Turn-on Coumarin Precursor: From Hydrazine Sensor to Covalent Inhibition and Fluorescence Detection of Rabbit Muscle Aldolase
Abstract
1. Introduction
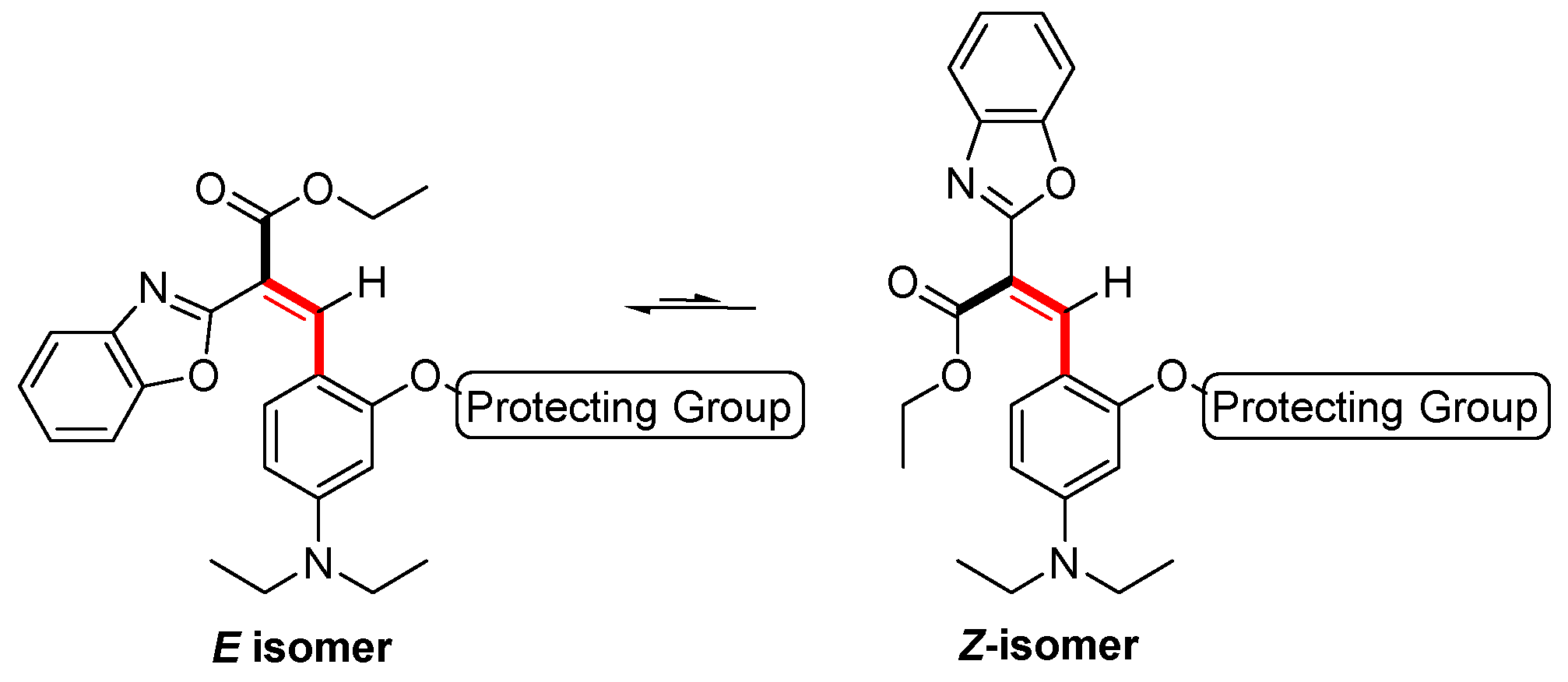
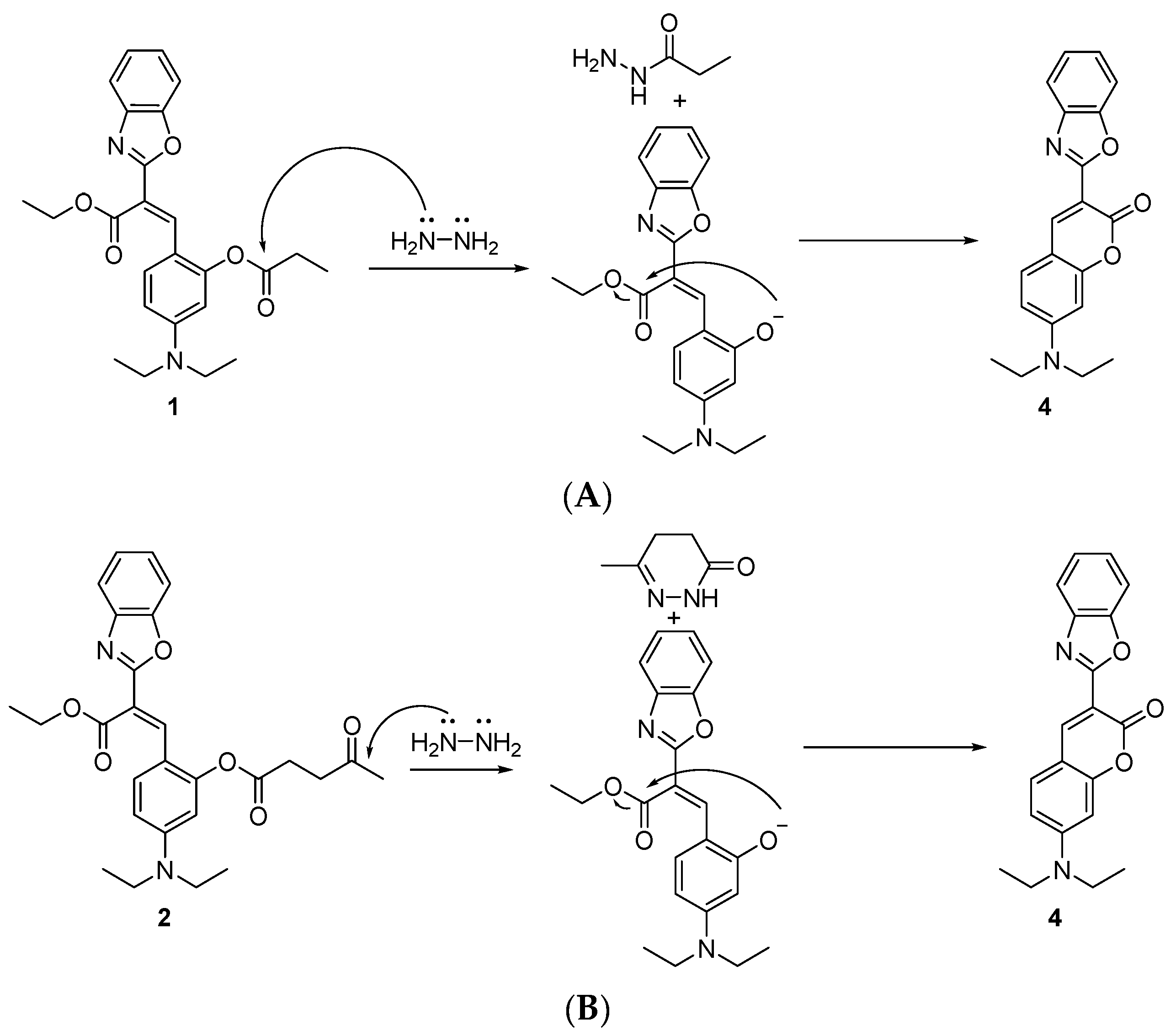
