Bioactive Dairy-Fermented Products and Phenolic Compounds: Together or Apart
Abstract
:1. Introduction
2. Immunoreactivity of Dairy-Fermented Products
3. Bioactive Milk Products with Pro-Health Properties
4. Polyphenols as Valuable Food Additives
5. Protein–Polypeptide Interactions
6. Protein–Polyphenol Products
7. The Role of Milk Bioactive Peptides on the Organisms
8. The Effects of Bioactive Polyphenols and Their Metabolites in Organisms during their Passage through the Gastrointestinal Tract
9. Docking: Potential Impact Mechanism of the Protein–Polyphenol Complex Reaction on Organisms
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savaiano, D.A.; Hutkins, R.W. Yogurt, Cultured Fermented Milk, and Health: A Systematic Review. Nutr. Rev. 2021, 79, 599–614. [Google Scholar] [CrossRef] [PubMed]
- García-Burgos, M.; Moreno-Fernández, J.; Alférez, M.J.M.; Díaz-Castro, J.; López-Aliaga, I. New Perspectives in Fermented Dairy Products and Their Health Relevance. J. Funct. Foods 2020, 72, 104059. [Google Scholar] [CrossRef]
- Wróblewska, B.; Kaliszewska-Suchodoła, A.; Fuc, E.; Markiewicz, L.H.; Ogrodowczyk, A.M.; Złotkowska, D.; Wasilewska, E. Effect of Low-Immunogenic Yogurt Drinks and Probiotic Bacteria on Immunoreactivity of Cow’s Milk Proteins and Tolerance Induction—In Vitro and In Vivo Studies. Nutrients 2020, 12, 3390. [Google Scholar] [CrossRef] [PubMed]
- Ogrodowczyk, A.M.; Kalicki, B.; Wróblewska, B. The Effect of Lactic Acid Fermentation with Different Bacterial Strains on the Chemical Composition, Immunoreactive Properties, and Sensory Quality of Sweet Buttermilk. Food Chem. 2021, 353, 129512. [Google Scholar] [CrossRef]
- Manzanarez-Quín, C.G.; Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; González-Córdova, A.F.; Vallejo-Cordoba, B. Invited Review: Potential Antiobesity Effect of Fermented Dairy Products. J. Dairy Sci. 2021, 104, 3766–3778. [Google Scholar] [CrossRef]
- Koksal, Z.G.; Uysal, P.; Mercan, A.; Bese, S.A.; Erge, D. Does Maternal Fermented Dairy Products Consumption Protect against Cow’s Milk Protein Allergy in Toddlers? Ann. Allergy Asthma Immunol. 2023, 130, 333–339. [Google Scholar] [CrossRef]
- Baruah, R.; Ray, M.; Halami, P.M. Preventive and Therapeutic Aspects of Fermented Foods. J. Appl. Microbiol. 2022, 132, 3476–3489. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Hui, X.; Gong, X.; Tran, K.N.; Stipkovits, L.; Mohan, M.S.; Brennan, M.A.; Brennan, C.S. Functionalization of Bovine Whey Proteins by Dietary Phenolics from Molecular-Level Fabrications and Mixture-Level Combinations. Trends Food Sci. Technol. 2021, 110, 107–119. [Google Scholar] [CrossRef]
- Hansen, M.M.; Hartel, R.W.; Roos, Y.H. Bioactives and Extracts Affect the Physico-Chemical Properties of Concentrated Whey Protein Isolate Dispersions. Food Prod. Process. Nutr. 2022, 4, 2. [Google Scholar] [CrossRef]
- Diep, T.T.; Yoo, M.J.Y.; Rush, E. Effect of In Vitro Gastrointestinal Digestion on Amino Acids, Polyphenols and Antioxidant Capacity of Tamarillo Yoghurts. Int. J. Mol. Sci. 2022, 23, 2526. [Google Scholar] [CrossRef]
- Pineda-Vadillo, C.; Nau, F.; Dubiard, C.G.; Cheynier, V.; Meudec, E.; Sanz-Buenhombre, M.; Guadarrama, A.; Tóth, T.; Csavajda, É.; Hingyi, H.; et al. In Vitro Digestion of Dairy and Egg Products Enriched with Grape Extracts: Effect of the Food Matrix on Polyphenol Bioaccessibility and Antioxidant Activity. Food Res. Int. 2016, 88, 284–292. [Google Scholar] [CrossRef]
- Wasilewska, E.; Zlotkowska, D.; Wroblewska, B. Yogurt Starter Cultures of Streptococcus thermophilus and Lactobacillus bulgaricus Ameliorate Symptoms and Modulate the Immune Response in a Mouse Model of Dextran Sulfate Sodium-Induced Colitis. J. Dairy Sci. 2019, 102, 37–53. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.; Awad, S. Milk Bioactive Peptides: Antioxidant, Antimicrobial and Anti-Diabetic Activities. Adv. Biochem. 2019, 7, 22. [Google Scholar] [CrossRef]
- Marcone, S.; Belton, O.; Fitzgerald, D.J. Milk-derived Bioactive Peptides and Their Health Promoting Effects: A Potential Role in Atherosclerosis. Brit. J. Clin. Pharm. 2017, 83, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Sponseller, J.; Schmidt, D.; Cohen, O.; Tzipori, S. Hyperimmune Bovine Colostrum for Treatment of GI Infections: A Review and Update on Clostridium difficile. Hum. Vaccines Immunother. 2013, 9, 1565–1568. [Google Scholar] [CrossRef]
- Kashyap, R.; Narayan, K.S.; Vij, S. Evaluation of the Antimicrobial Attribute of Bioactive Peptides Derived from Colostrum Whey Fermented by Lactobacillus against Diarrheagenic E. coli Strains. J. Food Sci. Technol. 2023, 60, 211–221. [Google Scholar] [CrossRef]
- Godhia, M.; Patel, N. Colostrum—Its Composition, Benefits as a Nutraceutical: A Review. Curr. Res. Nutr. Food Sci. 2013, 1, 37–47. [Google Scholar] [CrossRef]
- Ayar, A.; Sıçramaz, H.; Çetin, İ. The Effect of Bovine Colostrum on the Lactic Flora of Yogurt and Kefir. JSM Biotech. Biomed. Eng. 2016, 4, 1063. [Google Scholar]
- Abdel-Ghany, A.S.; Zaki, D.A. Production of Novel Functional Yoghurt Fortified with Bovine Colostrum and Date Syrup for Children. Alex. Sci. Exch. 2018, 39, 651–662. [Google Scholar] [CrossRef]
- Owczarek, K.; Fichna, J.; Lewandowska, U. Anti-Inflammatory Activity of Polyphenolic Compounds. Borgis-Postępy Fitoter. 2017, 1, 17–23. [Google Scholar]
- Yang, L.; Jia, L.; Li, X.; Zhang, K.; Wang, X.; He, Y.; Hao, M.; Rayman, M.P.; Zhang, J. Prooxidant Activity-Based Guideline for a Beneficial Combination of (−)-Epigallocatechin-3-Gallate and Chlorogenic Acid. Food Chem. 2022, 386, 132812. [Google Scholar] [CrossRef] [PubMed]
- Piekarska-Radzik, L.; Klewicka, E. Mutual Influence of Polyphenols and Lactobacillus spp. Bacteria in Food: A Review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Fukutomi, R.; Ohishi, T.; Koyama, Y.; Pervin, M.; Nakamura, Y.; Isemura, M. Beneficial Effects of Epigallocatechin-3-O-Gallate, Chlorogenic Acid, Resveratrol, and Curcumin on Neurodegenerative Diseases. Molecules 2021, 26, 415. [Google Scholar] [CrossRef] [PubMed]
- Tosif, M.M.; Najda, A.; Bains, A.; Krishna, T.C.; Chawla, P.; Dyduch-Siemińska, M.; Klepacka, J.; Kaushik, R. A Comprehensive Review on the Interaction of Milk Protein Concentrates with Plant-Based Polyphenolics. Int. J. Mol. Sci. 2021, 22, 13548. [Google Scholar] [CrossRef] [PubMed]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the Immune Response. J. Allergy Clin. Immunol. 2010, 125, 3–23. [Google Scholar] [CrossRef]
- Wildner, S.; Elsässer, B.; Stemeseder, T.; Briza, P.; Soh, W.T.; Villalba, M.; Lidholm, J.; Brandstetter, H.; Gadermaier, G. Endolysosomal Degradation of Allergenic Ole e 1-Like Proteins: Analysis of Proteolytic Cleavage Sites Revealing T Cell Epitope-Containing Peptides. Int. J. Mol. Sci. 2017, 18, 1780. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Bøgh, K.L.; Dupont, D.; Egger, L.; Gadermaier, G.; Larré, C.; Mackie, A.; Menard, O.; Adel-Patient, K.; Picariello, G.; et al. The Relevance of a Digestibility Evaluation in the Allergenicity Risk Assessment of Novel Proteins. Opinion of a Joint Initiative of COST Action ImpARAS and COST Action INFOGEST. FCT 2019, 129, 405–423. [Google Scholar] [CrossRef]
