Potential Immunoregulatory Mechanism of Plant Saponins: A Review
Abstract
1. Introduction
2. Saponins Promote the Growth and Development of Immune Organs
3. Saponins Enhance Immune Cell Activity
3.1. Enhancement of Macrophage Activity by Saponins
3.2. Upregulation of NK Cell Killing Ability by Saponins
3.3. Promotion of Dendritic Cell Maturation by Saponins
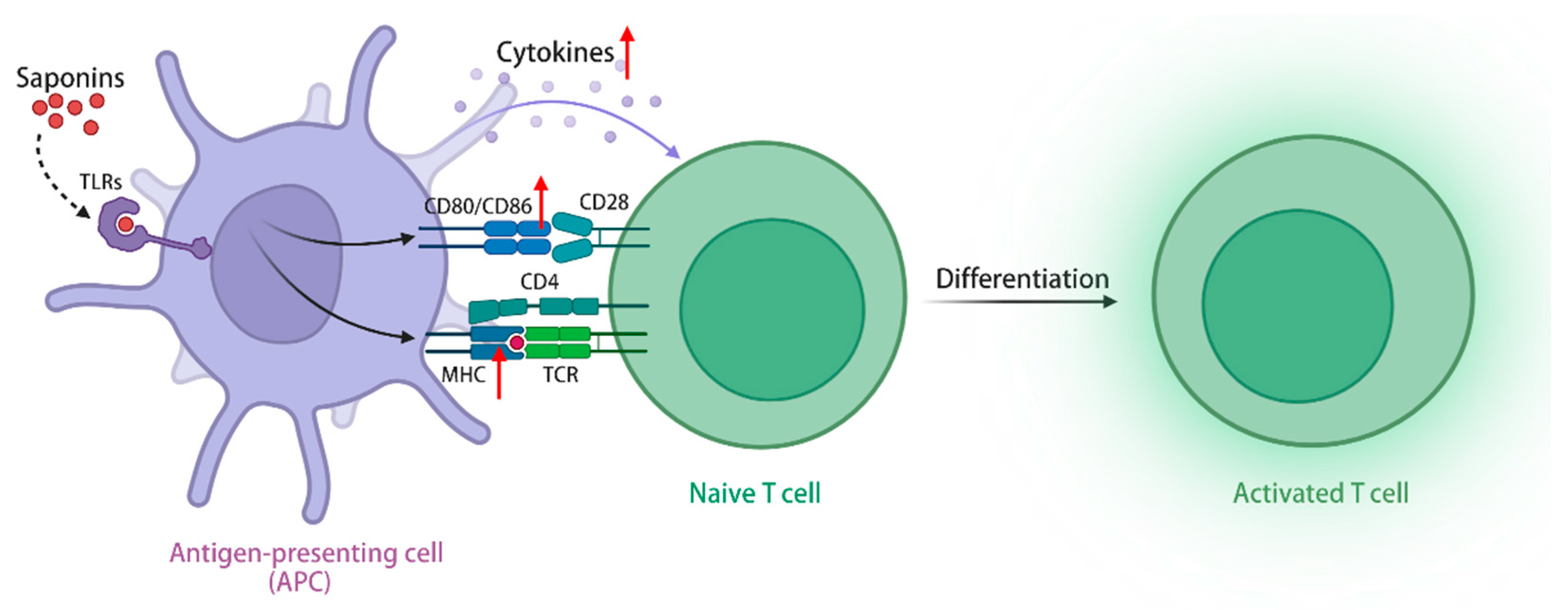
3.4. Activation of T and B Lymphocytes by Saponins
4. Saponins Upregulate the Expression of Immunomodulatory Molecules
4.1. Regulation of Cytokine and Chemokine Expression by Saponins
4.2. Promotion of Antibody Secretion by Saponins
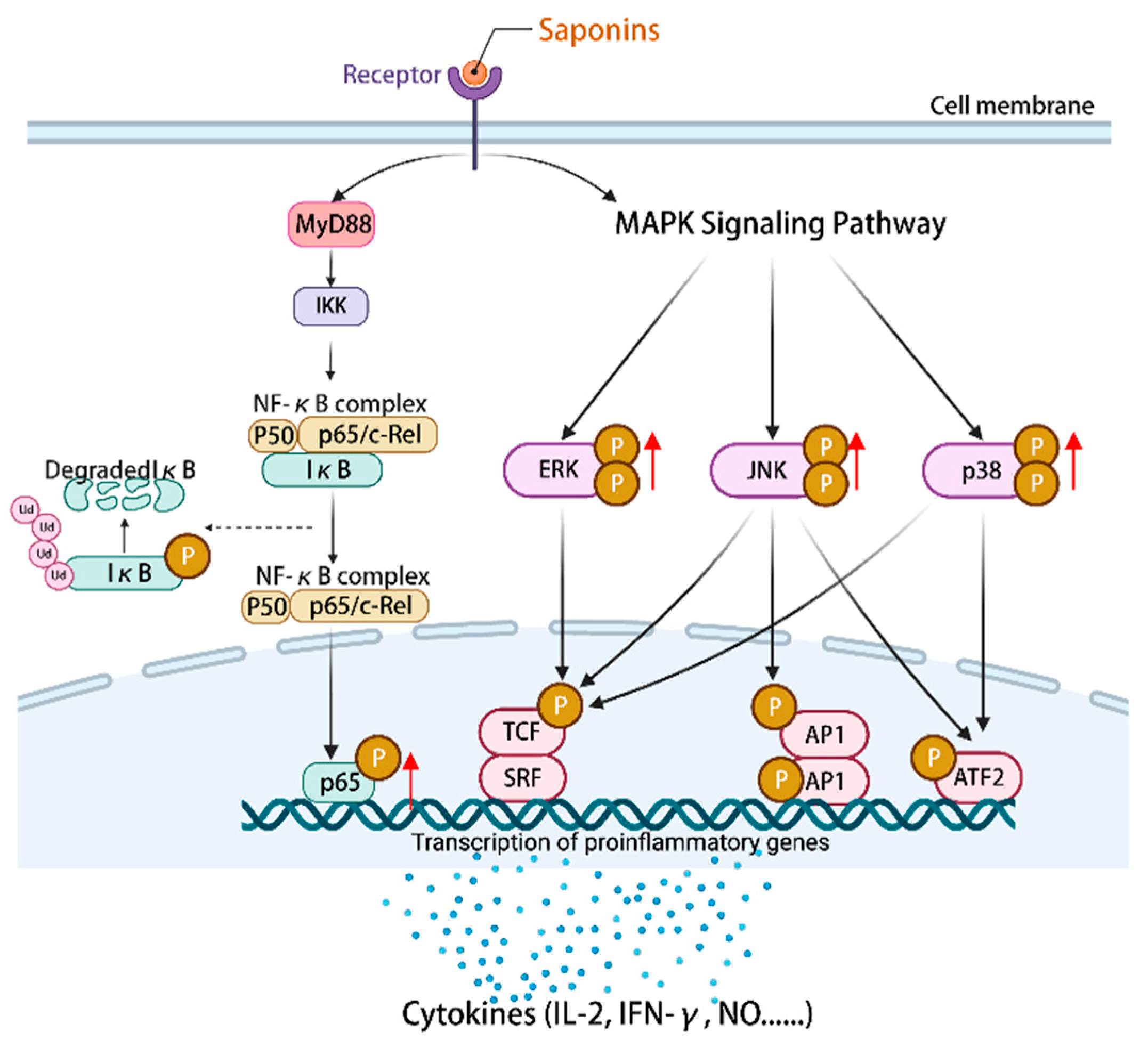
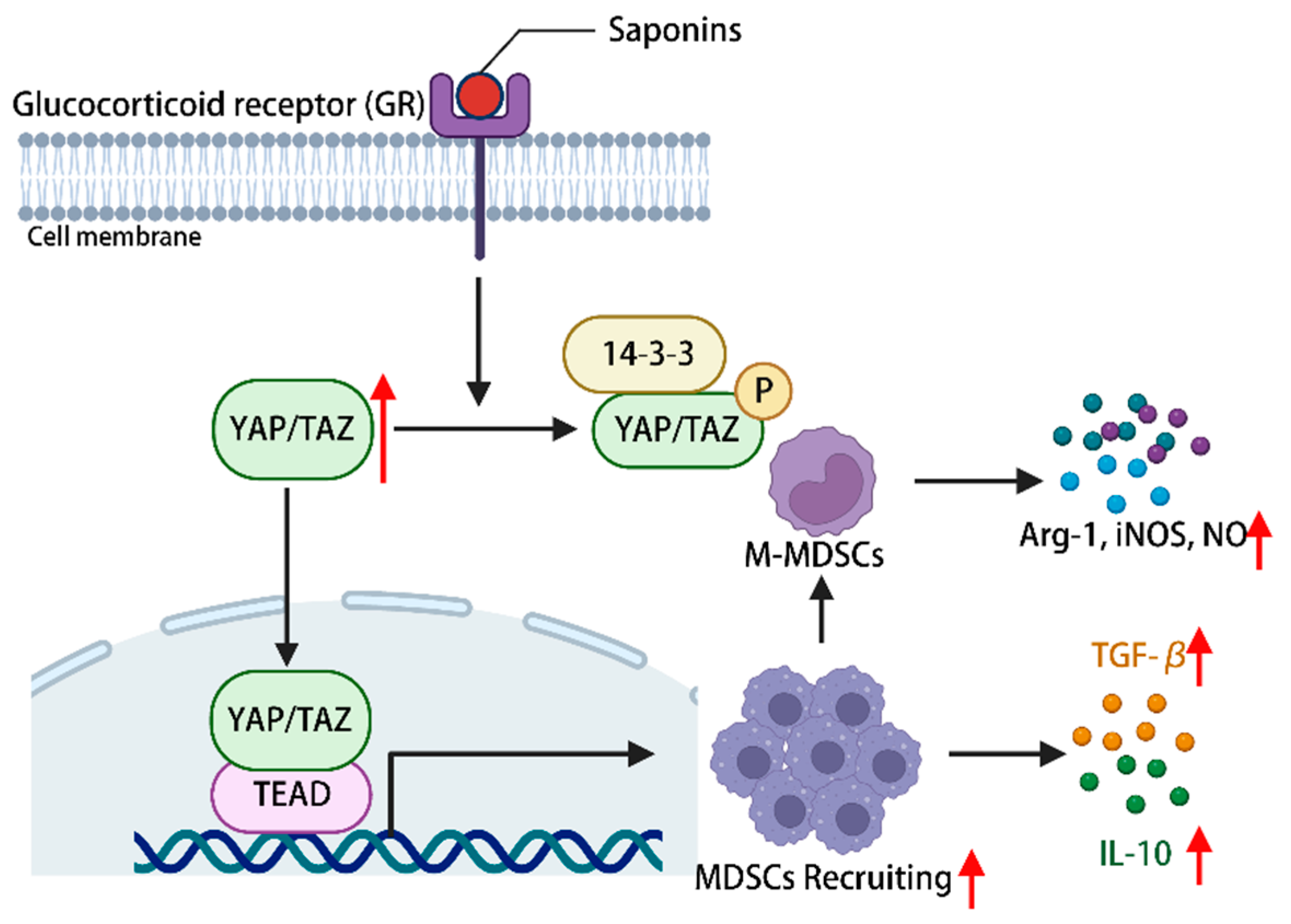
5. Saponins Modulate Immune-Related Signaling Pathways
6. Limitations of Saponins as Potential Immunomodulators
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, R.; Xiao, Y.; Yang, Q.; Pan, M.; Hao, Y.; He, X.; Peng, J.; Qian, Z. Ag (2)s nanoparticle-mediated Multiple Ablations Reinvigorates the Immune Response for Enhanced Cancer Photo-immunotherapy. Biomaterials 2021, 264, 120451. [Google Scholar] [CrossRef] [PubMed]
- Karkossa, I.; Raps, S.; von Bergen, M.; Schubert, K. Systematic Review of Multi-omics Approaches to Investigate Toxicological Effects in Macrophages. Int. J. Mol. Sci. 2020, 21, 9371. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Kumar, K.; Brisc, C.; Rus, M.; Nistor-Cseppento, D.C.; Bustea, C.; Aron, R.; Pantis, C.; Zengin, G.; Sehgal, A.; et al. Exploring the Multifocal Role of Phytochemicals as Immunomodulators. Biomed. Pharmacother. 2021, 133, 110959. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; You, D.J.; Lee, K.W. Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria Pinnatifida in Cyclophosphamide-induced Immunosuppressed mice. Mar. Drugs 2019, 17, 447. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dong, Q.; Kan, X.; Peng, L.; Xu, X.; Fang, Y.; Yang, J. Immunomodulatory Activity of a Novel Polysaccharide from Lonicera Japonica in Immunosuppressed Mice Induced by Cyclophosphamide. PLoS ONE 2018, 13, e204152. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Cui, J.; Song, W.; Liang, Y.; Hu, Y.; Guo, Y. Epidemiological Survey of Antinuclear Antibodies in Healthy Population and Analysis of Clinical Characteristics of Positive Population. J. Clin. Lab. Anal. 2019, 33, e22965. [Google Scholar] [CrossRef] [PubMed]
- Ramanadham, M.; Nageshwari, B. Anti-proliferative Effect of Levamisole on Human Myeloma Cell Lines in Vitro. J. Immunotoxicol. 2010, 7, 327–332. [Google Scholar] [CrossRef]
- Tong, L.X.; Sun, G.S.; Teng, J.M. Pediatric Lichen Sclerosis: A Review of the Epidemiology and Treatment Options. Pediatr. Dermatol. 2015, 32, 593–599. [Google Scholar] [CrossRef]
- Jantan, I.; Haque, M.A.; Ilangkovan, M.; Arshad, L. An Insight into the Modulatory Effects and Mechanisms of Action of Phyllanthus Species and Their Bioactive Metabolites on the Immune System. Front. Pharmacol. 2019, 10, 878. [Google Scholar] [CrossRef]
- Ren, W.; Sun, H.; Gao, G.F.; Chen, J.; Sun, S.; Zhao, R.; Gao, G.; Hu, Y.; Zhao, G.; Chen, Y.; et al. Recombinant SARS-CoV-2 spike S1-fc Fusion Protein Induced High Levels of Neutralizing Responses in Nonhuman Primates. Vaccine 2020, 38, 5653–5658. [Google Scholar] [CrossRef]
- Fleck, J.D.; Kauffmann, C.; Spilki, F.; Lencina, C.L.; Roehe, P.M.; Gosmann, G. Adjuvant Activity of Quillaja Brasiliensis Saponins on the Immune Responses to Bovine Herpesvirus Type 1 in Mice. Vaccine 2006, 24, 7129–7134. [Google Scholar] [CrossRef] [PubMed]
- de Costa, F.; Yendo, A.C.; Cibulski, S.P.; Fleck, J.D.; Roehe, P.M.; Spilki, F.R.; Gosmann, G.; Fett-Neto, A.G. Alternative Inactivated Poliovirus Vaccines Adjuvanted with Quillaja Brasiliensis or Quil-A Saponins are Equally Effective in Inducing Specific Immune Responses. PLoS ONE 2014, 9, e105374. [Google Scholar] [CrossRef] [PubMed]
- Yendo, A.C.; de Costa, F.; Cibulski, S.P.; Teixeira, T.F.; Colling, L.C.; Mastrogiovanni, M.; Soule, S.; Roehe, P.M.; Gosmann, G.; Ferreira, F.A.; et al. A Rabies Vaccine Adjuvanted with Saponins from Leaves of the Soap Tree (Quillaja Brasiliensis) Induces Specific Immune Responses and Protects Against Lethal Challenge. Vaccine 2016, 34, 2305–2311. [Google Scholar] [CrossRef] [PubMed]
- Juang, Y.P.; Liang, P.H. Biological and Pharmacological Effects of Synthetic Saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef] [PubMed]
