Abstract
A new series of trifluoromethylated pyrimido[1,2-b]indazol-4(1H)-one derivatives was synthesized with good to excellent yields through a simple condensation of 3-aminoindazole derivatives with ethyl 4,4,4-trifluoro 3-oxobutanoate. The functionalization of the corresponding chlorinated fused tricyclic scaffolds via Suzuki-Miyaura and aromatic nucleophilic substitution reactions led to the synthesis of highly diverse trifluoromethylated pyrimido[1,2-b]indazole derivatives with good yields.
1. Introduction
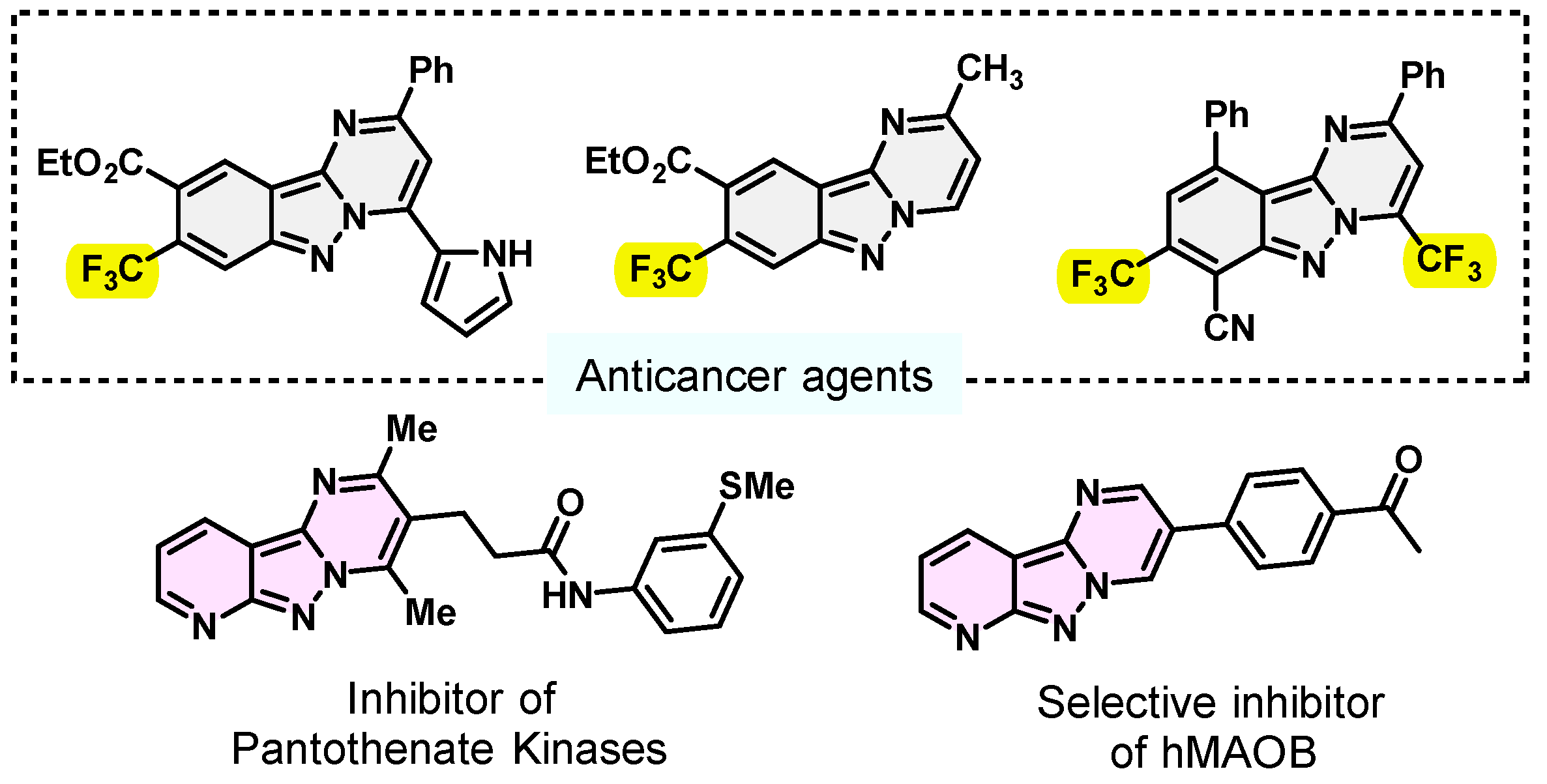
Nitrogen-containing heterocycles are of great and immense research interest due to their highly chemical, biological and pharmaceutical significance [1,2,3,4,5,6]. They play an essential role in natural and synthetic organic chemistry [7]. Among them, pyrimido[1,2-b]indazoles are known to exhibit a wide range of prominent biological and pharmaceutical activities, such as anticancer [8,9], MAO-B [10] and antibacterial [11] activity, as well as the inhibition of phosphodiesterase 10A (PDE10A) [2] and pantothenate kinases [12] (Figure 1).
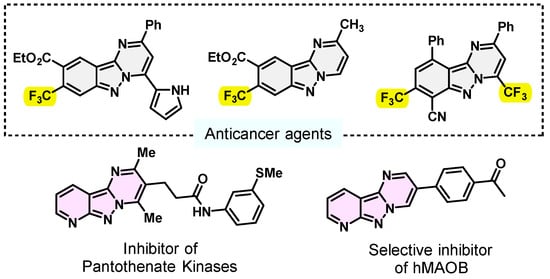
Figure 1.
Some representatively bioactive pyrimido[1,2-b]indazole derivatives.
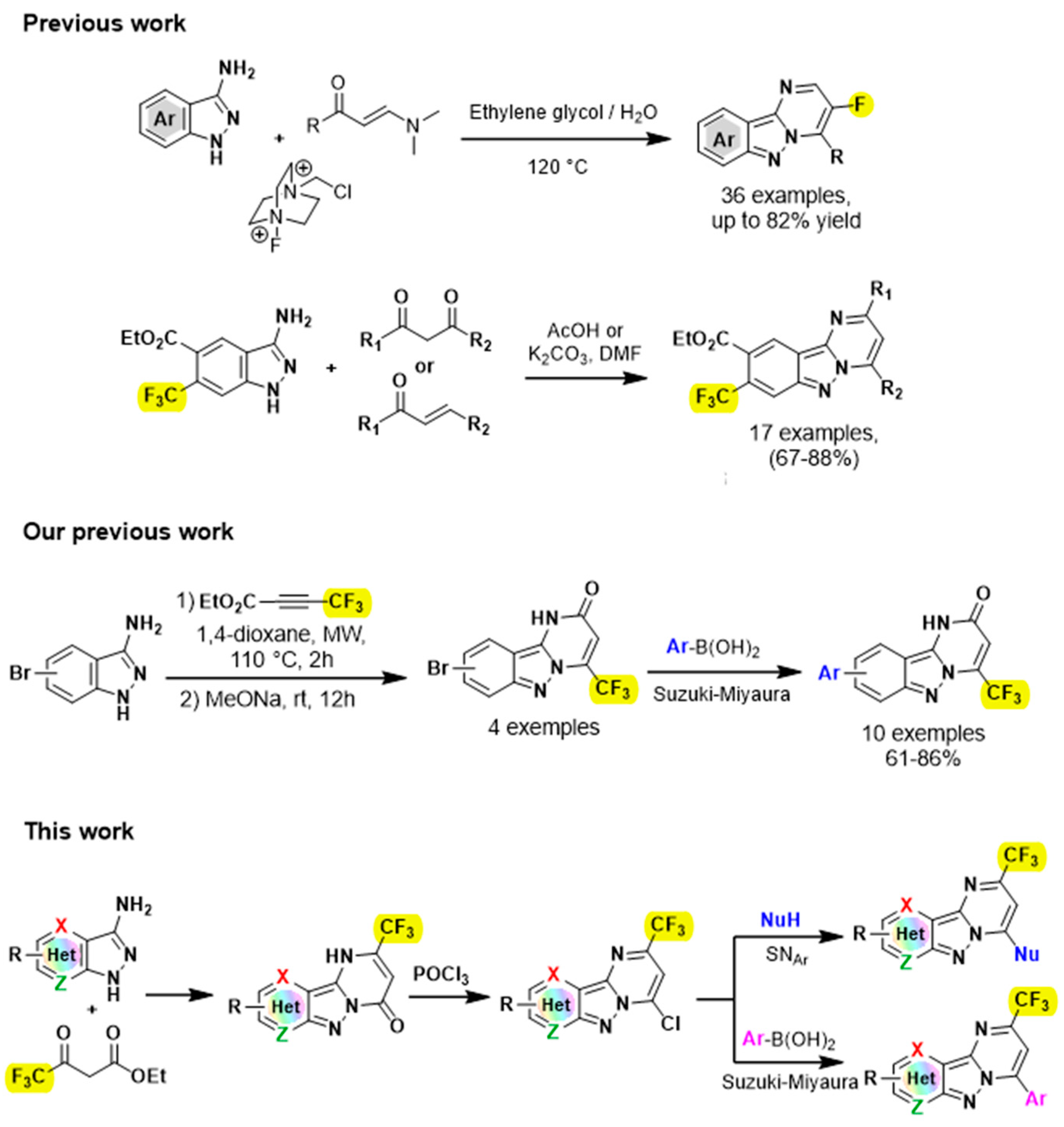
Unfortunately, despite their importance, a limited number of syntheses for the construction of these fused pyrimido[1,2-b]indazoles have been reported. A well-known procedure for the synthesis of this rigid tricyclic N-fused moiety is cyclocondensation between 3-aminoindazoles and carbonyl compounds [13,14,15,16,17]. For example, Song’s group reported BF3.Et2O-promoted intermolecular cyclocondensation between 3-aminoindazoles and 3-ethoxycyclobutanones [17]. Gao and his group developed a three-component reaction mediated by NH4I between 3-aminoindazoles, aromatic aldehydes and ethylamines [18]. Other excellent approaches were developed by Cao’s group by performing either cascade cyclization reactions for the synthesis of a series of chalcogens facilitated by pyrimido[1,2-b]indazoles [19], or by condensing the 3-aminoindazoles on the ynals [20]. Surprisingly, despite the pharmaceutical and synthetic importance of fluorine-containing heterocycles [21,22,23,24], methods for synthesizing fluorinated pyrimido[1,2-b]indazoles remain scarce. Only a few publications dealing with the synthesis of fluorinated pyrimido[1,2-b]indazoles has been reported recently in the literature (Figure 2) [9,25], notably by Wang’s group who disclosed an interesting multicomponent reaction of enaminones, 3-aminoindazoles and selectfluor for the synthesis of fluorinated pyrimido[1,2-b]indazoles [25]. Therefore, the development of an efficient and facile method to approach fluorinated tricyclic compounds is highly desirable.
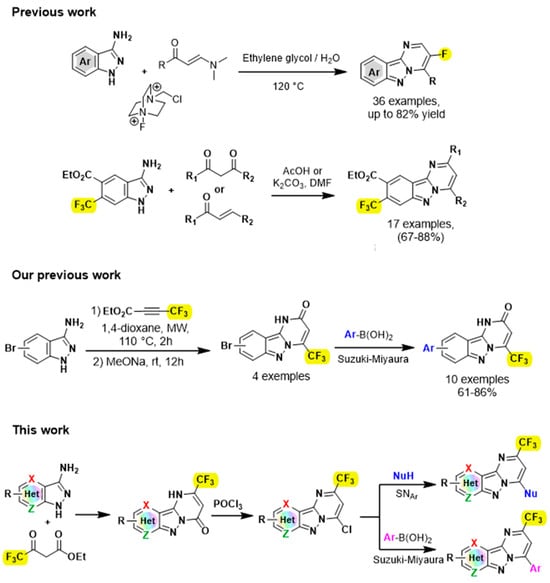
Figure 2.
Convergent approach for the synthesis of fluorinated pyrimido[1,2-b]indazole derivatives [9,25,26].
As part of our on-going effort in the synthesis of novel fluorinated heterocycles [27,28,29,30,31], we report herein a convenient and efficient synthetic strategy that enables the construction of trifluoromethylated pyrimido[1,2-b]indazole derivatives.
2. Results and Discussion
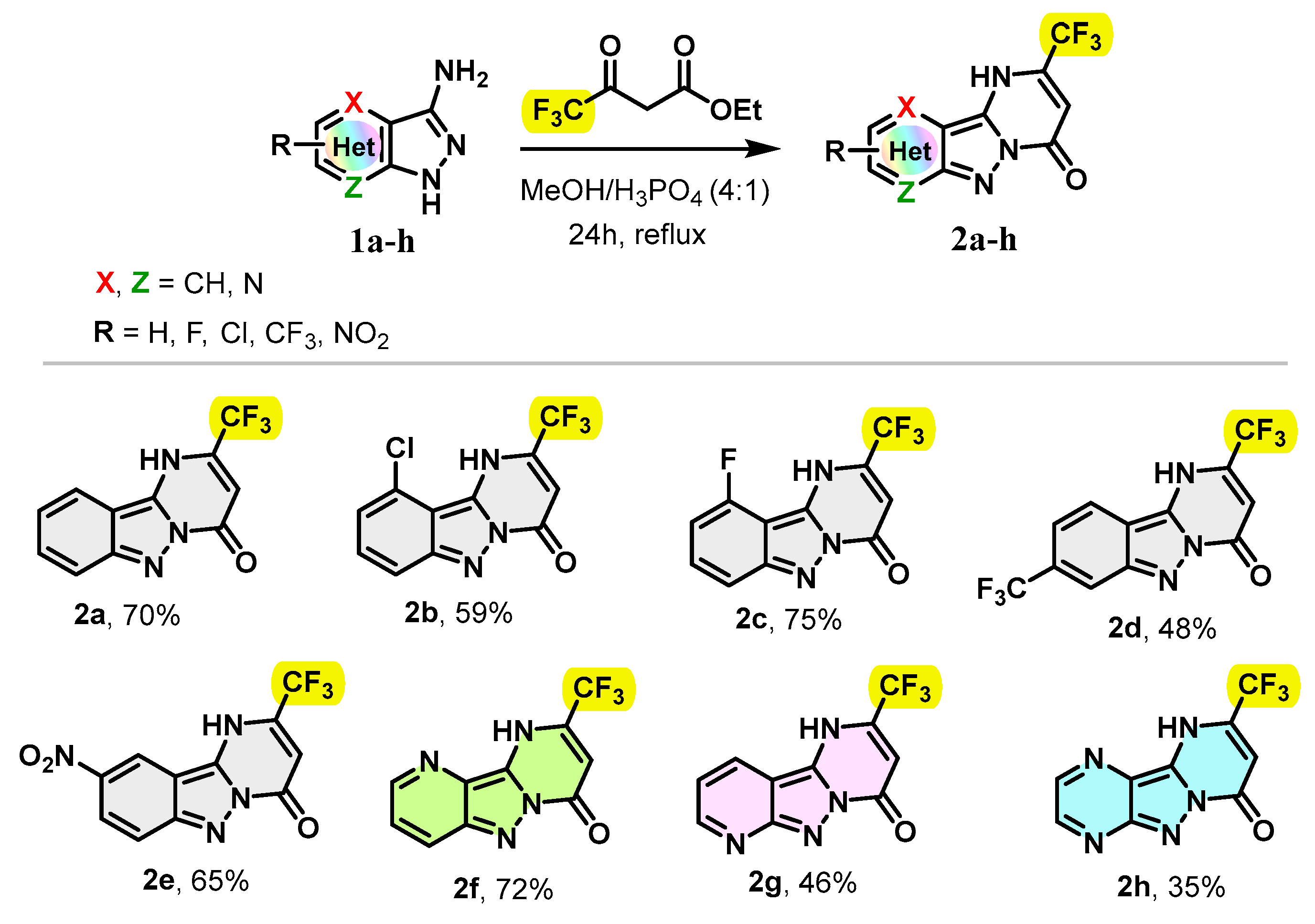
Our strategy started with the gram-scale synthesis of 2-(trifluoromethyl)pyrimido[1,2-b]indazol-4(1H)-one derivatives 2 by cyclocondensation of 3-aminoindazole derivatives 1 on ethyl 4,4,4-trifluoroacetoacetate. The starting materials 1a–h were synthesized according to well-known procedure [30,31]. The reaction between 3-aminoindazole derivatives 1 and ethyl 4,4,4-trifluoroacetoacetate was carried out in a MeOH/H3PO4 mixture (4/1) at reflux for 24 h. Note that the use of methanol as the sole solvent led to the formation of the desired product 2a with 27% of yield, but unfortunately with only 36% conversion of the starting material 1a after 72 h. A mixture of methanol/AcOH (4/1) was also screened but was found to be ineffective, giving the expected product 2a in a yield of 39% with a partial conversion of 78% after 24 h.
