Abstract
The food sector’s interest in sustainability and the demand for novel bioactive compounds are increasing. Many fruits are wasted every year before ripening due to various climatic conditions and harsh weather. Unripe mangoes, grapes, and black lemons could be rich sources of phenolic compounds that need to be fully elucidated. Using fruit waste as a source of bioactive chemicals has grown increasingly appealing as it may have significant economic benefits. Polyphenols are beneficial for human health to inhibit or minimize oxidative stress and can be used to develop functional and nutraceutical food products. In this context, this study aimed to characterize and screen unripe mangoes, grapes, and black lemons for phenolic compounds using LC-ESI-QTOF-MS/MS and their antioxidant activities. Unripe mangoes were quantified with higher total phenolic content (TPC, 58.01 ± 6.37 mg GAE/g) compared to black lemon (23.08 ± 2.28 mg GAE/g) and unripe grapes (19.42 ± 1.16 mg GAE/g). Furthermore, unripe mangoes were also measured with higher antioxidant potential than unripe grapes and black lemons. A total of 85 phenolic compounds (70 in black lemons, 49 in unripe grapes, and 68 in unripe mango) were identified, and 23 phenolic compounds were quantified using LC-MS/MS. Procyanidin B2, gallic acid, epicatechin, caffeic acid, quercetin, and chlorogenic acid were measured with higher concentration in these selected unripe fruits. A positive correlation was found between phenolic contents and the antioxidant activities of unripe fruits. Furthermore, chemometric analysis was conducted to validate the results. This study will explore the utilization of these unripe fruits to develop functional and therapeutic foods.
Keywords:
unripe mango; black lemon; unripe grapes; polyphenols; flavonoids; antioxidants; human health 1. Introduction
Recently, the demand for natural products of superior quality has increased, which is beneficial for human health. Fruits are a rich source of vitamins, minerals, dietary fiber, and antioxidant bioactive compounds. Apple, citrus, lime, grape, banana, and mango are the most popular fruits. Globally, citrus (124.8 million metric tons (MMT)), banana (114.1 MMT), grapes (74.5 MMT), and mangoes (45.2 MMT) are produced in large quantities each year. The need for fruit production has expanded globally over the last few decades due to population growth and shifting consumer preferences for healthful diets [1]. Sustainability in food processing and waste reduction are major issues facing the modern food business [1,2]. To reduce waste and provide new sources of bioactive compounds—which are thought to act as protective agents against certain diseases like diabetes, cancer, and cardiovascular disease—researchers have concentrated on recovering the bioactive components from fruit and vegetable waste, where a significant number of phytochemicals still exist in the agro-industrial wastes [2,3,4]. Because these fruit wastes contain sizable amounts of bioactive compounds, there is currently a dearth of information regarding the use of these fruit wastes in the development of functional ingredients and food items, as well as their effects on the sensory quality of food products [2,4]. Concurrent with the increasing demand for healthier and functional foods, the food industry realized how important it was to identify natural food additives to create value-added goods with positive health effects.
Grapes (Vitis vinifera L). are the most prominent fruits on earth, with an annual production of over 75 million tons. The industry primarily uses grates to screen out many unripe grapes, which are then dumped into agricultural land [5,6]. Several potentially bioactive compounds, such as organic acids, phenolic compounds, vitamins, and minerals that are highly beneficial to human health, cosmetics, and biomedical areas, are thought to have been recently discovered in unripe grapes [7]. Unripe grapes are used to make verjuice and sour grape sauces, which are typically used as seasonings or acidifiers. Using immature grape waste, abundant in bioactive compounds, in agricultural areas is crucial to producing high-value foods, cosmetics, and pharmaceuticals [8,9]. Research conducted in vitro and on animals has shown that unripe grapes have anti-inflammatory, cardioprotective, anticancer, and antidiabetic qualities [9]. Recently, a renewed focus has been on the special potential applications of unripe grape extract in food and beverages, such as preservation, enrichment, or direct use in the human diet [10]. Mango (Mangifera indica L.) is a widely grown fruit known for its taste and nutritional value. It is a rich source of carbohydrates, vitamins, minerals, dietary fiber, and bioactive compounds like xanthonoids (mangiferin) [11,12,13]. Mangiferin is well known for its antioxidant, anticancer, antimicrobial, antiviral, antidiabetic, and antiallergic properties [14,15]. Black lemons are dried products of unripe lemons. In Middle Eastern countries these are also called dried limes. These are produced by boiling unripe limes in brine and then leaving them to dry (oven or sun drying) which gives them a dark black appearance. They are abundantly utilized in various dishes and food products to imbue a tangy flavor. Black lime tea has a unique citrusy flavor and is well-known for various health properties due to its high concentration of bioactive compounds. Black lemons are enriched with alkaloids, polyphenols, terpenes, organic acids, and vitamins [16,17].
These substances are significant because of their capacity to function as antioxidants or free-radical scavengers. This capacity is prompted by hydrogen- or electron-donating properties that are influenced by the quantity and location of hydroxyl groups in the compounds’ structure [18,19,20]. They are effective as antidiabetic, anticancer, antihypertensive, anti-inflammatory, antiaging, cardioprotective, and neuroprotective medicines because of their antioxidant qualities [18,19,20]. From this vantage point, much research has been conducted on the fortification of fruit wastes in extruded snacks, short-dough biscuits, yoghurt, kefir, and functional cookies to create unique functional food products [1]. Therefore, it is vital to recover phenolic compounds from wasted unripe fruits, particularly when searching for sustainable and affordable bioactive compounds that could be added to different food matrices to increase their nutritional value and used as a natural food coloring [21]. Even though the food sector produces enormous volumes of trash, seasonal production and the changeable composition of waste products provide a significant barrier to its industrial use.
So, using fruit waste as a source of bioactive chemicals has grown increasingly appealing as it may have significant economic benefits [4,21]. To extract value-added byproducts from agro-industrial wastes, identifying bioactive components—specifically, phenolics—has drawn interest [22]. There is a wealth of information regarding the efficacy of phenolic compounds as natural antioxidants and their ability to prevent disease, making them one of the most significant categories of natural antioxidants of interest [23,24]. Several studies have been conducted on industrial waste, but studies on unripe fruit waste are scarce. Therefore, this study aimed to screen out the selected sour fruit waste with high antioxidant activities, which could function as readily available and affordable sources of bioactive compounds for use in the food and pharmaceutical industries. This study aimed to identify and quantify phenolic compounds and their antioxidant capacity in unripe grapes, mangoes, and black lemons using state-of-the-art analytical tools, including LC-ESI-QTOF-MS/MS. We measured total phenolic contents (TPC), total flavonoid contents (TFC), and total condensed tannins (TCT). At the same time, antioxidant activities, including 2,2-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 2,2-diphenyl-1-picryl-hydrazyl-hydrate radical scavenging activity (DPPH), hydroxyl radical scavenging assay (OH-RSA), and ferrous ion chelating activity (FICA), were also analyzed. Chemometric analysis further validates the results of this study. This study provides evidence that these wasted fruits have the potential to be used in pharmaceuticals, cosmetics, nutraceuticals, and food sectors.
2. Results and Discussion
2.1. Measurement of Phenolic Contents of Unripe Fruits
The distinct qualities and advantageous potential of phenolic compounds, such as their anti-inflammatory, antioxidant, antidiabetic, antimicrobial, and anticarcinogenic qualities, have drawn the attention of scientists [25]. Table 1 displays black lemons’, unripe mangoes’, and unripe grapes’ phenolic content and antioxidant examinations. Reports have shown that sour fruits are high in phenolic compounds [23]. TPC, TFC, and TTC were used in this study to measure the phenolic content of the three sour fruits, namely black lemon, unripe mango, and unripe grapes (Table 1). Unripe mangoes had the highest phenolic content of all the samples since they had significantly greater TPC and TFC and higher TCT (p < 0.05) than the other samples.

Table 1.
Measurement of phenolic contents in unripe fruits.
