Chemical Analysis of the Antihyperglycemic, and Pancreatic α-Amylase, Lipase, and Intestinal α-Glucosidase Inhibitory Activities of Cannabis sativa L. Seed Extracts
Abstract
:1. Introduction

2. Results
2.1. High-Performance Liquid Chromatography HPLC
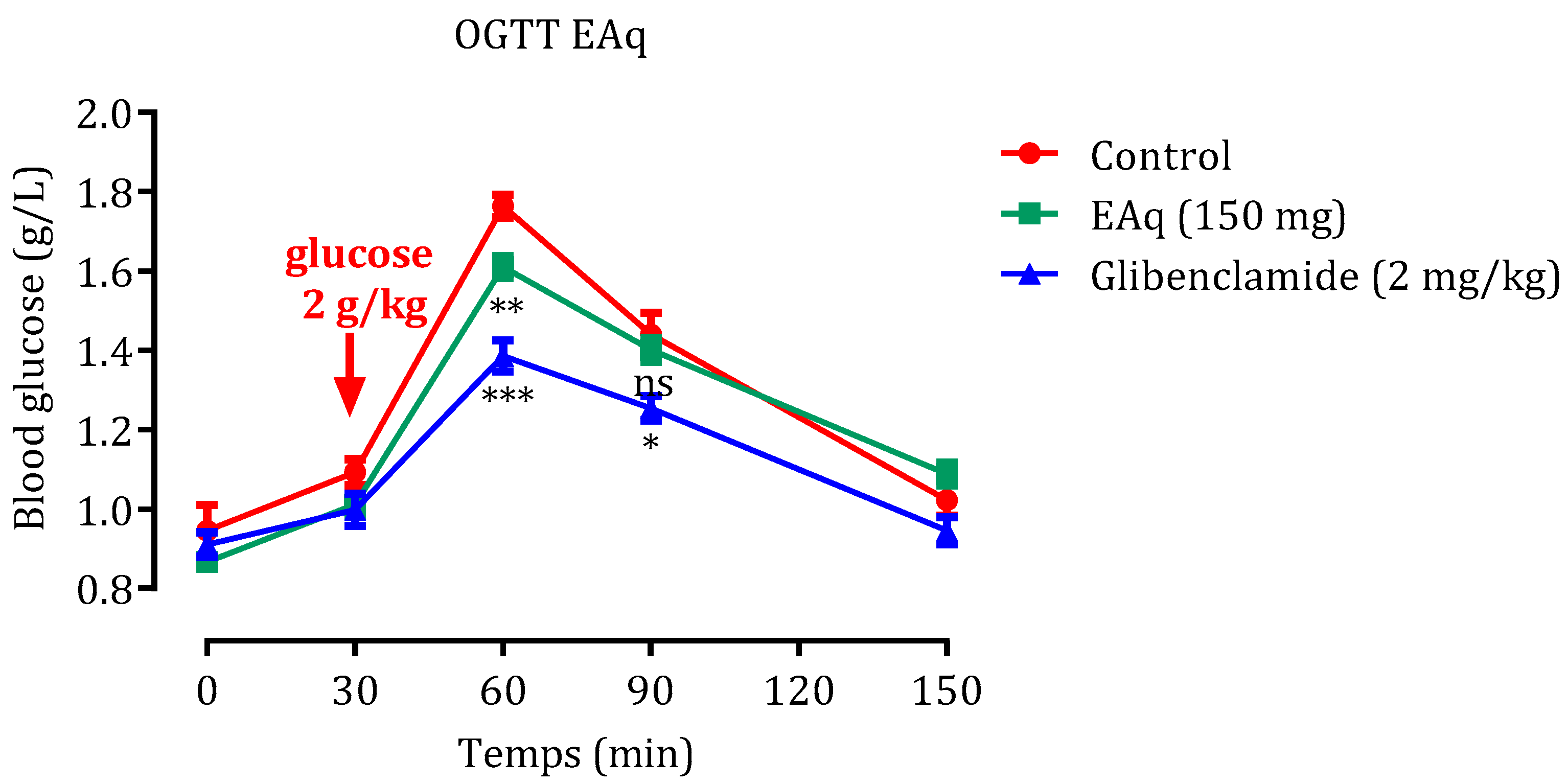
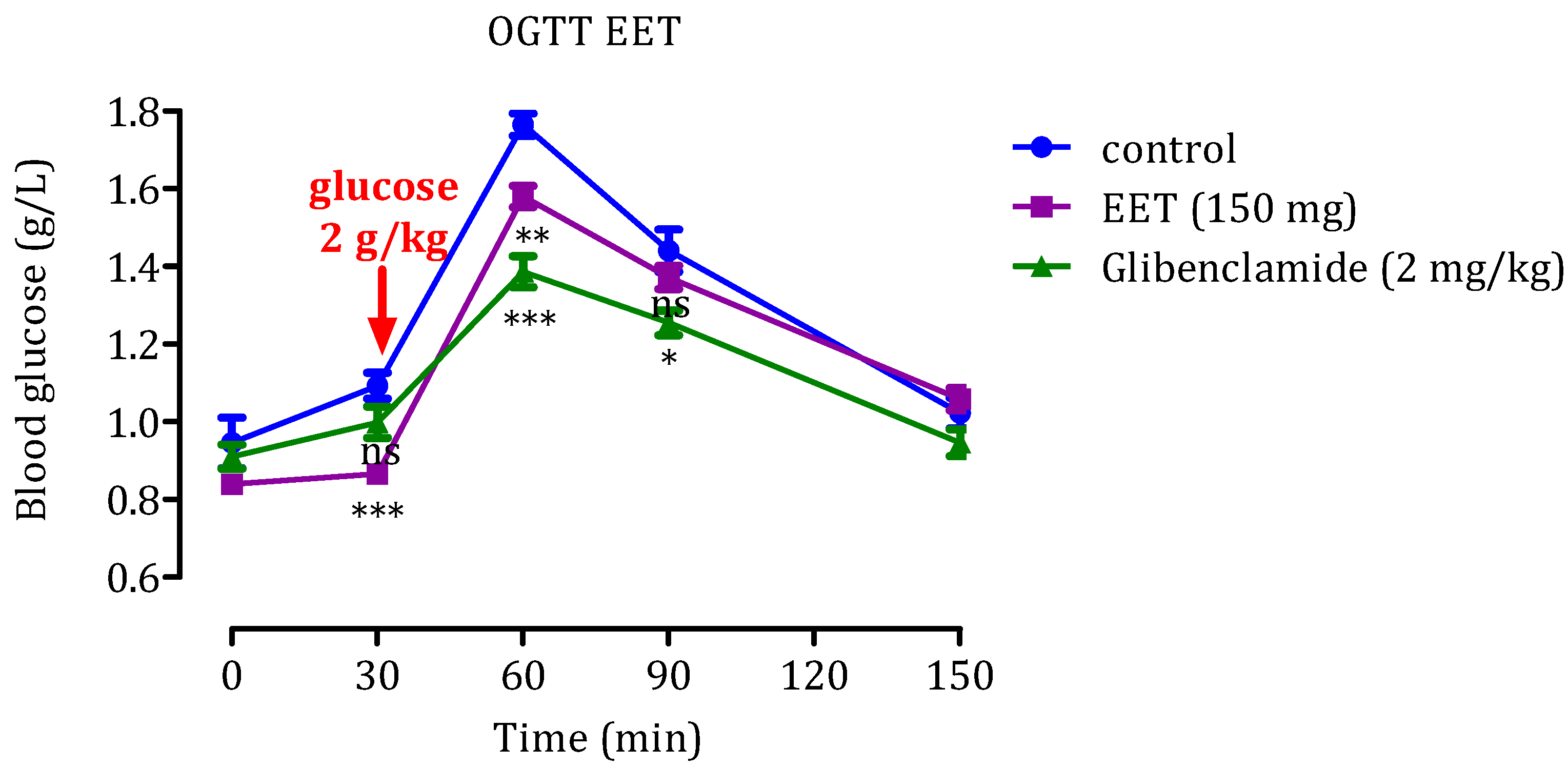
2.2. Oral Glucose Tolerance Test (OGTT)
2.2.1. Effect of EAq
2.2.2. Effect of EET
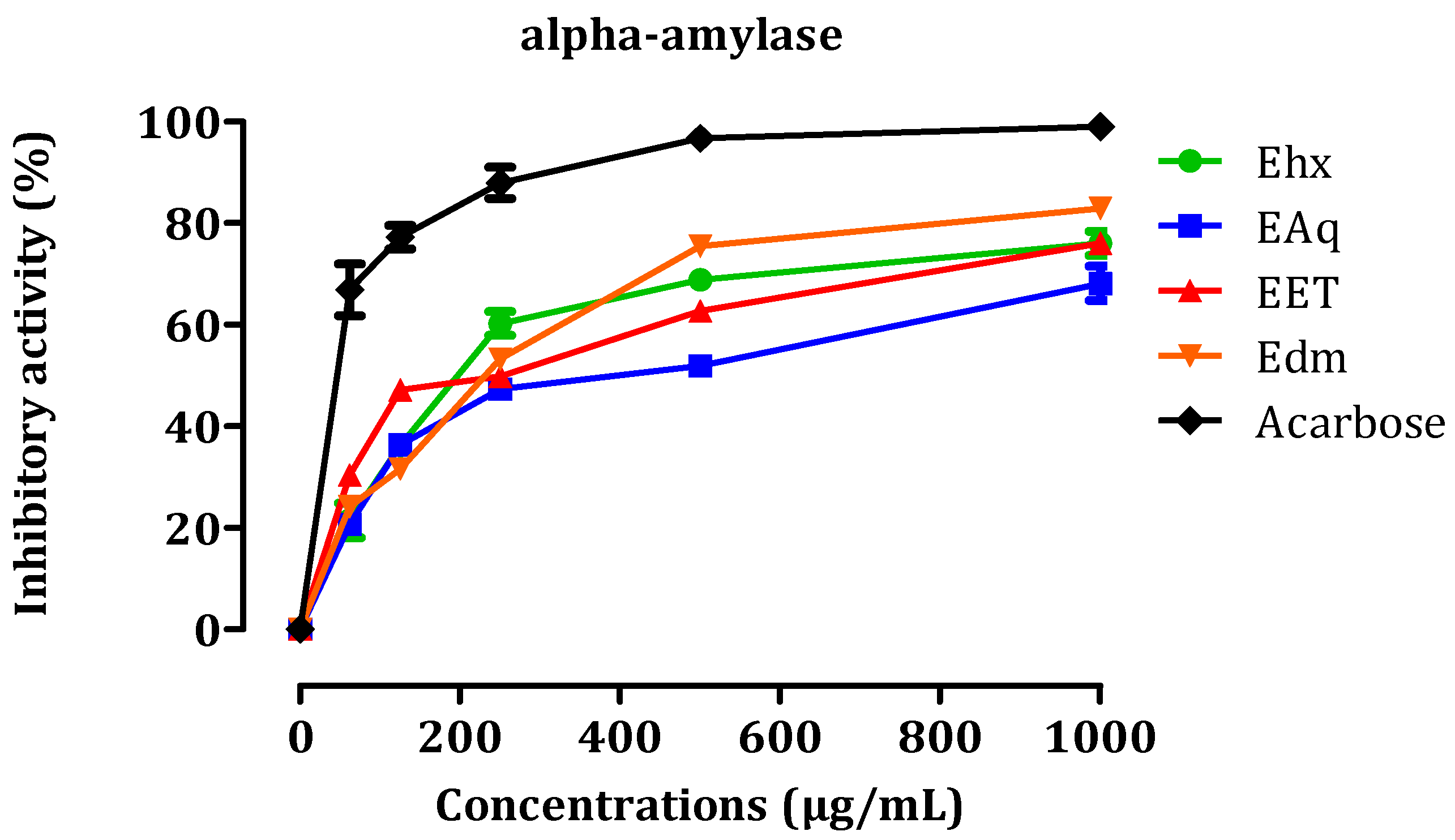
2.3. In Vitro Inhibition of Pancreatic α-Amylase and Lipase and Intestinal α-Glucosidase Enzymatic Activities
2.3.1. In Vitro, α-Amylase Inhibitory Activity
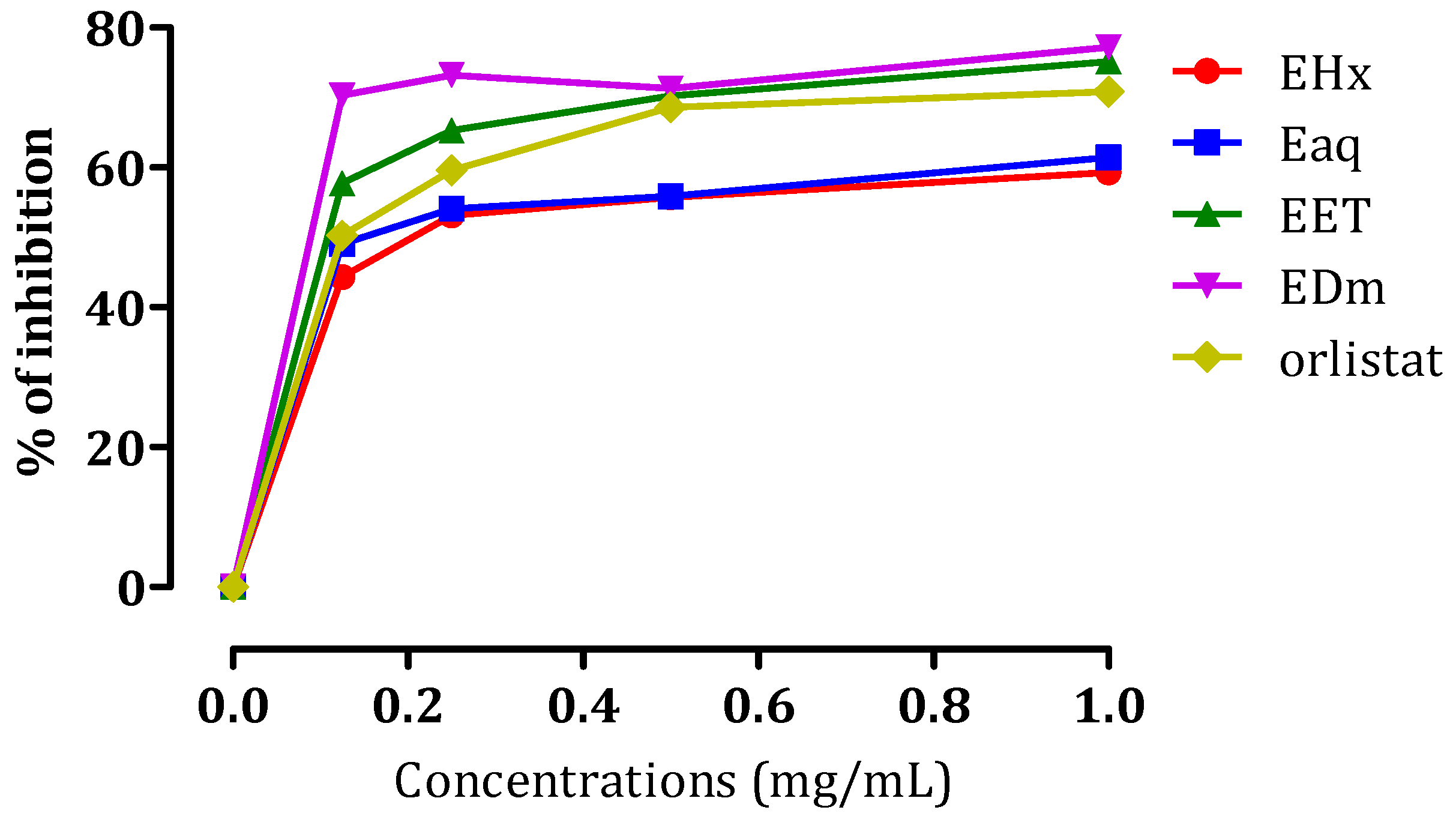
2.3.2. In Vitro Pancreatic Lipase Inhibitory Effect
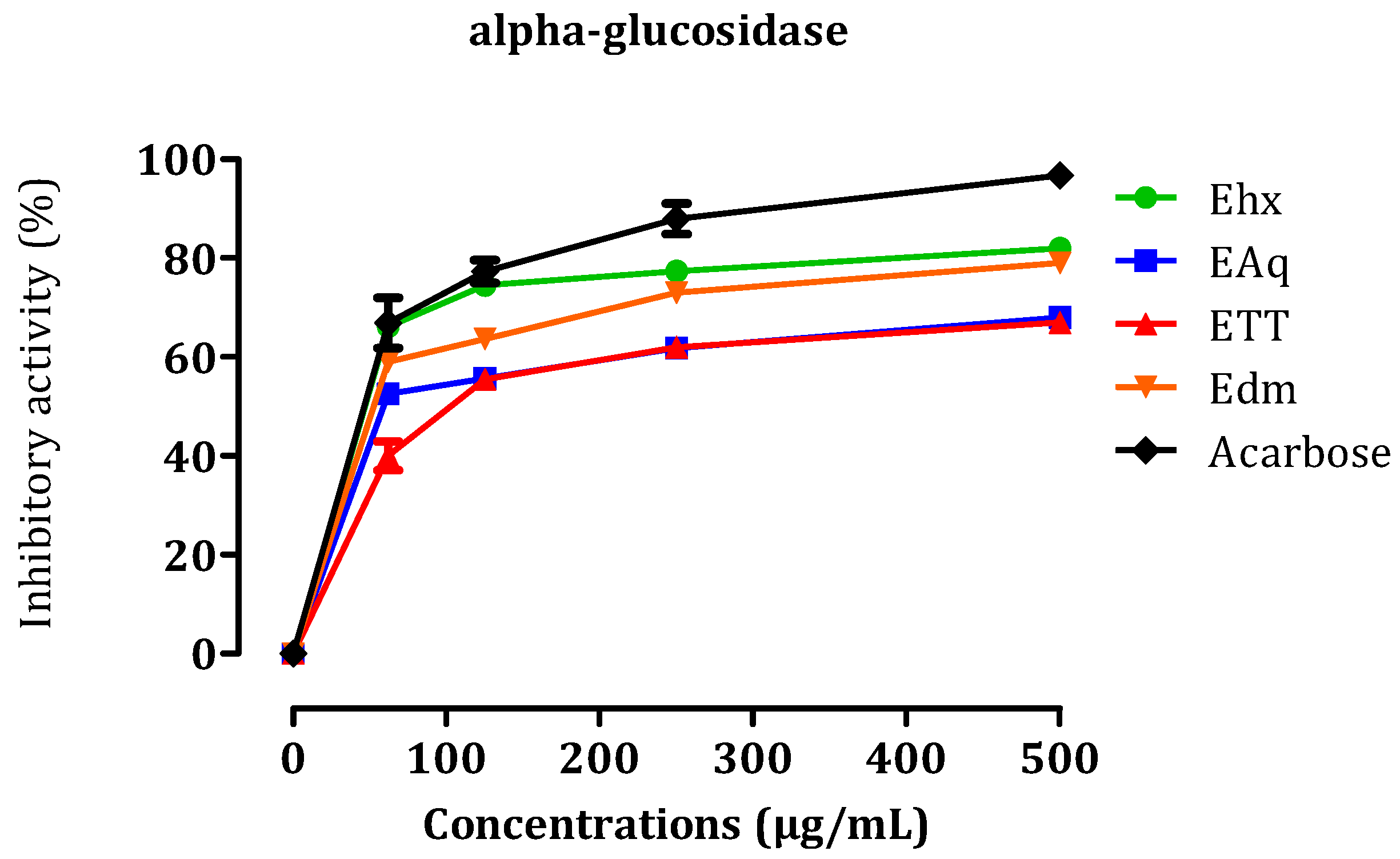
2.3.3. In Vitro α-Glucosidase Inhibitory Effect
2.4. Inhibition of Pancreatic α-Amylase and Intestinal α-Glucosidase In Vivo
2.4.1. Effect of the EAq
2.4.2. Effect of the EET
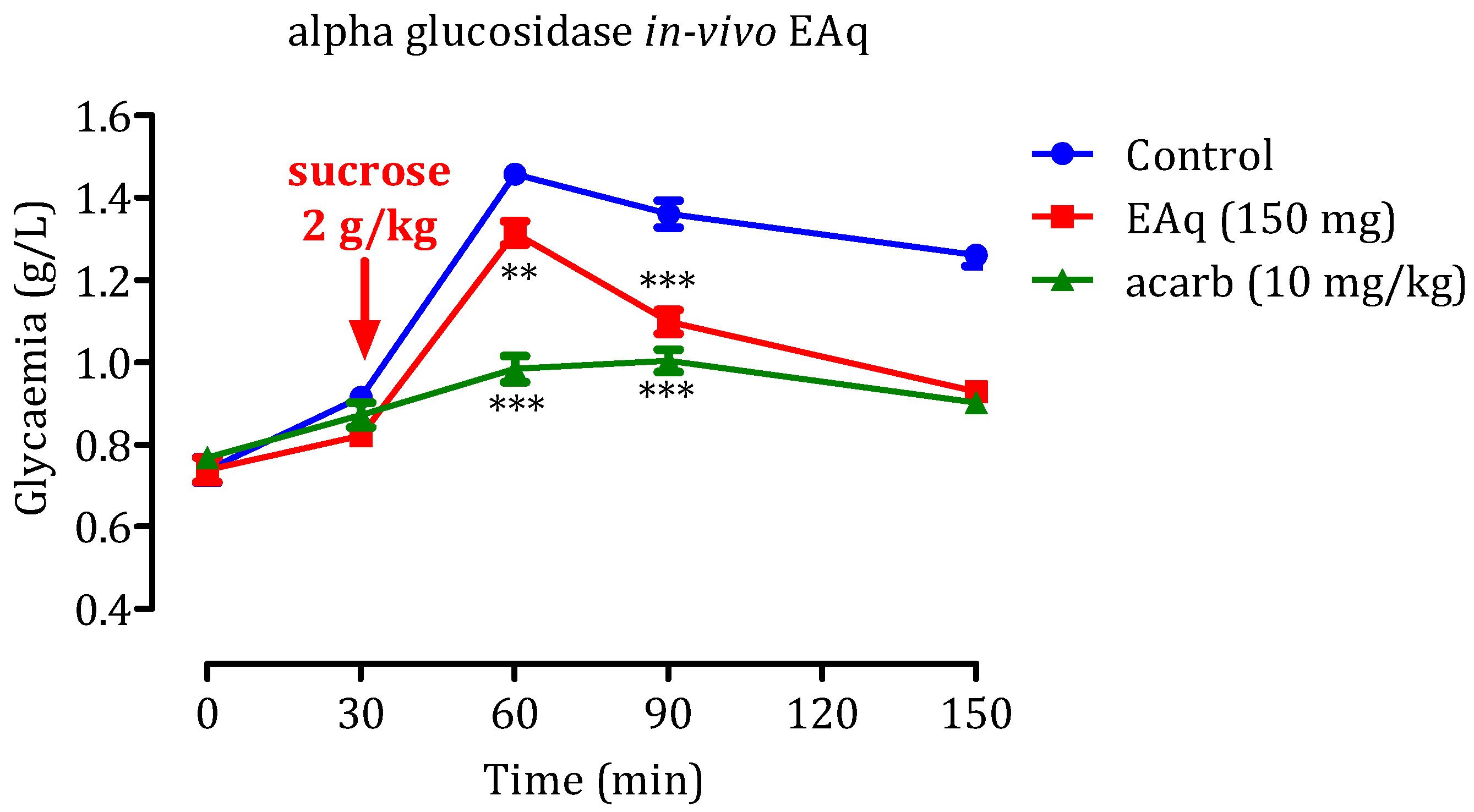
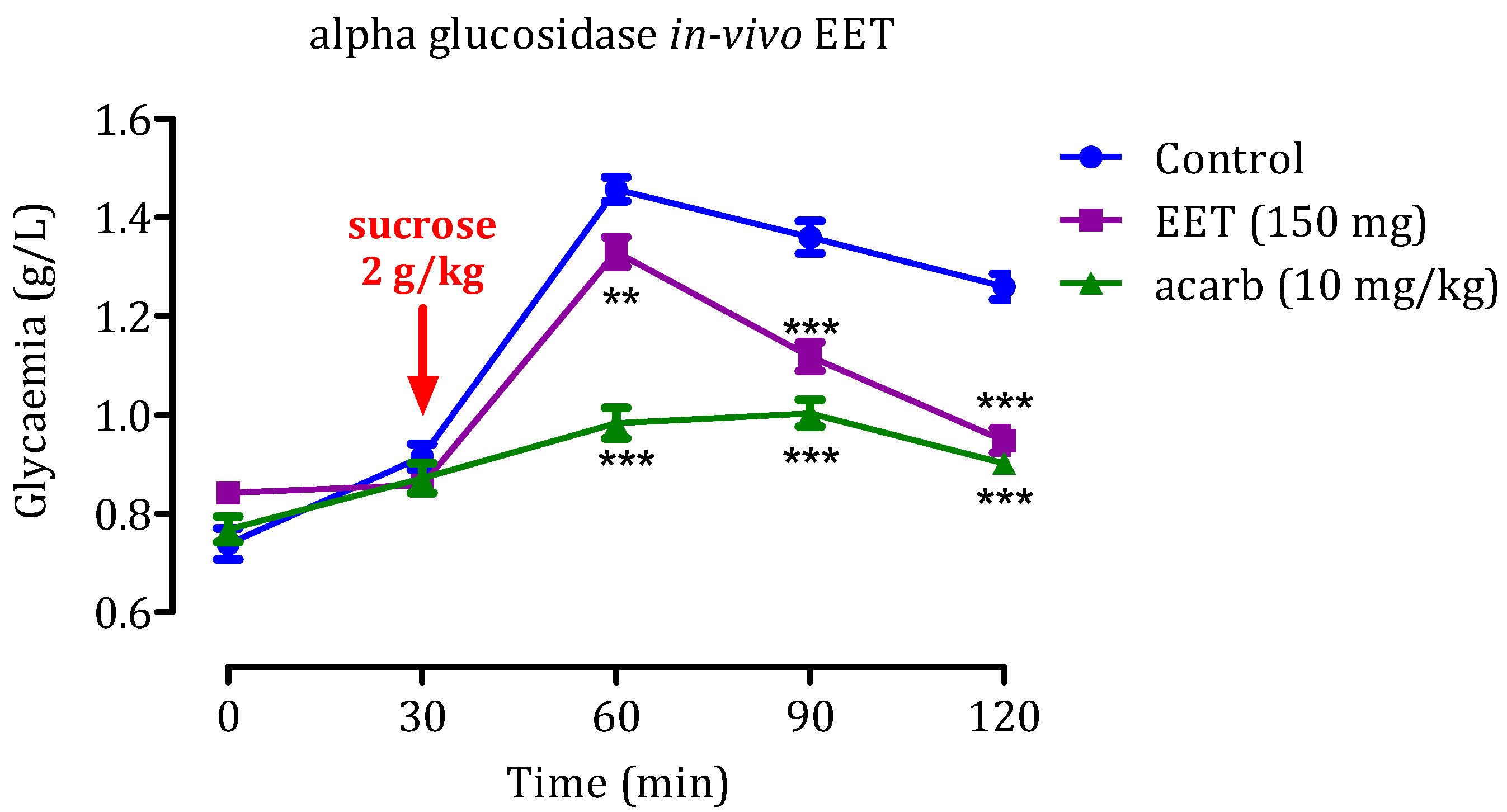
2.5. In Vivo α-Glucosidase Inhibitory Effect
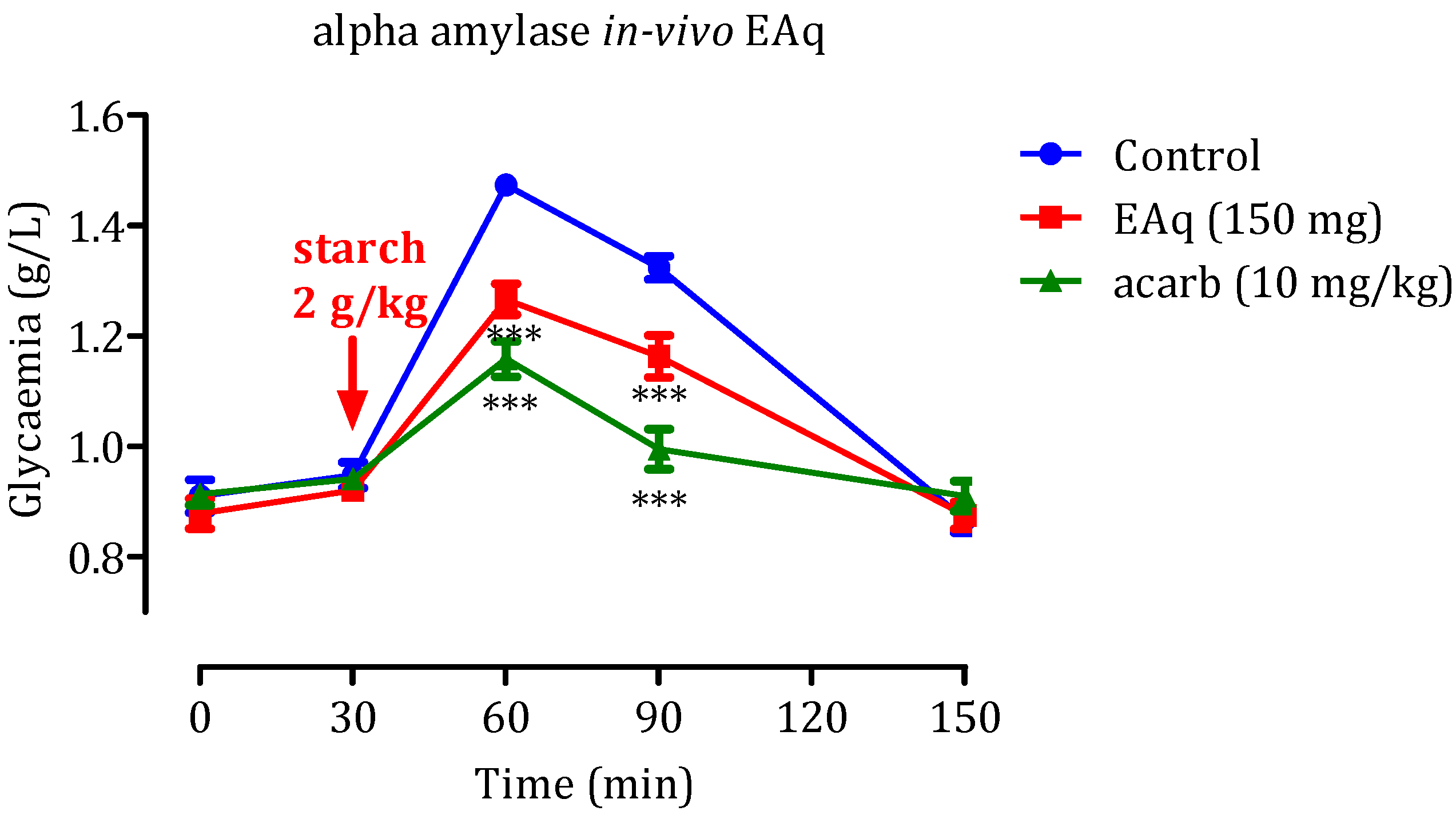
2.5.1. Effect of the EAq
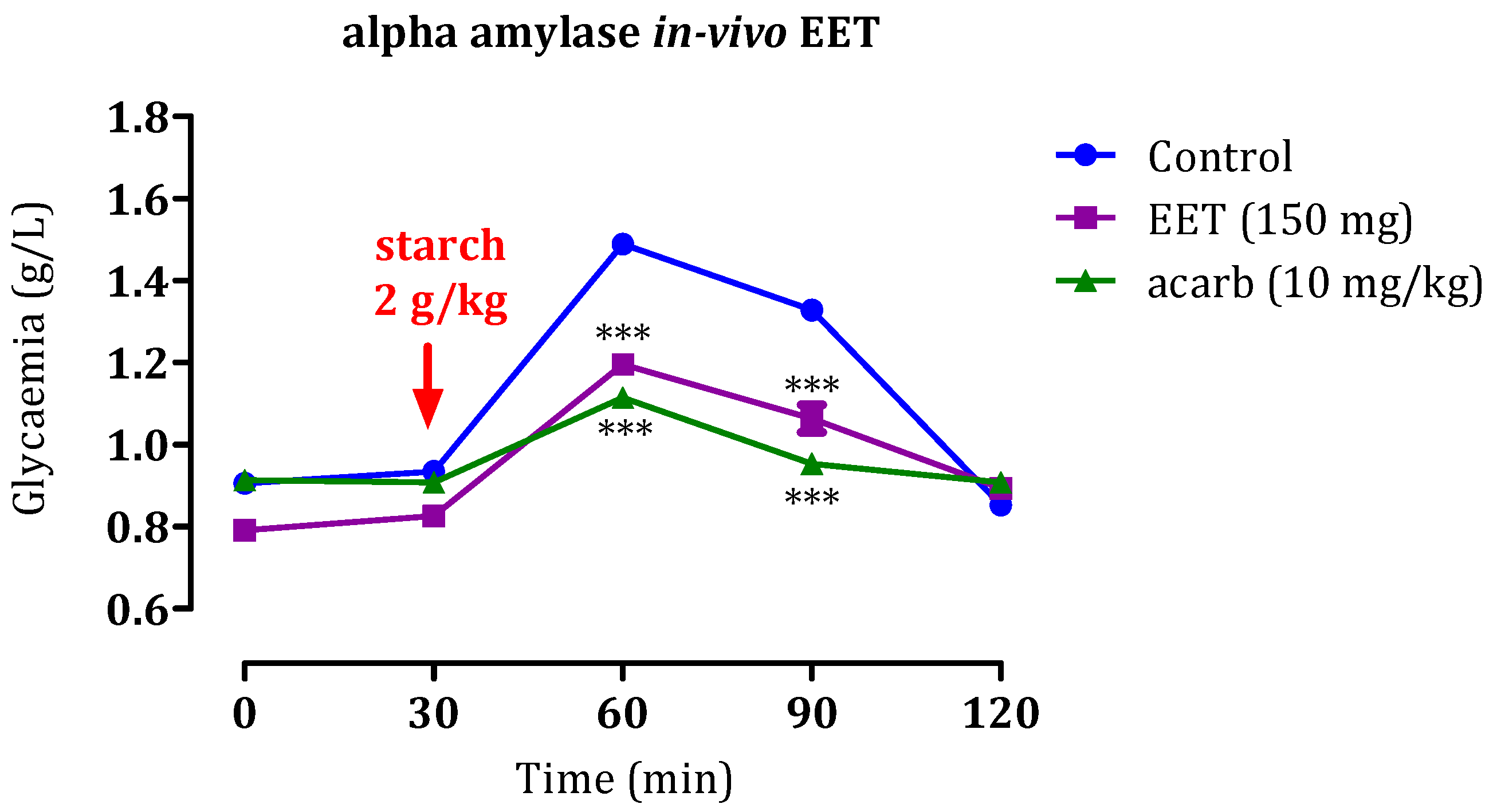
2.5.2. Impact of EET
3. Materials and Methods
3.1. Plant Materials and Extraction
3.2. Experimental Animals
3.3. High-Performance Liquid Chromatography Analysis
3.4. Antihyperglycemic Effect of C. sativa L.
Oral Glucose Tolerance Test
3.5. In Vitro Inhibition of Carbohydrate Hydrolase Enzymes
Inhibition of Pancreatic α-Amylase Assessed In Vitro
3.6. Inhibition of Intestinal α-Glucosidase Assessed In Vitro
Pancreatic Lipase Inhibitory Test
3.7. In Vivo Inhibition of Carbohydrate Hydrolase Enzymes
In Vivo Pancreatic α-Amylase Inhibitory Assay
3.8. Inhibition of Intestinal α-Glucosidase In Vivo
