Network Proximity Analysis Deciphers the Pharmacological Mechanism of Osthole against D-Galactose Induced Cognitive Disorder in Rats
Abstract
:1. Introduction
2. Results
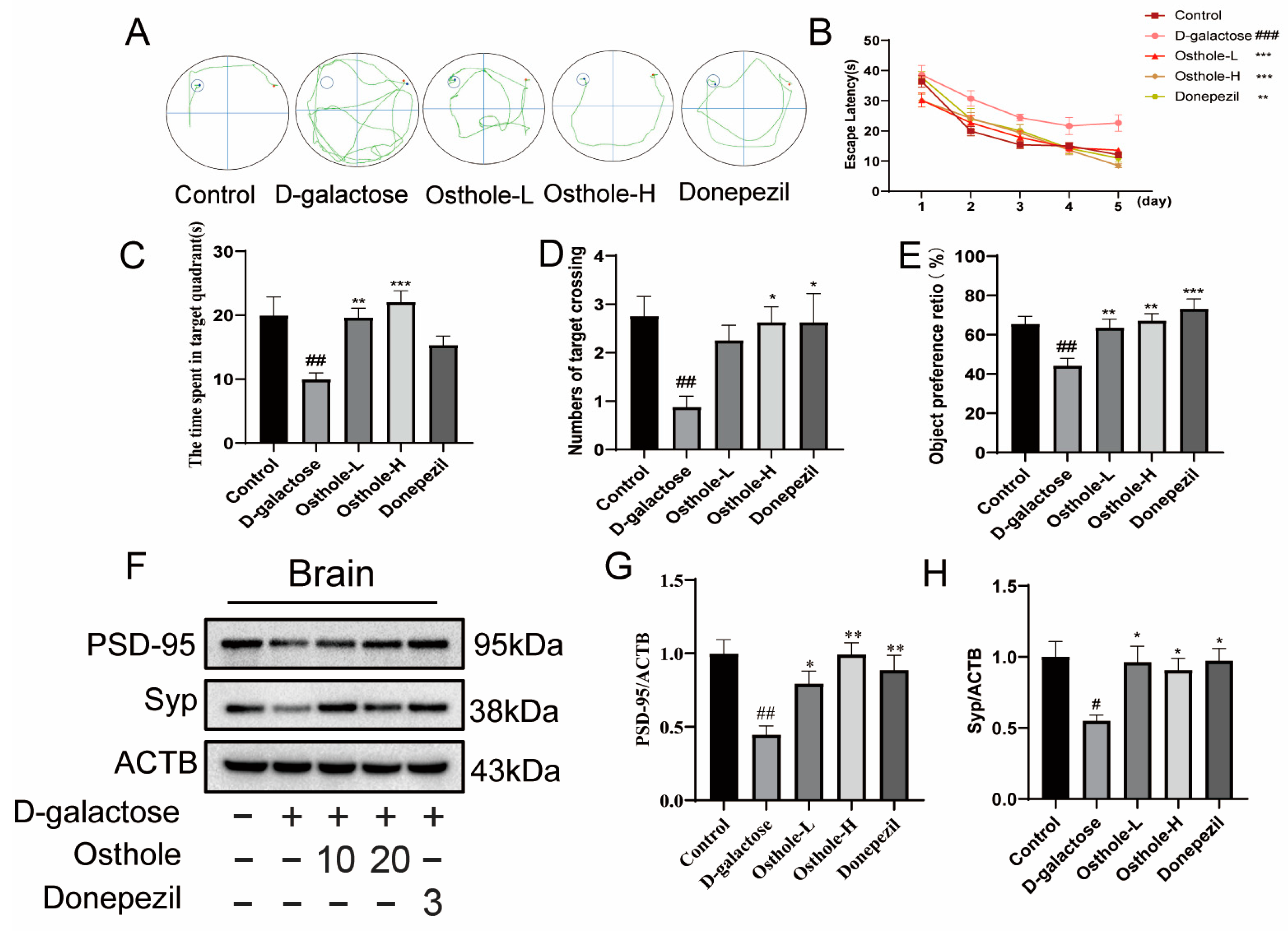
2.1. Osthole Alleviates D-Galactose-Induced Cognition and Synaptic Dysfunction
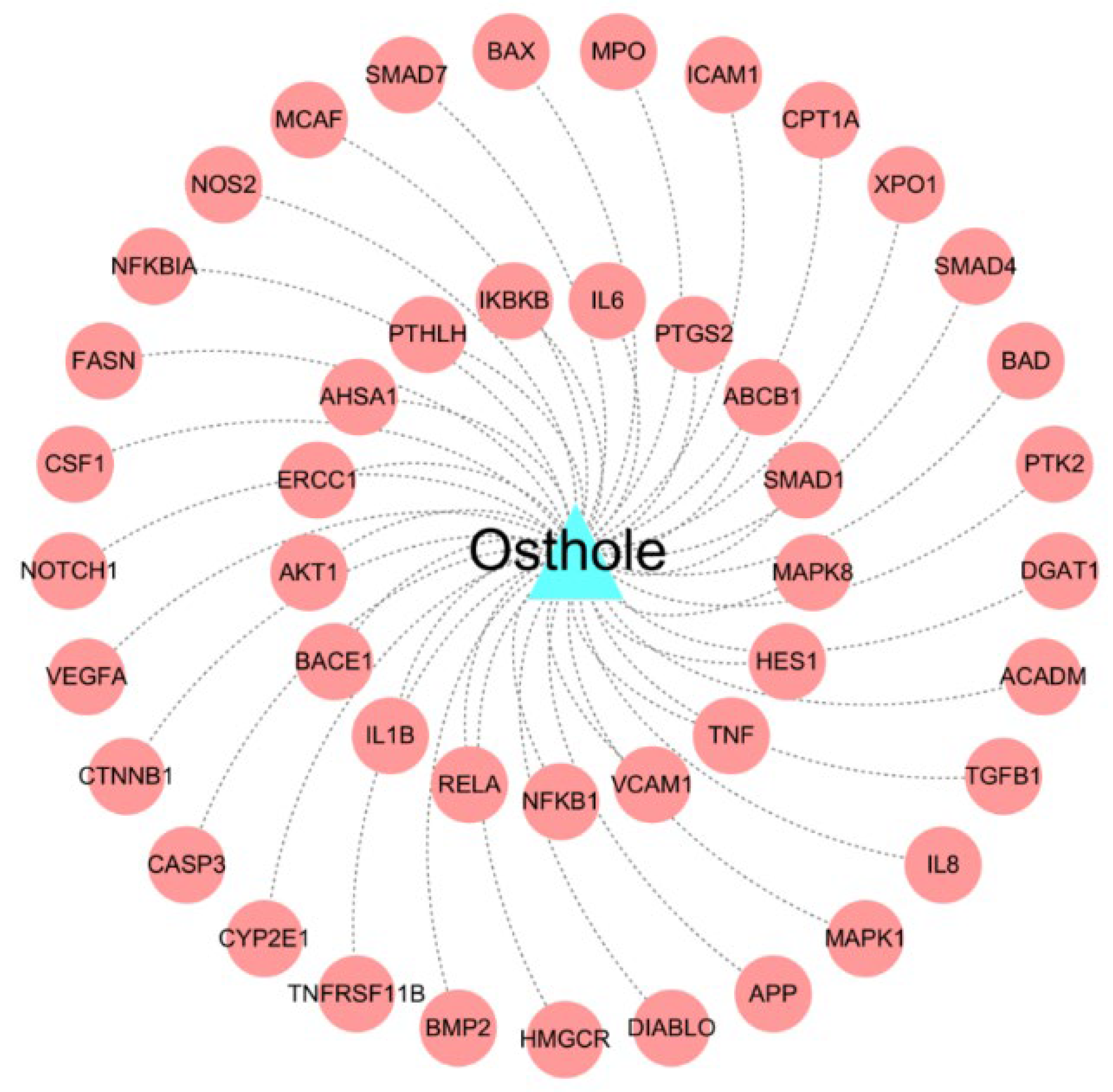
2.2. Construction of the Drug-Target Network for Osthole
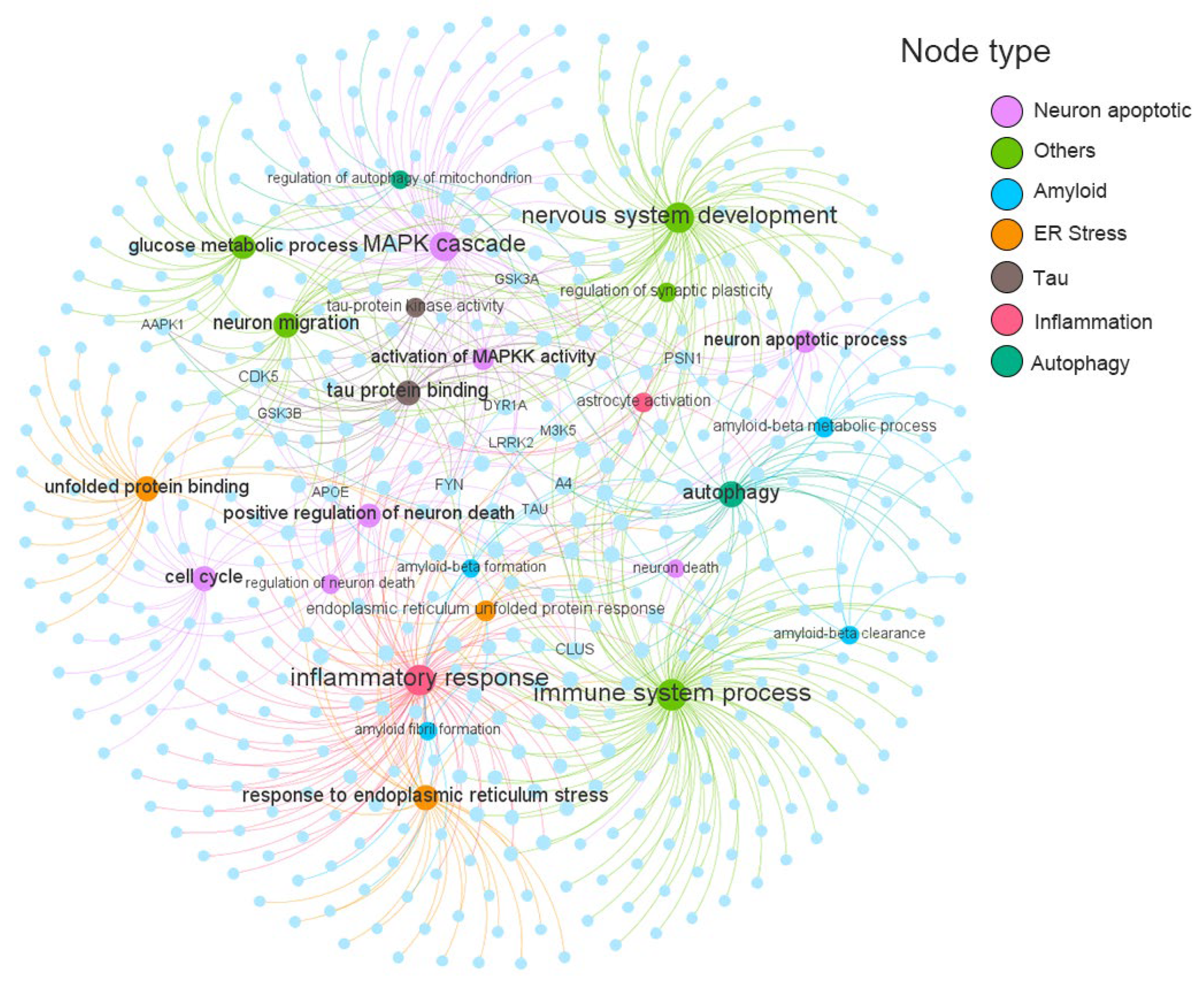
2.3. Construction of Endophenotype Network for AD
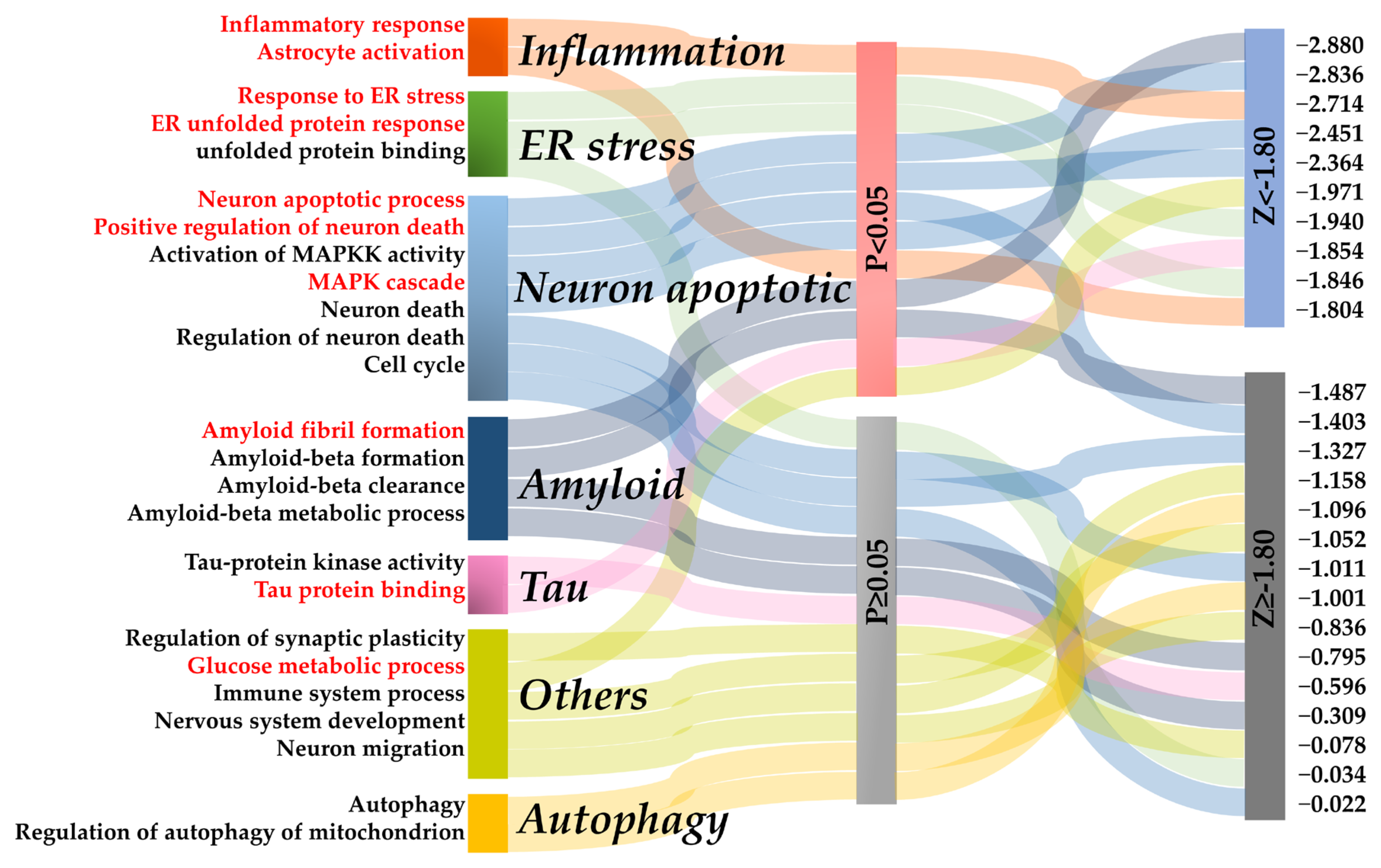
2.4. Network Proximity Identified Potential AD Pathological Mechanisms Regulated by Osthole
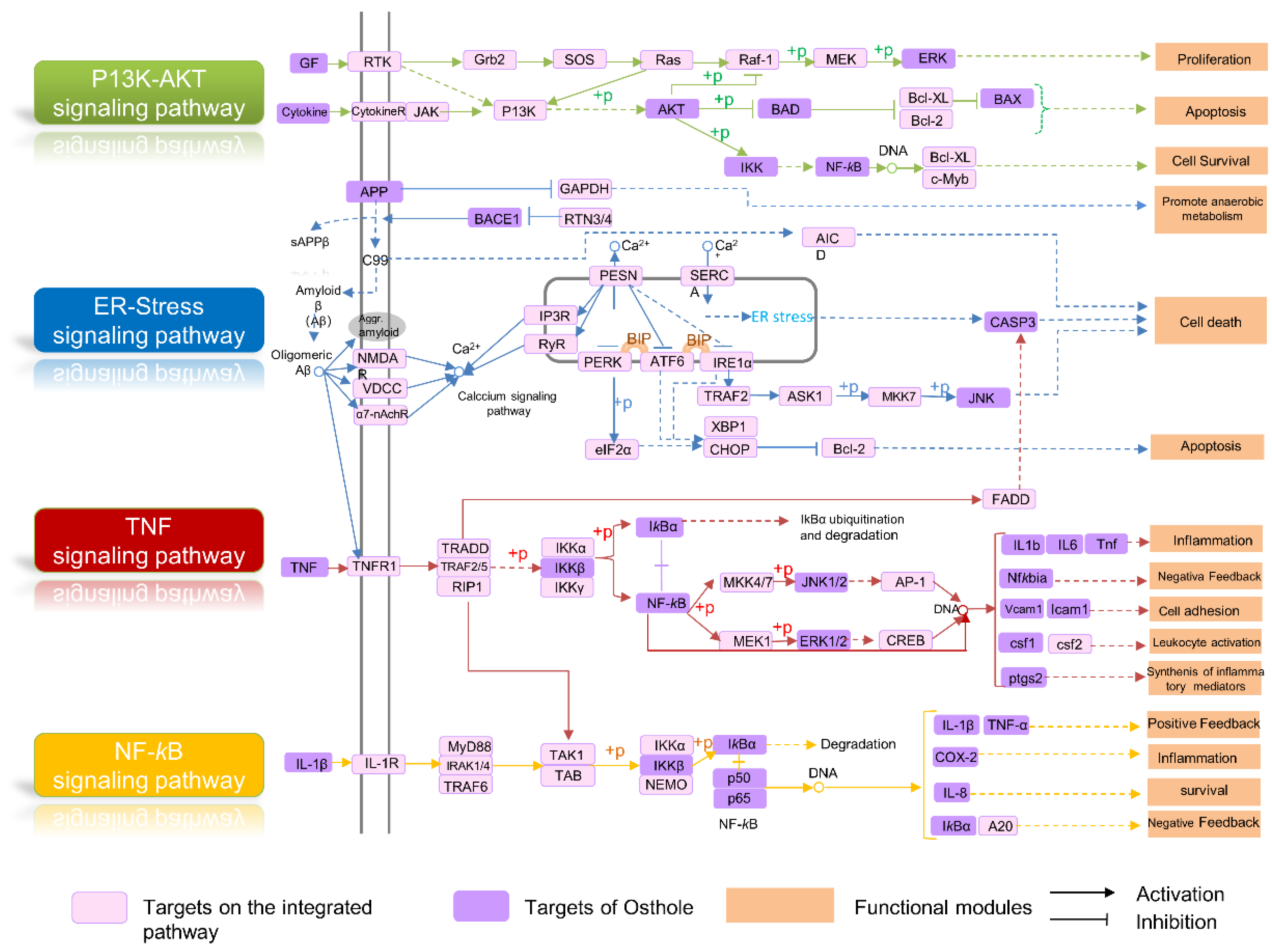
2.5. Integrated Pathway Map Analysis
2.5.1. Apoptosis Module
2.5.2. Neuroinflammation Regulation Module
2.5.3. Endoplasmic Reticulum Stress Regulation Module
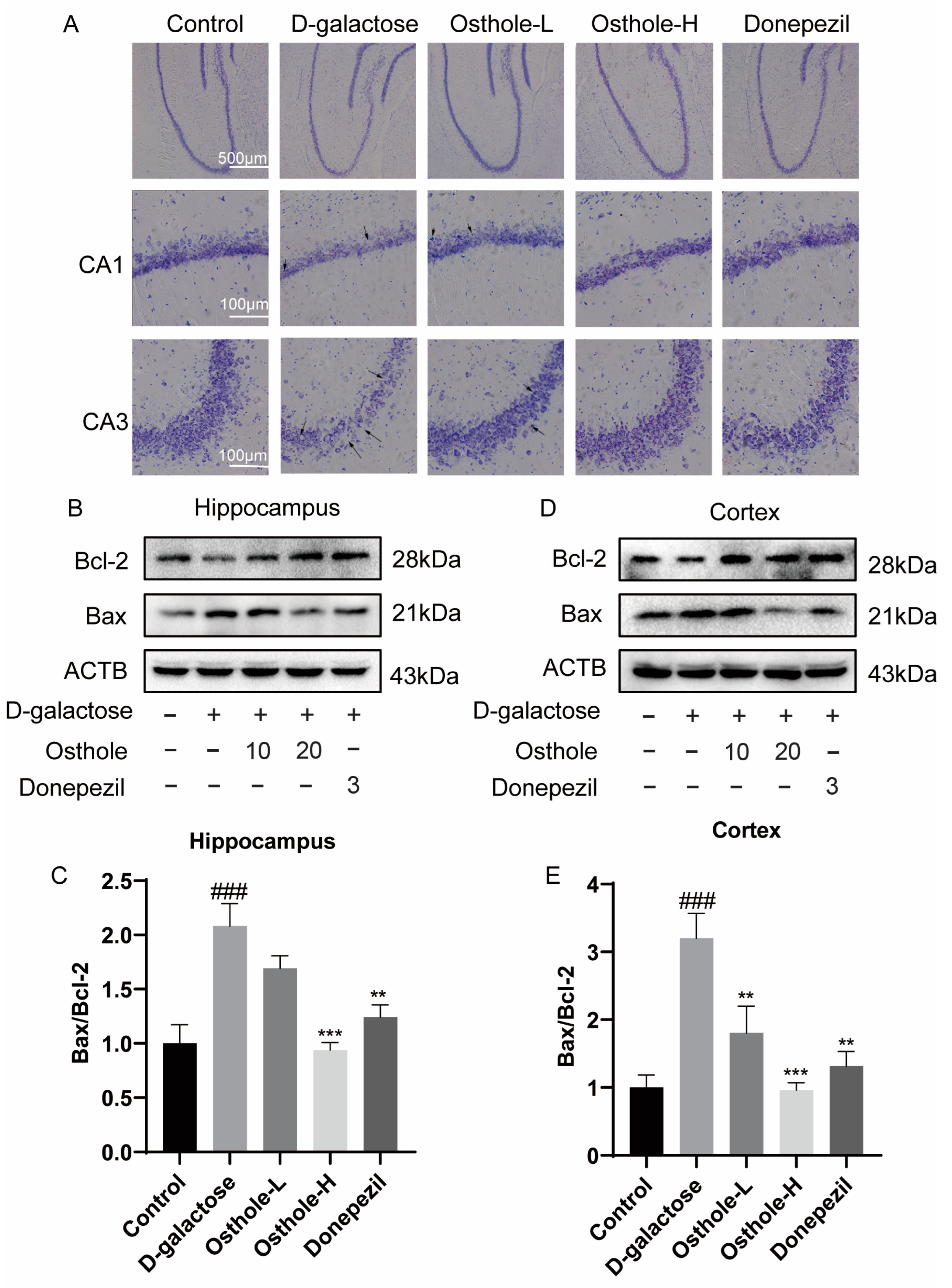
2.6. Osthole Inhibited D-Galactose-Induced Neuron Loss and Neuron Apoptosis
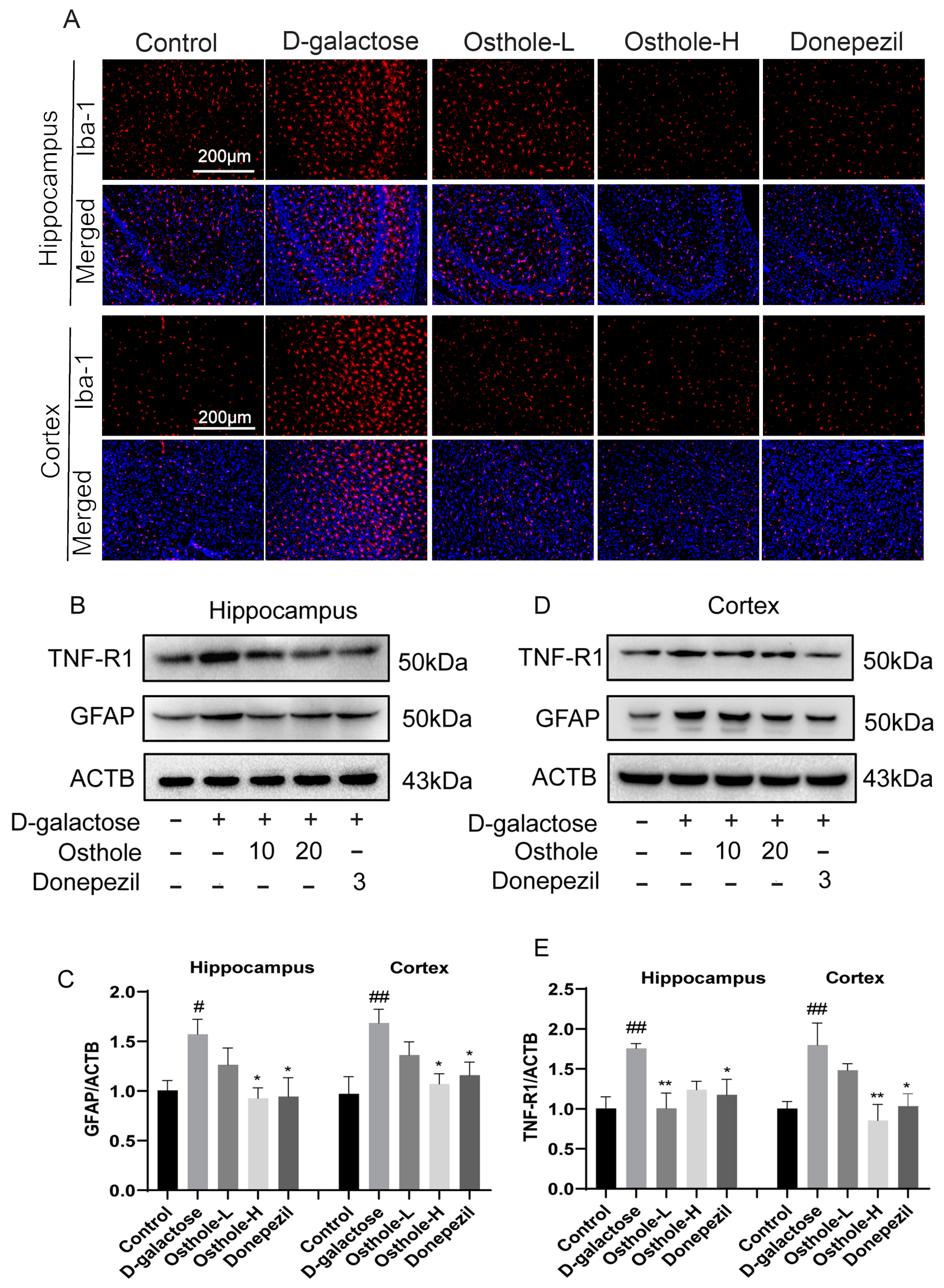
2.7. Osthole Inhibited D-Galactose-Induced Neuroinflammation
3. Discussion and Conclusions
4. Materials and Methods
4.1. Animals and Drug Administrations
4.2. Behavior Analysis
4.2.1. Morris Water Maze Test (MWM)
4.2.2. Novel Object Recognition Test (NORT)
4.3. Nissl Staining
4.4. Immunofluorescence
4.5. Western Blot
4.6. Endophenotype Network Strategy
4.6.1. Collection of Interacted Protein Targets of Osthole
4.6.2. Collection of Genes for Building Endophenotype Modules for AD
4.6.3. Construction of the Human Protein–Protein Interactome