The Folin–Ciocalteu method was used to determine total phenolic contents [26]. Every sample differed considerably (p < 0.05) in terms of TPC. The unripe mango (58.01 ± 6.37 mg GAE/g) had the highest content of phenolic compounds, with black lemon (23.08 ± 2.28 mg GAE/g) following closely behind. Among all samples, unripe grapes showed the lowest TPC value of 19.42 ± 1.16 mg GAE/g. The variance in fruit maturity levels used in this study and subsequent investigations for measuring phenolic contents may have contributed to the differences in TPC results. In a previous study by Arshad Mehmood et al. [27] the TPC of ripe mango cultivars assessed in the peel and pulp samples showed that the peel samples had a higher TPC than the pulp. The TPC results of unripe mango samples are comparable to those of reports for the TPC (62.45 d ± 1.25 mg GAE/g) and (51.68 ± 0.66 mg GAE/g) of mango pulp of the F2-Narcissus mango and F9-Egg mango, respectively [27]. Previously, Dorta et al. [28] measured TPC between 35 and 98 mg GAE/g in the peel of various mango cultivars in Spain. This variation in the concentration of polyphenols was observed in different studies [12].
Black lemon (16.41 ± 1.02 mg GAE/g) and unripe grapes (15.01 ± 1.12 mg GAE/g) did not substantially differ from one another in TFC, while unripe mango (44.94 ± 5.02 mg QE/g) did demonstrate a significantly (p < 0.05) higher concentration above the others. On the other hand, unripe mango was found to have a considerably higher phenolic compound profile, which makes it a beneficial natural antioxidant or functional ingredient in food preparation. The previous study provided estimated values of the total flavonoid content in the examined mango samples and found that the peel samples had a much higher level of TFC than the pulp samples, which is opposite to their results for TPC. The TFC value of the unripe mango in our study is consistent with the peeled mango variety, F2-Narcissus mango (48.87 ± 1.50) [27].
In the case of TCT, at 11.41 ± 0.91, the unripe mango cultivar had the highest total tannin content. In contrast, unripe grapes and black lemon cultivars come after it with a nominal difference among their TCT values, as 4.28 ± 0.62 and 4.01 ± 0.32, respectively. In a previous study, the highest concentration of tannins was found in lemon (7.4 ± 0.14 mg tannic acid equivalent (TAE)), with citrus lemon leaf extract coming in second (5.9 ± 0.20 mg TAE). The least amount of tannins (4.8 ± 0.18 mg TAE) is found in citrus lemon root, comparable to our study results [29].
Flavonoids are an abundant class of polyphenols; they are vital secondary metabolites in fruits, herbs, and vegetables, having a positive impact on human health. Previously, no single study was conducted on these unripe fruits in such a comprehensive way. This study will explore the therapeutic and functional utilization of these unripe fruits in the food industry.
2.2. Antioxidant Potential of Unripe Fruits
Antioxidants are essential components of many products that serve as metal chelators, radical oxygen scavengers, reducing agents, and H+ ion donors. The antioxidant capacity of unripe mango, black lemon extracts, and unripe grapes was assessed using a variety of assays, such as DPPH, ABTS, ferrous ion chelating activity (FICA), and OH-RSA. Table 2 shows the antioxidant potential results for the three samples (unripe mango, unripe grapes, and black lemon).

Table 2.
Quantification of antioxidant potential in unripe fruits.
Sour fruits are included in the human diet as essential sources of antioxidant compounds capable of preventing free radical damage. The main active antioxidants in these fruits are generally phenolic compounds, which are thought to provide a variety of health advantages. These substances are multipurpose agents that work in biological systems as hydrogen ion donors, strong reducers, metal chelators, and scavengers of free radicals [4]. The exceptional antioxidant capacity of sour fruits is crucial for evaluating their dietary benefits. Numerous components found in these fruits are known to have antioxidant qualities. Many bioactive substances, primarily phenolic compounds, are potential therapeutic agents for reducing the harmful impacts of free radicals. These compounds include flavonoids, tannins, procyanidins, coumarins, phenolic acids, stilbenes, xanthones, lignans, and other polyphenols [30].
Antioxidants possess the ability to either inhibit or stop other molecules from oxidizing. An approach for determining antioxidant capacity is the DPPH radical scavenging assay, which measures a decrease in absorbance following oxidation processes [31]. Antioxidants can retard color loss which has allowed scientists to calculate the scavenging activity [31]. The DPPH values of unripe mango (114.27 ± 9.42 mg AAE/g) are estimated to be significantly higher, at a p < 0.05, than the two listed sour fruits. The research results demonstrated a significant difference between the DPPH values of black lemon (32.53 ± 2.47 mg AAE/g) and unripe grapes samples (23.71 ± 2.17 mg AAE/g) (Table 2). The significant differences in the DPPH results between our study and the earlier study could have several causes, particularly regarding unripe mango. The fruit samples used in our study may have come from different sources and are of higher quality than those used in the previous research, which could substantially affect their antioxidant content [32]. Our prior study addressed the mango peel and pulp separately [32], while our sample in this study is an entire unripe mango. The amount of antioxidants in the fruit may vary depending on its parts [33]. The second reason might be that the antioxidant extraction process of the fruit samples had a significant impact on the outcomes [33]. Different concentrations of antioxidants in the extracts can result from variations in extraction conditions, solvents, and efficiency [34].
Most people agree that the ABTS assay is the most affordable. This is such that, in comparison to the other assays, the ABTS assay can afford to produce its synthetic chromogenic substrate [35]. For assessing the antioxidant potential of a range of materials, including foods, dietary supplements, and biological samples, the ABTS (2,20-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)) assay is regarded as a versatile and efficient technique [36]. Since the ABTS assay relies on the interaction of ABTS with an agent that exhibits antioxidant activity, it is also comparatively easy to conduct [37]. According to the results of the ABTS assay, unripe mango (134.12 ± 9.63 mg AAE/g) exhibited significantly (p < 0.05) higher activities than black lemon (69.25 ± 5.17 mg AAE/g) and unripe grapes (53.44 ± 4.79 mg AAE/g). In a previous study by Deepa Madalageri et al., mango peel had a significantly higher mean total antioxidant activity (24.782 mg TE/g DM or 99.128 μmol TE/g DM) than mango pulp (1.964 mg TE/g DM or 7.856 μmol TE/g DM) when the means of the ABTS assay were compared [38]. Le (2012) reported that the dehydrated mango’s ABTS scavenging activity ranged from 46.7 to 73.8 μmol TE/g DM [39]. Using the ABTS assay, dried mango pulp’s antioxidant activity was 27.1 μmol/g db ascorbic acid equivalent [40]. According to Sogi et al. (2014), the antioxidant properties of dried mango samples ranged from 50.7 to 103.8 μmol TE/g db [41].
A ferrous ion chelating assay was also performed to assess the antioxidant potential of the selected sample [42]. The results indicated that, compared to black lemon (14.24 ± 1.07 mg EDTA/g) and unripe grapes (11.49 ± 0.87 mg EDTA/g), unripe mango had a significantly (p < 0.05) higher FICA potential (33.16 ± 2.08 mg EDTA/g). Furthermore, the scavenging activity of OH radicals was conducted to estimate the antioxidant profile. The results showed that unripe mangoes had the highest FICA (57.31 ± 4.85 mg AAE/g) while unripe grapes had the lowest FICA (19.57 ± 1.42 mg AAE/g).
2.3. Pearson Correlation
Pearson’s correlation was conducted between phenolic contents and their antioxidant activities. The results of Pearson’s correlation analyses are presented in Table 3.

Table 3.
Pearson’s correlation between phenolic contents and antioxidant activities of unripe fruits.
The results show that TPC and TFC have very strong correlations (p < 0.05), indicating that flavonoids are the main class of phenolic compounds in selected unripe sour fruits. TFC and TCT are also significantly correlated, which means that flavonoids, particularly condensed tannins, are very potent in these sour fruits. Overall, TPC and TFC are highly correlated with antioxidant activities (DPPH and FICA). The results are supported by previous studies where it has been reported that total phenolics and flavonoids are the main compounds responsible for the antioxidant capacity of fruits, vegetables, and herbs [25,43,44]. Moreover, a biplot analysis (Figure 1) was conducted to further understand the contribution of selected sour fruits in phenolic contents and their antioxidant activities.
