobina M, Łyczko J, Szumny A, Galek R. Plant In Vitro Cultures of Coleus scutellarioides (L.) Benth. “Electric Lime” and Possibilities of Modification in the Biosynthesis of Volatile Compounds. Molecules. 2024; 29(10):2193. https://doi.org/10.3390/molecules29102193
Chicago/Turabian StyleJakobina, Maciej, Jacek Łyczko, Antoni Szumny, and Renata Galek. 2024. "Plant In Vitro Cultures of Coleus scutellarioides (L.) Benth. “Electric Lime” and Possibilities of Modification in the Biosynthesis of Volatile Compounds" Molecules 29, no. 10: 2193. https://doi.org/10.3390/molecules29102193
APA StyleJakobina, M., Łyczko, J., Szumny, A., & Galek, R. (2024). Plant In Vitro Cultures of Coleus scutellarioides (L.) Benth. “Electric Lime” and Possibilities of Modification in the Biosynthesis of Volatile Compounds. Molecules, 29(10), 2193. https://doi.org/10.3390/molecules29102193