- Vincken, J.P.; Heng, L.; de Groot, A.; Gruppen, H. Saponins, Classification and Occurrence in the Plant Kingdom. Phytochemistry 2007, 68, 275–297. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent Advances in Biotransformation of Saponins. Molecules 2019, 24, 2365. [Google Scholar] [CrossRef] [PubMed]
- Lorent, J.H.; Quetin-Leclercq, J.; Mingeot-Leclercq, M.P. The Amphiphilic Nature of Saponins and Their Effects on Artificial and Biological Membranes and Potential Consequences for Red Blood and Cancer Cells. Org. Biomol. Chem. 2014, 12, 8803–8822. [Google Scholar] [CrossRef] [PubMed]
- Bafundo, K.W.; Duerr, I.; Mcnaughton, J.L.; Johnson, A.B. The Effects of a Quillaja and Yucca Combination on Performance and Carcass Traits of Coccidia-Vaccinated Broilers Exposed to an Rnteric Disease Challenge. Poult. Sci. 2021, 100, 101391. [Google Scholar] [CrossRef]
- Warshakoon, H.J.; Hood, J.D.; Kimbrell, M.R.; Malladi, S.; Wu, W.Y.; Shukla, N.M.; Agnihotri, G.; Sil, D.; David, S.A. Potential Adjuvantic Properties of Innate Immune Stimuli. Hum. Vaccin. 2009, 5, 381–394. [Google Scholar] [CrossRef]
- Richou, R.; Jensen, R.; Belin, C. Research on Saponin, an Adjuvant Substance Which Stimulates Immunity. Rev. Immunol. Ther. Antimicrob. 1964, 28, 49–62. [Google Scholar]
- Qi, Z.; Chen, L.; Li, Z.; Shao, Z.; Qi, Y.; Gao, K.; Liu, S.; Sun, Y.; Li, P.; Liu, J. Immunomodulatory Effects of (24r)-Pseudo-Ginsenoside HQ and (24s)-Pseudo-Ginsenoside HQ on Cyclophosphamide-induced Immunosuppression and Their Anti-Tumor Effects Study. Int. J. Mol. Sci. 2019, 20, 836. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.L.; Dou, D.Q.; Chen, X.H.; Yang, H.Z.; Guo, N.; Cheng, G.F. Protopanaxatriol-type Ginsenosides Differentially Modulate Type 1 and Type 2 Cytokines Production from Murine Splenocytes. Planta Med. 2005, 71, 202–207. [Google Scholar] [CrossRef]
- Yuan, D.; Yuan, Q.; Cui, Q.; Liu, C.; Zhou, Z.; Zhao, H.; Dun, Y.; Wang, T.; Zhang, C. Vaccine Adjuvant Ginsenoside Rg1 Enhances Immune Responses Against Hepatitis B Surface Antigen in Mice. Can. J. Physiol. Pharmacol. 2016, 94, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.F.; Yu, H.J.; Liu, Z.; Zhang, D.F.; Zhou, Q.J.; Zhang, H.L.; Du, A.F. Ginsenoside Rg1 Enhances Immune Response Induced by Recombinant Toxoplasma Gondii Sag1 Antigen. Vet. Parasitol. 2011, 179, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Bi, S.; Xu, W.; Zhang, C.; Lu, Y.; Zhai, L.; Hu, S. Improved Immune Response to an Attenuated Pseudorabies Virus Vaccine by Ginseng Stem-leaf Saponins (GSLS) in Combination with Thimerosal (TS). Antiviral Res. 2016, 132, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, A.; Sun, H.; Ye, Y.; Fang, W. Ginsenoside Rd Elicits Th1 and Th2 Immune Responses to Ovalbumin in Mice. Vaccine 2007, 25, 161–169. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Yuan, L.; Maqbool, B.; Xu, W.; He, S.; Guan, R.; Hu, S. A Solution with Ginseng Saponins and Selenium as Vaccine Diluent to Increase Th1/Th2 Immune Responses in Mice. J. Immunol. Res. 2020, 2020, 2714257. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kuang, N.; Hu, W.Y.; Yin, D.; Wei, Y.Y.; Hu, T.J. The Effect of Panax Notoginseng Saponins on Oxidative Stress Induced by PCV2 Infection in Immune Cells: In Vitro and in Vivo Studies. J. Vet. Sci. 2020, 21, e61. [Google Scholar] [CrossRef]
- Sun, H.; Ye, Y.; Pan, Y. Immunological-Adjuvant Saponins from the Roots of Panax Notoginseng. Chem. Biodivers. 2005, 2, 510–515. [Google Scholar] [CrossRef]
- Yang, Z.G.; Ye, Y.P.; Sun, H.X. Immunological Adjuvant Effect of Ginsenoside Rh4 from the Roots of Panax Notoginseng on Specific Antibody and Cellular Response to Ovalbumin in Mice. Chem. Biodivers. 2007, 4, 232–240. [Google Scholar] [CrossRef]
- Sun, H.X.; Ye, Y.P.; Pan, H.J.; Pan, Y.J. Adjuvant Effect of Panax Notoginseng Saponins on the Immune Responses to Ovalbumin in Mice. Vaccine 2004, 22, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Pan, H.J.; Pan, Y.J. Haemolytic Activities and Immunologic Adjuvant Effect of Panax Notoginseng Saponins. Acta Pharmacol. Sin. 2003, 24, 1150–1154. [Google Scholar] [PubMed]