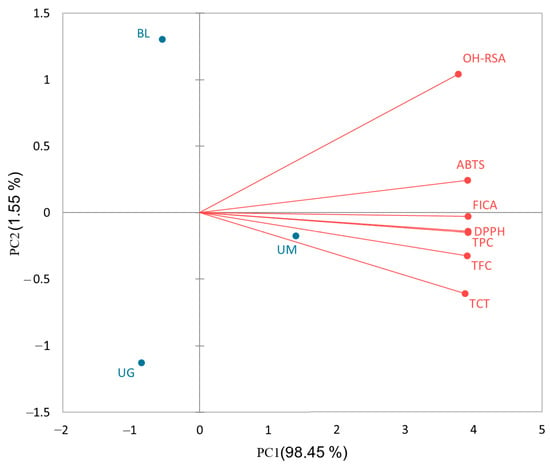
Figure 1.
Biplot analysis of phenolic contents and antioxidant activities of sour fruits (black lemon (BL), unripe mangoes (UM), and unripe grapes (UG)).
The biplot indicates that PC1 (principal component 1) shares a 98.5% contribution in this study, showing that unripe mango has the highest concentration of phenolic compounds and strong antioxidant activities. The phenolic contents (TPC, TFC, and TCT) are positively correlated with antioxidant activities (DPPH, FICA, ABTS, and OH-RSA).
2.4. LC-MS/MS Analysis
An untargeted analysis was conducted to screen and characterize phenolic compounds in complex unripe mangoes, grapes, and black lemon extracts using LC-ESI-QTOF-MS/MS. Only the compounds with less than 10 ppm error and more than 80 PCDL scores were reported in this study (Table 4).

Table 4.
LC-MS/MS Characterization of phenolic compounds in unripe fruits.
2.4.1. Flavonoids
Flavonoids are the most abundant class of phenolic compounds having strong antioxidant, free-radical-scavenging properties [45]. Thirty-five flavonoids were detected in the selected plants. Compound 3 (cyanidin, m/z 287) was seen in the positive mode in all three samples. Compounds 1 and 2 were tentatively identified as cyanidin 3-rhamnoside 5-glucoside and petunidin 3-rhamnoside 5-glucoside in a positive mode in the black lemon sample, which made product ions at m/z 449 and 287, and m/z 480 and 317, respectively. Compounds 1, 2, and 3 were characterized as anthocyanins. Procyanidin trimmer C1 (compound 6) and (−)-epigallocatechin 7-O-glucuronide (compound 8) at ESI+ m/z 867.2131 and m/z 483.1133 produced fragment ions at m/z 867 and 483, respectively. Three more compounds, epicatechin (compound 4, m/z 289.0717), procyanidin B2 (compound 5, m/z 577.1351), and procyanidin dimmer B2 (compound 7, m/z 579.1497) were detected in unripe mango, unripe grapes, and black lemon, which made product ions at m/z 245 and 205; m/z 451, 425, 407, and 289; and m/z 579. Procyanidin trimmer C1 and procyanidin dimmer B2 have previously been found in native Australian flora [46,47]. 6-Geranylnaringenin (compound 10) was found in black lemon by displaying the product ions at m/z 287, 243, 159, and 119 at ESI− m/z 407.1864. Compound 9 (didymin) and compound 11 (hesperidin) were detected in unripe mango, unripe grapes, and black lemon and produced fragment ions at m/z 577 and 287 and m/z 303. Hesperidin and hesperetin were previously detected and measured by Velamuri et al. [48] and Sharma et al. [49] in sage (Salvia officinalis) and rosemary (Rosmarinus officinalis). Compound 12, identified as hesperetin 3′-O-glucuronide (m/z 479.1184) with product ions at m/z 301, was found in unripe grapes with the positive ionization mode. Hesperetin-3-O-glucuronide was previously discovered in the Exocarpium Citri grandis (ECG) extract, according to Zeng et al. [50]. Nobiletin (compound 13), apigenin 6-C-glucoside (compound 14), and diosmin (compound 18) at ESI− were putatively identified in unripe mango, unripe grapes, and black lemon, which produced daughter ions at m/z 401, m/z 269, and m/z 301, respectively. One of the most well-known characteristics of the flavonoid nobiletin is its neuroprotective properties [51,52]. Research has also investigated its antioxidant, anti-inflammatory, and antiobesity properties, making it a promising natural substance for various medical applications [52]. Compound 16 (m/z 595.1658) and compound 17 (m/z 449.1079) at ESI+ produced fragment ions at m/z 595, 303, and 285. Compound 15 was tentatively identified as apigenin 7-O-glucoside with product ions at m/z 417 and was found in unripe grapes and unripe mangoes samples in the negative ionization mode.
Kaempferol 3,7,4′-O-triglucoside (m/z 773.2135), isorhamnetin 3-O-glucuronide (m/z 493.0977), and kaempferol 7-O-glucoside (m/z 448.1000) were recognized as the compounds 19, 20, and 21, and each of them displayed the product ions at m/z 773, 493, and 448; they existed in unripe mango and black lemon sample, respectively, with the positive ionization mode. Myricetin (compound 22) and quercetin (compound 26) at ESI+ m/z 319.0449 and m/z 303.0499, respectively, which were tentatively identified in all three samples and generated product ions at m/z 319 and m/z 285 and 169, respectively, were detected in MS/MS. In a previous study, a quercetin compound with m/z 301.0332 was identified in mint, basil, and fenugreek leaves [10]. Dihydroquercetin (compound 23) and kaempferol 3-O-glucuronide (compound 24) produced fragment ions at m/z 305 and 463, respectively. Dihydromyricetin 3-O-rhamnoside (m/z 467.1184) was observed as compound 25 in the positive mode and produced product ions at m/z 467 that were identified in the black lemon and unripe grape sample. Dihydromyricetin 3-O-rhamnoside is unique in that it can lessen the harmful effects of alcohol consumption and enhance liver function. This distinctive substance is widely recognized for its hepatoprotective properties. Four more compounds, 6″-O-acetylglycitin (compound 27), 6″-O-acetylgenistin (compound 28), 3′-O-methylequol (compound 32), and dihydrobiochanin A (compound 33) in the positive ionization mode produced fragment ions at m/z 489, 475, 273, 269, 203, 201, and 175, respectively. Compound 30 (violanone) and compound 31 (dihydroformononetin) were detected in black lemon, unripe grape, and mango [40]. Compound 29 (4′,7-dihydroxyisoflavan) at ESI− m/z 241.0870 produced fragment ions at m/z 241. Previously, it had been identified in mint, rosemary, sage, basil, and oregano [3]. Compound 34, identified as phloridzin (m/z 435.1297) with product ions at m/z 435, was found in black lemon and unripe mango with the negative ionization mode. Phloretin 2′-O-glucuronide (compound 35) at ESI− m/z 451.1235 generated a product ion at m/z 275.