Under the conditions indicated above, substituted 3-amino-1H-pyrazolo[4,3-b]pyridine, 3-amino-1H-pyrazolo[3,4-b]pyridine and 3-amino-1H-pyrazolo[3,4-b]pyrazine were successfully condensed and yielded regioselectively the trifluoromethylated pyrimido[1,2-b]indazol-4(1H)-one derivatives 2f–h with yields ranging from 35 to 75% (Scheme 1). Overall, the 3-amino indazole derivatives were found to exhibit comparable reactivities toward ethyl 4,4,4-trifluoro-3-oxobutanoate, except in the case of 3-amino-1H-pyrazolo[3,4-b]pyrazine which afforded the cyclocondensation product 2h with a yield of 35%. Furthermore, the presence of the halo groups, including the chloro-, fluoro- and trifluoromethyl groups, in substrates 1 was compatible under this synthetic protocol, affording the desired tricyclic compounds 2b–d with yields of 59, 75 and 48%, respectively, while, 3-aminoindazole with the strongly electron-withdrawing nitro group was condensed to generate the targeted product 2e in 65% yield.
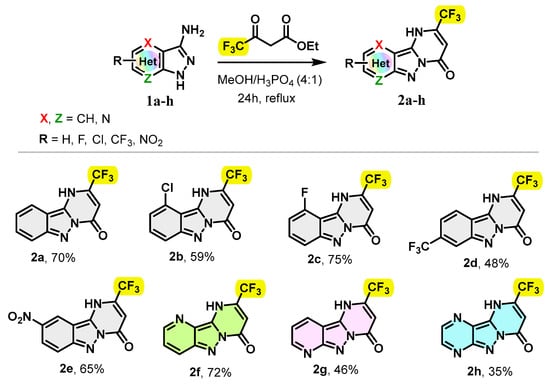
Scheme 1.
Scope of 3-aminoindazole derivatives.
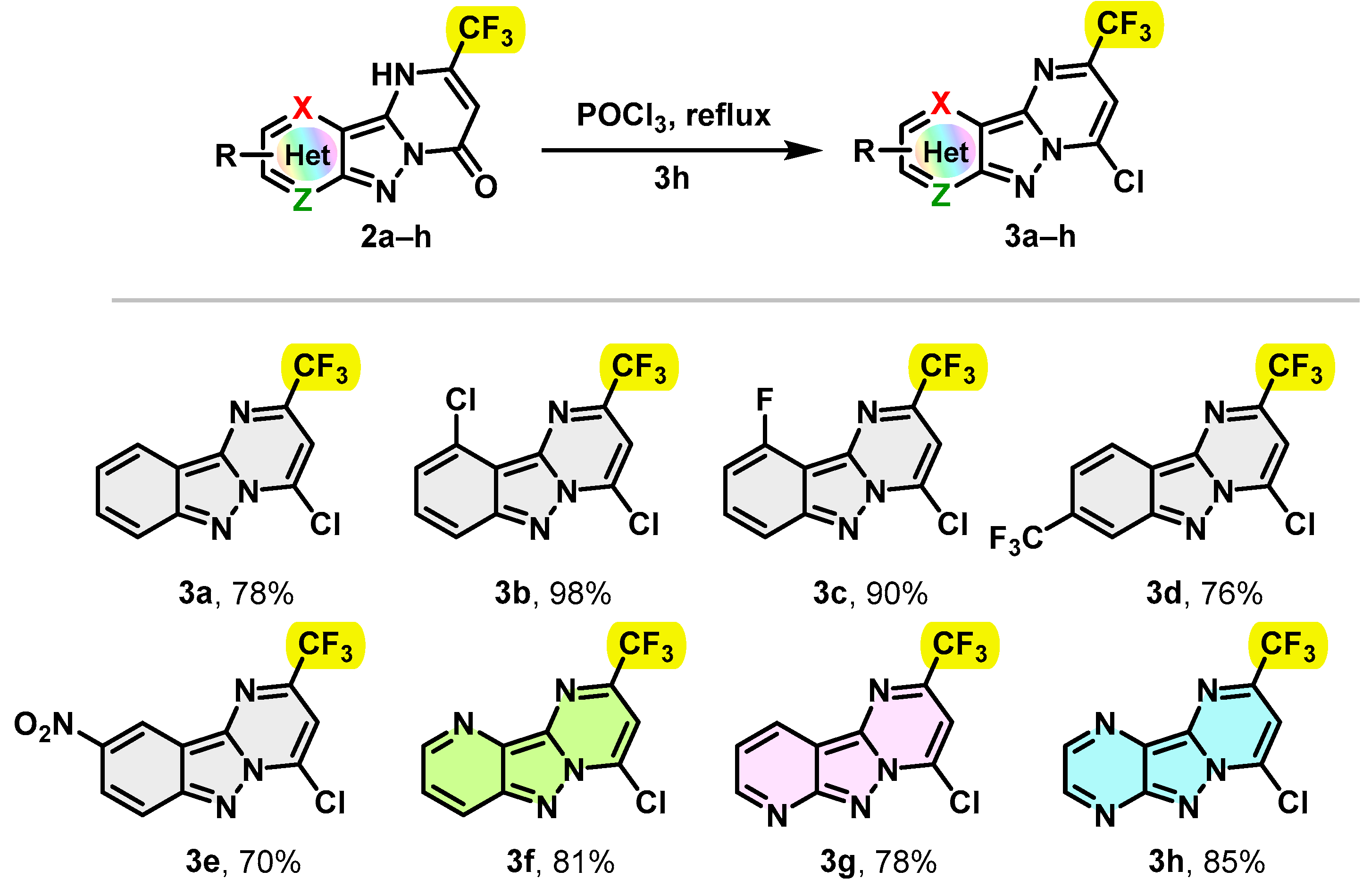
In the next step, some 2-trifluromethyl-1H-pyrimido[1,2-b]indazole-4-one derivatives 2 were converted into the corresponding 4-chloro-2-(trifluoromethyl)pyrimido[1,2-b]indazole derivatives 3a–h in gram-scale synthesis. The reactions were performed in POCl3 at reflux. The conversion was found to be complete after 3 h, providing the desired chlorinated products 3a–h in good to excellent yields. Notably, the unsubstituted 4-chloro-2-(trifluoromethyl)pyrimido[1,2-b]indazole derivatives 3a and 3f–h were successfully isolated with yields ranging from 78 to 85%. Similarly, 4-chloro-2-(trifluoromethyl)pyrimido[1,2-b]indazole derivatives 3b–e substituted with withdrawing groups such as Cl, F, CF3 and NO2 were easily accessible with yields of 98, 90, 76 and 70%, respectively. The results are summarized in Scheme 2.
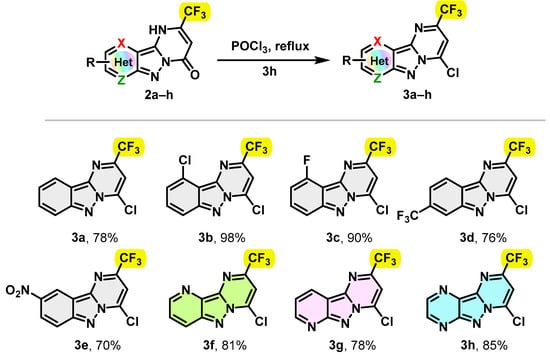
Scheme 2.
Synthesis of trifluoromethylated 4-chloro-2-(trifluoromethyl)pyrimido[1,2-b]indazole derivatives 3a–h.
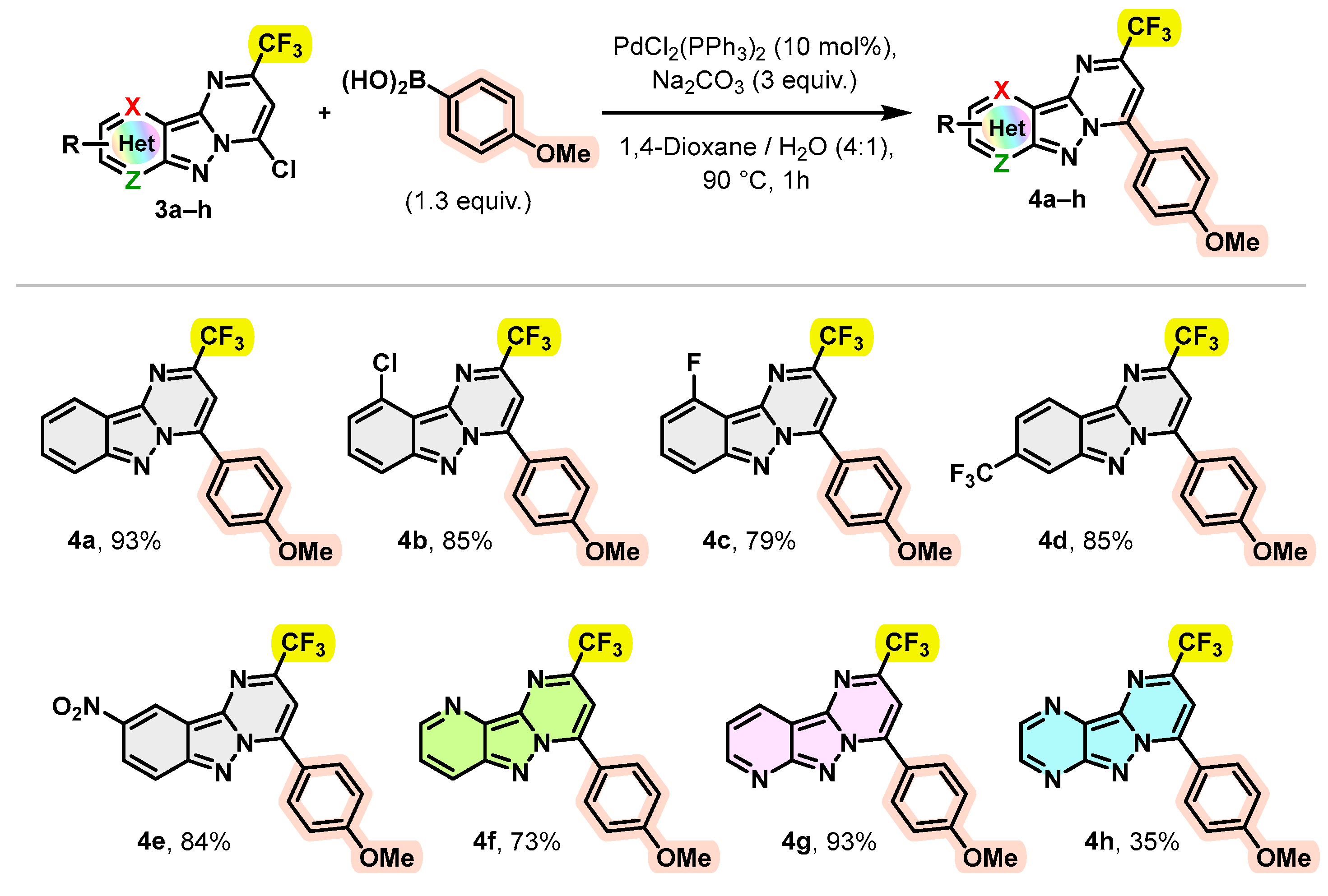
Subsequently, compounds 3a–h were used as building blocks to generate a novel chemical library of substituted pyrimido[1,2-b]indazole derivatives. As products, 3a–h carry a reactive carbon–chloride bond in position C-4, it seemed important to us to test the reactivity of this bond in order to achieve further transformations. Hence, the chlorine at the C-4 position of 3a–h was subjected to Suzuki–Miyaura cross-coupling using 4-methoxyphenylboronic acid and typical conditions [32,33,34], i.e., PdCl2(PPh3)2 (10 mol%) as catalyst, Na2CO3 (3 equivalents) as base in dioxane/H2O (4:1) at 90 °C for 1 h. The synthetic scope of this coupling reaction was examined using a variety of trifluoromethylated pyrimido[1,2-b]indazole derivatives 3a–h. The results are summarized in Scheme 3.
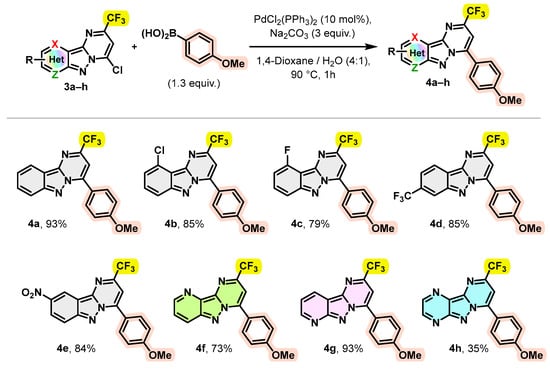
Scheme 3.
Behavior of 4-chloro-2-(trifluoromethyl)pyrimido[1,2-b]indazole derivatives 3a–h in the Suzuki–Miyaura cross coupling.
As shown in Scheme 3, a number of trifluoromethylated pyrimido[1,2-b]indazoles and pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidines 3a–g were efficiently arylated at position 4 by 4-methoxy phenyl, leading to the desired products 4a–g with excellent yields (>73%). However, this arylation appeared to be less effective in the case of trifluoromethylated pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine 3h, since the expected product 4h was isolated with a yield not exceeding 35%. We believe that complexation of boronic acid with a polynitrogen system would reduce the yield by inactivation of the boronic acid.
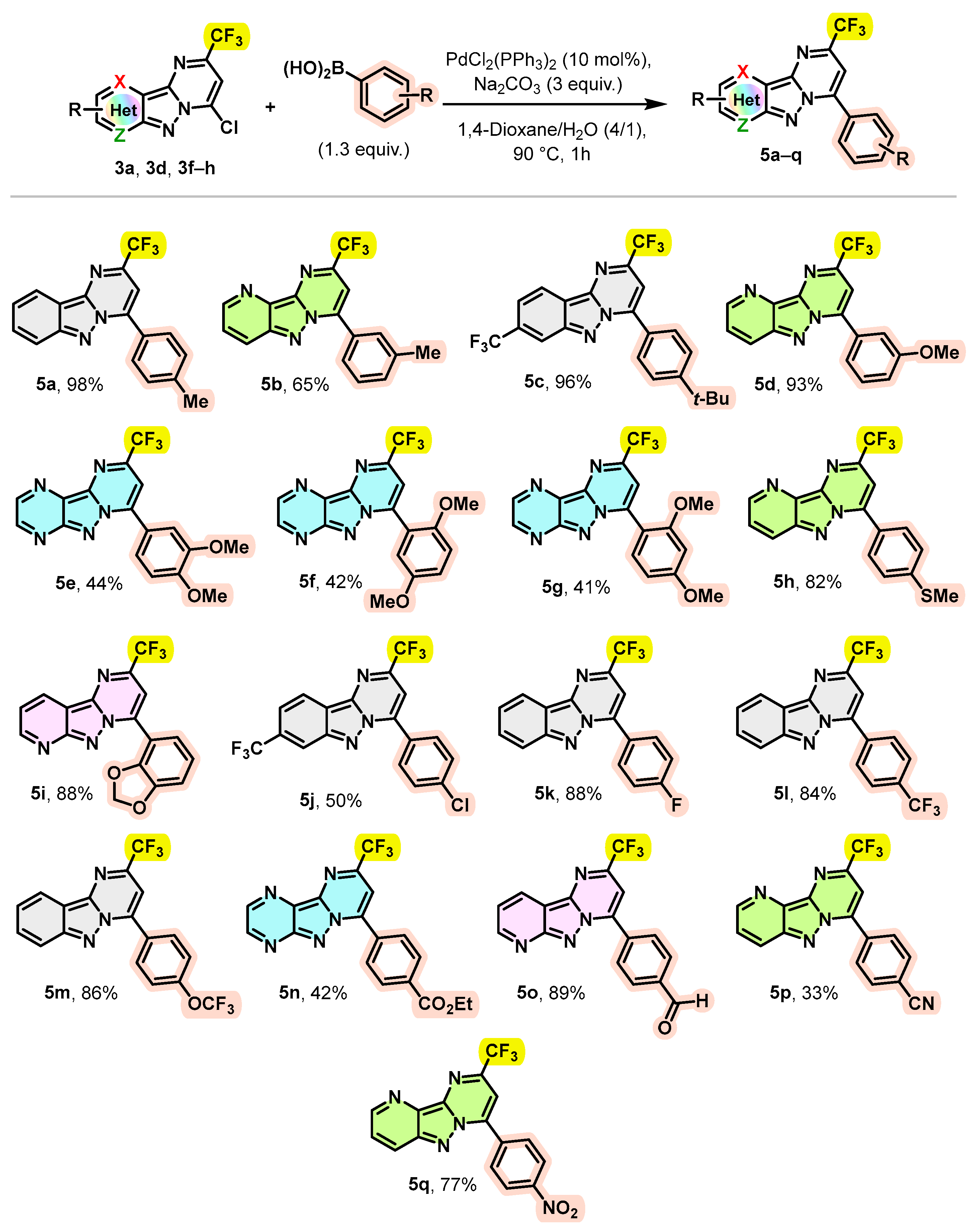
Encouraged by these results, we explored the scope of this coupling reaction using a wide variety of commercially available arylboronic acids to access new trifluoromethylated pyrimido[1,2-b]indazole derivatives with a high structural diversity. The obtained results are summarized in Scheme 4.