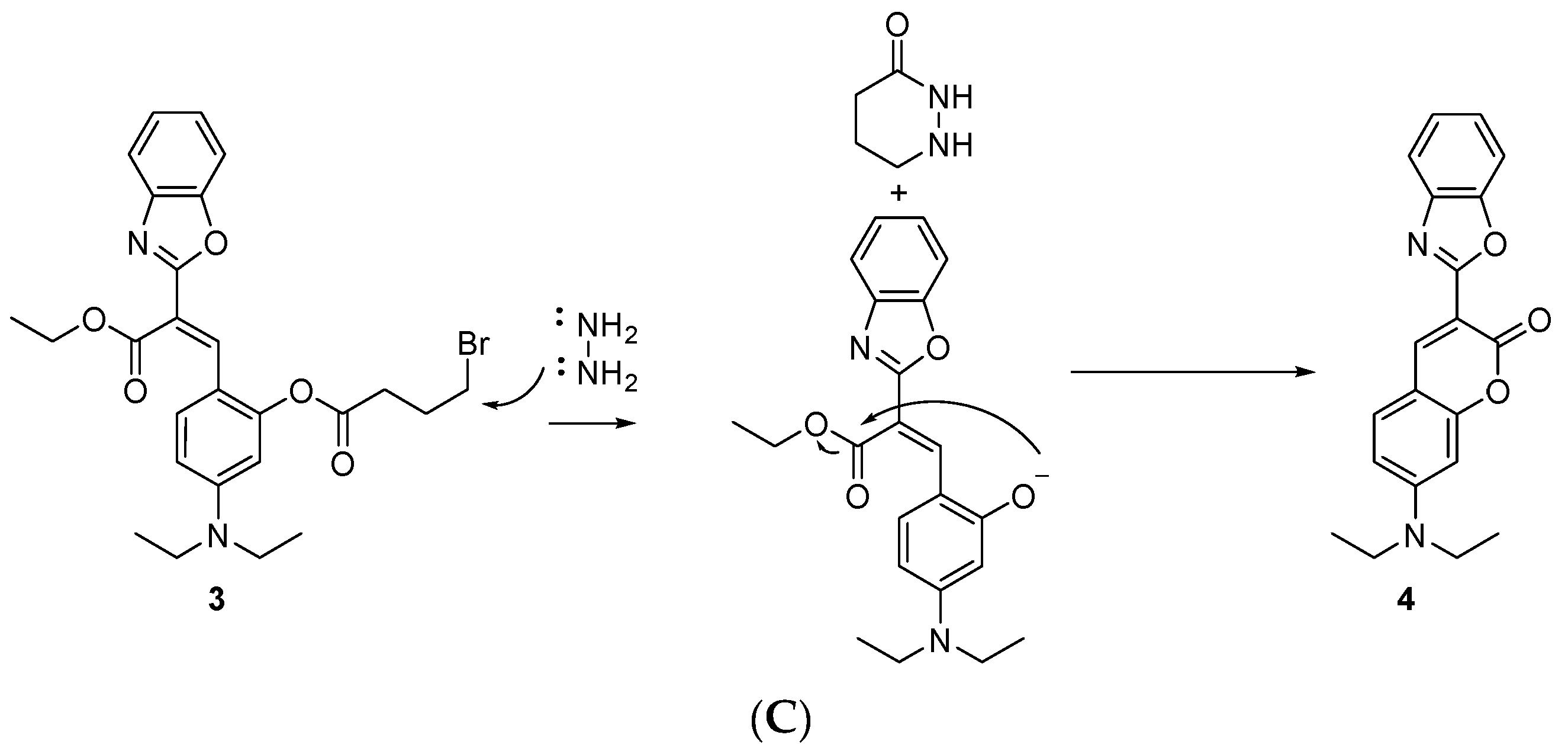
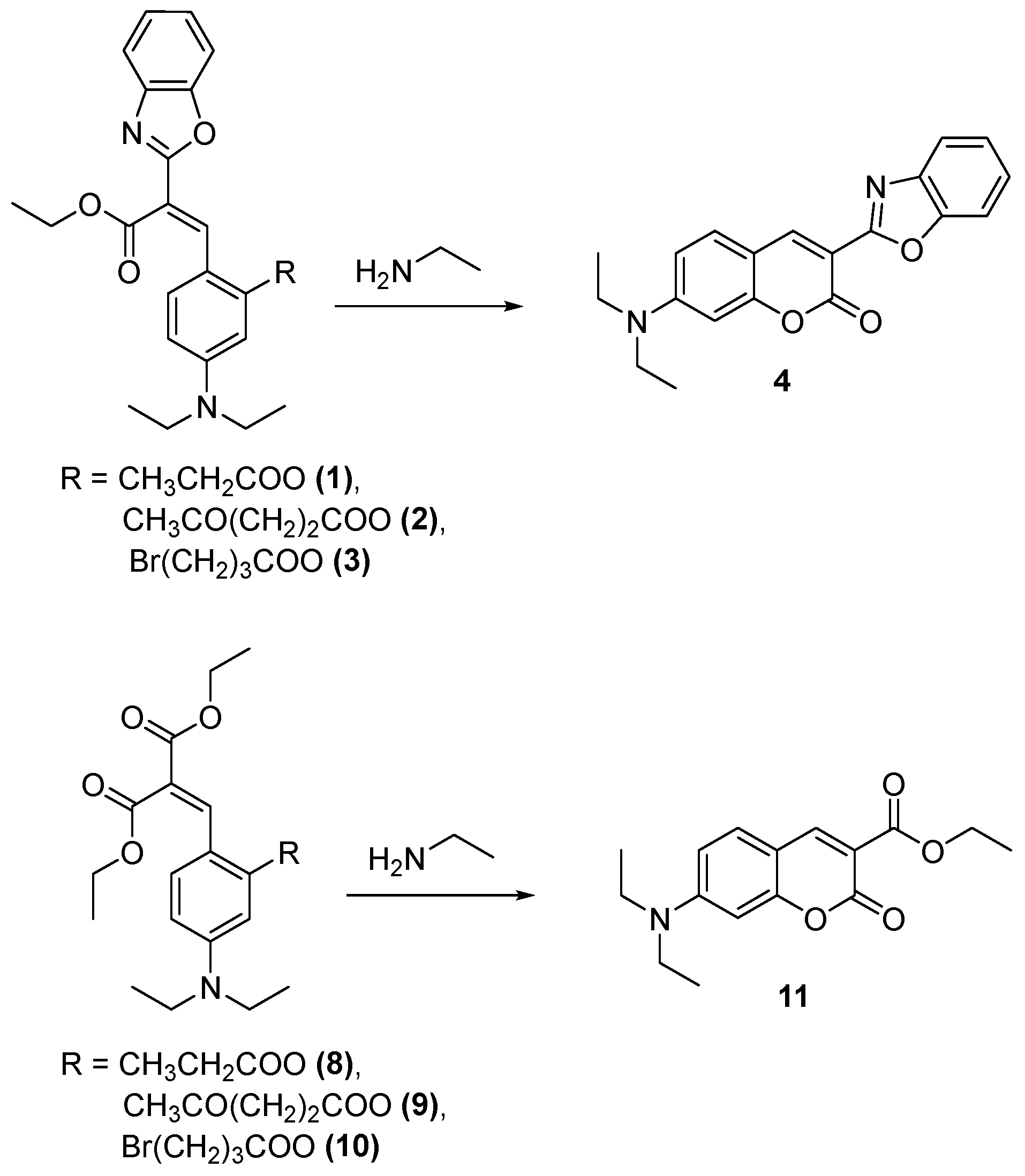
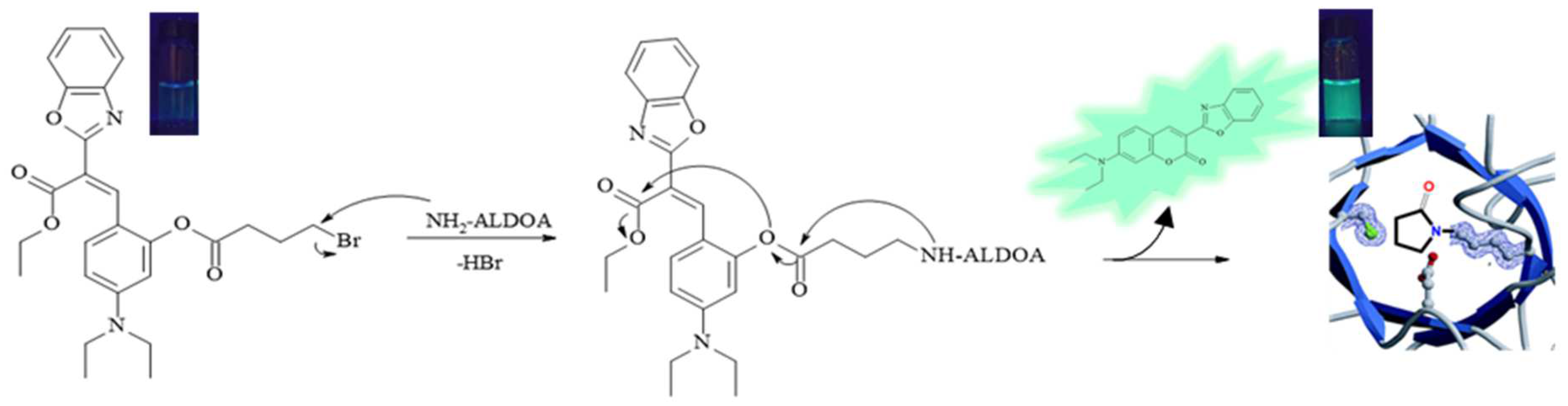
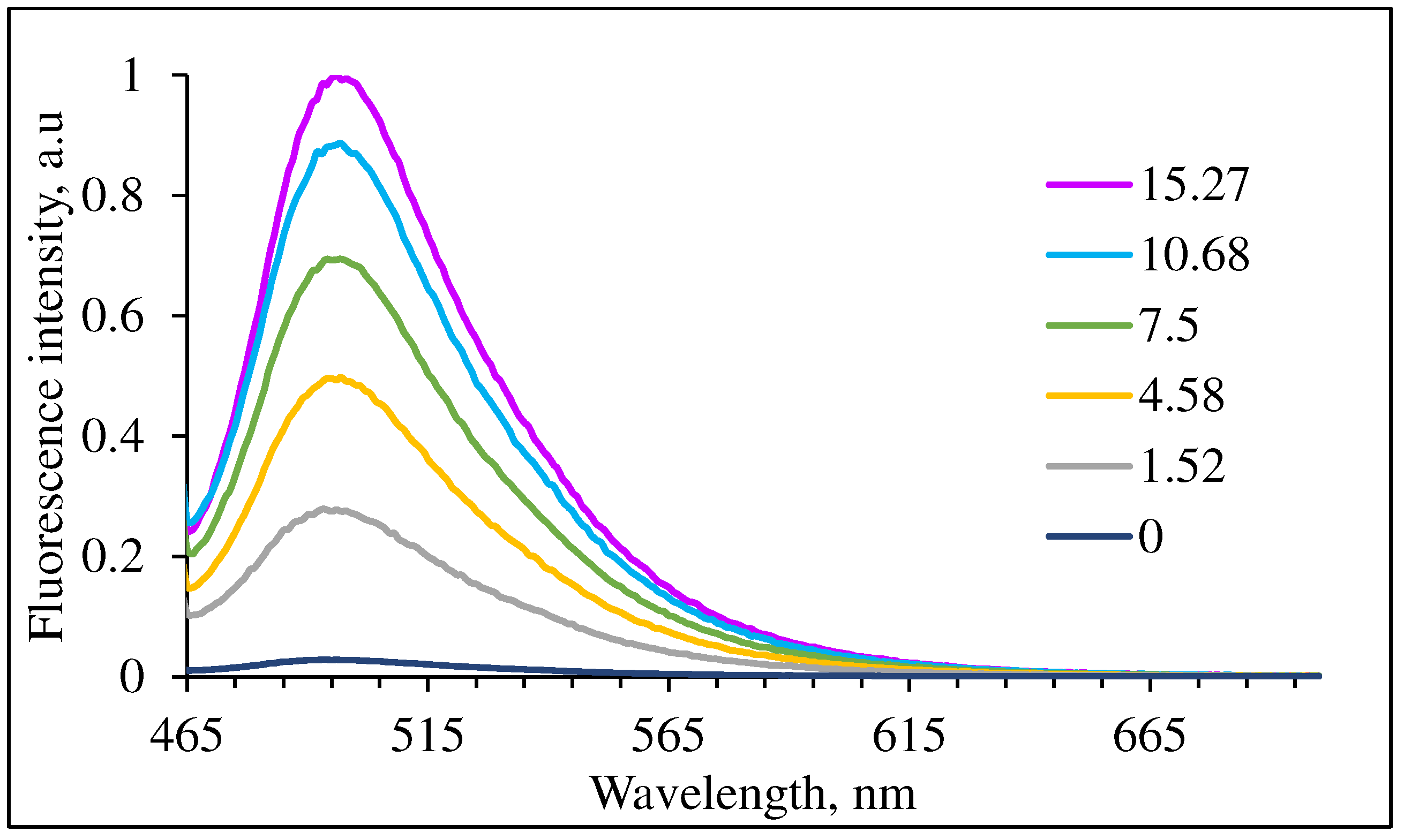
2. Results and Discussion

3. Materials and Methods
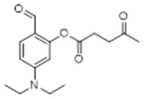