- Pessato, T.B.; De Morais, F.P.R.; De Carvalho, N.C.; Figueira, A.C.M.; Fernandes, L.G.R.; Zollner, R.D.L.; Netto, F.M. Protein Structure Modification and Allergenic Properties of Whey Proteins upon Interaction with Tea and Coffee Phenolic Compounds. J. Funct. Foods 2018, 51, 121–129. [Google Scholar] [CrossRef]
- Tian, R.; Han, X.; Tian, B.; Li, G.; Sun, L.; Tian, S.; Qin, L.; Wang, S. Effects of Covalent Binding of Different Polyphenols on Structure, Rheology and Functional Properties of Whey Protein Isolate. LWT 2023, 184, 114968. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Y.; He, S.; Wu, Z.; Jin, R. Food Allergenic Protein Conjugation with Plant Polyphenols for Allergenicity Reduction. Curr. Opin. Food Sci. 2022, 43, 36–42. [Google Scholar] [CrossRef]
- Bessa, C.; Francisco, T.; Dias, R.; Mateus, N.; Freitas, V.D.; Pérez-Gregorio, R. Use of Polyphenols as Modulators of Food Allergies. From Chemistry to Biological Implications. Front. Sustain. Food Syst. 2021, 5, 623611. [Google Scholar] [CrossRef]
- Huang, C.H.; Lin, Y.C.; Jan, T.R. Lactobacillus Reuteri Induces Intestinal Immune Tolerance Against Food Allergy in Mice. J. Funct. Foods 2017, 31, 44–51. [Google Scholar] [CrossRef]
- Hadjimbei, E.; Botsaris, G.; Chrysostomou, S. Beneficial Effects of Yoghurts and Probiotic Fermented Milks and Their Functional Food Potential. Foods 2022, 11, 2691. [Google Scholar] [CrossRef] [PubMed]
- Geiselhart, S.; Podzhilkova, A.; Hoffmann-Sommergruber, K. Cow’s Milk Processing—Friend or Foe in Food Allergy? Foods 2021, 10, 572. [Google Scholar] [CrossRef]
- Miciński, J.; Kowalski, I.M.; Zwierzchowski, G.; Szarek, J.; Pierożyński, B.; Zabłocka, E. Characteristics of Cow’s Milk Proteins Including Allergenic Properties and Methods for Its Reduction. Pol. Ann. Med. 2013, 20, 69–76. [Google Scholar] [CrossRef]
- Jensen, S.A.; Fiocchi, A.; Baars, T.; Jordakieva, G.; Nowak-Wegrzyn, A.; Pali-Schöll, I.; Passanisi, S.; Pranger, C.L.; Roth-Walter, F.; Takkinen, K.; et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) Guidelines Update-III-Cow’s Milk Allergens and Mechanisms Triggering Immune Activation. WAO J. 2022, 15, 100668. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Tomé, D. Milk Proteins: Digestion and Absorption in the Gastrointestinal Tract. In Milk Proteins, 3rd ed.; Boland, M., Singh, H., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2020; pp. 701–714. [Google Scholar] [CrossRef]
- De Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein Micelles and Their Internal Structure. Adv. Colloid Interface Sci. 2012, 171–172, 36–52. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, C.I.; Gomes, A.M.P.; Pintado, M.E.; Xavier Malcata, F. Bovine Whey Proteins—Overview on Their Main Biological Properties. Food Res. Int. 2007, 40, 1197–1211. [Google Scholar] [CrossRef]
- Wróblewska, B. Food Allergens. In Chemical and Functional Properties of Food Components, 4th ed.; Staroszczyk, H., Sikorski, Z.E., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2023; pp. 339–362. [Google Scholar]
- Teneva-Angelova, T.; Balabanova, T.; Boyanova, P.; Beshkova, D. Traditional Balkan Fermented Milk Products. Eng. Life Sci. 2018, 18, 807–819. [Google Scholar] [CrossRef]
- Abdullah, R.; Arshad, H.; Kaleem, A.; Iqtedar, M.; Aftab, M.; Saleem, F. Assessment of Angiotensin Converting Enzyme Inhibitory Activity and Quality Attributes of Yoghurt Enriched with Cinnamomum verum, Elettaria cardamomum, Beta vulgaris and Brassica oleracea. Saudi J. Biol. Sci. 2023, 30, 103556. [Google Scholar] [CrossRef]
- Shori, A.B. Storage Quality and Antioxidant Properties of Yogurt Fortified with Polyphenol Extract from Nutmeg, Black Pepper, and White Pepper. Electron. J. Biotechnol. 2022, 57, 24–30. [Google Scholar] [CrossRef]
- Tamime, A.Y.; Robinson, R.K. Microbiology of Yoghurt and Related Starter Cultures. In Tamime and Robinson’s Yoghurt, 3rd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing Ltd.: Sawston, UK, 2007; pp. 468–534. [Google Scholar] [CrossRef]
- Oberg, T.S.; McMahon, D.J.; Culumber, M.D.; McAuliffe, O.; Oberg, C.J. Invited Review: Review of Taxonomic Changes in Dairy-Related lactobacilli. J. Dairy Sci. 2022, 105, 2750–2770. [Google Scholar] [CrossRef]
- Dong, J.; Ping, L.; Cao, T.; Sun, L.; Liu, D.; Wang, S.; Huo, G.; Li, B. Immunomodulatory Effects of the Bifidobacterium Longum BL-10 on Lipopolysaccharide-Induced Intestinal Mucosal Immune Injury. Front. Immunol. 2022, 13, 947755. [Google Scholar] [CrossRef]
- Wróblewska, B.; Kaliszewska-Suchodoła, A.; Kołakowski, P.; Troszyńska, A. The Effect of Microbial Transglutaminase on the Immunoreactive and Sensory Properties of Fermented Milk Beverages. Int. J. Food Sci. Technol. 2013, 48, 1007–1017. [Google Scholar] [CrossRef]
- Jeon, H.-Y.; Kim, K.-S.; Kim, S. Effects of Yogurt Containing Probiotics on Respiratory Virus Infections: Influenza H1N1 and SARS-CoV-2. J. Dairy Sci. 2023, 106, 1549–1561. [Google Scholar] [CrossRef]
- Huang, M.; Tan, H.; Xiong, Z.; Hu, W.; Wu, Y.; Meng, X.; Chen, H.; Li, X. Allergenicity Evaluation of Peptides from Milk and Yogurt after Gastrointestinal Digestion Based on Epitopes. Food Funct. 2022, 13, 10769–10789. [Google Scholar] [CrossRef]
- Chuang, K.-C.; Lai, Y.-W.; Ko, C.-H.; Yen, C.-C.; Chen, H.-L.; Lan, Y.-W.; Chen, C.-F.; Chen, W.; Chen, C.-M. Therapeutic Effects of Kefir Peptides on Adjuvant-Induced Arthritis in Rats through Anti-Inflammation and Downregulation of Matrix Metalloproteinases. Life Sci. 2023, 317, 121411. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, X.; Jiang, H.; Dong, M. Analysis of the Microflora in Tibetan Kefir Grains Using Denaturing Gradient Gel Electrophoresis. Food Microbiol. 2009, 26, 770–775. [Google Scholar] [CrossRef]
- Wróblewska, B.; Kołakowski, P.; Pawlikowska, K.; Troszyńska, A.; Kaliszewska, A. Influence of the Addition of Transglutaminase on the Immunoreactivity of Milk Proteins and Sensory Quality of Kefir. Food Hydrocoll. 2009, 23, 2434–2445. [Google Scholar] [CrossRef]
- Hochwallner, H.; Schulmeister, U.; Swoboda, I.; Spitzauer, S.; Valenta, R. Cow’s Milk Allergy: From Allergens to New Forms of Diagnosis, Therapy and Prevention. Methods 2014, 66, 22–33. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, X.; Yue, Y.; Du, G.; Chen, H.; Ning, M.; Yuan, Y.; Yue, T. Metagenomic Features of Tibetan Kefir Grains and Its Metabolomics Analysis during Fermentation. LWT 2023, 175, 114502. [Google Scholar] [CrossRef]
