Extraction of Isoflavones, Alpha-Hydroxy Acids, and Allantoin from Soybean Leaves—Optimization by a Mixture Design of the Experimental Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Metabolite Content in Soybean Leaves
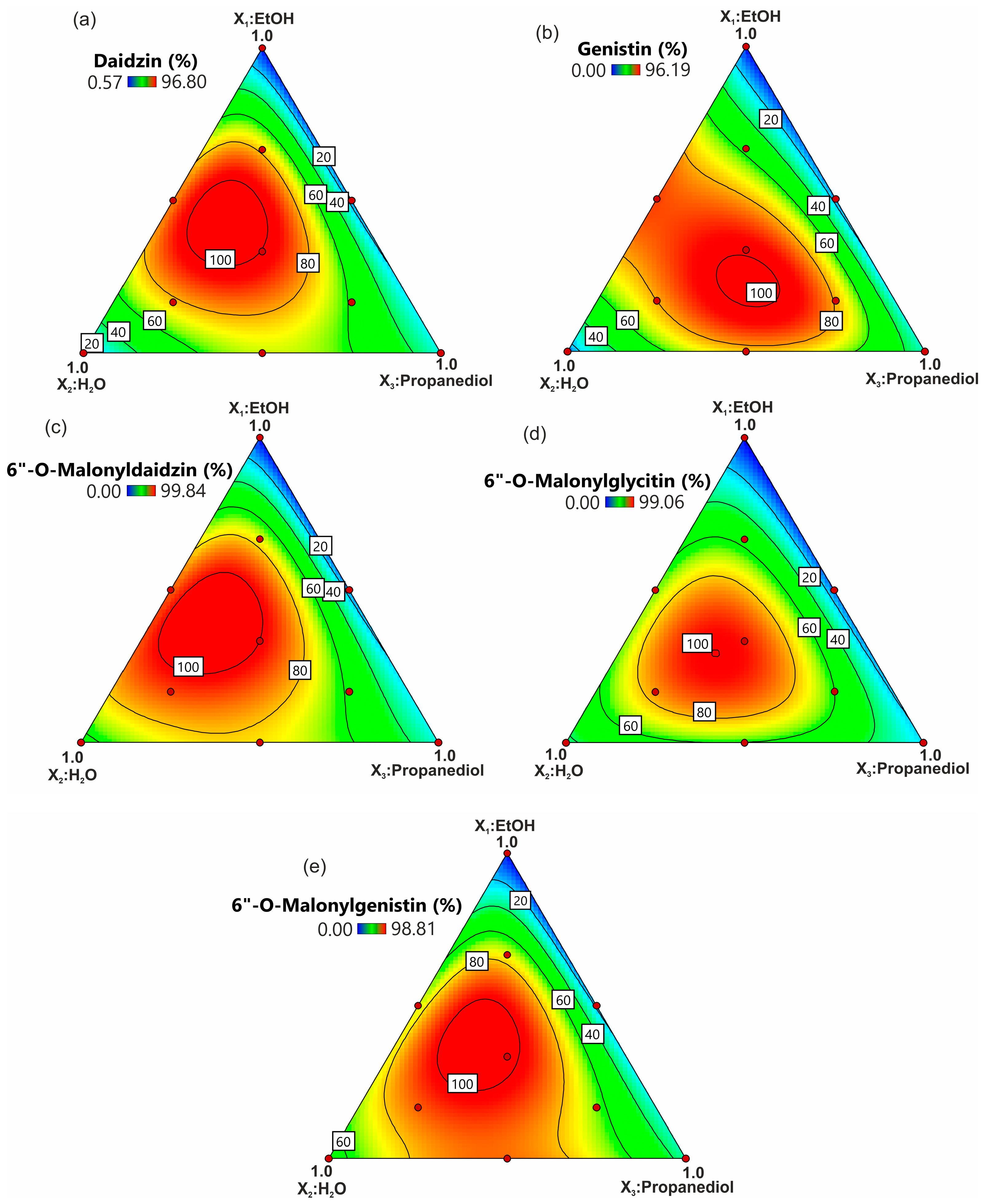
2.2. Polynomial Regression Model Development
2.3. Solvents’ Effect on Extraction Efficiency
2.4. Antioxidant Capacity and Soluble Phenol Content
2.5. Response Prediction and Model Confirmation
3. Materials and Methods
3.1. Chemicals and Reference Standard
3.2. Plant Materials and Extraction Procedure
3.3. Secondary Metabolite Analysis
3.4. Antioxidant Properties and Soluble Phenol Assay
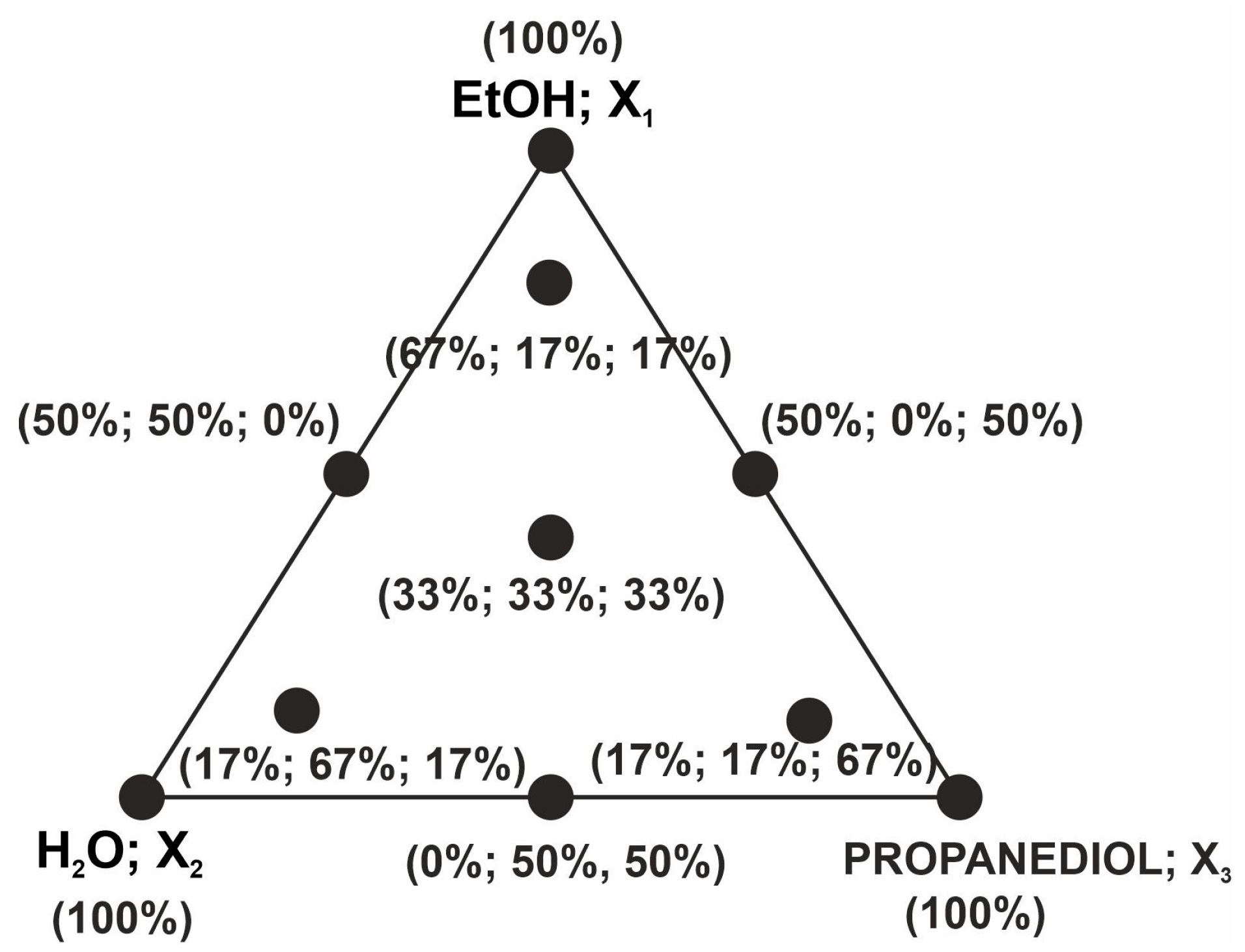
3.5. Experimental Design and Optimization of Solvent Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Sowa, I.; Wójciak-Kosior, M.; Strzemski, M.; Rokicka, K.; Blicharski, T.; Kocjan, R. Analysis of Compounds with Phytoestrogenic Activity in Dietary Supplements with Use of HPTLC-Densitometry Method. Acta Pol. Pharm. 2014, 71, 265–269. [Google Scholar] [PubMed]
- Dresler, S.; Wójciak-Kosior, M.; Sowa, I.; Strzemski, M.; Sawicki, J.; Kováčik, J.; Blicharski, T. Effect of Long-Term Strontium Exposure on the Content of Phytoestrogens and Allantoin in Soybean. Int. J. Mol. Sci. 2018, 19, 3864. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, G.; Wójciak-Kosior, M.; Sowa, I.; Zapała, K.; Strzemski, M.; Kocjan, R. Evaluation of Isoflavone Content and Antioxidant Activity of Selected Soy Taxa. J. Food Comp. Anal. 2017, 57, 40–48. [Google Scholar] [CrossRef]
- Waqas, M.K.; Akhtar, N.; Mustafa, R.; Jamshaid, M.; Khan, H.M.S.; Murtaza, G. Dermatological and Cosmeceutical Benefits of Glycine max (Soybean) and Its Active Components. Acta Pol. Pharm. 2015, 72, 3–11. [Google Scholar] [PubMed]
- Polito, F.; Marini, H.; Bitto, A.; Irrera, N.; Vaccaro, M.; Adamo, E.B.; Micali, A.; Squadrito, F.; Minutoli, L.; Altavilla, D. Genistein Aglycone, a Soy-Derived Isoflavone, Improves Skin Changes Induced by Ovariectomy in Rats. Br. J. Pharm. 2012, 165, 994–1005. [Google Scholar] [CrossRef]
- Kim, M.-S.; Hong, C.Y.; Lee, S.H. The Phytoestrogenic Effect of Daidzein in Human Dermal Fibroblasts. J. Soc. Cosmet. Sci. Korea 2014, 40, 279–287. [Google Scholar] [CrossRef]
- Huang, C.-C.; Hsu, B.-Y.; Wu, N.-L.; Tsui, W.-H.; Lin, T.-J.; Su, C.-C.; Hung, C.-F. Anti-Photoaging Effects of Soy Isoflavone Extract (Aglycone and Acetylglucoside Form) from Soybean Cake. Int. J. Mol. Sci. 2010, 11, 4782–4795. [Google Scholar] [CrossRef]