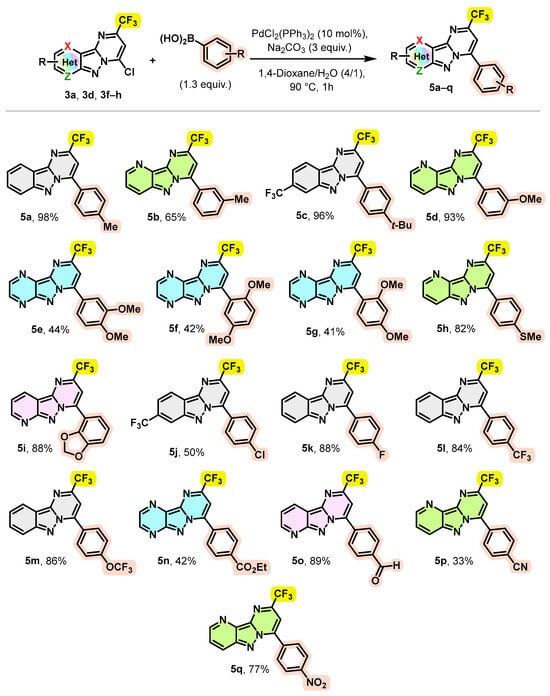
Scheme 4.
Scope of the Suzuki–Miyaura cross-coupling.
As illustrated in Scheme 4, different boronic acids bearing electron-donating or electron-withdrawing groups on the aromatic ring provided the arylated products 5a–q in good to excellent yields. Notably, 4-methyl and 3-methylphenylboronic acids were successfully coupled with compounds 3a and 3f leading to the arylation products 5a and 5b with yields of 98 and 65%, respectively. Likewise, phenyl boronic acid with a tert-butyl substituent at the para position also smoothly underwent reaction with 3d, giving the desired product 5c in a 96% yield. Arylboronic acids bearing strong electron-donating groups, such as a methoxy group in the meta-position or a thiomethyl group in the para-position, were readily coupled with 3f to provide the corresponding products 5d (93%) and 5h (82%), respectively. As observed previously, trifluoromethylated pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine 3h afforded poorer yields again when it was engaged in a Suzuki–Miyaura coupling reaction; the coupling of compound 3h with 3,4-, 2,5- and 2,4-dimethoxyphenylboronic acids provided the desired arylation products 5e g in yields of only 44%, 42% and 41%, respectively. These results seem to indicate that steric hindrance does not play a significant role in the efficiency of this coupling reaction. Moreover, the coupling reaction of compound 3g with 1,3-benzodioxol-4-ylboronic acid was easily converted to the desired product 5i with a yield of 88%. Likewise, phenylboronic acid substituted at the para position with an electron-withdrawing group such as Cl, F, CF3, OCF3, CO2Et, CHO, CN or NO2 provided the expected products 5j–q with yields ranging from 33% to 89%.
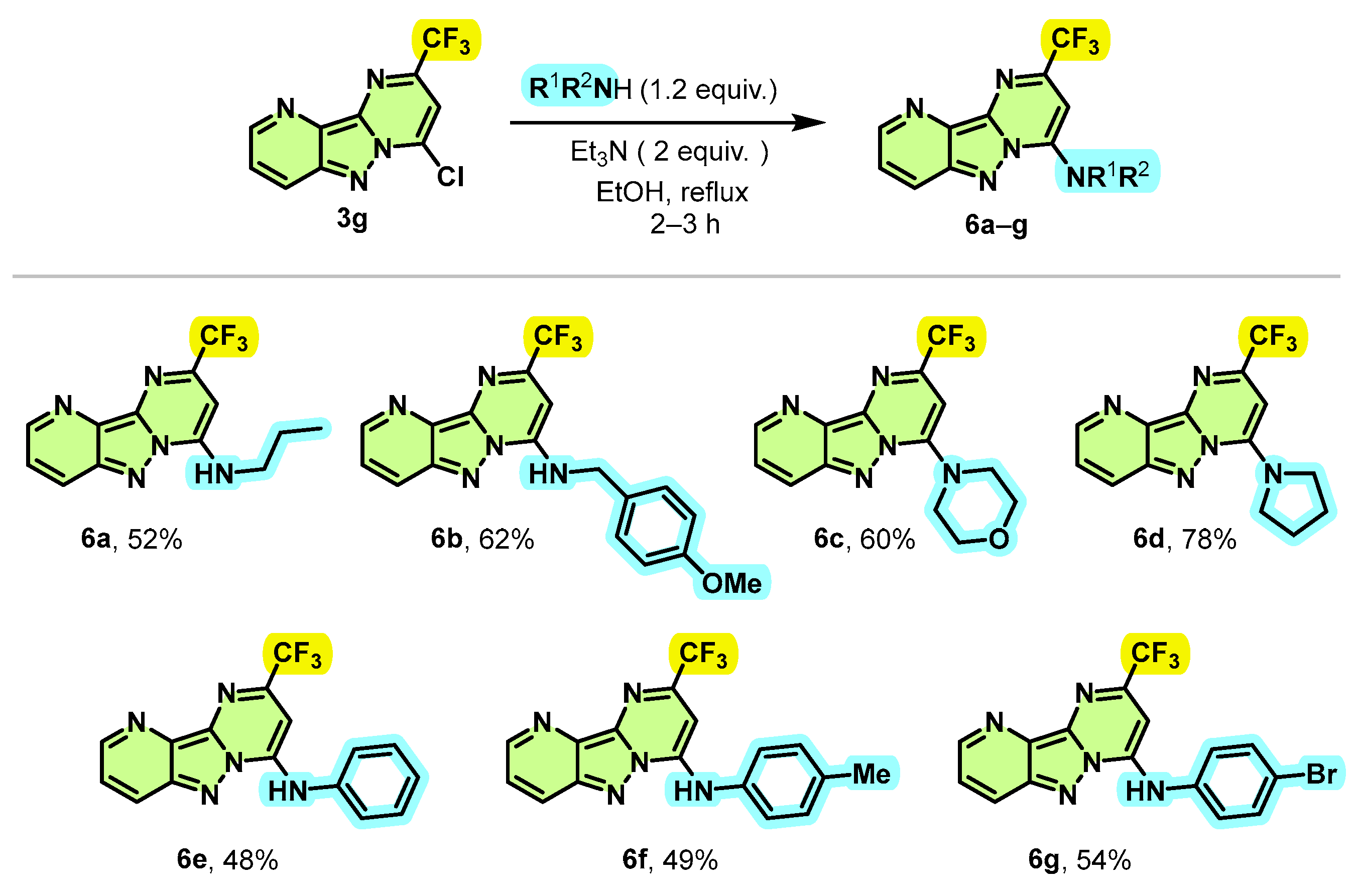
Given the success of this Suzuki–Miyaura coupling reaction allowing the introduction of aryl groups at position 4 of 2-(trifluoromethyl)pyrimido[1,2-b]indazole derivatives, and in order to introduce more functional diversity into these compounds, we therefore focused our investigations synthesizing new trifluoromethylated pyrimido[1,2-b]indazole derivatives by aromatic nucleophilic substitution (SNAr) displacement of the chloride moiety. To validate the feasibility of our hypothesis, we first sought to test the amination of compound 3g by using morpholine as the model substrate. Initially, when the reaction was carried out under reflux of EtOH in the absence of base, the desired compound 6c was obtained in 12% of yield with a very low conversion of 25% after 24 h. The desired product 6c was obtained this time in 31% of the yield with a conversion of 67% after 12 h when equivalent 1 of Et3N was used as a base. To our delight, we discovered that equivalent 2 of Et3N enables the total conversion of the starting material 3g into 6c with a yield of 60% after 2 h. Another base, such as K2CO3, was also effective for the reaction, but less efficient than Et3N, providing 6c in 55% yield after 2 h. Various amines were incorporated at the C-4 position of substrate 3f, as a representative example of chlorinated products, to provide the expected products 6a–g in moderate to good yields (Scheme 5). Primary amines such as n-propylamine and benzylamine reacted effectively to give the aminated products 6a and 6b with yields of 52% and 62%, respectively. This reaction was also extended to secondary amines, such as morpholine and pyrrolidine, leading to the new trifluoromethylated pyrimido[1,2-b]indazole derivatives 6c and 6d in yields of 60% and 78%, respectively.
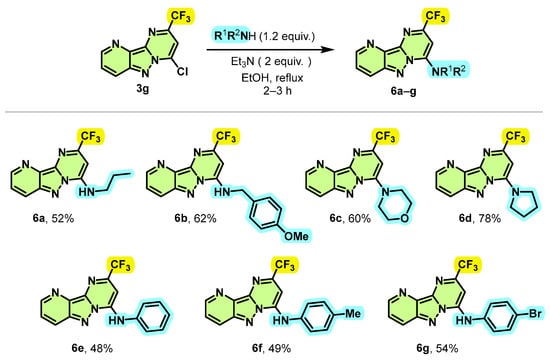
Scheme 5.
Synthesis route of 4-amino-2-trifluoromethyl pyrimido[1,2-b]indazole derivatives 6a–g.
Interestingly, this SNAr reaction proved to be compatible with aromatic amines, since unsubstituted or substituted aniline by electron-donating or electron-withdrawing groups such as methyl and bromine led to N-arylation products 6e–g with acceptable yields (48% to 54%).
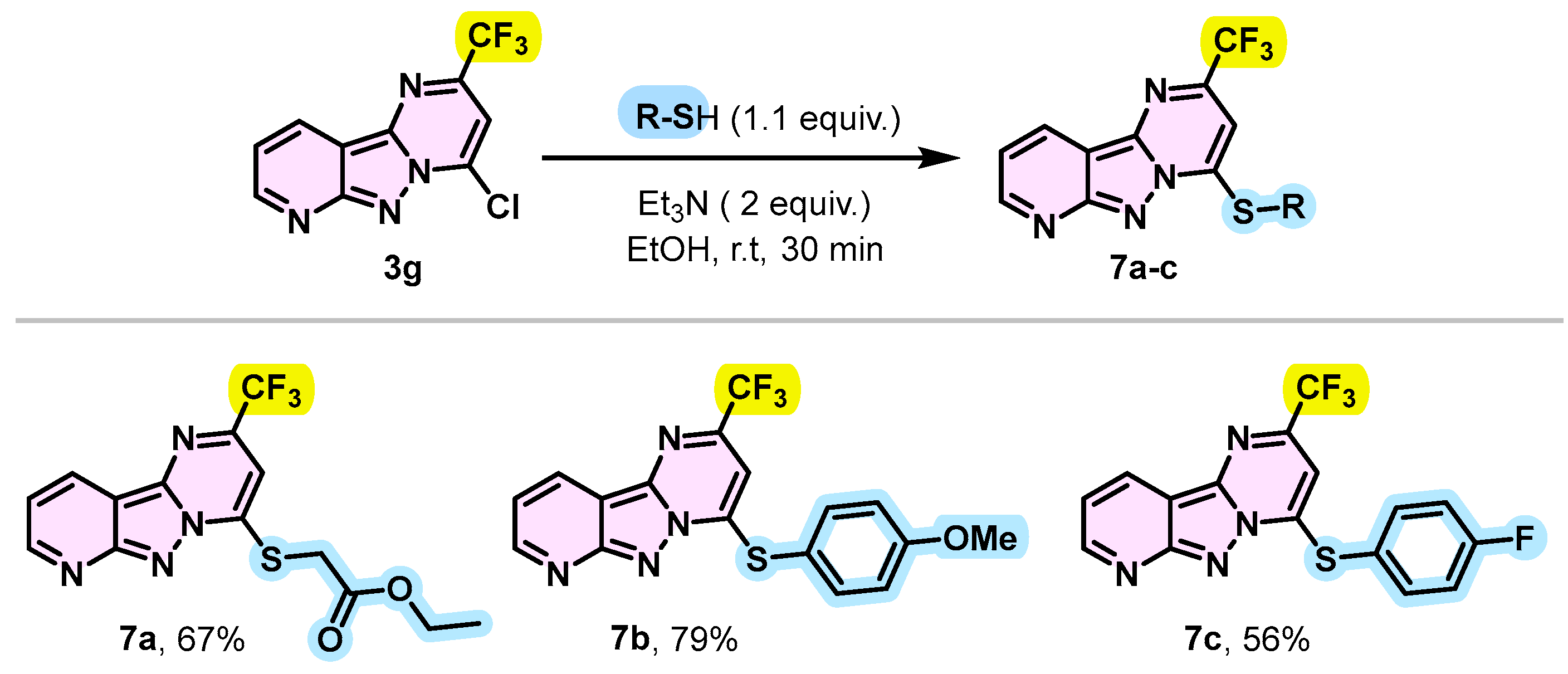
To demonstrate other practical applications of SNAr, an alkylthiol such as ethyl 2-mercapto acetate was found to be reactive towards 4-chloro-2-(trifluoromethy)pyrido[2′,3′;3,4]pyrazolo[1,5-a]pyrimidine 3g, providing the desired thiolation product 7a in 67% yield. The reaction was carried out in the presence of triethylamine (2 equivalents) in ethanol (EtOH) at room temperature for 30 min. Under the same conditions, thiophenols were also successfully introduced into the C-4 position. In fact, thiophenol substituted by an electron-donating group such as methoxy or an electron-withdrawing group such as fluorine led to the expected products 7b and 7c with respective yields of 79% and 56% (Scheme 6). This observed difference in yields could be explained by the difference in nucleophilicity of the thiols caused by the nature of the substituents on the aromatic ring.
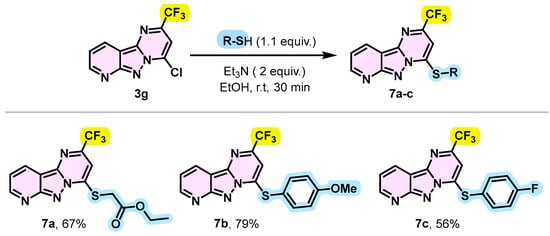
Scheme 6.
Synthesis route of 4-(alkylthio or arylthiol)-2-(trifluoromethyl)pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine 7a–c.
