Abstract
Background: Folk medicines are attractive therapeutic agents for treating type 2 diabetes mellitus (T2DM). Most plant extracts that have been suggested to restore β-cells function were tested in vivo. Some only have been tested in vitro to determine whether they have a direct effect on β-cells islets of Langerhans. Currently, there are no defined criteria for screening of β-cell-directed plant-based remedies as potential antidiabetic agents. Summary: In this review, we have identified certain criteria/characteristics that can be used to generate a “screening portfolio” to identify plant extracts as potential β-cell-directed agents for the treatment of T2DM. To validate our screening method, we studied the potential therapeutic efficacy of a Gymnema sylvestre (GS) extract using the screening criteria detailed in the review. Six criteria have been identified and validated using OSA®, a GS extract. By using this screening method, we show that OSA® fulfilled most of the criteria identified for an effective β-cell-directed antidiabetic therapy, being an effective insulin-releasing agent at nontoxic concentrations; maintaining β-cell insulin content by stimulating a concomitant increase in insulin gene transcription; maintaining β-cell mass by protecting against apoptosis; and being effective at maintaining normoglycemia in vivo in a mouse model and a human cohort with T2DM. Key messages: The present review has highlighted the importance of having a screening portfolio for plant extracts that have potential antidiabetic effects in the treatment of T2DM. We propose that this screening method should be adopted for future studies to identify new β-cell-directed antidiabetic plant derived agents.
1. Introduction
The area of alternative and complementary medicine, herbal and plant-based remedies in particular, has been the focus of attention of scientists in the past few decades, and it has been estimated that the percentage of people who have used herbal medicine reached 35–48% worldwide [1,2]. Diabetes mellitus is a health problem affecting all populations globally. The prevalence of the disease is increasing, and by the year 2045, the estimated number of adult people who will develop diabetes will rise to 629 million [3]. Type 2 diabetes mellitus (T2DM), which is characterized by two important pathophysiological characteristics, namely β-cell dysfunction and impaired insulin responsiveness in insulin target tissues, will affect 95% of all diabetes cases.
It is clear that β-cell dysfunction is the cornerstone in the development of T2DM and symptoms of hyperglycemia appear when β-cell function is no longer sufficient to maintain glucose homeostasis. Several studies have reported that the primary trigger for overt T2DM is impaired β-cell function and that reduced β-cell function/loss of β-cell mass is a determinant of the severity of T2DM [4,5,6]. Furthermore, variations in several genetic loci have been reported, and these loci are associated with an increased risk of developing T2DM [7,8,9,10,11,12]. The involvement of most genes in controlling β-cell function and insulin secretion supports the role of β-cell dysfunction in the progression of T2DM. Therefore, enhancement of β-cell function is one of the major drug targets for the reduction in hyperglycemia and prevention of microvascular and macrovascular complications. Examples of currently available therapies that target β-cell function are sulphonylureas and glucagon-like peptide 1 (GLP-1)-based therapy. However, serious adverse effects are associated with some of these therapies, and complete glycemic control is difficult to achieve. Therefore, introducing and developing new drugs that target β-cell function, especially those of plant origin, continues to be an active area of research. Metformin, which was originally isolated from a plant named Galega officinalis, is one of the most widely used frontline antidiabetic drugs in the management of T2DM [13]. Thus, identifying novel, effective extracts of plant origin to modulate β-cell function and thus treat T2DM could offer more affordable and easily accessible antidiabetic alternatives in the developing world.
Many plant species have been claimed to be effective in treating T2DM [14]. However, there are currently no defined criteria for identifying and screening herbal remedies that have positive activities on β-cell function as potential antidiabetic agents. Therefore, in this review, we have identified a number of characteristics/properties that can be used as screening tools to identify and investigate plant extracts with potential effects on β-cells as potential therapeutic agents for the treatment of T2DM.
2. Screening Plant Extracts for Therapeutic Potential
Here, we propose specific criteria that can be applied as a “screening portfolio” to identify potential β-cell-focused therapeutic agents of plant origin (Figure 1). Each plant extract will have an individualized profile that can act as a fingerprint for that extract, allowing rapid evaluation, comparison between extracts, and future identification of the most promising extracts that have the potential to be progressed to clinical trials and market.
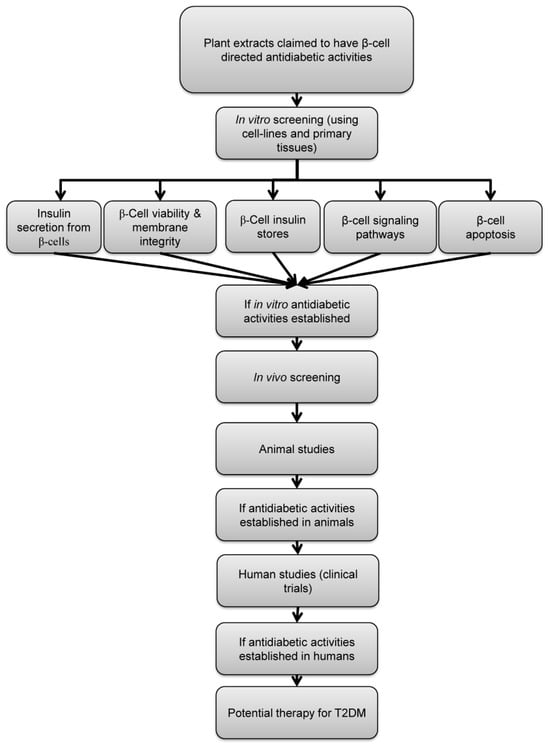
Figure 1.
Schematic representation of the proposed screening criteria of plant-derived antidiabetic agents. Plants claimed to have antidiabetic activities by acting through modulation of β-cell function should be first screened in vitro for the following properties: stimulation of insulin secretion, maintaining β-cell viability, preservation of β-cell insulin store, activation of known β-cell signaling pathways and preservation of β-cell mass. If antidiabetic effect of a certain plant extract is established, confirmation of efficacy in animal models of diabetes and human subjects is required.