- Liu, T.; Li, N.; Yan, Y.; Liu, Y.; Xiong, K.; Liu, Y.; Xia, Q.; Zhang, H.; Liu, Z. Recent Advances in the Anti-Aging Effects of Phytoestrogens on Collagen, Water Content, and Oxidative Stress. Phytother. Res. 2020, 34, 435–447. [Google Scholar] [CrossRef]
- Fedoreyev, S.A.; Inyushkina, Y.V.; Bulgakov, V.P.; Veselova, M.V.; Tchernoded, G.K.; Gerasimenko, A.V.; Zhuravlev, Y.N. Production of Allantoin, Rabdosiin and Rosmarinic Acid in Callus Cultures of the Seacoastal Plant Mertensia Maritima (Boraginaceae). Plant Cell Tiss. Organ Cult. 2012, 110, 183–188. [Google Scholar] [CrossRef]
- Araújo, L.U.; Grabe-Guimarães, A.; Mosqueira, V.C.F.; Carneiro, C.M.; Silva-Barcellos, N.M. Profile of Wound Healing Process Induced by Allantoin. Acta Cir. Bras. 2010, 25, 460–466. [Google Scholar] [CrossRef]
- Dresler, S.; Szymczak, G.; Wójcik, M. Comparison of Some Secondary Metabolite Content in the Seventeen Species of the Boraginaceae Family. Pharm. Biol. 2017, 55, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Sowa, I.; Paduch, R.; Strzemski, M.; Zielińska, S.; Rydzik-Strzemska, E.; Sawicki, J.; Kocjan, R.; Polkowski, J.; Matkowski, A.; Latalski, M.; et al. Proliferative and Antioxidant Activity of Symphytum Officinale Root Extract. Nat. Prod. Res. 2018, 32, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Hanaka, A.; Dresler, S.; Wójciak-Kosior, M.; Strzemski, M.; Kováčik, J.; Latalski, M.; Zawiślak, G.; Sowa, I. The Impact of Long- and Short-Term Strontium Treatment on Metabolites and Minerals in Glycine max. Molecules 2019, 24, 3825. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.J.; Van Scott, E.J. Alpha-Hydroxyacids and Carboxylic Acids. J. Cosmet. Derm. 2004, 3, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.-C.; Yang, J.-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef]
- Yoshiara, L.Y.; Madeira, T.B.; Delaroza, F.; da Silva, J.B.; Ida, E.I. Optimization of Soy Isoflavone Extraction with Different Solvents Using the Simplex-Centroid Mixture Design. Int. J. Food Sci. Nutr. 2012, 63, 978–986. [Google Scholar] [CrossRef]
- Strzemski, M.; Dresler, S.; Podkościelna, B.; Skic, K.; Sowa, I.; Załuski, D.; Verpoorte, R.; Zielińska, S.; Krawczyk, P.; Wójciak, M. Effectiveness of Volatile Natural Deep Eutectic Solvents (VNADESs) for the Green Extraction of Chelidonium Majus Isoquinoline Alkaloids. Molecules 2022, 27, 2815. [Google Scholar] [CrossRef]