- Barukčić, I.; Lisak Jakopović, K.; Božanić, R. Valorisation of Whey and Buttermilk for Production of Functional Beverages—An Overview of Current Possibilities. Food Technol. Biotechnol. 2019, 57, 448–460. [Google Scholar] [CrossRef]
- Buchanan, D.; Martindale, W.; Romeih, E.; Hebishy, E. Recent Advances in Whey Processing and Valorisation: Technological and Environmental Perspectives. Int. J. Dairy Technol. 2023, 76, 291–312. [Google Scholar] [CrossRef]
- Pires, A.F.; Marnotes, N.G.; Rubio, O.D.; Garcia, A.C.; Pereira, C.D. Dairy By-Products: A Review on the Valorization of Whey and Second Cheese Whey. Foods 2021, 10, 1067. [Google Scholar] [CrossRef]
- Jeong, G.-T. Valorization of Dairy By-Products: Optimized Synthesis of 5-Hydroxymethylfurfural and Levulinic Acid from Lactose and Whey. J. Environ. Chem. Eng. 2022, 10, 108413. [Google Scholar] [CrossRef]
- Pessione, E.; Cirrincione, S. Bioactive Molecules Released in Food by Lactic Acid Bacteria: Encrypted Peptides and Biogenic Amines. Front. Microbiol. 2016, 7, 879. [Google Scholar] [CrossRef]
- Tamang, J.P.; Shin, D.H.; Jung, S.J.; Chae, S.W. Functional Properties of Microorganisms in Fermented Foods. Front. Microbiol. 2016, 7, 578. [Google Scholar] [CrossRef]
- Pescuma, M.; Hébert, E.M.; Bru, E.; De Valdez, G.F.; Mozzi, F. Diversity in Growth and Protein Degradation by Dairy Relevant Lactic Acid Bacteria Species in Reconstituted Whey. J. Dairy Res. 2012, 79, 201–208. [Google Scholar] [CrossRef]
- Gomes-Santos, A.C.; Fonseca, R.C.; Lemos, L.; Reis, D.S.; Moreira, T.G.; Souza, A.L.; Silva, M.R.; Silvestre, M.P.C.; Cara, D.C.; Faria, A.M.C. Hydrolyzed Whey Protein Prevents the Development of Food Allergy to β-Lactoglobulin in Sensitized Mice. Cell. Immunol. 2015, 298, 47–53. [Google Scholar] [CrossRef]
- Wróblewska, B.; Kaliszewska-Suchodoła, A.; Markiewicz, L.H.; Szyc, A.; Wasilewska, E. Whey Prefermented with Beneficial Microbes Modulates Immune Response and Lowers Responsiveness to Milk Allergens in Mouse Model. J. Funct. Foods 2019, 54, 41–52. [Google Scholar] [CrossRef]
- Tarapata, J.; Maciejczyk, M.; Zulewska, J. Microfiltration of Buttermilk: Partitioning of Proteins and Modelling Using a Resistance-in-Series Model. Int. Dairy J. 2022, 134, 105445. [Google Scholar] [CrossRef]
- Ali, A.H. Current Knowledge of Buttermilk: Composition, Applications in the Food Industry, Nutritional and Beneficial Health Characteristics. Int. J. Dairy Technol. 2019, 72, 169–182. [Google Scholar] [CrossRef]
- García-Cano, I.; Yeh, P.-W.; Rocha-Mendoza, D.; Jiménez-Flores, R. Supercritical CO2 Treatment Reduces the Antigenicity of Buttermilk β-Lactoglobulin and Its Inflammatory Response in Caco-2 Cells. JDS Commun. 2021, 2, 1–6. [Google Scholar] [CrossRef]
- Azarcoya-Barrera, J.; Goruk, S.; Lewis, E.D.; Pouliot, Y.; Curtis, J.M.; Steele, R.; Wadge, E.; Field, C.J.; Jacobs, R.L.; Richard, C. Feeding Buttermilk-Derived Choline Forms During Gestation and Lactation Modulates Ex Vivo T-Cell Response in Rat Dams. J. Nutr. 2020, 150, 1958–1965. [Google Scholar] [CrossRef]
- Azarcoya-Barrera, J.; Field, C.J.; Goruk, S.; Makarowski, A.; Curtis, J.M.; Pouliot, Y.; Jacobs, R.L.; Richard, C. Buttermilk: An Important Source of Lipid Soluble Forms of Choline That Influences the Immune System Development in Sprague–Dawley Rat Offspring. Eur. J. Nutr. 2021, 60, 2807–2818. [Google Scholar] [CrossRef]
- Liutkevičius, A.; Kaminskas, A.; Abaravičius, J.A.; Vitkus, D.; Jablonskienė, V.; Sekmokienė, D.; Narkevičius, R.; Speiciene, V.; Alencikiene, G. Fermented Buttermilk-Based Beverage: Impact on Young Volunteers’ Health Parameters. Czech J. Food Sci. 2016, 34, 143–148. [Google Scholar] [CrossRef]
- Wróblewska, B.; Markiewicz, L.H.; Szyc, A.M.; Dietrich, M.A.; Szymkiewicz, A.; Fotschki, J. Lactobacillus casei LcY Decreases Milk Protein Immunoreactivity of Fermented Buttermilk but Also Contains IgE-Reactive Proteins. Food Res. Int. 2016, 83, 95–101. [Google Scholar] [CrossRef]
- Kondybayev, A.; Loiseau, G.; Achir, N.; Mestres, C.; Konuspayeva, G. Fermented Mare Milk Product (Qymyz, Koumiss). Int. Dairy J. 2021, 119, 105065. [Google Scholar] [CrossRef]
- Pieszka, M.; Łuszczyński, J.; Zamachowska, M.; Augustyn, R.; Długosz, B.; Hędrzak, M. Is Mare Milk an Appropriate Food for People?—A Review. Ann. Anim. Sci. 2016, 16, 33–51. [Google Scholar] [CrossRef]
- Uniacke-Lowe, T.; Fox, P.F. Equid Milk. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: Oxford, UK, 2011; Volume 3, pp. 518–529. [Google Scholar] [CrossRef]
- Fotschki, J.; Szyc, A.M.; Laparra, J.M.; Markiewicz, L.H.; Wróblewska, B. Immune-Modulating Properties of Horse Milk Administered to Mice Sensitized to Cow Milk. J. Dairy Sci. 2016, 99, 9395–9404. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xie, Q.; Chen, Q.; Evivie, S.E.; Liu, D.; Dong, J.; Huo, G.; Li, B. Cow, Goat, and Mare Milk Diets Differentially Modulated the Immune System and Gut Microbiota of Mice Colonized by Healthy Infant Feces. J. Agric. Food Chem. 2020, 68, 15345–15357. [Google Scholar] [CrossRef] [PubMed]
- Barreto, Í.M.L.G.; Rangel, A.H.D.N.; Urbano, S.A.; Bezerra, J.D.S.; Oliveira, C.A.D.A. Equine Milk and Its Potential Use in the Human Diet. Food Sci. Technol. 2019, 39, 1–7. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; García-Nebot, M.J.; Fernández-Tomé, S.; Amigo, L.; Recio, I. Dairy Protein Hydrolysates: Peptides for Health Benefits. Int. Dairy J. 2014, 38, 82–100. [Google Scholar] [CrossRef]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Córdoba, B. Invited Review: Fermented Milk as Antihypertensive Functional Food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef]
- Pérez-Escalante, E.; Jaimez-Ordaz, J.; Castañeda-Ovando, A.; Contreras-López, E.; Añorve-Morga, J.; González-Olivares, L.G. Antithrombotic Activity of Milk Protein Hydrolysates by Lactic Acid Bacteria Isolated from Commercial Fermented Milks. Braz. Arch. Biol. Technol. 2018, 61, e18180132. [Google Scholar] [CrossRef]
- Guha, S.; Sharma, H.; Deshwal, G.K.; Rao, P.S. A Comprehensive Review on Bioactive Peptides Derived from Milk and Milk Products of Minor Dairy Species. Food Prod. Process. Nutr. 2021, 3, 2. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Darewicz, M.; Iwaniak, A.; Borawska, J.; Bucholska, J.; Hrynkiewicz, M. Biologically Active Peptides from Food Proteins: In Silico, In Vitro and In Vivo Studies, Application Aspects, and Safety Evaluation. ŻNTJ 2015, 5, 5–22. [Google Scholar] [CrossRef]
- Chen, X.; Zheng, R.; Liu, R.; Li, L. Goat Milk Fermented by Lactic Acid Bacteria Modulates Small Intestinal Microbiota and Immune Responses. J. Funct. Foods 2020, 65, 103744. [Google Scholar] [CrossRef]
- Mehla, R. Bioactive Peptides in Fermented Milk Products and Their Functionality. J. Pharmacogn. Phytochem. 2020, 9, 2123–2126. [Google Scholar]