A very promising plant extract should possess some or all of the following criteria/properties, with the most promising extracts possessing more of the properties, thus enabling the ranking of extracts for therapeutic potential:
- Potentiating nutrient-induced insulin secretion to reduce postprandial hyperglycemia [15,16,17,18,19];
- Stimulating insulin secretion while maintaining β-cell viability and preserving β-cell membrane integrity [17,18,19,20];
- Stimulating insulin secretion while preserving β-cell insulin stores [15];
- Activating identifiable steps in the β-cell stimulus–secretion coupling pathway [17,19,21];
- Preserving β-cell mass by protecting against apoptosis [19,20];
- Efficacy in animal models of diabetes and in human subjects in vivo [15,16,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
We have applied these criteria to a plant that has been widely used in herbal medicine to treat the symptoms of T2DM, Gymnema sylvestre.
3. Methodology
In this review, the authors aimed at identifying potential β-cell-directed plant extract by recognizing some characteristics as a “screening portfolio” using Gymnema sylvestre as an example. A literature review was carried out. Central PubMed was searched to retrieve relevant scientific publications, using Gymnema sylvestre or its extract OSA® as the main searched keyword combined with one or more of the following keywords: diabetes, β-cells, MIN6 cells, human study, animal model of diabetes, insulin secretion, cell viability, apoptosis, signaling pathway, mouse islets, human islets. Research articles published from inception until January 2023 were selected for data collection. Table 1 summarizes the effects of OSA® and other GS extracts in vitro and in vivo.

Table 1.
Summary of pharmacological effects of OSA® and other Gymnema Sysvestre extracts on β-cells function in vitro and in vivo.
4. Gymnema sylvestre as an Antidiabetic Agent
Gymnema is a genus in the Asclepiadaceae family. A family that normally contains 100 species, among which the species Gymnema sylvestre (GS) has been repeatedly reported to be an effective ayurvedic medicine in treating various diseases, particularly T2DM [40,41]. The reported antidiabetic effect of GS has been shown to be linked to extracts of GS leaves. To validate our screening method, we studied the potential therapeutic efficacy of a GS extract named OSA® (Santal Solution, India) [42,43] using the criteria described above. OSA® is a high molecular weight GS extract (>3000 Da), and it is prepared by extracting fresh GS leaves with aqueous alcohol according to protocols described in the US Patents 6,949,261 and 6,946,151 [42,43].
4.1. Direct Stimulation of Insulin Release from β-Cell Line and Primary Islets
The first obvious target of an antidiabetic plant extract is to directly stimulate insulin secretion from β-cell in the islets of Langerhans in an analogous manner to currently used therapeutic agents, such as sulphonylureas, meglitinides, and glucagon-like peptide-1 (GLP-1) analogs [44,45]. In vitro experiments using β-cell lines and isolated primary islets are required to establish and validate this effect. Numerous β-cell lines have been used in insulin secretion experiments for decades, including RIN, BRIN-BD11 and MIN6 cells. Each cell line has experimental advantages and disadvantages, which have been reviewed in detail elsewhere [46], but the MIN6 cell line offers a useful model, with relatively high insulin content and glucose responsiveness, to screen for effective insulinotropic agents. MIN6 cells can be grown and used as adherent monolayers or configured as three-dimensional islet like structures (pseudoislets, (PIs)) to more closely mimic primary islets [47].
Two types of secretion experiments can be performed, depending on the level of information required, these being static incubations and perifusions. Static incubations measure the accumulation of insulin secretion from a fixed number of cells over a single given time period (usually 30 or 60 min), whereas perifusion experiments measure insulin secretion in sequential samples collected from perifused cells or PIs at a given frequency (usually every one or two minutes for up to 2 h). Perifusions are much more labor-intensive, but they have the advantage of detecting changes in the rate, reversibility and duration of the insulin output over a minute-to-minute time scale. The optimum results are obtained when a plant extract reversibly and sustainably stimulates insulin secretion under hyperglycemic conditions with no effect on insulin output at basal (<5 mM) glucose levels, thus avoiding potential hypoglycemic side-effects, which are common with the currently used sulphonylurea class of antidiabetic drugs. Most of our static and perifusion secretion experiments use a definite substimulatory (basal, 2 mM) or supramaximal stimulatory (20 mM) concentration of glucose to clearly identify those agents which stimulate insulin release under postabsorptive conditions and are thus potentially hypoglycemic and those which only enhance glucose-induced insulin secretion and are thus therapeutically more desirable.
In static incubations using MIN6 monolayers and at a substimulatory glucose concentration, we found OSA® to cause dose-dependent increases in insulin secretion [17]. Other GS extracts have also been reported to stimulate insulin secretion from MIN6 cells and other β-cell lines [18,19].
The kinetic profile of the OSA® insulin secretory responses was evaluated in perifusion experiments with MIN6 PIs. When MIN6 PIs were perifused with substimulatory glucose solution, a sustained increase in insulin secretion was induced by OSA®, and this increase was reversible upon withdrawal of OSA®. The sustainability and maintenance of insulin secretion following OSA® exposure may provide therapeutic benefits in long-term glycemic control and thus reduce dosing frequency, although careful dosage would be needed to avoid potential hypoglycemia.
Although β-cell lines provide a more readily accessible alternative to primary islets, all β-cell lines are derived from transformed insulinoma cells, which may differ phenotypically from normal cells, so the insulinotropic activities of any plant extracts should also be tested in primary tissues. The insulin-releasing effects of OSA® were also seen in primary mouse and human islets, demonstrating a direct activity on primary β-cells. In mouse and human islets, OSA® caused a similar effect to that seen in MIN6 cells. OSA® stimulated insulin secretion, which was again maintained in the presence of OSA® [15,16]. However, a major risk of increasing insulin secretion at substimulatory glucose concentrations could be the development of hypoglycemic episodes. Despite this risk, it is well known that sulphonylureas and meglitinides are still being used successfully in the treatment of T2DM despite the fact that both stimulate insulin release at basal glucose concentrations [48]. It indicates that OSA® may be at least as effective as these commonly used antidiabetic drug classes.