- Felix, A.C.S.; Novaes, C.G.; Rocha, M.P.; Barreto, G.E.; Junior, M.F.; Alvarez, L.D.G. An optimized alternative for phenolic compound-extraction of strawberry bagasse agro-industrial residues. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 815–820. [Google Scholar]
- Jacyna, J.; Kordalewska, M.; Markuszewski, M.J. Design of Experiments in Metabolomics-Related Studies: An Overview. J. Pharm. Biomed. Anal. 2019, 164, 598–606. [Google Scholar] [CrossRef]
- Dresler, S.; Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Zagórska-Dziok, M.; Bujak, T.; Skic, K.; Feldo, M.; Hanaka, A.; Wójciak, M.; Sowa, I.; et al. A Design-of-Experiment Approach for Obtaining Symphytum Officinale L. Extracts for Cosmetic Purposes. Ind. Crops Prod. 2023, 199, 116768. [Google Scholar] [CrossRef]
- Hutabarat, L.S.; Greenfield, H.; Mulholland, M. Isoflavones and Coumestrol in Soybeans and Soybean Products from Australia and Indonesia. J. Food Compos. Anal. 2001, 14, 43–58. [Google Scholar] [CrossRef]
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products. Off. J. Eur. Union 2009, L342, 59–209.
- Andres, A.I.; Petron, M.J.; Lopez, A.M.; Timon, M.L. Optimization of Extraction Conditions to Improve Phenolic Content and In Vitro Antioxidant Activity in Craft Brewers’ Spent Grain Using Response Surface Methodology (RSM). Foods 2020, 9, 1398. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Calhelha, R.C.; Barros, L.; Coutinho, J.A.P.; Ferreira, I.C.F.R.; Ferreira, O. Insights on the Extraction Performance of Alkanediols and Glycerol: Using Juglans regia L. Leaves as a Source of Bioactive Compounds. Molecules 2020, 25, 2497. [Google Scholar] [CrossRef] [PubMed]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Propylene Glycol, Tripropylene Glycol, and PPGs as Used in Cosmetics. Int. J. Toxicol. 2012, 31, 245S–260S. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A. Recovery of Biomolecules from Food Wastes—A Review. Molecules 2014, 19, 14821–14842. [Google Scholar] [CrossRef]
- Ganzera, M.; Sturm, S. Recent Advances on HPLC/MS in Medicinal Plant Analysis—An Update Covering 2011–2016. J. Pharm. Biomed. Anal. 2018, 147, 211–233. [Google Scholar] [CrossRef]
