A Benzil- and BODIPY-Based Turn-On Fluorescent Probe for Detection of Hydrogen Peroxide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Principle of the Benzil- and BODIPY-Based H2O2 Assay
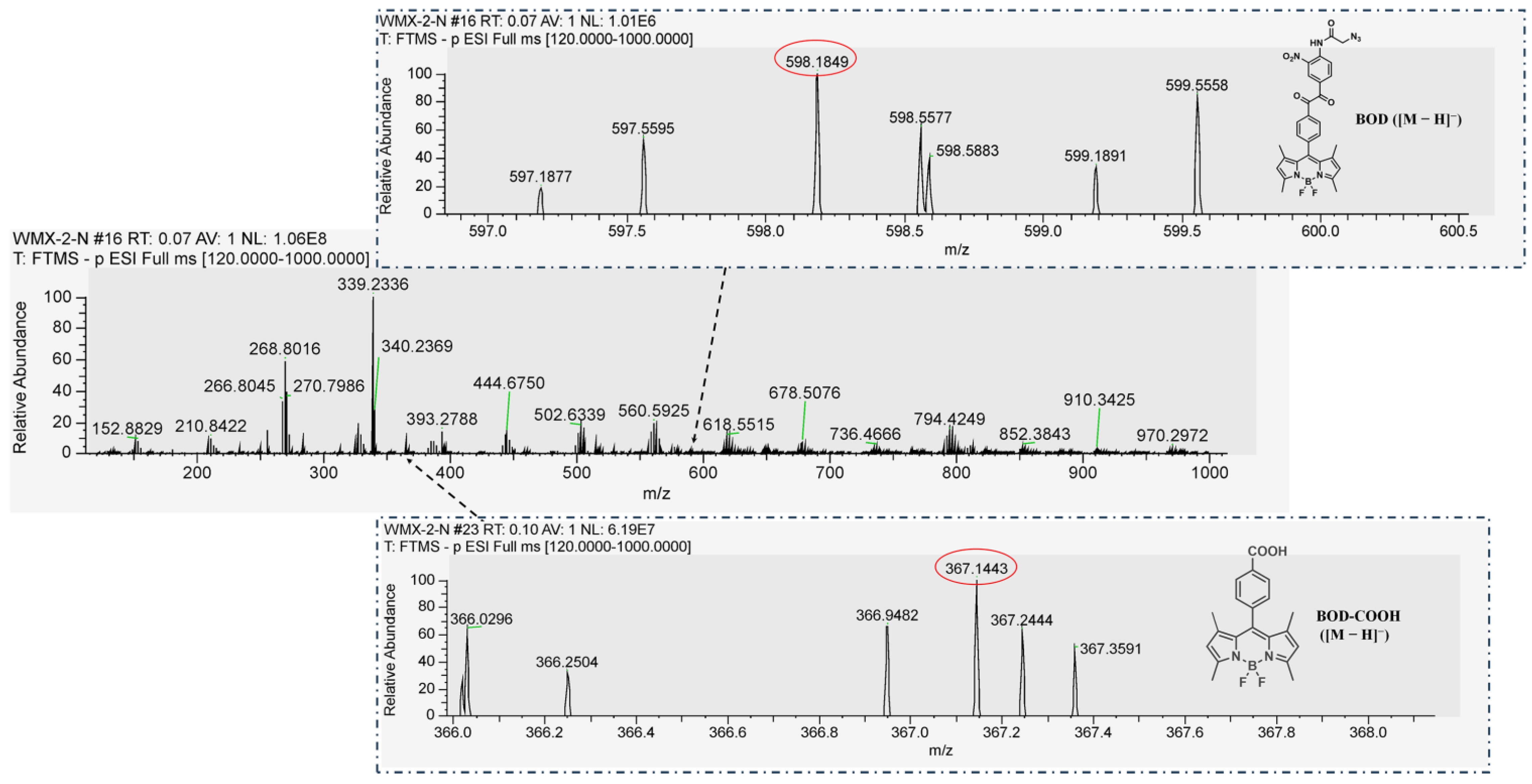
2.2. Synthesis and Characterizations of the BOD
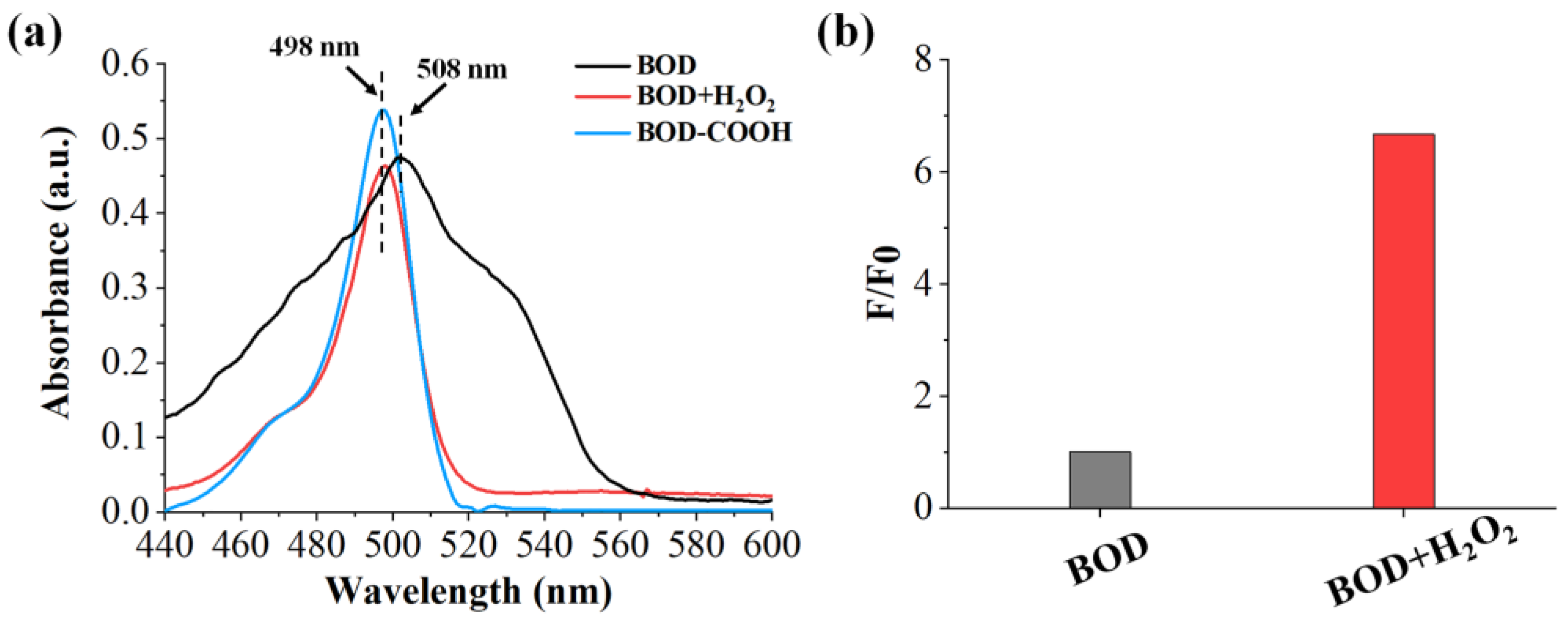
2.3. Feasibility of the Benzil- and BODIPY-Based H2O2 Assay
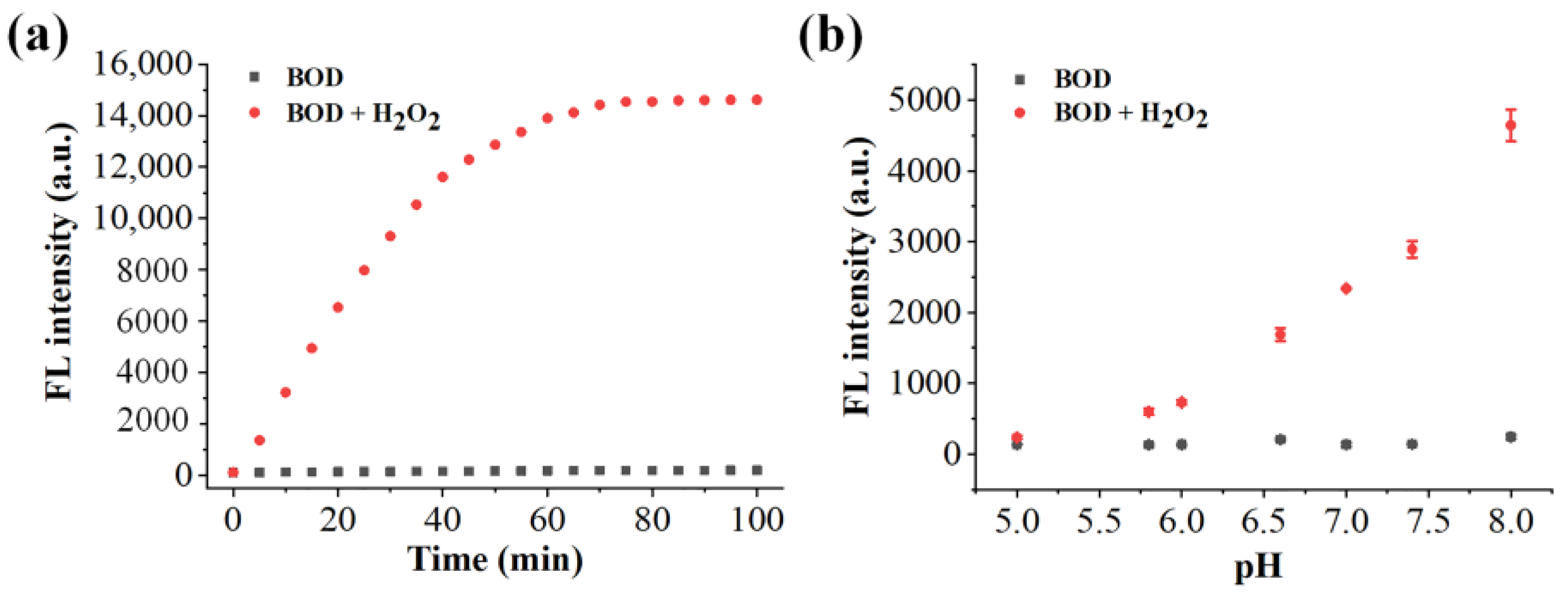
2.4. Optimization of the Benzil- and BODIPY-Based H2O2 Assay Conditions
2.5. Photostability Study of the BOD Probe
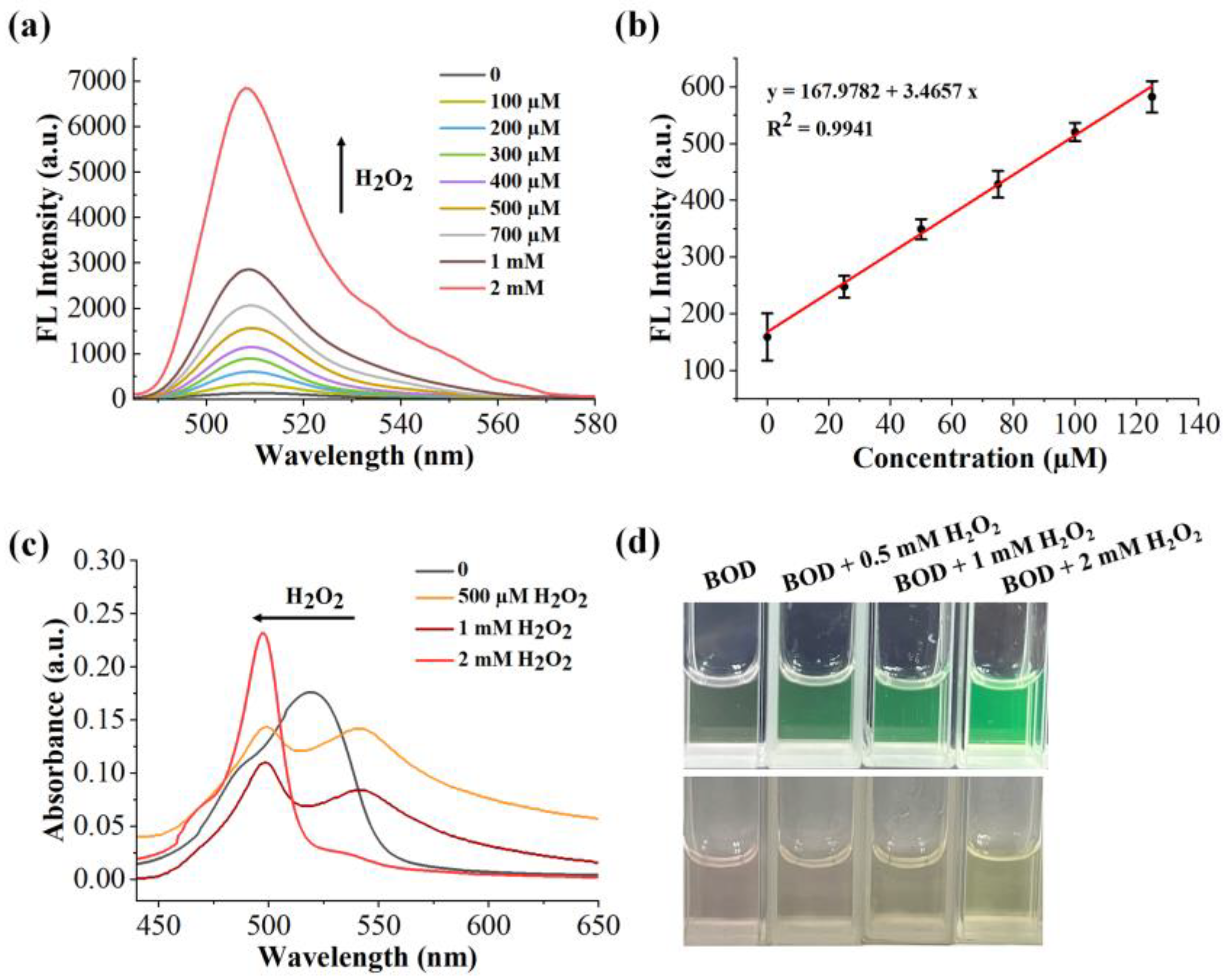
2.6. Sensitivity of the Benzil- and BODIPY-Based H2O2 Assay
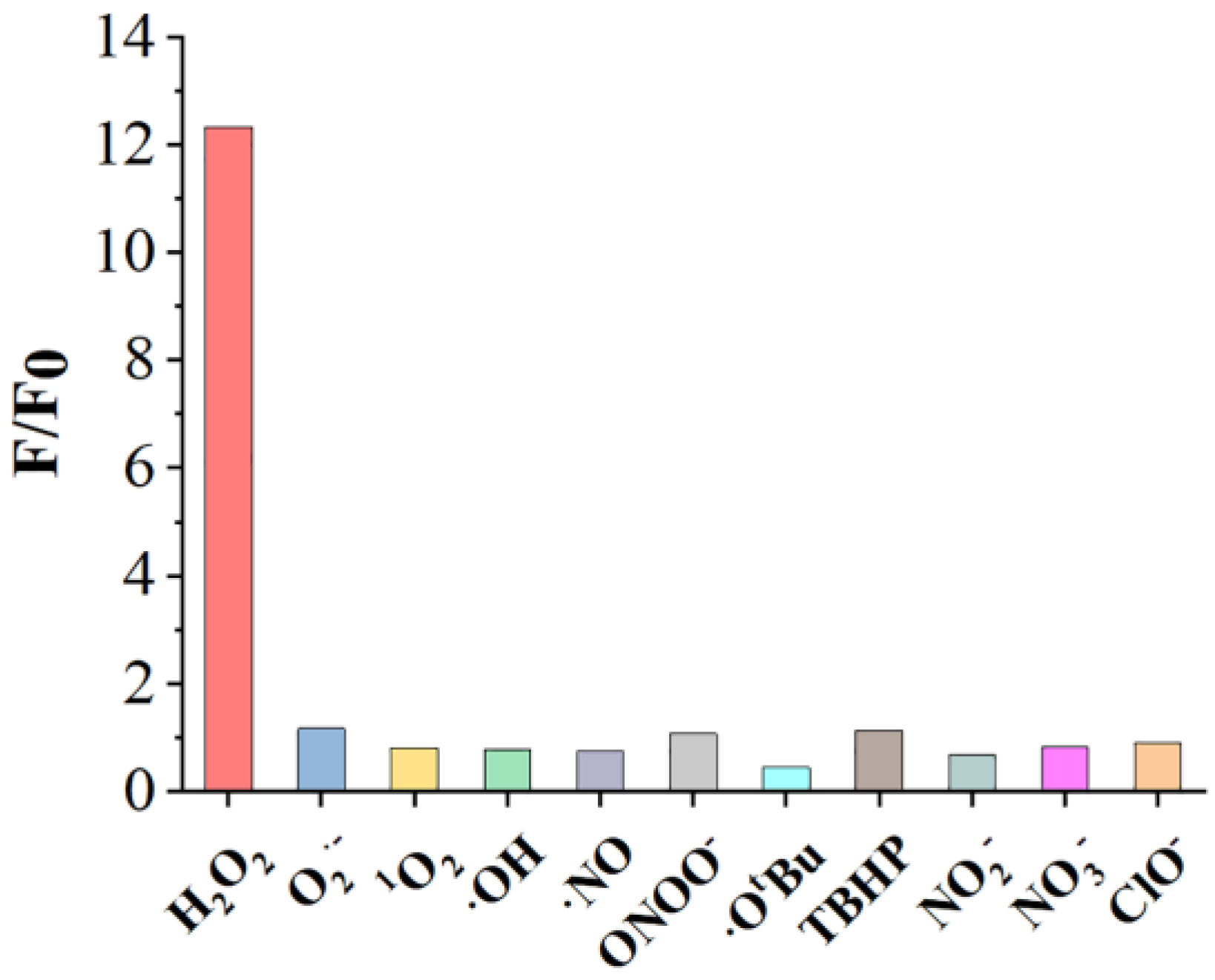
2.7. Selectivity of the Benzil- and BODIPY-Based H2O2 Assay
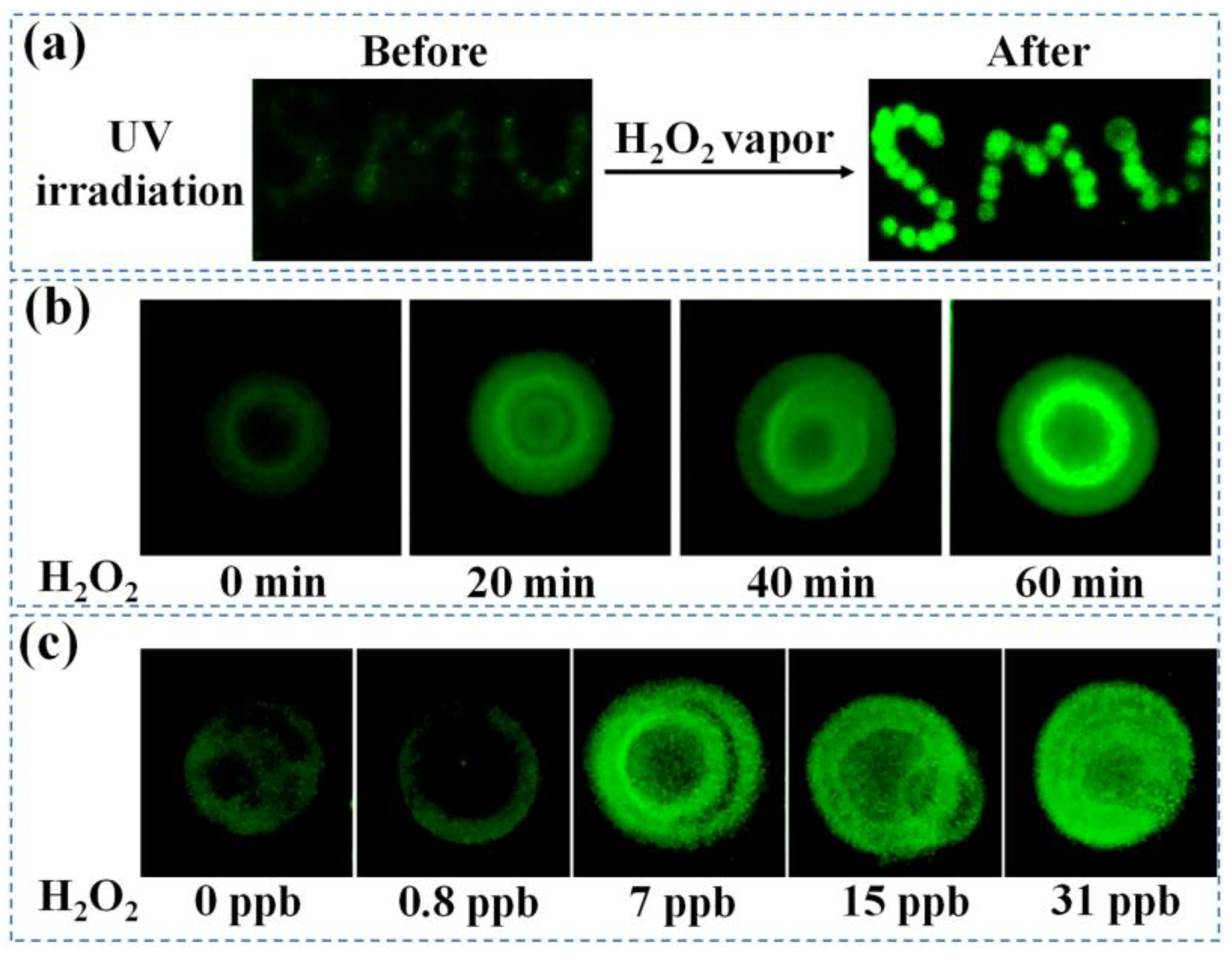
2.8. Detection of H2O2 Vapor Based on the Proposed BOD Probe
3. Materials and Methods
3.1. Materials
3.2. Apparatus
3.3. Synthesis of BOD
3.4. Feasibility of the BOD-Based H2O2 Assay
3.5. Sensitivity and Selectivity of the BOD-Based H2O2 Assay
3.6. Detection of H2O2 Vapor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schulte-Ladbeck, R.; Vogel, M.; Karst, U. Recent methods for the determination of peroxide-based explosives. Anal. Bioanal. Chem. 2006, 386, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fu, Y.; Gao, Y.; Yao, J.; Fan, T.; Zhu, D.; He, Q.; Cao, H.; Cheng, J. A simple but highly efficient multi-formyl phenol-amine system for fluorescence detection of peroxide explosive vapour. Chem. Commun. 