In primary mouse and human islets, OSA® also augmented insulin secretion induced by glucose in addition to its ability to initiate insulin secretion at substimulatory glucose concentrations [15,16]. At 20 mM glucose, a supramaximal stimulatory glucose concentration, OSA® stimulated insulin release. The ability of OSA® to increase insulin secretion over 20 mM glucose suggests that OSA® may work irrespective of nutrient metabolism and, therefore, may stimulate insulin secretion by bypassing glucose metabolism and thus being effective in glucose unresponsive β-cells. This action of OSA® is similar to exendin-4, a GLP-1 agonist. However, unlike exendin-4, OSA® has the advantage of not being broken down in the GI tract and is thus bioavailable after oral administration. Other GS extracts were also reported to stimulate insulin secretion from isolated rat islets incubated in the presence of 2, 10 and 20 mM glucose, in agreement with results obtained from OSA® [18].
4.2. Maintenance of Cell Viability
Some plant extracts have been reported to have a deleterious effect on cell viability because of disruptions in plasma membrane integrity caused by some of the active constituents of the plant, increasing membrane pore formation and, thus, the plasma membrane permeability [18,49,50]. Maintaining the integrity of the plasma membrane is essential for regulated exocytosis of insulin and in allowing meaningful in vitro tests to be conducted, so it is important to screen extracts for deleterious effects on cell viability. The Trypan Blue exclusion test is a rapid and simple qualitative test used to measure the integrity of a cell’s plasma membrane and, hence, viability. Trypan blue dye (MW ≈ 1000 Da) can enter permeable cells and has the ability to stain nuclei. Therefore, under a light microscope, non-viable cells appear blue. In addition to the Trypan Blue exclusion test, other viability tests such as ATP viability test (in our lab, we used CellTiter-Glo® Luminescent Cell Viability Assay, Promega, Madison, WI, USA) can quantify ATP content in metabolically active viable cells and thus can determine the number of viable cells in a fast and accurate fashion. The higher the ATP levels, the higher the number of viable cells.
OSA®, at the therapeutic concentrations used to induce insulin secretion, did not cause any noticeable damage to the plasma membrane, as shown by the Trypan Blue exclusion test and ATP viability test [17,20]. Other GS extracts have shown similar results [18,19]
4.3. Preservation of β-Cell Insulin Stores
The gradual loss of insulin secretory responses associated with the continued use of insulin-secreting drugs over extended periods of time can be avoided by maintaining and sustaining insulin reserves in β-cells after prolonged stimulation of insulin secretion [32]. Therefore, to sustain β-cell insulin stores, a successful antidiabetic therapy for T2DM should both promote insulin secretion and insulin biosynthesis. Sulphonylureas eventually failed as a first-line treatment for T2DM due to their inability to promote insulin gene production, which causes β-cell depletion and necessitates the use of insulin replacement therapy.
Measurement of (pre)proinsulin (PPI) mRNA or protein expression, using RT-PCR techniques and immunoassay measurements, respectively, can be used to establish the effects of plant extracts on the maintenance of β-cell stores of insulin. Increasing insulin gene transcription and PPI mRNA levels, or increased mRNA translation in response to the plant, will maintain total insulin content and thus preserve insulin stores within β-cells to prevent exhaustion in response to prolonged stimulation.
OSA® fulfills the requirements of promoting both insulin secretion and insulin production. OSA® significantly stimulated the genetic expression of PPI at the mRNA levels in chronically treated mouse islets. In addition, in spite of OSA® causing prolonged insulin secretion stimulation, the total intraislet insulin content of mouse islets treated with OSA® remained unchanged in comparison to vehicle-treated mouse islets [15]. The elevations in PPI mRNA levels and maintained intraislet insulin stores documented in OSA®-treated mouse islets suggest that OSA® may be advantageous over some of the insulin secretagogue medications in use today, such as sulphonylureas.
4.4. Activation of Identifiable Steps in β-Cell Stimulus–Secretion Coupling Pathways
The stimulus–secretion coupling pathways in β-cells have been studied since the 1970s and are now fairly well understood. Briefly, glucose is phosphorylated within β-cells by the low affinity, high specificity glucokinase and metabolized largely by oxidative phosphorylation to generate ATP. The subsequent changes in the ATP/ADP ratio result in the closure of an inwardly rectifying K+ channel (KATP) in the plasma membrane with the consequent depolarization of the cell and the opening of L-type voltage-gated Ca2+ channels (VGCC). This allows a rapid influx of extracellular Ca2+, which triggers the exocytosis of insulin-containing secretory granules, thus initiating the glucose-induced insulin secretory response [51]. Other second messenger systems, such as the adenylate cyclase/cyclic AMP (cAMP), phosphatidylinositol 3-kinases (PI3K)/phosphatidylinositol (3,4,5)-trisphosphate (PIP3), Ca2+/Calmodulin and the phospholipase C (PLC)/Inositol trisphosphate (IP3)/diacylglycerol (DAG) pathways, are also activated by glucose and other secretagogues to contribute to the insulin secretory response through the activation of specific classes of serine/threonine protein kinase enzymes.
Although it is not essential to understand the exact cellular mode of action of insulinotropic drugs, it is very useful when considering the therapeutic implications of their use. For example, sulphonylurea drugs were in use for many decades before the identification of the KATP channel as their major site of action in the β-cell, but this mode of action explains how sulphonylureas can stimulate insulin secretion from glucose-unresponsive β-cells and why sulphonylureas can induce hypoglycemia. Thus, measuring the effects of plant extracts on elements of these stimulus–secretion coupling pathways can offer insight into their cellular modes of action.
In our lab, changes in intracellular Ca2+ ([Ca2+]i) in single β-cells are routinely measured by Ca2+ microfluorimetry using Ca2+-sensitive fluorescent dyes such as Fura-2. This technique depends on ratiometric estimations of [Ca2+]i in response to differences in excitation spectra following fluorophore binding. Elevations in [Ca2+]i concentrations in Fura-2-loaded β-cells were associated with the insulin secretory responses to OSA® as assessed by Ca2+ microfluorimetry, and these [Ca2+]i increases caused by OSA® were inhibited in the presence of ethylene glycol tetraacetic acid (EGTA) or nifedipine demonstrating that OSA® increases cytosolic Ca2+ by enabling Ca2+ entry via L-type VGCC [17,21]. Other GS extracts have shown similar results [18].