- Zanutto-Elgui, M.R.; Vieira, J.C.S.; Prado, D.Z.D.; Buzalaf, M.A.R.; Padilha, P.D.M.; Elgui De Oliveira, D.; Fleuri, L.F. Production of Milk Peptides with Antimicrobial and Antioxidant Properties through Fungal Proteases. Food Chem. 2019, 278, 823–831. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic Hydrolysis and Microbial Fermentation: The Most Favorable Biotechnological Methods for the Release of Bioactive Peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef]
- Ong, L.; Shah, N.P. Release and Identification of Angiotensin-Converting Enzyme-Inhibitory Peptides as Influenced by Ripening Temperatures and Probiotic Adjuncts in Cheddar Cheeses. LWT 2008, 41, 1555–1566. [Google Scholar] [CrossRef]
- Giacometti Cavalheiro, F.; Parra Baptista, D.; Domingues Galli, B.; Negrão, F.; Nogueira Eberlin, M.; Lúcia Gigante, M. High Protein Yogurt with Addition of Lactobacillus helveticus: Peptide Profile and Angiotensin-Converting Enzyme ACE-Inhibitory Activity. Food Chem. 2020, 333, 127482. [Google Scholar] [CrossRef] [PubMed]
- Ashokbhai, J.K.; Basaiawmoit, B.; Das, S.; Sakure, A.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Padhi, S.; Rai, A.K.; Liu, Z.; et al. Antioxidative, Antimicrobial and Anti-Inflammatory Activities and Release of Ultra-Filtered Antioxidative and Antimicrobial Peptides during Fermentation of Sheep Milk: In-Vitro, In-Silico and Molecular Interaction Studies. Food Biosci. 2022, 47, 101666. [Google Scholar] [CrossRef]
- Dharmisthaben, P.; Basaiawmoit, B.; Sakure, A.; Das, S.; Maurya, R.; Bishnoi, M.; Kondepudi, K.K.; Hati, S. Exploring Potentials of Antioxidative, Anti-Inflammatory Activities and Production of Bioactive Peptides in Lactic Fermented Camel Milk. Food Biosci. 2021, 44, 101404. [Google Scholar] [CrossRef]
- Cirrincione, S.; Luganini, A.; Lamberti, C.; Manfredi, M.; Cavallarin, L.; Giuffrida, M.G.; Pessione, E. Donkey Milk Fermentation by Lactococcus lactis subsp. cremoris and Lactobacillus rhamnosus Affects the Antiviral and Antibacterial Milk Properties. Molecules 2021, 26, 5100. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.D.H.; Liang, N.; Rathish, H.; Kim, B.J.; Lueangsakulthai, J.; Koh, J.; Qu, Y.; Schulz, H.J.; Dallas, D.C. Bioactive Milk Peptides: An Updated Comprehensive Overview and Database. Crit. Rev. Sci. Nutr. 2023, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ianni, F.; Altomare, A.A.; Cenci-Goga, B.T.; Blasi, F.; Grispoldi, L.; Regazzoni, L.; Cossignani, L. Chromatographic Characterization and In Vitro Bioactivity Evaluation of Lactobacillus helveticus Hydrolysates upon Fermentation of Different Substrates. Appl. Sci. 2021, 11, 811. [Google Scholar] [CrossRef]
- Sozańska, B. Raw Cow’s Milk and Its Protective Effect on Allergies and Asthma. Nutrients 2019, 11, 469. [Google Scholar] [CrossRef]
- Van Neerven, R.J.J.; Knol, E.F.; Heck, J.M.L.; Savelkoul, H.F.J. Which Factors in Raw Cow’s Milk Contribute to Protection against Allergies? J. Allergy Clin. Immunol. 2012, 130, 853–858. [Google Scholar] [CrossRef]
- Beaulieu, J.; Dupont, C.; Lemieux, P. Whey Proteins and Peptides: Beneficial Effects on Immune Health. Therapy 2006, 3, 69–78. [Google Scholar] [CrossRef]
- Naito, Y.; Marotta, F.; Kantah, M.K.; Zerbinati, N.; Kushugulova, A.; Zhumadilov, Z.; Illuzzi, N.; Sapienza, C.; Takadanohara, H.; Kobayashi, R.; et al. Gut-Targeted Immunonutrition Boosting Natural Killer Cell Activity Using Saccharomyces boulardii Lysates in Immuno-Compromised Healthy Elderly Subjects. Rejuvenation Res. 2014, 17, 184–187. [Google Scholar] [CrossRef]
- Juríková, T. Polyphenols and Their Mechanism of Action in Allergic Immune ResponseImmune Response. Glob. J. Allergy 2015, 1, 037–039. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Mutha, R.E.; Tatiya, A.U.; Surana, S.J. Flavonoids as Natural Phenolic Compounds and Their Role in Therapeutics: An Overview. Futur. J. Pharm. Sci. 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent Advances on the Roles of Flavonoids as Plant Protective Molecules after UV and High Light Exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Satchanska, G. Antibacterial Activity of Plant Polyphenols. In Secondary Metabolites—Trends and Reviews; Vijayakumar, R., Selvapuram Sudalaimuthu Raja, S., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Regolo, L.; Alvarez-Suarez, J.M.; Navarro-Hortal, M.D.; Xiao, J.; Quiles, J.L.; Battino, M.; Giampieri, F. The Reciprocal Interaction between Polyphenols and Other Dietary Compounds: Impact on Bioavailability, Antioxidant Capacity and Other Physico-Chemical and Nutritional Parameters. Food Chem. 2022, 375, 131904. [Google Scholar] [CrossRef]
- Yadav, L.; Singh, S.; Maurya, N.K. Polyphenols in Health and Diseases, 1st ed.; Dharshan Publishers: Tamil Nadu, India, 2022. [Google Scholar]
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Anti-Oxidative Action of Resveratrol: Implications for Human Health. Arab. J. Chem. 2011, 4, 293–298. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Higbee, J.; Solverson, P.; Zhu, M.; Carbonero, F. The Emerging Role of Dark Berry Polyphenols in Human Health and Nutrition. Food Front. 2022, 3, 3–27. [Google Scholar] [CrossRef]
- Zimmermann, C.; Wagner, A.E. Impact of Food-Derived Bioactive Compounds on Intestinal Immunity. Biomolecules 2021, 11, 1901. [Google Scholar] [CrossRef] [PubMed]
- Mężyńska, M.; Brzóska, M.M. Polyphenolic Compounds in Prevention and Treatmentof Selected Civilization Diseases—Evidences from Epidemiological Studies. Pol. Prz. Nauk Zdr. 2016, 3, 269–276. [Google Scholar]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is There Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Patanè, P.; Laganà, P.; Devi, P.; Vig, A.; Haddad, M.A.; Natalello, S.; Cava, M.A.; Ameen, S.M.; Hashim, H.A. Polyphenols and Functional Foods from the Regulatory Viewpoint. J. AOAC Int. 2019, 102, 1373–1377. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Scientific Opinion on the Safety of Green Tea Catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
- Martin, K.R.; Appel, C.L. Polyphenols as Dietary Supplements: A Double-Edged Sword. Nutr. Diet. Suppl. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of Aronia Berry (Poly)Phenols on Vascular Function and Gut Microbiota: A Double-Blind Randomized Controlled Trial in Adult Men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Pap, N.; Fidelis, M.; Azevedo, L.; Do Carmo, M.A.V.; Wang, D.; Mocan, A.; Pereira, E.P.R.; Xavier-Santos, D.; Sant’Ana, A.S.; Yang, B.; et al. Berry Polyphenols and Human Health: Evidence of Antioxidant, Anti-Inflammatory, Microbiota Modulation, and Cell-Protecting Effects. Curr. Opin. Food Sci. 2021, 42, 167–186. [Google Scholar] [CrossRef]
- Habza-Kowalska, E.; Kaczor, A.A.; Żuk, J.; Matosiuk, D.; Gawlik-Dziki, U. Thyroid Peroxidase Activity Is Inhibited by Phenolic Compounds—Impact of Interaction. Molecules 2019, 24, 2766. [Google Scholar] [CrossRef] [PubMed]