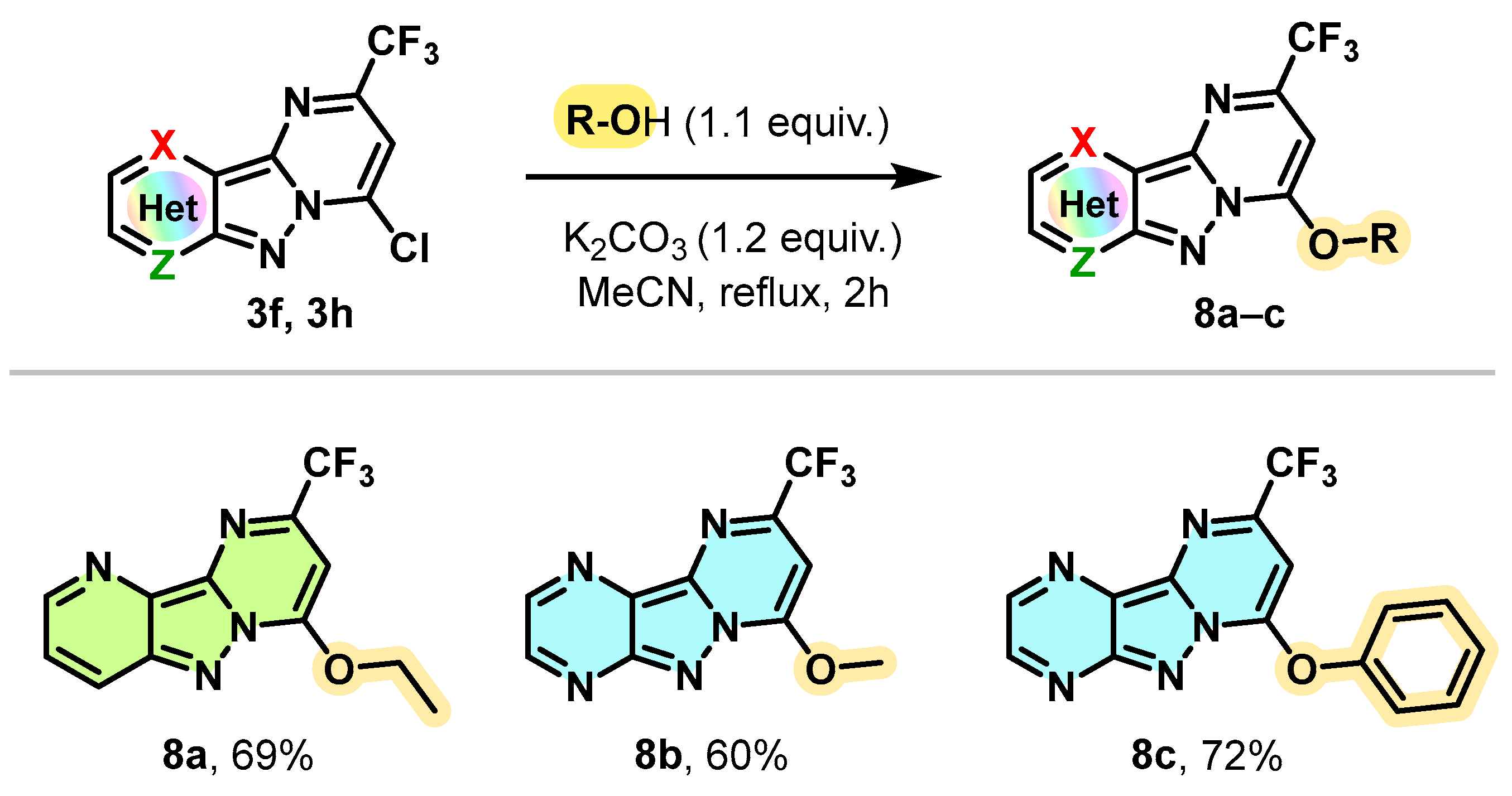
However, under the same conditions, alcohols and phenol showed no reactivity in the SNAr reaction (Scheme 7). Under modified conditions and operating this time in the presence of K2CO3 as a base (1.2 equivalent) at the reflux of acetonitrile for 2 h, EtOH and methanol reacted efficiently with the chlorinated derivatives 3f and 3h to provide the O-alkylation products 8a and 8b with yields of 69% and 60%, respectively. The conditions used were also compatible with the use of phenol as a nucleophile by allowing the O-arylation of compound 3h with a yield of 72%.
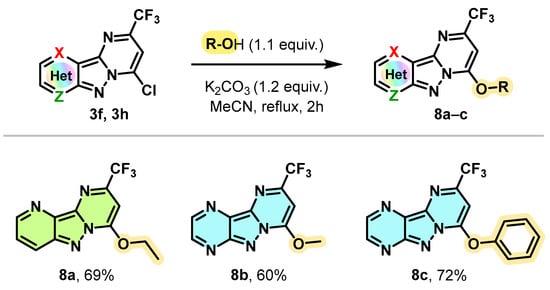
Scheme 7.
Synthesis of 7-alkoxy (or 7-phenoxy)-2-trifluoromethyl pyrimido[1,2-b]indazole derivatives 7a–c.
The flexibility of our strategy allows the synthesis of an original library of trifluoromethylated pyrimido[1,2-b]indazole derivatives with a large substrate scope and with reasonable to good yields (46 examples). All the synthesized trifluoromethylated pyrimido[1,2-b]indazole derivatives are new and have been fully characterized by 1H, 13C, 19F NMR spectroscopy and HRMS (Supplementary Materials).
3. Materials and Methods
3.1. General Methods
The reagents used were purchased from commercial suppliers and were used without further purification. The following solvents were distilled as follows: Triethylamine and MeCN on calcium hydride, and 1,4-dioxane on sodium and benzophenone. The reactions were monitored by thin layer chromatography (TLC) analysis using silica gel plates (60 F254). Compounds were visualized by long-wave (365 nm) or short-wave (254 nm) UV light. Purification of the products by column chromatography was carried out using silica gel 60 (230 to 400 mesh, 0.040 to 0.063 mm)
All 1H NMR, 13C NMR and 19F NMR spectra were recorded with a Bruker Avance FT-NMR spectrometer at 300 MHz (300 MHz, 75 MHz or 282 MHz, respectively). Chemical shifts are reported in ppm and tetramethylsilane (TMS) is used as an internal standard. 1H NMR assignment abbreviations are given as follows: singlet(s), doublet (d), triplet (t), quartet (q), wide singlet (br s), doublet of doublets (dd), triplet of doublets (td), doublet of a triplet (dt) and a multiplet (m). Coupling constants (J) are expressed in Hertz (Hz). High-resolution electrospray ionization mass spectrometry experiments were performed with a hybrid tandem quadrupole/time-of-flight (Q-TOF) instrument, equipped with a pneumatically assisted electrospray (Zspray) ion source (Micromass, Manchester, United Kingdom) operating in positive mode. The melting points were determined on a Kofler Bench apparatus.
3.2. General Procedure for the Synthesis of 2-Trifluromethyl-1H-pyrimido[1,2-b]indazole-4-one Derivatives 2a–h
To a mixture of aminoindazole derivative 1 (2 g, 1 equiv.) and ethyl 4,4,4-trifluoroacetoacetate (2 equiv.) in 4 mL of dry methanol was added dropwise 1 mL of polyphosphoric acid (H3PO4). The solution was refluxed for 24 h under Argon. The progress of the reaction was monitored by TLC (eluent: petroleum ether/ethyl acetate, 5:5). After cooling to room temperature, the solvent was removed under reduced pressure. The crude solid was suspended in a small quantity of water. The resulting precipitate was collected by filtration and washed with Et2O to provide the pure desired product. This procedure was employed to prepare all the 2-trifluromethyl-1H-pyrimido[1,2-b]indazole-4-one derivatives 2a–h. (1H-NMR, 19F-NMR and 13C-NMR of compounds 2a–h are shown in Supplementary Materials).
2-Trifluoromethyl-1H-pyrimido[1,2-b]indazol-4-one (2a).
Compound 2a was obtained as a white solid with a yield of 70%; m.p. 274–276 °C; 1H NMR (300 MHz, DMSO-d6): δ 8.20 (d, J = 8.1 Hz, 1H), 7.81 (t, J = 7.5 Hz, 1H), 7.58 (d, J = 8.1 Hz, 1H), 7.39 (t, J = 7.5 Hz, 1H), 6.77 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 154.4, 149.8 (q, J = 33.8 Hz), 147.3, 141.7, 133.9, 122.9, 122.8, 121.8 (q, J = 273.5 Hz), 115.2, 111.6, 101.0 (q, J = 2.8 Hz); 19F NMR (282 MHz, DMSO-d6): δ −67.54; HRMS (ESI) m/z [M+H]+ calcd for C11H7F3N3O: 254.0535; found: 254.0531.
10-Chloro-2-trifluoromethyl-1H-pyrimido[1,2-b]indazol-4-one (2b).
Compound 2b was obtained as a white solid with a yield of 59%; m.p. 289–291 °C; 1H NMR (300 MHz, DMSO-d6): δ 7.75 (t, J = 7.5 Hz, 1H), 7.54 (d, J = 8.4 Hz, 1H), 7.58 (d, J = 8.4 Hz, 1H), 6.81 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 154.2, 149.8 (q, J = 33,8 Hz), 146.1, 143.0, 134.3, 128.8, 123.0, 121.8 (q, J = 273.5 Hz), 112.5, 110.4, 101.3 (q, J = 2.7 Hz); 19F NMR (282 MHz, DMSO-d6): δ −67.47; HRMS (ESI) m/z [M+H]+ calcd for C11H6ClF3N3O: 288.0146; found: 288.0141.
10-Fluoro-2-trifluoromethyl-1H-pyrimido[1,2-b]indazol-4-one (2c).
Compound 2c was obtained as a beige powder with a yield of 75%; m.p. 304–306 °C; 1H NMR (300 MHz, DMSO-d6): δ 7.79 (dd, J = 12.9, 7.8 Hz, 1H), 7.40 (d, J = 8.4 Hz, 1H), 7.16 (dd, J = 12.9, 7.8 Hz, 1H), 6.80 (s, 1H); 13C NMR (75 MHz, THF-d8): δ 158.0 (d, J = 258.4 Hz), 153.4, 151.1 (q, J = 34.4 Hz), 145.7 (d, J = 3.7 Hz), 143.2 (d, J = 6.3 Hz), 134.6 (d, J = 8.5 Hz), 121.4 (q, J = 273.1 Hz), 107.3 (d, J = 17.7 Hz), 106.3 (d, J = 4.7 Hz), 105.6 (d, J = 19.2 Hz), 101.4 (q, J = 3.0 Hz); 19F NMR (282 MHz, DMSO-d6): δ −67.47, −115.32; HRMS (ESI) m/z [M+H]+ calcd for C11H6 F4N3O: 272.0441; found: 272.0436.
2,8-Bis-trifluoromethyl-1H-pyrimido[1,2-b]indazol-4-one (2d).
Compound 2d was obtained as a white crystal with a yield of 48%; m.p. 277–279 °C; 1H NMR (300 MHz, DMSO-d6): δ 8.46 (d, J = 8.4 Hz, 1H), 7.89 (s, 1H), 7.62 (d, J = 8.4 Hz, 1H), 6.83 (s, 1H); 13C NMR (75 MHz, THF-d8): δ 153.6, 151.0 (q, J = 34.4 Hz), 147.3, 140.8, 134.3 (q, J = 32.3 Hz), 124.2, 123.9 (q, J = 271.2 Hz), 121.4 (q, J = 273.1 Hz), 118.6, 118.5 (q, J = 3.4 Hz), 108.2 (q, J = 4.4 Hz), 102.2 (q, J = 2.9 Hz); 19F NMR (282 MHz, DMSO-d6): δ −61.07, −67.38; HRMS (ESI) m/z [M+H]+ calcd for C12H6F6N3O: 322.0409; found: 322.0403.
9-Nitro-2-trifluoromethyl-1H-pyrimido[1,2-b]indazol-4-one (2e).
Compound 2e was obtained as a yellow solid with a yield of 65%; m.p. 300–302 °C; 1H NMR (300 MHz, DMSO-d6): δ 8.93 (s, 1H), 8.43 (d, J = 9.0 Hz, 1H), 7.66 (d, J = 9.0 Hz, 1H), 6.76 (s, 1H); 13C NMR (75 MHz, THF-d8): δ 153.6, 151.0 (q, J = 34.5 Hz), 147.9, 143.4, 143.0, 127.9, 121.4 (q, J = 273.1 Hz), 119.8, 115.5, 111.0, 102.7 (q, J = 3.0 Hz); 19F NMR (282 MHz, DMSO-d6): δ −67.40; HRMS (ESI) m/z [M+H]+ calcd for C11H6F3N4O3: 299.0386; found: 299.0382.
9-(Trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidin-7(10H)-one (2f).
Compound 2f was obtained as a yellow solid with a yield of 72%; m.p. 296–298 °C; 1H NMR (300 MHz, DMSO-d6): δ 8.74 (dd, J = 4.5, 1.2 Hz, 1H), 8.23 (dd, J = 8.7, 1.2 Hz, 1H), 7.82 (dd, J = 8.7, 4.5 Hz, 1H), 6.77 (s, 1H); 13C NMR (75 MHz, DMSO): δ 155.0, 149.2 (q, J = 35.5 Hz), 145.5, 145.2, 138.2, 126.9, 123.3, 123.3, 122.0 (q, J = 273.1 Hz), 100.5 (q, J = 2.1 Hz); 19F NMR (282 MHz, DMSO-d6): δ −67.37; HRMS (ESI) m/z [M+H]+ calcd for C10H6F3N4O: 255.0488; found: 255.0484.
2-(Trifluoromethyl)pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidin-4(1H)-one (2g).
Compound 2g was obtained as an orange-yellow solid with a yield of 46%; m.p. 366–368 °C; 1H NMR (300 MHz, DMSO-d6): δ 9.04 (dd, J = 7.8, 0.9 Hz, 1H), 8.79 (dd, J = 5.7, 0.9 Hz, 1H), 7.28 (dd, J = 7.8, 5.7 Hz, 1H), 6.65 (s, 1H); 13C NMR (75 MHz, CDCl3/TFA-d1): δ 157.6, 148.3 (q, J = 36.2 Hz), 145.3, 144.6, 143.5, 135.6, 130.3, 120.1 (q, J = 274.4 Hz), 114.9, 102.6 (q, J = 2.1 Hz); 19F NMR (282 MHz, DMSO-d6): δ −67.25; HRMS (ESI) m/z [M+H]+ calcd for C10H6F3N4O: 255.0488; found: 255.0483.
9-(Trifluoromethyl)pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidin-7(10H)-one (2h).
Compound 2h was obtained as a red solid with a yield of 35%; m.p. 261–263 °C; 1H NMR (300 MHz, DMSO-d6): δ 8.83 (d, J = 2.1 Hz, 1H), 8.69 (d, J = 2.1 Hz, 1H), 6.75 (s, 1H); 13C NMR (75 MHz, THF-d8): δ 153.2, 148.3, 147.4, 145.8, 141.9, 127.5, 124.4, 121.4 (q, J = 273.1 Hz), 103.6 (q, J = 2.8 Hz); 19F NMR (282 MHz, DMSO-d6): δ −67.32; HRMS (ESI) m/z [M+H]+ calcd for C9H5F3N5O: 256.0440; found: 256.0436.
3.3. General Procedure for the Synthesis of 4-Chloro-2-trifluromethyl-1H-pyrimido[1,2-b]indazole-4-one Derivatives 3a–h
A solution of 2-trifluromethyl-1H-pyrimido[1,2-b]indazole-4-one derivative 2 (2 g, 0.005 mol) and phosphorus oxychloride (POCl3) (0.107 mol) was heated under reflux for 3 h. The progress of the reaction was monitored by TLC (eluent: petroleum ether/ethyl ac-etate, 7:3). The reaction mixture was brought to room temperature and the excess of POCl3 was removed in vacuo. Water was added and the chlorinated product was extracted with dichloromethane. The organic layer was separated, dried over anhydrous MgSO4 and filtered. The filtrate was concentrated and purified by column chromatography on silica gel to provide the desired compounds 3a–h. (1H-NMR, 19F-NMR and 13C-NMR of compounds 3a–h are shown in Supplementary Materials).