The involvement of second messengers and/or their downstream protein kinases can be investigated by evaluating the effects of either pharmacological inhibitors or siRNA knockdown of specific cellular targets involved in the stimulus–secretion coupling processes on extract-induced insulin secretory responses. These approaches have been applied to OSA®, but the downstream elements of the signaling pathway of OSA®-induced insulin secretion are still not fully understood.
Opening KATP channels by using diazoxide did not block OSA®-induced insulin secretion, suggesting that it was independent of β-cell depolarization. However, OSA®-induced insulin secretion was inhibited, albeit only partially, by nifedipine, a VGCC blocker, suggesting a partial involvement of L-type VGCC, and thus Ca2+ influx in the secretory responses. Similarly, OSA®-induced insulin secretion was also partially inhibited by staurosporine, a non-selective serine/threonine protein kinase inhibitor, implicating that protein kinase activation was also involved in the OSA®-induced insulin secretion [21]. It is unknown which type of protein kinase is responsible for the stimulation of insulin secretion by OSA®. Protein kinase Cαβ (PKCαβ) and calcium-calmodulin kinase II (CamKII) have been reported to be involved in insulin secretion regulation from β-cells. However, both kinases were demonstrated not to be implicated in OSA®-induced insulin secretion by the use of selective kinase inhibitors.
Cyclic adenosine monophosphate (cAMP) has been shown to stimulate insulin secretion by sensitizing the secretory machinery to [Ca2+]i without changing [Ca2+]i and, therefore, may suggest its involvement in OSA®’s ability to partially increase insulin secretion independently of [Ca2+]i. However, because OSA® surprisingly decreased [cAMP]i levels in β-cells in conjunction with enhancing insulin secretion, the insulin secretion produced by OSA® was separated from cAMP production [21].
4.5. Preserving β-Cell Mass
Reduction in β-cell mass is a hallmark of T2DM. It can be precipitated by β-cell death through caspase-dependent and -independent pathways leading to induction of apoptosis. Induction of apoptosis is triggered by a constant increase in cytokines, glucose and free fatty acids [52,53,54,55,56,57,58]. Preserving β-cell mass through compacting apoptosis may provide an important technique to prevent T2DM deterioration and progression. One successful example that could maintain β-cell mass is exendin-4, a GLP-1 agonist, which increased neogenesis and inhibited apoptosis induced by interleukin-1 beta (IL-1β) in β-cells in vitro, indicating that exendin-4 could have a protective role of in β-cells [59]. Two classes of antidiabetic drugs, namely exendin analogs or dipeptidyl peptidase IV (DPPIV) inhibitors, which inhibit the degradation and thus the metabolism of endogenous GLP-1, are now being used as a promising T2DM treatment because they have the ability to reduce hyperglycemia by concomitantly stimulating insulin secretion and maintaining β-cell mass [60].
There are many colorimetric, luminescence and fluorescence assays that are commercially available to measure apoptosis levels in cells or tissues. We have used a luminescent luciferase assay (Caspase Glo 3/7® assay, Promega) to measure the activation of caspase-3/7 in β-cells as an indicator of apoptosis and have shown that OSA® may offer similar protective effects to exendin-4. Thus, OSA® significantly reduced caspase-3/7 levels that were induced by cytokine in MIN6 cells and in primary mouse islets. Consistent with this protective effect against β-cell loss via apoptosis, OSA® also reduced the expression of pro-apoptotic effectors and enhanced the expression of key antiapoptotic effectors [20]. The findings of these studies suggest that OSA® may possess an additional potential mechanism of action as an antiapoptotic agent that may enable it to be an effective treatment for T2DM. Furthermore, the combination of OSA®’s cytoprotective properties and its insulin secretagogue function may allow its use as an alternative therapy in T2DM. Other GS extracts were also reported to reduce the levels of reactive oxygen species and oxidative stress in vitro [19].
4.6. Improvement of Glycemia In Vivo
If in vitro measurements of the properties described above suggest that a plant extract has therapeutic potential, it is then necessary to test its efficacy in lowering and/or maintaining blood glucose in vivo, first in animal models of T2DM and subsequently in human cohorts. Although it is very important at this stage to test the toxicity of plant extracts in vivo, experiments that are designed to examine these effects have been reviewed elsewhere [61]. In our study using ob/ob mice, a T2DM model characterized by hyperglycemia and profound obesity, a single oral dose of OSA® (500 mg/kg) dramatically improved the glucose intolerance characteristic of these animals during an intraperitoneal glucose tolerance test [15]. Similarly, our studies in a small cohort of human subjects with T2DM produced promising results. Improvements in blood glucose levels pre- and postprandially were noticeable following 60 days of oral OSA® (1 g/day) administration to patients with T2DM. Normalization of both fasting and postprandial blood glucose concentrations after OSA® treatment was observed, with elevations in C-peptide and plasma insulin levels indicating that such improvement in glycemic control induced by OSA® was because of a direct effect of OSA® on β-cells [16]. The use of OSA® as a β-cell-directed and insulin-releasing therapy for T2DM is strongly supported by these findings. The OSA® benefits shown in both our animal and human investigations were consistent with other GS extracts’ known antihyperglycemic properties [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
5. Conclusions and Future Directions
The work described in this review highlights the potential importance of plant-based β-cell-targeted remedies for the treatment of T2DM. Despite a vast literature on a variety of different plant extracts that have been suggested to be beneficial in treating T2DM, there are few structured, sequential studies providing comprehensive evidence for the efficacy of a specific extract. Here, we have devised a set of screening criteria and a sequential process through which to identify likely plant-based candidates that modulate β-cell function for the treatment of T2DM (Figure 1). We have identified six major characteristics that should be present in any β-cell-directed plant-derived therapy. These characteristics include the ability to stimulate insulin secretion through activating known stimulus–secretion coupling pathways while maintaining β-cell viability, mass and insulin stores. Thus, we have plugged into this framework a number of our recent experimental studies using an aqueous GS extract, OSA®, to validate this screening process as a means of identifying antidiabetic extracts with β-cell-directed therapy. Although we have focused here on OSA®, similar screening characteristics are valid for our studies using other plant extracts such as Costus pictus and Commiphora Myrrha [62,63,64,65,66].