- Espina, A.; Cañamares, M.V.; Jurašeková, Z.; Sanchez-Cortes, S. Analysis of Iron Complexes of Tannic Acid and Other Related Polyphenols as Revealed by Spectroscopic Techniques: Implications in the Identification and Characterization of Iron Gall Inks in Historical Manuscripts. ACS Omega 2022, 7, 27937–27949. [Google Scholar] [CrossRef] [PubMed]
- Białek, M.; Rutkowska, J.; Hallmann, E. Aronia Czarnoowocowa (Aronia melanocarpa) Jako Potencjalny Składnik Żywności Funkcjonalnej. ŻNTJ 2012, 6, 21–30. [Google Scholar]
- Sójka, M.; Kołodziejczyk, K.; Milala, J. Polyphenolic and Basic Chemical Composition of Black Chokeberry Industrial By-Products. Ind. Crops Prod. 2013, 51, 77–86. [Google Scholar] [CrossRef]
- Ciska, E.; Karamać, M.; Kosinska, A. Antioxidant Activity of Extracts of White Cabbage and Sauerkraut. Pol. J. Food Nutr. Sci. 2005, 55, 367–373. [Google Scholar]
- Płatosz, N.; Sawicki, T.; Wiczkowski, W. Profile of Phenolic Acids and Flavonoids of Red Beet and Its Fermentation Products. Does Long-Term Consumption of Fermented Beetroot Juice Affect Phenolics Profile in Human Blood Plasma and Urine? Pol. J. Food Nutr. Sci. 2020, 70, 55–65. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Leite, I.B.; Martins, A.B.N.; Fialho, E.; Tomás-Barberán, F.A.; Perrone, D.; Monteiro, M. Jaboticaba Berry: A Comprehensive Review on Its Polyphenol Composition, Health Effects, Metabolism, and the Development of Food Products. Food Res. Int. 2021, 147, 110518. [Google Scholar] [CrossRef]
- Lenquiste, S.A.; De Almeida Lamas, C.; Da Silva Marineli, R.; Moraes, É.A.; Borck, P.C.; Camargo, R.L.; Quitete, V.H.A.C.; Carneiro, E.M.; Junior, M.R.M. Jaboticaba Peel Powder and Jaboticaba Peel Aqueous Extract Reduces Obesity, Insulin Resistance and Hepatic Fat Accumulation in Rats. Food Res. Int. 2019, 120, 880–887. [Google Scholar] [CrossRef]
- Hryhorenko, N.; Krupa-Kozak, U.; Bączek, N.; Rudnicka, B.; Wróblewska, B. Gluten-Free Bread Enriched with Whole-Grain Red Sorghum Flour Gains Favourable Technological and Functional Properties and Consumers Acceptance. J. Cereal Sci. 2023, 110, 103646. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Feo, V.; Battistelli, A.; Da Cruz, A.G.; Coppola, R. Polyphenols, the New Frontiers of Prebiotics. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 94, pp. 35–89. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols—Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. In Vivo Formed Metabolites of Polyphenols and Their Biological Efficacy. Food Funct. 2019, 10, 6999–7021. [Google Scholar] [CrossRef] [PubMed]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic Effect of Dietary Polyphenols: A Systematic Review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, H.; He, S.; Lou, Q.; Yu, M.; Tang, M.; Tu, L. Metabolism and Prebiotics Activity of Anthocyanins from Black Rice (Oryza sativa L.) In Vitro. PLoS ONE 2018, 13, 0195754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Wu, Z.; Weng, P. The Modulatory Effect of Anthocyanins from Purple Sweet Potato on Human Intestinal Microbiota In Vitro. J. Agric. Food Chem. 2016, 64, 2582–2590. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Mazcorro, J.F.; Lage, N.N.; Mertens-Talcott, S.; Talcott, S.; Chew, B.; Dowd, S.E.; Kawas, J.R.; Noratto, G.D. Effect of Dark Sweet Cherry Powder Consumption on the Gut Microbiota, Short-Chain Fatty Acids, and Biomarkers of Gut Health in Obese Db/Db Mice. PeerJ 2018, 6, 4195. [Google Scholar] [CrossRef] [PubMed]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A Polyphenol-Rich Cranberry Extract Protects from Diet-Induced Obesity, Insulin Resistance and Intestinal Inflammation in Association with Increased Akkermansia spp. Population in the Gut Microbiota of Mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic Evaluation of Cocoa-Derived Flavanols in Healthy Humans by Using a Randomized, Controlled, Double-Blind, Crossover Intervention Study. AJCN 2011, 93, 62–72. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry Polyphenols Extract as a Potential Prebiotic with Anti-Obesity Effects on C57BL/6 J Mice by Modulating the Gut Microbiota. JNB 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Rodríguez-Costa, S.; Cardelle-Cobas, A.; Roca-Saavedra, P.; Porto-Arias, J.J.; Miranda, J.M.; Cepeda, A. In Vitro Evaluation of the Prebiotic Effect of Red and White Grape Polyphenolic Extracts. J. Physiol. Biochem. 2018, 74, 101–110. [Google Scholar] [CrossRef]
- Dolara, P.; Luceri, C.; Filippo, C.D.; Femia, A.P.; Giovannelli, L.; Caderni, G.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Red Wine Polyphenols Influence Carcinogenesis, Intestinal Microflora, Oxidative Damage and Gene Expression Profiles of Colonic Mucosa in F344 Rats. Mut. Res.–Fundam. Mol. 2005, 591, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary Protein-Phenolic Interactions: Characterization, Biochemical-Physiological Consequences, and Potential Food Applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 3589–3615. [Google Scholar] [CrossRef]
- Li, Y.; He, D.; Li, B.; Lund, M.N.; Xing, Y.; Wang, Y.; Li, F.; Cao, X.; Liu, Y.; Chen, X.; et al. Engineering Polyphenols with Biological Functions via Polyphenol-Protein Interactions as Additives for Functional Foods. Trends Food Sci. Technol. 2021, 110, 470–482. [Google Scholar] [CrossRef]
- Czubinski, J.; Dwiecki, K. A Review of Methods Used for Investigation of Protein–Phenolic Compound Interactions. Int. J. Food. Sci. Technol. 2017, 52, 573–585. [Google Scholar] [CrossRef]
- Rohn, S. Possibilities and Limitations in the Analysis of Covalent Interactions between Phenolic Compounds and Proteins. Food Res. Int. 2014, 65, 13–19. [Google Scholar] [CrossRef]
- Mejbaum-Katzenellenbogen, W. Insoluble Protein-Tannin Compounds. Acta Biochim. Pol. 1959, 6, 385. [Google Scholar]
- O’Connell, J.E.; Fox, P.F. Significance and Applications of Phenolic Compounds in the Production and Quality of Milk and Dairy Products: A Review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between Polyphenols and Macromolecules: Quantification Methods and Mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Yildirim-Elikoglu, S.; Erdem, Y.K. Interactions between Milk Proteins and Polyphenols: Binding Mechanisms, Related Changes, and the Future Trends in the Dairy Industry. Food Rev. Int. 2018, 34, 665–697. [Google Scholar] [CrossRef]
- Van De Langerijt, T.M.; O’Mahony, J.A.; Crowley, S.V. Structural, Binding and Functional Properties of Milk Protein-Polyphenol Systems: A Review. Molecules 2023, 28, 2288. [Google Scholar] [CrossRef]