- Kumar, B.R. Application of HPLC and ESI-MS Techniques in the Analysis of Phenolic Acids and Flavonoids from Green Leafy Vegetables (GLVs). J. Pharm. Anal. 2017, 7, 349–364. [Google Scholar] [CrossRef]
- Gumułka, P.; Żandarek, J.; Dąbrowska, M.; Starek, M. UPLC Technique in Pharmacy—An Important Tool of the Modern Analyst. Processes 2022, 10, 2498. [Google Scholar] [CrossRef]
- Hsu, B.-Y.; Inbaraj, B.S.; Chen, B.-H. Analysis of Soy Isoflavones in Foods and Biological Fluids: An Overview. J. Food Drug Anal. 2020, 18, 8. [Google Scholar] [CrossRef]
- Daud, A.; Sulistyarti, H.; Retnowati, R.; Ginting, E. High Performance Liquid Chromatography (HPLC) Method for Determination of Isoflavones Content in Shade-Tolerant Soybean Dena I. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 032004. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Poppenberger, B.; Rozhon, W. Quantification of Sugars and Organic Acids in Tomato Fruits. MethodsX 2018, 5, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Dresler, S.; Zubel, R.; Baczewska, I.; Karakuła, M.; Sawicki, J.; Hanaka, A.; Zielińska, S.; Płachno, B.J.; Sowa, I.; Wójciak, M.; et al. Is There Any Direct Link between Hazardous Trace Metals and the Allantoin Content in Some Moss Species? Sci. Total Environ. 2023, 864, 160653. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Du, J.; Li, J.; Li, M. Quantification of the Organic Acids in Hawthorn Wine: A Comparison of Two HPLC Methods. Molecules 2019, 24, 2150. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.J.; Song, Y.H.; Kim, J.Y.; Baiseitova, A.; Lee, K.W.; Kim, K.D.; Park, K.H. Comparative Investigation on Metabolites Changes in Soybean Leaves by Ethylene and Activation of Collagen Synthesis. Ind. Crops Prod. 2020, 154, 112743. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Sowa, I.; Blicharski, T.; Strzemski, M.; Dresler, S.; Szymczak, G.; Wnorowski, A.; Kocjan, R.; Świeboda, R. The Stimulatory Effect of Strontium Ions on Phytoestrogens Content in Glycine max (L.) Merr. Molecules 2016, 21, 90. [Google Scholar] [CrossRef]
- Dresler, S.; Hawrylak-Nowak, B.; Strzemski, M.; Wójciak-Kosior, M.; Sowa, I.; Hanaka, A.; Gołoś, I.; Skalska-Kamińska, A.; Cieślak, M.; Kováčik, J. Metabolic Changes Induced by Silver Ions in Carlina Acaulis. Plants 2019, 8, 517. [Google Scholar] [CrossRef]
- Dritsa, V.; Rigas, F.; Doulia, D.; Avramides, E.J.; Hatzianestis, I. Optimization of Culture Conditions for the Biodegradation of Lindane by the Polypore Fungus Ganoderma Australe. Water Air Soil Pollut. 2009, 204, 19–27. [Google Scholar] [CrossRef]