We show that OSA® fulfilled almost all of the criteria identified for an effective antidiabetic agent through targeting β-cell function, being (i) an effective insulin-releasing agent at nontoxic concentrations; (ii) maintaining β-cell insulin content by stimulating a simultaneous increase in insulin gene transcription to avoid β-cell exhaustion; (iii) maintaining β-cell mass by protecting against apoptosis induced by cytokines in an inflammatory T2DM-like environment; and (iv) being effective at maintaining normoglycemia in vivo in a mouse model and a human cohort with T2DM after delivery by the enteral route. Investigating the portfolio of other Gymnema species, such as Gymnema montanum (GM) and Gymnema yunnanense (GY), extracts fulfilled some of the properties for having antidiabetic therapeutic potential through being able to (i) preserve β-cell mass via protecting against apoptosis [67] and (ii) reduce blood glucose levels, increase plasma insulin concentrations and maintain beta cell mass in animal model of diabetes in vivo [68,69,70,71,72,73]. However, more studies are required to assess the other properties of the “screening portfolio” for GM and GY.
The schematic diagram in Figure 2 shows proposed mechanisms through which OSA®, and possibly other GS extracts, improve β-cell function and viability. We suggest that future studies of plant extracts as β-cell-targeted therapeutic agents for T2DM should adopt this “portfolio” approach of identifying likely candidates to take forward to clinical trials. In addition, we propose that the outcomes of our studies using OSA® have emphasized the therapeutic potential of these extracts as an inexpensive and readily available adjunctive therapy for the treatment of T2DM.
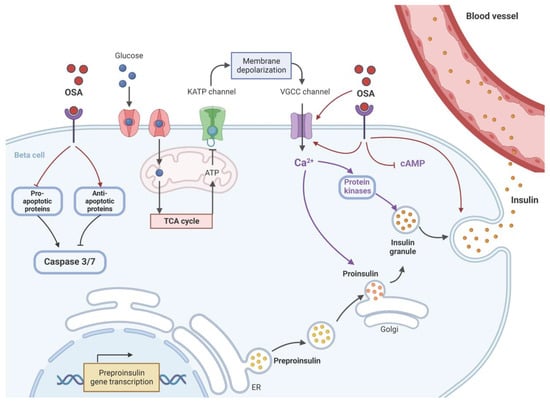
Figure 2.
The proposed mechanism of OSA® on the restoration of β-cell function. OSA®, either directly or through a receptor, activates voltage-gated calcium channels (VGCC) to increase intracellular Ca2+ ([Ca2+]i) concentrations, which, in turn, stimulate protein kinase activation. This should trigger insulin exocytosis and release. OSA® may also act directly to stimulate fusion of insulin vesicles and exocytosis independently from elevations of ([Ca2+]i and activation of protein kinases. OSA® also stimulates the expression of preproinsulin to maintain a constant supply of insulin vesicles during the secretion process and to prevent the depletion of β-cells stores. To preserve β-cell mass, OSA® protects against apoptosis by activating key antiapoptotic proteins while inhibiting key pro-apoptotic proteins. Both effects could reduce the levels of caspases 3 and 7 inside the β-cells. ER: endoplasmic reticulum, KATP: ATP-sensitive potassium channel, TCA: tricarboxylic acid cycle (Krebs cycle). Created with BioRender.com.
Author Contributions
Conceptualization, A.A.-R. and P.M.J.; methodology, A.A.-R.; writing—original draft preparation, A.A.-R.; writing—review and editing, S.J.P. and P.M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| [Ca2+]i | Intracellular Ca2+ |
| ATP | Adenosine triphosphate |
| CamKII | Calcium-calmodulin kinase II |
| cAMP | cyclic adenosine monophosphate |
| DAG | diacylglycerol |
| DPPIV | Dipeptidyl peptidase IV |
| EGTA | Ethylene glycol tetraacetic acid |
| GLP-1 | Glucagon-like peptide 1 |
| GS | Gymnema sylvestre |
| IL-1β | Interleukin-1 beta |
| IP3 | Inositol trisphosphate |
| KATP | ATP-sensitive potassium channel |
| mRNA | Messenger RNA |
| PI3K | Phosphatidylinositol 3-kinases |
| PIP3 | Phosphatidylinositol (3,4,5)-trisphosphate |
| PIs | pseudoislets |
| PKCαβ | Protein kinase Cαβ |
| PLC | Phospholipase C |
| PPI | Preproinsulin |
| T2DM | Type 2 diabetes mellitus |
| VGCC | Voltage-gated calcium channel |
| β-cell | Beta cells |
References
- Chang, H.Y.; Wallis, M.; Tiralongo, E. Use of complementary and alternative medicine among people living with diabetes: Literature review. J. Adv. Nurs. 2007, 58, 307–319. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, M.M.; Gibson, J.M.; Siminerio, L.; Morell, A.R.; Lee, E.S. Complementary and alternative medicine in diabetes care. Curr. Diabetes Rep. 2012, 12, 749–761. [Google Scholar] [CrossRef] [PubMed]
- IDF. The Global Picture. In Diabetes Atlas, 8th ed.; International Diabetes Federation: Brussels, Belgium, 2017; pp. 40–65. [Google Scholar]
- Pimenta, W.; Korytkowski, M.; Mitrakou, A.; Jenssen, T.; Yki-Jarvinen, H.; Evron, W.; Dailey, G.; Gerich, J. Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA 1995, 273, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Vaag, A.; Henriksen, J.E.; Madsbad, S.; Holm, N.; Beck-Nielsen, H. Insulin secretion, insulin action, and hepatic glucose production in identical twins discordant for non-insulin-dependent diabetes mellitus. J. Clin. Investig. 1995, 95, 690–698. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van Haeften, T.W.; Dubbeldam, S.; Zonderland, M.L.; Erkelens, D.W. Insulin secretion in normal glucose-tolerant relatives of type 2 diabetic subjects. Assessments using hyperglycemic glucose clamps and oral glucose tolerance tests. Diabetes Care 1998, 21, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M. Genome-wide association studies provide new insights into type 2 diabetes aetiology. Nat. Rev. Genet. 2007, 8, 657–662. [Google Scholar] [CrossRef]