2.4.2. Stilbenes and Lignans
The health benefits of stilbenes and lignans make them essential phenolic compounds. In this study, we reportedly discovered nine phenolic metabolites in various therapeutic sour fruits. 4′-hydroxy-3,4,5-trimethoxystilbene (compound 36) and resveratrol (compound 39) at ESI+ m/z 287.1278 and 229.0859, respectively, generated a product ion at m/z 287, 211, 167, and 127 and were found in unripe mango and unripe grape. Dihydroresveratrol and piceatannol were identified in unripe mango and black lemon by displaying product ions at m/z 229.0872 and 245.0823 in the positive ionization mode. Compound 37 (piceatannol 3-O-glucoside and m/z 405.1191) produced a product ion at m/z 245 and was identified in all three samples in the negative ionization mode. Compound 41 (7-hydroxymatairesinol) was identified with the positive ionization mode in unripe mango and black lemon. Lu and Yeap Foo [44] also reported the antioxidant activity of sagerinic acid. Sagerinic acid is widely distributed in herbs and spices. Compounds 42 (schisantherin A) and 46 (schisanhenol) at ESI+ m/z 535.1973 and 401.1969 were tentatively identified with the negative ionization mode in unripe mango and black lemon. Lariciresinol (compound 43) and 2-hydroxyenterodiol (compound 45) formed product ions at m/z 329, 192, 178, and 175 and m/z 299, 287, 269, and 257, respectively. 2-Hydroxyenterolactone (m/z 313.1081) and secoisolariciresinol (m/z 361.1656) were recognized as the compounds 44 and 47, and each of them displayed product ions at m/z 255 and 346, 315, 223, and 165, which existed in unripe grapes, unripe mango, and black lemon, respectively. Previously, whole-grain rye bran was discovered as containing secoisolariciresinol, 7-hydroxymatairesinol, and lariciresino through MS/MS analysis, as Hanhineva et al. [11] reported.
2.4.3. Phenolic Acids
Twenty-four compounds were recognized as phenolic acids. Phenolic acids are said to have greater sensitivity in the negative mode. Compounds 48, 49, 50, 53, 55, 56, and 57 were putatively characterized as protocatechuic acid 4-O-glucoside (ESI− m/z 153), gallic acid (ESI− m/z 125), ellagic acid (ESI− m/z 284, 257), protocatechuic acid (ESI− m/z 109), p-hydroxybenzoic acid (ESI− m/z 93), syringic acid (ESI− m/z 182, 153), and benzoic acid (ESI− m/z 103, 93); each were identified in black lemon, unripe grapes, and unripe mangoes, confirmed through the MS/MS pure standard. Serrano et al. reported species (Salviinae, Mentheae, and Lamiaceae) containing protocatechuic acid [12]. Previously, p-coumaric acid hexoside, ferulic acid, gallic acid, and chlorogenic acid were reported by Palafox-Carlos et al. [53]. Syringic acid lowers blood pressure, reduces the risk of blood clots, and raises lipid levels, all of which may benefit heart health. It may also provide health benefits such as anti-inflammatory and antioxidant properties. Syringic acid may also have antiviral, antibacterial, and anticancer effects and exhibits potential use as a natural food preservative [54]. 3-O-Methylgallic acid (compound 51) and gallic acid 3-monogallate (compound 54) were identified in black lemon and unripe mangoes at m/z 183.0299 and 323.0398, confirmed through the MS/MS product ion at m/z 169 and 125 in the negative ionization mode. Furthermore, paeoniflorin (compound 52) produced fragments at ESI+ m/z 481.
Caffeic acid 4-O-glucuronide, chlorogenic acid, p-coumaric acid, caffeic acid, cinnamic acid, and ferulic acid at ESI− were putatively identified in unripe mango, unripe grapes, and black lemon, which produced daughter ions at m/z 179; m/z 191, 179, and 161; and m/z 119, m/z 135, m/z 103, m/z 178, 149, and 134, respectively. Prior research on native Australian flora revealed the presence of p-coumaric acid, caffeic acid, cinnamic acid, and ferulic acid, all of which have strong antioxidant potential [46,47]. Caffeic acid has antibacterial, anti-inflammatory, and antioxidant properties. It also has anticancer and antidiabetic effects. Numerous potential health advantages of cinnamic acid include its antibacterial, antidiabetic, anticancer, anti-inflammatory, and antioxidant properties [55]. According to some research, cinnamic acid helps protect skin from sunburn by absorbing UV rays such that it may be helpful as a natural sunblock ingredient [55,56,57]. Ferulic acid has a wide range of known medical benefits, including anticancer, antioxidant, antidiabetic, antiaging, radioprotective, hypotensive, neuroprotective, antiatherogenic, and pulmonary protective effects [58,59]. Compounds 58 (cinnamoyl glucose—C15H18O7) and 62 (p-coumaric acid 4-O-glucoside—C15H18O8) were putatively identified in black lemon and unripe mango in the negative mode at m/z 311 and m/z 163. Sinapic acid (compound 68) was confirmed through the pure standard at m/z 223.0612 in unripe mango and black lemon. Furthermore, rosmarinic acid (compound 70) was detected only in black lemon, producing product ions at m/z 197 and 179. Compound 65 (dihydroferulic acid, m/z 197.0809), compound 67 (1,2,2′-triferuloylgentiobiose, m/z 871.2655), compound 69 (verbascoside A, m/z 669.2389) and compound 71 (p-coumaroyl glycolic acid, m/z 223.0601) were identified in the positive mode of ionization after producing a product ion at ESI+ m/z 197, 871, 669, and 223, respectively. Rosmarinic acids have antibacterial, anti-inflammatory, antioxidant, and antidiabetic qualities that may benefit human health. Rosmarinic acid may be beneficial as a natural allergy treatment because it reduces airway inflammation and improves respiratory function [60,61,62].
2.4.4. Other Compounds
p-Anisaldehyde (compound 80) and scopoletin (compound 85) were detected in black lemon, unripe grapes, and unripe mangoes in the positive ionization mode. Compound 72 (m/z 319.1187) and compound 75 (m/z 191.0561) were identified as 3,4-DHPEA-EDA and quinic acid after producing a product ion at ESI− m/z 275 and 195 and m/z 171, 127, and 85, respectively. Mangiferin, as well as mangiferin 6′-gallate, produced a characteristic fragment at m/z 331, 301, 259, and 421, and each was observed to be found in unripe mango. Previously, it was also identified and quantified in oregano, rosemary, mint, basil, bay, and thyme. Furthermore, p-HPEA-AC (compound 74) and mellein (compound 79) were detected in black lemon and unripe grapes, while each compound produced product ions at m/z 137, 119, and 133. Compounds 83 and 84 (coumarin and 4-hydroxycoumarin) generated fragment ions at ESI+ m/z 103 and 91 and m/z 163 in black lemon and unripe mango. Three more compounds, pyrogallol (compound 73), carvacrol (compound 81), and esculetin (compound 82), form product ions at m/z 127, m/z 151, and m/z 179, respectively.
2.5. Venn Distribution of Phenolic Compounds in Unripe Fruits
The results are presented as a Venn distribution in Figure 2. Figure 2A shows the total number of phenolic compounds in black lemon (blue), unripe mangoes (yellow), and unripe grapes (green). It indicates that black lemon contains the highest number of unique phenolic compounds (four; 4.7%), while unripe mangoes and grapes contain two and three unique phenolic compounds, respectively. A total of 26 (30.6%) phenolic compounds overlapped in all three sour fruits, while 30 (35.3%) compounds overlapped in black lemon and unripe mangoes. Figure 2B displays the total number of phenolic acids in unripe mangoes, grapes, and black lemons. A total of 12 (50%) phenolic acids overlapped in all three fruits, while a total of 10 (41.7%) compounds overlapped in black lemon and unripe mangoes. Figure 2C shows the total number of flavonoids in the selected sour fruit waste. Three unique flavonoids were identified in each black lemon and unripe grape. A total of 11 (31.4%) flavonoids overlapped in black lemon and unripe mangoes, while a total of 8 (22.9%) flavonoids overlapped in all three fruits. Figure 2D depicts the total number of other compounds in the selected fruit waste. A total of nine (42.9%) compounds overlapped in black lemon and unripe mangoes, while a total of six (28.6%) compounds overlapped in all three fruits’ waste. Overall, unripe mangoes, grapes, and black lemons contain diverse compounds with therapeutic and pharmaceutical properties.