- Shinde, M.N.; Talware, R.B.; Hudge, P.G.; Joshi, Y.S.; Kumbharkhane, A.C. Dielectric Relaxation and Hydrogen Bonding Studies of 1,3-Propanediol–Dioxane Mixtures Using Time Domain Reflectometry Technique. Pramana J. Phys. 2012, 78, 297–308. [Google Scholar] [CrossRef]
- Horii, K.; Adachi, T.; Matsuda, T.; Tanaka, T.; Sahara, H.; Shibasaki, S.; Ogino, C.; Hata, Y.; Ueda, M.; Kondo, A. Improvement of Isoflavone Aglycones Production Using β-Glucosidase Secretory Produced in Recombinant Aspergillus Oryzae. J. Mol. Catal. B Enzym. 2009, 59, 297–301. [Google Scholar] [CrossRef]
- Harjo, B.; Wibowo, C.; Zhang, E.J.N.; Luo, K.Q.; Ng, K.M. Development of Process Alternatives for Separation and Purification of Isoflavones. Ind. Eng. Chem. Res. 2007, 46, 181–189. [Google Scholar] [CrossRef]
- Genovese, M.I.; Lajolo, F.M. Determinação de isoflavonas em derivados de soja. Food Sci. Technol. 2001, 21, 86–93. [Google Scholar] [CrossRef]
- Rodríguez De Luna, S.L.; Ramírez-Garza, R.E.; Serna Saldívar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar] [CrossRef] [PubMed]
- Daneshfar, A.; Baghlani, M.; Sarabi, R.S.; Sahraei, R.; Abassi, S.; Kaviyan, H.; Khezeli, T. Solubility of Citric, Malonic, and Malic Acids in Different Solvents from 303.2 to 333.2K. Fluid Phase Equilibria 2012, 313, 11–15. [Google Scholar] [CrossRef]
- Oliveira, M.L.N.; Malagoni, R.A.; Franco, M.R. Solubility of Citric Acid in Water, Ethanol, n-Propanol and in Mixtures of Ethanol+water. Fluid Phase Equilibria 2013, 352, 110–113. [Google Scholar] [CrossRef]
- Harrington, E. The Desirability Function. Qual. Control 1965, 21, 494–498. [Google Scholar]
- Lazic, Z.R. Design of Experiments in Chemical Engineering: A Practical Guide; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nizioł-Łukaszewska, Z. Assessment of Cytotoxicity and Antioxidant Properties of Berry Leaves as By-Products with Potential Application in Cosmetic and Pharmaceutical Products. Sci. Rep. 2021, 11, 3240. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant Activity and Phenolic Compounds in 32 Selected Herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Dresler, S.; Hawrylak-Nowak, B.; Kováčik, J.; Pochwatka, M.; Hanaka, A.; Strzemski, M.; Sowa, I.; Wójciak-Kosior, M. Allantoin Attenuates Cadmium-Induced Toxicity in Cucumber Plants. Ecotoxicol. Environ. Saf. 2019, 170, 120–126. [Google Scholar] [CrossRef]