- Gerich, J.E. The Genetic Basis of Type 2 Diabetes Mellitus: Impaired Insulin Secretion versus Impaired Insulin Sensitivity. Endocr. Rev. 1998, 19, 491–503. [Google Scholar] [CrossRef]
- Horikoshi, M.; Hara, K.; Ito, C.; Shojima, N.; Nagai, R.; Ueki, K.; Froguel, P.; Kadowaki, T. Variations in the HHEX gene are associated with increased risk of type 2 diabetes in the Japanese population. Diabetologia 2007, 50, 2461–2466. [Google Scholar] [CrossRef]
- Sladek, R.; Rocheleau, G.; Rung, J.; Dina, C.; Shen, L.; Serre, D.; Boutin, P.; Vincent, D.; Belisle, A.; Hadjadj, S.; et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 2007, 445, 881–885. [Google Scholar] [CrossRef]
- Zeggini, E.; Scott, L.J.; Saxena, R.; Voight, B.F.; Marchini, J.L.; Hu, T.; de Bakker, P.I.; Abecasis, G.R.; Almgren, P.; Andersen, G.; et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat. Genet. 2008, 40, 638–645. [Google Scholar] [CrossRef]
- Zeggini, E.; Weedon, M.N.; Lindgren, C.M.; Frayling, T.M.; Elliott, K.S.; Lango, H.; Timpson, N.J.; Perry, J.R.B.; Rayner, N.W.; Freathy, R.M.; et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007, 316, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Witters, L.A. The blooming of the French lilac. J. Clin. Investig. 2001, 108, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Alarcon-Aguilara, F.J.; Roman-Ramos, R.; Perez-Gutierrez, S.; Aguilar-Contreras, A.; Contreras-Weber, C.C.; Flores-Saenz, J.L. Study of the anti-hyperglycemic effect of plants used as antidiabetics. J. Ethnopharmacol. 1998, 61, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Al-Romaiyan, A.; King, A.J.; Persaud, S.J.; Jones, P.M. A novel extract of Gymnema sylvestre improves glucose tolerance in vivo and stimulates insulin secretion and synthesis in vitro. Phytother. Res. 2013, 27, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Al-Romaiyan, A.; Liu, B.; Asare-Anane, H.; Maity, C.R.; Chatterjee, S.K.; Koley, N.; Biswas, T.; Chatterji, A.K.; Huang, G.-C.; Amiel, S.A.; et al. A novel Gymnema sylvestre extract stimulates insulin secretion from human islets in vivo and in vitro. Phytother. Res. 2010, 24, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Asare-Anane, H.; Al-Romaiyan, A.; Huang, G.; Amiel, S.A.; Jones, P.M.; Persaud, S.J. Characterisation of the insulinotropic activity of an aqueous extract of Gymnema sylvestre in mouse β-cells and human islets of Langerhans. Cell. Physiol. Biochem. 2009, 23, 125–132. [Google Scholar] [CrossRef]
- Persaud, S.; Al-Majed, H.; Raman, A.; Jones, P. Gymnema sylvestre stimulates insulin release in vitro by increased membrane permeability. J. Endocrinol. 1999, 163, 207–212. [Google Scholar] [CrossRef]
- Shenoy, R.S.; Prashanth, K.V.H.; Manonmani, H.K. In Vitro Antidiabetic Effects of Isolated Triterpene Glycoside Fraction from Gymnema sylvestre. Evid.-Based Complement. Altern. Med. Ecam 2018, 2018, 7154702. [Google Scholar] [CrossRef]
- Al-Romaiyan, A.; Liu, B.; Persaud, S.; Jones, P. A novel Gymnema sylvestre extract protects pancreatic beta-cells from cytokine-induced apoptosis. Phytother. Res. 2020, 34, 161–172. [Google Scholar] [CrossRef]
- Al-Romaiyan, A.; Liu, B.; Docherty, R.; Huang, G.C.; Amiel, S.; Persaud, S.J.; Jones, P.M. Investigation of intracellular signalling cascades mediating stimulatory effect of a Gymnema sylvestre extract on insulin secretion from isolated mouse and human islets of Langerhans. Diabetes Obes. Metab. 2012, 14, 1104–1113. [Google Scholar] [CrossRef]
- Balasubramaniam, K.; Seevaratnam, S.; Ageswaran, A.; Arasaratnam, V.; Thirumagal, K. Hypoglycaemic effect of Gymnema sylvestre on diabetic patients. Jaffna Med. J. 1988, 23, 49–53. [Google Scholar]
- Baskaran, K.; Kizar Ahamath, B.; Radha Shanmugasundaram, K.; Shanmugasundaram, E.R. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J. Ethnopharmacolology 1990, 30, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Daisy, P.; Eliza, J.; Mohamed Farook, K.A. A novel dihydroxy gymnemic triacetate isolated from Gymnema sylvestre possessing normoglycemic and hypolipidemic activity on STZ-induced diabetic rats. J. Ethnopharmacolology 2009, 126, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Gaytán Martínez, L.A.; Sánchez-Ruiz, L.A.; Zuñiga, L.Y.; González-Ortiz, M.; Martínez-Abundis, E. Effect of Gymnema sylvestre Administration on Glycemic Control, Insulin Secretion, and Insulin Sensitivity in Patients with Impaired Glucose Tolerance. J. Med. Food 2021, 24, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Gholap, S.; Kar, A. Effects of Inula racemosa root and Gymnema sylvestre leaf extracts in the regulation of corticosteroid induced diabetes mellitus: Involvement of thyroid hormones. Die Pharm.-Int. J. Pharm. Sci. 2003, 58, 413–415. [Google Scholar]