4-Chloro-2-(trifluoromethyl)pyrimido[1,2-b]indazole (3a).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9.6/0.4) to afford 3a as a yellow solid with a yield of 78%; m.p. 165–167 °C; 1H NMR (300 MHz, CDCl3): δ 8.43 (d, J = 8.4 Hz, 1H), 8.05 (d, J = 8.4 Hz, 1H), 7.80 (td, J = 8.7, 1.2 Hz, 1H), 7.74 (s, 1H), 7.51 (td, J = 8.7, 1.2 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 152.1, 143.7, 141.3 (q, J = 37.2 Hz), 137.7, 131.5, 123.4, 121.3, 120.6 (q, J = 273.1 Hz), 117.3, 114.9, 107.9 (q, J = 2.4 Hz); 19F NMR (282 MHz, CDCl3): δ −66.78; HRMS (ESI) m/z [M+H]+ calcd for C11H6ClF3N3: 272.0196; found: 272.0192.
4,10-Dichloro-2-(trifluoromethyl)pyrimido[1,2-b]indazole (3b).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9/1) to afford 3b as a yellow solid with a yield of 98%; m.p. 136–138 °C; 1H NMR (300 MHz, CDCl3): δ 7.94 (dd, J = 8.7, 0.6 Hz, 1H), 7.79 (s, 1H), 7.69 (dd, J = 8.7, 7.2 Hz, 1H), 7.47 (dd, J = 7.2, 0.6 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 152.7, 143.0, 142.2 (q, J = 37.1 Hz), 138.0, 131.5, 128.2, 123.6, 120.4 (q, J = 273.1 Hz), 115.8, 112.9, 108.5 (q, J = 2.3 Hz); 19F NMR (282 MHz, CDCl3): δ −66.98; HRMS (ESI) m/z [M+H]+ calcd for C11H5Cl2F3N3: 305.9807; found: 305.9801.
4-Chloro-10-fluoro-2-(trifluoromethyl)pyrimido[1,2-b]indazole (3c).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 3c as a yellow solid with a yield of 90%; m.p. 138–140 °C; 1H NMR (300 MHz, CDCl3): δ 7.84 (d, J = 8.4 Hz, 1H), 7.79 (s, 1H), 7.77–7.20 (m, 1H), 7.13 (dd, J = 9.6, 7.5 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 156.6 (d, J = 258.7 Hz), 153.3 (d, J = 4.0 Hz), 142.4 (q, J = 37.6 Hz), 141.9 (d, J = 5.2 Hz), 138.0, 132.0 (d, J = 7.6 Hz), 120.4 (q, J = 273.2 Hz), 113.2 (d, J = 4.9 Hz), 108.4 (q, J = 2.4 Hz), 107.2 (d, J = 17.3 Hz), 105.5 (d, J = 18.2 Hz); 19F NMR (282 MHz, CDCl3): δ −66.86, −114.27; HRMS (ESI) m/z [M+H]+ calcd for C11H5ClF4N3: 290.0102; found: 290.0097.
4-Chloro-2,8-bis-trifluoromethyl-pyrimido[1,2-b]indazole (3d).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9/1) to afford 3d as a yellow solid with a yield of 76%; m.p. 119–121 °C; 1H NMR (300 MHz, CDCl3): δ 8.56 (d, J = 8.7 Hz, 1H), 8.37 (s, 1H), 7.83 (s, 1H), 7.69 (d, J = 8.7 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 190.7, 143.5, 142.6 (q, J = 37.7 Hz), 138.7, 133.3 (q, J = 32.2 Hz), 123.9 (q, J = 271.2 Hz), 122.7, 120.4 (q, J = 273.2 Hz), 119.2 (q, J = 3.0 Hz), 116.3, 115.6 (q, J = 4.7 Hz), 109.0 (q, J = 2.4 Hz); 19F NMR (282 MHz, CDCl3): δ −66.61, −66.96; HRMS (ESI) m/z [M+H]+ calcd for C12H5ClF6N3: 340.0070; found: 340.0065.
4-Chloro-9-nitro-2-(trifluoromethyl)pyrimido[1,2-b]indazole (3e).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9.2/0.8) to afford 3e as a brown solid with a yield of 70%; m.p. 206–208 °C; 1H NMR (300 MHz, CDCl3): δ 9.41 (dd, J = 2.1, 0.6 Hz, 1H), 8.58 (dd, J = 9.3, 2.1 Hz, 1H), 8.09 (dd, J = 9.3, 0.6 Hz, 1H), 7.90 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 153.1, 145.7, 144.2 (q, J = 37.1 Hz), 143.4, 139.8, 125.8, 120.2 (q, J = 273.3 Hz), 119.9, 118.3, 113.7, 109.9 (q, J = 2.3 Hz); 19F NMR (282 MHz, CDCl3): δ −67.11; HRMS (ESI) m/z [M+H]+ calcd for C11H5ClF3N4O2: 317.0047; found: 317.0041.
7-Chloro-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidine (3f).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8.4/0.6) to afford 3f as a green solid with a yield of 81%; m.p. 181–183 °C; 1H NMR (300 MHz, DMSO-d6): δ 8.92 (dd, J = 4.2, 1.2 Hz, 1H), 8.60 (s, 1H), 8.53 (dd, J = 8.7, 1.2 Hz, 1H), 7.81 (dd, J = 8.7, 4.2 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 149.1, 145.9, 143.2 (q, J = 37.8 Hz), 143.0, 139.3, 131.0, 125.8, 125.6, 120.3 (q, J = 273.5 Hz), 109.2 (q, J = 2.3 Hz); 19F NMR (282 MHz, DMSO-d6): δ −66.62; HRMS (ESI) m/z [M+H]+ calcd for C10H5ClF3N4: 273.0149; found: 273.0143.
4-Chloro-2-(trifluoromethyl)pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (3g).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 3g as a light brown solid with a yield of 78%; m.p. 168–170 °C; 1H NMR (300 MHz, DMSO-d6): δ 9.07 (dd, J = 4.2, 1.8 Hz, 1H), 8.89 (dd, J = 8.4, 1.8 Hz, 1H), 8.62 (s, 1H), 7.54 (dd, J = 8.4, 4.2 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 160.8, 156.0, 142.9 (q, J = 37.6 Hz), 142.8, 139.4, 131.2, 120.4 (q, J = 273.3 Hz), 119.0, 109.2 (q, J = 2.4 Hz), 107.8; 19F NMR (282 MHz, DMSO-d6): δ −66.52; HRMS (ESI) m/z [M+H]+ calcd for C10H5ClF3N4: 273.0149; found: 273.0143.
7-Chloro-9-(trifluoromethyl)pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (3h).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 3h as a brown solid with a yield of 85%; m.p. 181–183 °C; 1H NMR (300 MHz, DMSO-d6): δ 9.12 (d, J = 2.1 Hz, 1H), 8.99 (d, J = 2.1 Hz, 1H), 8.76 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 155.0, 149.9, 144.9 (q, J = 38.2 Hz), 144.0, 142.4, 140.7, 124.2, 120.1 (q, J = 273.8 Hz), 110.4 (q, J = 2.2 Hz); 19F NMR (282 MHz, DMSO-d6): δ −66.84; HRMS (ESI) m/z [M+H]+ calcd for C9H4ClF3N5: 274.0101; found: 274.0096.
3.4. General Procedure for the Suzuki–Miyaura Cross-Coupling Reaction: Synthesis of 4-Arylated 2-Trifluoromethyl Pyrimido[1,2-b]indazole Derivatives 4a–h and 5a–q
A solution of compounds 3 (1.0 equiv.) in the mixture of 1,4-dioxane/H2O (4/1) was degassed using argon bubbling. Sodium carbonate (3 equiv.), 4-methoxyphenyl boronic acid (1.3 equiv.) and PdCl2(PPh3)2 (0.1 equiv.) were then added and the reaction mixture was heated to 90 °C for 1h in a sealed tube. After the completion of the reaction monitored using TLC analysis (eluent: petroleum ether/ethyl acetate, 8:2), the solvents were evaporated under reduced pressure, and the crude residue was purified by silica gel column chromatography to provide the desired compounds 4a–h and 5a–q. (1H-NMR, 19F-NMR and 13C-NMR of compounds 4a–h and 5a–q are shown in the Supplementary Materials).
4-(4-Methoxy-phenyl)-2-(trifluoromethyl)pyrimido[1,2-b]indazole (4a).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9/1) to afford 4a as an orange solid with a yield of 93%; m.p. 155–157 °C; 1H NMR (300 MHz, CDCl3): δ 8.45 (dt, J = 8.4, 0.9 Hz, 1H), 8.33 (d, J = 9.0 Hz, 2H), 7.94 (dt, J = 8.4, 0.9 Hz, 1H), 7.72 (ddd, J = 8.4, 6.9, 1.2 Hz, 1H), 7.64 (s, 1H), 7.43 (ddd, J = 8.4, 6.9, 1.2 Hz, 1H), 7.18 (d, J = 9.0 Hz, 2H), 3.97 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 162.4, 151.9, 145.6, 144.1, 142.0 (q, J = 36.2 Hz), 131.4 (2C), 130.6, 122.7, 122.1, 121.3 (q, J = 272.8 Hz), 121.2, 116.9, 114.4 (2C), 114.0, 106.0 (q, J = 2.4 Hz), 55.6; 19F NMR (282 MHz, CDCl3): δ −66.93; HRMS (ESI) m/z [M+H]+ calcd for C18H13F3N3O: 344.1005; found: 344.0998.
10-Chloro-4-(4-methoxy-phenyl)-2-(trifluoromethyl)pyrimido[1,2-b]indazole (4b).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9.8/0.2) to afford 4b as a yellow solid with a yield of 85%; m.p. 145–147 °C; 1H NMR (300 MHz, CDCl3): δ 8.29 (d, J = 8.7 Hz, 2H), 7.85 (d, J = 8.7 Hz, 1H), 7.68 (s, 1H), 7.61 (t, J = 7.5 Hz, 1H), 7.40 (d, J = 7.5 Hz, 1H), 7.17 (d, J = 8.7 Hz, 2H), 3.97 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 162.5, 152.6, 145.9, 143.5, 142.9 (q, J = 36.5 Hz), 131.5 (2C), 130.6, 128.2, 122.4, 122.4, 121.2 (q, J = 273.0 Hz), 115.5, 114.5 (2C), 111.8, 106.6 (q, J = 2.2 Hz), 55.6; 19F NMR (282 MHz, CDCl3): δ −67.17; HRMS (ESI) m/z [M+H]+ calcd for C18H12ClF3N3O: 378.0615; found: 378.0608.
10-Fluoro-4-(4-methoxy-phenyl)-2-(trifluoromethyl)pyrimido[1,2-b]indazole (4c).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9/1) to afford 4c as a yellow solid with a yield of 79%; m.p. 172–174 °C; 1H NMR (300 MHz, CDCl3): δ 8.31 (d, J = 9.0 Hz, 2H), 7.73 (dd, J = 9.9, 7.2 Hz, 1H), 7.68 (s, 1H), 7.66–7.61 (m, 1H), 7.18 (d, J = 9.0 Hz, 2H), 7.05 (dd, J = 9.9, 7.2 Hz, 1H), 3.97 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 162.5, 157.1 (d, J = 257.5 Hz), 153.4 (d, J = 4.2 Hz), 145.9, 143.2 (q, J = 36.5 Hz), 142.5, 131.5 (2C), 131.0 (d, J = 7.7 Hz), 122.3, 121.1 (q, J = 273.0 Hz), 114.4 (2C), 112.9 (d, J = 4.7 Hz), 106.5 (q, J = 2.2 Hz), 106.0 (d, J = 17.6 Hz), 104.6 (d, J = 17.7 Hz), 55.6; 19F NMR (282 MHz, CDCl3): δ −67.05, −114.97; HRMS (ESI) m/z [M+H]+ calcd for C18H12F4N3O: 362.0911; found: 362.0904.
4-(4-Methoxy-phenyl)-2,8-bis-trifluoromethyl-pyrimido[1,2-b]indazole (4d).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9.6/0.4) to afford 4d as a yellow solid with a yield of 85%; m.p. 186–188 °C; 1H NMR (300 MHz, CDCl3): δ 8.56 (dd, J = 8.7, 0.9 Hz, 1H), 8.33 (d, J = 9.0 Hz, 2H), 8.29 (br s, 1H), 7.72 (s, 1H), 7.60 (dd, J = 8.7, 0.9 Hz, 1H), 7.19 (d, J = 9.0 Hz, 2H), 3.98 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 162.7, 150.5, 146.5, 144.0, 143.3 (q, J = 36.7 Hz), 132.4 (q, J = 32.0 Hz), 131.6 (2C), 124.2 (q, J = 271.0 Hz), 122.6, 122.1, 121.1 (q, J = 272.9 Hz), 117.91 (q, J = 3.0 Hz), 115.4, 115.2 (q, J = 4.8 Hz), 114.5 (2C), 106.9 (q, J = 2.4 Hz), 55.6; 19F NMR (282 MHz, CDCl3): δ −62.41, −67.12; HRMS (ESI) m/z [M+H]+ calcd for C19H12F6N3O: 412.0879; found: 412.0872.
4-(4-Methoxy-phenyl)-9-nitro-2-(trifluoromethyl)pyrimido[1,2-b]indazole (4e).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9/1) to afford 4e as an orange solid with a yield of 84%; m.p. 201–203 °C; 1H NMR (300 MHz, CDCl3): δ 9.47 (d, J = 1.8 Hz, 1H), 8.53 (dd, J = 9.6, 2.1 Hz, 1H), 8.34 (d, J = 9.0 Hz, 2H), 7.78 (dd, J = 9.6, 2.1 Hz, 1H), 7.80 (s, 1H), 7.21 (d, J = 9.0 Hz, 2H), 3.99 (s, 3H); 13C NMR (75 MHz, DMSO-d6): δ 163.0, 153.0, 147.6, 146.3, 144.9 (q, J = 36.8 Hz), 142.5, 131.8 (2C), 125.0, 121.6, 120.8 (q, J = 273.4 Hz), 120.1, 117.7, 114.6 (2C), 112.8, 107.7 (q, J = 2.3 Hz), 55.7; 19F NMR (282 MHz, CDCl3): δ −67.32; HRMS (ESI) m/z [M+H]+ calcd for C18H12F3N4O3: 389.0856; found: 389.0849.
7-(4-Methoxyphenyl)-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidine (4f).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 4f as a yellow solid with a yield of 73%; m.p. 186–188 °C; 1H NMR (300 MHz, CDCl3): δ 8.92 (dd, J = 4.2, 1.2 Hz, 1H), 8.31 (d, J = 9.0 Hz, 2H), 8.30 (dd, J = 8.7, 1.2 Hz, 1H), 7.72 (s, 1H), 7.63 (dd, J = 8.7, 4.2 Hz, 1H), 7.20 (d, J = 9.0 Hz, 2H), 3.97 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 162.6, 147.9, 146.8, 145.4, 143.9 (q, J = 36.7 Hz), 143.3, 131.6 (2C), 130.6, 125.0, 122.2, 121.9, 121.0 (q, J = 273.4 Hz), 114.5 (2C), 107.1 (q, J = 2.2 Hz), 55.6; 19F NMR (282 MHz, CDCl3): δ −67.04; HRMS (ESI) m/z [M+H]+ calcd for C17H12F3N4O: 345.0957; found: 345.0950.
4-(4-Methoxyphenyl)-2-(trifluoromethyl)pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (4g).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 4g as a yellow solid with a yield of 93%; m.p. 184–186 °C; 1H NMR (300 MHz, CDCl3): δ 9.06 (dd, J = 4.2, 1.5 Hz, 1H), 8.80 (dd, J = 8.7, 1.5 Hz, 1H), 8.47 (d, J = 9.0 Hz, 2H), 7.78 (s, 1H), 7.40 (dd, J = 8.7, 4.2 Hz, 1H), 7.17 (d, J = 9.0 Hz, 2H), 3.97 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 162.7, 161.1, 155.3, 146.6, 143.6 (q, J = 36.4 Hz), 143.4, 132.0 (2C), 130.9, 121.9, 121.1 (q, J = 273.4 Hz), 117.9, 114.4 (2C), 106.8 (q, J = 2.3 Hz), 106.6, 55.6; 19F NMR (282 MHz, CDCl3): δ −67.22; HRMS (ESI) m/z [M+H]+ calcd for C17H12F3N4O: 345.0957; found: 345.0951.