- Gus’kov, E.P.; Shkurat, T.P.; Milyutina, N.P.; Prokof’ev, V.N.; Pokudina, I.O.; Mashkina, E.V.; Timofeeva, I.V. Effect of Allantoin on the Activity of Enzymes Providing Regulation of the ROS-Dependent Status of an Organism. Dokl. Biochem. Biophys. 2001, 379, 239–242. [Google Scholar] [CrossRef]
- Kowalska, G.; Baj, T.; Kowalski, R.; Szymańska, J. Optimization of Glycerol–Water Extraction of Selected Bioactive Compounds from Peppermint and Common Nettle. Antioxidants 2021, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Dresler, S.; Rutkowska, E.; Bednarek, W.; Stanisławski, G.; Kubrak, T.; Bogucka-Kocka, A.; Wójcik, M. Selected Secondary Metabolites in Echium vulgare L. Populations from Nonmetalliferous and Metalliferous Areas. Phytochemistry 2017, 133, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B.; Dresler, S.; Stasińska-Jakubas, M.; Wójciak, M.; Sowa, I.; Matraszek-Gawron, R. NaCl-Induced Elicitation Alters Physiology and Increases Accumulation of Phenolic Compounds in Melissa officinalis L. Int. J. Mol. Sci. 2021, 22, 6844. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.T.; Chew, K.W.; Bui, X.V.; Nguyen, T.D.P.; Le, T.T.A.; Truong, T.M.H.; Show, P.L. Optimization of Isoflavones Extraction from Soybeans Using Full Factorial Design. J. Food Process. Preserv. 2019, 43, e14078. [Google Scholar] [CrossRef]
- Wang, M.; Guo, J.; Qi, W.; Su, R.; He, Z. An Effective and Green Method for the Extraction and Purification of Aglycone Isoflavones from Soybean. Food Sci. Biotechnol. 2013, 22, 705–712. [Google Scholar] [CrossRef]
- Lakshmi, M.C.; Rao, L.J.; Ravi, R.; Raghavarao, K.S.M.S. Extraction and Concentration of Isoflavones from Soybean (Glycine max). Sep. Sci. Technol. 2013, 58, 166–174. [Google Scholar] [CrossRef]
- Zhou, W.-H.; Guo, M.-M.; Bi, Y.-H.; Wang, Z.-Y.; Duan, Z.-Q. Optimization of Extraction Technology of Isoflavones from Soybean Umbilicus. Mod. Food Sci. Technol. 2020, 36, 218–223. [Google Scholar]
- Nan, G.; Gao, Y.; Guo, L.; Meng, X.; Yang, G. Solid-liquid Extraction of Daidzein and Genistein from Soybean: Kinetic Modeling of Influential Factors. Prep. Biochem. 2018, 48, 946–953. [Google Scholar] [CrossRef]




| Isoflavones (mg·g−1 DW) | Allantoin (mg·g−1 DW) | ||
|---|---|---|---|
| Daidzin | |||
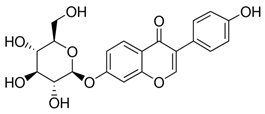 | 0.310 | 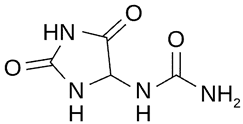 | 5.31 |