4.6.4. Network Proximity Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Nahed, P.; Kambar, M.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2022. Alzheimers Dement. 2022, 8, e12295. [Google Scholar] [CrossRef] [PubMed]
- Bendlin, B.B.; Zetterberg, H. The iterative process of fluid biomarker development and validation in Alzheimer’s disease. Alzheimers Dement. 2022, 14, e12341. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef]
- Zafar, S.; Sarfraz, I.; Rasul, A.; Shah, M.A.; Hussain, G.; Zahoor, M.K.; Shafiq, N.; Riaz, A.; Selamoglu, Z.; Sarker, S.D. Osthole: A Multifunctional Natural Compound with Potential Anticancer, Antioxidant and Anti-inflammatory Activities. Mini Rev. Med. Chem. 2021, 21, 2747–2763. [Google Scholar] [CrossRef]
- Yao, Y.; Gao, Z.; Liang, W.; Kong, L.; Jiao, Y.; Li, S.; Tao, Z.; Yan, Y.; Yang, J. Osthole promotes neuronal differentiation and inhibits apoptosis via Wnt/β-catenin signaling in an Alzheimer’s disease model. Toxicol. Appl. Pharmacol. 2015, 289, 474–481. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, D.; Zhang, L.; Li, W.; Meng, X. Osthole improves synaptic plasticity in the hippocampus and cognitive function of Alzheimer’s disease rats via regulating glutamate. Neural Regen. Res. 2012, 7, 2325–2332. [Google Scholar]
- Chu, Q.; Zhu, Y.; Cao, T.; Zhang, Y.; Chang, Z.; Liu, Y.; Lu, J.; Zhang, Y. Studies on the Neuroprotection of Osthole on Glutamate-Induced Apoptotic Cells and an Alzheimer’s Disease Mouse Model via Modulation Oxidative Stress. Appl. Biochem. Biotechnol. 2020, 190, 634–644. [Google Scholar] [CrossRef]