7-(4-Methoxyphenyl)-9-(trifluoromethyl)pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (4h).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 4h as a yellow solid with a yield of 35%; m.p. 199–201 °C; 1H NMR (300 MHz, CDCl3): δ 8.99 (d, J = 1.8 Hz, 1H), 8.89 (d, J = 1.8 Hz, 1H), 8.43 (d, J = 8.7 Hz, 2H), 7.86 (s, 1H), 7.19 (d, J = 8.7 Hz, 2H), 3.98 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 163.1, 154.7, 149.2, 147.8, 146.1, 145.4 (q, J = 37.6 Hz), 142.8, 132.1 (2C), 123.7, 121.3, 120.8 (q, J = 273.6 Hz), 114.5 (2C), 108.0 (q, J = 2.3 Hz), 55.7; 19F NMR (282 MHz, CDCl3): δ −67.28; HRMS (ESI) m/z [M+H]+ calcd for C16H11F3N5O: 346.0910; found: 346.0903.
4-p-Tolyl-2-(trifluoromethyl)pyrimido[1,2-b]indazole (5a).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9.8/0.2) to afford 5a as a yellow solid with a yield of 98%; m.p. 159–161 °C; 1H NMR (300 MHz, CDCl3): δ 8.46 (d, J = 8.7 Hz, 1H), 8.18 (d, J = 8.1 Hz, 2H), 7.96 (d, J = 8.7 Hz, 1H), 7.73 (td, J = 6.9, 1.2 Hz, 1H), 7.64 (s, 1H), 7.49 (d, J = 8.1 Hz, 2H), 7.43 (td, J = 6.9, 1.2 Hz, 1H), 2.53 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 151.9, 146.0, 144.0, 142.5, 142.0 (q, J = 36.4 Hz), 130.6, 129.7 (2C), 129.5 (2C), 127.7, 122.2, 121.3 (q, J = 272.8 Hz), 121.2, 117.0, 114.1, 106.6 (q, J = 2.4 Hz), 21.7; 19F NMR (282 MHz, CDCl3): δ −66.94; HRMS (ESI) m/z [M+H]+ calcd for C18H13F3N3: 328.1056; found: 328.1051.
7-(3-Methylphenyl)-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidine (5b).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9/1) to afford 5b as a yellow solid with a yield of 65%; m.p. 139–141 °C; 1H NMR (300 MHz, CDCl3): δ 8.93 (dd, J = 4.2, 1.5 Hz, 1H), 8.93 (dd, J = 8.7, 1.5 Hz, 1H), 8.04–8.00 (m, 2H), 7.72 (s, 1H), 7.64 (dd, J = 8.7, 4.2 Hz, 1H), 7.59 (t, J = 7.5 Hz, 1H), 7.51 (d, J = 7.5 Hz, 1H), 2.55 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 148.1, 147.3, 145.5, 143.9 (q, J = 36.7 Hz), 143.2, 139.0, 132.9, 130.7, 130.0, 129.9, 129.0, 126.8, 125.2, 125.1, 121.0 (q, J = 273.4 Hz), 108.2 (q, J = 2.2 Hz), 21.6; 19F NMR (282 MHz, CDCl3): δ −67.04; HRMS (ESI) m/z [M+H]+ calcd for C17H12F3N4: 329.1008; found: 329.1003.
4-(4-Tert-Butyl-phenyl)-2,8-bis-trifluoromethyl-pyrimido[1,2-b]indazole (5c).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9.8/0.2) to afford 5c as a yellow solid with a yield of 96%; m.p. 170–172 °C; 1H NMR (300 MHz, CDCl3): δ 8.58 (dd, J = 8.4, 0.9 Hz, 1H), 8.30 (s, 1H), 8.23 (d, J = 8.4 Hz, 2H), 7.74 (s, 1H), 7.72 (d, J = 8.4 Hz, 2H), 7.62 (dd, J = 8.4, 0.9 Hz, 1H), 1.44 (s, 9H); 13C NMR (75 MHz, CDCl3): δ 156.0, 150.5, 146.8, 143.9, 143.1 (q, J = 36.7 Hz), 132.4 (q, J = 31.9 Hz), 129.4 (2C), 127.2, 126.2 (2C), 124.1 (q, J = 271.0 Hz), 122.6, 121.0 (q, J = 273.1 Hz), 118.0 (q, J = 3.0 Hz), 115.5, 115.3 (q, J = 4.8 Hz), 107.6 (q, J = 2.2 Hz), 35.2, 31.1 (3C); 19F NMR (282 MHz, CDCl3): δ −62.42, −67.13; HRMS (ESI) m/z [M+H]+ calcd for C22H18F6N3: 438.1399; found: 438.1397.
7-(3-Methoxyphenyl)-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidine (5d).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 5d as a yellow solid with a yield of 93%; m.p. 189–191 °C; 1H NMR (300 MHz, CDCl3): δ 8.94 (dd, J = 4.2, 1.5 Hz, 1H), 8.93 (dd, J = 8.7, 1.5 Hz, 1H), 7.81–7.74 (m, 2H), 7.74 (s, 1H), 7.64 (dd, J = 8.7, 4.2 Hz, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.23 (ddd, J = 8.4, 2.7, 0.9 Hz, 1H), 3.96 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 159.8, 148.2, 146.9, 145.5, 143.9 (q, J = 36.9 Hz), 143.2, 131.1, 130.7, 130.2, 125.2, 125.1, 121.9, 120.9 (q, J = 272.3 Hz), 117.6, 115.3, 108.2 (q, J = 2.2 Hz), 55.6; 19F NMR (282 MHz, CDCl3): δ −67.02; HRMS (ESI) m/z [M+H]+ calcd for C17H12F3N4O: 345.0957; found: 345.0952.
7-(3,4-Dimethoxyphenyl)-9-(trifluoromethyl)pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (5e).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 7/3) to afford 5e as a yellow solid with a yield of 44%; m.p. 200–202 °C; 1H NMR (300 MHz, CDCl3): δ 9.0 (d, J = 1.8 Hz, 1H), 8.89 (d, J = 1.8 Hz, 1H), 8.03 (d, J = 2.4 Hz, 1H), 7.99 (dd, J = 8.4, 2.4 Hz, 1H), 7.87 (s, 1H), 7.15 (d, J = 8.4 Hz, 1H), 4.06 (s, 3H), 4.05 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 154.7, 152.8, 149.2, 149.1, 147.8, 145.4 (q, J = 37.0 Hz), 143.3, 142.8, 124.3, 123.7, 121.5, 120.8 (q, J = 273.6 Hz), 112.7, 111.2, 108.3 (q, J = 2.2 Hz), 56.4, 56.2; 19F NMR (282 MHz, CDCl3): δ −67.24; HRMS (ESI) m/z [M+H]+ calcd for C17H13F3N5O2: 376.1015; found: 376.1008
7-(2,5-Dimethoxyphenyl)-9-(trifluoromethyl)pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (5f).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 7/3) to afford 5f as a yellow solid with a yield of 42%; m.p. 208–210 °C; 1H NMR (300 MHz, CDCl3): δ 8.97 (d, J = 1.8 Hz, 1H), 8.88 (d, J = 1.8 Hz, 1H), 7.86 (s, 1H), 7.33 (d, J = 3.0 Hz, 1H), 7.20 (dd, J = 9.0, 3.0 Hz, 1H), 7.15 (d, J = 9.0 Hz, 1H), 3.86 (s, 3H), 3.80 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 154.7, 153.5, 151.8, 148.9, 146.5, 144.8 (q, J = 37.3 Hz), 142.7, 142.1, 123.9, 120.8 (q, J = 273.6 Hz), 118.9, 118.8, 116.0, 113.2, 111.4 (q, J = 2.2 Hz), 56.3, 56.0; 19F NMR (282 MHz, CDCl3): δ −67.18; HRMS (ESI) m/z [M+H]+ calcd for C17H13F3N5O2: 376.1015; found: 376.1008.
7-(2,4-dimethoxyphenyl)-9-(trifluoromethyl)pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (5g).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 7/3) to afford 11g as a yellow solid with a yield of 41%; m.p. 206–208 °C; 1H NMR (300 MHz, CDCl3): δ 8.95 (d, J = 1.5 Hz, 1H), 8.85 (d, J = 1.5 Hz, 1H), 7.95 (dd, J = 8.4, 2.1 Hz, 1H), 7.89 (s, 1H), 6.74 (dd, J = 8.4, 2.1 Hz, 1H), 6.68 (d, J = 2.1 Hz, 1H), 3.95 (s, 3H), 3.85 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 164.1, 159.2, 154.5, 148.9, 146.3, 144.8 (q, J = 37.0 Hz), 142.4, 142.3, 132.5, 124.0, 120.9 (q, J = 273.6 Hz), 111.2 (q, J = 2.3 Hz), 110.9, 105.3, 99.2, 55.9, 55.7; 19F NMR (282 MHz, CDCl3): δ −67.24; HRMS (ESI) m/z [M+H]+ calcd for C17H13F3N5O2: 376.1015; found: 376.1009.
7-[4-(Methylsulfanyl)phenyl]-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidine (5h).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 5h as a yellow solid with a yield of 82%; m.p. 187–189 °C; 1H NMR (300 MHz, CDCl3): δ 8.93 (dd, J = 3.9, 1.2 Hz, 1H), 8.30 (dd, J = 8.7, 1.2 Hz, 1H), 8.24 (d, J = 8.4 Hz, 2H), 7.73 (s, 1H), 7.64 (dd, J = 8.7, 3.9 Hz, 1H), 7.51 (d, J = 8.4 Hz, 2H), 2.62 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 148.1, 146.5, 145.5, 145.0, 143.9 (q, J = 36.9 Hz), 143.3, 130.6, 129.9 (2C), 125.7, 125.6 (2C), 125.1, 125.1, 121.0 (q, J = 273.4 Hz), 107.5 (q, J = 2.2 Hz), 14.9; 19F NMR (282 MHz, CDCl3): δ −67.03; HRMS (ESI) m/z [M+H]+ calcd for C17H12F3N4S: 361.0729; found: 361.0724.
4-(2H-1,3-Benzodioxol-4-yl)-2-(trifluoromethyl)pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (5i).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 5i as a yellow solid with a yield of 88%; m.p. 212–214 °C; 1H NMR (300 MHz, CDCl3): δ 9.07 (dd, J = 4.2, 1.8 Hz, 1H), 8.80 (dd, J = 8.1, 1.8 Hz, 1H), 8.00 (d, J = 1.8 Hz, 1H), 7.93 (dd, J = 8.4, 1.8 Hz, 1H), 7.75 (s, 1H), 7.41 (dd, J = 8.1, 4.2 Hz, 1H), 7.08 (dd, J = 8.4, 1.8 Hz, 1H), 6.16 (s, 2H); 13C NMR (75 MHz, CDCl3): δ 161.0, 155.4, 151.0, 148.1, 146.4, 143.6 (q, J = 36.6 Hz), 143.4, 130.9, 125.6, 123.4, 121.0 (q, J = 273.1 Hz), 118.0, 110.0, 108.8, 107.2 (q, J = 2.4 Hz), 106.7, 102.1; 19F NMR (282 MHz, CDCl3): δ −67.22; HRMS (ESI) m/z [M+H]+ calcd for C17H10F3N4O2: 359.0750; found: 359.0746.
4-(4-Chloro-phenyl)-2,8-bis-trifluoromethyl-pyrimido[1,2-b]indazole (5j).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9.8/0.2) to afford 5j as a yellow solid with a yield of 50%; m.p. 137–139 °C; 1H NMR (300 MHz, CDCl3): δ 8.57 (dd, J = 8.7, 1.2 Hz, 1H), 8.27 (s, 1H), 8.25 (d, J = 8.7 Hz, 2H), 7.23 (s, 1H), 7.68 (d, J = 8.7 Hz, 2H), 7.63 (dd, J = 8.7, 1.2 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 150.5, 145.4, 143.9, 143.9, 143.0 (q, J = 36.8 Hz), 132.6 (q, J = 32.0 Hz), 131.0 (2C), 129.4 (2C), 128.4, 124.1 (q, J = 271.0 Hz), 122.6, 120.9 (q, J = 273.1 Hz), 118.4 (q, J = 3.1 Hz), 115.5, 115.3 (q, J = 4.7 Hz), 107.6 (q, J = 2.3 Hz); 19F NMR (282 MHz, CDCl3): δ −62.45, −67.08; HRMS (ESI) m/z [M+H]+ calcd for C18H9ClF6N3: 416.0383; found: 416.0379
4-(4-Fluoro-phenyl)-2-(trifluoromethyl)pyrimido[1,2-b]indazole (5k).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9.9/0.1) to afford 5k as an orange solid with a yield of 88%; m.p. 186–188 °C; 1H NMR (300 MHz, CDCl3): δ 8.47 (d, J = 8.4 Hz, 1H), 8.33 (dd, J = 8.7, 5.4 Hz, 2H), 7.95 (d, J = 8.4 Hz, 1H), 7.74 (td, J = 6.9, 1.2 Hz, 1H), 7.64 (s, 1H), 7.47 (td, J = 6.9, 1.2 Hz, 1H), 7.38 (dd, J = 8.7, 5.4 Hz, 2H); 13C NMR (75 MHz, CDCl3): δ 164.6 (d, J = 252.4 Hz), 151.9, 144.7, 144.0, 142.0 (q, J = 36.4 Hz), 132.0, 131.9, 130.8, 126.7 (d, J = 3.2 Hz), 122.5, 121.2, 121.2 (q, J = 272.8 Hz), 117.0, 116.4, 116.1, 114.1, 106.7 (q, J = 2.2 Hz); 19F NMR (282 MHz, CDCl3): δ −66.93, −106.56; HRMS (ESI) m/z [M+H]+ calcd for C17H10F4N3: 332.0805; found: 332.0799.
2-Trifluoromethyl-4-(4-trifluoromethyl-phenyl)-pyrimido[1,2-b]indazole (5l).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9.8/0.2) to afford 5l as an orange solid with a yield of 84%; m.p. 180–182 °C; 1H NMR (300 MHz, CDCl3): δ 8.47 (d, J = 8.4 Hz, 1H), 8.39 (d, J = 8.1 Hz, 2H), 7.95 (d, J = 8.4 Hz, 3H), 7.75 (td, J = 7.8, 0.9 Hz, 1H), 7.67 (s, 1H), 7.48 (td, J = 7.8, 0.9 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 152.0, 144.1, 144.0, 141.9 (q, J = 36.6 Hz), 134.0, 133.4 (q, J = 32.3 Hz), 131.0, 130.0 (2C), 129.0, 126.0 (q, J = 3.5 Hz), 123.6 (q, J = 271.4 Hz), 122.8, 121.2, 121.1 (q, J = 272.2 Hz), 117.0, 114.2, 107.3 (q, J = 1.6 Hz); 19F NMR (282 MHz, CDCl3): δ −63.10, −66.92; HRMS (ESI) m/z [M+H]+ calcd for C18H10F6N3: 382.0773; found: 382.0767.