- Wu, X.; Lu, Y.; Xu, H.; Lin, D.; He, Z.; Wu, H.; Liu, L.; Wang, Z. Reducing the Allergenic Capacity of β-Lactoglobulin by Covalent Conjugation with Dietary Polyphenols. Food Chem. 2018, 256, 427–434. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Chen, R.; Wang, Y.; Miao, S.; Li, Z.; Wang, S.; Fu, L. Covalent and Non-Covalent Interactions of Cyanidin-3- O-Glucoside with Milk Proteins Revealed Modifications in Protein Conformational Structures, Digestibility, and Allergenic Characteristics. Food Funct. 2021, 12, 10107–10120. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.; Tomas, M.; et al. Functional Implications of Bound Phenolic Compounds and Phenolics–Food Interaction: A Review. Comp. Rev. Food Sci. Food Safe 2022, 21, 811–842. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Zeng, Z.; McClements, D.J.; Gong, X.; Yu, P.; Xia, J.; Gong, D. A Review of the Structure, Function, and Application of Plant-based Protein–Phenolic Conjugates and Complexes. Comp. Rev. Food Sci. Food Safe 2023, 22, 1312–1336. [Google Scholar] [CrossRef] [PubMed]
- Chima, B.; Mathews, P.; Morgan, S.; Johnson, S.A.; Van Buiten, C.B. Physicochemical Characterization of Interactions between Blueberry Polyphenols and Food Proteins from Dairy and Plant Sources. Foods 2022, 11, 2846. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chang, Y.; Britten, M.; St-Gelais, D.; Champagne, C.P.; Fustier, P.; Lacroix, M. Interactions of Phenolic Compounds with Milk Proteins. Eur. Food Res. Technol. 2019, 245, 1881–1888. [Google Scholar] [CrossRef]
- Pandey, P.; Grover, K.; Dhillon, T.S.; Kaur, A.; Javed, M. Evaluation of Polyphenols Enriched Dairy Products Developed by Incorporating Black Carrot (Daucus carota L.) Concentrate. Heliyon 2021, 7, e06880. [Google Scholar] [CrossRef]
- Zygmantaitė, G.; Keršienė, M.; Jasutienė, I.; Šipailienė, A.; Venskutonis, P.R.; Leskauskaitė, D. Extract Isolated from Cranberry Pomace as Functional Ingredient in Yoghurt Production: Technological Properties and Digestibility Studies. LWT 2021, 148, 111751. [Google Scholar] [CrossRef]
- Kosińska, A.; Karamać, M.; Penkacik, K.; Urbalewicz, A.; Amarowicz, R. Interactions between Tannins and Proteins Isolated from Broad Bean Seeds (Vicia Faba Major) Yield Soluble and Non-Soluble Complexes. Eur. Food Res. Technol. 2011, 233, 213–222. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, T.; Xu, Z.; Jin, L.; McAllister, T.A.; Acharya, S.; Zeller, W.E.; Mueller-Harvey, I.; Wang, Y. Composition and Protein Precipitation Capacity of Condensed Tannins in Purple Prairie Clover (Dalea purpurea Vent.). Front. Plant Sci. 2021, 12, 715282. [Google Scholar] [CrossRef]
- Sánchez-Bravo, P.; Zapata, P.; Martínez-Esplá, A.; Carbonell-Barrachina, Á.; Sendra, E. Antioxidant and Anthocyanin Content in Fermented Milks with Sweet Cherry Is Affected by the Starter Culture and the Ripening Stage of the Cherry. Beverages 2018, 4, 57. [Google Scholar] [CrossRef]
- Szajnar, K.; Pawlos, M.; Znamirowska, A. The Effect of the Addition of Chokeberry Fiber on the Quality of Sheep’s Milk Fermented by Lactobacillus rhamnosus and Lactobacillus acidophilus. Int. J. Food Sci. 2021, 2021, 7928745. [Google Scholar] [CrossRef] [PubMed]
- Lila, M.A.; Schneider, M.; Devlin, A.; Plundrich, N.; Laster, S.; Foegeding, E.A. Polyphenol-Enriched Berry Extracts Naturally Modulate Reactive Proteins in Model Foods. Food Funct. 2017, 8, 4760–4767. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.M.; Santos, L. Incorporation of Phenolic Extracts from Different By-Products in Yoghurts to Create Fortified and Sustainable Foods. Food Biosci. 2023, 51, 102293. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, T.; Lu, Y.; Lin, X.; Hu, X.; Liu, L.; He, Z.; Wu, X. Effect of Chlorogenic Acid Covalent Conjugation on the Allergenicity, Digestibility and Functional Properties of Whey Protein. Food Chem. 2019, 298, 125024. [Google Scholar] [CrossRef] [PubMed]
- Osman, D.; Noureldin, H.; El-Gazzar, F.; Salman, K. Fortification of Ice Milk with Purslane (Portulaca oleracea) Bioactive Compounds. AJAS 2023, 54, 34–49. [Google Scholar] [CrossRef]
- Sahingil, D.; Hayaloglu, A.A. Enrichment of Antioxidant Activity, Phenolic Compounds, Volatile Composition and Sensory Properties of Yogurt with Rosehip (Rosa canina L.) Fortification. Int. J. Gastron. Food Sci. 2022, 28, 100514. [Google Scholar] [CrossRef]
- Ahmed, S.; Noor, A.; Tariq, M.; Zaidi, A. Functional Improvement of Synbiotic Yogurt Enriched with Lacticaseibacillus rhamnosus and Aloe Vera Gel Using the Response Surface Method. Food Prod. Process. Nutr. 2023, 5, 38. [Google Scholar] [CrossRef]
- Theocharidou, A.; Psomas, E.; Koliouskas, A.; Ritzoulis, C. Yogurt Products Fortified with Microwave-Extracted Peach Polyphenols. Gels 2023, 9, 266. [Google Scholar] [CrossRef]
- Wicks, C.J.; Bolling, B.W.; Hartel, R.W. Effects of Tannic Acid on Proteins and Fat in Cream. Food Prod. Process. Nutr. 2023, 5, 51. [Google Scholar] [CrossRef]
- Kandyliari, A.; Potsaki, P.; Bousdouni, P.; Kaloteraki, C.; Christofilea, M.; Almpounioti, K.; Moutsou, A.; Fasoulis, C.K.; Polychronis, L.V.; Gkalpinos, V.K.; et al. Development of Dairy Products Fortified with Plant Extracts: Antioxidant and Phenolic Content Characterization. Antioxidants 2023, 12, 500. [Google Scholar] [CrossRef]
- He, W.; Xu, H.; Lu, Y.; Zhang, T.; Li, S.; Lin, X.; Xu, B.; Wu, X. Function, Digestibility and Allergenicity Assessment of Ovalbumin–EGCG Conjugates. J. Funct. Foods 2019, 61, 103490. [Google Scholar] [CrossRef]
- Bansode, R.R.; Randolph, P.D.; Plundrich, N.J.; Lila, M.A.; Williams, L.L. Peanut Protein-Polyphenol Aggregate Complexation Suppresses Allergic Sensitization to Peanut by Reducing Peanut-Specific IgE in C3H/HeJ Mice. Food Chem. 2019, 299, 125025. [Google Scholar] [CrossRef]
- Jolji, M.; Pecsenye, B.; Mposula, Z.; Aleya, A.; Kiss, T.; Mathé, E. Development and Comparative Analysis of Protein-Polyphenol-Fibre Bars as Nutritional Supplements Suitable for Healthy Senior Consumers. Acta Univ. Sapientiae Aliment. 2023, 16, 103–125. [Google Scholar] [CrossRef]
- Diaz, J.T.; Foegeding, E.A.; Lila, M.A. Whey Protein-Polyphenol Aggregate Particles Mitigate Bar Hardening Reactions in High Protein Bars. LWT 2021, 138, 110747. [Google Scholar] [CrossRef]
- Geng, S.; Li, Q.; Zhou, X.; Zheng, J.; Liu, H.; Zeng, J.; Yang, R.; Fu, H.; Hao, F.; Feng, Q.; et al. Gut Commensal E. coli Outer Membrane Proteins Activate the Host Food Digestive System through Neural-Immune Communication. Cell Host Microbe 2022, 30, 1401–1416.e8. [Google Scholar] [CrossRef] [PubMed]
- Mulet-Cabero, A.-I.; Mackie, A.R.; Brodkorb, A.; Wilde, P.J. Dairy Structures and Physiological Responses: A Matter of Gastric Digestion. Crit. Rev. Food Sci. Nutr. 2020, 60, 3737–3752. [Google Scholar] [CrossRef]
- Ye, A.; Roy, D.; Singh, H. Structural Changes to Milk Protein Products during Gastrointestinal Digestion. In Milk Proteins, 3rd ed.; Boland, M., Singh, H., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2020; pp. 671–700. [Google Scholar] [CrossRef]
- Horne, D.S.; Lucey, J.A. Revisiting the Temperature Dependence of the Coagulation of Renneted Bovine Casein Micelles. Food Hydrocoll. 2014, 42, 75–80. [Google Scholar] [CrossRef]