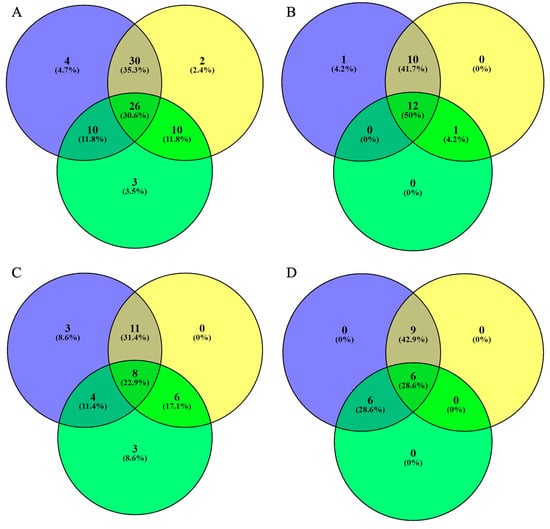
Figure 2.
Venn distribution of phenolic compounds (total number of phenolic compounds (A), total number of phenolic acids (B), total number of flavonoids (C), and total number of other compounds (D)) in black lemon (blue), unripe mango (yellow), and unripe grapes (green).
2.6. Heatmap Clustering of Quantified Phenolic Compounds in Unripe Fruits
The results of quantified phenolic compounds in unripe mangoes, grapes, and black lemons are presented in Table 5. A total of ten phenolic acids were quantified in the selected unripe fruits. Gallic acid (221.17 ± 9.69 μg/g), caffeic acid (177.31 ± 5.01 μg/g), chlorogenic acid (115.98 ± 8.79 μg/g), and ellagic acid (113.93 ± 8.31 μg/g) are the most abundant phenolic acids in unripe grapes and in unripe mangoes while ellagic acid (134.91 ± 11.42 μg/g) and syringic acid (113.42 ± 9.01 μg/g) are quantified in higher concentrations in black lemons. Chlorogenic acid, ellagic acid, and caffeic acid are abundant in unripe grapes. A total of eight flavonoids and four other compounds were quantified in the selected unripe fruits. Epicatechin (224.31 ± 13.41 μg/g) was quantified in higher concentration in unripe mangoes while the lowest concentration of epicatechin (88.52 ± 6.02 μg/g) was quantified in unripe grapes. Procyanidin B2 is a condensed tannin whose concentration was higher in black lemons (224.15 ± 11.34 μg/g) while the lowest concentration of procyanidin B2 was measured in unripe grapes (131.18 ± 10.06 μg/g). A small concentration of mangiferin was quantified only in unripe mangoes (44.93 ± 3.67 μg/g). Previously, mangiferin was measured in higher concentration in the peel (221 μg/g) than in the pulp (32 μg/g) [63]. The variation in concentration of polyphenols has been observed in different studies [12].

Table 5.
Targeted quantification/semi-quantification of phenolic compounds (μg/g).
At the same time, heatmap clustering was also conducted for the quantified phenolic compounds in these fruits (Figure 3). The heatmap shows that procyanidin B2 has the highest concentration in black lemon, while it is also quantified in unripe mangoes and grapes. Gallic acid, epicatechin, caffeic acid, chlorogenic acid, syringic acid, mangiferin, and ellagic acid have higher concentrations than other quantified compounds in unripe mangoes, while chlorogenic acid, procyanidin B2, caffeic acid, quercetin, ellagic acid, and ferulic acid were quantified in higher concentrations in unripe grapes than other compounds. Previously, gallic acid, chlorogenic acid, protocatechuic acid, vanillic acid, and ferulic acid in mango at different ripening stages were reported by Palafox-Carlos et al. [53].
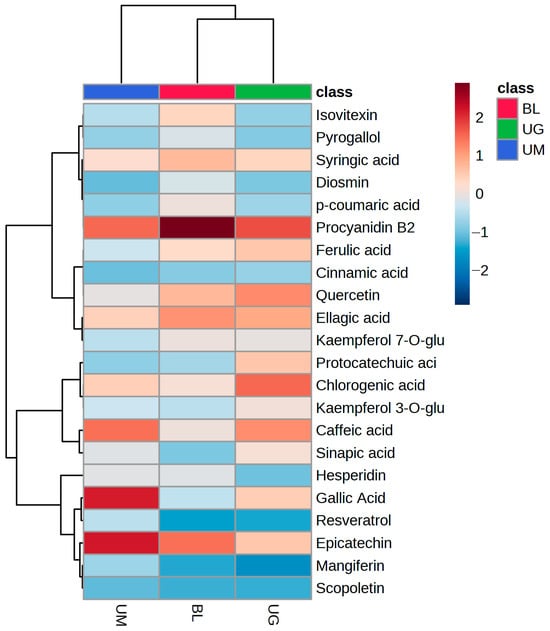
Figure 3.
Pearson heatmap clustering of quantified phenolic compounds in wasted sour fruits.
2.7. Chemometric Analysis of Phenolic Compounds in Unripe Fruits
Chemometrics analysis is a versatile science used to figure out the importance variables and observations in a complex dataset. We computed a partial-least-squares-discriminant-analysis derived variable’s importance in projection (VIP) score (4A), Pearson correlations heatmap (4B), and a principal component analysis 2D score plot (4C), as displayed in Figure 4. Figure 4A depicts that gallic acid, caffeic acid, epicatechin, resveratrol, sinapic acid, and diosmin are the biomarker phenolic compounds in the selected unripe sour fruits. Figure 4B illustrates that quercetin has the strongest correlation with ferulic acid and chlorogenic acid. The cell with dense red color shows the higher correlation between phenolic compounds. Figure 4C displays the importance of individual samples based on the concentration of phenolic compounds and depicts that unripe mangoes have the highest contribution of phenolic compounds which share almost 69%.
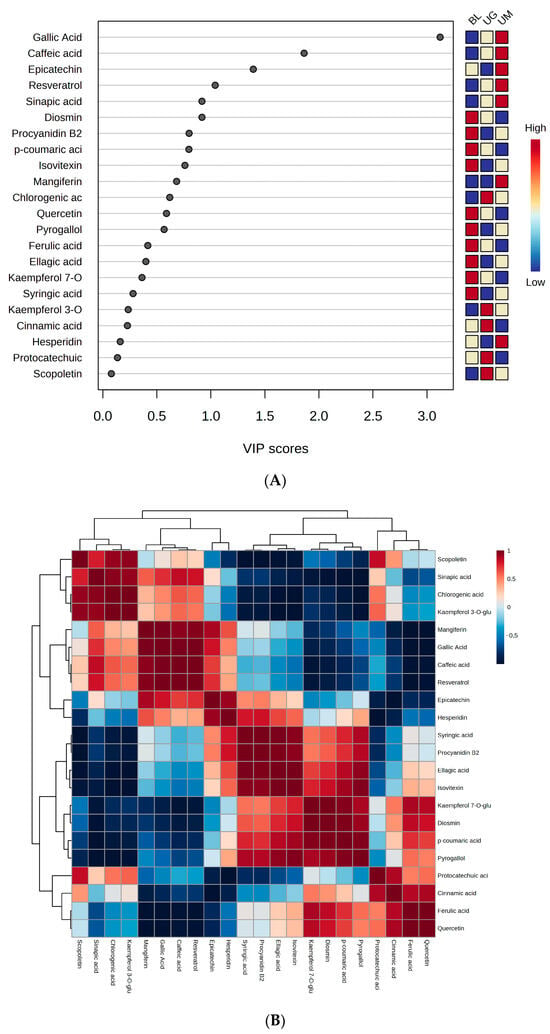
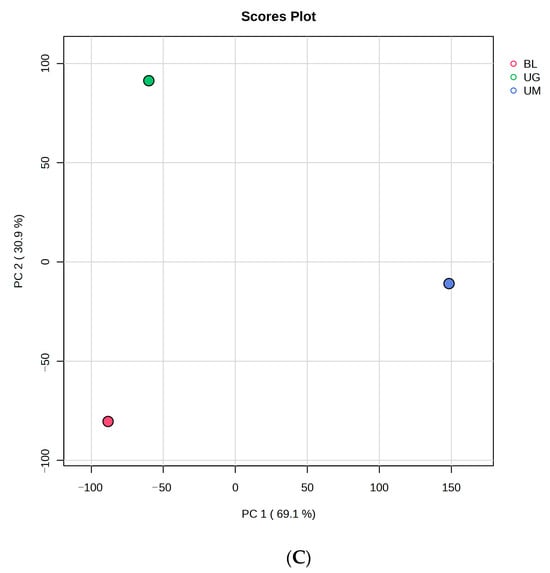
Figure 4.