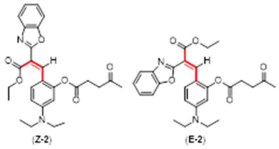
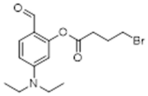
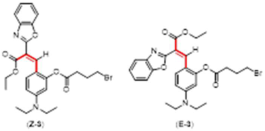
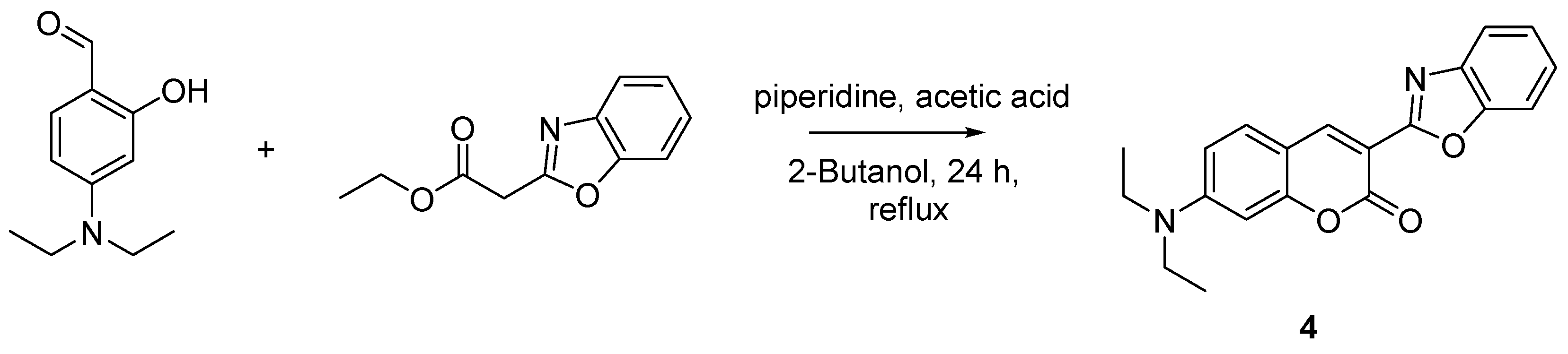
Synthetic Procedures
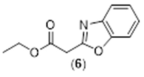
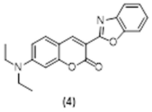
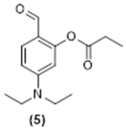
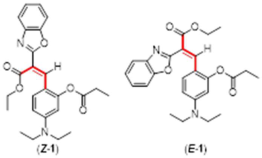
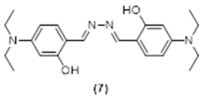
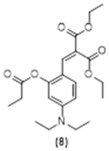
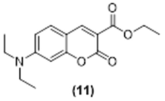
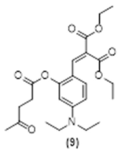

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Available online: https://wwwn.cdc.gov/TSP/ToxFAQs/ToxFAQsDetails.aspx?faqid=500&toxid=89 (accessed on 5 April 2024).