- Roy, D.; Ye, A.; Moughan, P.J.; Singh, H. Composition, Structure, and Digestive Dynamics of Milk from Different Species—A Review. Front. Nutr. 2020, 7, 577759. [Google Scholar] [CrossRef]
- Ye, A.; Cui, J.; Dalgleish, D.; Singh, H. Formation of a Structured Clot during the Gastric Digestion of Milk: Impact on the Rate of Protein Hydrolysis. Food Hydrocoll. 2016, 52, 478–486. [Google Scholar] [CrossRef]
- Wang, X.; Ye, A.; Lin, Q.; Han, J.; Singh, H. Gastric Digestion of Milk Protein Ingredients: Study Using an In Vitro Dynamic Model. J. Dairy Sci. 2018, 101, 6842–6852. [Google Scholar] [CrossRef]
- Lacroix, M.; Bos, C.; Léonil, J.; Airinei, G.; Luengo, C.; Daré, S.; Benamouzig, R.; Fouillet, H.; Fauquant, J.; Tomé, D.; et al. Compared with Casein or Total Milk Protein, Digestion of Milk Soluble Proteins Is Too Rapid to Sustain the Anabolic Postprandial Amino Acid Requirement. Am. J. Clin. Nutr. 2006, 84, 1070–1079. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Torcello-Gómez, A.; Saha, S.; Mackie, A.R.; Wilde, P.J.; Brodkorb, A. Impact of Caseins and Whey Proteins Ratio and Lipid Content on In Vitro Digestion and Ex Vivo Absorption. Food Chem. 2020, 319, 126514. [Google Scholar] [CrossRef]
- Yang, M.; Ye, A.; Yang, Z.; Everett, D.W.; Gilbert, E.P.; Singh, H. Kinetics of Pepsin-Induced Hydrolysis and the Coagulation of Milk Proteins. J. Dairy Sci. 2022, 105, 990–1003. [Google Scholar] [CrossRef]
- Hooton, D.; Lentle, R.; Monro, J.; Wickham, M.; Simpson, R. The Secretion and Action of Brush Border Enzymes in the Mammalian Small Intestine. In Reviews of Physiology, Biochemistry and Pharmacology; Nilius, B., Gudermann, T., Jahn, R., Lill, R., Petersen, O.H., De Tombe, P.P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; Volume 168, pp. 59–118. [Google Scholar] [CrossRef]
- Stevens, B.R. Amino Acid Transport by Epithelial Membranes. In Epithelial Transport Physiology; Gerencser, G.A., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 353–378. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Stevens, B.R.; Moughan, P.J. Are Intact Peptides Absorbed from the Healthy Gut in the Adult Human? Nutr. Res. Rev. 2014, 27, 308–329. [Google Scholar] [CrossRef]
- Sélo, I.; Clément, G.; Bernard, H.; Chatel, J.; Créminon, C.; Peltre, G.; Wal, J. Allergy to Bovine β-Lactoglobulin: Specificity of Human IgE to Tryptic Peptides: Bovine β-Lactoglobulin Allergy. Clin. Exp. Allergy 1999, 29, 1055–1063. [Google Scholar] [CrossRef]
- Exl, B.-M. A Review of Recent Developments in the Use of Moderately Hydrolyzed Whey Formulae in Infant Nutrition. Nutr. Res. 2001, 21, 355–379. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, Y.; Liu, J.; Tu, Z. Investigation into Predominant Peptide and Potential Allergenicity of Ultrasonicated β-Lactoglobulin Digestion Products. Food Chem. 2021, 361, 130099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, F.; Zheng, Y.; Xue, Y.; Kang, Z.; Zulewska, J.; Li, H.; Li, H.; Yu, J. Relationship between Antigenicity and Allergenic Epitopes of Extensive Hydrolyzed Whey Protein: LC-MS/MS-Based Analysis. Food Biosci. 2023, 54, 102870. [Google Scholar] [CrossRef]
- Benedé, S.; López-Expósito, I.; Giménez, G.; Grishina, G.; Bardina, L.; Sampson, H.A.; López-Fandiño, R.; Molina, E. Mapping of IgE Epitopes in In Vitro Gastroduodenal Digests of β-Lactoglobulin Produced with Human and Simulated Fluids. Food Res. Int. 2014, 62, 1127–1133. [Google Scholar] [CrossRef]
- Zarzosa-Moreno, D.; Avalos-Gómez, C.; Ramírez-Texcalco, L.S.; Torres-López, E.; Ramírez-Mondragón, R.; Hernández-Ramírez, J.O.; Serrano-Luna, J.; De La Garza, M. Lactoferrin and Its Derived Peptides: An Alternative for Combating Virulence Mechanisms Developed by Pathogens. Molecules 2020, 25, 5763. [Google Scholar] [CrossRef]
- Gifford, J.L.; Hunter, H.N.; Vogel, H.J. Lactoferricin: Lactoferricin: A Lactoferrin-Derived Peptide with Antimicrobial, Antiviral, Antitumor and Immunological Properties. Cell. Mol. Life Sci. 2005, 62, 2588–2598. [Google Scholar] [CrossRef]
- Van Der Kraan, M.I.A.; Groenink, J.; Nazmi, K.; Veerman, E.C.I.; Bolscher, J.G.M.; Nieuw Amerongen, A.V. Lactoferrampin: A Novel Antimicrobial Peptide in the N1-Domain of Bovine Lactoferrin. Peptides 2004, 25, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Kim, B.J.; Dallas, D.C. Bioavailability of Peptides Derived from the In Vitro Digestion of Human Milk Assessed by Caco-2 Cell Monolayers. J. Agric. Food Chem. 2022, 70, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.W.; Nam, M.S. Bioactive Peptides in Milk and Dairy Products: A Review. Korean J. Food Sci. Anim. Resour. 2015, 35, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Samtiya, M.; Samtiya, S.; Badgujar, P.C.; Puniya, A.K.; Dhewa, T.; Aluko, R.E. Health-Promoting and Therapeutic Attributes of Milk-Derived Bioactive Peptides. Nutrients 2022, 14, 3001. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, M.J.; Ezzatpanah, H.; Ghahderijani, M. In Vitro and In Silico Studies for the Identification of Anti-Cancer and Antibacterial Peptides from Camel Milk Protein Hydrolysates. PLoS ONE 2023, 18, e0288260. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.R.; Ahmed, A.S.; Miyata, T. Novel Angiotensin-Converting Enzyme Inhibitory Peptides from Caseins and Whey Proteins of Goat Milk. J. Adv. Res. 2017, 8, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W.; Jiang, L.; Du, M. Identification of a Novel ACE-Inhibitory Peptide from Casein and Evaluation of the Inhibitory Mechanisms. Food Chem. 2018, 256, 98–104. [Google Scholar] [CrossRef]
- Jia, C.; Hussain, N.; Joy Ujiroghene, O.; Pang, X.; Zhang, S.; Lu, J.; Liu, L.; Lv, J. Generation and Characterization of Dipeptidyl Peptidase-IV Inhibitory Peptides from Trypsin-Hydrolyzed α-Lactalbumin-Rich Whey Proteins. Food Chem. 2020, 318, 126333. [Google Scholar] [CrossRef]
- Lingua, M.S.; Wunderlin, D.A.; Baroni, M.V. Effect of Simulated Digestion on the Phenolic Components of Red Grapes and Their Corresponding Wines. J. Funct. Foods 2018, 44, 86–94. [Google Scholar] [CrossRef]
- Eran Nagar, E.; Okun, Z.; Shpigelman, A. Digestive Fate of Polyphenols: Updated View of the Influence of Chemical Structure and the Presence of Cell Wall Material. Curr. Opin. Food Sci. 2020, 31, 38–46. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic Compounds: Their Journey After Intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Ray, S.K.; Mukherjee, S. Evolving Interplay Between Dietary Polyphenols and Gut Microbiota—An Emerging Importance in Healthcare. Front. Nutr. 2021, 8, 634944. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Ifie, I.; Gonzalez-Aguilar, G.A.; Roopchand, D.E. The Gastrointestinal Tract as Prime Site for Cardiometabolic Protection by Dietary Polyphenols. Adv. Nutr. 2019, 10, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Lafay, S.; Morand, C.; Manach, C.; Besson, C.; Scalbert, A. Absorption and Metabolism of Caffeic Acid and Chlorogenic Acid in the Small Intestine of Rats. Br. J. Nutr. 2006, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Den Hartogh, D.J.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the Stability of Plant Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 2101–2110. [Google Scholar] [CrossRef]