We computed partial-least-squares-discriminant-analysis derived variable’s importance in projection (VIP) score (A), Pearson correlation heatmap (B), and principal component analysis 2D scores plot (C) of quantified compounds in black lemon (BL), unripe grapes (UG) and unripe mangoes (UM).
3. Materials and Methods
3.1. Materials
Analytical-grade chemicals were the only ones utilized in all the tests. Chemicals for identifying and characterizing compounds were supplied by Sigma Aldrich, located in Darmstadt, Germany. To estimate polyphenols and antioxidant capacity, the following materials were purchased from Sigma Aldrich (Castle Hill, NSW, Australia): potassium ferrocyanide (III), hydrated sodium acetate, trichloroacetic acid, vanillin, ammonium molybdate, sodium phosphate dibasic heptahydrate, Folin–Ciocalteu’s reagent, 2,2′-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS), ethylenediaminetetraacetic acid (EDTA), catechin, 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH), 2,4,6 tripyridyl-s-triazine (TPTZ), and 3-hydrobenzoic acid, as well as quercetin.
3.2. Sample Preparation and Method Optimization for Extraction of Phenolic Compounds
The samples of unripe mangoes, grapes, and black lemons were collected from the local market of Victoria, Australia. The unripe mangoes and grapes were collected in dried powder form, while the black lemon was collected in dried whole form. A laboratory grinder was used to grind the material into a fine powder. The process used to extract the phenolic compounds was as follows: Three separate extracts of the selected unripe sour fruits waste were prepared using one gram of powder and twenty milliliters of an 80% solvent (methanol) with 0.1 formic acid in Milli-Q water in triplicate. Samples were shaken for 16 h at 150 rpm and 4 °C in an orbital shaker (ZWYR-240) before being centrifuged. Following a 20 min centrifugation at 8000 rpm, the samples were separated into their supernatant and filtered through a 0.45 µm syringe filter. Prior to LC-MS/MS as well as spectrophotometric analysis, samples were stored at −20 °C for a maximum duration of fifteen days.
3.3. Quantification of Phenolic Contents in Unripe Fruits
3.3.1. Determination of Total Phenolic Content
Using the previously published methodology by Ali et al. [46], the phenolic chemical profile of the samples was examined. First, 200 µL of distilled water was combined with 25 µL of the Folin–Ciocalteu reagent (25% v/v). After adding 25 µL of the sample extract, the mixture was incubated for 5 min at 27 °C. Finally, 25 µL of 10% w/w sodium carbonate was incorporated into the reaction mixture, and it was incubated for a further hour at 27 °C in the dark. The measured absorbance of the samples was 760 nm. A standard curve was created against gallic acid to quantify the total phenolic content, ranging from 0 to 200 µg/mL in methanol. Milligram gallic acid equivalents, or GAE units, were used to record the results per gram of sample.
3.3.2. Total Flavonoid Content
A modified version of the procedure outlined by Ali et al. [64] was used to determine the flavonoid content of the samples. The TFC was determined using the aluminum chloride colorimetric technique in which 96-well plates were filled with an 80 µL sample extract, 80 µL AlCl3 solution, and 120 µL sodium acetate aqueous solution (50%). Following reaction mixture preparation, the prepared product was incubated for 2.5 h at 27 °C in the dark, and absorbance at 440 nm was measured using a spectrophotometer. A standard curve was created to determine flavonoid concentration, plotted against 0–50 µg/mL of quercetin in methanol. We expressed the results in milligram quercetin equivalents per gram of the sample unit.
3.3.3. Total Condensed Tannin
Initially, 150 µL of a 4% vanillin solution and 25 µL of the sample mixture were combined. The mixture was then added to 25 µL of 32% H2SO4. The last sample was incubated for 15 min at 25 °C. After calculating the absorbance at 500 nm, the standard catechin curve (0–1000 µg/mL) was created. Data are expressed in mg CE/g.
3.4. Antioxidant Activities of Unripe Fruits
3.4.1. ABTS Radical Scavenging Assay
The ABTS assay was carried out using the procedures outlined by Kiani et al. [65]. A mixture of 7 mM ABTS solution and 140 mM potassium persulfate solution was incubated for 16 h in the dark to create an ABTS+ solution. After diluting the solution with ethanol, the absorbance value at 734 nm was 0.70 ± 0.02. After adding 10 µL of sample extract and 290 µL of ABTS+ solution to a 96-well plate, it remained at room temperature for six minutes. At 734 nm, the absorbance was measured. To accomplish the measurement (mg AAE/g), a standard curve was generated against ascorbic acid concentrations ranging from 0 to 150 µg/mL in water.
3.4.2. DPPH Radical Scavenging Assay
A triplicate DPPH assay was conducted for every sample using the modified protocol outlined by Kiani et al. [65]. To begin, a 96-well plate technique was used to combine 25 µL sample extracts with 275 µL of DPPH dye (0.1 M) in methanol. Following preparation, the reaction mixture was incubated for 30 min at 25 °C in a dark environment. The spectrophotometer was then used to record the result at 517 nm. A standard curve was created against ascorbic acid (0–50 µg/mL) in water to assess the radical scavenging capabilities. Ascorbic acid equivalents in milligrams (mg AAE/g) were used to record the observed results.
3.4.3. Hydroxyl Radical Scavenging Assay
The procedure of Sharifi-Rad et al. [66] uses the Fenton-type reaction method to determine the •OH-RSA (hydroxyl radical scavenging activity) of the extracts. In this procedure, 50 µL of sample extract was mixed with 50 µL of 6 mM FeSO4·7H2O and 50 µL of 30% hydrogen peroxide. The mixture was then incubated for 10 min at 25 °C. The solution was then treated with 50 µL of 6 mM 3-hydroxybenzoic acid, and the absorbance was measured at 510 nm. The results are presented in mg AAE/g. A standard curve was constructed using ascorbic acid concentrations ranging from 0 to 300 µg/mL.
3.4.4. Fe2+ Chelating Activity (FICA)
Using this approach, 15 µL of the extract was combined with 85 µL of water, 50 µL of ferrozine (5 mM), and 50 µL of ferrous chloride (2 mM); later, the mixture was incubated at 25 °C for 10 min. The wavelength at which the absorbance was measured was 562 nm. The data are given as mg EDTA/g and were quantified using a standard curve created with EDTA at 0 to 50 µg/mL.
3.5. LC-MS/MS Characterization of Phenolic Compounds
The procedure previously reported by Ali et al. [47,67] was used to separate and identify phenolic compounds from plant samples. Using Agilent’s MassHunter Workstation Software (version B.06.00), located in Santa Clara, CA, USA, phytochemicals were extracted and identified. The untargeted phenolic metabolites of selected unripe mangoes, grapes, and black lemon were analyzed using an Agilent 6520 LC-ESI-Q-TOF-MS/MS (Accurate-Mass Q-TOF LC/MS) fitted with an Agilent HPLC 1200 series. A guard column (C18 ODS, 4.0 × 2.0 mm) shielded the Synergi 4 µm Hydro-RP 80 LC column (250 × 4.6 mm), which was used to screen the phenolic extracts. The column was manufactured by Phenomenex, located in Torrance, CA, USA. The following gradient was observed during the injection procedure of an aliquot of 10 µL from each phenolic extract: the flow rates of mobile phase B (0.1% formic acid in acetonitrile) and mobile phase A (0.1% formic acid in Milli-Q water) were 600 µL/min. Zero to ten minutes (10–20% B), ten to twenty minutes (20–25% B), twenty to thirty minutes (25–30% B), thirty to forty minutes (30–45% B), forty to fifty minutes (45–60% B), fifty to sixty-five minutes (60–90% B), sixty-five to sixty-seven minutes (90–100% B), sixty-seven to sixty-eight minutes (100–10% B), and sixty-eight to seventy minutes (10% B). Using the auto MS/MS mode, the following LC parameters were used: scan mode 50–1300 amu, capillary voltage (3500 V), nebulization 45 pressure, nitrogen gas flow rate (9 L/min) at 325 °C, and collision energies (10, 20, and 40 eV). Phenolic metabolites were identified and characterized using the Personal Compounds Database Library (PCDL) for metabolites and the Agilent MassHunter Workstation Software Quality Analysis (version B.06.00). This study used semi-quantification to analyze 23 phenolic compounds, with two runs of each sample. Additionally, 40 MS/MS spectra of commercial standards were collected in this experiment. LC-MS/MS, in conjunction with 26 commercial standards, were used to create equations [47]. Comprehensive help was provided from the Human Metabolome Database (HMDB) [68] for the identification of phenolic compounds.
