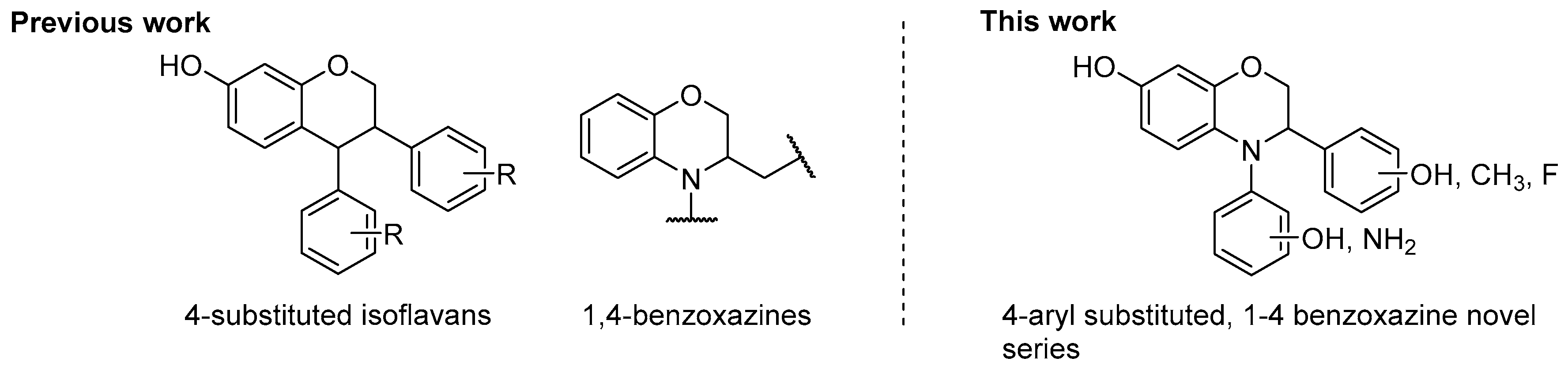
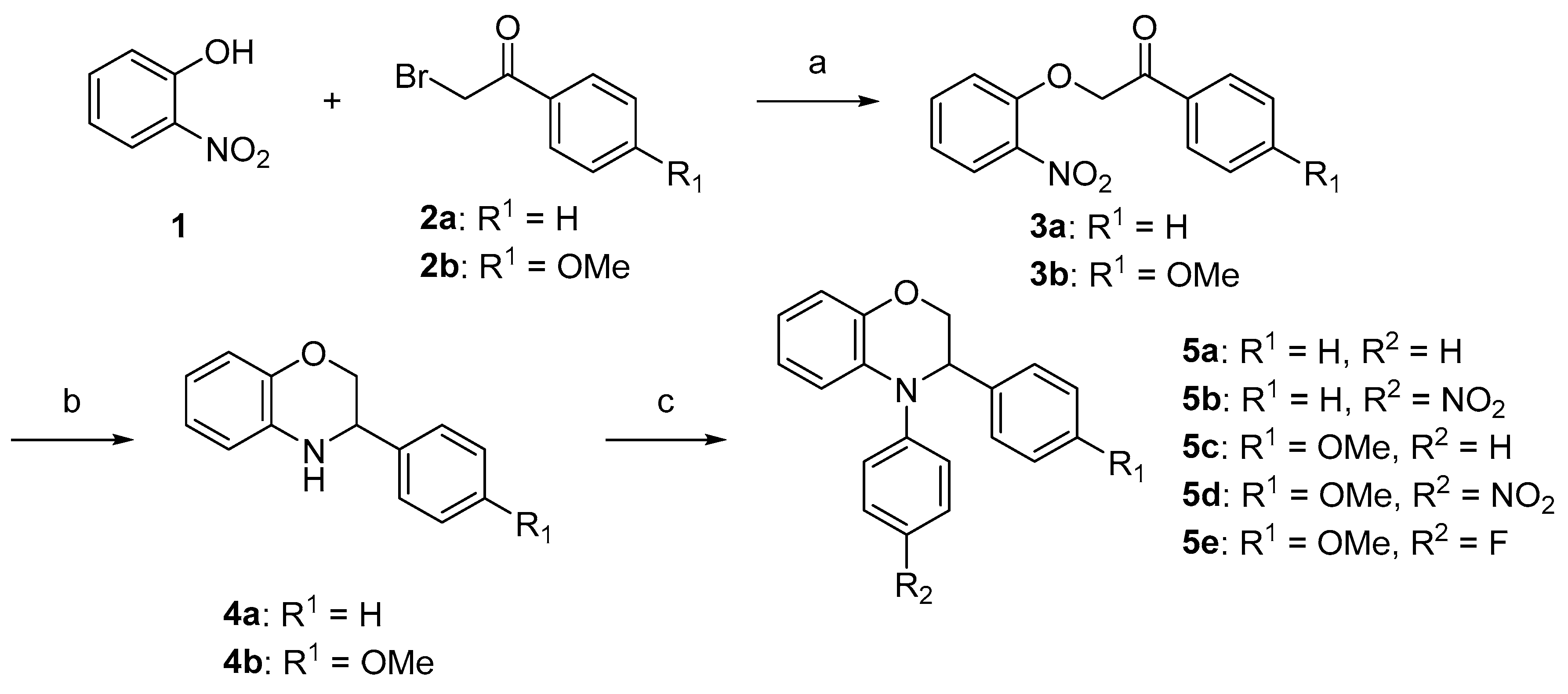
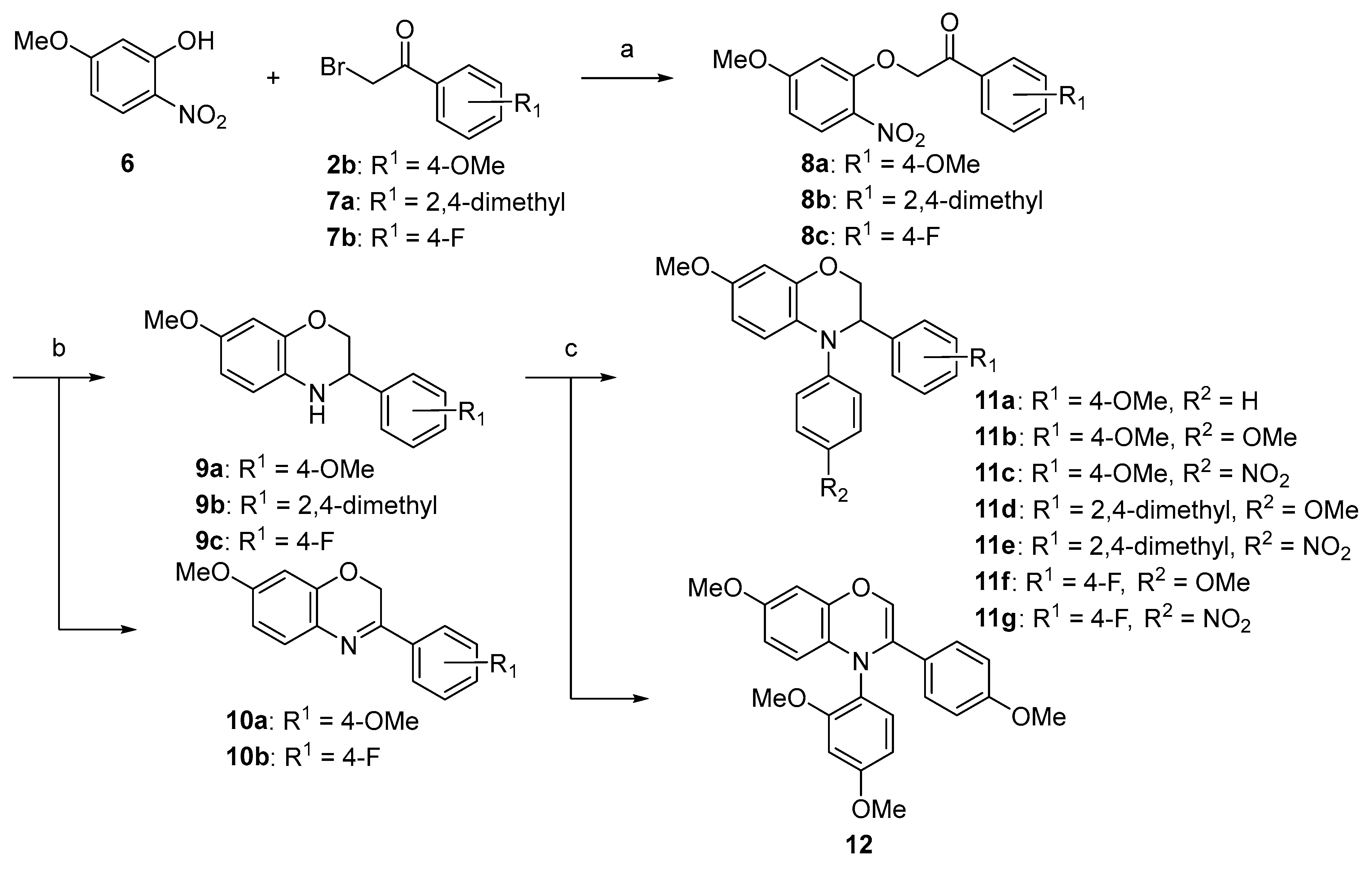
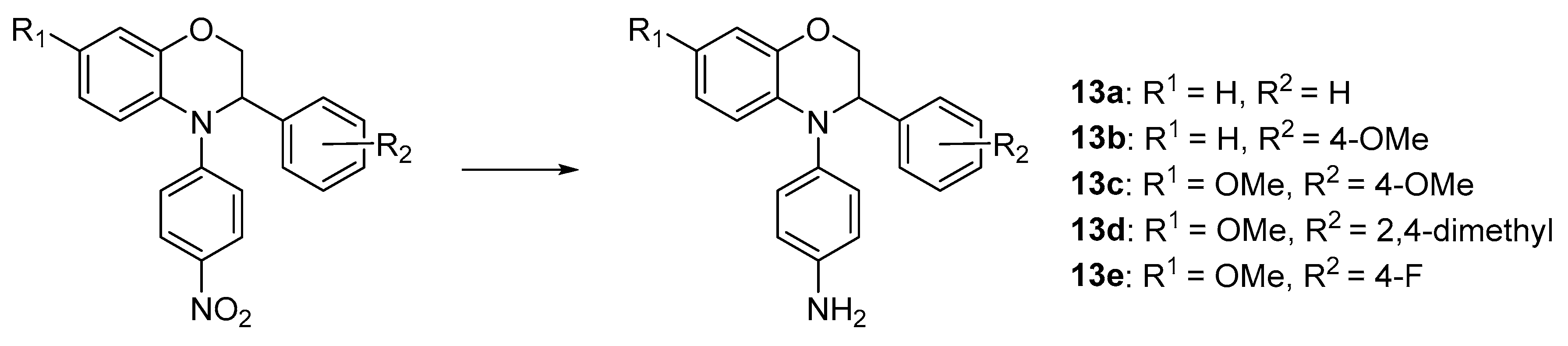
Experimental Protocols and Analytical Details
General procedure for the synthesis of 2-nitrophenoxyphenylethanones
Anhydrous K2CO3 (1.8 eq.) was added to a stirred solution of the corresponding 2-nitrophenol in acetone at room temperature and stirred for 30 min. The corresponding 2-bromo-1-phenylethanone (1.1 eq.) was added to the reaction mixture and stirred at room temperature until the reaction showed signs of completion as indicated by TLC. The reaction mixture was filtered, and the residue was washed with acetone. The combined filtrate was concentrated in vacuo to obtain the crude product. Acetone was added followed by the slow addition of H2O until solid precipitated out. The solid thus obtained was filtered and dried to obtain the target 2-nitrophenoxyphenylethanone.
2-(2-Nitrophenoxy)-1-phenylethan-1-one (
3a). Pale brown solid, yield: 83%.
1H NMR (400 MHz, DMSO-
d6) δ 8.05–7.98 (m, 2H), 7.90 (dd,
J = 8.1, 1.7 Hz, 1H), 7.76–7.67 (m, 1H), 7.63–7.54 (m, 3H), 7.30 (dd,
J = 8.6, 1.1 Hz, 1H), 7.13 (m, 1H), 5.87 (s, 2H).
13C NMR (101 MHz, DMSO-
d6) δ 194.06, 151.17, 140.15, 134.53, 134.46, 129.32, 128.38, 125.36, 121.38, 115.85, 71.54. Data consistent with reported literature [
28].
1-(4-Methoxyphenyl)-2-(2-nitrophenoxy)ethan-1-one (
3b). White solid, yield: 72%.
1H NMR (400 MHz, DMSO-
d6) δ 8.03–7.94 (m, 2H), 7.89 (dd,
J = 8.1, 1.7 Hz, 1H), 7.58 (ddd,
J = 8.6, 7.4, 1.7 Hz, 1H), 7.25 (dd,
J = 8.6, 1.1 Hz, 1H), 7.17–7.05 (m, 3H), 5.79 (s, 2H), 3.87 (s, 3H).
13C NMR (101 MHz, DMSO-
d6) δ 192.32, 164.21, 151.28, 140.12, 134.51, 130.76, 127.41, 125.35, 121.29, 115.83, 114.57, 71.23, 56.14. Data consistent with reported literature [
28].
