Lipase-Assisted Synthesis of Alkyl Stearates: Optimization by Taguchi Design of Experiments and Application as Defoamers
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Materials and Instruments
3.2. Synthesis of Alkyl Stearates
3.3. Evaluation of Emulsion Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vinod, N.; Tiwari, R.; Bhat, N.S.; Mal, S.S.; Dutta, S. High-yielding synthesis of alkyl stearates from stearic acid within a closed batch reactor using heteropolyacids as efficient and recyclable catalyst. AIP Conf. Proc. 2020, 2225, 070004. [Google Scholar] [CrossRef]
- Xie, T.; Zeng, C.; Wang, C.; Zhang, L. Preparation of Methyl Ester Sulfonates Based on Sulfonation in a Falling Film Microreactor from Hydrogenated Palm Oil Methyl Esters with Gaseous SO3. Ind. Eng. Chem. Res. 2013, 52, 3714–3722. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, Z.; Zhang, W.; Sun, H.; Liu, S. Cu-Zn/Al2O3 Catalyst for the Hydrogenation of Esters to Alcohols. Chin. J. Catal. 2010, 31, 769–775. [Google Scholar] [CrossRef]
- Baker, I.J.A.; Matthews, B.; Suares, H.; Krodkiewska, I.; Furlong, D.N.; Grieser, F.; Drummond, C.I. Sugar fatty acid ester surfactants: Structure and ultimate aerobic biodegradability. J. Surfactants Deterg. 2000, 3, 1–11. [Google Scholar] [CrossRef]
- Aoshima, H.; Miyagisnima, A.; Nozawa, Y.; Sadzuka, Y.; Sonobe, T. Glycerin fatty acid esters as a new lubricant of tablets. Int. J. Pharm. 2005, 293, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, E.D.C.; Aguieiras, É.C.G.; da Silva, P.R.; Duarte, J.G.; Cipolatti, E.P.; Fernandez-Lafuente, R.; da Silva, J.A.C.; Freire, D.M.G. Improved production of biolubricants from soybean oil and different polyols via esterification reaction catalyzed by immobilized lipase from Candida rugosa. Fuel 2018, 215, 705–713. [Google Scholar] [CrossRef]
- Maag, H. Fatty acid derivatives: Important surfactants for household, cosmetic and industrial purposes. J. Am. Oil Chem. Soc. 1984, 61, 259–267. [Google Scholar] [CrossRef]
- Allen, D.K.; Tao, B.Y. Carbohydrate-alkyl ester derivatives as biosurfactants. J. Surfactants Deterg. 1999, 2, 383–390. [Google Scholar] [CrossRef]
- Koshima, H.; Miyazaki, K.; Ishii, S.; Asahi, T.; Koshima, H.; Miyazaki, K.; Ishii, S.; Asahi, T. Microwave Effect on Fischer Esterification. Chem. Lett. 2016, 45, 505–507. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin, J.G., Jr. Effect of carbon chain length on esterification of carboxylic acids with methanol using acid catalysis. J. Catal. 2006, 243, 221–228. [Google Scholar] [CrossRef]
- Pereira, G.N.; Holz, J.P.; Giovannini, P.P.; Oliveira, J.V.; de Oliveira, D.; Lerin, L.A. Enzymatic esterification for the synthesis of butyl stearate and ethyl stearate. Biocatal. Agric. Biotechnol. 2018, 16, 373–377. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current developments in esterification reaction: A review on process and parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Puterbaugh, W.H.; Vanselow, C.H.; Nelson, K.; Shrawder, E.J. Esterification for the introductory organic laboratory course: A modified Dean-Stark trap. J. Chem. Educ. 1963, 40, 349–350. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Cardoso, A.L.; Natalino, R. Bioenergy II: Tin Catalysed Esterification of Free Fatty Acids. Int. J. Chem. React. Eng. 2010, 8, 1–12. [Google Scholar] [CrossRef]