- Zou, Q.; Wu, X.; Wang, J.; Xia, D.; Deng, M.; Ding, Y.; Dai, Y.; Zhao, S.; Chen, T. [Therapeutic Effect of Panax Notoginseng Saponins Combined with Cyclophosphamide in Mice Bearing Hepatocellular Carcinoma H22 Cell Xenograft]. Nan Fang. Yi Ke Da Xue Xue Bao 2022, 42, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Yakubogullari, N.; Cagir, A.; Bedir, E.; Sag, D. Astragalus Saponins, Astragaloside VII and Newly Synthesized Derivatives, Induce Dendritic Cell Maturation and T Cell Activation. Vaccines 2023, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.D.; You, C.G.; Zhang, R.L.; Gao, P.; Wang, Z.R. Effects of Astragalus Polysaccharides and Astragalosides on the Phagocytosis of Mycobacterium Tuberculosis by Macrophages. J. Int. Med. Res. 2007, 35, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Yakubogullari, N.; Coven, F.O.; Cebi, N.; Coven, F.; Coven, N.; Genc, R.; Bedir, E.; Nalbantsoy, A. Evaluation of Adjuvant Activity of Astragaloside VII and its Combination with Different Immunostimulating Agents in Newcastle Disease Vaccine. Biologicals 2021, 70, 28–37. [Google Scholar] [CrossRef]
- Yakubogullari, N.; Genc, R.; Coven, F.; Nalbantsoy, A.; Bedir, E. Development of Adjuvant Nanocarrier Systems for Seasonal Influenza a (H3N2) Vaccine Based on Astragaloside VII and Gum Aragacanth (APS). Vaccine 2019, 37, 3638–3645. [Google Scholar] [CrossRef]
- Nalbantsoy, A.; Nesil, T.; Erden, S.; Calis, I.; Bedir, E. Adjuvant Effects of Astragalus Saponins Macrophyllo Saponin B and Astragaloside VII. J. Ethnopharmacol. 2011, 134, 897–903. [Google Scholar] [CrossRef]
- Wan, C.P.; Gao, L.X.; Hou, L.F.; Yang, X.Q.; He, P.L.; Yang, Y.F.; Tang, W.; Yue, J.M.; Li, J.; Zuo, J.P. Astragaloside II Triggers T Cell Activation Through Regulation of CD45 Protein Tyrosine Phosphatase Activity. Acta Pharmacol. Sin. 2013, 34, 522–530. [Google Scholar] [CrossRef]
- Huang, P.; Lu, X.; Yuan, B.; Liu, T.; Dai, L.; Liu, Y.; Yin, H. Astragaloside IV Alleviates E. Coli-caused Peritonitis via Upregulation of Neutrophil Influx to the Site of Infection. Int. Immunopharmacol. 2016, 39, 377–382. [Google Scholar] [CrossRef]
- Sharma, R.; Palanisamy, A.; Dhama, K.; Mal, G.; Singh, B.; Singh, K.P. Exploring the Possible Use of Saponin Adjuvants in COVID-19 Vaccine. Human. Vaccines Immunother. 2020, 16, 2944–2953. [Google Scholar] [CrossRef] [PubMed]
- Morein, B.; Sundquist, B.; Hoglund, S.; Dalsgaard, K.; Osterhaus, A. ISCOM, a Novel Structure for Antigenic Presentation of Membrane Proteins from Enveloped Viruses. Nature 1984, 308, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; O’Hagan, D.T. Recent Advances in Veterinary Vaccine Adjuvants. Int. J. Parasit. 2003, 33, 469–478. [Google Scholar] [CrossRef]
- Sun, H.X.; Xie, Y.; Ye, Y.P. Advances in Saponin-based Adjuvants. Vaccine 2009, 27, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Garcon, N.; Van Mechelen, M. Recent Clinical Experience with Vaccines Using MPL- and QS-21-containing Adjuvant Systems. Expert. Rev. Vaccines 2011, 10, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Garcon, N.; Chomez, P.; Van Mechelen, M. GlaxoSmithKline Adjuvant Systems in Vaccines: Concepts, Achievements and Perspectives. Expert. Rev. Vaccines 2007, 6, 723–739. [Google Scholar] [CrossRef]
- Song, X.; Hu, S. Adjuvant Activities of Saponins from Traditional Chinese Medicinal Herbs. Vaccine 2009, 27, 4883–4890. [Google Scholar] [CrossRef]
- Wang, P.; Skalamera, D.; Sui, X.; Zhang, P.; Michalek, S.M. Synthesis and Evaluation of QS-7-based Vaccine Adjuvants. ACS Infect. Dis. 2019, 5, 974–981. [Google Scholar] [CrossRef]
- Liu, G.; Anderson, C.; Scaltreto, H.; Barbon, J.; Kensil, C.R. QS-21 Structure/Function Studies: Effect of Acylation on Adjuvant Activity. Vaccine 2002, 20, 2808–2815. [Google Scholar] [CrossRef]
- Deng, K.; Adams, M.M.; Gin, D.Y. Synthesis and Structure Verification of the Vaccine Adjuvant QS-7-api. Synthetic Access to Homogeneous Quillaja Saponaria Immunostimulants. J. Am. Chem. Soc. 2008, 130, 5860–5861. [Google Scholar] [CrossRef]
- Ouyang, K.; Chen, L.; Sun, H.; Du, J.; Shi, M. Screening and Appraisal for Immunological Adjuvant-Active Fractions from Platycodon Grandiflorum Total Saponins. Immunopharmacol. Immunotoxicol. 2012, 34, 126–134. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, H.X.; Li, D. Platycodin D is a Potent Adjuvant of Specific Cellular and Humoral Immune Responses Against Recombinant Hepatitis B Antigen. Vaccine 2009, 27, 757–764. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, H.X.; Li, D. Platycodin D Improves the Immunogenicity of Newcastle Disease Virus-Based Recombinant Avian Influenza Vaccine in Mice. Chem. Biodivers. 2010, 7, 677–689. [Google Scholar] [CrossRef]
- Xie, Y.; He, S.W.; Sun, H.X.; Li, D. Platycodin D2 Improves Specific Cellular and Humoral Responses to Hepatitis B Surface Antigen in Mice. Chem. Biodivers. 2010, 7, 178–185. [Google Scholar] [CrossRef]