- Qin, W.; Ketnawa, S.; Ogawa, Y. Effect of Digestive Enzymes and pH on Variation of Bioavailability of Green Tea during Simulated In Vitro Gastrointestinal Digestion. Food Sci. Hum. Wellness 2022, 11, 669–675. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A. Structure-Activity Relationships Analysis of Monomeric and Polymeric Polyphenols (Quercetin, Rutin and Catechin) Obtained by Various Polymerization Methods. Chem. Biodivers. 2019, 16, e1900426. [Google Scholar] [CrossRef]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary Flavonoid and Isoflavone Glycosides Are Hydrolysed by the Lactase Site of Lactase Phlorizin Hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid Metabolism: The Interaction of Metabolites and Gut Microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Villa-Rodriguez, J.A.; Montiel-Herrera, M.; Pacheco-Ordaz, R.; Roopchand, D.E.; Venema, K.; González-Aguilar, G.A. Phenolic Compounds Promote Diversity of Gut Microbiota and Maintain Colonic Health. Dig. Dis. Sci. 2021, 66, 3270–3289. [Google Scholar] [CrossRef]
- Santhakumar, A.B.; Battino, M.; Alvarez-Suarez, J.M. Dietary Polyphenols: Structures, Bioavailability and Protective Effects against Atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Cremonini, E.; Fraga, C.G. Anthocyanin Actions at the Gastrointestinal Tract: Relevance to Their Health Benefits. Mol. Asp. Med. 2023, 89, 101156. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Cheung, F.; Hong, M.; Feng, Y. The Potential and Action Mechanism of Polyphenols in the Treatment of Liver Diseases. Oxid. Med. Cell. Longev. 2018, 2018, 8394818. [Google Scholar] [CrossRef]
- Li, S.; Yin, S.; Ding, H.; Shao, Y.; Zhou, S.; Pu, W.; Han, L.; Wang, T.; Yu, H. Polyphenols as Potential Metabolism Mechanisms Regulators in Liver Protection and Liver Cancer Prevention. Cell Prolif. 2023, 56, e13346. [Google Scholar] [CrossRef]
- Van Duynhoven, J.; Vaughan, E.E.; Jacobs, D.M.; Kemperman, R.A.; Van Velzen, E.J.J.; Gross, G.; Roger, L.C.; Possemiers, S.; Smilde, A.K.; Doré, J.; et al. Metabolic Fate of Polyphenols in the Human Superorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 4531–4538. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Catalkaya, G.; Venema, K.; Lucini, L.; Rocchetti, G.; Delmas, D.; Daglia, M.; De Filippis, A.; Xiao, H.; Quiles, J.L.; Xiao, J.; et al. Interaction of Dietary Polyphenols and Gut Microbiota: Microbial Metabolism of Polyphenols, Influence on the Gut Microbiota, and Implications on Host Health. Food Front. 2020, 1, 109–133. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Wang, J.; Chai, Z.; Zhang, X.; Wang, J.; Wang, N.; Huang, W. Impact of In Vitro Gastrointestinal Digestion on Rabbiteye Blueberry Anthocyanins and Their Absorption Efficiency in Caco-2 Cells. Food Biosci. 2023, 52, 102424. [Google Scholar] [CrossRef]
- Pan, F.; Li, J.; Zhao, L.; Tuersuntuoheti, T.; Mehmood, A.; Zhou, N.; Hao, S.; Wang, C.; Guo, Y.; Lin, W. A Molecular Docking and Molecular Dynamics Simulation Study on the Interaction between Cyanidin-3-O-glucoside and Major Proteins in Cow’s Milk. J. Food Biochem. 2021, 45, e13570. [Google Scholar] [CrossRef] [PubMed]
- Baruah, I.; Kashyap, C.; Guha, A.K.; Borgohain, G. Insights into the Interaction between Polyphenols and β-Lactoglobulin through Molecular Docking, MD Simulation, and QM/MM Approaches. ACS Omega 2022, 7, 23083–23095. [Google Scholar] [CrossRef]
- Li, X.; Dai, T.; Hu, P.; Zhang, C.; Chen, J.; Liu, C.; Li, T. Characterization the Non-Covalent Interactions between Beta Lactoglobulin and Selected Phenolic Acids. Food Hydrocoll. 2020, 105, 105761. [Google Scholar] [CrossRef]
- Xu, L.; Li, W.; Chen, Z.; Guo, Q.; Wang, C.; Santhanam, R.K.; Chen, H. Inhibitory Effect of Epigallocatechin-3-O-Gallate on α-Glucosidase and Its Hypoglycemic Effect via Targeting PI3K/AKT Signaling Pathway in L6 Skeletal Muscle Cells. Int. J. Biol. Macromol. 2019, 125, 605–611. [Google Scholar] [CrossRef]
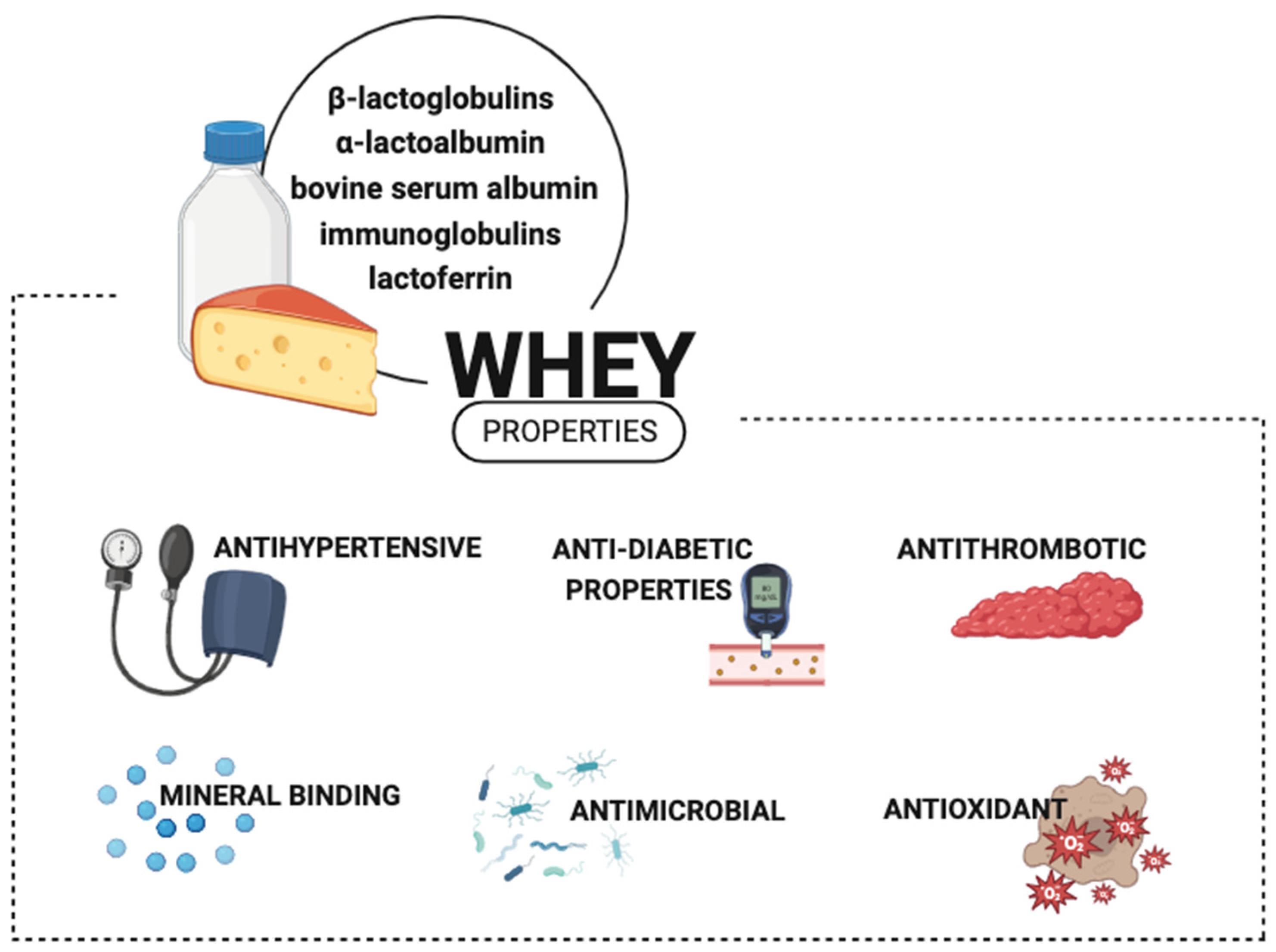
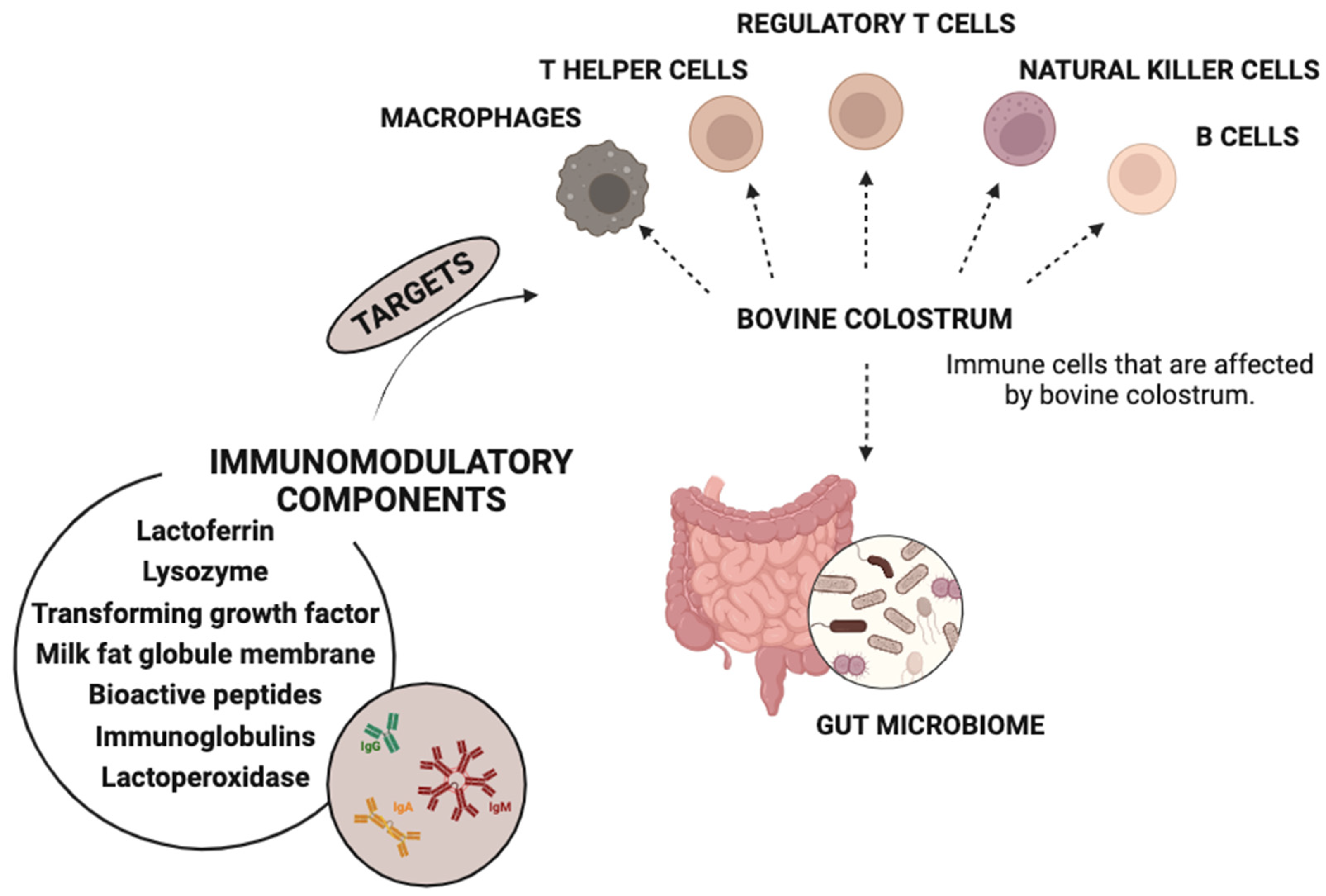

| Source | Properties | Reference |
|---|---|---|
| MILK | ||
| Raw cow’s milk |
| [94,95] |
| Mare’s milk |
| [73,75] |
| Fermented Milk Beverages | ||
| Camel milk |
| [90] |
| Donkey milk |
| [91] |
| Sheep milk |
| [89] |
| Yogurt |
| [49,50] |
| Kefir |
| [51,54] |
| Whey |
| [63,96] |
| Buttermilk |
| [70] |
| Kumis |
| [77] |
| Colostrum |
| [16,97] |
| Source | Properties | Type of Research | Reference |
|---|---|---|---|
| Anthocyanins | |||
| Black rice | Bifidobacterium bifidum ↑ Bifidobacterium adolescentis ↑ Bifidobacterium infantis ↑ Lactobacillus acidophilus ↑ | In vitro | [131] |
| Purple sweet potato | Bifidobacterium spp. ↑ Lactobacillus/Enterococcus spp.↑ Bacteroides-Prevotella ↓ Clostridium histolyticum ↓ | In vitro | [132] |
| Dark sweet cherry powder | Akkermansia ↑ | In vivo | [133] |
| Cranberry extract | Akkermansia spp. ↑ Reduced triglycerides ↓ Reduced oxidative stress ↓ Reduced intestinal inflammation ↓ | In vivo | [134] |
| Flavonols | |||
| Beverage with a high content of cocoa flavonols | Lactobacillus spp. ↑ Bifidobacterium spp. ↑ | Human study | [135] |
| Cocoa extract | Lactobacillus spp. ↑ Bifidobacterium spp. ↑ | In vitro | [135] |
| Other Polyphenols | |||
| Blueberry extract * | Inhibited weight gain Restored normal lipid metabolism Modulation of the composition of the intestinal microbiota | In vivo | [136] |
| Red and white grapes * | Lactobacillus spp. ↑ Bifidobacterium spp. ↑ | In vitro | [137] |
| Non-alcoholic extract of red wine ** | Lactobacillus spp. ↑ Bifidobacterium spp. ↑ Clostridium spp. ↓ Propionibacterium spp. ↓ Bacteroides spp. ↓ | In vivo | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewska, B.; Kuliga, A.; Wnorowska, K. Bioactive Dairy-Fermented Products and Phenolic Compounds: Together or Apart. Molecules 2023, 28, 8081. https://doi.org/10.3390/molecules28248081
Wróblewska B, Kuliga A, Wnorowska K. Bioactive Dairy-Fermented Products and Phenolic Compounds: Together or Apart. Molecules. 2023; 28(24):8081. https://doi.org/10.3390/molecules28248081
Chicago/Turabian StyleWróblewska, Barbara, Aleksandra Kuliga, and Kinga Wnorowska. 2023. "Bioactive Dairy-Fermented Products and Phenolic Compounds: Together or Apart" Molecules 28, no. 24: 8081. https://doi.org/10.3390/molecules28248081
APA StyleWróblewska, B., Kuliga, A., & Wnorowska, K. (2023). Bioactive Dairy-Fermented Products and Phenolic Compounds: Together or Apart. Molecules, 28(24), 8081. https://doi.org/10.3390/molecules28248081