3.6. Statistical Analysis
The analysis of variance (ANOVA), biplot analysis, and Pearson correlations were performed using Minitab (version 18.0, Minitab, LLC, State College, PA, USA) and XLSTAT-2019.1.3. We report phenolic content and biological activity results as mean + standard deviation. MetaboAnalyst 5.0 was used for chemometric analysis and heatmap clustering.
4. Conclusions
A total of 85 phenolic compounds (70 in black lemons, 49 in unripe grapes, and 68 in unripe mango) were identified, and 23 phenolic compounds were quantified using LC-ESI-QTOF-MS/MS. Unripe mangoes were measured as exhibiting the highest concentration of total phenolic content and antioxidant potential compared to unripe grapes and black lemon. This study confirmed that these unripe fruits are abundant with phenolic acids and flavonoids. There is notable variation in total phenolic and flavonoids recognized in unripe sour fruit waste. Procyanidin B2, gallic acid, epicatechin, caffeic acid, quercetin, and chlorogenic acid were measured as exhibiting higher concentrations in these selected unripe fruits. A positive correlation was found between phenolic contents and antioxidant activities of unripe fruits. The complementary profile of phenolic compounds in these unripe fruits makes them attractive, especially as dietary supplements in functional foods. The results of this research confirmed that unripe mangoes, grapes, and black lemons have vital polyphenols beneficial for human health.
Author Contributions
Conceptualization, A.A. and F.R.D.; methodology, A.A.; software, A.A. and Z.A.; validation, A.A. and F.R.D.; formal analysis, A.A.; visualization, A.A. and Z.A.; investigation, A.A.; resources, F.R.D.; data curation, A.A. and Z.A.; writing—original draft preparation, A.A.; writing—review and editing, supervision, project administration, J.J.C. and F.R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the University of Melbourne, Australia and Higher Education Commission of Pakistan for providing scholarship support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kandemir, K.; Piskin, E.; Xiao, J.; Tomas, M.; Capanoglu, E. Fruit juice industry wastes as a source of bioactives. J. Agric. Food Chem. 2022, 70, 6805–6832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Luan, Y.; Zhao, Y.; Liu, J.; Duan, Z.; Ruan, R. Current technologies and uses for fruit and vegetable wastes in a sustainable system: A review. Foods 2023, 12, 1949. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Barreca, D.; Rapisarda, A.; Cirmi, S.; Navarra, M. The second life of citrus fruit waste: A valuable source of bioactive compounds. Molecules 2021, 26, 5991. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.F.; Shen, C.; Xu, X.R.; Kuang, R.D.; Guo, Y.J.; Zeng, L.S.; Gao, L.L.; Lin, X.; Xie, J.F.; Xia, E.Q.; et al. Potential of fruit wastes as natural resources of bioactive compounds. Int. J. Mol. Sci. 2012, 13, 8308–8323. [Google Scholar] [CrossRef] [PubMed]
- Fia, G.; Bucalossi, G.; Proserpio, C.; Vincenzi, S. Unripe grapes: An overview of the composition, traditional and innovative applications, and extraction methods of a promising waste of viticulture. Aust. J. Grape Wine Res. 2022, 28, 8–26. [Google Scholar] [CrossRef]
- Bayram, Y.; Elgin Karabacak, C. Characterization of unripe grapes (Vitis vinifera L.) and its use to obtain antioxidant phenolic compounds by green extraction. Front. Sustain. Food Syst. 2022, 6, 909894. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape bioactive molecules, and the potential health benefits in reducing the risk of heart diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef]
- Fia, G.; Bucalossi, G.; Gori, C.; Borghini, F.; Zanoni, B. Recovery of bioactive compounds from unripe red grapes (cv. Sangiovese) through a green extraction. Foods 2020, 9, 566. [Google Scholar] [CrossRef]
- Zhou, K.; Raffoul, J.J. Potential anticancer properties of grape antioxidants. J. Oncol. 2012, 2012, 803294. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted health benefits of Mangifera indica L. (mango): The inestimable value of orchards recently planted in sicilian rural areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef] [PubMed]
- Lebaka, V.R.; Wee, Y.J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gurjar, P.S.; Beer, K.; Pongener, A.; Ravi, S.C.; Singh, S.; Verma, A.; Singh, A.; Thakur, M.; Tripathy, S.; et al. A review on valorization of different byproducts of mango (Mangifera indica L.) for functional food and human health. Food Biosci. 2022, 48, 101783. [Google Scholar] [CrossRef]
- Imran, M.; Arshad, M.S.; Butt, M.S.; Kwon, J.H.; Arshad, M.U.; Sultan, M.T. Mangiferin: A natural miracle bioactive compound against lifestyle related disorders. Lipids Health Dis. 2017, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Liu, H.; Lei, T.; Xie, X.; Wang, H.; He, X.; Tong, R.; Wang, Y. Mangiferin: An effective therapeutic agent against several disorders. Mol. Med. Rep. 2018, 18, 4775–4786. [Google Scholar]
- Al-Qudah, T.S.; Zahra, U.; Rehman, R.; Majeed, M.I.; Sadique, S.; Nisar, S.; Tahtamouni, R.; Tahtamouni, R.W. Lemon as a source of functional and medicinal ingredient: A review. Int. J. Chem. Biochem. Sci. 2018, 14, 55–61. [Google Scholar]
- Patrón-Vázquez, J.; Baas-Dzul, L.; Medina-Torres, N.; Ayora-Talavera, T.; Sánchez-Contreras, Á.; García-Cruz, U.; Pacheco, N. The effect of drying temperature on the phenolic content and functional behavior of flours obtained from lemon wastes. Agronomy 2019, 9, 474. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Bernini, R.; Campo, M.; Vita, C.; Souto, E.B.; Lombardi-Boccia, G.; Ramadan, M.F.; Santini, A.; Romani, A. Fruit wastes as a valuable source of value-added compounds: A collaborative perspective. Molecules 2021, 26, 6338. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Burò, I.; Consoli, V.; Vanella, L. Recent advances in health benefits of bioactive compounds from food wastes and by-products: Biochemical aspects. Int. J. Mol. Sci. 2023, 24, 2019. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Kiani, H.S.; Ali, B.; Al-Sadoon, M.K.; Al-Otaibi, H.S.; Ali, A. Lc-ms/ms and gc-ms identification of metabolites from the selected herbs and spices, their antioxidant, anti-diabetic potential, and chemometric analysis. Processes 2023, 11, 2721. [Google Scholar] [CrossRef]
- Pérez, M.; Dominguez-López, I.; Lamuela-Raventós, R.M. The chemistry behind the folin–ciocalteu method for the estimation of (poly)phenol content in food: Total phenolic intake in a mediterranean dietary pattern. J. Agric. Food Chem. 2023, 71, 17543–17553. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.M.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R.H. Comparative assessment of phenolic content and in vitro antioxidant capacity in the pulp and peel of mango cultivars. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; González, M. Using drying treatments to stabilise mango peel and seed: Effect on antioxidant activity. LWT Food Sci. Technol. 2012, 45, 261–268. [Google Scholar] [CrossRef]
- Rathod, Z.; Sharma, S.; Saraf, M. Identification and estimation of total phenol as a bioactive natural product from Citrus limon L. Burm. F. (lemon) and it’s endophytes. Curr. Trends Biomed. Eng. Biosci. 2022, 20, 144. [Google Scholar]