- Available online: https://www.epa.gov/sites/default/files/2016-09/documents/hydrazine.pdf (accessed on 5 April 2024).
- Narayanan, S.S.; Scholz, F. A Comparative Study of the Electrocatalytic Activities of Some Metal Hexacyanoferrates for the Oxidation of Hydrazine. Electroanalysis 1999, 11, 465–469. [Google Scholar] [CrossRef]
- Yamada, K.; Yasuda, K.; Fujiwara, N.; Siroma, Z.; Tanaka, H.; Miyazaki, Y.; Kobayashi, T. Potential Application of Anion-Exchange Membrane for Hydrazine Fuel Cell Electrolyte. Electrochem. Commun. 2003, 5, 892–896. [Google Scholar] [CrossRef]
- Ragnarsson, U. Synthetic Methodology for Alkyl Substituted Hydrazines. Chem. Soc. Rev. 2001, 30, 205–213. [Google Scholar] [CrossRef]
- Garrod, S.; Bollard, M.E.; Nicholls, A.W.; Connor, S.C.; Connelly, J.; Nicholson, J.K.; Holmes, E. Integrated Metabonomic Analysis of the Multiorgan Effects of Hydrazine Toxicity in the Rat. Chem. Res. Toxicol. 2005, 18, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Reilly, C.A.; Aust, S.D. Peroxidase Substrates Stimulate the Oxidation of Hydralazine to Metabolites Which Cause Single-Strand Breaks in DNA. Chem. Res. Toxicon. 1997, 10, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.W. Hydrazine and Its Derivatives: Preparation, Properties, Applications, 2 Volume Set, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Zhang, L.; Cheng, L. Advances in Optical Probes for the Detection of Hydrazine in Environmental and Biological Systems. Crit. Rev. Anal. Chem. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Yu, S.; Duan, L.; Meng, S.; Ding, S.; Dong, T. Fluorescence detection of hydrazine in an aqueous environment by a corrole derivative. Luminescence 2023, 38, 1968–1976. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.; Zhang, B.; Wang, J.; Fan, G.; Lu, X.; Zhang, J.; Zhao, W. Resorufin-based fluorescent probe for hydrazine detection and its application in environmental analysis and bioimaging. Anal. Methods 2023, 15, 6412–6416. [Google Scholar] [CrossRef]
- Xiao, L.; Tu, J.; Sun, S.; Pei, Z.; Pei, Y.; Pang, Y.; Xu, Y. A fluorescent probe for hydrazine and its in vivo applications. RSC Adv. 2014, 4, 41807–41811. [Google Scholar] [CrossRef]
- Ye, H.; Chen, L.; Wang, X.; Lu, D. A highly sensitive fluorescent probe for hydrazine detection: Synthesis, characterisation and application in living cells. Int. J. Environ. Anal. Chem. 2021, 101, 1086–1098. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Song, L.; Li, Z.; Qi, Q.; Huang, W. A hemicyanine-based fluorescent probe for hydrazine detection in aqueous solution and its application in real time bioimaging of hydrazine as a metabolite in mice. J. Mater. Chem. B 2019, 7, 3197–3200. [Google Scholar] [CrossRef]
- Tiensomjitr, K.; Noorat, R.; Chomngam, S.; Wechakorn, K.; Prabpai, S.; Kanjanasirirat, P.; Pewkliang, Y.; Borwornpinyo, S.; Kongsaeree, P. A Chromogenic and Fluorogenic Rhodol-Based Chemosensor for Hydrazine Detection and Its Application in Live Cell Bioimaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 195, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Aich, K.; Das, S.; Basu Roy, S.; Pakhira, B.; Sarkar, S. A Reaction Based Colorimetric as Well as Fluorescence “turn on” Probe for the Rapid Detection of Hydrazine. RSC Adv. 2014, 4, 14210–14214. [Google Scholar] [CrossRef]
- Ju, Z.; Li, D.; Zhang, D.; Li, D.; Wu, C.; Xu, Z. An ESIPT-Based Fluorescent Probe for Hydrazine Detection in Aqueous Solution and Its Application in Living Cells. J. Fluoresc. 2017, 27, 679–687. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, W.; Yuan, L. Development of a Near-Infrared Fluorescent Probe for Monitoring Hydrazine in Serum and Living Cells. Anal. Methods 2013, 5, 3450–3453. [Google Scholar] [CrossRef]