- Barabási, A.L.; Gulbahce, N.; Loscalzo, J. Network medicine: A network-based approach to human disease. Nat. Rev. Genet. 2011, 12, 56–68. [Google Scholar] [CrossRef]
- Jarrell, J.T.; Gao, L.; Cohen, D.S.; Huang, X. Network Medicine for Alzheimer’s Disease and Traditional Chinese Medicine. Molecules 2018, 23, 1143. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Pieper, A.A.; Nussinov, R.; Lee, G.; Bekris, L.; Leverenz, J.B.; Cummings, J.; Cheng, F. Harnessing endophenotypes and network medicine for Alzheimer’s drug repurposing. Med. Res. Rev. 2020, 40, 2386–2426. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhang, P.; Zhou, Y.; Chiang, C.W.; Tan, J.; Hou, Y.; Stauffer, S.; Li, L.; Pieper, A.A.; Cummings, J.; et al. Endophenotype-based in silico network medicine discovery combined with insurance record data mining identifies sildenafil as a candidate drug for Alzheimer’s disease. Nat. Aging 2021, 1, 1175–1188. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cai, C.; Liao, Y.; Wu, Q.; Ke, H.; Guo, P.; Wang, Q.; Ding, B.; Fang, J.; Fang, S. Systems pharmacology approach uncovers the therapeutic mechanism of medicarpin against scopolamine-induced memory loss. Phytomedicine 2021, 91, 153662. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. D-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef]