2-(5-Methoxy-2-nitrophenoxy)-1-(4-methoxyphenyl)ethan-1-one (8a). Pale brown solid, yield: 62%, mp: 147–149 °C. IR (neat) 1681, 1290, 1172 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.03–7.93 (m, 3H), 7.14–7.05 (m, 2H), 6.75 (d, J = 2.5 Hz, 1H), 6.69 (dd, J = 9.1, 2.5 Hz, 1H), 5.80 (s, 2H), 3.87 (s, 3H), 3.82 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 192.26, 164.64, 164.19, 154.26, 133.21, 130.81, 128.23, 127.49, 114.55, 106.47, 101.69, 71.39, 56.63, 56.13. HRMS (+ESI): Found m/z 340.07910, [M + Na]+. C16H15NO6Na [340.07971].
1-(2,4-Dimethylphenyl)-2-(5-methoxy-2-nitrophenoxy)ethan-1-one (8b). Pale yellow solid, yield: 90%, mp: 124–126 °C. IR (neat) 2925, 1679, 1261 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.97 (d, J = 9.1 Hz, 1H), 7.87 (d, J = 7.7 Hz, 1H), 7.19 (d, J = 8.9 Hz, 2H), 6.78–6.63 (m, 2H), 5.70 (s, 2H), 3.82 (s, 3H), 2.39 (s, 3H), 2.34 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 196.66, 164.63, 154.19, 143.05, 138.76, 133.09, 131.94, 129.79, 128.22, 126.89, 106.68, 101.35, 72.42, 56.63, 21.45, 21.28. HRMS (+ESI): Found m/z 338.09992, [M + Na]+. C17H17NO5Na [338.10044].
1-(4-Fluorophenyl)-2-(5-methoxy-2-nitrophenoxy)ethan-1-one (8c). White solid, yield: 79%, mp: 173–175 °C. IR (neat) 1684, 1592, 1501 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.15–8.05 (m, 2H), 7.99 (d, J = 9.1 Hz, 1H), 7.48–7.37 (m, 2H), 6.80 (d, J = 2.5 Hz, 1H), 6.70 (dd, J = 9.1, 2.5 Hz, 1H), 5.85 (s, 2H), 3.82 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 192.64, 167.14, 164.68, 164.63, 154.10, 133.21, 131.58, 131.48, 131.41, 131.38, 128.24, 116.49, 116.27, 106.57, 101.72, 71.61, 56.66. HRMS (+ESI): Found m/z 328.05918, [M + Na]+. C15H12FNO5Na [328.05972].
General procedure for the synthesis of 1,4-benzoxazines
Palladium on carbon (10% wt, 0.1 eq) was added to a solution of the corresponding 2-nitrophenoxyphenylethanone in methanol at room temperature and stirred for 16 h. The reaction mixture was filtered through celite and the filtrate was concentrated in vacuo to obtain crude product, which was purified by flash chromatography (hexane/ethyl acetate) to obtain the target 1,4-benzoxazine.