2015, 51, 10868–10870. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Han, J.M.; Wang, C.; Yang, X.; Pei, J.; Zang, L. Fluorescence ratiometric sensor for trace vapor detection of hydrogen peroxide. ACS Appl. Mater. Interfaces 2014, 6, 8708–8714. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Hu, Z.; Xu, Q.; Liu, B.; Zhu, Q.; Guan, J.; Liu, C.; Pan, Y.; Hu, L.; Fang, J.; et al. Improving quantification of hydrogen peroxide by synchrotron vacuum ultraviolet photoionization mass spectrometry. Combust. Flame 2022, 242, 112214. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, W.; Fu, L.; Lorenzo, J.M.; Hao, Y. Fabrication and application of electrochemical sensor for analyzing hydrogen peroxide in food system and biological samples. Food Chem. 2022, 385, 132555. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, K.; Yu, S.; Zhang, J.; Jin, L. The preparation of MnO2/BSA/CdTe quantum dots complex for ratiometric fluorescence/ T(1)-weighted MRI detection of H2O2. Talanta 2023, 252, 123774. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Yang, Z.; Wang, Y.; Rao, H.; Yue, G.; Wu, C.; Lu, C.; Wang, X. A ratiometric fluorescence and colorimetric dual-mode assay for H2O2 and xanthine based on Fe, N co-doped carbon dots. Dye. Pigment. 2020, 180, 108486. [Google Scholar] [CrossRef]