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abuzaytoun, R.; Shahidi, F. Oxidative stability of flax and hemp oils. J. Am. Oil Chem. Soc. 2006, 83, 855–861. [Google Scholar] [CrossRef]
- Karche, T. The application of hemp (Cannabis sativa L.) for a green economy: A review. Turk. J. Bot. 2019, 43, 710–723. [Google Scholar] [CrossRef]
- Zuardi, A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006, 28, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Essien, E.E. Effect of extraction conditions on total polyphenol contents antioxidant antimicrobial activities of Cannabis sativa L. Electron. J. Environ. Agric. Food Chem. 2011, 11, 300–307. [Google Scholar]
- Wasim, K.; Haq, I.; Ashraf, M. Antimicrobial studies of the leaf of Cannabis sativa L. Pak. J. Pharm. Sci. 1995, 8, 29–38. [Google Scholar] [PubMed]
- Formukong, E.A.; Evans, A.T.; Evans, F.J. Analgesic and antiinflammatory activity of constituents of Cannabis sativa L. Inflammation 1988, 12, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.M.M.; Almagboul, A.Z.I.; Khogali, S.M.E.; Gergeir, U.M.A. Antimicrobial Activity of Cannabis sativa L. Chin. Med. 2012, 3, 61–64. [Google Scholar] [CrossRef]
- Ramer, R.; Hinz, B. Cannabinoids as anticancer drugs. Adv. Pharmacol. 2017, 80, 397–436. [Google Scholar]
- Velasco, G.; Sánchez, C.; Guzmán, M. Towards the use of cannabinoids as antitumour agents. Nat. Rev. Cancer 2012, 12, 436–444. [Google Scholar] [CrossRef]
- McAllister, S.D.; Abood, M.E.; Califano, J.; Guzmán, M. Cannabinoid Cancer Biology and Prevention. JNCI Monogr. 2021, 2021, 99–106. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Bautista, J.L.; Yu, S.; Tian, L. Flavonoids in Cannabis sativa: Biosynthesis, bioactivities, and biotechnology. ACS Omega 2021, 6, 5119–5123. [Google Scholar] [CrossRef] [PubMed]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Retinoic Acids in the Treatment of Most Lethal Solid Cancers. J. Clin. Med. 2020, 9, 360. [Google Scholar] [CrossRef]