4-(4-Trifluoromethoxy-phenyl)-2-(trifluoromethyl)pyrimido[1,2-b]indazole (5m).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9.8/0.2) to afford 5m as an orange solid with a yield of 86%; m.p. 118–120 °C; 1H NMR (300 MHz, CDCl3): δ 8.46 (d, J = 8.7 Hz, 1H), 8.36 (d, J = 9.0 Hz, 2H), 7.95 (d, J = 8.7 Hz, 1H), 7.75 (td, J = 7.5, 0.9 Hz, 1H), 7.65 (s, 1H), 7.53 (d, J = 9.0 Hz, 2H), 7.49 (td, J = 7.5, 0.9 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 152.0, 151.5, 151.4, 144.3, 144.0, 142.0 (q, J = 36.6 Hz), 131.5 (2C), 130.9, 129.0, 122.6, 121.2, 121.2 (q, J = 272.8 Hz), 121.1, 120.4 (q, J = 257.3 Hz), 117.0, 114.2, 107.0 (q, J = 2.2 Hz); 19F NMR (282 MHz, CDCl3): δ −57.53, −66.93; HRMS (ESI) m/z [M+H]+ calcd for C18H10F6N3O: 398.0722; found: 398.0718.
Ethyl 4-[9-(trifluoromethyl)pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidin-7-yl]benzoate (5n).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9/1) to afford 5n as a yellow solid with a yield of 42%; m.p. 148–150 °C; 1H NMR (300 MHz, CDCl3): δ 9.02 (d, J = 1.8 Hz, 1H), 8.93 (d, J = 1.8 Hz, 1H), 8.38 (q, J = 7.8 Hz, 4H), 7.91 (s, 1H), 4.49 (q, J = 7.8 Hz, 2H), 1.48 (t, J = 6.9 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 165.4, 154.9, 149.4, 147.0, 145.4 (q, J = 36.0 Hz), 143.3, 142.6, 133.9, 133.1, 130.1 (2C), 130.0 (2C), 123.8, 120.6 (q, J = 273.7 Hz), 109.5 (q, J = 2.2 Hz), 61.6, 14.3; 19F NMR (282 MHz, CDCl3): δ −67.22; HRMS (ESI) m/z [M+H]+ calcd for C18H13F3N5O2: 388.1015; found: 388.1010.
4-[2-(Trifluoromethyl)pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidin-4-yl]benzaldehyde (5o).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 5o as a yellow solid with a yield of 89%; m.p. 237–239 °C; 1H NMR (300 MHz, CDCl3): δ 10.20 (s, 1H), 9.10 (dd, J = 4.2, 1.8 Hz, 1H), 8.84 (dd, J = 8.1, 1.8 Hz, 1H), 8.55 (d, J = 8.4 Hz, 2H), 8.18 (d, J = 8.4 Hz, 2H), 7.85 (s, 1H), 7.47 (dd, J = 8.1, 4.2 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 191.3, 161.2, 155.7, 145.3, 143.5 (q, J = 37.0 Hz), 143.2, 138.3, 135.2, 130.9, 130.6 (2C), 129.9 (2C), 120.9 (q, J = 273.1 Hz), 118.6, 108.3 (q, J = 2.4 Hz), 106.9; 19F NMR (282 MHz, CDCl3): δ −67.15; HRMS (ESI) m/z [M+H]+ calcd for C17H10F3N4O: 343.0801; found: 343.0798.
4-[9-(Trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidin-7-yl]benzonitrile (5p).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8.4/1.6) to afford 5p as a yellow solid with a yield of 33%; m.p. 201–203 °C; 1H NMR (300 MHz,CDCl3): δ 8.98 (dd, J = 3.9, 1.2 Hz, 1H), 8.40 (d, J = 8.7 Hz, 2H), 8.31 (dd, J = 8.7, 1.2 Hz, 1H), 8.00 (d, J = 8.7 Hz, 2H), 7.77 (s, 1H), 7.67 (dd, J = 8.7, 3.9 Hz, 1H); 13C NMR (75 MHz, CDCl3): δ 148.7, 145.5, 144.6, 143.8 (q, J = 37.4 Hz), 143.2, 134.1, 132.7 (2C), 130.7, 130.4 (2C), 125.5, 125.2, 120.7 (q, J = 273.4 Hz), 117.8, 115.6, 108.5 (q, J = 2.2 Hz); 19F NMR (282 MHz, CDCl3): δ −66.99; HRMS (ESI) m/z [M+H]+ calcd for C17H9F3N5: 340.0804; found: 340.0800
7-(4-Nitrophenyl)-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidine (5q).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 5q as a yellow orange solid with a yield of 77%; m.p. 284–286 °C; 1H NMR (300 MHz, CDCl3): δ 8.99 (dd, J = 4.2, 1.5 Hz, 1H), 8.56 (d, J = 9.0 Hz, 2H), 8.47 (d, J = 9.0 Hz, 2H), 8.32 (dd, J = 8.7, 1.5 Hz, 1H), 7.80 (s, 1H), 7.69 (dd, J = 8.7, 4.2 Hz, 1H); 13C NMR (75 MHz, DMSO-d6): δ 149.4, 148.5, 145.3, 145.2, 143.0, 142.4 (q, J = 37.8 Hz), 136.4, 132.2 (2C), 130.6, 125.9, 125.5, 124.0 (2C), 121.6 (q, J = 273.2 Hz), 116.3, 110.2 (q, J = 1.9 Hz); 19F NMR (282 MHz, CDCl3): δ −66.97; HRMS (ESI) m/z [M+H]+calcd for C16H9F3N5O2: 360.0702; found: 360.0698.
3.5. General Procedure for the Synthesis of 4-Amino-2-trifluoromethyl Pyrimido[1,2-b]indazole Derivatives 6a–g
A mixture of compounds 3f (1mmol, 1.0 equiv.) and the corresponding amine (1.2 mmol, 1.2 equiv.) was dissolved in absolute EtOH (5 mL), Et3N (2 mmol) was then added and the mixture was refluxed for 2–3 h. The progress of the reaction was monitored by TLC analysis (eluent: petroleum ether/ethyl acetate, 8:2). After cooling to room temperature, the solvent was evaporated under reduced pressure and the crude residue was tritured in water and then filtered, dried and recrystallized from EtOH to give the pure products 6a–g. (1H-NMR, 19F-NMR and 13C-NMR of compounds 6a–g are shown in Supplementary Materials).
N-Propyl-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidin-7-amine (6a).
Compound 6a was obtained as a light brown solid with a yield of 52%; m.p. 187–189 °C; 1H NMR (300 MHz, CDCl3): δ 8.78 (dd, J = 3.9, 1.2 Hz, 1H), 8.14 (dd, J = 8.7, 1.2 Hz, 1H), 7.56 (dd, J = 8.7, 3.9 Hz, 1H), 7.00 (br s, 1H), 6.74 (s, 1H), 3.59 (q, J = 6.9 Hz, 2H), 1.93 (sext, J = 7.2 Hz, 2H), 1.16 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 146.8, 146.3, 146.0 (q, J = 35.8 Hz), 144.5, 142.1, 130.6, 124.8, 124.3, 121.2 (q, J = 273.6 Hz), 85.9 (q, J = 2.6 Hz), 44.3, 22.3, 11.4; 19F NMR (282 MHz, CDCl3): δ −67.53; HRMS (ESI) m/z [M+H]+ calcd for C13H13F3N5: 296.1117; found: 296.1113.
N-[(4-Methoxyphenyl)methyl]-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidin-7-amine (6b).
Compound 6b was obtained as a light brown solid with a yield of 62%; m.p. 159–161 °C; 1H NMR (300 MHz, CDCl3): δ 8.80 (dd, J = 4.2, 0.9 Hz, 1H), 8.15 (dd, J = 8.7, 0.9 Hz, 1H), 7.75 (dd, J = 8.7, 4.2 Hz, 1H), 7.37 (d, J = 8.4 Hz, 2H), 6.97 (d, J = 8.4 Hz, 2H), 6.80 (s, 1H), 4.73 (d, J = 5.4 Hz, 2H), 3.85 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 159.8, 146.5, 145.9 (q, J = 35.8 Hz), 144.6, 142.2, 132.0, 130.8, 129.0 (2C), 126.9, 124.9, 124.2, 121.1 (q, J = 273.6 Hz), 114.6 (2C), 86.3 (q, J = 2.4 Hz), 55.4, 46.2; 19F NMR (282 MHz, CDCl3): δ −67.52; HRMS (ESI) m/z [M+H]+ calcd for C18H15F3N5O: 374.1223; found: 374.1221.
7-(Morpholin-4-yl)-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidine (6c).
Compound 6c was obtained as a gray solid with a yield of 60%; m.p. 270–272 °C; 1H NMR (300 MHz, CDCl3): δ 8.84 (dd, J = 3.9, 1.2 Hz, 1H), 8.22 (dd, J = 8.7, 1.2 Hz, 1H), 7.59 (dd, J = 8.7, 3.9 Hz, 1H), 6.91 (s, 1H), 4.10–4.08 (m, 4H), 4.03–4.01 (m, 4H); 13C NMR (75 MHz, CDCl3): δ 150.1, 147.1, 145.2 (q, J = 36.1 Hz), 144.5, 144.1, 130.1, 125.0, 124.7, 121.0 (q, J = 273.7 Hz), 93.3, 66.2 (2C), 48.5 (2C); 19F NMR (282 MHz, CDCl3): δ −67.27; HRMS (ESI) m/z [M+H]+ calcd for C14H13F3N5O: 324.1066; found: 324.1064.
7-(Pyrrolidin-1-yl)-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidine (6d).
Compound 6d was obtained as a yellow solid with a yield of 78%; m.p. 271–273 °C; 1H NMR (300 MHz, CDCl3): δ 8.74 (dd, J = 3.9, 1.2 Hz, 1H), 8.10 (dd, J = 8.7, 1.2 Hz, 1H), 7.50 (dd, J = 8.7, 3.9 Hz, 1H), 6.50 (s, 1H), 4.25–4.21 (m, 4H), 2.19–2.15 (m, 4H); 13C NMR (75 MHz, CDCl3): δ 147.7, 146.0 (q, J = 35.2 Hz), 145.9, 144.7, 144.1, 129.2, 124.5, 124.4, 121.3 (q, J = 273.6 Hz), 89.7 (q, J = 2.5 Hz), 51.8 (2C), 25.5 (2C); 19F NMR (282 MHz, CDCl3): δ −67.77; HRMS (ESI) m/z [M+H]+ calcd for C14H13F3N5: 308.1117; found: 308.1115.
N-Phenyl-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidin-7-amine (6e).
Compound 6e was obtained as a light brown solid with a yield of 48%; m.p. 170–172 °C; 1H NMR (300 MHz, CDCl3): δ 8.85 (dd, J = 3.6, 1.2 Hz, 1H), 8.71 (s, 1H), 8.24 (d, J = 8.7, 1.2 Hz, 1H), 7.65 (dd, J = 8.7, 3.9 Hz, 1H), 7.60 (t, J = 7.8 Hz, 2H), 7.50 (d, J = 8.1 Hz, 2H), 7.44 (d, J = 8.1 Hz, 1H), 7.10 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 146.8, 146.0 (q, J = 36.0 Hz), 145.2, 144.7, 142.4, 135.2, 130.8, 130.4 (2C), 127.7, 125.1, 124.4, 124.1 (2C), 121.0 (q, J = 273.6 Hz), 87.4 (q, J = 2.5 Hz); 19F NMR (282 MHz, CDCl3): δ −67.46; HRMS (ESI) m/z [M+H]+ calcd for C16H11F3N5: 330.0961; found: 330.0956.
N-(4-Methylphenyl)-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidin-7-amine (6f).
Compound 6f was obtained as a light brown solid with a yield of 49%; m.p. 207–209 °C; 1H NMR (300 MHz, CDCl3): δ 8.83 (dd, J = 3.9, 1.2 Hz, 1H), 8.61 (s, 1H), 8.21 (dd, J = 9.0, 1.2 Hz, 1H), 7.60 (dd, J = 9.0, 3.9 Hz, 1H), 7.34 (br s, 4H), 7.02 (s, 1H), 2.47 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 146.7, 146.0 (q, J = 36.0 Hz), 145.5, 144.7, 142.4, 137.9, 132.4, 130.9 (2C), 130.8, 125.0, 124.3 (2C), 124.2, 121.1 (q, J = 273.6 Hz), 87.3 (q, J = 2.5 Hz), 21.1; 19F NMR (282 MHz, CDCl3): δ −67.47; HRMS (ESI) m/z [M+H]+ calcd for C17H13F3N5: 344.1117; found: 344.1113.
N-(4-Bromophenyl)-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidin-7-amine (6g).
Compound 6g was obtained as a light brown solid with a yield of 54%; m.p. 249–251 °C; 1H NMR (300 MHz, CDCl3): δ 8.84 (dd, J = 4.2, 1.2 Hz, 1H), 8.63 (s, 1H), 8.20 (dd, J = 8.7, 1.2 Hz, 1H), 7.72 (d, J = 8.7 Hz, 2H), 7.61 (dd, J = 8.7, 4.2 Hz, 1H), 7.39 (d, J = 8.7 Hz, 2H), 7.05 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 146.8, 146.4, 144.6, 144.4 (q, J = 34.8 Hz), 142.8, 136.4, 133.1 (2C), 130.4, 127.2 (2C), 125.6, 124.6, 121.7 (q, J = 273.3 Hz), 119.5, 88.3 (q, J = 2.3 Hz); 19F NMR (282 MHz, CDCl3): δ −67.44; HRMS (ESI) m/z [M+H]+ calcd for C16H10BrF3N5: 408.0066; found: 408.0066.
3.6. General Procedure for the Synthesis of 4-(Alkylthio or Arylthiol)-2-(trifluoromethyl)pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine 7a–c
A mixture of compound 3g (1 mmol, 1.0 equiv.) and the corresponding thiol (1.1 mmol, 1.1 equiv.) was dissolved in absolute EtOH (5 mL), Et3N (2 mmol) was then added and the mixture was stirred at room temperature for 30 min. Then, the solvent was evaporated under reduced pressure and the crude residue was filtered, washed with water, dried, and recrystallized from EtOH to give the pure products 7a–c. (1H-NMR, 19F-NMR and 13C-NMR of compounds 7a–c are shown in Supplementary Materials).
Ethyl{[2-(trifluoromethyl)pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidin-4-yl]sulfanyl}acetate (7a).
Compound 7a was obtained as a yellow solid with a yield of 67%; m.p. 176–178 °C; 1H NMR (300 MHz, DMSO-d6): δ 9.03 (dd, J = 4.2, 1.2 Hz, 1H), 8.85 (dd, J = 8.1, 1.2 Hz, 1H), 7.99 (s, 1H), 7.78 (dd, J = 8.1, 4.2 Hz, 1H), 4.65 (s, 2H), 4.21 (q, J = 7.2 Hz, 2H), 1.23 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, DMSO-d6): δ 168.3, 160.5, 155.7, 150.8, 141.7 (q, J = 35.8 Hz), 141.2, 131.4, 121.4 (q, J = 273.3 Hz), 118.6, 106.5, 105.7 (q, J = 2.1 Hz), 62.3, 32.9, 14.4; 19F NMR (282 MHz, DMSO-d6): δ −66.63; HRMS (ESI) m/z [M+H]+ calcd for C14H12F3N4O2S: 357.0627; found: 357.0622.
4-[(4-Methoxyphenyl)sulfanyl]-2-(trifluoromethyl)pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (7b).
Compound 7b was obtained as a beige solid with a yield of 79%; m.p. 247–249 °C; 1H NMR (300 MHz, CDCl3): δ 9.08 (dd, J = 4.2, 1.5 Hz, 1H), 8.75 (dd, J = 8.1, 1.5 Hz, 1H), 7.67 (d, J = 8.7 Hz, 2H), 7.39 (dd, J = 8.1, 4.2 Hz, 1H), 7.15 (d, J = 8.7 Hz, 2H), 6.82 (s, 1H), 3.96 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 162.3, 161.0, 155.4, 154.0, 142.7 (q, J = 36.4 Hz), 141.4, 137.7 (2C), 131.0, 120.8 (q, J = 273.4 Hz), 118.0, 116.5 (2C), 115.0, 106.6, 103.7 (q, J = 2.5 Hz), 55.6; 19F NMR (282 MHz, CDCl3): δ −67.23; HRMS (ESI) m/z [M+H]+ calcd for C17H12F3N4OS: 377.0678; found: 377.0676.
4-[(4-Fluorophenyl)sulfanyl]-2-(trifluoromethyl)pyrido[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (7c).