- Su, L.; Cai, Y.; Wang, L.; Dong, W.; Mao, G.; Li, Y.; Zhao, M.; Ma, Y.; Zhang, H. Hemin@carbon dot hybrid nanozymes with peroxidase mimicking properties for dual (colorimetric and fluorometric) sensing of hydrogen peroxide, glucose and xanthine. Mikrochim. Acta 2020, 187, 132. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Dong, B.; Kong, X.; Tian, M. Chameleon-Like Fluorescent Probe for Monitoring Interplays between Three Organelles and Reporting Cell Damage Processes through Dramatic Color Change. Small 2022, 18, e2205026. [Google Scholar] [CrossRef]
- Xue, H.; Ge, E.; Ge, W.; Li, J.; Tian, M. Single Fluorescent Probe for Zero-Crosstalk Discrimination of Lipid Droplets and the Endoplasmic Reticulum Based on Reversible Cyclization Reaction. Anal. Chem. 2022, 94, 9158–9165. [Google Scholar] [CrossRef]
- Fan, S.; Lai, J.; Burn, P.L.; Shaw, P.E. Solid-State Fluorescence-based Sensing of TATP via Hydrogen Peroxide Detection. ACS Sens. 2019, 4, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Xue, L.; Hu, D.; Li, G.; Jiang, H. Quinoline-based fluorescent probe for ratiometric detection of hydrogen peroxide in aqueous solution. Dye. Pigment. 2012, 95, 373–376. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, Z.; Xu, W.; Fu, Y.; Zhu, D.; Xu, J.; He, Q.; Cao, H.; Cheng, J. Design, synthesis and properties of a reactive chromophoric/fluorometric probe for hydrogen peroxide detection. New J. Chem. 2017, 41, 3790–3797. [Google Scholar] [CrossRef]