- Shen, P.; Gao, Z.; Fang, B.; Rao, J.; Chen, B. Ferreting out the secrets of industrial hemp protein as emerging functional food ingredients. Trends Food Sci. Technol. 2021, 112, 1–15. [Google Scholar] [CrossRef]
- House, J.D.; Neufeld, J.; Leson, G. Evaluating the Quality of Protein from Hemp Seed (Cannabis sativa L.) Products through the use of the Protein Digestibility-Corrected Amino Acid Score Method. J. Agric. Food Chem. 2010, 58, 11801–11807. [Google Scholar] [CrossRef]
- Ruman, M.; Klvaňová, L. Konopí: Staronový Přítel Člověka; Konopa; 2008; Available online: https://www.databazeknih.cz/knihy/konopi-staronovy-pritel-cloveka-57347 (accessed on 10 December 2023).
- Kakudidi, E.; Tugume, P.; Asiimwe, S.; Anywar, G. Traditional modern Health uses of Cannabis sativa L. In africa and its phytochemical and pharmacological profile. Cannabis Marijuana Healthc. 2022, 189–210. [Google Scholar]
- VanderJagt, T.; Ghattas, R.; VanderJagt, D.; Crossey, M.; Glew, R. Comparison of the total antioxidant content of 30 widely used medicinal plants of New Mexico. Life Sci. 2002, 70, 1035–1040. [Google Scholar] [CrossRef]
- Haddou, S.; Mounime, K.; Loukili, E.; Ou-Yahia, D.; Hbika, A.; Idrissi, M.Y.; Legssyer, A.; Lgaz, H.; Asehraou, A.; Touzani, R.; et al. Investigating the Biological Activities of Moroccan Cannabis sativa L. Seed Extracts: Antimicrobial, Anti-inflammatory, and Antioxidant Effects with Molecular Docking Analysis. Moroc. J. Chem. 2023, 11, 1116–1136. [Google Scholar]