- Chiroma, S.M.; Mohd Moklas, M.A.; Mat Taib, C.N.; Baharuldin, M.T.H.; Amon, Z. d-galactose and aluminium chloride induced rat model with cognitive impairments. Biomed. Pharmacother. 2018, 103, 1602–1608. [Google Scholar] [CrossRef]
- Evin, G.; Barakat, A.; Masters, C.L. BACE: Therapeutic target and potential biomarker for Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2010, 42, 1923–1926. [Google Scholar] [CrossRef]
- Singh, N.; Das, B.; Zhou, J.; Hu, X.; Yan, R. Targeted BACE-1 inhibition in microglia enhances amyloid clearance and improved cognitive performance. Sci. Adv. 2022, 8, eabo3610. [Google Scholar] [CrossRef]
- D’Amelio, M.; Cavallucci, V.; Middei, S.; Marchetti, C.; Pacioni, S.; Ferri, A.; Diamantini, A.; De Zio, D.; Carrara, P.; Battistini, L.; et al. Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2011, 14, 69–76. [Google Scholar] [CrossRef]
- Liu, S.L.; Wang, C.; Jiang, T.; Tan, L.; Xing, A.; Yu, J.T. The Role of Cdk5 in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 4328–4342. [Google Scholar] [CrossRef]
- Zhou, R.; Ji, B.; Kong, Y.; Qin, L.; Ren, W.; Guan, Y.; Ni, R. PET Imaging of Neuroinflammation in Alzheimer’s Disease. Front. Immunol. 2021, 12, 739130. [Google Scholar] [CrossRef] [PubMed]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Yu, W.S.; Lim, L.W. Exploring ER stress response in cellular aging and neuroinflammation in Alzheimer’s disease. Ageing Res. Rev. 2021, 70, 101417. [Google Scholar] [CrossRef] [PubMed]