- Reddy, C.R.; Iyengar, P.; Nagendrappa, G.; Jai Prakash, B.S. Esterification of succinic anhydride to di-(p-cresyl) succinate over Mn+ montmorillonite clay catalysts. J. Mol. Catal. A Chem. 2005, 229, 31–37. [Google Scholar] [CrossRef]
- Kiss, A.A.; Dimian, A.C.; Rothenberg, G. Solid Acid Catalysts for Biodiesel Production—Towards Sustainable Energy. Adv. Synth. Catal. 2006, 348, 75–81. [Google Scholar] [CrossRef]
- Langrand, G.; Baratti, J.; Buono, G.; Triantaphylides, C. Lipase catalyzed reactions and strategy for alcohol resolution. Tetrahedron Lett. 1986, 27, 29–32. [Google Scholar] [CrossRef]
- Hari Krishna, S.; Karanth, N.G. Lipases and Lipase-Catalyzed Esterification Reactions in Nonaqueous Media. Cat. Rev. Sci. Eng. 2002, 44, 499–591. [Google Scholar] [CrossRef]
- Tavizón-Pozos, J.A.; Ibarra, I.S.; Guevara-Lara, A.; Galán-Vidal, C.A. Application of Design of Experiments in Biofuel Production: A Review. In Design of Experiments for Chemical, Pharmaceutical, Food, and Industrial Applications, 1st ed.; Carrillo-Cedillo, E.G., Rodríguez-Ávila, J.A., Arredondo-Soto, K.C., Cornejo-Bravo, J.M., Eds.; IGI Global: Hershey, PA, USA, 2019; Volume 1, pp. 77–103. [Google Scholar] [CrossRef]
- García, R.; Martínez, M.; Aracil, J. Enzymatic esterification of an acid with an epoxide using an immobilized lipase from Mucor miehei as catalyst: Optimization of the yield and isomeric excess of ester by statistical analysis. J. Ind. Microbiol. Biotechnol. 2002, 28, 173–179. [Google Scholar] [CrossRef]
- García, E.; Ferrari, F.; García, T.; Martínez, M.; Aracil, J. Optimization of the enzymatic esterification of diglycerol and lauric acid. J. Surfactants Deterg. 2001, 4, 257–262. [Google Scholar] [CrossRef]
- Mibielli, G.M.; Fagundes, A.P.; Bohn, L.R.; Cavali, M.; Bueno, A.; Bender, J.P.; Oliveira, J.V. Enzymatic production of methyl esters from low-cost feedstocks. Biocatal. Agric. Biotechnol. 2020, 24, 101558. [Google Scholar] [CrossRef]
- Lee, A.; Chaibakhsh, N.; Rahman, M.B.A.; Basri, M.; Tejo, B.A. Optimized enzymatic synthesis of levulinate ester in solvent-free system. Ind. Crops. Prod. 2010, 32, 246–251. [Google Scholar] [CrossRef]
- Mohd Hussin, F.N.N.; Attan, N.; Wahab, R.A. Taguchi design-assisted immobilization of Candida rugosa lipase onto a ternary alginate/nanocellulose/montmorillonite composite: Physicochemical characterization, thermal stability and reusability studies. Enzyme Microb. Techol. 2020, 136, 109506. [Google Scholar] [CrossRef] [PubMed]
- Farn, R.J. Chemistry and Technology of Surfactants, 1st ed.; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 76–81. [Google Scholar] [CrossRef]
- Garrett, P.R. Defoaming: Antifoams and mechanical methods. Curr. Opin. Colloid Interface Sci. 2015, 20, 81–91. [Google Scholar] [CrossRef]
- Joshi, K.S.; Jeelani, S.A.K.; Blickenstorfer, C.; Naegeli, I.; Windhab, E.J. Influence of fatty alcohol antifoam suspensions on foam stability. Colloids Surf. A Physicochem. Eng. Asp. 2005, 263, 239–249. [Google Scholar] [CrossRef]