- Zielonka, J.; Kalyanaraman, B. Small-molecule luminescent probes for the detection of cellular oxidizing and nitrating species. Free Radic. Biol. Med. 2018, 128, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Abo, M.; Urano, Y.; Hanaoka, K.; Terai, T.; Komatsu, T.; Nagano, T. Development of a highly sensitive fluorescence probe for hydrogen peroxide. J. Am. Chem. Soc. 2011, 133, 10629–10637. [Google Scholar] [CrossRef]
- Purdey, M.S.; McLennan, H.J.; Sutton-McDowall, M.L.; Drumm, D.W.; Zhang, X.; Capon, P.K.; Heng, S.; Thompson, J.G.; Abell, A.D. Biological hydrogen peroxide detection with aryl boronate and benzil BODIPY-based fluorescent probes. Sens. Actuators B Chem. 2018, 262, 750–757. [Google Scholar] [CrossRef]
- Huang, X.; Li, Z.; Liu, Z.; Zeng, C.; Hu, L. A near-infrared fluorescent probe for endogenous hydrogen peroxide real-time imaging in living cells and zebrafish. Dye. Pigment. 2019, 165, 518–523. [Google Scholar] [CrossRef]
- Nguyen, Y.T.; Shin, S.; Kwon, K.; Kim, N.; Bae, S.W. BODIPY-based fluorescent sensors for detection of explosives. J. Chem. Res. 2023, 47, 17475198231168961. [Google Scholar] [CrossRef]