- Maino, B.; Paparone, S.; Severini, C.; Ciotti, M.T.; D’Agata, V.; Calissano, P.; Cavallaro, S. Drug target identification at the crossroad of neuronal apoptosis and survival. Expert. Opin. Drug Discov. 2017, 12, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Obulesu, M.; Lakshmi, M.J. Apoptosis in Alzheimer’s disease: An understanding of the physiology, pathology and therapeutic avenues. Neurochem. Res. 2014, 39, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dhapola, R.; Reddy, D.H. Apoptosis in Alzheimer’s disease: Insight into the signaling pathways and therapeutic avenues. Apoptosis 2023, 28, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Bansal, N. Implications of Phosphoinositide 3-Kinase-Akt (PI3K-Akt) Pathway in the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2022, 59, 354–385. [Google Scholar] [CrossRef] [PubMed]
- Saahene, R.O.; Agbo, E.; Barnes, P.; Yahaya, E.S.; Amoani, B.; Nuvor, S.V.; Okyere, P. A Review: Mechanism of Phyllanthus urinaria in Cancers-NF-kappaB, P13K/AKT, and MAPKs Signaling Activation. Evid. Based Complement. Altern. Med. 2021, 2021, 4514342. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Ju Hwang, C.; Choi, D.Y.; Park, M.H.; Hong, J.T. NF-kappaB as a Key Mediator of Brain Inflammation in Alzheimer’s Disease. CNS Neurol. Disord. Drug Targets 2019, 18, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.M.; Han, Y.W.; Han, X.H.; Zhang, K.; Chang, Y.N.; Hu, Z.M.; Qi, H.X.; Ting, C.; Zhen, Z.; Hong, W. Upstream regulators and downstream effectors of NF-κB in Alzheimer’s disease. J. Neurol. Sci. 2016, 366, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.S.; Tewari, D.; Sharma, G.; Kabir, M.T.; Barreto, G.E.; Bin-Jumah, M.N.; Perveen, A.; Abdel-Daim, M.M.; Ashraf, G.M. Molecular Mechanisms of ER Stress and UPR in the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 2902–2919. [Google Scholar] [CrossRef]
- Hashimoto, S.; Saido, T.C. Critical review: Involvement of endoplasmic reticulum stress in the aetiology of Alzheimer’s disease. Open Biol. 2018, 8, 180024. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.S.; Cummings, J.; Jack, C.R., Jr.; Morris, J.C.; Sperling, R.; Frölich, L.; Jones, R.W.; Dowsett, S.A.; Matthews, B.R.; Raskin, J.; et al. On the path to 2025: Understanding the Alzheimer’s disease continuum. Alzheimers Res. Ther. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Martinez, L.; Calfío, C.; Farias, G.A.; Vilches, C.; Prieto, R.; Maccioni, R.B. New Frontiers in the Prevention, Diagnosis, and Treatment of Alzheimer’s Disease. J. Alzheimers Dis. 2021, 82 (Suppl. S1), S51–S63. [Google Scholar] [CrossRef]
- Maccioni, R.B.; Calfío, C.; González, A.; Lüttges, V. Novel Nutraceutical Compounds in Alzheimer Prevention. Biomolecules 2022, 12, 249. [Google Scholar] [CrossRef]
- Banji, O.J.; Banji, D.; Ch, K. Curcumin and hesperidin improve cognition by suppressing mitochondrial dysfunction and apoptosis induced by D-galactose in rat brain. Food Chem. Toxicol. 2014, 74, 51–59. [Google Scholar] [CrossRef]