- Xie, Y.; Deng, W.; Sun, H.; Li, D. Platycodin D2 is a Potential Less Hemolytic Saponin Adjuvant Eliciting Th1 and Th2 Immune Responses. Int. Immunopharmacol. 2008, 8, 1143–1150. [Google Scholar] [CrossRef]
- Xie, Y.; Ye, Y.P.; Sun, H.X.; Li, D. Contribution of the Glycidic Moieties to the Hemolytic and Adjuvant Activity of Platycodigenin-type Saponins from the Root of Platycodon Grandiflorum. Vaccine 2008, 26, 3452–3460. [Google Scholar] [CrossRef]
- Xie, Y.; Pan, H.; Sun, H.; Li, D. A Promising Balanced Th1 and Th2 Directing Immunological Adjuvant, Saponins from the Root of Platycodon Grandiflorum. Vaccine 2008, 26, 3937–3945. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, M.; Mao, S. Adjuvant Effects of Platycodin D on Immune Responses to Infectious Bronchitis Vaccine in Chickens. J. Poult. Sci. 2020, 57, 160–167. [Google Scholar] [CrossRef]
- Sun, T.; Yan, X.; Guo, W.; Zhao, D. Evaluation of Cytotoxicity and Immune-modulatory Activities of Soyasaponin Ab: An in Vitro and in Vivo Study. Phytomedicine 2014, 21, 1759–1766. [Google Scholar] [CrossRef]
- Naveed, G.; Ehtisham-Ul-Haque, S.; Khan, I.; Rahman, S.U.; Anam, S.; Usman, M.; Shakir, M.Z.; Naveed, A.; Abbas, G.; Anjum, F.R. Enhancement in Humoral Response Against Inactivated Newcastle Disease Vaccine in Broiler Chickens Administered Orally with Plant-Derived Soyasaponin. Poult. Sci. 2020, 99, 1921–1927. [Google Scholar] [CrossRef]
- Li, P.; Liu, Y.; Gao, M.; Fu, J.; Guo, Y. Dietary Soy Saponin Improves Antioxidant and Immune Function of Layer Hens. J. Poult. Sci. 2022, 59, 197–205. [Google Scholar] [CrossRef]
- de Groot, C.; Musken, M.; Bleckmann, M.; Ebensen, T.; Guzman, C.A.; Muller-Goymann, C.C. Novel Colloidal Associations of Soyasaponins and Lipid Components (Dppc, Cholesterol) as Potential Adjuvants for Vaccines. Vaccine 2019, 37, 4975–4986. [Google Scholar] [CrossRef]
- Sun, H.; He, S.; Shi, M. Adjuvant-active Fraction from Albizia Julibrissin Saponins Improves Immune Responses by Inducing Cytokine and Chemokine at the Site of Injection. Int. Immunopharmacol. 2014, 22, 346–355. [Google Scholar] [CrossRef]
- Sun, H.X. Adjuvant Effect of Achyranthes Bidentata Saponins on Specific Antibody and Cellular Response to Ovalbumin in Mice. Vaccine 2006, 24, 3432–3439. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.; Varshney, R.; Mal, G.; Ghosh, M.; Singh, B. Evaluation of Immunological Adjuvant Activities of Saponin Rich Fraction from the Fruits of Asparagus Adscendens Roxb with Less Adverse Reactions. Drug Chem. Toxicol. 2023, 46, 557–565. [Google Scholar] [CrossRef]
- Kukhetpitakwong, R.; Hahnvajanawong, C.; Homchampa, P.; Leelavatcharamas, V.; Satra, J.; Khunkitti, W. Immunological Adjuvant Activities of Saponin Extracts from the Pods of Acacia Concinna. Int. Immunopharmacol. 2006, 6, 1729–1735. [Google Scholar] [CrossRef]
- Mao, G.H.; Zhang, Z.H.; Fei, F.; Ding, Y.Y.; Zhang, W.J.; Chen, H.; Ali, S.S.; Zhao, T.; Feng, W.W.; Wu, X.Y.; et al. Effect of Grifola Frondosa Polysaccharide on Anti-Tumor Activity in Combination with 5-Fu in Heps-Bearing Mice. Int. J. Biol. Macromol. 2019, 121, 930–935. [Google Scholar] [CrossRef]
- Zheng, S.; Zheng, H.; Zhang, R.; Piao, X.; Hu, J.; Zhu, Y.; Wang, Y. Immunomodulatory Effect of Ginsenoside Rb2 Against Cyclophosphamide-Induced Immunosuppression in Mice. Front. Pharmacol. 2022, 13, 927087. [Google Scholar] [CrossRef]
- Pislyagin, E.A.; Manzhulo, I.V.; Gorpenchenko, T.Y.; Dmitrenok, P.S.; Avilov, S.A.; Silchenko, A.S.; Wang, Y.M.; Aminin, D.L. Cucumarioside A(2)-2 Causes Macrophage Activation in Mouse Spleen. Mar. Drugs 2017, 15, 341. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Y.; Yan, S.; Song, B.; Liu, Y.; Gao, M.; Tang, D.; Guo, Y. Soya Saponin Improves Egg-Laying Performance and Immune Function of Laying Hens. J. Anim. Sci. Biotechnol. 2022, 12, 126. [Google Scholar] [CrossRef]
- Szulc-Dabrowska, L.; Bossowska-Nowicka, M.; Struzik, J.; Toka, F.N. Cathepsins in Bacteria-Macrophage Interaction: Defenders or Victims of Circumstance? Front. Cell. Infect. Microbiol. 2020, 10, 601072. [Google Scholar] [CrossRef]
- Chen, C.; Su, X.; Hu, Z. Immune Promotive Effect of Bioactive Peptides May be Mediated by Regulating the Expression of Socs1/Mir-155. Exp. Ther. Med. 2019, 18, 1850–1862. [Google Scholar] [CrossRef]
- Gemperle, C.; Schmid, M.; Herova, M.; Marti-Jaun, J.; Wuest, S.J.; Loretz, C.; Hersberger, M. Regulation of the Formyl Peptide Receptor 1 (Fpr1) Gene in Primary Human Macrophages. PLoS ONE 2012, 7, e50195. [Google Scholar] [CrossRef]