- Erande, Y.; Chemate, S.; More, A.; Sekar, N. PET governed fluorescence “Turn ON” BODIPY probe for selective detection of picric acid. RSC Adv. 2015, 5, 89482–89487. [Google Scholar] [CrossRef]
- Gao, J.; Chen, X.; Chen, S.; Meng, H.; Wang, Y.; Li, C.; Feng, L. The BODIPY-Based Chemosensor for Fluorometric/Colorimetric Dual Channel Detection of RDX and PA. Anal. Chem. 2019, 91, 13675–13680. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.; Xiong, Y.; Yang, X.; Zhao, C.; Sun, J. Development of turn-on fluorescent probes for the detection of H2O2 vapor with high selectivity and sensitivity. Sens. Actuators B Chem. 2018, 268, 475–484. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, F.; Wang, C.; Yin, N.; Wang, Y.; Qin, G.; Xu, Q.; Gong, J.; Liu, H.; Duan, X. Target-Binding Accelerated Response for Sensitive Detection of Basal H2O2 in Tumor Cells and Tissues via a Dual-Functional Fluorescence Probe. Anal. Chem. 2022, 94, 5962–5969. [Google Scholar] [CrossRef]
- Peng, J.; Hou, X.; Zeng, F.; Wu, S. Fluorescent nanoprobe for in-vivo ratiometric imaging of endogenous hydrogen peroxide resulted from drug-induced organ damages. Biosens. Bioelectron. 2017, 94, 278–285. [Google Scholar] [CrossRef]
- Schulz-Fincke, A.; Blaut, M.; Braune, A.; Gütschow, M. A BODIPY-Tagged Phosphono Peptide as Activity-Based Probe for Human Leukocyte Elastase. ACS Med. Chem. Lett. 2018, 9, 345–350. [Google Scholar] [CrossRef]