- Amer, S.; Joseph, V.; Oded, B.E.; Marks, V.; Grynszpan, F.; Levine, M. Shining light on fluoride detection: A comprehensive study exploring the potential of coumarin precursors as selective turn-on fluorescent chemosensors. Org. Biomol. Chem. 2023, 21, 9410–9415. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Liu, W.; Zhou, T.; Yang, Y.; Li, W. A Novel PBT-Based Fluorescent Probe for Hydrazine Detection and Its Application in Living Cells. J. Photochem. Photobiol. A Chem. 2018, 356, 610–616. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Dou, Y.; Zhang, F.; Liu, X.; Qu, J.; Zhu, Q. A Novel Chemiluminescent Probe for Hydrazine Detection in Water and HeLa Cells. Org. Biomol. Chem. 2019, 17, 6975–6979. [Google Scholar] [CrossRef]
- Blonski, C.; De Moissac, D.; Périé, J.; Sygusch, J. Inhibition of Rabbit Muscle Aldolase by Phosphorylated Aromatic Compounds. Biochem. J. 1997, 323, 71–77. [Google Scholar] [CrossRef]
- Gizak, A.; Wiśniewski, J.; Heron, P.; Mamczur, P.; Sygusch, J.; Rakus, D. Targeting a Moonlighting Function of Aldolase Induces Apoptosis in Cancer Cells. Cell Death Dis. 2019, 10, 712. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, C.; Luo, Y.; Lin, J.; Wang, F.; Sun, X.; Gao, Y.; Tan, W.; Xia, Q.; Kong, X. Aldolase A Enhances Intrahepatic Cholangiocarcinoma Proliferation and Invasion through Promoting Glycolysis. Int. J. Biol. Sci. 2021, 17, 1782–1794. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Lin, Z.; Wan, A.; Sun, L.; Yan, S.; Liang, H.; Zhan, S.; Chen, D.; Bu, X.; Liu, P.; et al. Loss-of-Function Genetic Screening Identifies Aldolase A as an Essential Driver for Liver Cancer Cell Growth Under Hypoxia. Hepatology 2021, 74, 1461–1479. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Verwilst, P.; Li, M.; Ma, D.; Singh, N.; Yoo, J.; Kim, Y.; Yang, Y.; Zhu, J.-H.; Huang, H.; et al. Theranostic Fluorescent Probes. Chem. Rev. 2024, 124, 2699–2804. [Google Scholar]
- Chen, X.W.; Huang, Z.X.; Huang, L.H.; Shen, Q.; Yang, N.D.; Pu, C.B.; Shao, J.J.; Li, L.; Yu, C.M.; Huang, W. Small molecule fluorescent probes based on covalent assembly strategy for chemoselective bioimaging. RSC Adv. 2022, 12, 1393–1415. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjugate Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Wutz, P.G.M. Greene’s Protective Groups in Organic Synthesis, 5th ed.; John Wiley and Sons: New York, NY, USA, 2014; ISBN 978-1-118-05748-3. [Google Scholar]
- Tang, Y.; Lee, D.; Wang, J.; Li, G.; Yu, J.; Lin, W.; Yoon, J. Development of fluorescent probes based on protection-deprotection of the key functional groups for biological imaging. Chem. Soc. Rev. 2015, 44, 5003–5015. [Google Scholar] [CrossRef]
- Maeda, H.; Yamamoto, K.; Kohno, I.; Hafsi, L.; Itoh, N.; Nakagawa, S.; Kanagawa, N.; Suzuki, K.; Uno, T. Design of a practical fluorescent probe for superoxide based on protection-deprotection chemistry of fluoresceins with benzenesulfonyl protecting groups. Chem. Eur. J. 2007, 13, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Li, Q.; Qin, J.; Li, Z. A new approach to design ratiometric fluorescent probe for mercury(II) based on the Hg2+-promoted deprotection of thioacetals. ACS Appl. Mater. Interfaces 2010, 2, 1066–1072. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Yang, Y.-S.; Wang, W.; Jiao, Q.-C.; Zhu, H.-L. Fluorescent sensors for the detection of hydrazine in environmental and biological systems: Recent advances and future prospects. Coord. Chem. Rev. 2020, 417, 213367. [Google Scholar] [CrossRef]
- Li, K.; Xu, H.R.; Yu, K.K.; Hou, J.T.; Yu, X.Q. A coumarin-based chromogenic and ratiometric probe for hydrazine. Anal. Methods 2013, 5, 2653–2656. [Google Scholar] [CrossRef]
- Choi, M.G.; Hwang, J.; Moon, J.O.; Sung, J.; Chang, S.K. Hydrazine-selective chromogenic and fluorogenic probe based on levulinated coumarin. Org. Lett. 2011, 13, 5260–5263. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Das, S.; Aich, K.; Pakhira, B.; Panja, S.; Mukherjee, S.K.; Sarkar, S. A chemodosimeter for the ratiometric detection of hydrazine based on return of ESIPT and its application in live-cell imaging. Org. Lett. 2013, 15, 5412–5415. [Google Scholar] [CrossRef]