3-Phenyl-3,4-dihydro-2H-benzo[b][1,4]oxazine (
4a). Pale yellow oil, yield: 50%.
1H NMR (400 MHz, DMSO-
d6) δ 7.49–7.26 (m, 5H), 6.78–6.66 (m, 3H), 6.59–6.47 (m, 1H), 6.26 (s, 1H), 4.47 (dt,
J = 7.3, 2.4 Hz, 1H), 4.22 (ddd,
J = 10.5, 3.1, 1.6 Hz, 1H), 3.90 (dd,
J = 10.5, 7.5 Hz, 1H).
13C NMR (101 MHz, DMSO-
d6) δ 142.70, 140.14, 134.92, 128.39, 127.63, 127.13, 121.19, 116.87, 115.74, 114.89, 69.91, 52.75. Data consistent with reported literature [
29].
3-(4-Methoxyphenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (
4b). Pale yellow oil, yield: 96%.
1H NMR (400 MHz, DMSO-
d6) δ 7.39–7.31 (m, 2H), 7.00–6.91 (m, 2H), 6.76–6.68 (m, 3H), 6.53 (ddd,
J = 7.6, 5.2, 3.8 Hz, 1H), 6.17 (d,
J = 1.7 Hz, 1H), 4.41 (dt,
J = 7.5, 2.3 Hz, 1H), 4.19 (ddd,
J = 10.4, 3.0, 1.7 Hz, 1H), 3.86 (dd,
J = 10.4, 7.9 Hz, 1H), 3.76 (s, 3H).
13C NMR (101 MHz, DMSO-
d6) δ 159.36, 143.19, 135.51, 132.36, 128.75, 121.58, 117.35, 116.19, 115.41, 114.29, 70.60, 55.57, 52.69. Data consistent with reported literature [
30].
7-Methoxy-3-(4-methoxyphenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (9a). Pale yellow solid, yield: 60%, mp: 147–149 °C. IR (neat) 3327, 2837, 1507 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.39–7.30 (m, 2H), 6.98–6.90 (m, 2H), 6.69–6.56 (m, 1H), 6.40–6.31 (m, 2H), 5.74 (t, J = 1.8 Hz, 1H), 4.31 (dt, J = 8.1, 2.3 Hz, 1H), 4.16 (ddd, J = 10.4, 2.9, 1.8 Hz, 1H), 3.84 (dd, J = 10.4, 8.2 Hz, 1H), 3.75 (s, 3H), 3.63 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.84, 151.65, 143.40, 131.91, 128.65, 128.31, 115.65, 113.79, 106.70, 102.11, 70.51, 55.27, 55.11, 52.33. HRMS (+ESI): Found m/z 272.12811, [M + H]+. C16H18NO3 [272.12867].
3-(2,4-Dimethylphenyl)-7-methoxy-3,4-dihydro-2H-benzo[b][1,4]oxazine (9b). White solid, yield: 95%, mp: 122–124 °C. IR (neat) 3348, 2872, 1504 cm−1. 1H NMR (400 MHz, DMSO) δ 7.31–7.24 (m, 1H), 7.06–6.99 (m, 2H), 6.65–6.58 (m, 1H), 6.39–6.31 (m, 2H), 5.61 (t, J = 1.9 Hz, 1H), 4.48 (dt, J = 8.2, 2.4 Hz, 1H), 4.18 (ddd, J = 10.6, 2.9, 2.0 Hz, 1H), 3.74 (dd, J = 10.5, 8.3 Hz, 1H), 3.63 (s, 3H), 2.33 (s, 3H), 2.25 (s, 3H). 13C NMR (101 MHz, DMSO) δ 151.64, 143.37, 136.30, 135.13, 134.64, 130.89, 128.96, 126.73, 126.70, 115.74, 106.70, 102.12, 69.19, 55.29, 49.24, 20.57, 18.66. HRMS (+ESI): Found m/z 270.14885, [M + H]+. C17H20NO2 [270.14940].
3-(4-Fluorophenyl)-7-methoxy-3,4-dihydro-2H-benzo[b][1,4]oxazine (9c). Brown solid: 92%, mp: 122–124 °C. IR (neat) 3363, 2860, 1507 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.51–7.41 (m, 2H), 7.26–7.15 (m, 2H), 6.63 (dt, J = 8.6, 1.2 Hz, 1H), 6.40–6.33 (m, 2H), 5.84 (t, J = 1.8 Hz, 1H), 4.40 (dt, J = 7.9, 2.4 Hz, 1H), 4.19 (ddd, J = 10.5, 2.9, 1.7 Hz, 1H), 3.87 (dd, J = 10.4, 7.8 Hz, 1H), 3.64 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 163.32, 160.90, 152.19, 143.88, 136.86, 136.84, 129.65, 129.57, 128.84, 116.17, 115.68, 115.47, 107.32, 102.63, 70.68, 55.74, 52.62. HRMS (+ESI): Found m/z 260.10818, [M + H]+. C15H15FNO2 [260.10868].
7-Methoxy-3-(4-methoxyphenyl)-2H-benzo[b][1,4]oxazine (10a). White solid: 40%, mp: 128–130 °C. IR (neat) 2970, 2921, 2842 cm−1. 1H NMR (400 MHz, DMSO) δ 7.98–7.90 (m, 2H), 7.25 (d, J = 8.5 Hz, 1H), 7.09–7.01 (m, 2H), 6.59 (dd, J = 8.5, 2.7 Hz, 1H), 6.53 (d, J = 2.7 Hz, 1H), 5.12 (s, 2H), 3.84 (s, 3H), 3.76 (s, 3H). 13C NMR (101 MHz, DMSO) δ 161.91, 159.61, 156.10, 147.58, 128.53, 128.21, 128.05, 114.55, 108.28, 101.45, 62.68, 55.92, 55.86. HRMS (+ESI): Found m/z 270.11239, [M + H]+. C16H16NO3 [270.11302].
3-(4-Fluorophenyl)-7-methoxy-2H-benzo[b][1,4]oxazine (10b). Yellow crystal: 5%, mp: 145–147 °C. IR (neat) 2966, 1587, 1306 cm−1. 1H NMR (400 MHz, DMSO) δ 8.09–7.97 (m, 2H), 7.38–7.31 (m, 2H), 7.28 (d, J = 8.6 Hz, 1H), 6.61 (dd, J = 8.6, 2.7 Hz, 1H), 6.54 (d, J = 2.6 Hz, 1H), 5.16 (s, 2H), 3.77 (s, 3H). 13C NMR (101 MHz, DMSO) δ 165.38, 162.90, 160.08, 155.60, 147.61, 132.11, 132.08, 129.28, 129.20, 128.59, 127.75, 116.27, 116.05, 108.41, 101.47, 62.78, 55.97. HRMS (+ESI): Found m/z 258.09252, [M + H]+. C15H13FNO2 [258.09303].
General procedure A for the synthesis of 3,4-diphenyl-1,4-benzoxazines
A Schlenk flask was charged with caesium carbonate (1.4 eq.), the corresponding 1,4-benzoxazine, and bromophenol (1.6 eq.). The flask was vacuumed and backfilled with nitrogen three times before the reaction mixture was dissolved in toluene and tert-butanol and stirred for 10 min at room temperature. Pd2(dba)3 (0.02 eq.) and XPhos (0.1 eq.) were added to the solution and the mixture was refluxed until the reaction showed signs of completion as indicated by TLC. The mixture was cooled to room temperature and diluted with ethyl acetate. The solution was washed with brine and water before being dried with sodium sulphate and concentrated in vacuo to obtain crude product, which was purified by flash chromatography (hexane/ethyl acetate) to obtain the target 3,4-diphenyl-1,4-benzoxazine.
General procedure B for the synthesis of 3,4-diphenyl-1,4-benzoxazines
A Schlenk flask was charged with potassium tert-butoxide (1.5 eq.), the corresponding 1,4-benzoxazine, and bromophenol (1.6 eq.). The flask was vacuumed and back filled with nitrogen for three times before the reaction mixture was dissolved in toluene and stirred for 10 min at room temperature. Palladium acetate (0.05 eq.) and tri-tert-butylphosphine (0.1 eq.) was added to the solution and the mixture was refluxed until the reaction showed signs of completion as indicated by TLC. The mixture was cooled to room temperature and diluted with ethyl acetate. The solution was washed with brine and water before drying with sodium sulphate and then concentrated in vacuo to obtain crude product, which was purified by flash chromatography (hexane/ethyl acetate) to obtain target 3,4-diphenyl-1,4-benzoxazine.