- Gupta, S. Inhibitory effect of Gymnema sylvestre (Gurmar) on adrenaline-induced hyperglycemia in rats. Indian J. Med. Sci. 1961, 15, 883. [Google Scholar]
- Gupta, S. Effect of Gymnema sylvestre and Pterocarpus marsupium on glucose tolerance in albino rats. Indian J. Med. Sci. 1963, 17, 501. [Google Scholar]
- Khare, A.K.; Tondon, R.N.; Tewari, J.P. Hypoglycaemic activity of an indigenous drug (Gymnema sylvestre, ‘Gurmar’) in normal and diabetic persons. Indian J. Physiol. Pharmacol. 1983, 27, 257–258. [Google Scholar]
- Kousar, S.; Aslam, B.; Muhammad, F.; Khan, J.A. Hepatoprotective and hypolipidemic activities of Caesalpinia bonduc seed kernels and Gymnema sylvestre leaves extracts in alloxan-induced diabetic rats. Pak J. Pharm Sci. 2021, 34 (Suppl. S1), 307–311. [Google Scholar] [PubMed]
- Li, Y.; Liu, Y.; Liang, J.; Wang, T.; Sun, M.; Zhang, Z. Gymnemic Acid Ameliorates Hyperglycemia through PI3K/AKT- and AMPK-Mediated Signaling Pathways in Type 2 Diabetes Mellitus Rats. J. Agric. Food Chem. 2019, 67, 13051–13060. [Google Scholar] [CrossRef]
- Okabayashi, Y.; Tani, S.; Fujisawa, T.; Koide, M.; Hasegawa, H.; Nakamura, T.; Fujii, M.; Otsuki, M. Effect of Gymnema sylvestre, R.Br. on glucose homeostasis in rats. Diabetes Res. Clin. Pract. 1990, 9, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Sathya, S.; Kokilavani, R.; Gurusamy, K. Hypoglycemic effect of Gymnema sylvestre (retz.,) R.Br leaf in normal and alloxan induced diabetic rats. Anc. Sci. Life 2008, 28, 12–14. [Google Scholar] [PubMed]
- Shanmugasundaram, E.R.; Gopinath, K.L.; Radha Shanmugasundaram, K.; Rajendran, V.M. Possible regeneration of the islets of Langerhans in streptozotocin-diabetic rats given Gymnema sylvestre leaf extracts. J. Ethnopharmacol. 1990, 30, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, E.R.; Rajeswari, G.; Baskaran, K.; Rajesh Kumar, B.R.; Radha Shanmugasundaram, K.; Kizar Ahmath, B. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J. Ethnopharmacol. 1990, 30, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, E.R.; Venkatasubrahmanyam, M.; Vijendran, N.; Shanmugasundaram, K.R. Effect of an isolate from Gymnema sylvestre, R. Br. In the control of diabetes mellitus and the associated pathological changes. Anc. Sci. Life 1988, 7, 183–194. [Google Scholar] [PubMed]
- Shanmugasundaram, K.R.; Panneerselvam, C.; Samudram, P.; Shanmugasundaram, E. The insulinotropic activity of Gymnema sylvestre, R. Br. An Indian medical herb used in controlling diabetes mellitus. Pharmacol. Res. Commun. 1981, 13, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, Y.; Nigam, S.; Bhatt, H.; Verma, Y.; Prem, A. Hypoglycemic and life-prolonging properties of Gymnema sylvestre leaf extract in diabetic rats. Isr. J. Med. Sci. 1985, 21, 540–542. [Google Scholar]
- Sugihara, Y.; Nojima, H.; Matsuda, H.; Murakami, T.; Yoshikawa, M.; Kimura, I. Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema sylvestre leaves in streptozotocin-diabetic mice. J. Asian Nat. Prod. Res. 2000, 2, 321–327. [Google Scholar] [CrossRef]
- Benny, M. Insulin plant in gardens. Nat. Prod. Radiance 2004, 3, 349–350. [Google Scholar]
- Porchezhian, E.; Dobriyal, R.M. An overview on the advances of Gymnema sylvestre: Chemistry, pharmacology and patents. Die Pharm. 2003, 58, 5–12. [Google Scholar] [CrossRef]
- Chatterji, A.K. Therapeutic Compositions. USA patent US20040151783A1, 20 September 2005. [Google Scholar]
- Chatterji, A.K. Composition for diabetes treatment and prophylaxis. USA patent US20050119220A1, 27 September 2005. [Google Scholar]
- Brietzke, S.A. Oral antihyperglycemic treatment options for type 2 diabetes mellitus. Med. Clin. N. Am. 2015, 99, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Irwin, N.; Flatt, P.R. New perspectives on exploitation of incretin peptides for the treatment of diabetes and related disorders. World J. Diabetes 2015, 6, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Persaud, S.J. Pancreatic β-cell lines: Their roles in β-cell research and diabetes therapy. Adv. Mol. Cell Biol. 1999, 29, 21–46. [Google Scholar]
- Hauge-Evans, A.C.; Squires, P.E.; Persaud, S.J.; Jones, P.M. Pancreatic beta-cell-to-beta-cell interactions are required for integrated responses to nutrient stimuli: Enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 1999, 48, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Persaud, S.; Jones, P. Beta-cell-based therapies for Type 2 Diabetes. Eur. Endocrinol. 2008, 4, 36–39. [Google Scholar] [CrossRef]
- Ahnert-Hilger, G.; Gratzl, M. Controlled manipulation of the cell interior by pore-forming proteins. Trends Pharm. Sci. 1988, 9, 195–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schulz, I. Permeabilizing cells: Some methods and applications for the study of intracellular processes. Methods Enzymol. 1990, 192, 280–300. [Google Scholar][Green Version]