| Genistin | |||
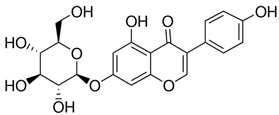 | 0.358 | ||
| Alpha-hydroxy acids (mg·g−1 DW) | |||
| 6″-O-malonyldaidzin | Malic acid | ||
 | 0.719 | 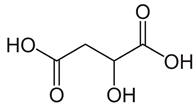 | 13.38 |
| 6″-O-malonylglycitin | Citric acid | ||
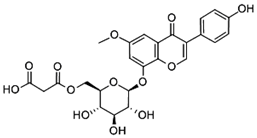 | 0.179 | 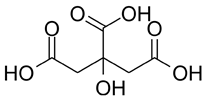 | 7.98 |
| 6″-O-malonylgenistin | |||
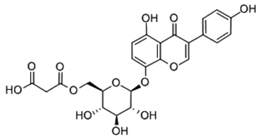 | 0.840 | ||
| Independent Variables | Responses | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EtOH X1 (%) | H2O X2 (%) | Propanediol X3 (%) | Daidzin (%) | Genistin (%) | 6″-O-Malonyldaidzin (%) | 6″-O-Malonylglycitin (%) | 6″-O-Malonylgenistin (%) | Allantoin (%) | Malic Acid (%) | Citric Acid (%) | ABTS Scavenging (%) | Soluble Phenols (mg GAE g−1) |
| 100 | 0 | 0 | 0.9 ± 0.43 | 0.3 ± 0.37 | nd | nd | nd | 19.9 ± 2.73 | 0.8 ± 0.09 | nd | 31.7 ± 0.42 | 1.07 ± 0.24 |
| 0 | 100 | 0 | 12.6 ± 1.35 | 11.8 ± 1.09 | 54.9 ± 1.91 | 37.2 ± 3.14 | 51.5 ± 3.45 | 48.3 ± 3.23 | 51.6 ± 1.89 | 83.5 ± 2.12 | 17.9 ± 0.03 | 4.28 ± 0.48 |
| 0 | 0 | 100 | 26.1 ± 4.32 | 27.7 ± 3.35 | 28.5 ± 2.42 | 22.2 ± 3.11 | 32.8 ± 2.09 | 37.2 ± 1.12 | 6.8 ± 0.76 | nd | 16.6 ± 0.74 | 0.62 ± 0.23 |
| 50 | 50 | 0 | 85.8 ± 0.62 | 90.5 ± 1.22 | 92.8 ± 1.50 | 67.9 ± 4.24 | 71.3 ± 4.21 | 76.4 ± 0.96 | 82.2 ± 5.24 | 91.0 ± 1.41 | 59.4 ± 0.91 | 2.34 ± 0.12 |
| 0 | 50 | 50 | 68.8 ± 2.49 | 74.2 ± 4.83 | 79.4 ± 4.22 | 60.1 ± 5.12 | 88.2 ± 5.26 | 66.5 ± 3.97 | 39.9 ± 2.43 | 49.4 ± 0.81 | 37.8 ± 3.18 | 2.99 ± 0.44 |
| 50 | 0 | 50 | 14.7 ± 4.90 | 16.1 ± 2.52 | 12.5 ± 2.30 | 8.9 ± 2.35 | 14.4 ± 2.45 | 37.6 ± 2.98 | 7.5 ± 0.39 | 1.1 ± 0.31 | 37.5 ± 2.97 | 0.33 ± 0.02 |
| 33.3 | 33.3 | 33.3 | 96.1 ± 1.01 | 94.8 ± 2.04 | 99.1 ± 1.04 | 98.1 ± 1.33 | 95.6 ± 4.06 | 56.6 ± 3.21 | 53.4 ± 2.50 | 32.7 ± 0.89 | 68.3 ± 2.40 | 2.09 ± 0.39 |
| 66.7 | 16.7 | 16.7 | 79.9 ± 0.86 | 52.2 ± 5.30 | 76.3 ± 4.04 | 53.8 ± 2.55 | 79.5 ± 3.26 | 56.2 ± 4.12 | 23.8 ± 0,98 | nd | 60.9 ± 1.34 | 2.33 ± 0.33 |
| 16.7 | 66.7 | 16.7 | 78.2 ± 5.29 | 81.3 ± 5.56 | 89.1 ± 5.37 | 81.6 ± 4.63 | 91.9 ± 0.30 | 64.8 ± 1.79 | 54.7 ± 3.65 | 99.3 ± 5.65 | 48.9 ± 1.48 | 2.80 ± 0.35 |
| 16.7 | 16.7 | 66.7 | 60.9 ± 4.47 | 82.2 ± 1.13 | 56.4 ± 4.45 | 58.3 ± 3.75 | 69.9 ± 6.15 | 52.4 ± 5.03 | 23.7 ± 2.71 | 3.9 ± 0.05 | 42.0 ± 5.42 | 1.52 ± 0.21 |