3,4-Diphenyl-3,4-dihydro-2H-benzo[b][1,4]oxazine (5a). White solid, yield: 50%, mp: 166–168 °C. IR (neat) 2936, 2167, 1493 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.37–7.26 (m, 6H), 7.27–7.16 (m, 3H), 7.12–7.03 (m, 1H), 6.87 (dd, J = 8.1, 1.6 Hz, 1H), 6.83–6.72 (m, 2H), 6.69 (td, J = 7.5, 1.6 Hz, 1H), 5.05 (t, J = 3.1 Hz, 1H), 4.48 (dd, J = 11.0, 3.3 Hz, 1H), 4.30 (dd, J = 11.0, 2.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 146.25, 144.65, 140.33, 132.83, 129.93, 128.87, 127.67, 127.40, 124.50, 124.48, 121.74, 119.73, 117.22, 116.19, 68.86, 60.66. HRMS (+ESI): Found m/z 288.13830, [M + H]+. C20H18NO [288.13884].
4-(4-Nitrophenyl)-3-phenyl-3,4-dihydro-2H-benzo[b][1,4]oxazine (5b). Orange solid, yield: 43%, mp: 167–169 °C. IR (neat) 2928, 1583, 1490 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.23–8.14 (m, 2H), 7.45–7.36 (m, 1H), 7.40–7.30 (m, 6H), 7.27 (ddd, J = 9.7, 5.1, 2.3 Hz, 1H), 6.96–6.86 (m, 2H), 6.87–6.79 (m, 1H), 5.37 (d, J = 2.4 Hz, 1H), 4.78 (dd, J = 11.4, 2.0 Hz, 1H), 4.39 (dd, J = 11.4, 2.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 152.11, 146.10, 141.02, 138.60, 129.16, 128.01, 127.88, 126.95, 126.08, 123.47, 121.63, 119.66, 119.57, 117.87, 68.32, 59.16. HRMS (+ESI): Found m/z 333.12349, [M + H]+. C20H17N2O3 [333.12392].
3-(4-Methoxyphenyl)-4-phenyl-3,4-dihydro-2H-benzo[b][1,4]oxazine (5c). Pale yellow crystal, yield: 47%, mp: 152–154 °C. IR (neat) 2906, 1489, 1248 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.38–7.27 (m, 2H), 7.21 (ddd, J = 15.6, 7.6, 1.7 Hz, 4H), 7.12–7.03 (m, 1H), 6.89–6.64 (m, 6H), 4.97 (t, J = 3.3 Hz, 1H), 4.42 (dd, J = 10.9, 3.8 Hz, 1H), 4.26 (dd, J = 10.9, 2.8 Hz, 1H), 3.70 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.86, 146.20, 144.63, 133.08, 131.96, 129.89, 128.63, 124.72, 124.53, 121.66, 119.65, 117.15, 116.15, 114.27, 69.04, 60.01, 55.46. HRMS (+ESI): Found m/z 318.14909, [M + H]+. C21H20NO2 [318.14940].
3-(4-Methoxyphenyl)-4-(4-nitrophenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (5d). Orange solid, yield: 50%, mp: 107–109 °C. IR (neat) 2833, 2161, 2044 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.23–8.14 (m, 2H), 7.43–7.31 (m, 3H), 7.30–7.23 (m, 2H), 6.92–6.81 (m, 5H), 5.28 (d, J = 2.6 Hz, 1H), 4.72 (dd, J = 11.4, 2.1 Hz, 1H), 4.36 (dd, J = 11.4, 2.8 Hz, 1H), 3.71 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.54, 151.62, 145.63, 140.48, 129.75, 127.68, 127.54, 125.59, 122.96, 121.08, 119.16, 119.06, 117.36, 114.10, 67.90, 58.15, 55.05. HRMS (+ESI): Found m/z 363.13424, [M + H]+. C21H19N2O4 [363.13448].
4-(4-Fluorophenyl)-3-(4-methoxyphenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (5e). Pale yellow solid, yield: 23%, mp: 101–103 °C. IR (neat) 2928, 1495, 1213 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.25–7.11 (m, 9H), 6.88–6.78 (m, 3H), 6.78–6.63 (m, 2H), 6.61 (dd, J = 7.9, 1.7 Hz, 1H), 4.91 (dd, J = 4.5, 2.9 Hz, 1H), 4.36 (dd, J = 10.9, 4.6 Hz, 1H), 4.27 (dd, J = 10.9, 2.9 Hz, 1H), 3.70 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.95, 144.43, 142.26, 142.23, 134.20, 131.59, 131.24, 131.16, 128.88, 128.11, 128.03, 121.76, 119.38, 117.06, 116.74, 116.52, 115.99, 115.78, 115.54, 114.27, 69.33, 60.26, 55.45. HRMS (+ESI): Found m/z 336.13957, [M + H]+. C21H19FNO2 [336.13998].
7-Methoxy-3-(4-methoxyphenyl)-4-phenyl-3,4-dihydro-2H-benzo[b][1,4]oxazine (11a). Orange oil, yield: 38%. IR (neat) 2935, 2836, 1502 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.86–7.76 (m, 1H), 7.52–7.42 (m, 1H), 7.31–7.24 (m, 3H), 7.17–7.09 (m, 2H), 6.99 (tt, J = 7.2, 1.2 Hz, 1H), 6.89–6.81 (m, 2H), 6.45–6.37 (m, 2H), 4.93 (t, 1H), 4.50 (dd, J = 11.0, 3.3 Hz, 1H), 4.21 (dd, J = 11.0, 2.8 Hz, 1H), 3.70 (s, 3H), 3.65 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.80, 153.82, 147.64, 145.91, 131.76, 129.81, 128.54, 125.26, 123.23, 122.84, 118.82, 114.26, 107.71, 102.75, 68.44, 59.80, 55.67, 55.47. HRMS (+ESI): Found m/z 348.15951, [M + H]+. C22H22NO3 [348.15997].
7-Methoxy-3,4-bis(4-methoxyphenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (11b). Red oil, yield: 82%. IR (neat) 2931, 1596, 1501 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.27–7.19 (m, 2H), 7.11–7.03 (m, 2H), 6.90–6.79 (m, 4H), 6.48 (d, J = 8.9 Hz, 1H), 6.42 (d, J = 2.8 Hz, 1H), 6.35 (dd, J = 8.9, 2.8 Hz, 1H), 4.79 (dd, J = 5.0, 2.8 Hz, 1H), 4.34 (dd, J = 10.9, 5.0 Hz, 1H), 4.21 (dd, J = 10.9, 2.9 Hz, 1H), 3.70 (d, J = 1.2 Hz, 6H), 3.64 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.86, 156.48, 153.01, 145.24, 140.07, 131.73, 128.97, 128.48, 127.17, 117.18, 115.06, 114.17, 107.52, 102.82, 69.37, 60.42, 55.71, 55.61, 55.44. HRMS (+ESI): Found m/z 378.16984, [M + H]+. C23H24NO4 [378.17053].
7-Methoxy-3-(4-methoxyphenyl)-4-(4-nitrophenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (11c). Red oil, yield: 24%. IR (neat) 2923, 1598, 1495 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.20–8.11 (m, 2H), 7.31–7.24 (m, 5H), 6.94–6.85 (m, 2H), 6.52 (dd, J = 9.0, 2.8 Hz, 1H), 6.42 (d, J = 2.8 Hz, 1H), 5.31 (s, 1H), 4.77 (dd, J = 11.5, 1.9 Hz, 1H), 4.33 (dd, J = 11.4, 2.8 Hz, 1H), 3.70 (d, J = 11.3 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 158.99, 156.12, 152.65, 147.36, 140.08, 129.91, 128.20, 126.20, 121.76, 120.43, 118.10, 114.54, 108.03, 102.68, 68.25, 58.23, 55.73, 55.53. HRMS (+ESI): Found m/z 393.14461, [M + H]+. C22H21N2O5 [393.14505].