- Hu, G.; Guo, M.; Xu, J.; Wu, F.; Fan, J.; Huang, Q.; Yang, G.; Lv, Z.; Wang, X.; Jin, Y. Nanoparticles Targeting Macrophages as Potential Clinical Therapeutic Agents Against Cancer and Inflammation. Front. Immunol. 2019, 10, 1998. [Google Scholar] [CrossRef]
- Cui, L.; Yang, G.; Ye, J.; Yao, Y.; Lu, G.; Chen, J.; Fang, L.; Lu, S.; Zhou, J. Dioscin Elicits Anti-Tumour Immunity by Inhibiting Macrophage M2 Polarization Via JNK And STAT3 Pathways in Lung Cancer. J. Cell. Mol. Med. 2020, 24, 9217–9230. [Google Scholar] [CrossRef]
- Yin, L.; Fan, Z.; Liu, P.; Chen, L.; Guan, Z.; Liu, Y.; Luo, Y. Anemoside A3 Activates TLR4-Dependent M1-Phenotype Macrophage Polarization to Represses Breast Tumor Growth and Angiogenesis. Toxicol. Appl. Pharmacol. 2021, 432, 115755. [Google Scholar] [CrossRef]
- Gotthardt, D.; Sexl, V. STATs in NK-cells: The Good, the Bad, and the Ugly. Front. Immunol. 2016, 7, 694. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural Killer Cells and Other Innate Lymphoid Cells in Cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Lee, Y.; Park, A.; Park, Y.J.; Jung, H.; Kim, T.D.; Noh, J.Y.; Choi, I.; Lee, S.; Ran, Y.S. Ginsenoside 20(r)-Rg3 Enhances Natural Killer Cell Activity by Increasing Activating Receptor Expression Through the MAPK/ERK Signaling Pathway. Int. Immunopharmacol. 2022, 107, 108618. [Google Scholar] [CrossRef]
- Nussing, S.; Sutton, V.R.; Trapani, J.A.; Parish, I.A. Beyond Target Cell Death—Granzyme Serine Proteases in Health and Disease. Mol. Asp. Med. 2022, 88, 101152. [Google Scholar] [CrossRef]
- Luo, L.; Qin, T.; Huang, Y.; Zheng, S.; Bo, R.; Liu, Z.; Xing, J.; Hu, Y.; Liu, J.; Wang, D. Exploring the Immunopotentiation of Chinese Yam Polysaccharide Poly (Lactic-Co-Glycolic Acid) Nanoparticles in an Ovalbumin Vaccine Formulation in Vivo. Drug Deliv. 2017, 24, 1099–1111. [Google Scholar] [CrossRef]
- Garg, R.; Shrivastava, P.; van Drunen, L.D.H.S. The Role of Dendritic Cells in Innate and Adaptive Immunity to Respiratory Syncytial Virus, and Implications for Vaccine Development. Expert. Rev. Vaccines 2012, 11, 1441–1457. [Google Scholar] [CrossRef]
- Welsby, I.; Detienne, S.; N’Kuli, F.; Thomas, S.; Wouters, S.; Bechtold, V.; De Wit, D.; Gineste, R.; Reinheckel, T.; Elouahabi, A.; et al. Lysosome-dependent Activation of Human Dendritic Cells by the Vaccine Adjuvant QS-21. Front. Immunol. 2016, 7, 663. [Google Scholar] [CrossRef]
- Ho, N.I.; Huis, I.T.V.L.; van Eck, V.D.S.J.; Heuts, B.; Looman, M.; Kers-Rebel, E.D.; van den Dries, K.; Dolstra, H.; Martens, J.; Hobo, W.; et al. Saponin-based Adjuvants Enhance Antigen Cross-Presentation in Human CD11c(+) CD1c(+) CD5(-) CD163(+) Conventional Type 2 Dendritic Cells. J. Immunother. Cancer 2023, 11, e007082. [Google Scholar] [CrossRef]
- Klingensmith, N.J.; Fay, K.T.; Lyons, J.D.; Chen, C.W.; Otani, S.; Liang, Z.; Chihade, D.B.; Burd, E.M.; Ford, M.L.; Coopersmith, C.M. Chronic Alcohol Ingestion Worsens Survival and Alters Gut Epithelial Apoptosis and CD8+ T Cell Function After Pseudomonas Aeruginosa Pneumonia-Induced Sepsis. Shock. 2019, 51, 453–463. [Google Scholar] [CrossRef]
- Zouggari, Y.; Ait-Oufella, H.; Bonnin, P.; Simon, T.; Sage, A.P.; Guerin, C.; Vilar, J.; Caligiuri, G.; Tsiantoulas, D.; Laurans, L.; et al. B Lymphocytes Trigger Monocyte Mobilization and Impair Heart Function After Acute Myocardial Infarction. Nat. Med. 2013, 19, 1273–1280. [Google Scholar] [CrossRef]
- He, F.; Ding, Y.; Liang, C.; Song, S.B.; Dou, D.Q.; Song, G.Y.; Kim, Y.H. Antitumor Effects of Dammarane-Type Saponins from Steamed Notoginseng. Pharmacogn. Mag. 2014, 10, 314–317. [Google Scholar] [CrossRef]
- Bengtsson, K.L.; Song, H.; Stertman, L.; Liu, Y.; Flyer, D.C.; Massare, M.J.; Xu, R.H.; Zhou, B.; Lu, H.; Kwilas, S.A.; et al. Matrix-M Adjuvant Enhances Antibody, Cellular and Protective Immune Responses of a Zaire Ebola/Makona Virus Glycoprotein (GP) Nanoparticle Vaccine in Mice. Vaccine 2016, 34, 1927–1935. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Choi, Y.S.; Eto, D.; Yang, J.A.; Lao, C.; Crotty, S. Cutting Edge: STAT1 is Required for IL-6-Mediated Bcl6 Induction for Early Follicular Helper Cell Differentiation. J. Immunol. 2013, 190, 3049–3053. [Google Scholar] [CrossRef]