- Pugh, R.J. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–142. [Google Scholar] [CrossRef]
- Owen, M.J. Defoamers, 1st ed.; Kirk-Othmer Encyclopedia of Chemical Technology: New York, NY, USA, 2001; pp. 236–254. [Google Scholar] [CrossRef]
- Touqeer, T.; Mumtaz, M.W.; Mukthar, H.; Irfan, A.; Akram, S.; Shabbir, A.; Rashid, U.; Nehdi, I.A.; Choong, T.S.Y. Fe3O4-PDA-Lipase as Surface Functionalized Nano Biocatalyst for the Production of Biodiesel Using Waste Cooking oil as Feedstock: Characterization and Process Optimization. Energies 2020, 13, 177. [Google Scholar] [CrossRef]
- Stergiou, P.Y.; Foukis, A.; Filippou, M.; Koukouritaki, M.; Parapouli, M.; Theodorou, L.G.; Hatziloukas, E.; Afendra, A.; Pandey, A.; Papamichael, E.M. Advances in lipase-catalyzed esterification reactions. Biotechnol. Adv. 2013, 31, 1846–1859. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Volpato, G.; Wada, K.; Ayub, M.A.Z. Enzymatic Synthesis of Biodiesel from Transesterification Reactions of Vegetable Oils and Short Chain Alcohols. J. Am. Oil Chem. Soc. 2008, 85, 925–930. [Google Scholar] [CrossRef]
- Yong, Y.P.; Al-Duri, B. Kinetic Studies on Immobilised Lipase Esterification of Oleic Acid and Octanol. J. Chem. Technol. Biotechnol. 1996, 65, 239–248. [Google Scholar] [CrossRef]
- Tsitsimpikou, C.; Daflos, H.; Kolisis, F.N. Comparative studies on the sugar esters synthesis catalysed by Candida antarctica and Candida rugosa lipases in hexane. J. Mol. Catal. B Enzym. 1997, 3, 189–192. [Google Scholar] [CrossRef]
- Boseley, J.A.; Peilow, A.D. Immobilization of Lipases on Porous Polypropylene: Reduction in Esterification Efficiency at Low Loading. J. Am. Oil Chem. Soc. 1997, 74, 107–111. [Google Scholar] [CrossRef]
- Deng, L.; Tan, T.; Wang, F.; Xu, X. Enzymatic production of fatty acid alkyl esters with a lipase preparation from Candida sp. 99-125. Eur. J. Lipid Sci. Technol. 2003, 105, 727–734. [Google Scholar] [CrossRef]
- Serrano-Arnaldos, M.; Máximo-Martín, M.F.; Montiel-Morte, M.C.; Ortega-Requena, S.; Gómez-Gómez, E.; Bastida-Rodríguez, J. Solvent-free enzymatic production of high quality cetyl esters. Bioprocess Biosyst. Eng. 2016, 39, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Foresti, M.L.; Galle, M.; Ferreira, M.L.; Briand, L.E. Enantioselective esterification of ibuprofen with ethanol as reactant and solvent catalyzed by immobilized lipase: Experimental and molecular modeling aspects. J. Chem. Technol. Biotechnol. 2009, 84, 1461–1473. [Google Scholar] [CrossRef]
- Kulschewski, T.; Sasso, F.; Secundo, F.; Lotti, M.; Pleiss, J. Molecular mechanism of deactivation of C. antarctica lipase B by methanol. J. Biochem. 2013, 168, 462–469. [Google Scholar] [CrossRef]
- Manjón, A.; Iborra, J.L.; Arocas, A. Short-chain flavour ester synthesis by immobilized lipase in organic media. Biotechnol. Lett. 1991, 13, 339–344. [Google Scholar] [CrossRef]
- Deng, L.; Wang, X.; Nie, K.; Wang, F.; Liu, J.; Wang, P.; Tan, T. Synthesis of Wax Esters by Lipase-catalyzed Esterification with Immobilized Lipase from Candida sp. 99-125*. Chin. J. Chem. Eng. 2011, 19, 978–982. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics. Available online: https://www.molinspiration.com (accessed on 31 October 2023).