- Gowd, V.; Bao, T.; Wang, L.; Huang, Y.; Chen, S.; Zheng, X.; Cui, S.; Chen, W. Antioxidant and antidiabetic activity of blackberry after gastrointestinal digestion and human gut microbiota fermentation. Food Chem. 2018, 269, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Uddin, N.; Hasan, M.R.; Hossain, M.M.; Sarker, A.; Hasan, A.N.; Islam, A.M.; Chowdhury, M.M.H.; Rana, M.S. In vitro α–amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit. Asian Pac. J. Trop. Biomed. 2014, 4, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Harrower, A.D.B. Comparison of efficacy, secondary failure rate, and complications of sulfonylureas. J. Diabetes Its Complicat. 1994, 8, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.K.; White, J.R., Jr.; Saulie, B.A. Metformin: A new oral biguanide. Clin. Ther. 1996, 18, 360–371, discussion 359. [Google Scholar] [CrossRef] [PubMed]
- Haddou, S.; Loukili, E.H.; Hbika, A.; Chahine, A.; Hammouti, B. Phytochemical study using HPLC-UV/GC–MS of different of Cannabis sativa L. seeds extracts from Morocco. Mater. Today Proc. 2023, 72, 3896–3903. [Google Scholar] [CrossRef]
- Benkirane, C.; Moumen, A.B.; Fauconnier, M.L.; Belhaj, K.; Abid, M.; Caid, H.S.; Elamrani, A.; Mansouri, F. Bioactive compounds from hemp (Cannabis sativa L.) seeds: Optimization of phenolic antioxidant extraction using simplex lattice mixture design and HPLC-DAD/ESI-MS 2 analysis. RSC Adv. 2022, 12, 25764–25777. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.M.; Choi, J.Y.; Bae, I.A.; Kim, H.T.; Hong, S.S.; Noah, J.K.; Boo, Y.C. Identification of p-coumaric acid and ethyl p-coumarate as the main phenolic components of hemp (Cannabis sativa L.) roots. Molecules 2022, 27, 2781. [Google Scholar] [CrossRef]
- Shobana, S.; Sreerama, Y.; Malleshi, N. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: Mode of inhibition of α-glucosidase and pancreatic amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Grussu, D.; Stewart, D.; McDougall, G.J. Berry Polyphenols Inhibit α-Amylase in Vitro: Identifying Active Components in Rowanberry and Raspberry. J. Agric. Food Chem. 2011, 59, 2324–2331. [Google Scholar] [CrossRef]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The α-amylase and α-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef]