- De Giovanni, M.; Cutillo, V.; Giladi, A.; Sala, E.; Maganuco, C.G.; Medaglia, C.; Di Lucia, P.; Bono, E.; Cristofani, C.; Consolo, E.; et al. Spatiotemporal Regulation of Type I Interferon Expression Determines the Antiviral Polarization of CD4+ T Cells. Nat. Immunol. 2020, 21, 321–330. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, W.; Xiong, Y.; Li, Y.; Wan, Q.; Zhou, W.; Zhao, H.; Xiao, Q.; Liu, D. Astragaloside IV Alleviates Ulcerative Colitis by Regulating the Balance of Th17/Treg Cells. Phytomedicine 2022, 104, 154287. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Zheng, X.; Lan, Z.; Shi, J.; Jiang, J.; Zwiep, T.; Li, Q.; Quan, D.; Zhang, Z.X.; et al. Prevention of Allograft Rejection in Heart Transplantation Through Concurrent Gene Silencing of TLR and Kinase Signaling Pathways. Sci. Rep. 2016, 6, 33869. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Y.; Ren, G.; Shao, Q.; Gao, W.; Sun, J.; Wang, H.; Ji, C.; Li, X.; Zhang, Y.; et al. Increased Expression of Surface CD44 in Hypoxia-Dcs Skews Helper T Cells Toward a Th2 Polarization. Sci. Rep. 2015, 5, 13674. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional Specialization of Interleukin-17 Family Members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef]
- Guo, M.F.; Dai, Y.J.; Gao, J.R.; Chen, P.J. Uncovering the Mechanism of Astragalus Membranaceus in the Treatment of Diabetic Nephropathy Based on Network Pharmacology. J. Diabetes Res. 2020, 2020, 5947304. [Google Scholar] [CrossRef]
- Hasan, A.; Nurunnabi, M.; Morshed, M.; Paul, A.; Polini, A.; Kuila, T.; Al, H.M.; Lee, Y.K.; Jaffa, A.A. Recent Advances in Application of Biosensors in Tissue Engineering. Biomed. Res. Int. 2014, 2014, 307519. [Google Scholar] [CrossRef]
- Kaushik, H.; Deshmukh, S.K.; Solanki, A.K.; Bhatia, B.; Tiwari, A.; Garg, L.C. Immunization with Recombinant Fusion of LTB and Linear Epitope (40-62) of Epsilon Toxin Elicits Protective Immune Response Against the Epsilon Toxin of Clostridium Perfringens Type D. AMB Express 2019, 9, 105. [Google Scholar] [CrossRef]
- Su, F.; Li, J.; Xue, Y.; Yu, B.; Ye, S.; Xu, L.; Fu, Y.; Yuan, X. Early Oral Administration of Ginseng Stem-Leaf Saponins Enhances the Peyer’s Patch-Dependent Maternal IgA Antibody Response to a Pedv Inactivated Vaccine in Mice, with Gut Microbiota Involvement. Vaccines 2023, 11, 830. [Google Scholar] [CrossRef]
- Long, K.K.; Pavlath, G.K.; Montano, M. Sca-1 Influences the Innate Immune Response During Skeletal Muscle Regeneration. Am. J. Physiol.-Cell Physiol. 2011, 300, C287–C294. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, J.; Zhang, Y.; Liu, Q.; Wang, Q.; Liu, X. A Compound Ginseng Stem Leaf Saponins and Aluminium Adjuvant Enhances the Potency of Inactivated Aeromonas Salmonicida Vaccine in Turbot. Fish. Shellfish. Immunol. 2022, 128, 60–66. [Google Scholar] [CrossRef]
- Blum, P.; Pircher, J.; Merkle, M.; Czermak, T.; Ribeiro, A.; Mannell, H.; Krotz, F.; Hennrich, A.; Spannagl, M.; Koppel, S.; et al. Arterial Thrombosis in the Context of HCV-Associated Vascular Disease can be Prevented by Protein C. Cell. Mol. Immunol. 2017, 14, 986–996. [Google Scholar] [CrossRef][Green Version]
- Byrd-Leifer, C.A.; Block, E.F.; Takeda, K.; Akira, S.; Ding, A. The Role of Myd88 and TLR4 in the LPS-Mimetic Activity of Taxol. Eur. J. Immunol. 2001, 31, 2448–2457. [Google Scholar] [CrossRef]
- Seth, S.; Oberdorfer, L.; Hyde, R.; Hoff, K.; Thies, V.; Worbs, T.; Schmitz, S.; Forster, R. CCR7 Essentially Contributes to the Homing of Plasmacytoid Dendritic Cells to Lymph Nodes Under Steady-State as well as Inflammatory Conditions. J. Immunol. 2011, 186, 3364–3372. [Google Scholar] [CrossRef]
- Ma, X.; Chi, X.; Yuan, L.; Wang, Y.; Li, Z.; Xu, W.; Rajput, Z.I.; Hu, S. Immunomodulatory Effect of Ginseng Stem-Leaf Saponins and Selenium on Harderian Gland in Immunization of Chickens to Newcastle Disease Vaccine. Vet. Immunol. Immunopathol. 2020, 225, 110061. [Google Scholar] [CrossRef]
- Su, F.; Yuan, L.; Zhang, L.; Hu, S. Ginsenosides Rg1 and React as Adjuvant Via TLR4 Signaling Pathway. Vaccine 2012, 30, 4106–4112. [Google Scholar] [CrossRef]
- Van Hoeven, N.; Wiley, S.; Gage, E.; Fiore-Gartland, A.; Granger, B.; Gray, S.; Fox, C.; Clements, D.E.; Parks, D.E.; Winram, S.; et al. A Combination of TLR-4 Agonist and Saponin Adjuvants Increases Antibody Diversity and Protective Efficacy of a Recombinant West Nile Virus Antigen. NPJ Vaccines 2018, 3, 39. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared Principles in NF-Kappa B Signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Xi, L.; Xiao, C.; Bandsma, R.H.; Naples, M.; Adeli, K.; Lewis, G.F. C-reactive Protein Impairs Hepatic Insulin Sensitivity and Insulin Signaling in Rats: Role of Mitogen-Activated Protein Kinases. Hepatology 2011, 53, 127–135. [Google Scholar] [CrossRef]