- Denkov, N.D. Mechanisms of Foam Destruction by Oil-Based Antifoams. Langmuir 2004, 20, 9463–9505. [Google Scholar] [CrossRef]


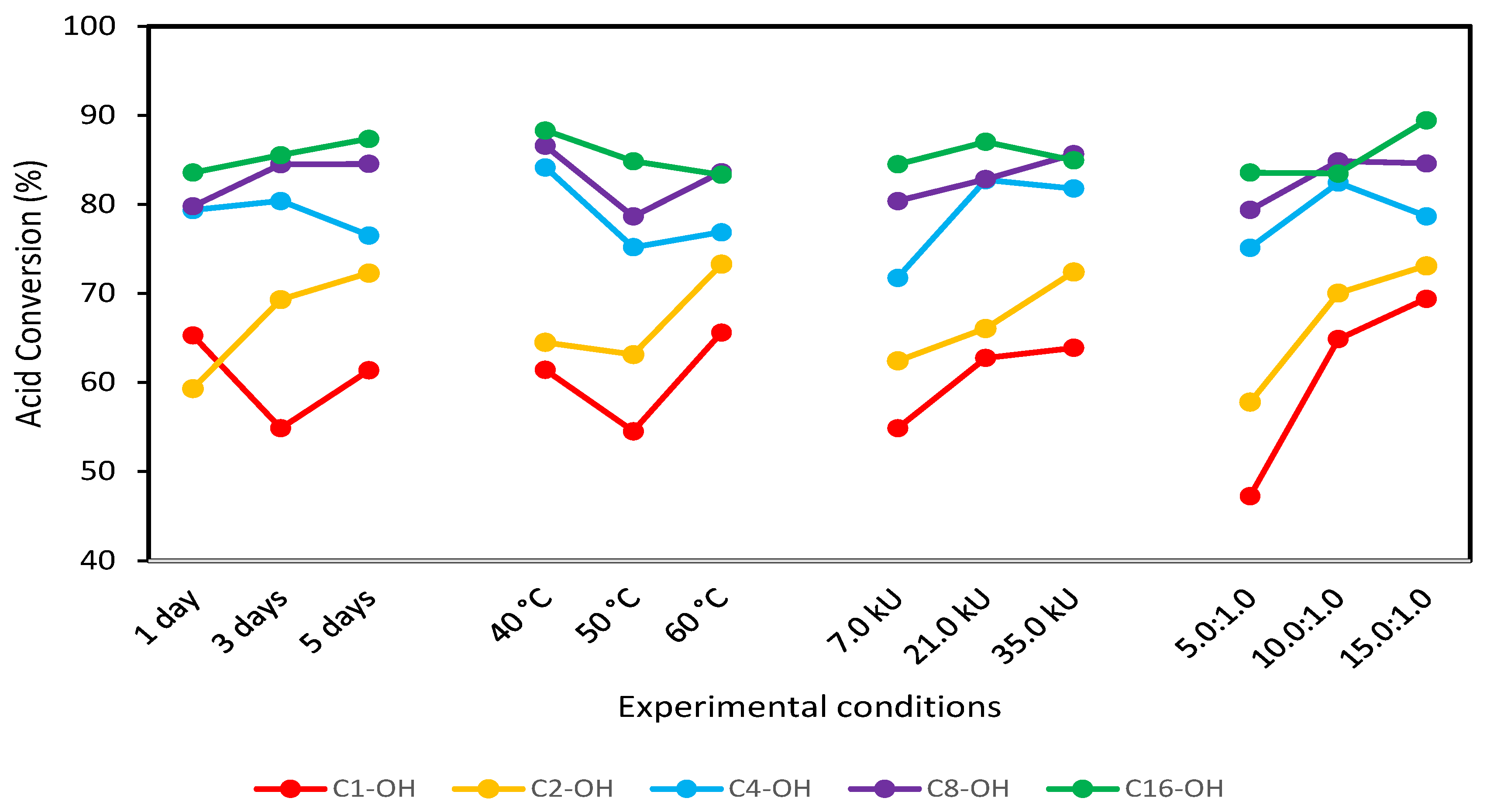
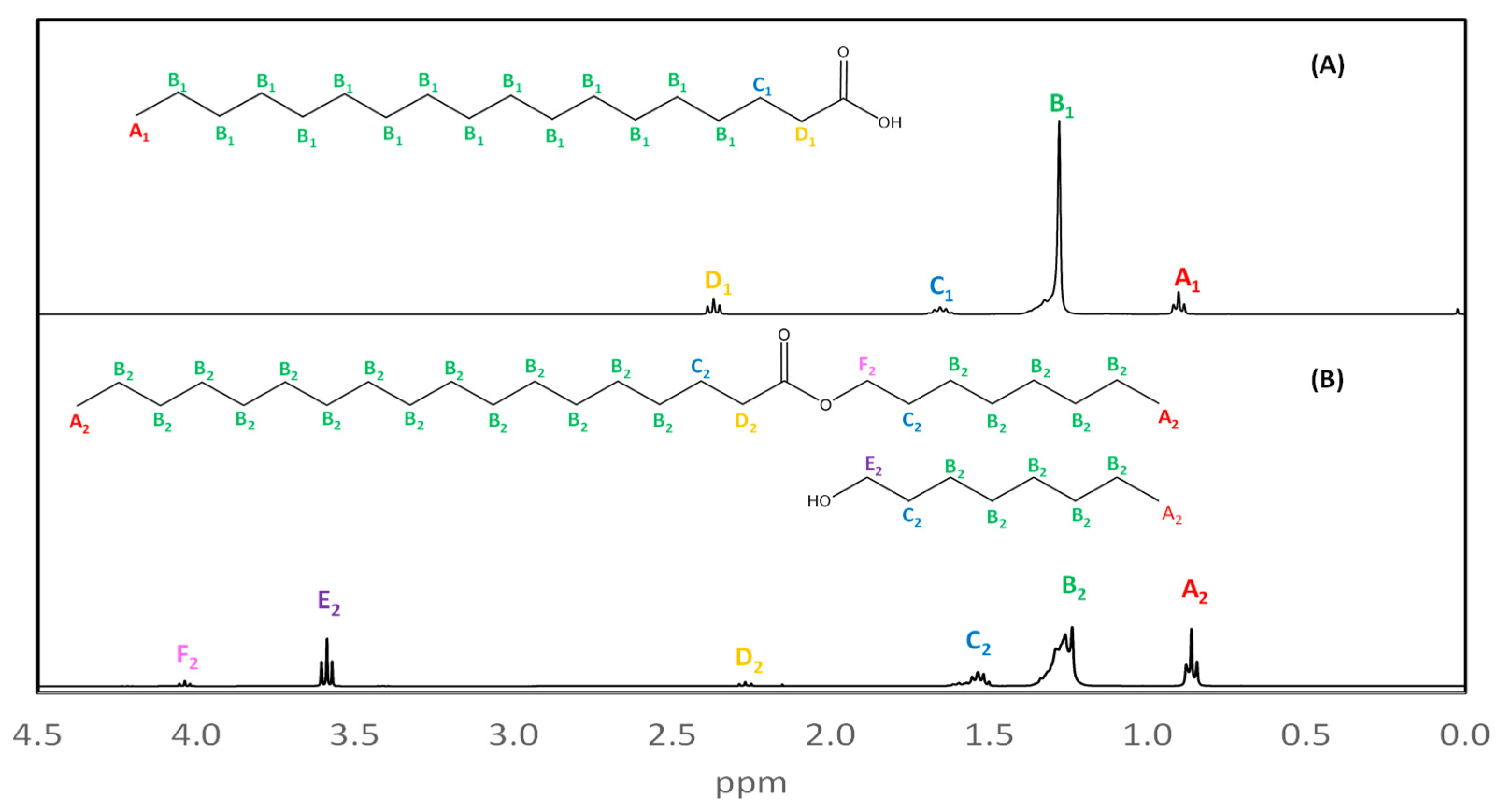
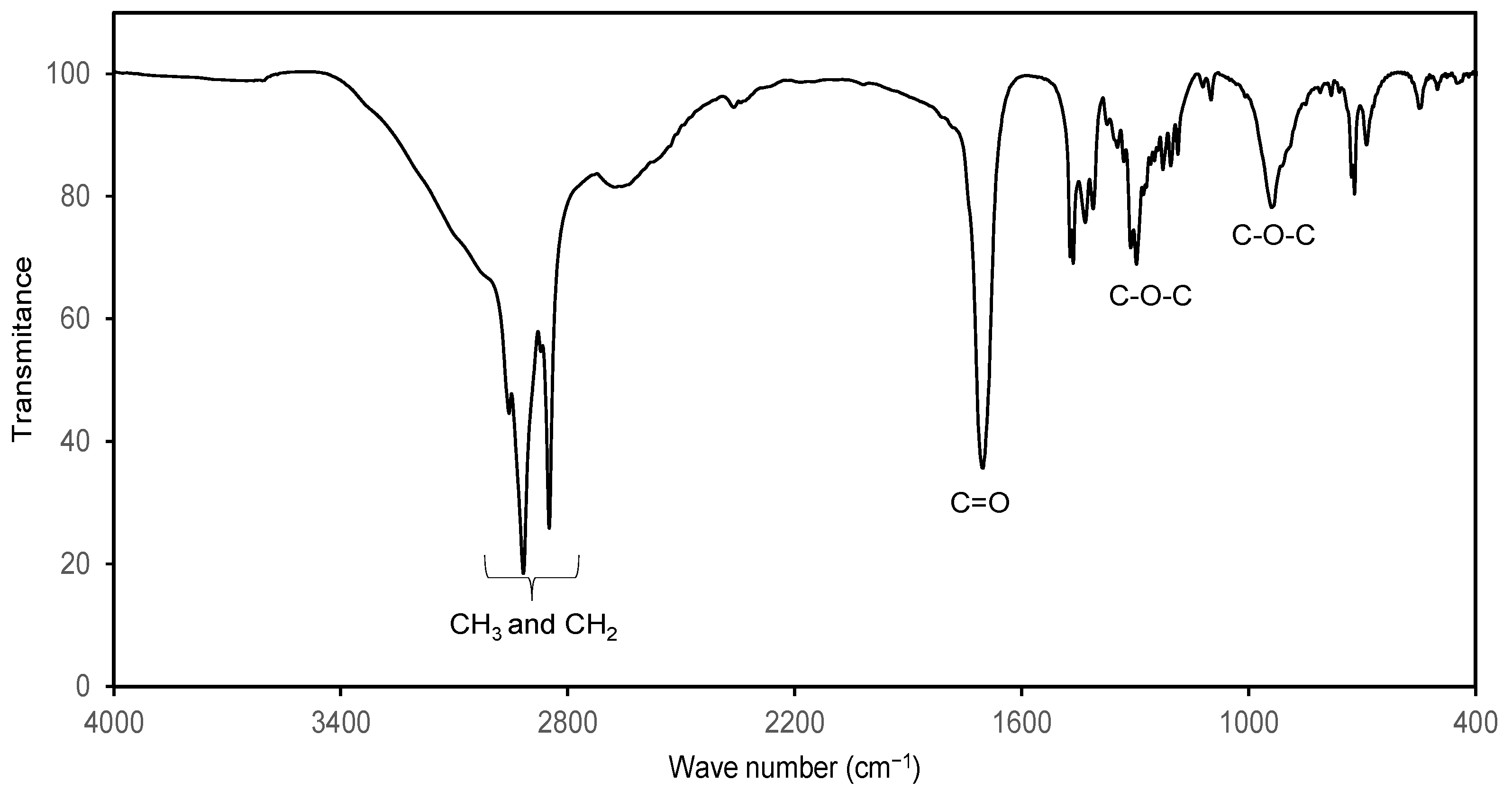
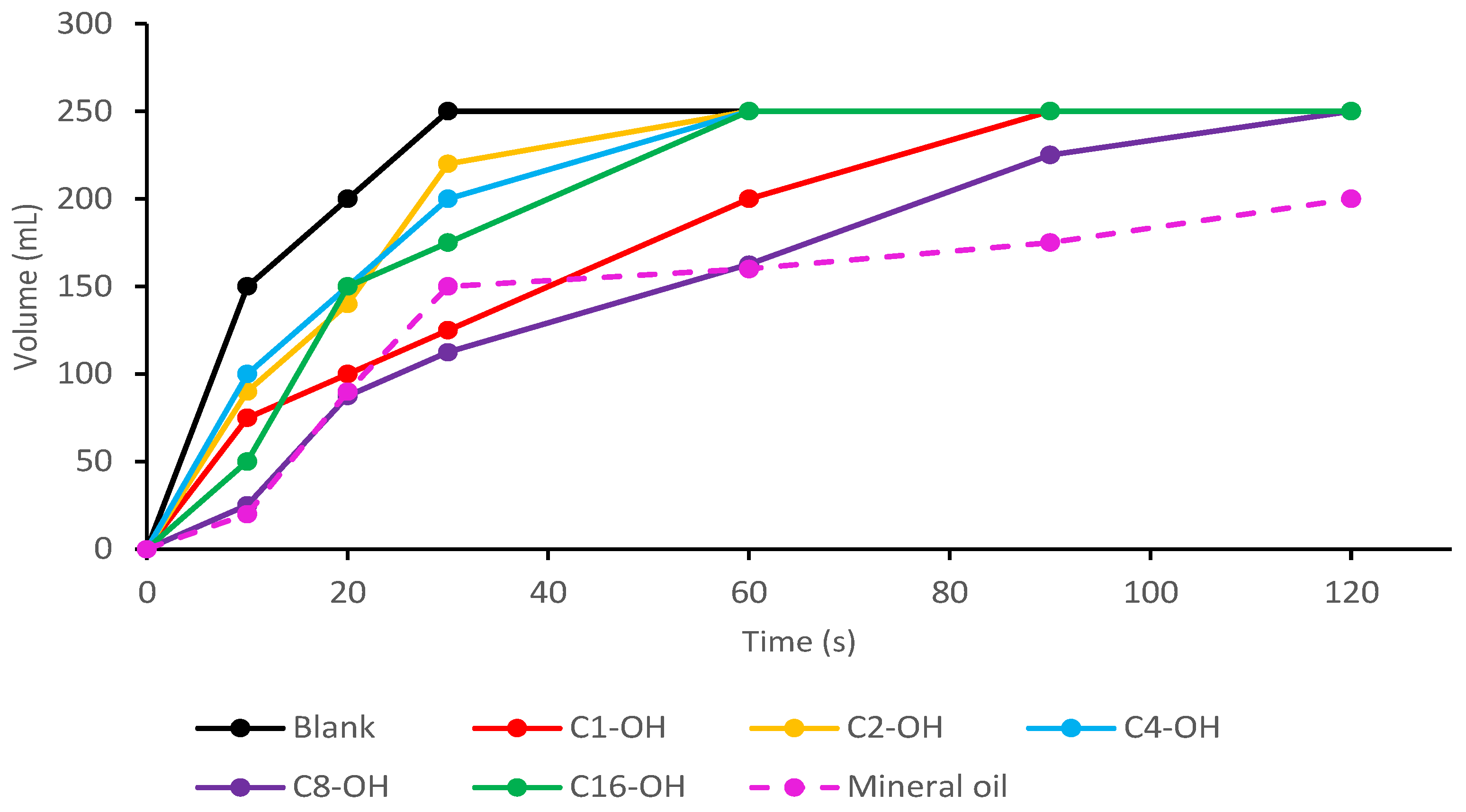
| Experiment | Time (Days) | Temperature (°C) | Lipase (kU) | Alcohol:Stearic Acid Molar Ratio | Mean Reaction Conversion (%RSD, n = 3) | ||||
|---|---|---|---|---|---|---|---|---|---|
| C1-OH | C2-OH | C4-OH | C8-OH | C16-OH | |||||
| 1 | 1 | 40 | 7.0 | 5:1 | 47.2 (2.2%) | 41.0 (2.4%) | 74.1 (1.4%) | 77.2 (0.8%) | 83.4 (1.5%) |