- Tehranipour, M.; Shahri, N.M.; Ekrami, A. Antidiabetic effect of Cannabis sativa extract in wister rat. Int. J. Chem. Pharm. Res. 2012, 1, 124–130. [Google Scholar]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic Analyses, In Vitro Biological Activities, and Cytotoxicity of Cannabis sativa L. Essential Oil: A Multidisciplinary Study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Aji, O.R.; Hudaya, R.A.; Putri, D.A. In Vitro Pancreatic Lipase Inhibitor Activity of Mangifera foetida Leaves Extract. J. Biodjati 2021, 6, 82–92. [Google Scholar] [CrossRef]
- Pirahanchi, Y.; Sharma, S. Lipase; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Bauer, E.; Jakob, S.; Mosenthin, R. Principles of Physiology of Lipid Digestion. Asian-Australas. J. Anim. Sci. 2005, 18, 282–295. [Google Scholar] [CrossRef]
- Liu, T.-T.; Liu, X.-T.; Chen, Q.-X.; Shi, Y. Lipase Inhibitors for Obesity: A Review. Biomed. Pharmacother. 2020, 128, 110314. [Google Scholar] [CrossRef]
- Bajes, H.R.; Almasri, I.; Bustanji, Y. Plant Products and Their Inhibitory Activity Against Pancreatic Lipase. Rev. Bras. Farm. 2020, 30, 321–330. [Google Scholar] [CrossRef]
- Mazzara, E.; Carletti, R.; Petrelli, R.; Mustafa, A.M.; Caprioli, G.; Fiorini, D.; Scortichini, S.; Dall’Acqua, S.; Sut, S.; Nuñez, S.; et al. Green extraction of hemp (Cannabis sativa L.) using microwave method for recovery of three valuable fractions (essential oil, phenolic compounds and cannabinoids): A central composite design optimization study. J. Sci. Food Agric. 2022, 102, 6220–6235. [Google Scholar] [CrossRef]