| Daidzin | Genistin | 6″-O-Malonyldaidzin | 6″-O-Malonylglycitin | 6″-O-Malonylgenistin | Allantoin | Citric Acid | Malic Acid | |
|---|---|---|---|---|---|---|---|---|
| ABTS scavenging | 0.81 *** | 0.77 *** | 0.67 ** | 0.71 *** | 0.58 ** | ns | ns | ns |
| Soluble phenols | ns | ns | 0.61 ** | 0.51 * | 0.58 ** | 0.64 * | 0.76 *** | 0.69 * |
| Response Variables | Predicted Value | Experimental Value | RD (%) |
|---|---|---|---|
| Daidzin (%) | 98.9 | 97.7 ± 2.31 | −1.21 |
| Genistin (%) | 90.9 | 90.7 ± 2.56 | −0.22 |
| 6″-O-Malonyldaidzin (%) | 106.3 | 99.1 ± 0.99 | −6.77 |
| 6″-O-Malonylglycitin (%) | 92.2 | 90.8 ± 3.11 | −1.52 |
| 6″-O-Malonylgenistin (%) | 99.1 | 99.3 ± 1.26 | 0.20 |
| Allantoin (%) | 70.9 | 70.1 ± 1.30 | −1.13 |
| Citric acid (%) | 92.3 | 91.2 ± 1.73 | −1.19 |
| Malic acid (%) | 68.9 | 68.3 ± 0.71 | −0.87 |
| ABTS (mg TE L−1) | 63.2 | 60.6 ± 2.21 | −4.11 |
| Soluble phenols (mg GAE L−1) | 2.52 | 2.30 ± 0.16 | −8.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dresler, S.; Strzemski, M.; Baczewska, I.; Koselski, M.; Hassanpouraghdam, M.B.; Szczepanek, D.; Sowa, I.; Wójciak, M.; Hanaka, A. Extraction of Isoflavones, Alpha-Hydroxy Acids, and Allantoin from Soybean Leaves—Optimization by a Mixture Design of the Experimental Method. Molecules 2023, 28, 3963. https://doi.org/10.3390/molecules28093963
Dresler S, Strzemski M, Baczewska I, Koselski M, Hassanpouraghdam MB, Szczepanek D, Sowa I, Wójciak M, Hanaka A. Extraction of Isoflavones, Alpha-Hydroxy Acids, and Allantoin from Soybean Leaves—Optimization by a Mixture Design of the Experimental Method. Molecules. 2023; 28(9):3963. https://doi.org/10.3390/molecules28093963
Chicago/Turabian StyleDresler, Sławomir, Maciej Strzemski, Izabela Baczewska, Mateusz Koselski, Mohammad Bagher Hassanpouraghdam, Dariusz Szczepanek, Ireneusz Sowa, Magdalena Wójciak, and Agnieszka Hanaka. 2023. "Extraction of Isoflavones, Alpha-Hydroxy Acids, and Allantoin from Soybean Leaves—Optimization by a Mixture Design of the Experimental Method" Molecules 28, no. 9: 3963. https://doi.org/10.3390/molecules28093963
APA StyleDresler, S., Strzemski, M., Baczewska, I., Koselski, M., Hassanpouraghdam, M. B., Szczepanek, D., Sowa, I., Wójciak, M., & Hanaka, A. (2023). Extraction of Isoflavones, Alpha-Hydroxy Acids, and Allantoin from Soybean Leaves—Optimization by a Mixture Design of the Experimental Method. Molecules, 28(9), 3963. https://doi.org/10.3390/molecules28093963