- Rurack, K.; Kollmannsberger, M.; Daub, J. Molecular Switching in the Near Infrared (NIR) with a Functionalized Boron-Dipyrromethene Dye. Angew. Chem. Int. Ed. Engl. 2001, 40, 385–387. [Google Scholar] [CrossRef]
- Panfilov, M.A.; Karogodina, T.Y.; Songyin, Y.; Karmatskih, O.Y.; Vorob’ev, A.Y.; Tretyakova, I.S.; Glebov, E.M.; Moskalensky, A.E. Photophysical properties of BODIPYs with sterically-hindered nitrophenyls in meso-position. J. Lumin. 2022, 246, 118837. [Google Scholar] [CrossRef]
- Bettinger, H.F.; Rabuck, A.D.; Scuseria, G.E.; Wang, N.-X.; Litosh, V.A.; Saini, R.K.; Billups, W.E. Pathways for the thermally induced dehydrogenation of C60H2. Chem. Phys. Lett. 2002, 360, 509–514. [Google Scholar] [CrossRef]
- Sunahara, H.; Urano, Y.; Kojima, H.; Nagano, T. Design and synthesis of a library of BODIPY-based environmental polarity sensors utilizing photoinduced electron-transfer-controlled fluorescence ON/OFF switching. J. Am. Chem. Soc. 2007, 129, 5597–5604. [Google Scholar] [CrossRef]
- Słowiński, D.; Świerczyńska, M.; Romański, J.; Podsiadły, R. Sensitive Detection of Various Forms of Hydrogen Sulfide via Highly Selective Naphthalimide-Based Fluorescent Probe. Molecules 2023, 28, 6299. [Google Scholar] [CrossRef]
- Lippert, A.R.; New, E.J.; Chang, C.J. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J. Am. Chem. Soc. 2011, 133, 10078–10080. [Google Scholar] [CrossRef]