- Suttithumsatid, W.; Shah, M.A.; Bibi, S.; Panichayupakaranant, P. α-Glucosidase inhibitory activity of cannabidiol, tetrahydrocannabinol and standardized cannabinoid extracts from Cannabis sativa. Curr. Res. Food Sci. 2022, 5, 1091–1097. [Google Scholar] [CrossRef]
- Grover, J.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Amalan, V.; Vijayakumar, N.; Indumathi, D.; Ramakrishnan, A. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacother. 2016, 84, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Peungvicha, P.; Thirawarapan, S.S.; Watanabe, H. Possible Mechanism of Hypoglycemic Effect of 4-Hydroxybenzoic Acid, a Constituent of Pandanus odorus Root. Jpn. J. Pharmacol. 1998, 78, 395–398. [Google Scholar] [CrossRef]
- Lu, Q.; Hao, M.; Wu, W.; Zhang, N.; Isaac, A.T.; Yin, J.; Zhu, X.; Du, L.; Yin, X. Antidiabetic cataract effects of GbE, rutin and quercetin are mediated by the inhibition of oxidative stress and polyol pathway. Acta Biochim. Pol. 2018, 65, 35–41. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US). Committee for the Update of the Guide for the Care and Use of Laboratory Animals. In Studies, Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Loukili, E.H.; Bouchal, B.; Bouhrim, M.; Abrigach, F.; Genva, M.; Zidi, K.; Bnouham, M.; Bellaoui, M.; Hammouti, B.; Addi, M.; et al. Chemical Composition, Antibacterial, Antifungal and Antidiabetic Activities of Ethanolic Extracts of Opuntia dillenii Fruits Collected from Morocco. J. Food Qual. 2022, 2022, 9471239. [Google Scholar] [CrossRef]
- Laaroussi, H.; Aouniti, A.; Hafez, B.; Mokhtari, O.; Sheikh, R.A.; Hamdani, I.; Rahhou, I.; Loukili, E.H.; Belbachir, C.; Hammouti, B.; et al. Argan leaves aqueous extract’s antioxidant activity and mild steel corrosion inhibition ability. Int. J. Corros. Scale Inhib. 