- Jones, P.; Persaud, S. Islet function and insulin secretion. In Textbook of Diabetes Chichester; Holt, R., Cockram, C., Flyvbjerg, A., Goldstein, B., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 87–103. [Google Scholar][Green Version]
- Cnop, M.; Welsh, N.; Jonas, J.C.; Jorns, A.; Lenzen, S.; Eizirik, D.L. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes 2005, 54 (Suppl. S2), S97–S107. [Google Scholar] [CrossRef]
- Eizirik, D.L.; Mandrup-Poulsen, T. A choice of death—The signal-transduction of immune-mediated beta-cell apoptosis. Diabtologia 2001, 44, 2115–2133. [Google Scholar] [CrossRef]
- Johnson, J.D.; Luciani, D.S. Mechanisms of pancreatic beta-cell apoptosis in diabetes and its therapies. Adv. Exp. Med. Biol. 2010, 654, 447–462. [Google Scholar]
- Lupi, R.; Del Prato, S. Beta-cell apoptosis in type 2 diabetes: Quantitative and functional consequences. Diabetes Metab. 2008, 34 (Suppl. S2), S56–S64. [Google Scholar] [CrossRef] [PubMed]
- Mandrup-Poulsen, T. beta-cell apoptosis: Stimuli and signaling. Diabetes 2001, 50 (Suppl. S1), S58–S63. [Google Scholar] [CrossRef] [PubMed]
- Poitout, V.; Robertson, R.P. Glucolipotoxicity: Fuel excess and beta-cell dysfunction. Endocr Rev. 2008, 29, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.P.; Harmon, J.; Tran, P.O.; Poitout, V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004, 53 (Suppl. S1), S119–S124. [Google Scholar] [CrossRef] [PubMed]
- Ferdaoussi, M.; Abdelli, S.; Yang, J.Y.; Cornu, M.; Niederhauser, G.; Favre, D.; Widmann, C.; Regazzi, R.; Thorens, B.; Waeber, G.; et al. Exendin-4 protects beta-cells from interleukin-1 beta-induced apoptosis by interfering with the c-Jun NH2-terminal kinase pathway. Diabetes 2008, 57, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Stoffers, D.A.; Habener, J.F.; Bonner-Weir, S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999, 48, 2270–2276. [Google Scholar] [CrossRef]
- Dunnick, J.K.; Nyska, A. The toxicity and pathology of selected dietary herbal medicines. Toxicol. Pathol. 2013, 41, 374–386. [Google Scholar] [CrossRef]
- Al-Romaiyan, A.; Huang, G.-C.; Jones, P.; Persaud, S. Commiphora myrrha stimulates insulin secretion from mouse and human islets of Langerhans. J. Ethnopharmacol. 2020, 264, 113075. [Google Scholar] [CrossRef]
- Al-Romaiyan, A.; Jayasri, M.A.; Mathew, T.L.; Huang, G.C.; Amiel, S.; Jones, P.M.; Persaud, S.J. Costus pictus extracts stimulate insulin secretion from mouse and human islets of Langerhans in vitro. Cell. Physiol. Biochem. 2010, 26, 1051–1058. [Google Scholar] [CrossRef]
- Al-Romaiyan, A.; Masocha, W.; Oyedemi, S.; Marafie, S.K.; Huang, G.-C.; Jones, P.M.; Persaud, S.J. Commiphora myrrha stimulates insulin secretion from β-cells through activation of atypical protein kinase C and mitogen-activated protein kinase. J. Ethnopharmacol. 2023, 302 Pt B, 115937. [Google Scholar] [CrossRef]
- Jayasri, M.; Gunasekaran, S.; Radha, A.; Mathew, T. Anti-diabetic effect of Costus pictus leaves in normal and streptozotocin-induced diabetic rats. Int. J. Diabetes Metab. 2008, 16, 117–122. [Google Scholar]
- Jayasri, M.; Lazar, M.; Radha, A. A report on the antioxidant activity of leaves and rhizomes of Costus pictus D. Don. Int. J. Integr. Biol. 2009, 5, 20–26. [Google Scholar]
- Ramkumar, K.M.; Lee, A.S.; Krishnamurthi, K.; Devi, S.S.; Chakrabarti, T.; Kang, K.P.; Lee, S.; Kim, W.; Park, S.K.; Lee, N.H.; et al. Gymnema montanum H. protects against alloxan-induced oxidative stress and apoptosis in pancreatic beta-cells. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2009, 24, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, R.; Baskar, C.; NarmathaBai, V.; Pari, L.; Latha, M.; Ramkumar, K.M. Antidiabetic effect of Gymnema montanum leaves: Effect on lipid peroxidation induced oxidative stress in experimental diabetes. Pharmacol. Res. 2003, 48, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Ananthan, R.; Latha, M.; Pari, L.; Ramkumar, K.M.; Baskar, C.G.; Bai, V.N. Effect of Gymnema montanum on blood glucose, plasma insulin, and carbohydrate metabolic enzymes in alloxan-induced diabetic rats. J. Med. Food 2003, 6, 43–49. [Google Scholar] [CrossRef]
- Ananthan, R.; Latha, M.; Ramkumar, K.M.; Pari, L.; Baskar, C.; Narmatha Bai, V. Effect of Gymnema montanum leaves on serum and tissue lipids in alloxan diabetic rats. Exp. Diabesity Res. 2003, 4, 183–189. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Rajaguru, P.; Latha, M.; Ananthan, R. Effect of Gymnema montanum leaves on red blood cell resistance to oxidative stress in experimental diabetes. Cell Biol. Toxicol. 2008, 24, 233–241. [Google Scholar] [CrossRef]
- Ramkumar, K.M.; Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Sujatha, J. Antidiabetic activity of alcoholic stem extract of Gymnema montanum in streptozotocin-induced diabetic rats. Food Chem. Toxicol. 2011, 49, 3390–3394. [Google Scholar] [CrossRef]
- Xie, J.T.; Wang, A.; Mehendale, S.; Wu, J.; Aung, H.H.; Dey, L.; Qiu, S.; Yuan, C.S. Anti-diabetic effects of Gymnema yunnanense extract. Pharmacol. Res. 2003, 47, 323–329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).