| 2 | 1 | 50 | 21.0 | 10:1 | 65.8 (1.6%) | 57.6 (1.8%) | 83.5 (1.2%) | 78.3 (2.7%) | 81.4 (1.3%) |
| 3 | 1 | 60 | 35.0 | 15:1 | 82.6 (0.5%) | 77.2 (1.4%) | 80.4 (0.8%) | 84.8 (0.9%) | 84.8 (0.8%) |
| 4 | 3 | 40 | 21.0 | 15:1 | 66.9 (0.6%) | 72.1(1.5%) | 89.6 (1.3%) | 89.6 (1.2%) | 93.8 (1.1%) |
| 5 | 3 | 50 | 35.0 | 5:1 | 38.9 (2.7%) | 61.7 (1.7%) | 76.2 (1.4%) | 79.3 (1.4%) | 82.4 (1.3%) |
| 6 | 3 | 60 | 7.0 | 10:1 | 58.7 (0.8%) | 74.1 (1.4%) | 75.2 (0.5%) | 84.5 (1.2%) | 80.3 (1.3%) |
| 7 | 5 | 40 | 35.0 | 10:1 | 70.0 (1.5%) | 78.3 (1.3%) | 88.6 (1.2%) | 92.8 (1.1%) | 87.6 (0.9%) |