- Saha, T.; Kand, D.; Talukdar, P. A colorimetric and fluorometric BODIPY probe for rapid, selective detection of H2S and its application in live cell imaging. Org. Biomol. Chem. 2013, 11, 8166–8170. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Lin, W.; Tan, L. A phenanthroimidazole-based fluorescent chemosensor for imaging hydrogen sulfide in living cells. Org. Biomol. Chem. 2012, 10, 9683–9688. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, Y.; Ren, X.; Wang, B.; Yang, Z.; Song, X.; Wang, W. Fluorescent Detection of Dynamic H2O2/H2S Redox Event in Living Cells and Organisms. Anal. Chem. 2020, 92, 4387–4394. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liu, K.; Yang, H.; Li, Y.; Lan, H.; Liu, Y.; Zhang, X.; Yi, T. A highly sensitive ratiometric fluorescent probe for the detection of cytoplasmic and nuclear hydrogen peroxide. Anal. Chem. 2014, 86, 9970–9976. [Google Scholar] [CrossRef] [PubMed]
- Pak, Y.L.; Park, S.J.; Xu, Q.; Kim, H.M.; Yoon, J. Ratiometric Two-Photon Fluorescent Probe for Detecting and Imaging Hypochlorite. Anal. Chem. 2018, 90, 9510–9514. [Google Scholar] [CrossRef] [PubMed]
- Manatt, S.L.; Manatt, M.R. On the analyses of mixture vapor pressure data: The hydrogen peroxide/water system and its excess thermodynamic functions. Chem. Eur. J. 2004, 10, 6540–6557. [Google Scholar] [CrossRef]