- Cao, P.; Zhang, J.; Huang, Y.; Fang, Y.; Lyu, J.; Shen, Y. The age-related changes and differences in energy metabolism and glutamate-glutamine recycling in the d-gal-induced and naturally occurring senescent astrocytes in vitro. Exp. Gerontol. 2019, 118, 9–18. [Google Scholar] [CrossRef]
- Chi, H.; Chang, H.Y.; Sang, T.K. Neuronal Cell Death Mechanisms in Major Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Singh, D. Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J. Neuroinflamm. 2022, 19, 206. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Fang, J.; Lu, W.; Wang, Z.; Wang, Q.; Hou, Y.; Jiang, X.; Reizes, O.; Lathia, J.; Nussinov, R.; et al. A Systems Pharmacology Approach Uncovers Wogonoside as an Angiogenesis Inhibitor of Triple-Negative Breast Cancer by Targeting Hedgehog Signaling. Cell Chem. Biol. 2019, 26, 1143–1158.e6. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Zheng, G.; Wang, C.; Chen, Z.; Mao, T.; Gao, J.; Yan, Y.; Chen, X.; Ji, X.; Yu, J.; et al. HIT 2.0: An enhanced platform for Herbal Ingredients’ Targets. Nucleic Acids Res. 2021, 50, D1238–D1243. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Lu, W.; Liu, C.; Fang, J.; Hou, Y.; Handy, D.E.; Wang, R.; Zhao, Y.; Yang, Y.; Huang, J.; et al. A genome-wide positioning systems network algorithm for in silico drug repurposing. Nat. Commun. 2019, 10, 3476. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, P.; Wang, Q.; Chiang, C.W.; Zhou, Y.; Hou, Y.; Xu, J.; Chen, R.; Zhang, B.; Lewis, S.J.; et al. Artificial intelligence framework identifies candidate targets for drug repurposing in Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 7. [Google Scholar] [CrossRef]