2022, 11, 1539–1556. [Google Scholar]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Mechchate, H.; Al-Salahi, R.; Nasr, F.A.; Bnouham, M.; et al. Artemisia absinthium L. Aqueous and ethyl acetate extracts: Antioxidant effect and potential activity in vitro and in vivo against pancreatic α-amylase and intestinal α-glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef]
- Ouahabi, S.; Loukili, E.H.; Daoudi, N.E.; Chebaibi, M.; Ramdani, M.; Rahhou, I.; Bnouham, M.; Fauconnier, M.-L.; Hammouti, B.; Rhazi, L.; et al. Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Gracilaria bursa-pastoris Extracts. Mar. Drugs 2023, 21, 372. [Google Scholar] [CrossRef]
- Elrherabi, A.; Bouhrim, M.; Abdnim, R.; Berraaouan, A.; Ziyyat, A.; Mekhfi, H.; Legssyer, A.; Bnouham, M. Antihyperglycemic potential of the Lavandula stoechas aqueous extract via inhibition of digestive enzymes and reduction of intestinal glucose absorption. J. Ayurveda Integr. Med. 2023, 14, 100795. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Al-Lahham, S.; Zaid, A.N.; Hussein, F.; Issa, L.; Abualhasan, M.N.; Hawash, M.; Yahya, A.; Shehadi, O.; Omair, R.; et al. Carlina curetum plant phytoconstituents, enzymes inhibitory and cytotoxic activity on cervical epithelial carcinoma and colon cancer cell lines. Eur. J. Integr. Med. 2019, 30, 100933. [Google Scholar] [CrossRef]
- Cheng, Q.; Cai, S.; Ni, D.; Wang, R.; Zhou, F.; Ji, B.; Chen, Y. In vitro antioxidant and pancreatic α-amylase inhibitory activity of isolated fractions from water extract of Qingzhuan tea. J. Food Sci. Technol. 2015, 52, 928–935. [Google Scholar] [CrossRef] [PubMed]
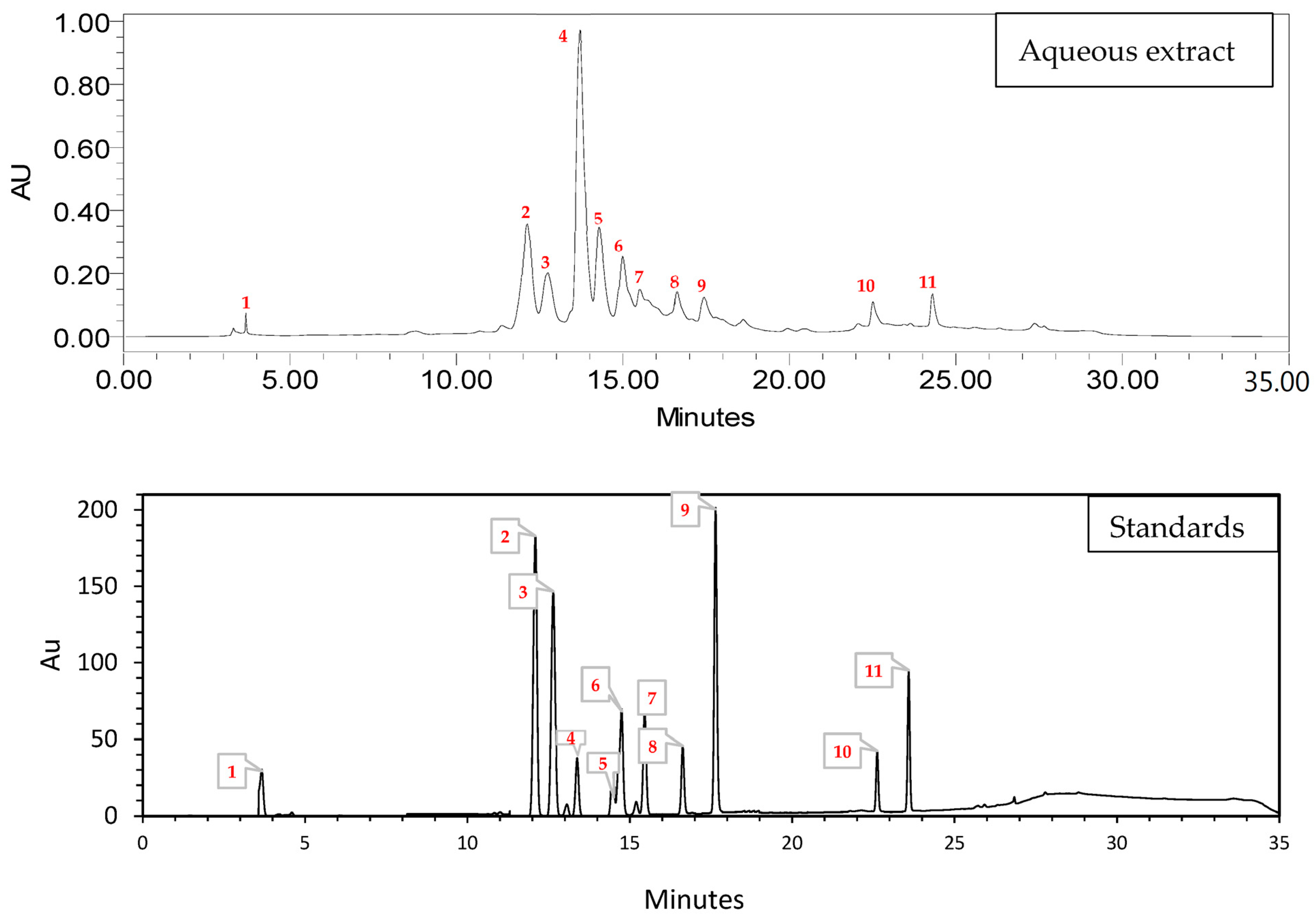
| No. | Compound | Tr (min) | % Area |
|---|---|---|---|
| 1 | Catechin | 3.674 | 0.39 |
| 2 | 4-Hydroxybenzoic acid | 12.123 | 17.89 |
| 3 | Vanillin | 12.729 | 7.81 |
| 4 | 3-hydroxycinnamic acid | 13.702 | 38.08 |
| 5 | Rutin | 14.276 | 11.51 |
| 6 | p-Coumaric acid | 14.983 | 8.49 |