- Jung, Y.; Jung, J.; Huh, Y.; Kim, D. Benzo[g]Coumarin-Based Fluorescent Probes for Bioimaging Applications. J. Anal. Methods Chem. 2018, 2018, 5249765. [Google Scholar] [CrossRef] [PubMed]
- da Hora Machado, A.E.; Severino, D.; Ribeiro, J.; De Paula, R.; Gehlen, M.H.; de Oliveira, H.P.M.; dos Santos Matos, M.; de Miranda, J.A. Effects on the Photophysics of 3-(Benzoxazol-2-Yl )-7-(N,N-Diethylamino) Chromen-2-One. Photochem. Photobiol. Sci. 2004, 3, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.K.; Gangopadhyay, A.; Maiti, K.; Mondal, S.; Mahapatra, A.K. Recent Developments in Fluorometric and Colorimetric Chemodosimeters Targeted towards Hydrazine Sensing: Present Success and Future Possibilities. Chem. Select 2019, 4, 7219–7245. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhuang, Z.; Song, L.L.; Lin, X.; Zhang, S.; Zheng, G.; Zhan, F. A FRET-Based Ratiometric Fluorescent Probe for Hydrazine and Its Application in Living Cells. J. Photochem. Photobiol. A Chem. 2018, 358, 10–16. [Google Scholar] [CrossRef]
- Chen, S.; Hou, P.; Wang, J.; Liu, L.; Zhang, Q. A Highly Selective Fluorescent Probe Based on Coumarin for the Imaging of N2H4 in Living Cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Xu, Z.; Song, C.; Qin, T.; Jia, T.; Zhao, C.; Wang, L.; Liu, B.; Peng, X. Naphthalene-based fluorescent probe for on-site detection of hydrazine in the environment. J. Hazard. Mat. 2023, 445, 130415. [Google Scholar] [CrossRef]
- Ma, X.; Cheng, J.; Liu, J.; Zhou, X.; Xiang, H. Ratiometric Fluorescent PH Probes Based on Aggregation-Induced Emission-Active Salicylaldehyde Azines. New J. Chem. 2015, 39, 492–500. [Google Scholar] [CrossRef]
- Stanton, C.L.; Houk, K.N. Benchmarking PK Prediction Methods for Residues in Proteins. J. Chem. Theory Comput. 2008, 4, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Crans, D.C.; Whitesides, G.M. Glycerol Kinase: Substrate Specificity. J. Am. Chem. Soc. 1985, 107, 7008–7018. [Google Scholar] [CrossRef]
- Hofer, F.; Kraml, J.; Kahler, U.; Kamenik, A.S.; Liedl, K.R. Catalytic Site pKa Values of Aspartic, Cysteine, and Serine Proteases: Constant PH MD Simulations. J. Chem. Inf. Model. 2020, 60, 3030–3042. [Google Scholar] [CrossRef]
- City, K. The Role of Cysteine Residues in the Catalytic Activity of Glycerol-3-Phosphate Dehydrogenase. Biochim. Biophys. Acta 1979, 567, 269–277. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; ISBN 978-0-387-31278-1. [Google Scholar]
- Brouwer, A.M. Standards for Photoluminescence Quantum Yield Measurements in Solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Fujita, K.; Urano, Y. Activity-Based Fluorescence Diagnostics for Cancer. Chem. Rev. 2024, 124, 4021–4078. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, S.; Miles, U.; Firer, M.; Grynszpan, F. Turn-on Coumarin Precursor: From Hydrazine Sensor to Covalent Inhibition and Fluorescence Detection of Rabbit Muscle Aldolase. Molecules 2024, 29, 2175. https://doi.org/10.3390/molecules29102175
Amer S, Miles U, Firer M, Grynszpan F. Turn-on Coumarin Precursor: From Hydrazine Sensor to Covalent Inhibition and Fluorescence Detection of Rabbit Muscle Aldolase. Molecules. 2024; 29(10):2175. https://doi.org/10.3390/molecules29102175
Chicago/Turabian StyleAmer, Sara, Uri Miles, Michael Firer, and Flavio Grynszpan. 2024. "Turn-on Coumarin Precursor: From Hydrazine Sensor to Covalent Inhibition and Fluorescence Detection of Rabbit Muscle Aldolase" Molecules 29, no. 10: 2175. https://doi.org/10.3390/molecules29102175
APA StyleAmer, S., Miles, U., Firer, M., & Grynszpan, F. (2024). Turn-on Coumarin Precursor: From Hydrazine Sensor to Covalent Inhibition and Fluorescence Detection of Rabbit Muscle Aldolase. Molecules, 29(10), 2175. https://doi.org/10.3390/molecules29102175