- Liu, T.; Wen, C.; Shen, X. Effect of Cisplatin Combined with Astragaloside on Inflammatory Factors and Immune Function in Rats with Breast Cancer (in Chinese). Chin. J. Gerontol. 2020, 40, 863–865. [Google Scholar] [CrossRef]
- Li, Y.; Meng, T.; Hao, N.; Tao, H.; Zou, S.; Li, M.; Ming, P.; Ding, H.; Dong, J.; Feng, S.; et al. Immune Regulation Mechanism of Astragaloside IV on Raw264.7 Cells Through Activating the NF-Kappa B/MAPK Signaling Pathway. Int. Immunopharmacol. 2017, 49, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Cui, W.Q.; Wei, Y.; Cui, J.; Qiu, J.; Hu, L.L.; Gong, W.Y.; Dong, J.C.; Liu, B.J. Correction: Astragaloside IV Inhibits Lung Cancer Progression and Metastasis by Modulating Macrophage Polarization Through AMPK Signaling. J. Exp. Clin. Cancer Res. 2023, 42, 70. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Tao, J.; Barbi, J.; Chen, Q.; Park, B.V.; Li, Z.; Zhang, N.; Lebid, A.; Ramaswamy, A.; Wei, P.; et al. Yap is Essential for Treg-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2018, 8, 1026–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lu, X.; Dey, P.; Deng, P.; Wu, C.C.; Jiang, S.; Fang, Z.; Zhao, K.; Konaparthi, R.; Hua, S.; et al. Targeting Yap-Dependent MDSC Infiltration Impairs Tumor Progression. Cancer Discov. 2016, 6, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, J.; Shi, Z.; Zhu, Y.; Yang, J.; Liu, X.; Que, R.; Lin, L.; Chen, Y.; Li, Y. Ginsenosides Regulates Innate Immunity to Affect Immune Microenvironment of AIH Through Hippo-Yap/TAZ Signaling Pathway. Front. Immunol. 2022, 13, 851560. [Google Scholar] [CrossRef] [PubMed]
- Manaargadoo-Catin, M.; Ali-Cherif, A.; Pougnas, J.L.; Perrin, C. Hemolysis by Surfactants—A Review. Adv. Colloid. Interface Sci. 2016, 228, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, S.; Yang, Y.; Wang, D.; Gao, H. Natural Barrigenol-Like Triterpenoids: A Comprehensive Review of Their Contributions to Medicinal Chemistry. Phytochemistry 2019, 161, 41–74. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, P.; Guo, W.; Huang, X.; Tian, X.; Wu, G.; Xu, B.; Li, F.; Yan, C.; Liang, X.J.; et al. Natural Berberine-Based Chinese Herb Medicine Assembled Nanostructures with Modified Antibacterial Application. ACS Nano 2019, 13, 6770–6781. [Google Scholar] [CrossRef]
- Sun, Y.; Fry, C.M.; Shieh, A.; Parquette, J.R. Self-Assembly of a Robust, Reduction-Sensitive Camptothecin Nanotube. Chem. Commun. 2020, 56, 10337–10340. [Google Scholar] [CrossRef]
- Kenth, S.; Sylvestre, J.P.; Fuhrmann, K.; Meunier, M.; Leroux, J.C. Fabrication of Paclitaxel Nanocrystals by Femtosecond Laser Ablation and Fragmentation. J. Pharm. Sci. 2011, 100, 1022–1030. [Google Scholar] [CrossRef]
- Campos, E.; Proenca, P.; Oliveira, J.L.; Pereira, A.; de Morais, R.L.; Fernandes, F.O.; Goncalves, K.C.; Polanczyk, R.A.; Pasquoto-Stigliani, T.; Lima, R.; et al. Carvacrol and Linalool Co-Loaded in Beta-Cyclodextrin-Grafted Chitosan Nanoparticles as Sustainable Biopesticide Aiming Pest Control. Sci. Rep. 2018, 8, 7623. [Google Scholar] [CrossRef] [PubMed]
- Cibulski, S.; Teixeira, T.F.; Varela, A.; de Lima, M.F.; Casanova, G.; Nascimento, Y.M.; Fechine, T.J.; Da, S.M.; Sesterheim, P.; Souza, D.O.; et al. IMXQB-80: A Quillaja Brasiliensis Saponin-Based Nanoadjuvant Enhances Zika Virus Specific Immune Responses in Mice. Vaccine 2021, 39, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhu, J.; Xu, Y.W.; Mou, F.F.; Shan, X.L.; Wang, Q.L.; Liu, B.N.; Ning, K.; Liu, J.J.; Wang, Y.C.; et al. Notoginsenoside R1-Loaded Mesoporous Silica Nanoparticles Targeting the Site of Injury Through Inflammatory Cells Improves Heart Repair After Myocardial Infarction. Redox Biol. 2022, 54, 102384. [Google Scholar] [CrossRef] [PubMed]
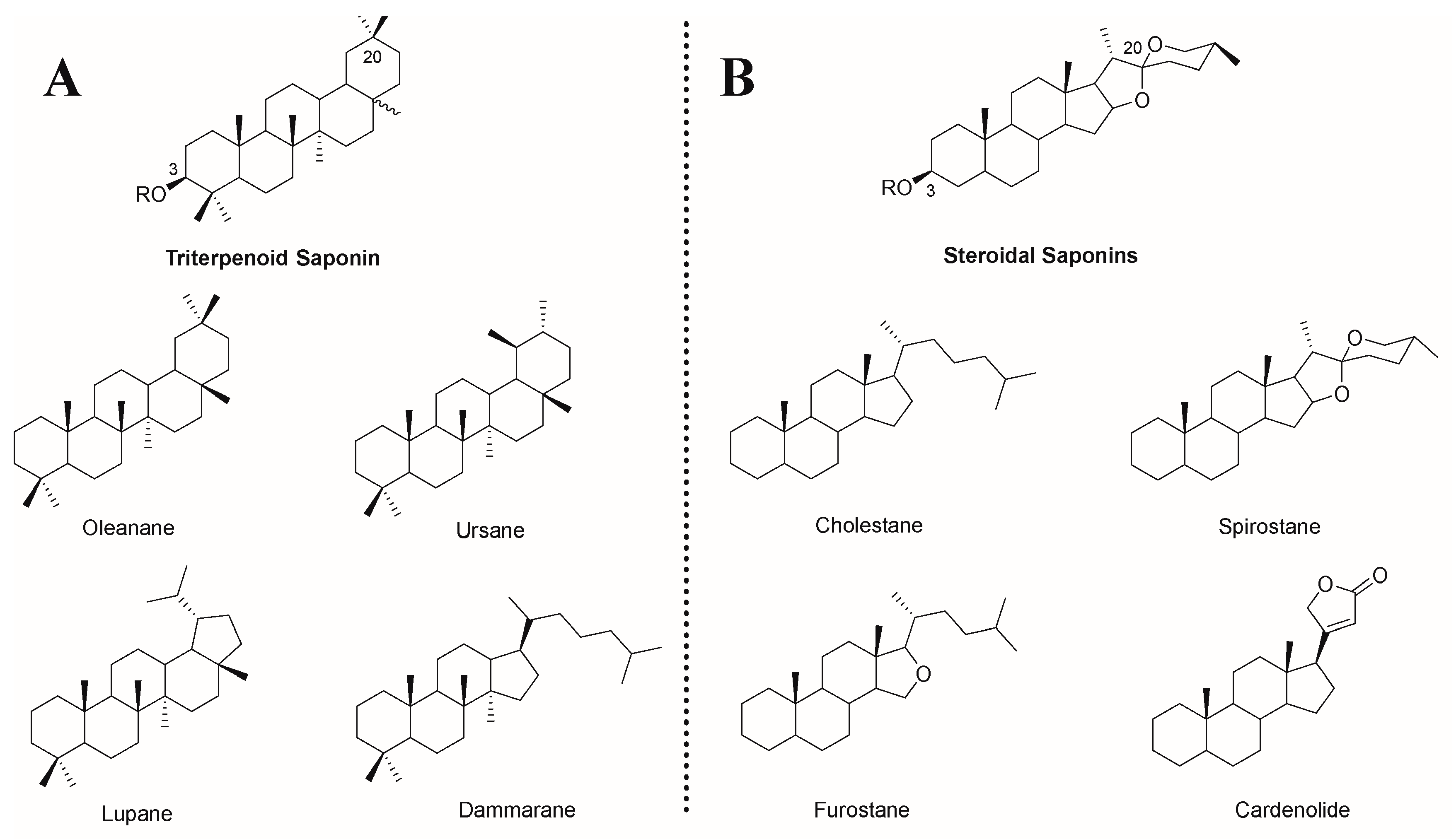
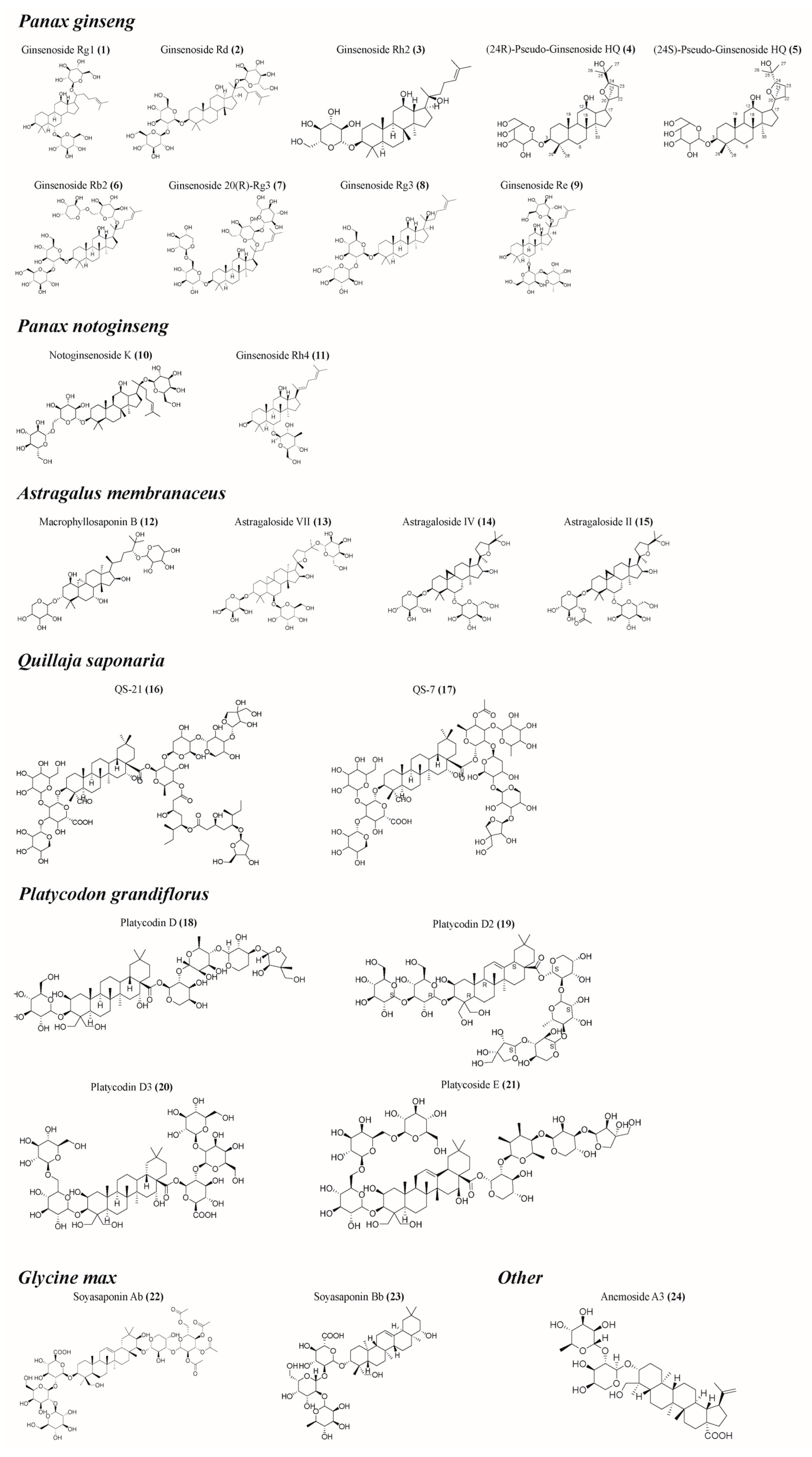
| Saponin Sources | Saponin Name | Immunoreaction |
|---|---|---|
| Panax ginseng | Ginsenoside Rg1 (1) Ginsenoside Rd (2) Ginsenoside Rh2 (3) (24R)-Pseudo-Ginsenoside HQ (4) (24S)-Pseudo-Ginsenoside HQ (5) Ginsenoside Rb2 (6) Ginsenoside 20(R)-Rg3 (7) Ginsenoside Rg3 (8) Ginsenoside Re (9) | Facilitating the upregulation of Th1 (IFN-γ, IL-2, T-bet, etc.) and Th2 (IL-4, IL-6, GATA3, etc.) cytokines and transcription factors [22,23,27]; Enhancing the levels of antigen-specific antibodies enhances the body’s immune defense against antigens [23,24,25]; Stimulating lymphocyte proliferation [21,24,25,26]; Preventing atrophy of immune organs [21]; Upregulating the CD4+/CD8+ T cell ratio [21]. |