- Wu, Q.; Fan, X.; Hong, H.; Gu, Y.; Liu, Z.; Fang, S.; Wang, Q.; Cai, C.; Fang, J. Comprehensive assessment of side effects in COVID-19 drug pipeline from a network perspective. Food Chem. Toxicol. 2020, 145, 111767. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Fu, X.; Luo, X.; Lai, Y.; Cai, C.; Liao, Y.; Dai, Z.; Fang, S.; Fang, J. Network Proximity Analysis Deciphers the Pharmacological Mechanism of Osthole against D-Galactose Induced Cognitive Disorder in Rats. Molecules 2024, 29, 21. https://doi.org/10.3390/molecules29010021
Wang X, Fu X, Luo X, Lai Y, Cai C, Liao Y, Dai Z, Fang S, Fang J. Network Proximity Analysis Deciphers the Pharmacological Mechanism of Osthole against D-Galactose Induced Cognitive Disorder in Rats. Molecules. 2024; 29(1):21. https://doi.org/10.3390/molecules29010021
Chicago/Turabian StyleWang, Xue, Xiaomei Fu, Xiurong Luo, Yiyi Lai, Chuipu Cai, Yanfang Liao, Zhao Dai, Shuhuan Fang, and Jiansong Fang. 2024. "Network Proximity Analysis Deciphers the Pharmacological Mechanism of Osthole against D-Galactose Induced Cognitive Disorder in Rats" Molecules 29, no. 1: 21. https://doi.org/10.3390/molecules29010021
APA StyleWang, X., Fu, X., Luo, X., Lai, Y., Cai, C., Liao, Y., Dai, Z., Fang, S., & Fang, J. (2024). Network Proximity Analysis Deciphers the Pharmacological Mechanism of Osthole against D-Galactose Induced Cognitive Disorder in Rats. Molecules, 29(1), 21. https://doi.org/10.3390/molecules29010021