| 8 | 5 | 50 | 7.0 | 15:1 | 58.6 (1.0%) | 70.0 (1.5%) | 65.8 (1.6%) | 79.3 (0.7%) | 89.7 (1.0%) |
| 9 | 5 | 60 | 21.0 | 5:1 | 55.4 (2.0%) | 68.5 (1.6%) | 75.0 (1.4%) | 81.5 (1.3%) | 84.8 (0.7%) |
| Alkyl Stearate | Time (Days) | Temperature (°C) | Lipase (kU) | Alcohol:Stearic Acid Molar Ratio | Conversion (%RSD n = 3) |
|---|---|---|---|---|---|
| C1-OH | 1 | 60 | 35.0 | 15:1 | 90.5 (0.6%) |
| C2-OH | 5 | 60 | 35.0 | 15:1 | 91.9 (0.7%) |
| C4-OH | 3 | 40 | 21.0 | 10:1 | 94.7 (0.3%) |
| C8-OH | 3 | 40 | 35.0 | 10:1 | 96.4 (0.3%) |
| C16-OH | 5 | 40 | 21.0 | 15:1 | 96.8 (0.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olvera-Ureña, E.; Lopez-Tellez, J.; Vizueto, M.M.; Hidalgo-Ledezma, J.G.; Martinez-Quiroz, B.; Rodriguez, J.A. Lipase-Assisted Synthesis of Alkyl Stearates: Optimization by Taguchi Design of Experiments and Application as Defoamers. Molecules 2024, 29, 195. https://doi.org/10.3390/molecules29010195
Olvera-Ureña E, Lopez-Tellez J, Vizueto MM, Hidalgo-Ledezma JG, Martinez-Quiroz B, Rodriguez JA. Lipase-Assisted Synthesis of Alkyl Stearates: Optimization by Taguchi Design of Experiments and Application as Defoamers. Molecules. 2024; 29(1):195. https://doi.org/10.3390/molecules29010195
Chicago/Turabian StyleOlvera-Ureña, Enoch, Jorge Lopez-Tellez, M. Monserrat Vizueto, J. Guadalupe Hidalgo-Ledezma, Baltazar Martinez-Quiroz, and Jose A. Rodriguez. 2024. "Lipase-Assisted Synthesis of Alkyl Stearates: Optimization by Taguchi Design of Experiments and Application as Defoamers" Molecules 29, no. 1: 195. https://doi.org/10.3390/molecules29010195
APA StyleOlvera-Ureña, E., Lopez-Tellez, J., Vizueto, M. M., Hidalgo-Ledezma, J. G., Martinez-Quiroz, B., & Rodriguez, J. A. (2024). Lipase-Assisted Synthesis of Alkyl Stearates: Optimization by Taguchi Design of Experiments and Application as Defoamers. Molecules, 29(1), 195. https://doi.org/10.3390/molecules29010195