- Dong, X.; Hu, Y.; Li, Y.; Zhou, Z. The maturity degree, phenolic compounds and antioxidant activity of eureka lemon [Citrus limon (L.) Burm. F.]: A negative correlation between total phenolic content, antioxidant capacity and soluble solid content. Sci. Hortic. 2019, 243, 281–289. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Dpph radical scavenging assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. Lc-esi-qtof/ms profiling of Australian mango peel by-product polyphenols and their potential antioxidant activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Arshiya, S. The antioxidant effect of certain fruits: A review. J. Pharm. Sci. Res. 2013, 5, 265. [Google Scholar]
- Venkatesan, T.; Choi, Y.W.; Kim, Y.K. Impact of different extraction solvents on phenolic content and antioxidant potential of Pinus densiflora bark extract. Biomed. Res. Int. 2019, 2019, 3520675. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. Abts/pp decolorization assay of antioxidant capacity reaction pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Reese, R.N.; Tulio, A.Z., Jr.; Scheerens, J.C.; Miller, A.R. Modified 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (abts) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (frap) and 2,2′-diphenyl-1-picrylhydrazyl (dpph) methods. J. Agric. Food Chem. 2006, 54, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Madalageri, D.; Bharati, P.C.; Orsat, V.; Raghavan, V.; Kage, U. Antioxidant activity in pulp and peel of three mango varieties. J. Hortic. Sci. 2015, 10, 199–209. [Google Scholar] [CrossRef]
- Le, H.M. Antioxidative Effects of Mango Wastes on Shelf Life of Pork Products. Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2012. [Google Scholar]
- Soong, Y.-Y.; Barlow, P.J. Antioxidant activity and phenolic content of selected fruit seeds. Food Chem. 2004, 88, 411–417. [Google Scholar] [CrossRef]
- Sogi, D.S.; Siddiq, M.; Dolan, K.D. Total phenolics, carotenoids and antioxidant properties of tommy Atkin mango cubes as affected by drying techniques. LWT—Food Sci. Technol. 2015, 62, 564–568. [Google Scholar] [CrossRef]
- Wong, F.C.; Yong, A.L.; Ting, E.P.; Khoo, S.C.; Ong, H.C.; Chai, T.T. Antioxidant, metal chelating, anti-glucosidase activities and phytochemical analysis of selected tropical medicinal plants. Iran. J. Pharm. Res. 2014, 13, 1409–1415. [Google Scholar] [PubMed]
- Ali, A.; Kiloni, S.M.; Cáceres-Vélez, P.R.; Jusuf, P.R.; Cottrell, J.J.; Dunshea, F.R. Phytochemicals, antioxidant activities, and toxicological screening of native Australian fruits using zebrafish embryonic model. Foods 2022, 11, 4038. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of phenolics profile in freeze-dried apple peel and their bioactivities during in vitro digestion and colonic fermentation. Int. J. Mol. Sci. 2023, 24, 1514. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Antioxidant, alpha-glucosidase inhibition activities, in silico molecular docking and pharmacokinetics study of phenolic compounds from native Australian fruits and spices. Antioxidants 2023, 12, 254. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Characterization, antioxidant potential, and pharmacokinetics properties of phenolic compounds from native Australian herbs and fruits. Plants 2023, 12, 993. [Google Scholar] [CrossRef] [PubMed]
- Velamuri, R.; Sharma, Y.; Fagan, J.; Schaefer, J. Application of UHPLC-ESI-QTOF-MS in phytochemical profiling of sage (Salvia officinalis) and rosemary (Rosmarinus officinalis). Planta Medica Int. Open 2020, 7, e133–e144. [Google Scholar] [CrossRef]
- Sharma, Y.; Velamuri, R.; Fagan, J.; Schaefer, J. Full-spectrum analysis of bioactive compounds in rosemary (Rosmarinus officinalis L.) as influenced by different extraction methods. Molecules 2020, 25, 4599. [Google Scholar] [CrossRef]
- Zeng, X.; Su, W.; Zheng, Y.; Liu, H.; Li, P.; Zhang, W.; Liang, Y.; Bai, Y.; Peng, W.; Yao, H. UFLC-Q-TOF-MS/MS-based screening and identification of flavonoids and derived metabolites in human urine after oral administration of Exocarpium citri grandis extract. Molecules 2018, 23, 895. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y. Potential benefits of nobiletin, a citrus flavonoid, against Alzheimer’s disease and Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef] [PubMed]
- Palafox-Carlos, H.; Yahia, E.M.; González-Aguilar, G.A. Identification and quantification of major phenolic compounds from mango (Mangifera indica, cv. Ataulfo) fruit by HPLC–DAD–MS/MS-ESI and their individual contribution to the antioxidant activity during ripening. Food Chem. 2012, 135, 105–111. [Google Scholar] [CrossRef]
- Xiang, S.; Xiao, J. Protective effects of syringic acid on inflammation, apoptosis and intestinal barrier function in caco-2 cells following oxygen-glucose deprivation/reoxygenation-induced injury. Exp. Ther. Med. 2022, 23, 66. [Google Scholar] [CrossRef] [PubMed]
- Rychlicka, M.; Rot, A.; Gliszczyńska, A. Biological properties, health benefits and enzymatic modifications of dietary methoxylated derivatives of cinnamic acid. Foods 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Savych, A.; Marchyshyn, S.; Kyryliv, M.; Bekus, I. Cinnamic acid and its derivatives in the herbal mixtures and their antidiabetic activity. Farmacia 2021, 69, 595–601. [Google Scholar] [CrossRef]
- Deng, H.; Xu, Q.; Guo, H.-Y.; Huang, X.; Chen, F.; Jin, L.; Quan, Z.-S.; Shen, Q.-K. Application of cinnamic acid in the structural modification of natural products: A review. Phytochemistry 2023, 206, 113532. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic acid: Therapeutic potential through its antioxidant property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Peres, D.D.A.; Sarruf, F.D.; de Oliveira, C.A.; Velasco, M.V.R.; Baby, A.R. Ferulic acid photoprotective properties in association with uv filters: Multifunctional sunscreen with improved SPF and UVA-PF. J. Photochem. Photobiol. B Biol. 2018, 185, 46–49. [Google Scholar] [CrossRef]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic acid-human pharmacokinetics and health benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Guan, H.; Luo, W.; Bao, B.; Cao, Y.; Cheng, F.; Yu, S.; Fan, Q.; Zhang, L.; Wu, Q.; Shan, M. A comprehensive review of Rosmarinic acid: From phytochemistry to pharmacology and its new insight. Molecules 2022, 27, 3292. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Simirgiotis, M.J. Antioxidant properties and hyphenated HPLC-PDA-MS profiling of chilean pica mango fruits (Mangifera indica L. Cv. Piqueño). Molecules 2014, 19, 438–458. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive profiling of most widely used spices for their phenolic compounds through LC-ESI-QTOF-MS2 and their antioxidant potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Kiani, H.S.; Ahmad, W.; Nawaz, S.; Farah, M.A.; Ali, A. Optimized extraction of polyphenols from unconventional edible plants: Lc-ms/ms profiling of polyphenols, biological functions, molecular docking, and pharmacokinetics study. Molecules 2023, 28, 6703. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Song, S.; Ali, A.; Subbiah, V.; Taheri, Y.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS characterization of phenolic compounds from pyracantha coccinea m. Roem. and their antioxidant capacity. Cell. Mol. Biol. 2021, 67, 201–211. [Google Scholar] [CrossRef]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and characterization of anthocyanins and non-anthocyanin phenolics from australian native fruits and their antioxidant, antidiabetic, and anti-Alzheimer potential. Food Res. Int. 2022, 162, 111951. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. Hmdb 5.0: The human metabolome database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).