3-(2,4-Dimethylphenyl)-7-methoxy-4-(4-methoxyphenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (11d). Light brown gum, yield: 38%. IR (neat) 2921, 1586, 1501 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.18–7.13 (m, 1H), 7.13–7.06 (m, 2H), 6.89 (d, J = 6.7 Hz, 2H), 6.87–6.81 (m, 2H), 6.46 (dd, J = 7.9, 2.8 Hz, 1H), 6.38 (d, J = 8.8 Hz, 1H), 6.33 (dd, J = 8.9, 2.7 Hz, 1H), 5.00 (dd, J = 5.9, 3.0 Hz, 1H), 4.25 (dd, J = 11.0, 3.1 Hz, 1H), 4.17 (dd, J = 11.0, 5.9 Hz, 1H), 3.69 (s, 3H), 3.65 (s, 3H), 2.24 (s, 3H), 2.18 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 156.90, 152.66, 144.90, 139.31, 136.50, 135.29, 134.41, 131.49, 130.19, 128.28, 128.09, 126.96, 115.96, 115.01, 107.40, 102.94, 69.02, 57.27, 55.75, 55.56, 20.97, 19.25. HRMS (+ESI): Found m/z 376.19085, [M + H]+. C24H26NO3 [376.19127].
3-(2,4-Dimethylphenyl)-7-methoxy-4-(4-nitrophenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (11e). Red solid, yield: 78%, mp: 121–123 °C. IR (neat) 2929, 1585, 1493 cm−1. 1H NMR (400 MHz, Chloroform-d) δ 8.15–8.06 (m, 2H), 7.30 (d, J = 8.6 Hz, 1H), 7.19–7.12 (m, 2H), 7.09–7.06 (m, 1H), 7.04 (d, J = 7.9 Hz, 1H), 6.94–6.87 (m, 1H), 6.61–6.53 (m, 2H), 5.11 (t, J = 3.6 Hz, 1H), 4.47–4.39 (m, 1H), 4.35 (dd, J = 11.0, 3.5 Hz, 1H), 3.81 (s, 3H), 2.42 (s, 3H), 2.31 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 155.74, 152.55, 147.71, 141.04, 137.72, 133.65, 133.30, 132.04, 127.42, 126.61, 125.55, 122.89, 119.79, 118.57, 108.20, 103.00, 68.33, 58.86, 55.60, 31.60, 22.67, 20.97, 19.29, 14.14. HRMS (+ESI): Found m/z 391.16525, [M + H]+. C23H23N2O4 [391.16578].
3-(2,4-Dimethylphenyl)-7-methoxy-4-(quinolin-3-yl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (15a). Yellow solid, yield: 55%, mp: 112–114 °C. IR (neat) 2923, 1587, 1505 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.79 (d, J = 2.6 Hz, 1H), 8.00 (d, J = 2.6 Hz, 1H), 7.91 (d, J = 8.4 Hz, 1H), 7.82 (dd, J = 8.3, 1.4 Hz, 1H), 7.63 (ddd, J = 8.4, 6.8, 1.5 Hz, 1H), 7.52 (ddd, J = 8.2, 6.8, 1.3 Hz, 1H), 7.13 (d, J = 7.9 Hz, 1H), 6.95 (d, J = 1.8 Hz, 1H), 6.91–6.82 (m, 1H), 6.76 (d, J = 8.9 Hz, 1H), 6.53 (d, J = 2.9 Hz, 1H), 6.43 (dd, J = 8.9, 2.9 Hz, 1H), 5.32 (d, J = 3.7 Hz, 1H), 4.41–4.26 (m, 2H), 3.69 (s, 3H), 2.34 (s, 3H), 2.17 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 153.93, 149.67, 145.91, 144.82, 140.22, 136.84, 135.37, 134.10, 131.80, 128.95, 128.71, 128.65, 128.36, 127.89, 127.63, 127.40, 127.24, 127.06, 116.96, 107.83, 103.22, 68.51, 57.39, 55.79, 20.94, 19.33. HRMS (+ESI): Found m/z 397.19073, [M + H]+. C26H25N2O2 [397.19160].
3-(4-Fluorophenyl)-7-methoxy-4-(4-methoxyphenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (11f). Yellow oil, yield: 83%. IR (neat) 2938, 2835, 1501 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.41–7.31 (m, 2H), 7.16–7.03 (m, 4H), 6.91–6.82 (m, 2H), 6.53 (d, J = 8.9 Hz, 1H), 6.44 (d, J = 2.8 Hz, 1H), 6.37 (dd, J = 8.9, 2.8 Hz, 1H), 4.88 (t, J = 3.7 Hz, 1H), 4.38 (dd, J = 10.9, 4.6 Hz, 1H), 4.24 (dd, J = 10.9, 2.8 Hz, 1H), 3.70 (s, 3H), 3.65 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 163.00, 160.58, 156.54, 153.13, 145.23, 140.07, 136.26, 136.23, 129.80, 129.72, 128.03, 127.03, 117.37, 115.65, 115.44, 115.13, 107.72, 102.86, 68.99, 60.48, 55.70, 55.62. HRMS (+ESI): Found m/z 388.13199, [M + Na]+. C22H20FNO3Na [388.13249].
3-(4-Fluorophenyl)-7-methoxy-4-(4-nitrophenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (11g). Yellow solid, yield: 33%, mp: 139–141 °C. IR (neat) 2912, 1589, 1494 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.21–8.08 (m, 2H), 7.45–7.36 (m, 2H), 7.35–7.24 (m, 3H), 7.24–7.12 (m, 2H), 6.53 (dd, J = 9.0, 2.8 Hz, 1H), 6.43 (d, J = 2.8 Hz, 1H), 5.38 (s, 1H), 4.81 (dd, J = 11.5, 1.9 Hz, 1H), 4.34 (dd, J = 11.5, 2.8 Hz, 1H), 3.69 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 163.10, 160.68, 156.18, 152.65, 147.29, 140.27, 134.45, 134.42, 129.15, 129.07, 126.19, 121.93, 120.24, 118.28, 116.03, 115.82, 108.20, 102.70, 68.09, 58.30, 55.73. HRMS (+ESI): Found m/z 403.10655, [M + Na]+. C21H17FN2O4Na [403.10700].
3-(4-Fluorophenyl)-7-methoxy-2H-benzo[b][1,4]oxazine (12). Dark purple solid: 35%, mp: 159–161 °C. IR (neat) 2989, 2935, 2834 cm−1. 1H NMR (400 MHz, DMSO) δ 7.83–7.72 (m, 2H), 7.34 (d, J = 8.6 Hz, 1H), 7.04–6.93 (m, 2H), 6.72 (d, J = 8.5 Hz, 2H), 6.69 (s, 2H), 6.65 (d, J = 2.4 Hz, 1H), 6.56 (dd, J = 8.6, 2.7 Hz, 1H), 6.36 (d, J = 2.7 Hz, 1H), 6.32 (dd, J = 8.6, 2.4 Hz, 1H), 3.95 (s, 3H), 3.77 (s, 3H), 3.69 (s, 6H). 13C NMR (101 MHz, DMSO) δ 162.12, 161.70, 160.02, 158.53, 156.13, 145.90, 129.79, 128.34, 128.12, 128.07, 127.76, 116.04, 114.59, 108.16, 105.44, 102.18, 99.64, 66.66, 56.49, 55.82, 55.80, 55.70. HRMS (+ESI): Found m/z 406.16517, [M + H]+. C24H24NO5 [406.16545].