| Panax notoginseng | Notoginsenoside K (10) Ginsenoside Rh4 (11) | Enhancing the levels of antigen-specific antibodies enhances the body’s immune defense against antigens [29,30,31,32,33]; Ameliorating antigen-induced oxidative stress in immune cells [28]; Improvement of monocyte and macrophage carbon scavenging [33]; Stimulating lymphocyte proliferation [30,31,32,33]; Promoting the secretion of cytokines such as TNF-α and IL-2 [33]. |
| Astragalus membranaceus | Macrophyllosaponin B (12) Astragaloside VII (13) Astragaloside IV (14) Astragaloside II (15) | Promoting the maturation and activation of APCs such as macrophages or dendritic cells [34,35]; Enhancing the levels of antigen-specific antibodies enhances the body’s immune defense against antigens [35,36,37]; Stimulating lymphocyte proliferation [38,39]; Stimulating the release of cytokines (IL-1β, IL-2, IL-4, IFN-γ, TNF-α, etc.) from immune cells to balance the Th1/Th2 of the organism [38,39]; Activation of CD4+ and CD8+ T cells [34]; Inhibition of TLR2 activator-induced reduction in CXCR4 expression and neutrophil migration improves the body’s antimicrobial immunity [40]. |
| Quillaja saponaria | QS-21 (16) QS-7 (17) | Enhancing the levels of antigen-specific antibodies enhances the body’s immune defense against antigens [43,44,45,46,47,48]; Stimulating the production of CTLs [43,44,49,50]; Promoting the secretion of relevant cytokines and inducing a balanced Th1/Th2 response [43,44,49,50]. |
| Platycodon grandiflorus | Platycodin D (18) Platycodin D2 (19) Platycodin D3 (20) Platycoside E (21) | Enhancing the levels of antigen-specific antibodies enhances the body’s immune defense against antigens [51,52,53,54,55,56,57]; Stimulating the proliferation of immune cells such as lymphocytes and monocytes [51,52,53,54,55,56,57,58]; Increasing the killing activity of NK cells and CTL [51,52,53]; Facilitating the upregulation of Th1 (IFN-γ, IL-2, T-bet, etc.) and Th2 (IL-4, IL-6, GATA3, etc.) cytokines and transcription factors for a better balance of Th1/Th2 immune responses [52,53,54,55,56,58]. |
| Glycine max | Soyasaponin Ab (22) Soyasaponin Bb (23) | Enhancing the levels of antigen-specific antibodies enhances the body’s immune defense against antigens [59,60]; Activating TRL4/NF-κB signaling [59]; Stimulating lymphocyte proliferation [61]; Stimulation of CD4+ and CD8+ T cells to bind to the antigen [62]; Promoting the secretion of cytokines such as TNF-α and IFN-γ [59,61]; Promoting elevated blood leukocyte, lymphocyte, and monocyte counts within the physiologic range [61]. |
| Other | Anemoside A3 (24) Achyranthes bidentata saponins Albizia julibrissin saponins Asparagus adscendens saponins Momordica charantia saponins Acacia concinna saponins | Enhancing the levels of antigen-specific antibodies enhances the body’s immune defense against antigens [63,64,65,66]; Stimulation of lymphocyte proliferation [63,64,65,66]; Enhancing natural killer (NK) cell killing activity [63]; Promoting the secretion of cytokines such as IL-12 [65]; Increasing CD3/CD19 expression in spleen and lymph nodes [65]; Induction of injection site cytokines (IL-12p40, IL-12p40/p70, IFN-γ, IL-1β, IL-3, IL-6, IL-9, IL-10, IL-13, TNF-α, sTNFR I, and sTNFR III) and chemokines (eotaxin, I-TAC, MIG, MIP-1α, RA, N T-E S, TECK, fractalkine, fasL, M-CSF, and GM-CSF) are expressed to promote immune cell recruitment at the injection site [63]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Luo, H.; Fan, L.; Tian, X.; Tang, A.; Wu, X.; Dong, K.; Su, Z. Potential Immunoregulatory Mechanism of Plant Saponins: A Review. Molecules 2024, 29, 113. https://doi.org/10.3390/molecules29010113
Shen L, Luo H, Fan L, Tian X, Tang A, Wu X, Dong K, Su Z. Potential Immunoregulatory Mechanism of Plant Saponins: A Review. Molecules. 2024; 29(1):113. https://doi.org/10.3390/molecules29010113
Chicago/Turabian StyleShen, Liuhong, Hao Luo, Lei Fan, Xinyu Tian, Anguo Tang, Xiaofeng Wu, Ke Dong, and Zhetong Su. 2024. "Potential Immunoregulatory Mechanism of Plant Saponins: A Review" Molecules 29, no. 1: 113. https://doi.org/10.3390/molecules29010113
APA StyleShen, L., Luo, H., Fan, L., Tian, X., Tang, A., Wu, X., Dong, K., & Su, Z. (2024). Potential Immunoregulatory Mechanism of Plant Saponins: A Review. Molecules, 29(1), 113. https://doi.org/10.3390/molecules29010113