Compound 7c was obtained as a yellow solid with a yield of 56%; m.p. 221–223 °C; 1H NMR (300 MHz, DMSO-d6): δ 9.06 (dd, J = 4.2, 1.5 Hz, 1H), 8.87 (dd, J = 8.1, 1.5 Hz, 1H), 7.96 (dd, J = 8.7, 5.4 Hz, 2H), 7.59 (t, J = 8.7 Hz, 2H), 7.50 (dd, J = 8.1, 4.2 Hz, 1H), 6.81 (s, 1H); 13C NMR (75 MHz, DMSO-d6): δ 164.6 (d, J = 249.3 Hz), 160.7, 155.9, 153.0, 152.9, 141.8 (q, J = 35.8 Hz), 141.4, 139.1 (d, J = 9.1 Hz), 131.5, 121.3 (q, J = 271.3 Hz), 120.8 (d, J = 3.1 Hz), 118.9, 118.8, 118.6, 106.5, 104.4 (q, J = 2.5 Hz); 19F NMR (282 MHz, DMSO-d6): δ −66.0.1, −107.87; HRMS (ESI) m/z [M+H]+ calcd for C16H9F4N4S: 365.0478; found: 365.0477.
3.7. General Procedure for the Synthesis of 7-Alkoxy (or 7-Phenoxy)-2-trifluoromethyl Pyrimido[1,2-b]indazole Derivatives 8a–c
A solution of the corresponding alcohol or phenol (1.1 equiv.) in the mixture of acetonitrile was degassed through argon bubbling. Potassium carbonate (1.3 equiv.) was then added and the mixture was stirred at room temperature for 15 min. Then, compound 3f (or 3h) (1 equivalent) was added and the reaction mixture was refluxed for 2–3 h. After the completion of the reaction monitored by TLC analysis (eluent: petroleum ether/ethyl acetate, 8:2), the solvent was evaporated under reduced pressure, and the crude residue was purified using silica gel column chromatography to give the desired products 8a–c. (1H-NMR, 19F-NMR and 13C-NMR of compounds 8a–c are shown in Supplementary Materials).
7-Ethoxy-9-(trifluoromethyl)pyrido[3′,2′:3,4]pyrazolo[1,5-a]pyrimidine (8a).
The purification of the crude product by chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 8a as a white crystal with a yield of 69%; m.p. 189–191 °C; 1H NMR (300 MHz, CDCl3): δ 8.87 (dd, J = 3.9, 1.2 Hz, 1H), 8.32 (dd, J = 8.7, 1.2 Hz, 1H), 7.61 (dd, J = 8.7, 3.9 Hz, 1H), 7.01 (s, 1H), 4.75 (q, J = 7.2 Hz, 2H), 1.79 (t, J = 7.2 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 155.3, 147.9, 146.0 (q, J = 37.3 Hz), 145.6, 143.6, 130.3, 125.3, 125.2, 120.8 (q, J = 272.0 Hz), 88.3 (q, J = 2.1 Hz), 68.2, 14.2; 19F NMR (282 MHz, CDCl3): δ −67.24; HRMS (ESI) m/z [M+H]+ calcd for C12H10F3N4O: 283.0801; found: 283.0799.
7-Methoxy-9-(trifluoromethyl)pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (8b).
The purification of the crude product by chromatography on silica gel was carried out using (PE/EtOAc: 8/2) to afford 8b as a brown solid with a yield of 60%; m.p. 145–147 °C; 1H NMR (300 MHz, CDCl3): δ 8.99 (d, J = 2.1 Hz, 1H), 8.84 (d, J = 2.1 Hz, 1H), 7.16 (s, 1H), 4.52 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 156.4, 154.8, 149.6, 147.5 (q, J = 37.2 Hz), 143.0, 142.9, 123.4, 120.5 (q, J = 274.0 Hz), 89.2 (q, J = 2.2 Hz), 58.5; 19F NMR (282 MHz, CDCl3): δ −67.47; HRMS (ESI) m/z [M+H]+ calcd for C10H7F3N5O: 270.0597; found: 270.0595.
7-Phenoxy-9-(trifluoromethyl)pyrazino[2′,3′:3,4]pyrazolo[1,5-a]pyrimidine (8c).
The purification of the crude product using chromatography on silica gel was carried out using (PE/EtOAc: 9/1) to afford 8c as a light brown solid with a yield of 72%; m.p. 183–185 °C; 1H NMR (300 MHz, CDCl3): δ 9.02 (d, J = 1.8 Hz, 1H), 8.87 (d, J = 1.8 Hz, 1H), 7.64 (t, J = 8.1 Hz, 2H), 7.53 (t, J = 7.5 Hz, 1H), 7.41 (d, J = 7.5 Hz, 2H), 6.85 (s, 1H); 13C NMR (75 MHz, CDCl3): δ 156.1, 155.0, 151.4, 149.8, 147.2 (q, J = 37.3 Hz), 143.4, 143.1, 131.2 (2C), 128.3, 123.5, 120.8 (2C), 120.3 (q, J = 273.8 Hz), 91.8 (q, J = 2.3 Hz); 19F NMR (282 MHz, CDCl3): δ −67.43; HRMS (ESI) m/z [M+H]+ calcd for C15H9F3N5O: 332.0753; found: 332.0750
4. Conclusions
We have developed a simple and general strategy for the regioselective synthesis of trifluoromethylated pyrimido[1,2-b]indazole derivatives starting from commercially available compounds. This strategy was based first on the condensation of the 3-amino indazole derivatives with ethyl 4,4,4-trifluoro-3-oxobutanoate providing trifluoromethylated pyrimido[1,2-b]indazol-4(1H)-one derivatives with good yields. The functionalization of the corresponding chlorinated derivatives, using the Suzuki–Miyaura coupling reaction and aromatic nucleophilic substitution, provided access to a new library of trifluoromethylated pyrimido[1,2-b]indazole derivatives.
The process shows promise as a valuable tool for constructing complex fluorinated heterocycles. This versatile strategy is applied to the production of a wide range of compounds currently under biological evaluation, in particular as novel monoamine oxidase (MAO) inhibitors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29010044/s1, 1H-NMR, 19F-NMR and 13C-NMR of compounds 2a–h, 3a–h, 4a–h, 5a–q, 6a–g, 7a–c and 8a–c.
Author Contributions
Methodology, S.T. and B.J.; Formal analysis, D.H.-O.; Investigation, M.A.; Resources, S.T.; Writing—review & editing, M.A.; Supervision, B.J. and M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank the ‘‘Département d’analyses Chimiques et Médicales’’ (Tours, France) for chemical analyses.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yakaiah, T.; Lingaiah, B.P.V.; Narsaiah, L.B.; Kumar, K.P.; Murthy, U.S.N. Synthesis of diverse nitrogen fused polycyclic dihydroisoquinoline (DHIQ derivatives via GBB-based cyclic iminium induced double-annulation cascade. Eur. J. Med. Chem. 2008, 43, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Chino, A.; Seo, R.; Amano, Y.; Namatame, I.; Hamaguchi, W.; Honbou, K.; Mihara, T.; Yamazaki, M.; Tomishima, M.; Masuda, N. Fragment-Based Discovery of Pyrimido-[1,2-b]indazole PDE10A Inhibitors. Chem. Pharm. Bull. 2018, 66, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Cerecetto, H.; Gerpe, A.; Gonzalez, M.; Aran, V.J.; Ochoa de Ocariz, C. Pharmacological properties of indazole derivatives: Recent developments. Mini-Rev. Med. Chem. 2005, 5, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Mc Gee, M.M.; Gemma, S.; Butini, S.; Ramunno, A.; Zisterer, D.M.; Fattorusso, C.; Catalanotti, B.; Kukreja, G.; Fiorini, I.; Pisano, C.; et al. Pyrrolo[1,5]benzoxa(thia)zepines as a New Class of Potent Apoptotic Agents. Biological Studies and Identification of an Intracellular Location of Their Drug Target. J. Med. Chem. 2005, 48, 4367–4377. [Google Scholar] [CrossRef] [PubMed]
- Flick, A.C.; Ding, H.X.; Leverett, C.A.; Kyne, R.E., Jr.; Liu, K.K.C.; Fink, S.J.; O’Donnell, C.J. Synthetic Approaches to the New Drugs Approved During 2015. J. Med. Chem. 2017, 60, 6480–6515. [Google Scholar] [CrossRef]
- Shah, K.; Chhabra, S.; Shrivastava, S.K.; Mishra, P. Benzimidazole: A promising pharmacophore. Med. Chem. Res. 2013, 22, 5077–5104. [Google Scholar] [CrossRef]
- Joule, J.A.; Mills, K. Heterocyclic Chemistry, 5th ed.; Blackwell Science: Oxford, UK, 2010. [Google Scholar]
- Yakaiah, T.; Lingaiah, B.P.V.; Narsaiah, B.; Shireesha, B.; Kumar, B.A.; Gururaj, S.; Parthasarathy, T.; Sridhar, B. Synthesis and structure-activity relationships of novel pyrimido[1,2-b]indazoles as potential anticancer agents against A-549 cell lines. Bioorg. Med. Chem. Lett. 2007, 17, 3445–3453. [Google Scholar] [CrossRef]
- Kumar, N.R.; Poornachandra, Y.; Swaroop, D.K.; Dev, G.J.; Kumar, C.G.; Narsaiah, B. Synthesis of novel ethyl 2,4-disubstituted 8-(trifluoromethyl)pyrido[2,3:3,4]pyrazolo[1,5-a]pyrimidine-9-carboxylate derivatives as promising anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 5203–5206. [Google Scholar] [CrossRef]
- Jismy, B.; El Qami, A.; Pislar, A.; Frlan, R.; Kos, J.; Gobec, S.; Knez, D.; Abarbri, M. Pyrimido[1,2-b]indazole derivatives: Selective inhibitors of human monoamine oxidase B with neuroprotective activity. Eur. J. Med. Chem. 2021, 209, 112911–112926. [Google Scholar] [CrossRef]
- Gurram, S.R.; Azam, M.A. GyrB inhibitors as potential antibacterial agents: A review. Monatsh. Chem. 2021, 152, 725–744. [Google Scholar] [CrossRef]
- Sharma, L.K.; Leonardi, R.; Lin, W.; Boyd, V.A.; Goktug, A.; Shelat, A.A.; Chen, T.; Jackowski, S.; Rock, C.O. A High-Throughput Screen Reveals New Small-Molecule Activators and Inhibitors of Pantothenate Kinases. J. Med. Chem. 2015, 58, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lou, Y.; Wang, Y.; Song, Q. Oxidant-controlled divergent transformations of 3-aminoindazoles for the synthesis of pyrimido[1,2-b]-indazoles and aromatic nitrile-derived dithioacetals. Org. Chem. Front. 2019, 6, 3355–3362. [Google Scholar] [CrossRef]
- Palaniraja, J.; Roopan, S.M.; Rayalu, G.M. One-pot synthesis of highly functionalized pyrimido[1,2-b]indazoles via 6-endo-dig cyclization. RSC Adv. 2016, 6, 24610–24616. [Google Scholar] [CrossRef]
- Shinde, V.V.; Jeong, Y.T. Organic-base-catalyzed diversity-oriented synthesis of novel pyrimido[1,2-b]indazole-3-carbonitrile. Tetrahedron 2016, 72, 4377–4382. [Google Scholar] [CrossRef]
- Palaniraja, J.; Roopan, S.M.; Rayalu, G.M.; Al-Dhabi, N.A.; Arasu, M.V. A Metal-Free Regioselective Multicomponent Approach for the Synthesis of Free Radical Scavenging Pyrimido-Fused Indazoles and Their Fluorescence Studies. Molecules 2016, 21, 1571. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Zhou, Y.; Song, Q. Lewis-acid Promoted Chemoselective Condensation of 2-Aminobenzimidazoles or 3-Aminoindazoles with 3-Ethoxycyclobutanones to Construct Fused Nitrogen heterocycles. Adv. Synth. Catal. 2018, 360, 1943–1948. [Google Scholar] [CrossRef]
- Gao, Q.; Han, X.; Tong, P.; Zhang, Z.; Shen, H.; Guo, Y.; Bai, S. Aerobic α,β-C(sp3)−H Bond Difunctionalization and C−N Bond Cleavage of Triethylamine: Difunctional Ammonium Iodide Enabling the Regioselective Synthesis of 4-Arylpyrimido[1,2-b]indazoles. Org. Lett. 2019, 21, 6074–6078. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, W.; Zheng, H.; Pei, Y.; Liu, X.; Cao, H. Visible Light-Induced Cascade Cyclization of 3-Aminoindazoles, Ynals, and Chalcogens: Access to Chalcogen-Containing Pyrimido[1,2-b]-indazoles. Org. Lett. 2021, 23, 2754–2759. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Lin, J.; Zhang, Z.; Wu, S.; He, Q.; Cao, H. Controllable Site-Selective Construction of 2- and 4-Substituted Pyrimido[1,2-b]indazole from Aminoindazoles and Ynals. J. Org. Chem. 2021, 86, 9107–9116. [Google Scholar] [CrossRef]
- Muller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001−2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Understanding Organofluorine Chemistry. An Introduction to the C−F Bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar]
- Xu, Z.; Geng, X.; Cai, Y.; Wang, L. A Straightforward Approach to Fluorinated Pyrimido[1,2-b]indazole Derivatives via Metal/Additive-Free Annulation with Enaminones, 3-Aminoindazoles, and Selectfluor. J. Org. Chem. 2022, 87, 6562–6572. [Google Scholar] [CrossRef] [PubMed]
- Jismy, B.; Guillaumet, G.; Akssira, M.; Tikad, A.; Abarbri, M. Efficient microwave-assisted Suzuki–Miyaura cross-coupling reaction of 3-bromo pyrazolo[1,5-a]pyrimidin-5(4H)-one: Towards a new access to 3,5-diarylated 7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine derivatives. RSC Adv. 2021, 11, 1287–1302. [Google Scholar] [CrossRef]
- Jismy, B.; Allouchi, H.; Guillaumet, G.; Akssira, M.; Abarbri, M. An Efficient Synthesis of New 7-Trifluoromethyl-2,5-disubstituted Pyrazolo[1,5-a]pyrimidines. Synthesis 2018, 50, 1675–1686. [Google Scholar]
- Jismy, B.; Guillaumet, G.; Akssira, M.; Knez, D.; Abarbri, M. A simple and a practical strategy to synthesize new 5-trifluoromethyl-7-substituted Imidazo[1,2-a]pyrimidines and benzimidazo[1,2-a]pyrimidines, and their biological evaluation test. New J. Chem. 2019, 43, 9961–9968. [Google Scholar] [CrossRef]
- Blancou, W.; Jismy, B.; Allouchi, H.; Abarbri, M. Simple and Expedient Access to Novel Fluorinated Thiazolo- and Oxazolo[3,2-a]pyrimidin-7-one Derivatives and Their Functionalization via Palladium-Catalyzed Reactions. Molecules 2022, 27, 3013. [Google Scholar] [CrossRef]
- Wheeler, R.C.; Baxter, E.; Campbell, I.B.; Macdonald, S.J.F. A general, One-Step Synthesis of Substituted Indazoles using a Flow Reactor. Org. Process Res. Dev. 2011, 15, 565–569. [Google Scholar] [CrossRef]
- Bagheria, M.; Shekarchia, M.; Jorjanib, M.; Hossein Ghahremanic, M.; Vosooghia, M.; Shafieea, A. Synthesis and Antihypertensive Activity of 1-(2-Thiazolyl)-3,5-disubstituted-2-Pyrazolines. Arch. Pharm. Int. J. Pharm. Med. Chem. 2004, 337, 25–34. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Stereoselective Synthesis of Arylated (E)-Alkenes by the Reaction of Alk-1-enylboranes with Aryl Halides in the Presence of Palladium Catalyst. J. Chem. Soc. Chem. Commun. 1979, 19, 866–867. [Google Scholar] [CrossRef]
- Kadu, B.S. Suzuki–Miyaura cross coupling reaction: Recent advancements in catalysis and organic synthesis. Catal. Sci. Technol. 2021, 11, 1186–1221. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).