3-(4-Fluorophenyl)-7-methoxy-4-(quinolin-3-yl)-3,4-dihydro-2H-benzo[b][1,4]oxazine (15b). Brown gum, yield: 67%. IR (neat) 3078, 3002, 1507 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.83 (d, J = 2.6 Hz, 1H), 7.93 (d, J = 8.4 Hz, 1H), 7.90 (d, J = 2.7 Hz, 1H), 7.82 (d, J = 7.5 Hz, 1H), 7.61 (ddd, J = 8.4, 6.9, 1.5 Hz, 1H), 7.55–7.44 (m, 3H), 7.21–7.11 (m, 2H), 7.02–6.95 (m, 1H), 6.51–6.44 (m, 2H), 5.26 (s, 1H), 4.64 (dd, J = 11.2, 3.1 Hz, 1H), 4.34 (dd, J = 11.2, 2.7 Hz, 1H), 3.69 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 163.07, 160.65, 154.77, 148.15, 146.49, 144.34, 141.22, 135.56, 135.53, 129.55, 129.47, 128.94, 128.79, 128.16, 127.70, 127.49, 125.34, 123.92, 119.52, 115.88, 115.67, 108.20, 102.96, 67.97, 59.71, 55.72. HRMS (+ESI): Found m/z 387.15037, [M + H]+. C24H20FN2O2 [387.15088].
General procedure for the synthesis of 3-phenyl-4-aniline-1,4-benzoxazine
Palladium on carbon (10% wt, 0.1 eq.) was added to a solution of the corresponding 3-phenyl-4-aniline-1,4-benzoxazine in methanol at room temperature and stirred for 16 h. The reaction mixture was filtered through celite and the filtrate removed in vacuo to obtain crude product, which was purified by flash chromatography (hexane/ethyl acetate) to obtain target 3-phenyl-4-aniline-1,4-benzoxazine.
4-(3-Phenyl-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)aniline (13a). Brown solid, yield: 85%, mp: 169–171 °C. IR (neat) 3454, 3374, 2920 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.33–7.15 (m, 5H), 6.89–6.81 (m, 2H), 6.76 (dd, J = 7.8, 1.5 Hz, 1H), 6.68 (ddd, J = 8.0, 7.3, 1.6 Hz, 1H), 6.57 (td, J = 7.6, 1.6 Hz, 1H), 6.53–6.41 (m, 3H), 5.00 (s, 2H), 4.83 (t, J = 3.8 Hz, 1H), 4.32–4.21 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 146.59, 143.41, 140.24, 135.86, 133.58, 128.20, 127.96, 127.38, 127.17, 121.34, 117.26, 116.16, 114.52, 114.15, 69.15, 61.04. HRMS (+ESI): Found m/z 303.14921, [M + H]+. C20H19N2O [303.14974].
4-(3-(4-Methoxyphenyl)-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)aniline (13b). Yellow solid, yield: 78%, mp: 170–172 °C. IR (neat) 3456, 3371, 2932 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.23–7.15 (m, 2H), 6.87–6.79 (m, 4H), 6.76 (dd, J = 7.8, 1.6 Hz, 1H), 6.71–6.62 (m, 1H), 6.56 (td, J = 7.5, 1.6 Hz, 1H), 6.52–6.44 (m, 2H), 6.41 (dd, J = 8.0, 1.5 Hz, 1H), 5.00 (s, 2H), 4.75 (t, J = 4.0 Hz, 1H), 4.22 (d, J = 4.0 Hz, 2H), 3.70 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.85, 147.04, 143.91, 136.60, 133.99, 132.29, 129.07, 128.59, 121.72, 117.74, 116.56, 114.98, 114.73, 114.07, 69.87, 60.74, 55.42. HRMS (+ESI): Found m/z 333.15971, [M + H]+. C21H21N2O2 [333.16030].
4-(7-Methoxy-3-(4-methoxyphenyl)-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)aniline (13c). Dark-brown solid, yield: 100%, mp: 142–144 °C. IR (neat) 3448, 2929, 2837 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.28–7.13 (m, 2H), 6.88–6.73 (m, 4H), 6.50–6.42 (m, 2H), 6.42–6.25 (m, 3H), 4.93 (s, 2H), 4.68 (dd, J = 5.6, 3.0 Hz, 1H), 4.32–4.13 (m, 2H), 3.70 (s, 3H), 3.63 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 158.82, 152.40, 146.54, 144.82, 135.40, 132.00, 130.17, 129.14, 127.96, 116.46, 114.98, 114.05, 107.33, 102.75, 69.89, 60.59, 55.72, 55.41. HRMS (+ESI): Found m/z 363.17036, [M + H]+. C22H23N2O3 [363.17087].
4-(3-(2,4-Dimethylphenyl)-7-methoxy-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)aniline (13d). Brown solid, yield: 75%, mp: 119–121 °C. IR (neat) 3466, 3377, 2883 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 7.17 (d, J = 7.8 Hz, 1H), 6.93–6.85 (m, 2H), 6.86–6.78 (m, 2H), 6.48–6.37 (m, 3H), 6.30 (d, J = 1.6 Hz, 2H), 4.94 (s, 2H), 4.91 (dd, J = 6.4, 3.0 Hz, 1H), 4.22 (dd, J = 10.9, 3.0 Hz, 1H), 4.12 (dd, J = 10.9, 6.4 Hz, 1H), 3.64 (s, 3H), 2.22 (s, 3H), 2.18 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 152.21, 146.82, 144.62, 136.36, 135.33, 134.85, 134.56, 131.40, 131.35, 128.52, 128.30, 126.89, 115.76, 114.95, 107.23, 102.83, 69.29, 57.17, 55.75, 20.99, 19.26. HRMS (+ESI): Found m/z 361.19108, [M + H]+. C23H25N2O2 [361.19160].
4-(3-(4-Fluorophenyl)-7-methoxy-2,3-dihydro-4H-benzo[b][1,4]oxazin-4-yl)aniline (13e).
Dark-brown solid, yield: 43%, mp: 128–130 °C. IR (neat) 3455, 3364, 2836 cm−1. 1H NMR (400 MHz, Chloroform-d) δ 7.32–7.23 (m, 2H), 7.04–6.93 (m, 2H), 6.97–6.89 (m, 2H), 6.65–6.57 (m, 3H), 6.52 (d, J = 2.8 Hz, 1H), 6.39 (dd, J = 8.9, 2.8 Hz, 1H), 4.68 (dd, J = 5.1, 3.1 Hz, 1H), 4.37 (dd, J = 10.8, 5.1 Hz, 1H), 4.31 (dd, J = 10.8, 3.1 Hz, 1H), 3.75 (s, 3H), 3.64 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 163.35, 160.91, 152.98, 144.97, 143.41, 138.35, 135.41, 135.38, 129.16, 129.08, 128.72, 127.54, 117.25, 116.08, 115.46, 115.24, 107.56, 102.61, 69.38, 61.52, 55.67. HRMS (+ESI): Found m/z 351.15036, [M + H]+. C21H20FN2O2 [351.15088].
General procedure for demethylation using BBr3
BBr3 (1M) in dichloromethane solution (2 eq. per methoxy group) was added to a solution of the corresponding methylated 3,4-diphenyl-1,4-benzoxazine in dichloromethane at 0 °C and stirred for 12 h. The reaction mixture was quenched with saturated sodium bicarbonate solution and diluted with ethyl acetate. The solution was washed with brine and water before drying with sodium sulphate and concentrated in vacuo to obtain crude product, which was purified by flash chromatography (dichloromethane/methanol) to obtain target demethylated 3,4-diphenyl-1,4-benzoxazine.