| 7 | Quercetin | 15.508 | 5.21 |
| 8 | Luteolin | 16.616 | 1.53 |
| 9 | Quercetin 3-O-β-d-glucoside | 17.428 | 5.46 |
| 10 | Kaempferol | 22.504 | 1.40 |
| 11 | Flavone | 24.291 | 2.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haddou, S.; Elrherabi, A.; Loukili, E.H.; Abdnim, R.; Hbika, A.; Bouhrim, M.; Al Kamaly, O.; Saleh, A.; Shahat, A.A.; Bnouham, M.; et al. Chemical Analysis of the Antihyperglycemic, and Pancreatic α-Amylase, Lipase, and Intestinal α-Glucosidase Inhibitory Activities of Cannabis sativa L. Seed Extracts. Molecules 2024, 29, 93. https://doi.org/10.3390/molecules29010093
Haddou S, Elrherabi A, Loukili EH, Abdnim R, Hbika A, Bouhrim M, Al Kamaly O, Saleh A, Shahat AA, Bnouham M, et al. Chemical Analysis of the Antihyperglycemic, and Pancreatic α-Amylase, Lipase, and Intestinal α-Glucosidase Inhibitory Activities of Cannabis sativa L. Seed Extracts. Molecules. 2024; 29(1):93. https://doi.org/10.3390/molecules29010093
Chicago/Turabian StyleHaddou, Salima, Amal Elrherabi, El Hassania Loukili, Rhizlan Abdnim, Asmae Hbika, Mohamed Bouhrim, Omkulthom Al Kamaly, Asmaa Saleh, Abdelaaty A. Shahat, Mohamed Bnouham, and et al. 2024. "Chemical Analysis of the Antihyperglycemic, and Pancreatic α-Amylase, Lipase, and Intestinal α-Glucosidase Inhibitory Activities of Cannabis sativa L. Seed Extracts" Molecules 29, no. 1: 93. https://doi.org/10.3390/molecules29010093
APA StyleHaddou, S., Elrherabi, A., Loukili, E. H., Abdnim, R., Hbika, A., Bouhrim, M., Al Kamaly, O., Saleh, A., Shahat, A. A., Bnouham, M., Hammouti, B., & Chahine, A. (2024). Chemical Analysis of the Antihyperglycemic, and Pancreatic α-Amylase, Lipase, and Intestinal α-Glucosidase Inhibitory Activities of Cannabis sativa L. Seed Extracts. Molecules, 29(1), 93. https://doi.org/10.3390/molecules29010093