- Xu, M.; Han, J.M.; Zhang, Y.; Yang, X.; Zang, L. A selective fluorescence turn-on sensor for trace vapor detection of hydrogen peroxide. Chem. Commun. 2013, 49, 11779–11781. [Google Scholar] [CrossRef]
- Matsumoto, A.; Nishiyabu, R.; Kubo, Y. Synthesis of a borylated boron–dibenzopyrromethene dye enabling the visual detection of H2O2 vapor. RSC Adv. 2014, 4, 37973–37978. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Y.; Liu, B.; Yuan, Y.; Wei, L.; Wang, M.; Chen, Z. A Benzil- and BODIPY-Based Turn-On Fluorescent Probe for Detection of Hydrogen Peroxide. Molecules 2024, 29, 229. https://doi.org/10.3390/molecules29010229
Wang Y, Liu Y, Liu B, Yuan Y, Wei L, Wang M, Chen Z. A Benzil- and BODIPY-Based Turn-On Fluorescent Probe for Detection of Hydrogen Peroxide. Molecules. 2024; 29(1):229. https://doi.org/10.3390/molecules29010229
Chicago/Turabian StyleWang, Yunxia, Ye Liu, Bo Liu, Yihua Yuan, Lixia Wei, Mingxiu Wang, and Zhe Chen. 2024. "A Benzil- and BODIPY-Based Turn-On Fluorescent Probe for Detection of Hydrogen Peroxide" Molecules 29, no. 1: 229. https://doi.org/10.3390/molecules29010229
APA StyleWang, Y., Liu, Y., Liu, B., Yuan, Y., Wei, L., Wang, M., & Chen, Z. (2024). A Benzil- and BODIPY-Based Turn-On Fluorescent Probe for Detection of Hydrogen Peroxide. Molecules, 29(1), 229. https://doi.org/10.3390/molecules29010229