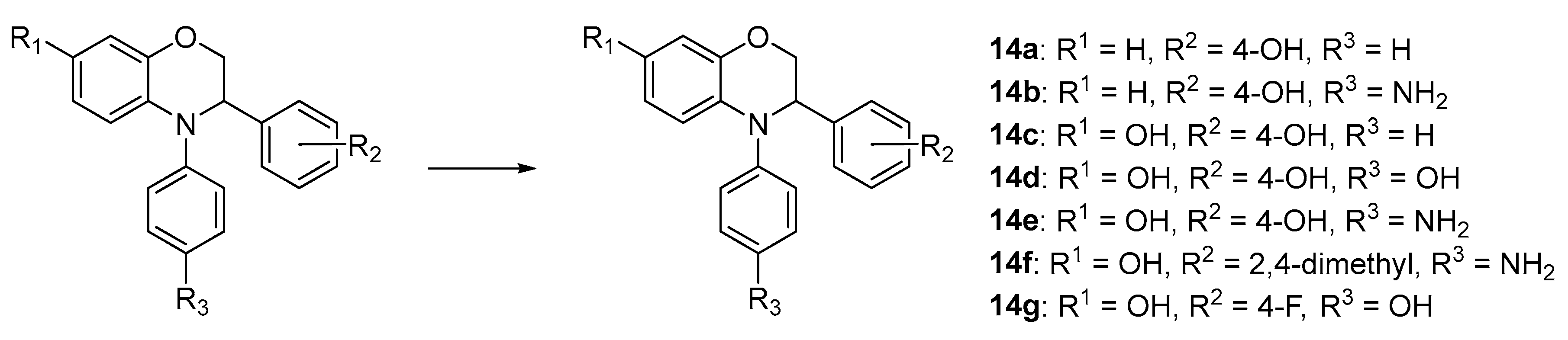
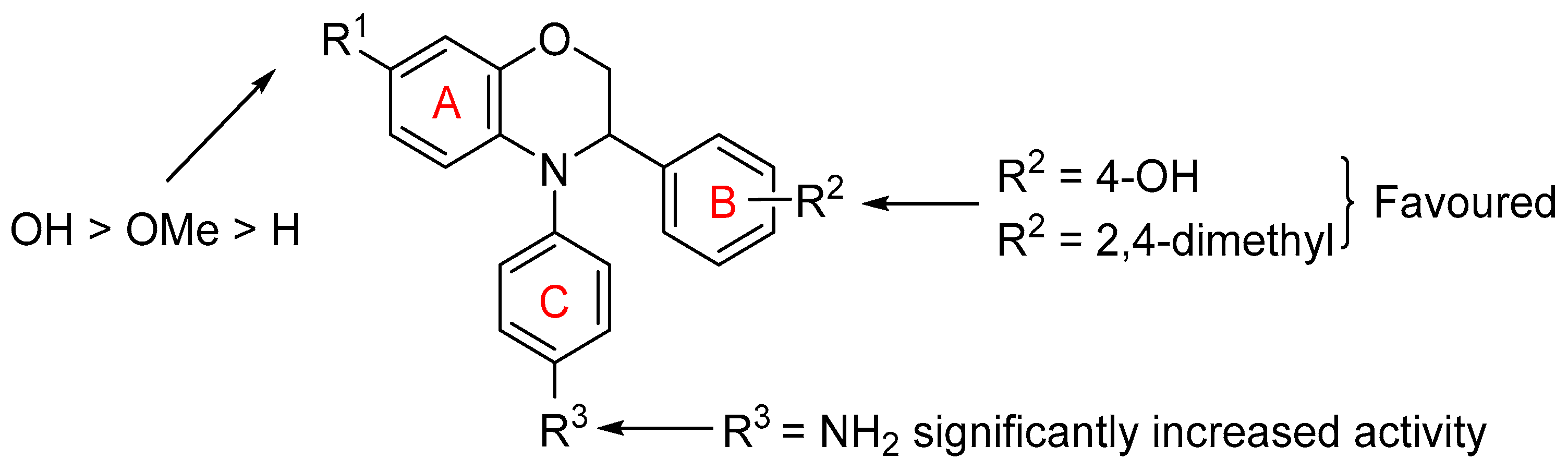
4-(4-Phenyl-3,4-dihydro-2H-benzo[b][1,4]oxazin-3-yl)phenol (14a). White solid, yield: 67%, mp: 150–152 °C. IR (neat) 3516, 1586, 1490 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.32 (s, 1H), 7.37–7.27 (m, 2H), 7.22–7.14 (m, 2H), 7.14–7.03 (m, 3H), 6.83–6.62 (m, 6H), 4.90 (t, J = 3.3 Hz, 1H), 4.38 (dd, J = 10.9, 3.9 Hz, 1H), 4.25 (dd, J = 10.9, 2.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 156.97, 146.22, 144.62, 133.29, 130.12, 129.85, 128.62, 124.92, 124.55, 121.61, 119.53, 117.10, 116.08, 115.59, 69.13, 60.10. HRMS (+ESI): Found m/z 304.13317, [M + H]+. C20H18NO2 [304.13375].
4-(4-(4-Aminophenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazin-3-yl)phenol (14b). Brown solid, yield: 62%, mp: 113–115 °C. IR (neat) 3438, 3352, 2884 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.28 (s, 1H), 7.10–7.02 (m, 2H), 6.85–6.77 (m, 2H), 6.75 (dd, J = 7.8, 1.6 Hz, 1H), 6.69–6.60 (m, 3H), 6.55 (td, J = 7.5, 1.6 Hz, 1H), 6.52–6.44 (m, 2H), 6.39 (dd, J = 8.1, 1.6 Hz, 1H), 4.99 (s, 2H), 4.68 (t, J = 4.1 Hz, 1H), 4.22–4.18 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 156.95, 146.98, 143.91, 136.72, 134.06, 130.49, 129.04, 128.68, 121.67, 117.65, 116.52, 115.42, 114.97, 114.70, 69.94, 60.84. HRMS (+ESI): Found m/z 319.14402, [M + H]+. C20H19N2O2 [319.14465].
3-(4-Hydroxyphenyl)-4-phenyl-3,4-dihydro-2H-benzo[b][1,4]oxazin-7-ol (14c). Brown solid, yield: 40%, mp: 129–131 °C. IR (neat) 3189, 3927, 2865 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.30 (s, 1H), 8.92 (s, 1H), 7.31–7.21 (m, 2H), 7.17–7.06 (m, 4H), 6.96 (tt, J = 7.1, 1.1 Hz, 1H), 6.73 (d, J = 8.5 Hz, 1H), 6.71–6.62 (m, 2H), 6.27–6.19 (m, 2H), 4.84 (t, J = 3.0 Hz, 1H), 4.43 (dd, J = 11.0, 3.4 Hz, 1H), 4.16 (dd, J = 11.0, 2.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 156.85, 151.84, 148.11, 146.04, 130.05, 129.69, 128.52, 123.77, 122.78, 122.58, 119.28, 115.54, 108.79, 103.97, 68.27, 59.93. HRMS (+ESI): Found m/z 342.11001, [M + Na]+. C20H17NO3Na [342.11061].
4,4′-(7-Hydroxy-2,3-dihydro-4H-benzo[b][1,4]oxazine-3,4-diyl)diphenol (14d). Black solid, yield: 70%, mp: 91–93 °C. IR (neat) 3023, 2814, 2692 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.28 (s, 1H), 9.22 (s, 1H), 8.74 (s, 1H), 7.12–7.04 (m, 2H), 6.95–6.87 (m, 2H), 6.69–6.59 (m, 4H), 6.30 (d, J = 8.7 Hz, 1H), 6.22 (d, J = 2.6 Hz, 1H), 6.15 (dd, J = 8.7, 2.7 Hz, 1H), 4.62 (dd, J = 5.5, 2.9 Hz, 1H), 4.24 (dd, J = 10.8, 5.5 Hz, 1H), 4.14 (dd, J = 10.8, 2.9 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 156.90, 154.62, 150.61, 145.15, 138.92, 130.08, 129.05, 127.88, 127.65, 117.31, 116.23, 115.42, 108.57, 103.88, 69.52, 60.68. HRMS (+ESI): Found m/z 358.10491, [M + Na]+. C20H17NO4Na [358.10553].
4-(4-Aminophenyl)-3-(4-hydroxyphenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazin-7-ol (14e). Black gum, yield: 75%. IR (neat) 2929, 1596, 1502 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.26 (s, 1H), 8.68 (s, 1H), 7.13–6.99 (m, 2H), 6.82–6.71 (m, 2H), 6.68–6.57 (m, 2H), 6.50–6.39 (m, 2H), 6.28–6.18 (m, 2H), 6.13 (dd, J = 8.7, 2.7 Hz, 1H), 4.89 (s, 2H), 4.56 (dd, J = 5.8, 3.1 Hz, 1H), 4.19 (dd, J = 10.8, 5.8 Hz, 1H), 4.13 (dd, J = 10.8, 3.1 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 156.87, 150.21, 146.30, 144.93, 135.98, 130.24, 129.15, 128.89, 127.94, 116.93, 115.36, 114.92, 108.46, 103.80, 69.87, 60.76. HRMS (+ESI): Found m/z 335.13894, [M + H]+. C20H19N2O3 [335.13957].
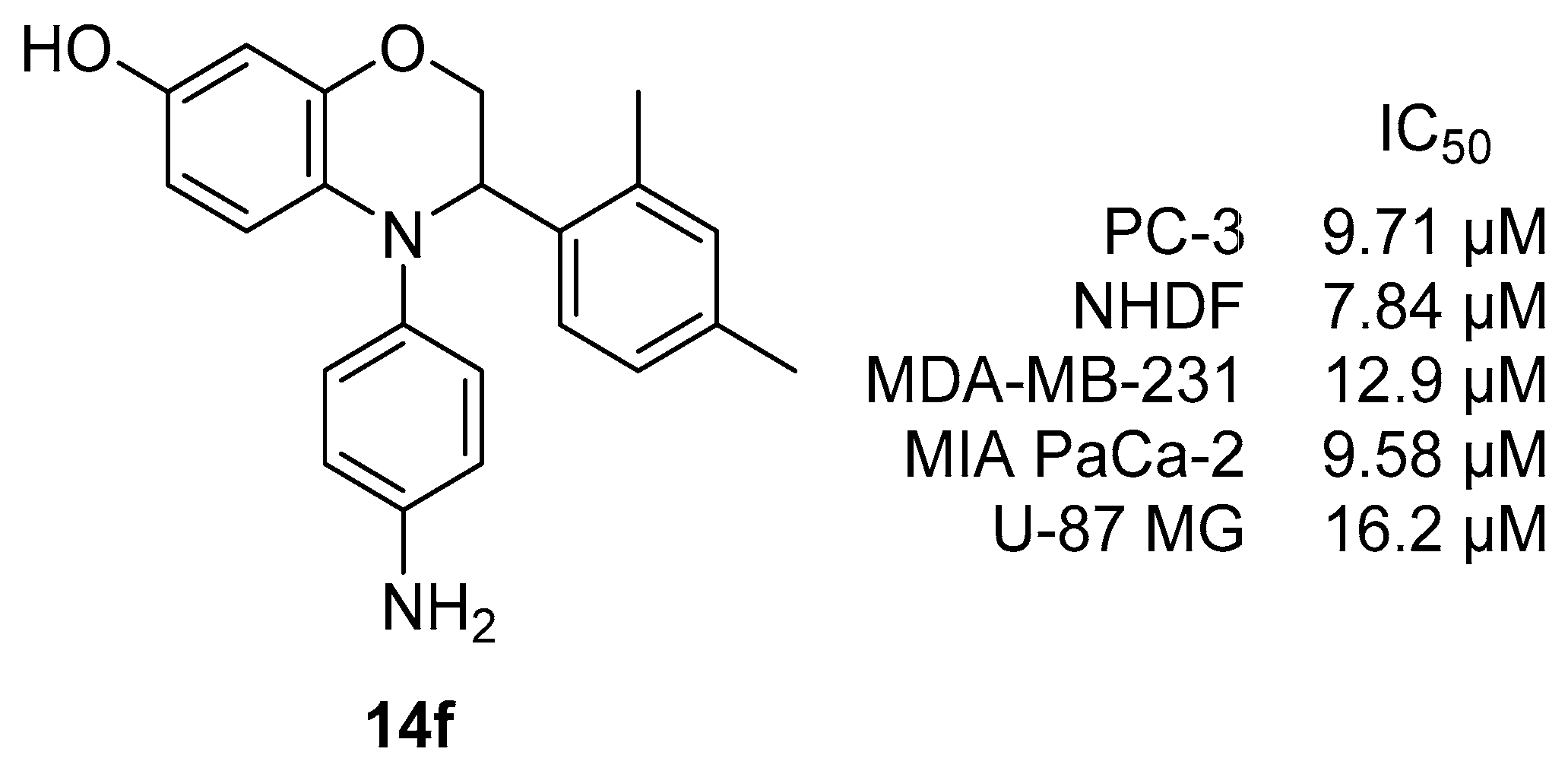
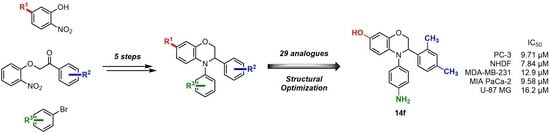
4-(4-Aminophenyl)-3-(2,4-dimethylphenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazin-7-ol (14f). Pale-brown Solid, yield: 89%, mp: 185–187 °C. IR (neat) 3373, 3304, 2920 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 8.67 (s, 1H), 7.18 (d, J = 7.8 Hz, 1H), 6.94–6.84 (m, 2H), 6.84–6.74 (m, 2H), 6.47–6.37 (m, 2H), 6.24 (d, J = 2.6 Hz, 1H), 6.20 (d, J = 8.7 Hz, 1H), 6.13 (dd, J = 8.7, 2.6 Hz, 1H), 4.91 (s, 2H), 4.86 (dd, J = 6.7, 3.0 Hz, 1H), 4.17 (dd, J = 10.9, 3.0 Hz, 1H), 4.08 (dd, J = 10.9, 6.7 Hz, 1H), 2.22 (s, 3H), 2.18 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 150.05, 146.65, 144.70, 136.28, 135.35, 135.29, 134.57, 131.30, 130.05, 128.47, 128.39, 126.87, 116.20, 114.90, 108.45, 103.85, 69.30, 57.18, 20.99, 19.28. HRMS (+ESI): Found m/z 347.17541, [M + H]+. C22H23N2O2 [347.17595].
3-(4-Fluorophenyl)-4-(4-hydroxyphenyl)-3,4-dihydro-2H-benzo[b][1,4]oxazin-7-ol (14g). Brown gum, yield: 95%. IR (neat) 3401, 1698, 1502 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.25 (s, 1H), 8.79 (s, 1H), 7.40–7.30 (m, 2H), 7.14–7.04 (m, 2H), 6.97–6.89 (m, 2H), 6.71–6.62 (m, 2H), 6.38 (d, J = 8.7 Hz, 1H), 6.24 (d, J = 2.6 Hz, 1H), 6.19 (dd, J = 8.7, 2.7 Hz, 1H), 4.78 (dd, J = 5.0, 2.8 Hz, 1H), 4.31 (dd, J = 10.9, 5.0 Hz, 1H), 4.18 (dd, J = 10.9, 2.8 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 162.95, 160.53, 154.68, 150.83, 145.16, 138.95, 136.41, 136.38, 129.89, 129.81, 127.32, 127.17, 117.59, 116.34, 115.55, 115.34, 108.84, 103.95, 68.98, 60.63. HRMS (+ESI): Found m/z 360.10063, [M + Na]+. C20H16FNO3Na [360.10119].
3-(4-Fluorophenyl)-4-(quinolin-3-yl)-3,4-dihydro-2H-benzo[b][1,4]oxazin-7-ol (15c). Brown gum, yield: 87%. IR (neat) 2923, 2798, 1598 cm−1. 1H NMR (400 MHz, DMSO-d6) δ 9.12 (s, 1H), 8.84 (d, J = 2.7 Hz, 1H), 7.99–7.85 (m, 2H), 7.88–7.78 (m, 1H), 7.60 (ddd, J = 8.4, 6.9, 1.5 Hz, 1H), 7.52 (ddd, J = 8.2, 6.9, 1.3 Hz, 1H), 7.51–7.42 (m, 2H), 7.21–7.07 (m, 2H), 6.90 (d, J = 8.7 Hz, 1H), 6.36–6.26 (m, 2H), 5.24 (d, J = 3.1 Hz, 1H), 4.61 (dd, J = 11.2, 3.0 Hz, 1H), 4.30 (dd, J = 11.2, 2.7 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 163.04, 160.63, 153.02, 147.79, 146.65, 143.82, 141.66, 135.59, 135.56, 129.56, 129.48, 128.86, 128.66, 128.07, 127.65, 127.54, 124.89, 122.10, 120.05, 115.83, 115.62, 109.37, 104.22, 67.68, 59.68. HRMS (+ESI): Found m/z 373.13476, [M + H]+. C23H18FN2O2 [373.13523].